Abstract
IMPORTANCE
Identifying novel factors that protect against age-related diseases and promote healthy aging is critical to public health. Higher levels of circulating very-long-chain saturated fatty acids (VLSFAs) are integrated biomarkers of diet and metabolism shown to have beneficial associations in cardiovascular disease and total mortality, but whether they are associated with overall healthy aging is unknown.
OBJECTIVE
The objective of the study was to examine the association of circulating levels of three VLSFAs with unhealthy aging events, including incident chronic disease (cardiovascular disease, cancer, lung disease or severe kidney disease), physical dysfunction and cognitive decline.
DESIGN.
The study design was a prospective cohort study utilizing 1992–2014 data from the Cardiovascular Health Study (CHS).
SETTING.
The CHS is a multicenter, population-based study of cardiovascular disease among older adults.
PARTICIPANTS.
Among the 4559 CHS participants with available fatty acid data, we excluded 1879 participants who had an age-related event before their first measurement. The remaining 2680 participants were included in the analyses.
MAIN OUTCOMES AND MEASURES.
Plasma phospholipid VLSFA levels were measured by thin layer chromatography followed by gas chromatography. The main outcome was the hazard ratio of an incident unhealthy aging event associated with serial measures of plasma arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0).
RESULTS
Among the 2680 study participants, the mean age was 74.7 years old at entry and 36.4% were male. During a median 7.5 years of follow up, 2484 participants experienced an unhealthy event. Compared with the lowest quintile, levels of 22:0 in the highest quintile of the fatty acid distribution were associated with lower risk of an unhealthy event (HR, 0.86; 95% CI, 0.74–0.97; P trend, 0.01), after adjustment for demographics, lifestyle factors, and clinical conditions. In analogous comparisons, levels of 24:0 were similarly associated with lower risk (HR, 0.84; 95% CI, 0.73–0.95; P trend, 0.001).
CONCLUSIONS AND RELEVANCE
Higher levels of circulating 22:0 and 24:0 are associated with lower risk of unhealthy aging events. These results highlight the need to explore determinants of circulating VLSFA for potential novel efforts to promote healthy aging.
Introduction
With increasing life expectancy, the population of older adults is growing rapidly worldwide.1 However, increased longevity does not necessarily translate into an increase in healthy life span, as older adults experience high rates of cardiovascular and other chronic diseases.2 Identifying novel factors that protect against age-related disease and promote healthy aging is critical to public health.
Higher levels of circulating very long-chain saturated fatty acids (VLSFAs), saturated fatty acids with 20 carbons or more, are integrated biomarkers of diet and metabolism that are associated with lower risk of heart failure,3,4 atrial fibrillation,5 and diabetes,6–8 which are chronic diseases that contribute to unhealthy aging, and lower risk of sudden cardiac arrest9 and total mortality.10 These widely beneficial associations led us to hypothesize that higher circulating levels of VLSFAs may be broadly associated with a greater likelihood of healthy aging.
We used data from the Cardiovascular Health Study (CHS), a prospective cohort study of risk factors for cardiovascular disease among older adults,11 to examine the associations of plasma phospholipid levels of three circulating VLSFAs, arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0), measured at the current study baseline and up to 2 more times during follow-up, with risk of an incident unhealthy aging event.
Methods
Study Population
CHS is a prospective, population-based cohort study of cardiovascular disease among older adults.11 Participants were recruited from four U.S. communities (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; Allegheny County, Pennsylvania) from a random sample generated from the Health Care Financing Administration files. Among eligible adults who were contacted, 57% agreed to participate. The cohort consists of 5201 non-institutionalized men and women, aged ≥65 y, recruited in 1989 through 1990, plus an additional 687 predominantly Black participants recruited in 1992 through 1993. The study was conducted using data from 1992 to 2014 and participants were followed for a median follow up of 7.5 years. Race was classified by self-identification. Each center’s institutional review board approved the study, and all participants provided informed written consent.
Plasma phospholipid fatty acids were measured up to 3 times: on blood drawn in 1992 through 1993, the study baseline (N = 3941), on blood drawn in 1998 through 1999 (N = 2609), and on blood drawn in 2005 through 2006 (N = 933). Among the 4559 participants with available fatty acid data from at least one time point, we excluded 1879 participants who had an age-related event before their first fatty acid measurement. The remaining 2680 participants were included in the analyses. Among these, 2434 had fatty acid data in 1992–1993, 1696 in 1998–1999, and 700 in 2005–2006.
