Abstract
Context
Lower sex hormone-binding globulin (SHBG) is associated with many diseases including cardiovascular disease, cancer, polycystic ovarian syndrome, arthritis, and liver disease. However, the definition of low SHBG and its prevalence in US adults are unknown.
Objective
To define low SHBG and to determine its prevalence and risk factors in US adults.
Design, Setting, and Participants
This cohort study included adults ≥20 years from the US National Health and Nutrition Examination Survey (NHANES) from 2013 to 2016 who had fasting serum SHBG.
Exposures
NHANES coverage during 2013-2016.
Main Outcomes Measures
Definition, prevalence, and risk factors of low SHBG.
Results
This study included 4093 adults (weighted sample size of 204 789 616) with a mean (SD) age of 47.5 (17.0) years. In a “healthy” reference sub-cohort of 1477 adults, low SHBG was defined as SHBG < 12.3 nmol/L in men < 50 years, <23.5 nmol/L in men ≥ 50 years, <14.5 nmol/L in women < 30 years, and <21.9 nmol/L in women ≥ 30 years. The estimated US national prevalence of low SHBG was 3.3% in men, 2.7% in women, and 3.0% overall. Risk factors for this condition in both men and women included higher body mass index, diabetes, ethnicity (being other than Hispanic, non-Hispanic black, or non-Hispanic white), chronic obstructive pulmonary disease, coronary heart disease, and smoking.
Conclusions
This study established the criteria for low SHBG among US adults. The estimated US national prevalence of low SHBG was 3.3% in men and 2.7% in women.
Keywords: sex hormone-binding globulin, prevalence, risk factor
Sex hormone-binding globulin (SHBG) is a plasma glycoprotein that can bind sex hormones. SHBG is important in transporting sex hormones and regulating their bioavailability (1). SHBG can also bind and activate its receptors on the cell surface to exert direct effects on cellular function (2,3). Recent studies indicate that SHBG may be involved in many diseases. In particular, lower levels of SHBG are reported to be associated with higher prevalence or incidence of hypertension (4), arterial stiffness (5), insulin resistance (3), type 2 diabetes (3), coronary heart disease (6), stroke (7), cancer (8,9), polycystic ovarian syndrome (10), arthritis (11), liver disease (12), and inflammation (13), highlighting the importance of investigating the condition of low SHBG. The mechanisms by which lower SHBG levels are related to an increased risk of these diseases are not well understood. It has been proposed that potential mechanisms include a combination of direct effects of SHBG on cellular function and indirect effects of SHBG through alterations in the balance between testosterone and estradiol (14).
However, low SHBG has not been defined for US adults. Previous SHBG-related studies conducted their analyses treating SHBG as either a continuous variable or a categorical variable with multiple categories (eg, quartiles). Due to the lack of a definition, the prevalence of low SHBG in the general population is unknown.
Using a representative cohort of US adults who participated in the US National Health and Nutrition Examination Survey (NHANES) from 2013 to 2016, this study aimed to define low SHBG among US adults through the determination of the reference interval of SHBG in a “healthy” sub-cohort. Subsequently, this study investigated the prevalence and risk factors of low SHBG.
Methods
Study Participants
The National Center for Health Statistics ethics review board approved the NHANES protocols, and written informed consent was obtained from all participants. This analysis of deidentified data did not involve direct interaction with participants and was not subject to institutional review board review. NHANES provides data from a representative sample of noninstitutionalized US population. From 2013 to 2016, a total of 4093 adults aged 20 years or older had fasting serum SHBG levels and were included in the analyses of this study.
SHBG Determination
SHBG data were obtained from the NHANES website. Fasting serum SHBG was measured at the National Center for Environmental Health. It was quantified based on the reaction of SHBG with immuno-antibodies and chemiluminescence measurements of the reaction products by a photomultiplier tube. The limit of detection was 0.80 nmol/L, well below the lowest SHBG reading of the cohort (ie, 6.70 nmol/L). The accuracy of the detection method was 97.6%.
Selection of a “Health” Reference Sub-cohort
A sub-cohort of “healthy” participants were selected based on the following exclusion criteria. The numbers of excluded participants were the number of participants who were excluded progressively: (1) those having a history of taking sex hormone medication including testosterone, progesterone, estrogen, or unspecified sex hormones (n = 400); (2) those having polycystic ovary syndrome or undergoing hysterectomy or ovary removal (n = 225); (3) those pregnant or breastfeeding (n = 46); and (4) those having a history of the following diseases: (a) cardiovascular diseases including heart attack, angina, congestive heart failure, coronary heart disease, or stroke (n = 334); (b) lung diseases including chronic bronchitis, chronic obstructive pulmonary disease, or emphysema (n = 162); (c) liver disease (n = 100); (d) kidney disease (n = 65); (e) thyroid disease (n = 183); (f) cancer (n = 139); (g) HIV (n = 7); (h) arthritis (n = 345); (i) diabetes (n = 237); or (j) hypertension (n = 373). The resulting 1477 individuals were included in the “healthy” reference sub-cohort.
Determination of Reference Intervals
Reference intervals of SHBG were determined using the method outlined by the Clinical and Laboratory Standards Institute for appropriate statistical determination of reference intervals (15,16). In brief, a cohort of healthy reference individuals (n = 1477) were selected as detailed in the previous section; the SHBG data were then normalized by natural log-transformation; the natural log-transformed SHBG data were truncated by excluding outliers (ie, outside of the range of mean ± 3 SD); the reference interval of natural log-transformed SHBG was established by calculating the 2.5th (mean − 2 SD) and 97.5th (mean + 2 SD) percentiles of the truncated natural log-transformed SHBG distribution (15); finally, inverse transformation of natural log-transformed SHBG values corresponding to mean ± 2 SD yielded the reference intervals for SHBG.
