Abstract
Aim:
To compare a glucagon-like peptide-1 receptor agonist with basal insulin at hospital discharge in patients with uncontrolled type 2 diabetes in a randomized clinical trial.
Methods:
A total of 273 patients with glycated haemoglobin (HbA1c) 7%–10% (53–86 mol/mol) were randomized to liraglutide (n = 136) or insulin glargine (n = 137) at hospital discharge. The primary endpoint was difference in HbA1c at 12 and 26 weeks. Secondary endpoints included hypoglycaemia, changes in body weight, and achievement of HbA1c <7% (53 mmol/mol) without hypoglycaemia or weight gain.
Results:
The between-group difference in HbA1c at 12 weeks and 26 weeks was −0.28% (95% CI −0.64, 0.09), and at 26 weeks it was −0.55%, (95% CI −1.01, −0.09) in favour of liraglutide. Liraglutide treatment resulted in a lower frequency of hypoglycaemia <3.9 mmol/L (13% vs 23%; P = 0.04), but there was no difference in the rate of clinically significant hypoglycaemia <3.0 mmol/L. Compared to insulin glargine, liraglutide treatment was associated with greater weight loss at 26 weeks (−4.7 ± 7.7 kg vs −0.6 ± 11.5 kg; P < 0.001), and the proportion of patients with HbA1c <7% (53 mmol/mol) without hypoglycaemia was 48% versus 33% (P = 0.05) at 12 weeks and 45% versus 33% (P = 0.14) at 26 weeks in liraglutide versus insulin glargine. The proportion of patients with HbA1c <7% (53 mmol/mol) without hypoglycaemia and no weight gain was higher with liraglutide at 12 (41% vs 24%, P = 0.005) and 26 weeks (39% vs 22%; P = 0.014). The incidence of gastrointestinal adverse events was higher with liraglutide than with insulin glargine (P < 0.001).
Conclusion:
Compared to insulin glargine, treatment with liraglutide at hospital discharge resulted in better glycaemic control and greater weight loss, but increased gastrointestinal adverse events.
Keywords: algorithm, glargine, GLP-1RA, hospital discharge, hospital hyperglycaemia, inpatient hyperglycaemia, type 2 diabetes
1 |. INTRODUCTION
Diabetes has reached epidemic proportions, with an estimated 30.4 million people affected in the United States and over 463 million worldwide.1,2 A number of studies have demonstrated that people with diabetes have hospital admission rates between two and six times higher than people without diabetes.3 In addition, several studies have reported that diabetes increases the risk of hospitalization and unplanned readmission, imposing a substantial burden on patients, caregivers, health systems, and the economy.4
The transition period after hospital discharge represents a period of risk for patients with diabetes due to the presence of coexisting chronic conditions, and the development of severe hyperglycaemia and/or iatrogenic hypoglycaemia, all of which may lead to increased readmission rates.5,6 Clinical guidelines and recent prospective studies have recommended using glycated haemoglobin (HbA1c) levels to tailor discharge treatment for patients with diabetes.7–9 At discharge, patients with HbA1c levels between 7% and 9% (53 – 74.9 mmol/mol) should restart preadmission antidiabetic therapy with the addition of basal insulin at 50% of the hospital daily dose. For patients with HbA1c >9% (74.9 mmol/mol), it is recommended to restart oral agents with 80% of the basal daily dose or discharge on a basal-bolus insulin regimen. In the Basal Plus Discharge trial,10 this strategy resulted in a ~ 1.5% to 2.5% reduction in HbA1c at 12 weeks of follow-up; however, it was associated with a high rate of postdischarge hypoglycaemia reported in 25% of patients on basal insulin alone, 30% on the combination of oral antidiabetic drugs (OADs) plus basal insulin, and 44% on a basal-bolus insulin regimen.10
Several ambulatory randomized controlled trials (RCTs) have compared the safety and efficacy of various daily and weekly glucagon-like peptide-1 receptor agonists (GLP-1RAs) and basal insulin for the management of patients with type 2 diabetes (T2D).11–13 These studies have shown that the use of GLP-1RAs results in similar improvement in HbA1c, with lower rates of hypoglycaemia and less weight gain, compared to use of basal insulin.11–13 In the hospital setting, our group recently reported the safety and efficacy of GLP-1RAs in general medicine and surgery patients with T2D,14 and reported comparable improvement in glycaemic control with lower rates of hypoglycaemia than basal-bolus insulin. Based on the available evidence, we hypothesized that the use of liraglutide, a daily GLP-1RA agent, could represent a valid alternative to the use of insulin treatment in patients with poorly controlled T2D. Accordingly, we conducted a prospective RCT to compare the safety and efficacy of liraglutide and basal insulin after hospital discharge in general medicine and surgery patients with T2D.
2 |. RESEARCH DESIGN AND METHODS
This multicentre, prospective, noninferiority open-label randomized study was conducted at four academic institutions in the United States, including: Emory University, Atlanta, Georgia; the Metro Health Medical Centre, Cleveland, Ohio; the State University of New York at Buffalo; the University of Miami, Miami, Florida; and the Cemediab Medical Center, in Buenos Aires, Argentina. The institutional review boards at Emory University and participating institutions approved the study protocol. Informed consent was obtained from all participants during hospitalization prior to discharge. This trial is registered with clinicaltrials.gov: NCT01919489.
2.1 |. Participant recruitment
We screened patients with T2D during their hospital admission from medical or general surgical services. We enrolled patients with glucose levels between 7.8 and 22.2 mmol/L and HbA1c levels >7% to 10%, aged 18 to 80 years, whose home diabetes regimen included diet and/or OADs including sulphonylureas, repaglinide, nateglinide, or metformin, either as monotherapy or in combination therapy, or taking a low total daily dose of insulin (≤0.4 unit/kg/d). Owing to the inpatient enrolment used in this study, there was no run-in period. We excluded patients with a history of diabetic ketoacidosis or hyperosmolar hyperglycaemic state or with laboratory evidence of ketoacidosis,15 patients with history of type 1 diabetes, medullary thyroid cancer or multiple endocrine neoplasia, acute or chronic pancreatitis, pancreatic cancer or gallbladder disease or previous treatment with GLP-1RAs during the past 3 months prior to admission. We also excluded patients admitted to or expected to require admission to an intensive care unit, corticosteroid therapy >5 mg/d of prednisone equivalent, clinically relevant hepatic disease or impaired renal function (estimated glomerular filtration rate <30 mL/min per 1.73 m2), body mass index (BMI) < 25 and > 45 kg/m2, pregnancy, parenteral nutrition, immunosuppressive treatment, or mental condition rendering the participant unable to understand the nature, scope and possible consequences of the study.