Plasma phospholipid fatty acids
Blood was drawn after ≥ 8-h fasting, and plasma specimens were stored at −70°C. Plasma lipids were extracted by the method of Folch.12 Phospholipids were separated from other lipids by thin-layer-chromatography. Fatty acid methyl esters were prepared by direct trans-esterification of the phospholipid fraction13 and separated by gas chromatography using a fused-silica 100-m capillary column as previously described.14 Fatty acids were expressed as a weight percentage of total fatty acids. Inter-assay coefficients of variation for the VLSFA measurements were ≤3.5%.
Ascertainment of unhealthy aging events
Participants were followed by means of annual study-clinic examinations with interim phone contacts for 10 years and telephone contacts every 6 months thereafter. Cardiovascular events were adjudicated by a centralized Event Committee. Unhealthy aging was defined based on the established definition in CHS, as previously described,15 and shown in Table S1. Briefly, we defined an unhealthy event as any of the following: incident cardiovascular disease (myocardial infarction, heart failure, stroke, transient ischemic attack, or claudication), incident severe kidney disease (estimated glomerular filtration rate <10 or dialysis), incident chronic obstructive pulmonary disease, incident cancer, a decrease in cognition (first Mini Mental State Examination score ≤80), or an increase in difficulties of activities of daily living (first record of difficulty with ≥1 daily living activity).
Risk Factors
Information on medical history, medications, life style, and clinical risk factors was collected at annual clinic visits as previously reported.11 Diabetes was defined by glucose ≥126 mg/dL when participants reported fasting ≥8 h before venipuncture, glucose ≥200 mg/dL when fasting was <8 h, or use of insulin or oral hypoglycemic medication. Body mass index was calculated as body weight (kg) divided by height squared (m2). Waist circumference was measured at the umbilicus. Lipids, glucose, insulin, and inflammatory biomarkers were assessed on fasting blood samples using enzymatic methods.11 The self-reported depression score of 1 to 10 was based on the 10-item Centers for Epidemiological Studies’ Depression Scale.16
Statistical Analysis
Baseline (unadjusted) demographic characteristics, cardiometabolic risk factors, and lifestyle habits for the study population were summarized according to quintiles of 24:0. We used a Cox proportional-hazards model to evaluate the association between time-varying VLSFA levels, adjusting for time-varying covariates (updated at each fatty acid measurement) and the likelihood of unhealthy aging. Time-to-event was calculated as the time elapsed between VLSFA measurement and the earliest of: ascertainment of first unhealthy event, death, or date of last follow-up in 2014. The functional forms of the associations between VLSFAs and unhealthy aging were evaluated using natural cubic splines, and tests for non-linearity were performed by comparing the spline model to a linear model using the likelihood ratio test. Quintiles of VLSFA levels were defined from the baseline distributions. Comparisons between quintiles of VLSFA levels were evaluated using time-dependent indicator variables, with tests for trend based on a time-dependent linear variable for quintile. The associations were adjusted for age, race (Black, non-Black), sex, field center, education (less than high school, high school graduates, some college, college graduates), physical activity (number of city blocks walked in the previous week), body mass index, waist circumference, alcohol consumption, smoking status (never, former, current), self-reported health (excellent/very good, good, fair/poor), prevalent diabetes, systolic blood pressure, use of hypertensive medications, and depression score. Modification of the association of VLSFA with the risk of an unhealthy event was evaluated for age (linear), sex, body mass index (BMI, linear), and prevalent diabetes with statistical significance assessed via the Wald test for the interaction term, in models where VLSFAs were modeled linearly.
Several sensitivity analyses were performed. To assess the role of potential mediators, we fit a model additionally including variables for triglycerides and LDL and one further adjusted for 16:0. To assess the impact of other factors that could influence the associations, we fit separate models adjusting for i.) plasma phospholipid eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA); ii) lipid-lowering medications; and iii.) the sum of plasma phospholipid long chain saturated fatty acids (14:0+16:0+18:0). Finally, to examine whether reverse causation might have created these associations, we performed analyses excluding the first two years of events.
