Abstract
A significant hurdle in obtaining biophysical information on membrane proteins is developing a successful strategy for their reconstitution into a suitable membrane mimic. In particular, utilization of the more ‘native-like’ membrane mimics such as bicelles is generally more challenging than simple micellar solubilization. Caveolin-1, an integral membrane protein involved in membrane curvature, endocytosis, mechano-protection, and signal transduction, has been shown to be particularly recalcitrant to standard reconstitution protocols due to its highly hydrophobic characteristics. Herein we describe a robust method to incorporate recombinantly produced full-length caveolin-1 into bicelles at levels needed for biophysical experimentation. The benchmark of successful reconstitution is the obtainment of protein in a homogeneous state; therefore, we developed a validation procedure to monitor the success of the reconstitution using analytical ultracentrifugation of density-matched bicelles. Our findings indicated that our protocol produces a very homogeneous preparation of caveolin-1 associated with bicelles, and that caveolin-1 is highly α-helical (by circular dichroism spectroscopy). We believe that this methodology will serve as a general strategy to facilitate biophysical studies on membrane proteins.
Keywords: caveolin-1, reconstitution, analytical ultracentrifugation, sedimentation equilibrium, bicelles, fluorescence spectroscopy
Graphical Abstract
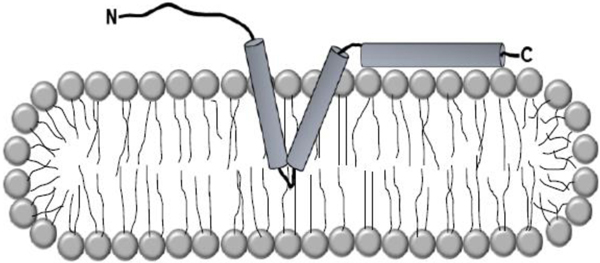
Introduction
Biophysical studies of homogeneously purified membrane proteins necessitate their reconstitution into membrane mimics that closely resemble the environment of the biological membrane. Most commonly, detergent micelles, liposomes, nanodiscs, or bicelles are employed with each system having particular strengths and weaknesses.1,2 The choice of a suitable membrane mimic is largely empirical; for some membrane proteins detergent micelles support full structure and function while others need more sophisticated mimics such as bicelles.3–6 Caveolin-1 is an integral membrane protein of great interest to the biological community as it is intimately involved in caveolae biogenesis, signal transduction, mechano-protection, and membrane curvature.7–9 The hallmark feature of caveolin is the intramembrane domain which is postulated to form an unusual hairpin structure within the hydrophobic core of the membrane.10 Thus, there is intense enthusiasm in characterizing caveolin structurally.11 However to date, most structural studies on caveolin have been done in micellar environments which have produced a wealth of secondary structure data, but have fallen woefully short of producing compelling tertiary structure data.12–16 This paucity of data suggests indirectly that micelles may not adequately support the tertiary structure of caveolin. Therefore, it is imperative to assess caveolin behavior in an environment which better resembles the native bilayer. However, the employment of more native-like membrane mimics such as bicelles tends to be much more technically demanding than standard micellar reconstitution.17–19
Phospholipid bicelles, are discoidal lipid aggregates comprised of mixtures short-chain phospholipids (DHPC) and long-chain phospholipids (DMPC), and have a number of features which make them particularly well-suited for biophysical studies on membrane proteins.20 One benefit is the presence of an actual bilayer, the size of which can be controlled by the molar ratio of DMPC to DHPC, q. In addition, bicelles formed at q values ≥ 1.0 have thermotropic behavior (gel to fluid main-phase transition temperatures) which is indistinguishable from that of pure DMPC vesicles, indicating that a protein residing in the center of the bicelle (i.e. not the rim) will experience a native-like environment in terms of membrane fluidity.21,22
Here, we present a facile protocol for reconstituting recombinantly produced full-length human caveolin-1 into bicelles for biophysical studies. We then employ analytical ultracentrifugation using density-matched bicelles to prove that the procedure allows for the procurement of the protein in a homogenous state. Furthermore, circular dichroism spectroscopy revealed that the reconstituted caveolin-1 was highly helical. We believe that the methodology developed in this work will serve as a general strategy for incorporating membrane proteins in bicelles and evaluating their homogeneity.
Materials and Methods
DMPC and NBD-DMPE were purchased from Avanti Polar Lipids (Alabaster, AL). DPH was purchased from Sigma-Aldrich (St. Louis, MO). D2O was purchased from Cambridge Isotope Laboratories (Tewksbury, MA). All other reagents were of standard laboratory grade.