Statistical Analysis
Data from two NHANES cycles (2013-2014 and 2015-2016) were combined using the appropriate weighting methods (17). Four-year weights were calculated by dividing the fasting subsample 2-year weights by 2 (18) and used in all analyses to adjust for unequal selection probability and nonresponse bias following NHANES analytical guidelines (17). Estimated population means, medians, and proportions were reported. Descriptive statistics were presented as weighted median and interquartile range (nonnormally distributed continuous data), weighted mean and SD (approximately normally distributed continuous data), or weighted percentages (categorical data). The differences in the prevalence of low SHBG between those with and without a specific condition or disease were analyzed using the weighted Pearson Chi-square test. The differences in circulating SHBG concentrations between men and women were analyzed using the weighted Mann-Whitney U test.
The analyses of risk factors for low SHBG were conducted using weighted multivariable binary logistic regression analysis. Risk factors included age (continuous variable), ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, or other), body mass index (natural log-transformed, continuous variable), physically active (yes, no, or unknown), past or current smoker (yes, no, or unknown), and past or current alcohol drinker (yes, no, or unknown). Self-reported comorbidities with 3 categories (yes, no, or unknown) included heart attack, angina, congestive heart failure, coronary heart disease, stroke, chronic bronchitis, chronic obstructive pulmonary disease, emphysema, liver disease, kidney disease, thyroid disease, cancer, HIV, and arthritis. Potential risk factors for low SHBG also included diabetes (yes or no) and hypertension (yes, no, or unknown). Diabetes was defined as fasting plasma glucose ≥126 mg/dL, taking hypoglycemic drugs, or self-reported diagnosis (19). Hypertension was defined as systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg, or prior diagnosis and treatment of hypertension (20). Female-specific risk factors included self-reported pregnancy, breastfeeding, parity (≥2 deliveries), and oophorectomy, with 3 categories: yes, no, or unknown. Hysterectomy did not affect SHBG (21,22) and therefore was not treated as a confounder for low SHBG. Thirty-two participants had missing body mass index and excluded from this analysis. Therefore, the risk factor analysis was conducted in the remaining 4061 participants (a weighted sample size of 203 635 433).
Correlation between low SHBG and low testosterone (total testosterone <200 ng/dL) (23) was analyzed using weighted multivariable binary logistic regression, with or without adjustment for demographic factors, lifestyle confounders, and comorbidities.
All tests were 2-sided and a P-value of <0.05 was regarded as statistically significant. All statistical analyses were performed using SPSS version 27.0 (IBM SPSS Statistics for Windows, Armonk, NY, USA).
Results
The Characteristics of the Cohort
This study included a total of 4093 participants (weighted sample size of 204 789 616) aged 20 to 80 years, with a mean (SD) age of 47.5 (17.0) years. Table 1 describes the characteristics of the cohort. Out of these 4093 participants, 1477 were included in the “healthy” reference sub-cohort (weighted sample size of 80 718 623).
Table 1.
Characteristics of the cohort
| Men | Women | Overall | |
|---|---|---|---|
| n, unweighted | 1977 | 2116 | 4093 |
| n, weighted | 99 576 283 | 105 213 333 | 204 789 616 |
| SHBG, median (IQR), nmol/L | 38.9 (26.9-55.5) | 62.2 (41.4-96.4) | 48.9 (32.6-75.5) |
| Age, mean (SD), years | 46.9 (16.7) | 48.1 (17.1) | 47.5 (17.0) |
| BMI, median (IQR), kg/m2 | 28.1 (24.9-32.0) | 28.3 (23.7-33.9) | 28.2 (24.3-33.0) |
| Ethnicity,% | |||
| Hispanic | 15.8 | 15.1 | 15.5 |
| Non-Hispanic white | 65.8 | 64.2 | 65.0 |
| Non-Hispanic black | 9.8 | 12.0 | 10.9 |
| Other | 8.6 | 8.7 | 8.7 |
| Physical active, % | 54.0 | 50.2 | 52.0 |
| Smoker, % | 51.2 | 36.9 | 43.8 |
| Alcohol drinker, % | 86.2 | 76.8 | 81.4 |
| Hysterectomy, % | NA | 20.1 | NA |
| Oophorectomy, % | NA | 10.7 | NA |
| Pregnant, % | NA | 0.8 | NA |
| Breastfeeding, % | NA | 1.8 | NA |
| Parity (≥2 deliveries), % | NA | 55.8 | NA |
| Heart attack, % | 4.2 | 2.6 | 3.4 |
| Angina, % | 2.1 | 1.7 | 1.9 |
| Congestive HF, % | 2.5 | 2.4 | 2.4 |
| CHD, % | 4.1 | 2.8 | 3.4 |
| Stroke, % | 2.6 | 3.1 | 2.9 |
| Chronic bronchitis, % | 3.4 | 6.8 | 5.2 |
| COPD, % | 3.7 | 2.9 | 3.3 |
| Emphysema, % | 2.1 | 1.6 | 1.8 |
| Liver disease, % | 4.4 | 3.4 | 3.9 |
| Kidney disease, % | 2.3 | 3.0 | 2.7 |
| Thyroid disease, % | 3.8 | 18.3 | 11.3 |
| Cancer, % | 9.3 | 10.1 | 9.7 |
| HIV, % | 0.5 | 0.0 | 0.2 |
| Arthritis, % | 20.7 | 30.9 | 25.9 |
| HTN,% | 34.0 | 34.8 | 34.4 |
| DM, % | 16.2 | 13.7 | 14.9 |
Abbreviations: BMI, body mass index; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HF, heart failure; HIV, human immunodeficiency virus disease; HTN, hypertension; IQR, interquartile range; NA, not applicable; SHBG, sex hormone-binding globulin.