2.2 |. Randomization procedure and study protocol
During the hospital stay, patients were managed with a standard basal or basal-bolus regimen according to hospital protocol.9,16 Participants were randomized to receive liraglutide or insulin glargine at the time of discharge, either as monotherapy in treatment-naïve patients or as add-on therapy to the participant’s preadmission diabetes regimen (Appendix, Table S1). Randomization tables for each site were created by Dr Limin Peng, the statistician for the group based at Emory University School of Public Health. Randomization was allocated by a research pharmacist at each site who was not involved in enrolment.
Liraglutide was started at 0.6 mg once daily by subcutaneous injections, with dose escalation every 2 weeks by increments of 0.6 mg until the maintenance dose of 1.8 mg was reached. Injections were given at any time of the day and irrespective of meals; however, it was recommended that the time of injection be consistent throughout the trial. Dose escalation was extended over 4 weeks at the discretion of the investigator in case of adverse events. Treatment-naïve patients with HbA1c < 8% (<64 mmol/mol) received liraglutide as monotherapy or in combination with metformin if HbA1c was > 8% (> 64 mmol/mol). Liraglutide therapy was given as add-on to the participant’s preadmission OAD regimen. The dose of insulin secretagogues (sulphonylureas, nateglinide and repaglinide) was reduced by 50% or stopped at the discretion of the investigator, otherwise, the dose of OAD remained unchanged throughout the trial.
Insulin glargine U-100/mL was provided in 3-mL pen cartridges. Treatment-naïve patients with HbA1c < 8% (<64 mmol/mol) received glargine as monotherapy or in combination with metformin if HbA1c was >8% (> 64 mmol/mol). Patients who were treated with OADs prior to admission with HbA1c between 7% and 9% (53 – 75 mmol/mol) were discharged on their preadmission OADs in combination with glargine at 50% of the hospital daily dose. Patients with HbA1c > 9% (≫ 75 mmol/mol) were discharged on 80% of the hospital glargine dose. Dipeptidyl peptidase-4 inhibitors were not used in combination with liraglutide during the study period. The total daily dose of insulin secretagogues was reduced by 50% or stopped at the discretion of the investigator.
After discharge, a member of the diabetes research team contacted patients via telephone call every 2 weeks to assess response to therapy and titrate study medications. Patients were asked to attend an outpatient clinic visit at 4, 12 and 26 weeks after hospital discharge. Data were collected during research visits in an electronic Health Insurance Portability and Accountability Act -compliant platform.
2.3 |. Measured outcomes
The study objective was to compare the efficacy and safety of liraglutide versus insulin glargine with regard to glycaemic control in general medicine and surgical patients with T2D after hospital discharge.
The primary efficacy endpoint of the study was change in HbA1c concentration at 12 and 26 weeks from discharge. Secondary endpoints were differences between treatment groups after hospital discharge in fasting and postprandial glucose concentration, incidence rate and number of hypoglycaemic events (3.9 mmol/L [<70 mg/dL]), clinically significant (<3 mmol/L [54 mg/dL]) and severe hypoglycaemic events (<2.2 mmol/L [40 mg/dL]), proportion of patients with 12- and 26-week HbA1c level <7.0% and no hypoglycaemia <3.9 mmol/L (<70 mg/dL), and proportion of patients with 12- and 26-week HbA1c <7.0%, no hypoglycaemia and no weight gain.
In addition, we compared differences between groups in cardiovascular risk factors including changes in blood pressure, heart rate, and lipid profile between treatment groups, as well as total daily dose of insulin, change in body weight and BMI, development of acute kidney injury, defined as a clinical diagnosis of acute renal failure with documented new-onset abnormal renal function (increment in creatinine ≥0.5 mg/dL (44.2 umol/L) from baseline), and number of emergency room visits and hospital readmissions.
2.4 |. Statistical analysis
The overall hypothesis was that patients with T2D discharged on liraglutide and glargine will experience similar improvement in glycaemic control (HbA1c level) at 12 and 26 weeks after discharge. To show the noninferiority of liraglutide to basal glargine insulin in terms of glycaemic control, we set the equivalence margin at 0.5%, given that an HbA1c difference < 0.5% is usually not considered clinically significant. Based on preliminary Basal Plus Discharge Trial results,8 we assumed a standard deviation of 26-week HbA1c to be bounded approximately 1.5%. We set the margin of equivalence as 0.5% and assumed the true difference between mean HbA1c to be 0. A sample size of 124 for each treatment group would achieve 80% power to reject the hypothesis that the mean HbA1c in patients treated with liraglutide is <0.5% more than that in patients treated with insulin glargine, based on a two-sample one-sided t-test, with α = 0.05.
The primary endpoint was difference in HbA1c at 12 and 26 weeks. Secondary endpoints included hypoglycaemia, changes in body weight, and achievement of HbA1c <7% without hypoglycaemia or weight gain. Nonparametric Wilcoxon tests were used to compare continuous outcomes, such as HbA1c at 12 and 26 weeks, between the liraglutide and insulin glargine groups. Chi-squared tests or Fisher’s exact tests were used to compare binary or categorical outcomes, such as the occurrence of hypoglycaemia. Normal approximations were used to compute the 95% confidence intervals (CIs) of the outcome differences between the two treatment groups. P values <0.05 were taken to indicate statistical significance. We performed statistical analyses using SAS 9.4 software.
3 |. RESULTS
A total of 306 patients gave consent to participate, of whom 19 were screen failures, 140 were randomized to liraglutide and 147 were randomized to insulin glargine. A total of 273 patients were included the intention-to-treat analysis, 136 patients in the liraglutide group and 137 in the basal insulin group. After randomization, in the liraglutide group, four patients withdrew consent to participation due to the need for injections, 10 patients withdrew as a result of gastrointestinal adverse events, two patients withdrew for hospital admissions, there were two deaths, and 30 patients were lost to follow-up at 12 weeks and eight patients at 26 weeks. In the insulin glargine group, seven patients discontinued participation due the need for daily injections, nine patients discontinued due to hospital readmission, there was one death, and 20 patients were lost to follow-up at 12 weeks and seven patients at 26 weeks (Appendix, Figure S1).