Caveolin-1 expression and purification
The DNA for full-length human caveolin-1 (1–178) with a C-terminal myc-tag was synthesized by Genscript Corporation (Piscataway, NJ). Caveolin-1 has three sites of cysteine palmitoylation (C133, C143, C156) that have been shown to be nonessential for proper caveolin-1 trafficking.23 Therefore, each cysteine was mutated to serine. The construct was cloned into the pET-24a vector which appends a C-terminal hexahistidine tag (EMD Millipore, Billerica, MA) yielding a final construct of caveolin-1-myc-H6. Next, the plasmid was transformed into BL21(DE3) E. coli cells, and over-expressed using the auto-induction method at 25°C for 24 hours.24 Please note, that the caveolin-1-myc-H6 construct has previously been shown to retain key functional properties which strongly suggests that the fold of the protein is not altered by the tags.25 Cells from a 1 L growth were harvested by centrifugation and washed with 0.9% (w/v) NaCl. The pellet was resuspended in 40 mL of lysis buffer (50 mM phosphate, 300 mM NaCl, pH 8, 2% (v/v) Empigen BB® detergent (Sigma, St. Louis, MO)). Cells were lysed at 4°C by sonication with magnetic stirring for 20 minutes (Bronson 450 watt sonifer, power level 4, duty cycle 30%). The lysate was cleared by centrifugation at 50,000 x g for 1.5 hours, and the supernatant was filtered through a 0.2 μm syringe filter and passed over a 25 mL Ni-Sepharose column (GE Healthcare, Piscataway, NJ) equilibrated with lysis buffer. The column was washed with 125 mL of wash buffer (50 mM phosphate, pH 8, 300 mM NaCl, 35 mM imidazole, 0.5% (v/v) Empigen BB®), and eluted using elution buffer (50 mM phosphate, pH 8, 300 mM NaCl, 250 mM imidazole, 0.5% (v/v) Empigen BB®). The protein-containing fractions were pooled and concentrated to 5 mL using ultrafiltration (MWCO 10,000). The concentrated sample was re-filtered through a 0.2 μm syringe filter and injected onto a Sephacryl 300 HR 16/60 column (GE Healthcare, Piscataway, NJ) equilibrated with gel filtration buffer (50 mM phosphate, pH 7.4, 150 mM NaCl, 0.5% (v/v) Empigen BB®). The purified protein was stored at 4°C and quantitated using the micro-BCA assay (Thermofisher Scientific, Waltham, MA). Protein concentrations were estimated by A280 measurements using a calculated extinction coefficient of 30,940 M−1cm−1 (Protparam, https://web.expasy.org/protparam/)
Bicelle Preparation Calculations
The DHPC component of a bicellar mixture is in rapid equilibrium between a free monomer in solution and the rim of the bicelle, and regardless of the concentration of the bicellar solution, the concentration of free monomeric DHPC is constant at ~7 mM. Therefore, the parameter q used to prepare a bicellar solution can deviate significantly from the the actual molar ratio of DMPC and DHPC within the bicelle (qeff). To prepare bicelles at a particular qeff, the following equation was used:
For this study, qeff = 1.0 bicelles were prepared which corresponds to a value of q = 0.7.
Incorporation of caveolin-1 into bicelles
To incorporate caveolin-1 (1–178) into bicelles, roughly 350 μg of purified caveolin-1 was applied to a Ni-NTA spin column (Qiagen, Valencia, CA) equilibrated with gel filtration buffer. The nickel spin column was washed twice with 600 μL of a qeff = 1.0 bicellar solution (2.3% (w/w) total lipid, 10 mM HEPES pH 7.4, and 100 mM NaCl). The protein was eluted with 2 × 200 μL of the above buffer containing 250 mM imidazole. The elution was then processed using a Zeba™ desalting column (Pierce, Rockford, IL.) to remove the imidazole. The desalting column was equilibrated with 10 mM HEPES pH 7.4, 100 mM NaCl, 7 mM DHPC. From this stock three samples at various concentrations (30 ± 2 μM, 15 ± 2 μM, and 7.5 ± 2 μM) were prepared for analytical ultracentrifugation by diluting with the desalting column equilibration buffer.
Verification of detergent removal
To determine that all of the Empigen BB® was removed under these conditions, a trial experiment was run. For this experiment, DPH was added to the 350 μg sample of caveolin-1 to yield a final concentration of ~1 μM. Next, the fluorescence was measured in a 1 x 0.1 cm cuvette held at 25 °C using an excitation wavelength of 355 nm and an emission wavelength of 430 nm. The excitation and emission slits were each set to 5 nm respectively. Next, the sample was reconstituted into bicelles using the method discussed above. The DPH fluorescence was then re-measured at the end of the reconstitution (post desalting step) to assess the completeness of detergent removal.