Reference Intervals of SHBG
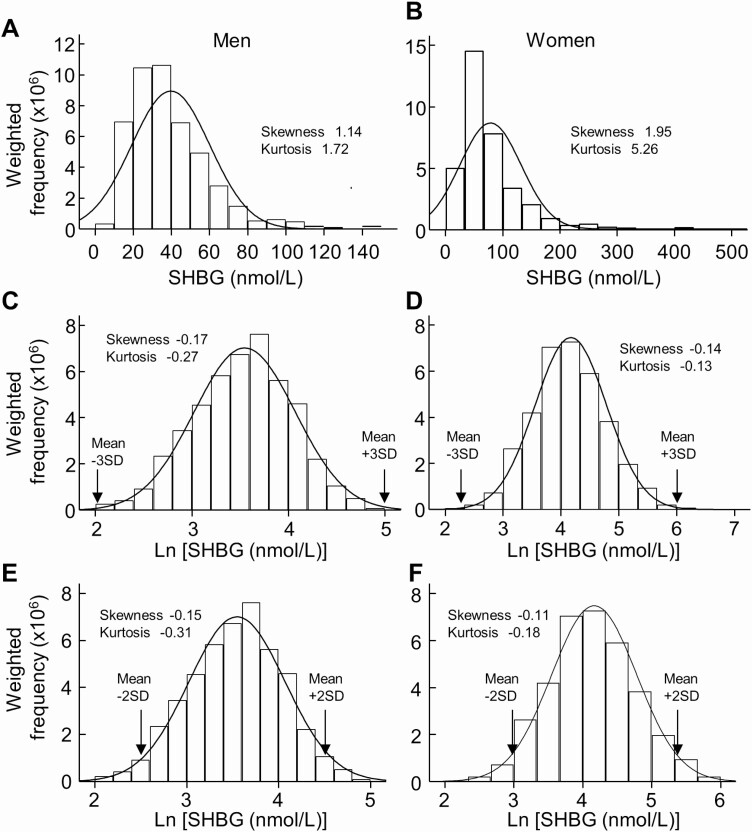
Serum levels of SHBG were higher in women than those in men in both the whole cohort and the reference sub-cohort (Fig. 1). Therefore, the reference interval of SHBG was determined separately for men and women. SHBG in the reference sub-cohort (n = 815 men, n = 662 women) showed a clear right-skewed shift from the Gaussian distribution for either sex (Fig. 2A and 2B). Natural log-transformed SHBG (Ln SHBG) appeared approximately normally distributed (Fig. 2C and 2D). After outlier rejection (2 cases were deemed as outliers with 1 from each sex, Fig. 2C and 2D), mean ± 2 SD of Ln SHBG values were calculated (Fig. 2E and 2F). Inverse transformation of Ln SHBG values corresponding to mean ± 2 SD yielded the reference intervals for SHBG: 12.6 to 92.4nmol/L in men and 18.4 to 211.5nmol/L in women (Reference Interval 1, Table 2).
Figure 1.
Serum levels of SHBG. Weighted levels of SHBG in the whole cohort (N = 4093, unweighted number (A) and the “healthy” sub-cohort (n = 1477, unweighted number (B). Error bars = SD. The difference between men and women was analyzed by the weighted Mann-Whitney U test. Abbreviation: SHBG, sex hormone-binding globulin.
Figure 2.
Determination of reference intervals of SHBG in men and women. Weighted data distribution of SHBG in the sub-cohort of “healthy” men (A) (n = 815, unweighted number) and women (B) (n = 662, unweighted number) and weighted data distribution of natural log-transformed SHBG (Ln SHBG) in the “healthy” men (C) and women (D). After truncation of Ln SHBG values, which were outside of mean ± 3 SD, mean ± 2 SD of Ln SHBG was calculated in the “healthy” men (E) (n = 814, unweighted number) and women (F) (n = 661, unweighted number). The resulting reference interval of SHBG in men was 12.6 to 92.4 nmol/L, and it was 18.4 to 211.5 nmol/L in women. Abbreviation: SHBG, sex hormone-binding globulin.
Table 2.
Reference intervals of SHBG in the “healthy” sub-cohort of men and women
| Number, unweighted | Mean (nmol/L) | Reference intervals (nmol/L) | ||
|---|---|---|---|---|
| Lower boundary | Upper boundary | |||
| Reference Interval 1: Sex-specific | ||||
| Men | 814 | 34.1 | 12.6 | 92.4 |
| Women | 661 | 62.4 | 18.4 | 211.5 |
| Reference Interval 2: Sex- and age-specific | ||||
| Men, years | ||||
| 20-29 | 257 | 30.2 | 12.2 | 74.6 |
| 30-39 | 223 | 30.7 | 11.3 | 83.3 |
| 40-49 | 164 | 34.1 | 14.4 | 81.2 |
| 50-59 | 95 | 49.7 | 21.8 | 113.3 |
| 60-80 | 74 | 55.9 | 27.4 | 114.1 |
| Women, years | ||||
| 20-29 | 229 | 60.2 | 14.5 | 249.8 |
| 30-39 | 192 | 64.0 | 21.4 | 191.5 |
| 40-49 | 139 | 68.2 | 23.8 | 195.5 |
| 50-59 | 62 | 54.9 | 20.8 | 144.9 |
| 60-80 | 38 | 62.6 | 22.6 | 173.5 |
| Reference Interval 3: Simplified sex- and age-specific | ||||
| Men, years | ||||
| 20-49 | 644 | 31.3 | 12.3 | 79.6 |
| 50-80 | 169 | 52.0 | 23.5 | 114.7 |
| Women, years | ||||
| 20-29 | 229 | 60.2 | 14.5 | 249.8 |
| 30-80 | 431 | 63.9 | 21.9 | 186.0 |
Abbreviations: SHBG, sex hormone-binding globulin.