There were no significant differences between groups with regard to clinical characteristics at randomization including age, BMI, body weight or duration of diabetes or diabetes treatment prior to admission.
Clinical characteristics and demographics in both groups are shown in Table 1. More patients were recruited from medicine services (87%) than surgery services (13%). Prior to admission, most patients in both groups were treated with OADs or OADs plus insulin therapy (Table 1).
TABLE 1.
Baseline clinical characteristics of the intention-to-treat population
| Characteristic | Liraglutide | Glargine | P |
|---|---|---|---|
| Age, years (liraglutide, n = 136; insulin glargine, n = 137) | 56.1 ± 9.5 | 55.9 ± 11.2 | 0.93 |
| Weight, kg (liraglutide, n = 136; insulin glargine, n = 137) | 101.0 ± 20.6 | 98.2 ± 18.0 | 0.24 |
| BMI, kg/m2 (liraglutide, n = 136; insulin glargine, n = 137) | 33.5 ± 5.3 | 33.3 ± 5.3 | 0.79 |
| Sex, n (%) | 0.09 | ||
| Female | 47 (35) | 61 (45) | |
| Male | 89 (65) | 76 (55) | |
| Race, n (%) | 0.83 | ||
| Black | 97 (71) | 95 (69) | |
| White | 23 (17) | 27 (20) | |
| Other | 16 (12) | 15 (11) | |
| Duration of diabetes, years (liraglutide, n = 135; insulin glargine, n = 137) | 9.5 ± 7.8 | 9.8 ± 9.1 | 0.77 |
| Hospital service, n (%) | 0.35 | ||
| Medicine | 116 (85%) | 122 (89%) | |
| Surgery | 20 (15) | 15 (11) | |
| Treatment prior to admission, n (%) | 0.90 | ||
| No diabetes medication | 22 (16) | 24 (18) | |
| Oral agents | 61 (45) | 69 (51) | |
| Insulin | 22 (16) | 19 (14) | |
| Oral agents + insulin | 30 (22) | 23 (17) | |
| Admission HbA1c, % (mmol/mol) | 8.3 ± 0.9 | 8.4 ± 0.8 | 0.41 |
| 67.2 ± −13.6 | 68.3 ± −14.7 | ||
| Admission blood glucose, mmol/L (liraglutide, n = 136; insulin glargine, n = 136) | 11.05 ± 3.7 | 10.77 ± 3.5 | 0.38 |
| Randomization blood glucose, mmol/L (liraglutide, n = 134; insulin glargine, n = 131) | 10.3 ± 3.1 | 10.1 ± 3.0 | 0.68 |
| Median (range) length of stay, days | 4.0 (1.0, 58) | 4.0 (1.0, 33) | 0.35 |
Note: Data are means ± SD, unless otherwise indicated.
Abbreviations: BMI, body mass index; HbA1c, glycated haemoglobin.
The mean daily dose of glargine at hospital discharge was 16 ± 7.9 U/d, and increased to 18 ± 9.8 U/d at 12 weeks and to 21 ± 11.1 U/d at 26 weeks. All patients in the liraglutide group were discharged on an initial daily dose of 0.6 mg. The mean daily dose of liraglutide was 1.53 ± 0.40 mg/d at 12 weeks and 1.65 ± 0.32 mg/d at 26 weeks. Among patients treated with OADs prior to admission, 140 (77%) were discharged on metformin, 17 (9%) were discharged on sulphonylureas, and no patients were discharged on thiazolidinediones. During follow-up, nine patients in the liraglutide group received additional insulin therapy as basal insulin (n = 5), pre-mixed insulin (n = 1) or prandial insulin (n = 3). Among those discharged on prandial insulin, three patients received added prandial (basal-bolus) regimen.
The primary endpoint analysis showed that mean HbA1c levels significantly decreased from baseline to end-of-study period in both treatment groups. The admission HbA1c in the liraglutide group was 8.3 ± 0.9% and decreased to 7.14 ± 1.3% at 12 weeks and 7.12 ± 1.3% at 26 weeks of follow-up. In the insulin glargine group, the admission HbA1c was 8.40 ± 0.8% and decreased to 7.42 ± 1.2% at 12 weeks and 7.68 ± 1.6% at 26 weeks of follow-up (Table 2). The between-group difference (liraglutide vs insulin glargine) at 12 weeks and 26 weeks was −0.28% (95% CI −0.64, 0.09), and −0.55% (95% CI −1.01, −0.09), respectively.
TABLE 2.