Density matching experiments
All sedimentation equilibrium experiments were performed at 25°C using a Beckman XL-A analytical ultracentrifuge and a 4-hole AnTi-60 rotor. Density matching samples were prepared on a 300 μL scale. First, 3.58 mg of DMPC and 0.43 μg of NBD-DMPE (1:10,000 mole ratio) were co-dissolved in chloroform followed by evaporation to dryness. To each sample, 7.5 μL of a 40× concentrated stock buffer solution was added which yielded final concentrations of 10 mM HEPES pH 7.4 and 100 mM NaCl. Next, the appropriate quantity of H2O and D2O were added to achieve the desired composition (0, 10, 25, 40, 50, 60, 70, 80, 90% D2O (v/v)). Following this, the samples were vortexed until a homogeneous milk-like suspension was obtained. Finally, 13.03 μL of a 25% (w/w) DHPC solution was added. After adding the DHPC, the solution became clear. The samples were loaded into a 6-channel charcoal-filled epon centerpiece using a reference solution containing only buffer. A volume of 120 μL of sample was loaded per channel. Equilibrium absorbance measurements (468 nm) were taken from 10,000 rpm to 35,000 rpm stepping up the speed in 1,000 rpm increments. Data was collected at each rotor speed. Achievement of equilibrium was determined using the computer program WinMatch. For each percentage of D2O, a plot of the natural log of the absorbance versus the square of the radius was generated, fitted to a linear function and the buoyant molecular weight (Mef f ) was extracted from the slope. Next, Mef f was averaged over all speeds. The density of the bicellar solutions at each percentage was measured using a Kyoto Electronics Density/Specific Gravity Meter (model #DA-210). Finally, Mef f was plotted versus the density of the solution at each amount of additive, fitted to a linear function, and interpolated to a buoyant molecular weight of zero to determine the density matched concentration of D2O.
Sedimentation equilibrium of caveolin-1 in bicelles
In a fashion similar to that stated for the density matching experiments above, a bicellar solution of caveolin-1 was analyzed at three different speeds (25,000, 30,000 and 35,000 rpm) and three different concentrations. Final scans at each speed (step-size 0.001 cm and 99 replicates) were acquired once equilibrium had been established using the match feature in the software Heteroanalysis (version 1.1.0.58, University of Connecticut, Storrs, CT). The reference was a sample which did not contain protein. All experiments were carried out at 25 °C. The data were fitted globally to a single-species model using a non-linear least squares approach using Heteroanalysis. During fitting, the molecular weight, baseline, and reference concentration were all allowed to float.
Circular Dichroism Spectroscopy Measurements
Circular dichroism measurements were performed at 25°C using a JASCO CD Spectrophotometer (Easton, MD). The bicellar caveolin-1 sample (qeff = 1.0, 2.3% (w/w) total lipid, 10 mM HEPES pH 7.4, and 100 mM NaCl) was at a concentration of 30 μM. A 0.1 mm path length quartz cuvette was utilized for measurements. Data were collected by scanning every wavelength from 260–190 nm in step mode at a speed of 200 nm/minute taking 16 accumulations for signal averaging. A background spectrum of DMPC-DHPC bicelles lacking protein was used as a blank. The data were converted from machine units (θ) to units of mean residue ellipticity (θ) using the following equation:
Here, MRW is the mean residue weight (protein MW/number of residues in the protein), P is the pathlength in cm, and C is the protein concentration in mg/mL yielding units of M−1 cm−1.
SDS-PAGE and Immunoblotting
Protein samples taken at various stages of the purification procedure were resolved on 15% polyacrylamide gels and transferred to a PVDF membrane. The blots were blocked with 1% (w/v) casein, and probed using an anti-myc antibody. Signals were detected using chemiluminescence. The immuno-blots were imaged using a BioRad ChemiDoc XRS+ system and Image Lab software (BioRad, Hercules, CA).