SHBG concentrations changed over decades of human life (Fig. 3). Therefore, age-specific reference intervals were determined for each decade of age (Reference Interval 2, Table 2). The results obtained (Fig. 3, Table 2) suggested that men may be regrouped as those aged 20 to 49 years and those aged 50 to 80 years, and women may be regrouped as those aged 20 to 29 years and those aged 30 to 80 years. Thus, these simplified sex- and age-specific reference intervals were determined (Reference Interval 3, Table 2).
Figure 3.
Serum levels of SHBG in the “healthy” sub-cohort of men (n = 814, unweighted number) and women (n = 661, unweighted number) over the years of human life. Box plots of natural log-transformed SHBG in “healthy” men (A) and women (B) in each decades of human life. Abbreviation: SHBG, sex hormone-binding globulin.
The lower boundary values of the SHBG reference intervals were used as criteria to define low SHBG. To choose the best criteria for low SHBG, the prevalence of low SHBG in the reference group (n = 1477) was calculated according to 3 criteria derived from those 3 reference intervals (Table 2). The results suggested that the optimal criteria were those from the simplified sex- and age-specific criteria (Criteria 3, Table 3), as this set of criteria resulted in a prevalence of low SHBG close to the expected 2.5% in the reference sub-cohort. Therefore, Criteria 3 was the chosen criteria for low SHBG by this study.
Table 3.
Criteria and prevalence of low SHBG in the “healthy” reference sub-cohort (n = 1477, unweighted)
| Criteria for low SHBG (nmol/L) | Prevalence of low SHBG (%) | |||
|---|---|---|---|---|
| Men | Women | Overall | ||
| Criteria 1: Sex-specific | Men: <12.6 Women: < 18.4 |
2.1 | 1.6 | 1.9 |
| Criteria 2: Sex- and age-specific | Men 20-29 years: < 12.2 30-39 years: < 11.3 40-49 years: < 14.4 50-59 years: < 21.8 60-80 years: < 27.4 Women 20-29 years: < 14.5 30-39 years: < 21.4 40-49 years: < 23.8 50-59 years: < 20.8 60-80 years: < 22.6 |
3.6 | 1.7 | 2.8 |
| Criteria 3: Simplified sex- and age-specific | Men 20-49 years: < 12.3 50-80 years: < 23.5 Women 20-29 years: < 14.5 30-80 years: < 21.9 |
2.9 | 1.8 | 2.4 |
Prevalence of low SHBG
According to Criteria 3 (Table 3), the estimated US national prevalence of low SHBG was 3.3% in men, 2.7% in women, and 3.0% overall. Prevalence of low SHBG in sub-cohorts with various conditions, status, or diseases are listed in Table 4. Of note, participants with diabetes or undergoing oophorectomy had a high prevalence of low SHBG, 7.1% and 4.8%, respectively (Table 4).
Table 4.
Prevalence of the condition of low SHBG in sub-cohorts with various conditions, status, or diseases
| Participants without the specified condition, status, or disease (control) | Participants with the specified condition, status, or disease | P valueb | ||||
|---|---|---|---|---|---|---|
| Unweighted (n) | Weighted prevalencea (%) | Number, unweighted | Weighted prevalencea (%) | Change from control (%) | ||
| Hysterectomy | 1508 | 2.7 | 420 | 3.2 | 19 | <0.001 |
| Oophorectomy | 1702 | 2.5 | 207 | 4.8 | 92 | <0.001 |
| Pregnant | 497 | 1.7 | 15 | 0.0 | −100 | <0.001 |
| Breastfeeding | 90 | 1.2 | 33 | 1.4 | 17 | <0.001 |
| Hispanic | 2964 | 2.9 | 1129 | 3.7 | 28 | <0.001 |
| Non-Hispanic white | 2502 | 3.3 | 1591 | 2.9 | −12 | <0.001 |
| Non-Hispanic black | 3335 | 3.1 | 758 | 2.2 | −29 | <0.001 |
| Non-Hispanic other | 3478 | 2.8 | 615 | 3.9 | 39 | <0.001 |
| Heart attack | 3921 | 3.0 | 169 | 3.3 | 10 | <0.001 |
| Angina | 3994 | 3.0 | 94 | 1.8 | −40 | <0.001 |
| Congestive HF | 3947 | 3.1 | 142 | 1.8 | −42 | <0.001 |
| CHD | 3911 | 3.0 | 171 | 3.2 | 7 | <0.001 |
| Stroke | 3944 | 3.0 | 142 | 3.0 | 0 | <0.001 |
| Chronic bronchitis | 3847 | 3.0 | 232 | 3.4 | 13 | <0.001 |
| COPD | 3947 | 3.0 | 143 | 3.3 | 10 | <0.001 |
| Emphysema | 4005 | 3.1 | 85 | 1.4 | −55 | <0.001 |
| Liver disease | 3903 | 3.0 | 184 | 3.6 | 20 | <0.001 |
| Kidney disease | 3936 | 3.0 | 151 | 1.8 | −40 | <0.001 |
| Thyroid disease | 3633 | 2.9 | 456 | 4.1 | 41 | <0.001 |
| Cancer | 3722 | 3.0 | 369 | 3.4 | 13 | <0.001 |
| HIV | 2700 | 3.3 | 14 | 0.0 | −100 | <0.001 |
| Arthritis | 3013 | 2.9 | 1072 | 3.3 | 14 | <0.001 |
| HTN | 2431 | 2.7 | 1598 | 3.4 | 26 | <0.001 |
| DM | 3325 | 2.3 | 768 | 7.1 | 209 | <0.001 |
Abbreviations: CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HF, heart failure; HIV, human immunodeficiency virus disease; HTN, hypertension.
