Abstract
Three patients developed severe ascending aortic aneurysm requiring surgical resection after heart transplantation. In all 3 cases, the donor aorta of the transplant remained normal in caliber, despite the development of a large aneurysm in the native upper ascending aorta. The aneurysmal disease did not cross the suture line. (Level of Difficulty: Advanced.)
Key Words: aorta, dissection, genetics
Abbreviations and Acronyms: TGF, transforming growth factor
Central Illustration
After heart transplantation, two segments of ascending aorta—donor and recipient—of different genetic origins—are exposed to the same physiologic milieu (the patient). This juxtaposition provides a unique opportunity to evaluate the role of genetics versus physiologic milieu in determining the fate of both donor and recipient aortic segments.
Learning Objectives
-
•
To become familiar with the problem of ascending aortic aneurysm in the upper remaining native aorta after heart transplantation.
-
•
To be aware of the importance of following the ascending aorta as cephalad as possible during routine post-transplantation echocardiography.
-
•
To be alert to the fact that some donor hearts may come from patients predisposed to post-transplantation aneurysm formation in the donor segment of aorta.
-
•
To recognize that the underlying genetics of the disparate aortic segments determine the aneurysmal fate, not the biologic milieu.
We report 3 cases of large ascending aneurysm developing only in the recipient aorta, all of which were operated safely for aneurysm resection. We review the scant pertinent literature and explore insights offered by these rare cases. This investigation was approved by the Human Investigation Committee of the Yale University School of Medicine.
Cases
Patient 1
This child was born with hypoplastic left heart syndrome. He underwent heart transplantation as an infant.
He was seen by our aortic team at the age of 14 years. He was 5 feet/5 inches tall and very thin. He was maintained only on tacrolimus for his transplanted heart.
Over the years, he had developed severe aortic enlargement of the native distal ascending aorta, aortic arch, and innominate artery. The aorta grew from 4.5 cm to 6.8 cm over 4 years. The transplanted (proximal) aorta remained normal in size. There was an abrupt transition between the normal donor aorta and the patient’s own diseased aorta, consistent with an inborn native connective tissue disorder (Figure 1).
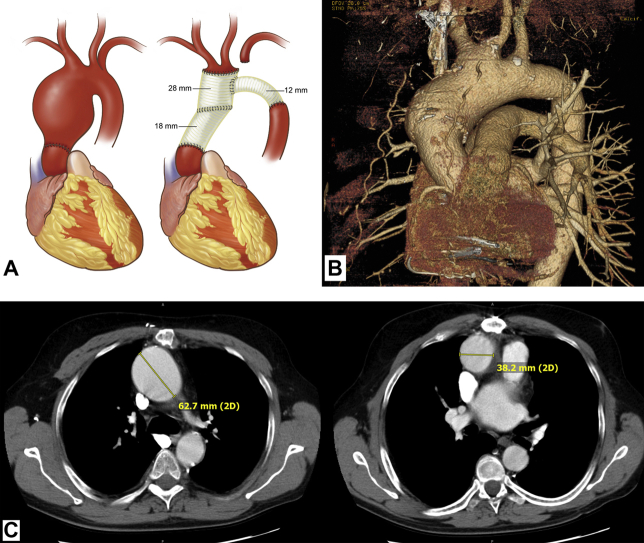
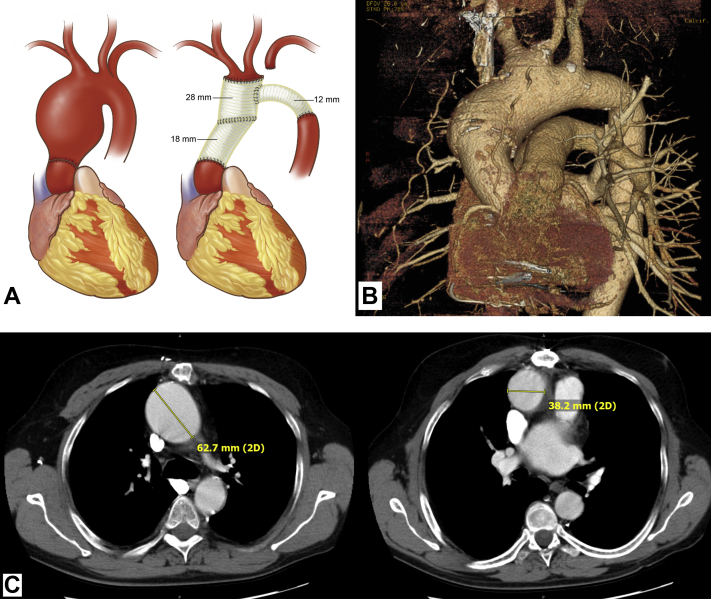
Figure 1.