Results and Discussion
Reconstitution of caveolin-1 into phospholipid bicelles
To date, no tertiary structural data has been collected for full-length caveolin-1. This is likely due to the exceptionally insoluble (hydrophobic) character of caveolin-1 which has resulted in most structural studies being carried out in strong detergent micelles.11 While micelles are advantageous because reconstitution is not technically demanding, it is becoming apparent that they may not adequately support the tertiary structure of caveolin-1.13 Bicelles on the other hand appear to be well suited to caveolin-1; they contain an actual lipid bilayer and they are composed of saturated phosphatidylcholine lipids which predominate in caveolae where caveolin-1 is natively found.26
We sought to develop a non-denaturing protocol for reconstituting recombinantly-produced homogeneously purified caveolin-1 efficiently into bicelles. As an expression source, E. coli is appropriate as over-expression of full-length caveolin-1 in the host has been shown to result in caveolae formation, a property that clearly indicates that the protein is properly folded and functional.27,28 A number of factors were considered when choosing a detergent for the initial lysis and purification steps. Ideally, the detergent would be native-like and non-denaturing. The zwitterionic detergent, Empigen BB® was identified as being an optimal choice for the purification as it has a zwitterionic headgroup that mimics phosphocholine, is not cost prohibitive, and most importantly it readily solubilized caveolin-1. Initial purification was done via nickel affinity chromatography followed by gel filtration chromatography (SI Figure 1). The gel filtration chromatography step was absolutely necessary to get a pure caveolin-1 sample as significant aggregates and/or impurities remained after the nickel affinity chromatography step (SI Figure 1B). The material sharply eluted from a sephacryl S-300 HR gel filtration column and at nearly the same volume in empigen micelles as was observed for prior studies utilizing dodecylphosphocholine micelles (DPC) where caveolin-1 was shown to be in a singular oligomeric state.29 In addition, this step removes the imidazole used to elute the protein in the first nickel affinity chromatography step. This allows for the reassociation of the protein with the Ni-NTA spin column in the subsequent step. After binding to the Ni-NTA spin column, the protein was eluted into qeff = 1.0 DMPC-DHPC bicelles, a choice that was based on studies which showed that bicelles with qeff values less than 1.0 showed a significant loss of lipid segregation and we did not desire to risk exposing caveolin-1 to the detergent-like DHPC which could disrupt its tertiary fold.19,22 A final step employing a mini-desalting column pre-equilibrated with DHPC at the critical bicelle concentration (7 mM) was used to remove the imidazole, while maintaining a minimal concentration of the DHPC to maintain the desired qeff of the caveolin loaded bicelles.30 Using A280 measurements, the amount of protein in the final bicellar preparation was estimated. It was found that nearly all of the protein initially loaded onto the column was present in the bicelle elution fraction, indicating that the reconstitution process was effective. Western blot analysis of the purification procedure confirmed that full length caveolin-1 was obtained in the bicelle elution, migrating to the approximate distance of a 23 kD molecular weight standard (SI Figure 2).
As Empigen BB® has no convenient handle for detection, we evaluated the ability of our method to remove diphenylhexatriene (DPH), a hydrophobic fluorescent probe which produces a strong signal when incorporated into membranes. This was spiked into the sample before applying it to the nickel-NTA spin column. Analysis of the DPH fluorescence before and after the bicelle reconstitution step showed that a negligible amount of probe was present in the final sample (Figure 1). This indicated that the wash steps utilized were sufficient to remove all of the Empigen BB® detergent. Finally, CD spectroscopy was used to probe the protein’s secondary structure in bicelles. The CD spectrum of caveolin-1 in bicelles had a maximum around 190 nm and two minima at 208 and 222 nm which indicate strong α-helical character for caveolin-1 in agreement with other biophysical studies (Figure 2).15,16,31 Analysis of the CD spectrum using the CDSSTR algorithm (Dichroweb) indicated that caveolin-1 is approximately 67% α-helical which compares favorably with determinations based on secondary structure evaluation of a functional caveolin-1 construct comprised of residues 62–178 reconstituted into lysomyristoylphosphatidylglycerol micelles by NMR (72%).15 This finding is also in agreement with studies performed in the presence of many other micelle types, including DPC which is structurally similar to Empigen BB®.11
Figure 1.
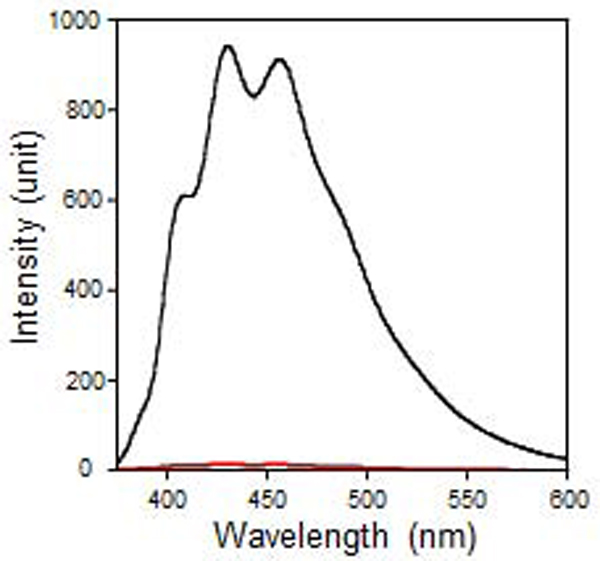
Fluorescence emission spectra of DPH before (black) and after exchange of the protein into bicelles (red). The signal is significantly reduced after the reconstitution into bicelles indicating that this method is effective for detergent exchange.
Figure 2.

Circular dichroism spectra of caveolin-1 reconstituted into q = 1.0 bicelles.