Missing covariates (<4% for all covariates) were imputed using data on age, gender, race, education, smoking, alcohol use, BMI, physical activity, self-reported health status, and prevalent coronary heart disease at baseline, as previously described.15 Results with imputed data are presented. The results were unchanged from analyses that excluded participants with missing values.
Analyses were performed using Stata version 14.0 (StataCorp, College Station, Texas) R version 4.05 (Vienna, Austria).
This report follows the STROBE guidelines for reporting methods, findings and study limitations for cohort studies.
Results
Participant mean age (SD) was 74.7 (4.8) years old at entry and 36.4% were male. Median levels of the plasma phospholipids 24:0, 22:0, and 20:0 were 1.38%, 1.66%, and 0.50% of total fatty acids, respectively. Baseline characteristics of the study participants across quintiles of 24:0 are shown in Table 1. Participants in the highest quintile of 24:0 were more likely to be male, Black, college graduates, and current smokers, with higher levels of LDL and physical activity, but they were less likely to have diabetes and showed lower average levels of triglycerides.
Table 1.
Baseline1 characteristics of the Cardiovascular Health Study participants by quintiles of plasma phospholipid levels of 24:0
| Q1 | Q2 | Q3 | Q4 | Q5 | |
|---|---|---|---|---|---|
|
| |||||
| Quintile cutpoints | <=1.17 | >1.17–1.31 | >1.31–1.45 | >1.45–1.63 | >1.63 |
| Quintile median | 1.07 | 1.25 | 1.38 | 1.53 | 1.78 |
| Number of participants | 546 | 532 | 530 | 540 | 532 |
|
| |||||
| Participant characteristics: | |||||
| Age, years | 75.51 ±5.03 | 75.01 ±4.88 | 74.56 ±4.70 | 74.50 ±4.83 | 73.93 ±4.62 |
| Male | 146 (26.7%) | 179 (33.6%) | 190 (35.8%) | 244 (45.2%) | 217 (40.8%) |
| Black | 47 (8.6%) | 49 (9.2%) | 54 (10.2%) | 60 (11.1%) | 98 (18.4%) |
| Field center | |||||
| NC | 122 (22.3%) | 126 (23.7%) | 134 (25.3%) | 140 (25.9%) | 146 (27.4%) |
| CA | 155 (28.4%) | 125 (23.5%) | 140 (26.4%) | 144 (26.7%) | 151 (28.4%) |
| MD | 128 (23.4%) | 127 (23.9%) | 119 (22.5%) | 108 (20.0%) | 112 (21.1%) |
| PA | 141 (25.8%) | 154 (28.9%) | 137 (25.8%) | 148 (27.4%) | 123 (23.1%) |
| Education | |||||
| <HS | 107 (19.6%) | 136 (25.6%) | 122 (23.0%) | 106 (19.6%) | 102 (19.2%) |
| HS graduate | 187 (34.2%) | 165 (31.0%) | 155 (29.2%) | 140 (25.9%) | 157 (29.5%) |
| Some coll. | 134 (24.5%) | 117 (22.0%) | 123 (23.2%) | 145 (26.9%) | 115 (21.6%) |
| College grad. | 118 (21.6%) | 114 (21.4%) | 130 (24.5%) | 149 (27.6%) | 158 (29.7%) |
| Body mass index (kg/m2) | 27.15 ±4.81 | 26.84 ±4.65 | 26.31 ±4.27 | 26.44 ±4.12 | 26.12 ±4.00 |