aLow SHBG was defined as SHBG < 12.3 nmol/L in men < 50 years, <23.5nmol/L in men ≥ 50 years, <14.5nmol/L in women < 30 years, and <21.9nmol/L in women ≥ 30 years.
bDifferences in the prevalence of low SHBG between those with and without the specified condition, status, or disease were analyzed using the weighted Pearson Chi-square test.
Risk Factors for Low SHBG
There were some similarities and differences in risk factor profiles between men and women (Table 5). Risk factors for low SHBG in both sexes included higher body mass index, diabetes, ethnicity (being other than Hispanic, non-Hispanic black, or non-Hispanic white), chronic obstructive pulmonary disease, coronary heart disease, and smoking. Female-specific risk factors for low SHBG included oophorectomy, thyroid disease, angina, breastfeeding, and hypertension. Male-specific risk factors for low SHBG included cancer, alcohol drinking, and heart attack (Table 5).
Table 5.
Odds ratios (95% CI) of various conditions, status, or diseases for low SHBG in 4061 participants analyzed by weighted multivariable binary logistic regression
| Men | Women | |||||
|---|---|---|---|---|---|---|
| ORa | 95% CI | P-value | ORa | 95% CI | P-value | |
| Pregnancy | NA | NA | NA | 0.00 | 0-1.4E+27 | 0.677 |
| Breastfeeding | NA | NA | NA | 1.94 | 1.91-1.97 | <0.001 |
| Parity(≥2 deliveries) | NA | NA | NA | 0.74 | 0.74-0.75 | <0.001 |
| Oophorectomy | NA | NA | NA | 2.13 | 2.13-2.14 | <0.001 |
| Age, y | 1.02 | 1.02-1.02 | <0.001 | 0.98 | 0.98-0.98 | <0.001 |
| Ethnicity | ||||||
| Hispanic | 1.00 | 1.00 | ||||
| Non-Hispanic white | 0.65 | 0.64-0.65 | <0.001 | 1.08 | 1.08-1.08 | <0.001 |
| Non-Hispanic black | 0.76 | 0.76-0.76 | <0.001 | 0.43 | 0.43-0.44 | <0.001 |
| Non-Hispanic other | 1.59 | 1.58-1.60 | <0.001 | 2.19 | 2.18-2.20 | <0.001 |
| Ln [BMI (kg/m2)] | 13.75 | 13.67-13.83 | <0.001 | 21.11 | 20.99-21.22 | <0.001 |
| Physically active | 0.68 | 0.68-0.68 | <0.001 | 1.07 | 1.06-1.07 | <0.001 |
| Smoker | 1.02 | 1.02-1.02 | <0.001 | 1.43 | 1.43-1.44 | <0.001 |
| Alcohol drinker | 1.90 | 1.89-1.91 | <0.001 | 0.92 | 0.91-0.92 | <0.001 |
| Heart attack | 1.63 | 1.62-1.64 | <0.001 | 0.00 | 0-1.4E+11 | 0.425 |
| Angina | 0.47 | 0.46-0.47 | <0.001 | 1.99 | 1.97-2.02 | <0.001 |
| Congestive HF | 0.28 | 0.28-0.28 | <0.001 | 0.64 | 0.63-0.65 | <0.001 |
| CHD | 1.68 | 1.67-1.69 | <0.001 | 1.27 | 1.26-1.28 | <0.001 |
| Stroke | 0.94 | 0.93-0.95 | <0.001 | 1.01 | 1.00-1.02 | 0.038 |
| Chronic bronchitis | 0.36 | 0.36-0.36 | <0.001 | 1.05 | 1.04-1.05 | <0.001 |
| COPD | 1.77 | 1.76-1.78 | <0.001 | 1.23 | 1.22-1.24 | <0.001 |
| Emphysema | 0.29 | 0.28-0.29 | <0.001 | 0.28 | 0.27-0.28 | <0.001 |
| Liver condition | 1.06 | 1.05-1.06 | <0.001 | 0.44 | 0.44-0.44 | <0.001 |
| Kidney disease | 0.81 | 0.81-0.82 | <0.001 | 0.12 | 0.11-0.12 | <0.001 |
| Thyroid disease | 0.6 | 0.6-0.61 | <0.001 | 2.41 | 2.40-2.41 | <0.001 |
| Cancer | 3.22 | 3.21-3.23 | <0.001 | 0.53 | 0.53-0.53 | <0.001 |
| HIV | 0.00 | 0-2.4E+39 | 0.749 | 0.00 | 0-2.9E+167 | 0.938 |
| Arthritis | 0.79 | 0.79-0.79 | <0.001 | 0.71 | 0.71-0.71 | <0.001 |
| DM | 2.65 | 2.64-2.65 | <0.001 | 3.49 | 3.49-3.51 | <0.001 |
| HTN | 0.46 | 0.46-0.46 | <0.001 | 1.40 | 1.39-1.40 | <0.001 |
Abbreviations: CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HF, heart failure; HIV, human immunodeficiency virus disease; HTN, hypertension; Ln [BMI (kg/m2)], natural log-transformed body mass index (kg/m2); NA, not applicable; OR, odds ratio.
aOR of the absence of the specified condition, status, or disease for low SHBG was regarded as 1.