Determining the dispersity of a membrane protein sample can be challenging due to the presence of the membrane mimic. For example, gel filtration is of little utility because the large size of the membrane mimic dominates the size determination and prevents one from assessing the aggregation state of the protein itself. On the other hand, analytical ultracentrifugation is particularly attractive because one can eliminate the contribution of the membrane mimic straightforwardly through density matching.32–34 Density matching represents a condition where the density of the solution is matched to the inverse of the partial specific volume of the membrane mimic. When this condition occurs, the buoyant molecular weight of the membrane mimic becomes zero; therefore it will have no influence on the determination of the aggregation state of the protein.
Density matching of the bicelles was carried out by increasing the density of solution using heavy water (i.e. D2O). Figure 3 shows the plot of the buoyant molecular weight (Meff ) versus solution density (i.e. various concentrations of D2O). The density matched condition for bicelles was determined by interpolation of the line equation in Figure 3 to a buoyant molecular weight of 0. The matched condition was determined to be a density of 1.084 g/cm3 (71.7% (v/v) D2O, SI Figure 3). Using this value, the partial specific volume of the bicelle was determined and yielded a value of 0.923 cm3/g. This data represents, to our knowledge, the first instance where q = 1.0 bicelles have been density-matched, and the partial specific volume of a bicelle determined. The amount of D2O required to density match the bicelles was close to the maximum amount of D2O that can practically be added. Therefore, if significant quantities of denser phospholipids (e.g. phosphatidylserine or glycophospholipids) need to be added, one may need to use D218O instead which will allow for higher solution densities.
Figure 3.

Linear plot of the buoyant molecular weight, Mef f, versus solution density for bicellar (q = 1.0) solutions prepared with various concentrations of D2O. The dashed line represents a Mef f of zero which is the amount of D2O required to density match q = 1.0 bicelles.
Although glycerol or sucrose were also convenient options for density matching experiments, for the study discussed here, we chose to use D2O for two reasons: 1) it is quite similar to H2O and 2) it simplifies data interpretation as glycerol and sucrose could distort the data by altering the bicelle partial specific volume in a concentration dependent manner due to density gradient formation and hydrostatic pressure.32
Sedimentation equilibrium analysis of caveolin-1 in bicelles
The sedimentation profile of caveolin-1 (Figure 4) was obtained at three different speeds (25,000, 30,000, and 35,000 rpm) and three different concentrations (30 ± 2 μM, 15 ± 2 μM, and 7.5 ± 2 μM). The three concentrations employed correspond to lipid to protein ratios of 1400:1, 2800:1, and 5600:1 which yields a theoretical density of caveolin-1 molecules per μm2 bicelle of 0.6 × 103 for the lowest concentration and 2.5 × 103 for the highest concentration when considering just the surface area of the planar region of bicelles.35 This compares favorably to the theoretical density of caveolin-1 in caveolae, which is estimated to be on the order of 103 caveolin molecules per μm2.26,36
Figure 4.
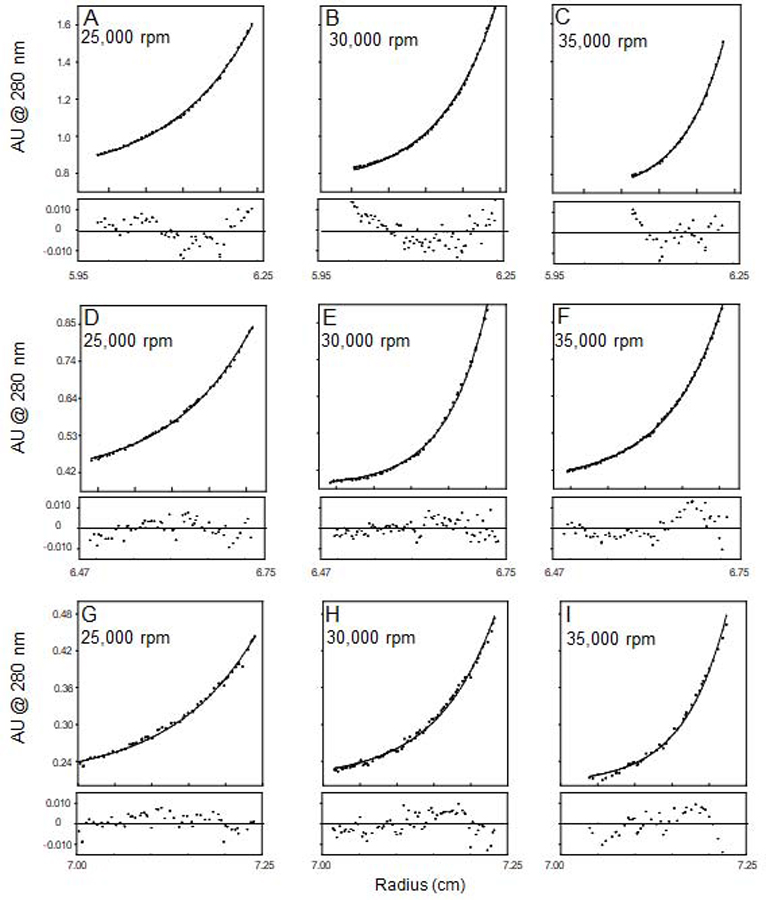
Sedimentation equilibrium profiles of caveolin-1 in density-matched (with D2O) bicellar solutions (q = 1.0). Panels A-C represent a concentration of 30 μM, D-F a concentration of 15 μM,and G-I a concentration of 7.5 μM. Residuals are displayed underneath each equilibrium profile.