| Waist circumference (cm) | 98.01 ±14.22 | 97.58 ±13.41 | 95.82 ±12.20 | 95.86 ±12.13 | 94.96 ±12.19 |
| Systolic bp (mm hg) | 138.94 ±21.59 | 135.67 ±20.18 | 135.00 ±19.33 | 132.99 ±20.26 | 134.17 ±21.55 |
| LDL (mg/dl) | 112.63 ±31.76 | 123.34 ±30.10 | 129.80 ±30.34 | 132.73 ±30.65 | 139.18 ±30.79 |
| HDL (mg/dl) | 53.75 ±15.89 | 53.92 ±14.19 | 54.13 ±13.83 | 54.17 ±14.12 | 55.48 ±13.15 |
| Triglycerides (mg/dl) | 192.32 ±121.45 | 147.61 ±72.07 | 132.62 ±61.97 | 123.53 ±54.57 | 107.91 ±46.45 |
| C-reactive protein (mg/l) | 5.95 ±10.67 | 4.67 ±7.65 | 4.20 ±8.54 | 4.28 ±8.81 | 3.91 ±5.81 |
| Diabetes | 143 (26.2%) | 103 (19.4%) | 86 (16.2%) | 81 (15.0%) | 91 (17.1%) |
| # Alcoholic beverages/wk | 2.00 ±5.33 | 2.35 ±10.81 | 1.94 ±4.56 | 2.10 ±4.55 | 2.16 ±4.58 |
| Blocks walked in previous week | 37.80 ±62.56 | 48.47 ±80.71 | 47.37 ±66.42 | 54.29 ±74.58 | 47.75 ±69.76 |
| CES-D score | 5.19 ±4.54 | 5.24 ±4.64 | 4.45 ±4.11 | 4.62 ±4.68 | 4.45 ±4.29 |
| Anti-hypertensive medication | 292 (53.5%) | 242 (45.5%) | 215 (40.6%) | 208 (38.5%) | 213 (40.0%) |
| Lipid-lowering medications | 69 (12.6%) | 44 (8.3%) | 35 (6.6%) | 32 (5.9%) | 27 (5.1%) |
| Smoking status | |||||
| Never | 299 (54.8%) | 246 (46.2%) | 274 (51.7%) | 263 (48.7%) | 250 (47.0%) |
| Former | 211 (38.6%) | 237 (44.5%) | 206 (38.9%) | 241 (44.6%) | 230 (43.2%) |
| Current | 36 (6.6%) | 49 (9.2%) | 50 (9.4%) | 36 (6.7%) | 52 (9.8%) |
| Self-reported health | |||||
| Excellent/VG | 193 (35.3%) | 221 (41.5%) | 249 (47.0%) | 282 (52.2%) | 237 (44.5%) |
| Good | 267 (48.9%) | 238 (44.7%) | 224 (42.3%) | 206 (38.1%) | 226 (42.5%) |
| Fair/Poor | 86 (15.8%) | 73 (13.7%) | 57 (10.8%) | 52 (9.6%) | 69 (13.0%) |
| Dietary intake2 | |||||
| Energy intake, kcal/day | 1922.53 ±607.18 | 2002.80 ±612.90 | 2098.88 ±681.90 | 1956.53 ±591.68 | 2035.40 ±642.27 |
| Protein, % energy | 19.44 ±3.32 | 19.17 ±3.04 | 18.99 ±3.04 | 19.01 ±3.19 | 19.01 ±3.03 |
| Carbohydrate, % energy | 53.58 ±8.39 | 52.75 ±7.95 | 52.52 ±7.81 | 52.45 ±7.66 | 52.42 ±7.60 |
| Total fat, % energy | 30.77 ±6.16 | 31.58 ±6.12 | 32.11 ±5.98 | 32.20 ±5.92 | 32.30 ±5.92 |
| Saturated fat, % energy | 9.81 ±2.37 | 10.13 ±2.30 | 10.18 ±2.15 | 10.14 ±2.13 | 10.12 ±2.16 |
| Polyunsaturated fat, % energy | 7.12 ±2.11 | 7.23 ±2.19 | 7.51 ±2.15 | 7.64 ±2.31 | 7.70 ±2.12 |
| Monounsaturated fat, % energy | 11.01 ±2.53 | 11.37 ±2.49 | 11.55 ±2.40 | 11.54 ±2.36 | 11.65 ±2.39 |
| Peanuts, servings/week | 1.02 ±1.47 | 1.18 ±1.50 | 1.40 ±1.66 | 1.47 ±1.73 | 2.01 ±2.03 |
Baseline is the time of the 1st VLSFA measurement
Diet was assessed in 1989–90 and available only in participants enrolled in 1989–90.