Post-Transplant Aneurysm Images
(A) Artist’s rendition of pre- and postoperative anatomy of patient 1, based on the surgeon’s intra-operative sketches. (Left) Preoperative. Note abrupt transition at transplant suture line. (Right) Postoperative. (B) Three-dimensional reconstruction of chest computed tomography scan of patient 2, showing abrupt enlargement at suture line of donor aorta, with enlargement extending into aortic arch. Note also the aneurysmal base of the bovine innominate artery. (C) Axial images of patient 3, showing (left) large upper ascending aneurysm (native aorta) and (right) normal-sized proximal aorta (donor). Drawing by Alexandra Webber of DNA Illustrations.
We performed redo cardiac surgery, replacing the ascending aorta from the top of the donor aorta to the aortic arch to the descending aorta under hypothermic circulatory arrest.
The native aorta showed “elastic wall artery with medial myxoid degeneration.”
In this case, the anatomic transition was abrupt between the normal proximal donor aorta and the aneurysmal distal native aorta. The transplanted aorta did not develop aneurysmal dilation despite 14 years of exposure to the patient’s aneurysmal milieu.
Patient 2
This patient underwent heart transplantation (for cardiomyopathy after long-standing bicuspid valve disease) at the age of 42 years, 17 years before his aortic presentation. The native aorta was noted to be enlarged and thin-walled at that time. Ultimately, he developed a large (5.6 cm) aortic arch aneurysm in the native aorta beyond the aortic suture line (Figure 1).
Redo surgery was performed at the age of 59 years under hypothermic circulatory arrest, with resection of the aortic arch and the innominate artery and surgical reimplantation of those vessels.
As in the patient 1, the transition was abrupt between the normal proximal donor aorta and the aneurysmal distal native aorta. The transplanted aorta did not develop aneurysmal dilation despite 17 years of exposure to the patient’s aneurysmal milieu.
Patient 3
This patient underwent heart transplantation 8 years earlier for congenital bicuspid aortic stenosis. He had had 2 previous aortic valve replacements with eventual end-stage heart failure.
His remaining aorta above the transplant grew to 6 cm (Figure 1). As in the patients 1 and 2, the ascending aortic aneurysm was located in the distal native ascending aorta, above the aortic suture line. The demarcation at the suture line was sharp and dramatic. Of note, the aortic arch was of bovine configuration.
At the patient’s age of 44 years, we performed re-re-redo sternotomy and resection of the remnant ascending aorta with deep hemiarch replacement. The proximal transplant ascending aorta remained mildly dilated at 4.3 cm over the remaining 7 years of his life.
As in patients 1 and 2, the anatomic transition was abrupt, with severe dilation confined to the native aorta above the transplanted heart’s aorta.
Discussion
Improved long-term survival after heart transplantation has permitted later complications to emerge.
Rupture of abdominal aortic aneurysms after heart or other transplantation is well known (1,2). Here we discuss the less commonly seen ascending aortic aneurysms after heart transplantation.
Our 3 cases (Table 1) demonstrated severe dilation of the native upper ascending aorta (above the aortic suture line), which had developed over the years after heart transplantation, sharply demarcated at the anastomotic line between the transplanted heart’s aorta and the native recipient aorta.
Table 1.
Literature Cases of Ascending Aortic Aneurysm or Dissection After Heart Transplantation (13, 14, 15, 16, 17, 18, 19, 20)
| Patient | First Author (Study Year) | Dilated Native or Donor Aorta? | Age at Transplantation, y | Age at Aneurysm Surgery, y | Sex | Aortic Size, cm | Post-Transplantation Exposure, y | Comments |
|---|---|---|---|---|---|---|---|---|
| This paper | ||||||||
| 1 | Anis (2021) | Native | 1 | 14 | M | 6.8 | 13 | CMN |
| 2 | Anis (2021) | Native | 42 | 59 | M | 17 | Arch replacement | |
| 3 | Anis (2021) | Native | 36 | 44 | M | 6 | 8 | 4th open heart surgery; bicuspid AS originally |
| Literature review | ||||||||
| 4 | d’Addese (2019) | Native | 6 wk | 17 (aortic dissection) | F | 7.5 | 17 | CMD; mutation in MYBPC3 (infantile CMP) |
| 5 | Pozzi (2018) | Transplant | 42 | 58 (aortic dissection) | M | 9 | 16 | CMD; macrophages in adventitia |
| 6 | Fukuhara (2016) | Native | 54 | 73 | M | 5.5 | 19 | |
| 7 | Fukuhara (2016) | Native | 54 | 67 | F | 5.2 | 13 | |
| 8 | Korkut (2003) | Transplant | 68 (aortic dissection) | M | 7 | Intimal fibrosis, medial atrophy; donor died of cerebral bleed | ||
| 9 | Chen (2008) | Transplant | 64 | 67 | M | 7.0 | 3 | CMD; marfanoid changes; donor tall, thin; donor aorta slightly dilated at transplant (3.5 cm) |
| 10 | Stephens (2017) | Transplant | 48 | 60 | F | 4.8 | 12 | Donor BAV |
| 11 | Vigano (1999) | Native | 57 | 59 | M | 10 | 2 | |
| 12 | Bojko (2019) | Native | 72 | 50 | M | 7.2 | 22 | Prior AVR for BAV; true aneurysm entirely in native tissue, not at suture line. |
Infectious aortic lesions, anastomotic pseudoaneurysms, and heterotopic heart transplant cases are excluded.