Using the computer program Heteroanalysis (University of Connecticut, Mansfield, CT), the data were fit simultaneously to an ideal single species model (This model provides an average molecular weight of all populations present in solution). After fitting the data, a molecular weight of 23.0 ± 0.1 kD was obtained. This agreed very well with the calculated molecular weight of caveolin-1 which was 22.7 kD. Employment of other models (i.e. monomer-dimer, etc) resulted in poor fits (data not shown) and confirmed the single species models was the best. Based on these results it was clear that caveolin-1 is present in a single state in the bicellar environment (monomer), and is not displaying any significant degree of sample heterogeneity.
Conclusions
To date, to our knowledge, there is no reported bicellar reconstitution strategy that has been presented for full-length caveolin-1. Our method is advantageous because it is both gentle and easily carried out. Importantly, we have circumvented strategies which would destabilize the protein’s tertiary fold by instead performing the reconstitution step “on-column” where the protein is immobilized. This method likely prevents excessive or biologically irrelevant self-association of the protein as it gently integrates into the bicelle. Additionally, we have found that our strategy is far more efficient and is likely to be both more general and reliable than other “mild” bicelle reconstitution strategies that first incorporate the protein into liposomes followed by the addition of DHPC at the desired molar ratio to generate bicelles, as vesicle reconstitution strategies often need to be systematically determined for a given protein.37 Our method is also useful as a diagnostic for protein aggregation because bicelles are very compatible with analytical ultracentrifugation and can be easily density-matched using the ultra-mild density modifier, D2O. It is conceivable that this reconstitution strategy will also be of use for those who wish to investigate protein-protein interactions.
Overall the studies presented here reveal that full-length caveolin-1 can be readily reconstituted into q = 1.0 bicelles, and that the protein exists in a singular aggregation state (monomer, >98%). To our knowledge this is the first report of the oligomeric state of caveolin-1 in a bilayered membrane-like environment, and our approach further highlights the importance of using biophysical methods, like analytical ultracentrifugation, to understand membrane protein behavior in cells. One caveat of this study is that the influence of reconstituted protein on the morphology of the bicelle is unknown and future efforts should be devoted to probing this issue. CD studies of reconstituted caveolin-1 show that the full-length protein is highly α-helical (Figure 2), which is consistent with previous studies of caveolin-1, and also show that caveolin-1 is likely properly folded in bicelles.12,15,16,31 With the successful reconstitution of caveolin-1 in hand, we can now focus our attention on the numerous important structural questions that can only be addressed in a bilayered environment.
Supplementary Material
SI Figure 1. A) Representative chromatogram of the nickel affinity column purification of caveolin-1. B) Representative chromatogram of the size exclusion purification of caveolin-1. The peak fraction containing caveolin is marked with a star.
SI Figure 2. Western blot analysis of caveolin-1 throughout the purification. Lane 1, cell lysate; lane 2, post Empigen solubilization pellet, lane 3, Empigen solubilized fraction, lane 4, nickel affinity elution peak, lane 5, size exclusion elution peak, lane 6, mini-nickel spin column bicelle wash, lane 7, mini-nickel spin column elution.
SI Figure 3. Plots of ln(concentration) versus radius2 for bicelle solutions at different concentration of D2O used to generate figure 3.
Highlights.
Reconstitution of full-length caveolin-1 into bicelles.
Caveolin-1 is in a highly homogeneous unaggregated state.
Caveolin-1 displays helical content.
Detergent removal can be monitored by fluorescence spectroscopy.
Acknowledgements
We thank Mike Kelly for laboratory assistance. We also thank Sarah Plucinsky and Jeffrey Julien for critical reading of the manuscript. This work was supported by NIH RO1 GM093258 awarded to K.J.G.