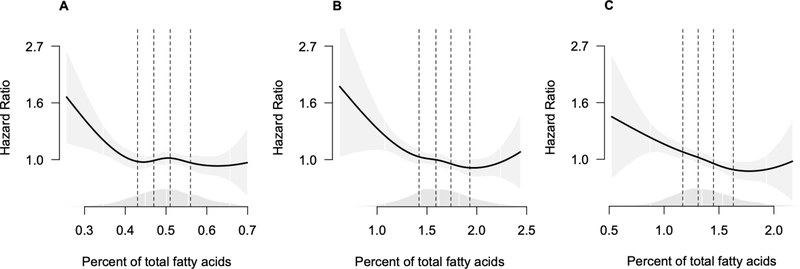
During up to 23 years of follow-up, 2484 participants in the previously healthy population experienced an event of unhealthy aging. The distribution of the first events is shown in Table 2, with the most frequent first event being CVD and the least frequent being severe kidney disease. Higher levels of plasma phospholipid 24:0 and 22:0 were each associated with lower risk of unhealthy aging after adjustment for demographics, adiposity measures, physical activity, smoking, alcohol, diabetes, self-reported health, hypertension, and depression score (Table 3). A one standard deviation higher value of 24:0 was associated with 15% lower risk of unhealthy aging (HR: 0.85, 95% CI: 0.77–0.94). Analyses using cubic splines show the associations with lower risk were linear over most of the data, as suggested from the quintile analyses shown in Table 3, with possibly higher risk at very high levels of 22:0 and 24:0 (Figure 1 and Figure S1). However, tests of non-linearity comparing cubic splines with linear models did not show strong evidence of non-linearity (Table S2). Further adjustments for levels of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA), which may be associated with healthier aging,17 for the potential mediators triglycerides and LDL, for lipid lowering medications and for the sum of plasma phospholipid long chain saturated fatty acids (14:0+16:0+18:0) did not appreciably change the results (Tables S3 – S6). Adjustment for the combination of triglycerides, LDL and plasma phospholipid 16:0 attenuated the associations slightly (Table 4). Finally, excluding the unhealthy aging events that occurred in the first two years to assess the possibility of reverse causation, did not change the study results (Table S7).
Table 2.
List of the Incident First Unhealthy Aging Events among 2680 CHS Participants
| First incident unhealthy aging event | Number of events |
|---|---|
|
| |
| Incident Cardiovascular diseasea | 880 |
| Increase in difficulties of activities of daily livingb | 680 |
| Incident Cancer | 403 |
| Cognitive declinec | 376 |
| Incident chronic obstructive pulmonary disease | 133 |
| Incident Kidney diseased | 12 |
| No event | 196 |
defined as myocardial infarction, heart failure, stroke, transient ischemic attack or claudication.
first record of difficulty with ≥1 activity of daily living.
first Mini Mental State Examination score ≤80.
defined as estimated glomerular filtration rate <10 or dialysis.
Table 3.
Hazard ratio (95% confidence interval) of unsuccessful aging associated with higher quintiles of plasma phospholipid VLSFAs.
| VLSFA | Q1 | Q2 | Q3 | Q4 | Q5 | P trend | Per SD increment |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 20:0 | |||||||
| Events/P-Y | 452/3662 | 434/3841 | 523/4461 | 522/4696 | 553/5152 | ||
| HR (95% CI) | 1.00 (Ref.) | 1.12 (0.98–1.27) | 1.03 (0.90–1.17) | 1.10 (0.98–1.25) | 1.03 (0.91–1.16) | 0.12 | 0.91 (0.81–1.00) |
| 22:0 | |||||||
| Events/P-Y | 563/4341 | 551/4562 | 495/4484 | 469/4298 | 406/4126 | ||
| HR (95% CI) | 1.00 (Ref.) | 0.93 (0.83–1.05) | 0.89 (0.79–1.01) | 0.90 (0.79–1.02) | 0.85 (0.74–0.97) | 0.01 | 0.87 (0.78–0.97) |
| 24:0 | |||||||
| Events/P-Y | 561/4278 | 530/4224 | 510/4597 | 462/4490 | 421/4223 | ||
| HR (95% CI) | 1.00 (Ref.) | 0.97 (0.86–1.10) | 0.88 (0.78–1.00) | 0.86 (0.76–0.97) | 0.84 (0.73–0.95) | 0.001 | 0.85 (0.77–0.94) |
Models are adjusted for age, race (Black, non-Black), sex, field center, education (<HS, HS, Some college, College), physical activity (#blocks walked last week), BMI, waist circumference, alcohol consumption, smoking status (never, former, current), self-reported health ((Excellent/Very good, Good, Fair/Poor), prevalent diabetes, systolic blood pressure, use of hypertensive medications, and depression score.