Primary and secondary outcomes at baseline, 12 and 26 weeks of follow-up*
| Outcome | Liraglutide | Glargine | P |
|---|---|---|---|
| HbA1c, % | |||
| 12 weeks (liraglutide, n = 88, 100) | 7.1 ± 1.3 | 7.4 ± 1.2 | 0.04 |
| 26 weeks (liraglutide, n = 80, 93) | 7.13 ± 1.3 | 7.68 ± 1.69 | 0.016 |
| Fasting blood glucose, mmol/L | |||
| 12 weeks (liraglutide, n = 60; insulin glargine, n = 79) | 7.96 ± 3.3 | 7.70 ± 2.6 | 0.40 |
| 26 weeks (liraglutide, n = 53; insulin glargine, n = 60) | 7.61 ± 2.2 | 8.56 ± 3.8 | 0.21 |
| Postprandial blood glucose, mmol/L | |||
| 12 weeks (liraglutide, n = 44; insulin glargine, n = 42) | 7.67 ± 1.6 | 9.32 ± 2.8 | 0.002 |
| 26 weeks | 8.23 ± 2.8 | 8.72 ± 2.3 | 0.21 |
| Body weight change from baseline, kg | |||
| 12 weeks (liraglutide, n = 87; insulin glargine, n = 89) | −1.1 ± 16 | −1.3 ± 7.8 | 0.09 |
| 26 weeks (liraglutide, n = 73; insulin glargine, n = 91) | −4.77 ± 8 | +0.6 ±11 | <0.01 |
| Hypoglycaemia, n (%) | |||
| <3.88 mmol/L, n (%) | 18 (13) | 31 (23) | 0.038 |
| <3.0 mmol/L, n (%) | 1 (1) | 4 (3) | 0.37 |
| <2.2 mmol/L, n (%) | 2 (1) | 3 (2) | 0.68 |
| HbA1c < 7% (53 mmol/mol) without hypoglycaemia, n (%) | |||
| 12 weeks | 40 (48) | 31 (33) | 0.05 |
| 26 weeks | 34 (45) | 29 (33) | 0.14 |
| HbA1c < 7% (53 mmol/mol) without weight gain, n (%) | |||
| 12 weeks | 40 (45) | 24 (25) | 0.005 |
| 26 weeks | 32 (41) | 21 (23) | 0.01 |
| HbA1c < 7% (53 mmol/mol) without hypoglycaemia and no weight gain, n (%) | |||
| 12 weeks | 35 (41) | 23 (24) | 0.02 |
| 26 weeks | 30 (39) | 20 (22) | 0.014 |
| Systolic BP, mm Hg | |||
| At discharge (liraglutide, n = 104; insulin glargine, n = 112) | 134 ± 17 | 130 ± 16 | 0.027 |
| 12 weeks (liraglutide, n = 86; insulin glargine, n = 89) | 135 ± 22 | 134 ± 21 | 0.47 |
| 26 weeks (liraglutide, n = 73; insulin glargine, n = 90) | 136 ± 22 | 134.9 ± 19 | 0.86 |
| Heart rate, beats/min | |||
| At discharge (liraglutide, n = 104; insulin glargine, n = 112) | 79 ± 14 | 79 ± 14 | 0.99 |
| 12 weeks (liraglutide, n = 86; insulin glargine, n = 87) | 82 ± 12 | 77.4 ± 13 | 0.004 |
| 26 weeks (liraglutide, n = 73; insulin glargine, n = 90) | 83 ± 13 | 79 ± 14 | 0.06 |
| Adverse events, n (%) | |||
| Nausea | 37 (46) | 3 (4) | <0.01 |
| Vomiting | 18 (24) | 0 (0) | <0.01 |
| Medication discontinued due to AEs | 13 (10) | 0 (0) | <0.001 |
| Emergency department visit | 31 (23) | 23 (17) | 0.24 |
| Congestive heart failure | 12 (9) | 13 (9) | 0.85 |
| Acute myocardial infarction | 0 (0) | 1 (1) | >0.99 |
| Acute kidney injury | 1 (1) | 3 (2) | 0.62 |
| Cerebrovascular event | 0 (0) | 3 (2) | 0.25 |
| Readmission | 35 (26) | 43 (31) | 0.30 |
| Death | 2 (1) | 1 (1) | 0.62 |
Note: Data are expressed as mean ± SD, unless otherwise indicated.
Abbreviations: AE, adverse event; BP, blood pressure; HbA1c, glycated haemoglobin.
The mean fasting glucose was 7.7 ± 2.2 mmol/L at discharge, 7.96 ± 3.3 mmol/L at 12 weeks and 7.61 ± 2.2 mmol/L at 26 weeks of follow-up in the liraglutide group, and 7.55 ± 2.2 mmol/L at discharge, 7.70 ± 2.6 mmol/L at 12 weeks and 8.56 ± 3.8 mmol/L at 26 weeks of follow-up in the glargine group (all P = nonsignificant).
Patients performed 2-hour postprandial glucose testing while on therapy, which was performed on the day prior to the 12- and 26-week follow-up visits. A lower postprandial blood glucose was observed at 12 weeks of follow-up in the liraglutide group than in the insulin glargine group: 7.67 ± 1.6 versus 9.32 ± 2.8 mmol/L (P = 0.002). A trend towards lower postprandial glucose levels continued at 26 weeks in the liraglutide group (8.23 vs 8.72 mmol/L; P = 0.21), although this was not significant.
Treatment with liraglutide was associated with a lower number of hypoglycaemic events (< 3.9 mmol/L) during follow-up compared to basal insulin therapy (Table 2, Figure 1). A total of 31 patients (23%) in the insulin glargine group and 18 patients (13%) in the liraglutide group had mild hypoglycaemia (P = 0.04), which represents a risk reduction with an odds ratio equal to 0.52 for liraglutide compared to insulin glargine. The number of individual hypoglycaemic episodes was also significantly lower in the liraglutide group (mean: 0.25 ± 0.71 events; total: 34 events) compared to the insulin glargine group (mean: 0.74 ± 2.18 events; total: 101 events; P = 0.04). However, there were no differences in clinically significant hypoglycaemia (<3 mmol/L) or severe hypoglycaemia (<2.2 mmol/L). Only three patients in the insulin glargine group and one patient in the liraglutide group reported clinically significant hypoglycaemia and two patients in the insulin glargine and one in the liraglutide group experienced severe hypoglycaemia (Table 2).
FIGURE 1.