Association of Low Testosterone With Low SHBG
Low testosterone was strongly associated with low SHBG with an odds ratio (95%CI) of 5.28 (5.25-5.29), after adjustment for all the tested confounders (Table 6).
Table 6.
Association of low testosterone (independent variable) with low SHBG (dependent variable) in 4061 participants analyzed by weighted multivariable binary logistic regression
| Odds ratio | 95% CI | P-value | |
|---|---|---|---|
| Model 1 | 1.34 | 1.34-1.34 | <0.001 |
| Model 2 | 9.85 | 9.82-9.87 | <0.001 |
| Model 3 | 5.44 | 5.43-5.46 | <0.001 |
| Model 4 | 5.28 | 5.25-5.29 | <0.001 |
Model 1: unadjusted. Model 2: adjusted for age, sex, and race/ethnicity. Model 3: adjusted for all the factors in Model 2 plus body mass index (natural log-transformed), physical activity, smoking status, and alcohol drinking status. Model 4: adjusted for all the factors in Model 3 plus comorbidities including heart attack, angina, congestive heart failure, coronary heart disease, stroke, chronic bronchitis, chronic obstructive pulmonary disease, emphysema, liver condition, kidney disease, thyroid disease, cancer, HIV, arthritis, diabetes, and hypertension.
Discussion
This study defined criteria for low SHBG in US adults, which were SHBG < 12.3 nmol/L in men < 50 years, <23.5 nmol/L in men ≥ 50 years, <14.5 nmol/L in women < 30 years, and <21.9 nmol/L in women ≥ 30 years. In addition, this study showed that the estimated US national prevalence of low SHBG was 3.3% in men, 2.7% in women, and 3.0% overall. Moreover, risk factors for this condition in both men and women included higher body mass index, diabetes, ethnicity (being other than Hispanic, non-Hispanic black, or non-Hispanic white), chronic obstructive pulmonary disease, coronary heart disease, and smoking.
To my knowledge, there are no prior studies reporting the definition of low SHBG for US adults. This deficiency becomes more problematic given that accumulating studies have shown that a lower level of SHBG, which was treated as a continuous variable or a multilevel categorical variable, is associated with many diseases including cardiovascular diseases (3-7), cancer (8,9), liver disease (12), arthritis (11), and polycystic ovarian syndrome (10). Therefore, the availability of the definition of low SHBG, provided by the current study, could facilitate future research to investigate the role of low SHBG in the pathogenesis of other diseases as well as the suitability of using low SHBG as a potential therapeutic target.
A few studies investigated the reference interval of SHBG with participants from other countries [eg, Chinese men with a low SHBG cutoff of 11.5 nmol/L (24) and Kuwait men with a low SHBG cutoff of 6.5nmol/L (25)]. The study with Kuwait men (25) also supported the current study’s approach of re-grouping men to <50 years and ≥50 years as that study similarly showed that the mean SHBG in men ≥ 50 years was higher than that in those <50 years. The cutoffs of low SHBG in Chinese and Kuwait men were lower than those for US men reported in the current study. This may be due to differences in ethnicity, location, lifestyle, or other factors.
In the United States, each laboratory or hospital uses its in-house criteria for low SHBG. For example, Mayo Clinic Laboratories use a low SHBG cutoff of 13.3 nmol/L for men and 18.2 nmol/L for women ≤ 46 years and 16.8nmol/L for women > 47 years, whereas the Department of Pathology from the University of Iowa uses a cutoff of 10 nmol/L for men and 20 nmol/L for women.
The current study has a few advantages in establishing the reference interval of SHBG for US adults. First, it used a representative US adult cohort selected by the National Center for Health Statistics from 15 different counties. Second, it used an extensive list of exclusion criteria to select the “healthy” reference group. Third, it investigated the reference intervals for different age groups, simplified the age-specific reference intervals, and compared and finally selected the best criteria for low SHBG.
SHBG is commonly measured by immunological and mass spectrometric assays (26). The detection limits of most assays range from 0.1 to 2.0 nmol/L (26-28), well below the cutoffs for low SHBG established by the current study. Immunological assays are indirect detection methods and employ a standard curve that has been set up with the use of a standard preparation. The accuracy of the assays may be affected by nonspecificity bias, as the preparation of the standard may differ from the individual serum composition in which SHBG locates (29). The mass spectrometric assays directly measure SHBG; however, the accuracy of the assays may be affected by various procedures such as sample purification.
Different methods or platforms for SHBG detection have different variability. For example, the mass spectrometry-IALGGLLFPASNLR peptide assay had a coefficient of variation (CV) of 9.0% at the control SHBG level of 10.7 nmol/L, whereas the mass spectrometry-stable isotope standards and capture by anti-peptide antibodies assay had a CV of 15.3% at the control SHBG level of 10.9 nmol/L. Immunological assays seemed to have a lower CV in general (26). For example, COBAS e411 had an intraassay CV of 2.1% and an interassay CV of 2.7% at the control SHBG level of 14 nmol/L, and Immulite 2000 had an intraassay CV of 2.7% and an interassay CV of 4.0% at the control SHBG level of 5.4 to 5.5nmol/L (26).