Figure 1.
Hazard ratio (95% pointwise confidence interval) of unsuccessful aging associated with plasma phospholipid levels of VLSFAs relative to the median value
A = 20:0, B = 22:0, C = 24:0.
Each fatty acid was fit as a cubic spline with 4 knots. The presentation excludes the top 1% of values of each FA to illustrate the shape of the association with most of the data. Dashed lines indicate the quintile cutpoints. The shading around the line indicates the 95% pointwise confidence interval. Density plot for the fatty acid values shown just above the horizontal x-axis.
Models are adjusted for age, race (Black, non-Black), sex, field center, education (<HS, HS, Some college, College), physical activity (#blocks walked last week), BMI, waist circumference, alcohol consumption, smoking status (never, former, current), self-reported health ((Excellent/Very good, Good, Fair/Poor), prevalent diabetes, systolic blood pressure, use of hypertensive medications, and depression score.
Table 4.
Hazard ratio (95% confidence interval) of unsuccessful aging associated with higher quintiles of plasma phospholipid VLSFAs with further adjustment for the potential mediators triglycerides, LDL and 16:0
| Q1 | Q2 | Q3 | Q4 | Q5 | p trend | Per std. increment | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 20:0 | 1.00 (Ref.) | 1.08 (0.93–1.25) | 1.01 (0.88–1.15) | 1.09 (0.96–1.23) | 1.02 (0.90–1.15) | 0.36 | 0.93 (0.83–1.05) |
| 22:0 | 1.00 (Ref.) | 0.94 (0.83–1.07) | 0.91 (0.80–1.04) | 0.92 (0.80–1.06) | 0.87 (0.75–1.02) | 0.11 | 0.89 (0.78–1.02) |
| 24:0 | 1.00 (Ref.) | 0.97 (0.86–1.11) | 0.89 (0.78–1.01) | 0.87 (0.76–1.00) | 0.85 (0.73–0.98) | 0.007 | 0.86 (0.76–0.97) |
Models are adjusted for age, race (Black, non-Black), sex, field center, education (<HS, HS, Some college, College), physical activity (#blocks walked last week), BMI, waist circumference, alcohol consumption, smoking status (never, former, current), self-reported health (Excellent/Very good, Good, Fair/Poor), systolic blood pressure, use of hypertensive medications, depression score, LDL, triglycerides, diabetes, and 16:0.
The associations of each plasma phospholipid VLSFA with unhealthy aging did not vary according to age, sex, and BMI (smallest observed P for interaction: 0.22). However, we found significant interactions between 20:0 and 22:0 and prevalent diabetes (p values for interaction: 0.002 and 0.01, respectively). We observed associations of 20:0 and 22:0 with lower risk of unhealthy aging in the larger group of participants without diabetes, but not in the smaller group of participants with diabetes (Table S8).
Discussion
For the first time, we have demonstrated in a large prospective cohort that higher levels of plasma phospholipid VLSFAs may be associated with improved healthy life span as shown by lower risk of unhealthy aging events. The strongest association was with 24:0, lignoceric acid. When compared with the lowest quintile, the quintile with the highest levels of 24:0 was associated with a 16% lower risk of an unhealthy aging event (95% CI, 5%−27%), after adjustment for demographics, lifestyle factors, and diabetes.
The primary driver of incident unhealthy aging events in this cohort was cardiovascular disease, followed by declining physical function, cancer, and declining cognition. Higher VLSFAs have beneficial associations with mortality risk,10 multiple metabolic and inflammatory parameters,18–21 and a wide range of incident cardiovascular and metabolic diseases such as heart failure,4 atrial fibrillation,5 and diabetes.7,8 Further studies will be needed to investigate potential relationships of VLSFAs to cognition and other age-related conditions. Our purpose here was to study healthy aging, considered as living without chronic disease and with intact physical and mental functions.
Identifying people resistant to the effects of aging compared to peers, and identifying potentially modifiable determinants of this resistance are both of great public health importance.