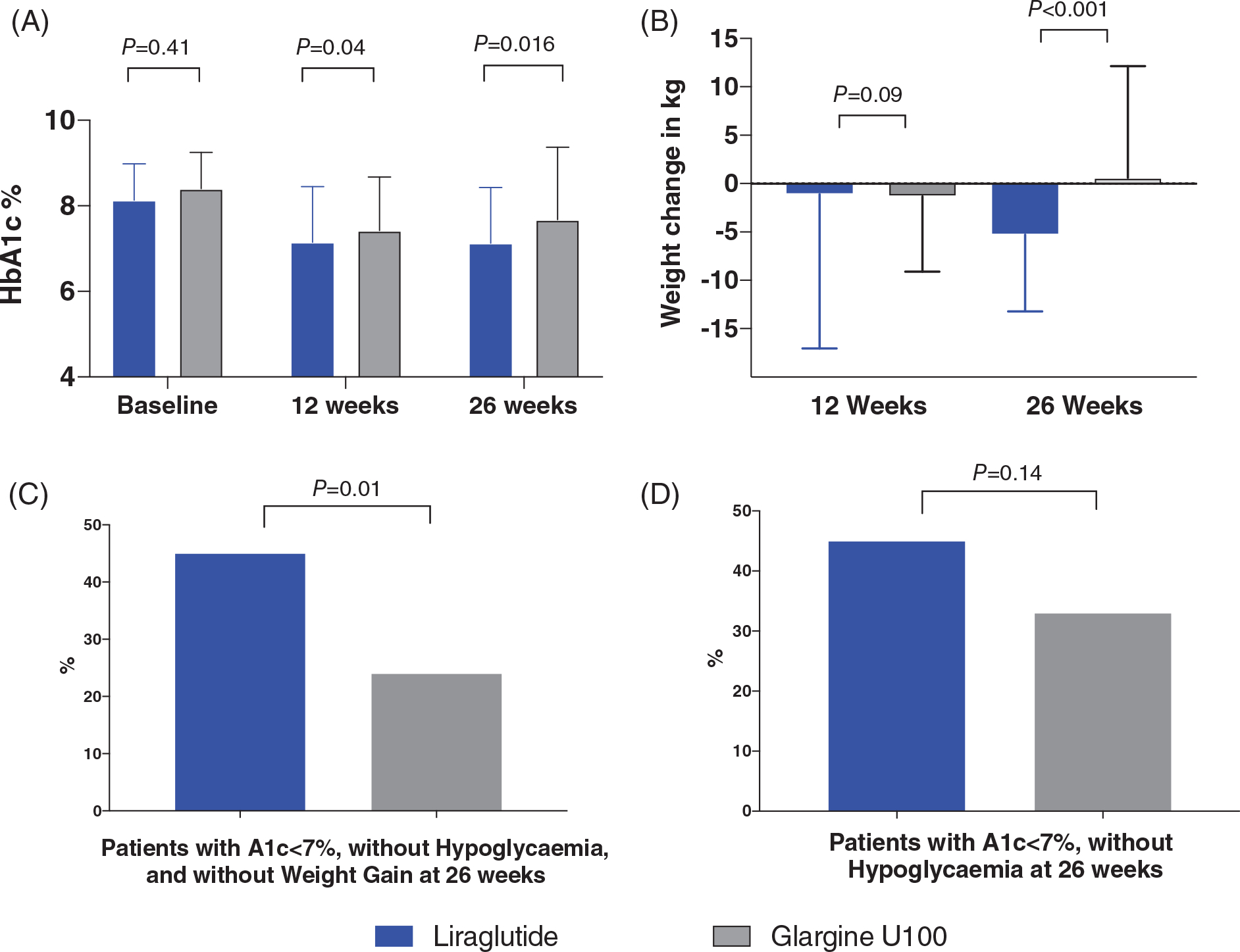
Primary and secondary outcomes in patients with type 2 diabetes discharged on liraglutide and insulin glargine. A, glycated haemoglobin (HbA1c) at baseline, 12 weeks, and 26 weeks. B, Change in body weight from baseline. C, HbA1c < 7% (53 mmol/mol) and no hypoglycaemia or weight loss. D, HbA1c < 7% (53 mmol/mol) and no hypoglycaemia
Differences in body weight change at 12 and 26 weeks are shown in Table 2. There was no difference in body weight change at 12 weeks with liraglutide or insulin glargine (−1.1 ± 16 vs 1.3 ± 7.8 kg, respectively; P = 0.69). The difference in body weight change between groups was significant at 26 weeks of follow-up. The mean weight loss from baseline of 4.8 ± 7.7 kg achieved in the liraglutide group was superior to the change of body weight (0.6 ± 11.5 kg) in the insulin glargine group (P < 0.001), resulting in a mean treatment difference of 5.38 (95% CI 2.28−8.48; Figure 2).
FIGURE 2.
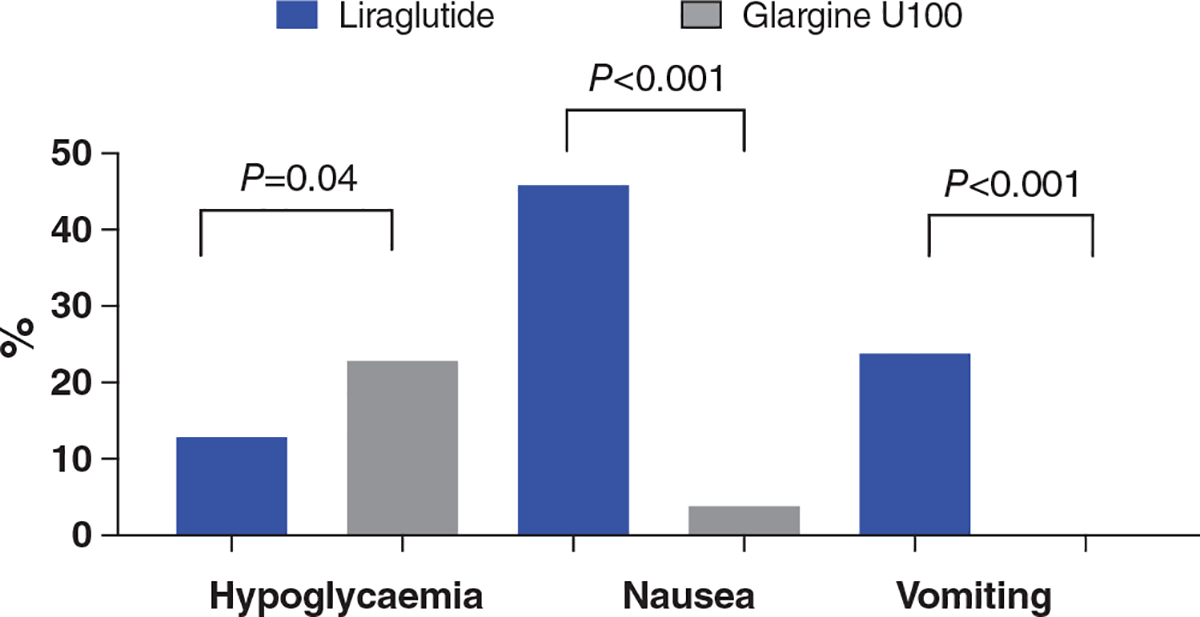
Adverse events including hypoglycaemia and gastrointestinal adverse events after hospital discharge
The liraglutide group had a greater number of patients with HbA1c <7% without hypoglycaemia at 12 weeks (48% vs 33%; P = 0.05) and 26 weeks (45% vs 33%; P = 0.14 [Figure 1C]. The liraglutide group also had a greater proportion of patients with an HbA1c < 7% without hypoglycaemia and no weight gain at 12 weeks (41% vs 24%; P = 0.02) and at 26 weeks (39% vs 22%; P = 0.014). As expected, the incidence of gastrointestinal adverse events was higher with liraglutide than with insulin glargine (P < 0.001; Figure 1D).