Different assay methods or platforms can produce different SHBG readings (26,27). For example, a recent study compared four commonly used immunoassay platforms (ie, Abbott Architect, Roche, Beckman, and Siemens) and found that the Roche platform produced the highest readings whereas the Abbott Architect platform produced the lowest (27). That study also showed that the major difference in SHBG readings was from the high end of SHBG concentrations (27). For example, different platforms could produce results that were 30% off from each other when SHBG was >100 nmol/L; however, when SHBG was <25 nmol/L, all the 4 platforms produced strikingly similar results, with ~3% variation among them (27). These results suggest that variation in cutoff values of low SHBG may be less likely due to the platforms used and rather may be due to differences in selection of the “healthy” reference group, composition of the reference group, thoroughness of the investigation, or other factors.
This study revealed that the prevalence of low SHBG was higher in people with diabetes (7.1%) compared to that in nondiabetic counterparts (2.3%). In addition, diabetes posted a 2.65-fold and 3.52-fold higher risk for low SHBG in men and women, respectively, after adjustment for all the tested confounders. These observations are consistent with the previous finding that lower levels of SHBG were associated with insulin resistance and type 2 diabetes (3). Genetic studies, which are less likely to be confounded, biased, or influenced by disease processes, showed that SHBG-raising alleles were associated with a reduced risk of type 2 diabetes (30), suggesting that SHBG may be involved in the etiology of diabetes. However, the causal relationship between low SHBG and diabetes cannot be established by the current study due to its cross-sectional nature. Indeed, diabetes may also lead to low SHBG, as insulin and carbohydrate can decrease SHBG production (3). The importance of low SHBG in the pathogenesis of diabetes warrants further investigation.
The study also found that women undergoing oophorectomy had a higher prevalence of low SHBG of 4.8%, compared to the prevalence of 2.5% in women without oophorectomy. The causal relationship between oophorectomy and low SHBG needs to be clarified in the future. SHBG is produced by the liver (31) and its concentration, as demonstrated by the current study, was relatively consistent over decades of age in women aged 30 to 80 years. Why ovary removal might affect SHBG production is unclear. Given that the estimated US national percentage of oophorectomy among women was high (10.7%), whether the association between oophorectomy and low SHBG has any clinical implication needs to be investigated in the future.
This study also showed that a higher body mass index was the biggest risk factor for low SHBG, with a 1-SD change in log-transformed value representing a 13-fold and 20-fold higher risk of low SHBG in men and women, respectively, after adjustment for all tested confounders. This is consistent with literature reports that obese people had low plasma SHBG levels compared to nonobese counterparts (32), and weight loss was associated with an increase in SHBG (33). The current study also found smoking was a risk factor for low SHBG in both men and women. These results suggest that weight loss and smoking cessation may be effective means to treat low SHBG.
This study confirmed that low SHBG was positively associated with many diseases in both men and women such as diabetes, chronic obstructive pulmonary disease, and coronary heart disease. However, there are sex differences in the associations between low SHBG and other diseases. For example, thyroid disease and hypertension were positively associated with low SHBG in women, whereas these associations were negative in men. On the other hand, cancer was positively associated with low SHBG in men, whereas the association was a negative one in women. The reasons underlying these sex differences are not clear and need to be investigated in the future.
Strengths and Limitations
A strength of this study is the large sample size which is representative of the US general population (a weighted sample size of 204 789 616). This study has a number of limitations. First, most diseases except for diabetes and hypertension were based on self-reported data. Second, this study defined diabetes as fasting plasma glucose ≥126 mg/dL, taking hypoglycemic drugs, or self-reported diagnosis. However, according to the American Diabetes Association guidelines (19), assessment of diabetes was defined according to the fulfillment of 1 of the following criteria: fasting plasma glucose ≥126 mg/dL, 2-h plasma glucose ≥200 mg/dL, hemoglobin A1c ≥ 6.5%, or a random plasma glucose ≥200mg/dL in a patient with classic symptoms of hyperglycemia. Therefore, the diabetes status in the current study could be underestimated. Third, this study is based on cross-sectional data; therefore, the causal relationship between low SHBG and other diseases cannot be established.
In conclusion, this study provided data on the definition, prevalence, and risk factors of low SHBG for US adults. The availability of these data would facilitate future research investigating the role of low SHBG in the pathogenesis of many other diseases.
Acknowledgments
Financial Support: Y.W. was supported by a grant from the National Health and Medical Research Council of Australia (1062671).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Contributions: Y.W. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Y.W. solely contributed to all aspects of this study.
Additional Information
Disclosures: None reported.
Data Availability
All data in the current analysis are publicly available on the NHANES website.