Abbreviations and Symbols
- CD
circular dichroism spectroscopy
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DHPC
1,2-dihexanoyl-sn-glycero-3-phosphocholine
- DPH
1,6-Diphenyl-1,3,5-hexatriene
- NBD-DMPE
1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2–1,3-benzoxadiazol-4-yl)
- HEPES
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- 1.Warschawski DE, Arnold AA, Beaugrand M, Gravel A, Chartrand É, Marcotte I. Choosing membrane mimetics for NMR structural studies of transmembrane proteins. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2011;1808(8):1957–1974. [DOI] [PubMed] [Google Scholar]
- 2.Ro SY, Ross MO, Deng YW, et al. From micelles to bicelles: Effect of the membrane on particulate methane monooxygenase activity. Journal of Biological Chemistry. 2018;293(27):10457–10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiles JA, Glover KJ, Vold RR, Komives EA. Methods for studying transmembrane peptides in bicelles: consequences of hydrophobic mismatch and peptide sequence. Journal of magnetic resonance. 2002, 158(1–2):149–156. [DOI] [PubMed] [Google Scholar]
- 4.Sanders CR, Sönnichsen F. Solution NMR of membrane proteins: practice and challenges. Magnetic Resonance in Chemistry. 2006;44(S1):S24–S40. [DOI] [PubMed] [Google Scholar]
- 5.Laguerre A, Löhr F, Henrich E, et al. From Nanodiscs to Isotropic Bicelles: A Procedure for Solution Nuclear Magnetic Resonance Studies of Detergent-Sensitive Integral Membrane Proteins. Structure. 2016;24(10):1830–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyukmanova EN, Shenkarev ZO, Khabibullina NF, et al. Lipid–protein nanodiscs for cell-free production of integral membrane proteins in a soluble and folded state: Comparison with detergent micelles, bicelles and liposomes. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2012;1818(3):349–358. [DOI] [PubMed] [Google Scholar]
- 7.Spisni E, Tomasi V, Cestaro A, Tosatto SCE. Structural insights into the function of human caveolin 1. Biochemical and Biophysical Research Communications. 2005;338(3):1383–1390. [DOI] [PubMed] [Google Scholar]
- 8.Drab M, Verkade P, Elger M, et al. Loss of Caveolae, Vascular Dysfunction, and Pulmonary Defects in Caveolin-1 Gene-Disrupted Mice. Science. 2001;293(5539):2449–2452. [DOI] [PubMed] [Google Scholar]
- 9.Park DS, Woodman SE, Schubert W, et al. Caveolin-1/3 Double-Knockout Mice Are Viable, but Lack Both Muscle and Non-Muscle Caveolae, and Develop a Severe Cardiomyopathic Phenotype. The American Journal of Pathology. 2002;160(6):2207–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia T v. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Molecular Biology of the Cell. 1995;6(7):911–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Root KT, Plucinsky SM, Glover KJ. Recent Progress in the Topology, Structure, and Oligomerization of Caveolin: A Building Block of Caveolae. Current Topics in Membranes. Vol 75.; 2015:305–336. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Glover KJ. The transmembrane domain of caveolin-1 exhibits a helix–break–helix structure. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2012;1818(5):1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang G, Dong Z, Xu H, et al. Structural study of caveolin-1 intramembrane domain by circular dichroism and nuclear magnetic resonance. Biopolymers. 2015;104(1):11–20. [DOI] [PubMed] [Google Scholar]
- 14.Hoop CL, Sivanandam VN, Kodali R, Srnec MN, van der Wel PCA. Struc tural Characterization of the Caveolin Scaffolding Domain in Association with Cholesterol-Rich Membranes. Biochemistry. 2012;51(1):90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plucinsky SM, Glover KJ. Secondary Structure Analysis of a Functional Construct of Caveolin-1 Reveals a Long C-Terminal Helix. Biophysical journal. 2015;109(8):1686–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J-H, Schlebach JP, Lu Z, Peng D, Reasoner KC, Sanders CR. A pH-Mediated Topological Switch within the N-Terminal Domain of Human Caveolin-3. Biophysical Journal. 2016;110(11):2475–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prosser RS, Evanics F, Kitevski JL, Al-Abdul-Wahid MS. Current applications of bicelles in NMR studies of membrane-associated amphiphiles and proteins. Biochemistry. 2006;45(28):8453–8465. [DOI] [PubMed] [Google Scholar]