Treatment-emergent adverse events are reported in Table 2. Participants in the liraglutide group experienced more episodes of nausea and vomiting compared to those in the insulin glargine group (P< 0.001; Table 2). No participants in the insulin glargine group and 13% of participants in the liraglutide group withdrew from the study because of a treatment-emergent adverse event (P < 0.001; Table 2). The rate of complications including infections, cardiovascular disease, acute kidney injury and mortality was similar in the two groups (Table 2). There was no difference in emergency room visits or hospital readmissions between groups.
Patient attrition was higher in the liraglutide group compared to the insulin glargine group (Appendix, Figure S1). Data on HbA1c, the primary outcome after hospital discharge, was available for 188 patients (68.8%) at 12 weeks and 173 (63.3%) at 26 weeks. Among patients in the liraglutide group, an HbA1c value was collected in 88 (64.7%) and 80 patients (58.8%) at 12 and 26 weeks, respectively. In the insulin glargine group, an HbA1c value was collected in 100 (72.99%) and 93 patients (67.88%) at 12 and 26 weeks, respectively. Treatment discontinuation was most common in patients not receiving injectable medications before enrolment, and in patients who were not previously treated by our clinical diabetes service prior to admission. There were also more patients in the liraglutide group (10%) who discontinued therapy due to adverse events compared to insulin glargine (0%). In addition, some patients were lost to follow-up during the COVID-19 pandemic due to fear and the closure of our clinical research programme between February and July 2020.
4 |. DISCUSSION
This is the first RCT comparing the efficacy and safety of a GLP-1RA with basal insulin treatment after hospital discharge in general medicine and surgery patients with T2D. Our results indicate that treatment with liraglutide and insulin glargine produced clinically meaningful improvements in glycaemic control after hospital discharge. The between-group HbA1c difference at 12 weeks was −0.28% (95% CI −0.64, 0.09), and at 26 weeks it was −0.55% (95% CI −1.01, −0.09) in favour of liraglutide. Analysis of secondary endpoints indicate that liraglutide treatment was associated with greater weight loss, and a higher percentage of patients achieving an HbA1c <7% without weight gain or hypoglycaemia. As expected, liraglutide therapy was associated with higher rates of gastrointestinal adverse events, leading to discontinuation in 10% of patients. These results indicate that the addition of liraglutide is a sound strategy with which to improve glycaemic control after hospital discharge in patients with poorly controlled T2D.
Extensive data exist regarding the importance of diabetes care during the hospital stay. A large number of observational trials and RCTs in hospitalized patients have shown that hyperglycaemia and diabetes increase the risk of complications and mortality,17–21 and that improved glycaemic control is associated with improved clinical outcome, shorter length of stay, as well as reduced risk of infections and overall complications.17,20,22–24 Clinical guidelines from professional organizations25–27 recommend the use of subcutaneous basal-bolus insulin as the preferred therapy for glycaemic control in general medical and surgical patients with T2D. The use of a basal-bolus regimen improves glycaemic control and can reduce the rate of hospital complications28–31; however, this regimen requires multiple insulin daily injections and is associated with a significant risk of hypoglycaemia, which has been reported in up to 32% of patients with T2D not treated in the intensive care unit.29–31 Increasing evidence indicates that incretin-based agents are safe and effective for the hospital management of patients with T2D.32–34 Recent RCTs comparing differences in glycaemic control between treatment with dipeptidyl peptidase-4 inhibitors, alone or in combination with basal insulin, compared to a basal-bolus regimen reported no differences in mean daily blood glucose, frequency of hypoglycaemia, length of hospital stays, and complications in general medicine and surgery patients with T2D.32–35 Similarly, we recently reported that treatment with exenatide improves glycaemic control and, in combination with basal insulin, resulted in a higher proportion of blood glucose levels in target range of 70–180 mg/dL (3.9–10 mmol/L) compared with a basal-bolus regimen.14
Adding basal insulin to a diabetes regimen is not equivalent to adding noninsulin agents such as GLP-1RAs or sodium-glucose co-transporter-2 inhibitors in the outpatient setting, particularly in high-risk patients with T2D. These newer classes are now preferred in patients with cardiorenal risk or those with excess weight.36 In addition, these agents do not increase the risk of hypoglycaemia compared to insulin or sulphonylureas. Given the known association with weight gain and increased risk of iatrogenic hypoglycaemia, the addition of basal insulin is now reserved for patients failing noninsulin agents, those with higher HbA1c levels, or those with symptomatic hyperglycaemia. Our results show that intensifying therapy with a noninsulin agent is acceptable and may be superior compared to adding insulin at discharge. A meta-analysis of comparing the clinical effects of short- or long-acting GLP-1RAs versus insulin treatment from head-to-head clinical trials showed slightly better glycaemic control can be achieved by adding GLP-1RAs to oral agents compared to adding insulin.37
Few prospective studies have assessed different treatment strategies during the transition period from the inpatient to the outpatient care setting. Following the recommendations of the Endocrine Society guideline for the management of hospitalized patients with diabetes, we conducted a prospective study on insulin titration according to the admission HbA1c levels.10 In that study, patients with HbA1c <7% were discharged on their preadmission diabetes therapy. Patients with HbA1c between 7% and 9% were discharged on a preadmission regimen plus basal insulin at 50% of hospital daily dose. Patients with HbA1c > 9% were discharged on oral antidiabetic agents plus basal insulin at 80% of the inpatient dose, or on a basal-bolus insulin regimen.10 This strategy resulted in a significant improvement in glycaemic control, but it also contributed to high rates of hypoglycaemia, reported in 30% and 44% of patients discharged on basal plus OADs and basal-bolus regimen, respectively.10
Recent studies have explored alternative therapies to insulin at hospital discharge, aiming to reduce hypoglycaemic risk. In one study patients were discharged on the combination of sitagliptin/metformin7 with or without insulin according to an HbA1c-based algorithm. This study reported significant improvement in glycaemic control with a low risk of clinically significant hypoglycaemia.7 In agreement with ambulatory studies comparing insulin glargine and liraglutide in individuals with T2D taking oral agents,12,38 we observed that treatment with liraglutide was as effective as basal insulin in reducing HbA1c by ~1.5% from baseline. The modest difference observed in HbA1c between groups may be related to a potential higher efficacy of adding a GLP-1RA versus adding insulin therapy.37 Another possibility may also be the fear by investigators aggressively uptitrate inulin doses given the known risk of iatrogenic hypoglycaemia in this population7,8 with glycaemic control relatively close to goal.