AS = aortic stenosis; AVR = aortic valve replacement; BAV = bicuspid aortic valve; CMD = cystic medial degeneration; CMN = cystic medial necrosis; CMP = cardiomyopathy.
After transplantation, the donor aorta is exposed to the same biologic environment as the native aorta. We know that a complex molecular interplay has been implicated in thoracic aortic aneurysm development, including up-regulation of transforming growth factor (TGF)-β, abnormal mechanosensing/transduction, and matrix metalloproteinase/tissue inhibitor of matrix metalloproteinases dysregulation (3). It is also clear that aortic aneurysm is part of a broader systemic process, as in Marfan, Ehlers-Danlos, and Loeys-Dietz diseases. Even nonsyndromic aneurysm patients often show systemic skin, joint, and skeletal irregularities. Serologic changes indicate systemic alterations in these pathways throughout the body, not just in the aorta (eg, altered RNA profiles [4]). Thoracic aortic aneurysm patients often harbor renal and hepatic cysts, with matrix metalloproteinases “dissolving” organ substance and leaving lacunae in their wake (5, 6, 7, 8, 9). After transplantation, the donor aorta is exposed to the same abnormal aneurysm-related systemic milieu as the native aorta, a milieu that produced an aneurysm in the native aorta.
But in our cases, the donor aorta did not dilate, despite exposure to the same “aneurysmogenic” biologic environment. The aneurysm disease does not cross the suture line to affect the transplanted heart, even when the native aneurysm has progressed to a high level of severity.
This suggests that the original genetics of aortic tissue origin dominate, above and beyond any impact of the abnormal biologic milieu. This interpretation is supported by other published studies, most describing single illustrative cases (Table 1). In those literature cases also, the aneurysm does not cross the suture line to affect the donor aorta.
Among the cases in the literature, there are several in which the donor aorta goes on to develop an aortic root pathology. In most of those cases, there are clues suggesting donor predisposing factors for aneurysm formation (eg, bicuspid aortic valve, cerebral bleed, and marfanoid features). Once again, the aneurysm disease does not cross the suture line, as the native aortas remain normal in those cases. Again, the innate genetic background of the aorta, not its post-transplantation milieu, appears to dominate.
This experience has implications for post-transplantation echocardiographic follow-up of the heart transplant patient. Because the lower (transplant) ascending aorta is normal in those patients with late dilation of the upper ascending (native) aorta, these upper ascending aneurysms can easily escape detection by echocardiography (10). In post-transplantation cardiac echocardiography, the echographer should make every effort to visualize the aortic anastomosis and the remnant native aorta, if possible. Occasional 3-dimensional imaging by computed tomography or magnetic resonance imaging should be considered as well.
Immunosuppressants may play a role in the development of these post-transplantation aneurysms. Many aortic surgeons feel that steroids are injurious to the aortic wall (11,12). Also, some have postulated that discrepant mechanical properties between recipient and donor aortas may engender unbalanced mechanical forces at the suture line, leading ultimately to aneurysmal degeneration (13).
The thrust of this report is that genes dominate over biologic environment in aneurysm disease after cardiac transplantation.