References
- 1. Mendel CM. The free hormone hypothesis. Distinction from the free hormone transport hypothesis. J Androl. 1992;13(2):107-116. [PubMed] [Google Scholar]
- 2. Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Sex hormone-binding globulin mediates steroid hormone signal transduction at the plasma membrane. J Steroid Biochem Mol Biol. 1999;69(1-6):481-485. [DOI] [PubMed] [Google Scholar]
- 3. Le TN, Nestler JE, Strauss JF 3rd, Wickham EP 3rd. Sex hormone-binding globulin and type 2 diabetes mellitus. Trends Endocrinol Metab. 2012;23(1):32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daka B, Rosen T, Jansson PA, Larsson CA, Råstam L, Lindblad U. Low sex hormone-binding globulin is associated with hypertension: a cross-sectional study in a Swedish population. BMC Cardiovasc Disord. 2013;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Han K, Chun H, Kim MJ, et al. Low levels of sex hormone-binding globulin constitute an independent risk factor for arterial stiffness in Korean women. Int J Endocrinol. 2017;2017:6956495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reinecke H, Bogdanski J, Woltering A, et al. Relation of serum levels of sex hormone binding globulin to coronary heart disease in postmenopausal women. Am J Cardiol. 2002;90(4):364-368. [DOI] [PubMed] [Google Scholar]
- 7. Madsen TE, Luo X, Huang M, et al. Circulating SHBG (sex hormone-binding globulin) and risk of ischemic stroke: findings from the WHI. Stroke. 2020;51(4):1257-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He XY, Liao YD, Yu S, Zhang Y, Wang R. Sex hormone binding globulin and risk of breast cancer in postmenopausal women: a meta-analysis of prospective studies. Horm Metab Res. 2015;47(7):485-490. [DOI] [PubMed] [Google Scholar]
- 9. Arthur RS, Xue X, Rohan TE. Prediagnostic circulating levels of sex steroid hormones and SHBG in relation to risk of ductal carcinoma in situ of the breast among UK women. Cancer Epidemiol Biomarkers Prev. 2020;29(5):1058-1066. [DOI] [PubMed] [Google Scholar]
- 10. Zhu JL, Chen Z, Feng WJ, Long SL, Mo ZC. Sex hormone-binding globulin and polycystic ovary syndrome. Clin Chim Acta. 2019;499:142-148. [DOI] [PubMed] [Google Scholar]
- 11. Qu Z, Huang J, Yang F, Hong J, Wang W, Yan S. Sex hormone-binding globulin and arthritis: a Mendelian randomization study. Arthritis Res Ther. 2020;22(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang X, Xie J, Pang J, et al. Serum SHBG is associated with the development and regression of nonalcoholic fatty liver disease: a prospective study. J Clin Endocrinol Metab. 2020;105(3): e791-e804. [DOI] [PubMed] [Google Scholar]
- 13. Maggio M, Ceda GP, Lauretani F, et al. SHBG, sex hormones, and inflammatory markers in older women. J Clin Endocrinol Metab. 2011;96(4):1053-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol. 2010;316(1):79-85. [DOI] [PubMed] [Google Scholar]
- 15. Yamada C, Mitsuhashi T, Hiratsuka N, Inabe F, Araida N, Takahashi E. Optimal reference interval for homeostasis model assessment of insulin resistance in a Japanese population. J Diabetes Investig. 2011;2(5):373-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clinical and Laboratory Standards Institute. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline. 3rd ed.Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 17. Chen TC, Parker JD, Clark J, et al. National health and nutrition examination survey: estimation procedures, 2011-2014. Vital Health Stat 2. 2018;177:1-26. [PubMed] [Google Scholar]
- 18. Chen TC, Clark J, Riddles MK, et al. National Health and Nutrition Examination Survey, 2015-2018: sample design and estimation procedures. Vital Health Stat 2. 2020;184:1-35. [PubMed] [Google Scholar]
- 19. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S13-S28. [DOI] [PubMed] [Google Scholar]
- 20. Chobanian AV, Bakris GL, Black HR, et al. ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee . Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206-1252. [DOI] [PubMed] [Google Scholar]
- 21. Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Mühlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85(2):645-651. [DOI] [PubMed] [Google Scholar]
- 22. Kotsopoulos J, Shafrir AL, Rice M, et al. The relationship between bilateral oophorectomy and plasma hormone levels in postmenopausal women. Horm Cancer. 2015;6(1):54-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92(2):405-413. [DOI] [PubMed] [Google Scholar]
- 24. Yu S, Qiu L, Liu M, et al. Establishing reference intervals for sex hormones and SHBG in apparently healthy Chinese adult men based on a multicenter study. Clin Chem Lab Med. 2018;56(7):1152-1160. [DOI] [PubMed] [Google Scholar]
- 25. Kehinde EO, Akanji AO, Al-Hunayan A, et al. Do differences in age specific androgenic steroid hormone levels account for differing prostate cancer rates between Arabs and Caucasians? Int J Urol. 2006;13(4):354-361. [DOI] [PubMed] [Google Scholar]
- 26. Veldhuis JD, Bondar OP, Dyer RB, et al. Immunological and mass spectrometric assays of SHBG: consistent and inconsistent metabolic associations in healthy men. J Clin Endocrinol Metab. 2014;99(1):184-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adaway J, Keevil B, Miller A, Monaghan PJ, Merrett N, Owen L. Ramifications of variability in sex hormone-binding globulin measurement by different immunoassays on the calculation of free testosterone. Ann Clin Biochem. 2020;57(1):88-94. [DOI] [PubMed] [Google Scholar]
- 28. Stegeman BH, Helmerhorst FM, Vos HL, Rosendaal FR, Van Hylckama Vlieg A. Sex hormone-binding globulin levels are not causally related to venous thrombosis risk in women not using hormonal contraceptives. J Thromb Haemost. 2012;10(10):2061-2067. [DOI] [PubMed] [Google Scholar]
- 29. Cekan SZ. Biases in the assays of steroids and their binding proteins. J Steroid Biochem. 1987;27(1-3):95-98. [DOI] [PubMed] [Google Scholar]
- 30. Perry JR, Weedon MN, Langenberg C, et al. ; MAGIC . Genetic evidence that raised sex hormone binding globulin (SHBG) levels reduce the risk of type 2 diabetes. Hum Mol Genet. 2010;19(3):535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simó R, Sáez-López C, Barbosa-Desongles A, Hernández C, Selva DM. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab. 2015;26(7):376-383. [DOI] [PubMed] [Google Scholar]
- 32. Glass AR, Swerdloff RS, Bray GA, Dahms WT, Atkinson RL. Low serum testosterone and sex-hormone-binding-globulin in massively obese men. J Clin Endocrinol Metab. 1977;45(6):1211-1219. [DOI] [PubMed] [Google Scholar]
- 33. Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab. 2004;6(3):208-215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in the current analysis are publicly available on the NHANES website.