In the present study, a higher proportion of patients in the insulin glargine group (23%) experienced hypoglycaemia <3.9 mmol/L compared to 13% in the liraglutide group, with an overall two-third reduction in the total frequency of hypoglycaemic events in the liraglutide group. However, there were no differences in the number of clinically significant and severe hypoglycaemic events between treatment groups, Thus, the modest difference in the number of mild hypoglycaemic events is not clinically significant and is unlikely to translate into measurable clinical outcomes.
The EAGLE study reported weight gain of 2.0 kg for insulin glargine and − 3.0 kg for liraglutide, and the LEAD 5 trial reported a weight increase of 1.6 kg with insulin glargine and weight loss of −1.8 kg with liraglutide, resulting in a mean treatment difference of −3.43 kg (95% CI −4.00, −2.86; P < 0.0001). Although we did not observe an expected difference in weight between groups at 12 weeks, we observed a significant reduction in weight at 26 weeks with liraglutide (−4.8 kg vs 0.6 kg; P < 0.001). The impact of liraglutide may reach its highest effect after 20 weeks of exposure as previously reported,39 and may explain a significant difference observed only at 26 weeks. The lack of significant weight gain in the glargine group, increased risk of mild hypoglycaemia, along with a decline in HbA1c from baseline, suggest additional factors may affect glycaemic control in this population after discharge. We speculate this may be related to changes in usual patterns of oral intake after an acute illness, and further research is needed to identify optimal treatment strategies in this vulnerable population.
In general, participants tolerated their injectable therapies during the 26 weeks of this trial. As expected, patients randomized to liraglutide experienced more gastrointestinal events. Fifty-five patients in the liraglutide group and three in the insulin glargine group experienced at least one gastrointestinal adverse event during the study period. There were no differences in the number of emergency room visits, hospital readmissions, infections, heart failure exacerbations, or cardiovascular, neurological and renal complications during the study period.
Limitations of this study include the open-label design of the trial. Masking treatment allocation was not feasible because insulin glargine and liraglutide have different titration requirements. The duration of the study was relatively short at 6 months, although this was similar to previous trials comparing diabetes drugs.12,38 The short study duration and fear of hypoglycaemia may have prevented some participants from reaching the target HbA1c. Approximately 40% of patients were lost to follow-up or discontinued participation in the study. The observed rate of treatment discontinuation was higher than the rate of discontinuation reported in previous hospital discharge studies.7,10 In the Basal Plus Discharge trial10 and in a sitagliptin discharge study7 we observed a 38% and 30% discontinuation rate after 3 and 6 months of follow-up, respectively. In addition, treatment discontinuation rates were higher than the 25% to 40% reported in ambulatory studies with the use of GLP-1RAs29,30 and basal insulin therapy at 6 months of therapy.31 Treatment discontinuation after hospital discharge was higher in patients not receiving injectable medications prior to admission and in patients who were not previously treated by our clinical diabetes service. There were also more patients in the liraglutide group (10%) who discontinued therapy due to adverse events compared to the insulin group (0%).
In conclusion, the results of our comparative effectiveness and safety study of a GLP-1RA and basal insulin after hospital discharge provide important and practical clinical information. Both basal insulin and GLP-1RA treatment result in improved glycaemic control after hospital discharge. While the transition from inpatient to outpatient is a high-risk period, the use of liraglutide was associated with better glycaemic control, greater weight loss, and a higher proportion of patients achieving the target of HbA1c <7% without weight gain and without hypoglycaemia. As expected, liraglutide treatment was associated with a higher rate of gastrointestinal adverse events. Based on these results, we conclude that the addition of liraglutide is a sound strategy to manage patients with poorly controlled diabetes after hospital discharge.
Supplementary Material
ACKNOWLEDGMENTS
FJP, AJ, GI, SC and MAU edited the protocol, conducted the study and edited the manuscript. KWZC, BA, MCPG, PG, IA, ALM, MF, RG, PV, JMF and GMD conducted the study, reviewed and edited the manuscript, and contributed to the discussion. LP performed the statistical analysis. GEU wrote the initial research proposal and the first draft of the manuscript. GEU is the guarantor of this work and takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
This work was an investigator-initiated grant, with unrestricted research support provided by Novo Nordisk to Emory University. The funding source had no input on the design of the study, interpretation of the results or manuscript preparation.
CONFLICT OF INTEREST
FJP is supported in part by the National Institute of Health (NIH) under grant awards 1K23GM128221–01A3 and P30DK111024–05S, and has received research support from Merck and Dexcom, and consulting fees from Boehringer Ingelheim. RJG is supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the NIH under Award Numbers P30DK11102 and 1K23DK123384–01. RJG received research support (to Emory University) for investigator-initiated studies from Novo Nordisk, and consulting fees from Abbott Diabetes Care, Sanofi, Novo Nordisk and Valeritas. PV is supported in part by NIH grant 1K23DK113241 and has received consulting fees from Merck and Boehringer-Ingelheim. JF received consulting and lecturer fees from Boehringer Ingelheim and Sanofi. MF is supported in part by NIH grant, K23-NIDDK 124647A. GMD is supported by NIH grant K23-NIDDK 122199–01. GEU is partly supported by research grants from the National Center for Advancing Translational Sciences of the NIH under Award Number UL1TR002378 from the Clinical and Translational Science Award programme and NIH grant U30, P30DK11102, and has received research grant support to Emory University for investigator-initiated studies from Novo Nordisk, Astra Zeneca and Dexcom. AC has been a consultant and speaker for Lilly, Novo Nordisk, Astra Zeneca, Boehringer-Ingelheim, Sanofi. MAU, SC, KWZ, BA, CPG, PG, IA, AM, GI and LP have nothing to disclose. This study was partially presented at the virtual American Diabetes Association Scientific Sessions, 2020.