Funding Support and Author Disclosures
Dr Elefteriades is a principal of Coolspine; on a data and safety monitoring board for Terumo; and a consultant for CryoLife. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Cron D.C., Coleman D.M., Sheetz K.H., Englesbe M.J., Waits S.A. Aneurysms in abdominal organ transplant recipients. J Vasc Surg. 2014;59:594–598. doi: 10.1016/j.jvs.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 2.Englesbe M.J., Wu A.H., Clowes A.W., Zierler R.E. The prevalence and natural history of aortic aneurysms in heart and abdominal organ transplantation. J Vasc Surg. 2002;37:27–31. doi: 10.1067/mva.2003.57. [DOI] [PubMed] [Google Scholar]
- 3.Elefteriades J.A., Ziganshin B.A., Halperin J.L. In: The Heart. 15th ed. Fuster V., Narula J., Vaishnava P., Leon M.B., Callans D.J., Rumsfeld J.S., Hurst’s, editors. McGraw-Hill; 2021. Diseases of the aorta. [in press] [Google Scholar]
- 4.Wang Y., Barbacioru C.C., Shiffman D. Gene expression signature in peripheral blood detects thoracic aortic aneurysm. PLoS One. 2007;17 doi: 10.1371/journal.pone.0001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziganshin B.A., Theodoropoulos P., Salloum M.N. Simple renal cysts as markers of thoracic aortic disease. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges L.F., Gomez D., Quintana M. Fibrinolytic activity is associated with presence of cystic medial degeneration in aneurysms of the ascending aorta. Histopathology. 2010;57:917–932. doi: 10.1111/j.1365-2559.2010.03719.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu B., Li C., Liu Z., Dai Z., Tao Y. Increasing extracellular matrix collagen level and MMP activity induces cyst development in polycystic kidney disease. BMC Nephrol. 2012;13:109. doi: 10.1186/1471-2369-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurihara T., Shimizu-Hirota R., Shimoda M. Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation. 2012;126:3070–3080. doi: 10.1161/CIRCULATIONAHA.112.097097. [DOI] [PubMed] [Google Scholar]
- 9.Georgiadis G.S., Antoniou G.A., Argyriou C. Correlation of baseline plasma and inguinal connective tissue metalloproteinases and their inhibitors with late high-pressure endoleak after endovascular aneurysm repair: long-term results. J Endovasc Ther. 2019;26:826–835. doi: 10.1177/1526602819871963. [DOI] [PubMed] [Google Scholar]
- 10.Elefteriades J.A., Mukherjee S.K., Mojibian H. Discrepancies in measurement of the thoracic aorta. J Am Coll Cardiol. 2020;76:201–217. doi: 10.1016/j.jacc.2020.03.084. [DOI] [PubMed] [Google Scholar]
- 11.Tajima Y., Goto H., Ohara M. Oral steroid use and abdominal aortic expansion—positive association. Circ J. 2017:1774–1782. doi: 10.1253/circj.CJ-16-0902. [DOI] [PubMed] [Google Scholar]
- 12.Smith D.C., Hirst A.E. Spontaneous aortic rupture associated with chronic steroid therapy for rheumatoid arthritis in two cases. AJR Am J Roentgenol. 1979;132:271–273. doi: 10.2214/ajr.132.2.271. [DOI] [PubMed] [Google Scholar]
- 13.Vagano M., Rinaldi M., D’Armini A.M. The spectrum of aortic complications after heart transplantation. Ann Thorac Surg. 1999;68:105–111. doi: 10.1016/s0003-4975(99)00471-3. [DOI] [PubMed] [Google Scholar]
- 14.d’Addese L., Komarlu R., Zahka K. Incidental finding of type A aortic dissection in a paediatric heart transplant recipient. Cardiol Young. 2019;29 doi: 10.1017/S1047951119001811. 1219-1212. [DOI] [PubMed] [Google Scholar]
- 15.Pozzi M., Hanna S., Sebbag L., Obadia J.F. Donor aortic dissection in a heart transplantation recipient. Interact Cardiovasc Thorac Surg. 2018;27:790–791. doi: 10.1093/icvts/ivy153. [DOI] [PubMed] [Google Scholar]
- 16.Fukuhara S., Stephens E.H., Glotzbach J.P., Borger M.A. Repair of ascending aortic aneurysms following cardiac transplantation. J Cardiac Surg. 2016;31:778–780. doi: 10.1111/jocs.12865. [DOI] [PubMed] [Google Scholar]
- 17.Korkut A.K., Wellens F., Foubert L., Goethals M. Successful treatment of acute dissection of the donor aorta after orthotopic heart transplantation. J Heart Lung Transplant. 2003;22:701–704. doi: 10.1016/s1053-2498(02)01151-8. [DOI] [PubMed] [Google Scholar]
- 18.Chen I.C., Wei J., Chang C.Y. Successful treatment of aortic root aneurysm after orthotopic heart transplantation: case report. Transplant Proc. 2008;40:2852–2853. doi: 10.1016/j.transproceed.2008.08.054. [DOI] [PubMed] [Google Scholar]
- 19.Stephens E.H., Fukuhara S., Neely R.C., Takayama H. Aortic root replacement for bicuspid aortopathy following heart transplantation. J Cardiac Surg. 2017;32:667–669. doi: 10.1111/jocs.13215. [DOI] [PubMed] [Google Scholar]
- 20.Bojko M., Eisen H., Mather P., Vallabhajosyula P. Delayed aneurysmal complication of bicuspid aortic valve disease after heart transplantation. J Thorac Cardiovasc Surg. 2019;158:e185–e186. doi: 10.1016/j.jtcvs.2019.02.113. [DOI] [PubMed] [Google Scholar]