- 18.Poget SF, Girvin ME. Solution NMR of membrane proteins in bilayer mimics: small is beautiful, but sometimes bigger is better. Biochimica et biophysica acta. 2007;1768(12):3098–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piai A, Fu Q, Dev J, Chou JJ. Optimal Bicelle Size q for Solution NMR Studies of the Protein Transmembrane Partition. Chemistry (Weinheim an der Bergstrasse, Germany). 2017;23(6):1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dürr UHN, Gildenberg M, Ramamoorthy A. The magic of bicelles lights up membrane protein structure. Chemical reviews. 2012;112(11):6054–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaugrand M, Arnold AA, Hénin J, Warschawski DE, Williamson PTF, Marcotte I. Lipid concentration and molar ratio boundaries for the use of isotropic bicelles. Langmuir : the ACS journal of surfaces and colloids. 2014;30(21):6162–6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caldwell TA, Baoukina S, Brock AT, et al. Low- q Bicelles Are Mixed Micelles. The Journal of Physical Chemistry Letters. 2018;9(15):4469–4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietzen DJ, Hastings WR, Lublin DM. Caveolin Is Palmitoylated on Multiple Cysteine Residues. Journal of Biological Chemistry. 1995;270(12):6838–6842. [DOI] [PubMed] [Google Scholar]
- 24.Studier FW. Protein production by auto-induction in high density shaking cultures. Protein expression and purification. 2005;41(1):207–234. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Song KS, & Lisanti MP (1996). Expression and characterization of recombinant caveolin. Purification by polyhistidine tagging and cholesterol-dependent incorporation into defined lipid membranes. The Journal of Biological Chemistry, 271(1), 568–573. [PubMed] [Google Scholar]
- 26.Pike LJ, Han X, Chung K-N, Gross RW. Lipid Rafts Are Enriched in Arachidonic Acid and Plasmenylethanolamine and Their Composition Is Independent of Caveolin-1 Expression: A Quantitative Electrospray Ionization/Mass Spectrometric Analysis †. Biochemistry. 2002;41(6):2075–2088. [DOI] [PubMed] [Google Scholar]
- 27.Walser PJ, Ariotti N, Howes M, et al. Constitutive Formation of Caveolae in a Bacterium. Cell. 2012;150(4):752–763. [DOI] [PubMed] [Google Scholar]
- 28.Ariotti N, Rae J, Leneva N, et al. Molecular Characterization of Caveolin-induced Membrane Curvature. Journal of Biological Chemistry. 2015;290(41):24875–24890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieth MD, Lee J, Glover KJ. Probing the caveolin-1 P132L mutant: critical insights into its oligomeric behavior and structure. Biochemistry. 2012;51(18):3911–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glover KJ, Whiles JA, Wu G, Yu N, Deems R, Struppe JO, Stark RE, Komives EA, Vold RR. Structural evaluation of phospholipid bicelles for solution-state studies of membrane-associated biomolecules. Biophysical Journal. 2001. October;81(4):2163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez I, Ying Y, Albanesi J, Anderson RGW. Mechanism of caveolin filament assembly. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(17):11193–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer G, Ludwig B, Müller H-W, Broek JA, Friesen RHE, Schubert D. Studying membrane proteins in detergent solution by analytical ultracentrifugation: different methods for density matching. In: Analytical Ultracentrifugation Berlin V, Heidelberg: Springer Berlin Heidelberg; 1999:176–181. [Google Scholar]
- 33.Reynolds JA, Tanford C. Determination of molecular weight of the protein moiety in protein-detergent complexes without direct knowledge of detergent binding. Proceedings of the National Academy of Sciences of the United States of America. 1976;73(12):4467–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleming KG. Determination of Membrane Protein Molecular Weight Using Sedimentation Equilibrium Analytical Ultracentrifugation. In: Current Protocols in Protein Science. Vol Chapter 7. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2008;7.12.1–7.12.13. [DOI] [PubMed] [Google Scholar]
- 35.Dulhunty AF, & Franzini-Armstrong C The relative contributions of the folds and caveolae to the surface membrane of frog skeletal muscle fibres at different sarcomere lengths. The Journal of Physiology, 1975;250(3), 513–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vold RR, & Prosser RS (1996). Magnetically Oriented Phospholipid Bilayered Micelles for Structural Studies of Polypeptides. Does the Ideal Bicelle Exist? Journal of Magnetic Resonance, Series B, 1996;113(3), 267–271. [Google Scholar]
- 37.Wang L, Tonggu L. Membrane protein reconstitution for functional and structural studies. Science China Life Sciences. 2015;58(1):66–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SI Figure 1. A) Representative chromatogram of the nickel affinity column purification of caveolin-1. B) Representative chromatogram of the size exclusion purification of caveolin-1. The peak fraction containing caveolin is marked with a star.
SI Figure 2. Western blot analysis of caveolin-1 throughout the purification. Lane 1, cell lysate; lane 2, post Empigen solubilization pellet, lane 3, Empigen solubilized fraction, lane 4, nickel affinity elution peak, lane 5, size exclusion elution peak, lane 6, mini-nickel spin column bicelle wash, lane 7, mini-nickel spin column elution.
SI Figure 3. Plots of ln(concentration) versus radius2 for bicelle solutions at different concentration of D2O used to generate figure 3.