Funding information
The study was an investigator-initiated study funded by Novo Nordisk. The funding source was not involved in the study design, data collection, interpretation, statistical analysis, manuscript preparation, or the decision to submit the manuscript for publication.
Footnotes
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14347.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020. [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas teB, Belgium: 2019. https://www.diabetesatlas.org. Accessed January 6, 2021.
- 3.De Berardis G, D’Ettorre A, Graziano G, et al. The burden of hospitalization related to diabetes mellitus: a population-based study. Nutr Metab Cardiovasc Dis. 2012;22(7):605–612. [DOI] [PubMed] [Google Scholar]
- 4.McCoy RG, Lipska KJ, Herrin J, Jeffery MM, Krumholz HM, Shah ND. Hospital readmissions among commercially insured and Medicare advantage beneficiaries with diabetes and the impact of severe hypoglycemic and hyperglycemic events. J Gen Intern Med. 2017;32(10): 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shepperd S, McClaran J, Phillips CO, et al. Discharge planning from hospital to home. Cochrane Database Syst Rev. 2010;1:CD000313. [DOI] [PubMed] [Google Scholar]
- 6.Griffith ML, Boord JB, Eden SK, Matheny ME. Clinical inertia of discharge planning among patients with poorly controlled diabetes mellitus. J Clin Endocrinol Metab. 2012;97(6):2019–2026. [DOI] [PubMed] [Google Scholar]
- 7.Gianchandani RY, Pasquel FJ, Rubin DJ, et al. The efficacy and safety of co-Administration of Sitagliptin with metformin in patients with type 2 diabetes at hospital discharge. Endocr Pract. 2018;24(6): 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umpierrez GE, Reyes D, Smiley D, et al. Hospital discharge algorithm based on admission HbA1c for the management of patients with type 2 diabetes. Diabetes Care. 2014;37(11):2934–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38. [DOI] [PubMed] [Google Scholar]
- 10.Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103–112. [DOI] [PubMed] [Google Scholar]
- 12.D’Alessio D, Haring HU, Charbonnel B, et al. Comparison of insulin glargine and liraglutide added to oral agents in patients with poorly controlled type 2 diabetes. Diabetes Obes Metab. 2015;17(2): 170–178. [DOI] [PubMed] [Google Scholar]
- 13.Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617. [DOI] [PubMed] [Google Scholar]
- 14.Fayfman M, Galindo RJ, Rubin DJ, et al. A randomized controlled trial on the safety and efficacy of Exenatide therapy for the inpatient Management of General Medicine and Surgery Patients with Type 2 diabetes. Diabetes Care. 2019;42:450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6(1):40. [DOI] [PubMed] [Google Scholar]
- 16.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002; 87(3):978–982. [DOI] [PubMed] [Google Scholar]
- 18.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. JAMA. 2003;290(15):2041–2047. [DOI] [PubMed] [Google Scholar]
- 19.Van den Berghe G, Wouters PJ, Bouillon R, et al. Outcome benefit of intensive insulin therapy in the critically ill: insulin dose versus glycemic control. Crit Care Med. 2003;31(2):359–366. [DOI] [PubMed] [Google Scholar]
- 20.Pomposelli JJ, Baxter JK 3rd, Babineau TJ, et al. Early postoperative glucose control predicts nosocomial infection rate in diabetic patients. JPEN J Parenter Enteral Nutr. 1998;22(2):77–81. [DOI] [PubMed] [Google Scholar]
- 21.Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26(1):57–65. [DOI] [PubMed] [Google Scholar]
- 22.Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553–597. [DOI] [PubMed] [Google Scholar]
- 23.McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810–815. [DOI] [PubMed] [Google Scholar]
- 24.Baker EH, Janaway CH, Philips BJ, et al. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006; 61(4):284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnipper JL, Magee M, Larsen K, Inzucchi SE, Maynard G. Society of Hospital Medicine Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts. J Hosp Med. 2008;3(5 Suppl):66–75. [DOI] [PubMed] [Google Scholar]
- 27.Seley JJ, D’Hondt N, Longo R, et al. Position statement: inpatient glycemic control. Diabetes Educ. 2009;35(Suppl 3):65–69. [Google Scholar]
- 28.Korytkowski MT, Salata RJ, Koerbel GL, et al. Insulin therapy and glycemic control in hospitalized patients with diabetes during enteral nutrition therapy: a randomized controlled clinical trial. Diabetes Care. 2009;32(4):594–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umpierrez GE, Hor T, Smiley D, et al. Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(2):564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120(7):563–567. [DOI] [PubMed] [Google Scholar]
- 31.Umpierrez GE, Simley D, Jacobs S, et al. RAndomized study of basal bolus insulin therapy in the inpatient Management of Patients with type 2 diabetes undergoing general surgery (RABBIT surgery). Diabetes Care. 2011;34(2):256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasquel FJ, Gianchandani R, Rubin DJ, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, noninferiority randomised trial. Lancet Diabetes Endocrinol. 2017;5(2):125–133. [DOI] [PubMed] [Google Scholar]
- 33.Vellanki P, Rasouli N, Baldwin D, et al. Glycaemic efficacy and safety of Linagliptin compared to basal-bolus insulin regimen in patients with type 2 diabetes undergoing non-cardiac surgery: a multicenter randomized clinical trial. Diabetes Obes Metab. 2019;21(4):837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garg R, Schuman B, Hurwitz S, Metzger C, Bhandari S. Safety and efficacy of saxagliptin for glycemic control in non-critically ill hospitalized patients. BMJ Open Diabetes Res Care. 2017;5(1):e000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hulst AH, Visscher MJ, Godfried MB, et al. Study protocol of the randomised placebo-controlled GLOBE trial: GLP-1 for bridging of hyperglycaemia during cardiac surgery. BMJ Open. 2018;8(6):e022189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marx N, Davies MJ, Grant PJ, et al. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol. 2021;9(1):46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abd El Aziz MS, Kahle M, Meier JJ, Nauck MA. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes Metab. 2017;19(2):216–227. [DOI] [PubMed] [Google Scholar]
- 38.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36(6):843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