The univariate association of higher levels of VLSFAs with male sex, Black race, smoking, and an increase in LDL is surprising, as none of these factors is associated with healthy aging. Considering that LDL has a causal effect on CAD,22 it is interesting that higher levels of VLSFAs have consistent and beneficial associations with risk of heart disease, including CAD,23,24 despite their LDL association. There are also strong negative associations between VLSFA levels and levels of triglycerides and 16:0 palmitate, markers of de novo lipogenesis,20 and we previously reported that these markers mediate the associations of VLSFAs with lower risk of diabetes.7,8 However, adjusting for levels of triglycerides, 16:0 and LDL or prevalent diabetes did not negate the associations between VLSFAs and healthy aging. Interestingly, we found significant effect modification by diabetes, and no benefits of VLSFAs on healthy aging in patients with prevalent diabetes. While this interaction should be interpreted cautiously until replicated, it further illustrates the complex physiological associations of these compounds.
VLSFAs may influence the aging process through multiple mechanisms. They are major components of ceramides and sphingomyelins, lipids that contain one fatty acid acylated to a sphingoid backbone.25 Ceramides, specifically, are known for their role in apoptosis26 and inflammation27 and appear to contribute to the pathogenesis of a broad range of disorders including insulin resistance and atherosclerosis.28–30 Interestingly, in experimental cell and animal studies, ceramides that contain a VLSFA may actually counteract this tendency by having biological activities opposite from ceramides that contain the shorter saturated fatty acid 16:0, palmitate.31,32 We speculate that VLSFAs may have a positive effect on healthy aging by lowering endogenous levels of shorter-chain ceramides.
The sources of circulating VLSFAs are both dietary and metabolic. Dietary sources include peanuts, macadamia nuts, and canola oil.33 Small short-term feeding trials have shown an increase in circulating levels of VLSFAs with consumption of both macadamia nuts34 and peanut butter,35 illustrating that dietary intake may directly influence circulating levels of VLSFAs. We reported previously that higher levels of 22:0 and 24:0 were associated with greater peanut intake measured 3 years earlier.8 VLSFA may also be synthesized endogenously from 18:0 by elongases, such as the ubiquitously distributed ELOVL1.36 In agreement with the predominant occurrence of VLSFAs in ceramides and other sphingolipids, ELOVL1 appears to be co-regulated with ceramide synthase 2, which produces ceramide by addition of a VLSFA to sphingosine.37 Further, we have previously shown an association of circulating VLSFAs with genetic variation in the sphingolipid synthesis pathway.38 The extent to which dietary intake and endogenous metabolism contribute to circulating levels of VLSFA is currently unknown.
Study Limitations and strengths.
The study has several limitations and strengths. This is an observational study and causality cannot be established, as residual confounding by unknown factors is possible. However, the results were robust to adjustment for multiple characteristics. Reverse causality is also of potential concern in association studies39 and would operate for example if frailty led to low levels of VLSFAs. However, we eliminated participants with a wide range of medical conditions and symptoms in this study of incident unhealthy aging, and examined the association of VLSFAs in a particularly healthy group at entry. We also observed stronger associations in the healthier subgroup without prevalent diabetes. Furthermore, we did not see a weakening of the associations over time, and exclusion of the first two years of follow-up did not remove or attenuate the observed associations, suggesting reverse causation does not account in large part for the observed associations. The study was conducted among older adults and the results may not be generalizable to other populations; however, healthy aging is of high relevance to this older population. The study was limited to White and Black participants. Strengths include the prospective design, population-based enrollment, use of an objective marker of diet and metabolism, repeated measurements, and the rich information available on demographics, risk factors, and lifestyle habits.
Conclusions
In conclusion, we report for the first time an association of higher levels of plasma phospholipid 24:0 and 22:0 with lower risk of incident unhealthy aging events. Together with our previous reports of inverse associations of VLSFA with incident heart failure,4 incident atrial fibrillation,5 incident sudden cardiac arrest,9 and total mortality,10 the study findings should prompt further research on determinants of VLSFA levels and may lead to novel approaches to promote healthy aging.
Supplementary Material
Key Points.
Question:
Are higher levels of circulating VLSFAs (20:0, 22:0 and 24:0) associated with healthy aging?
Findings:
In this large prospective cohort study of 2680 older individuals, participants in the highest quintile of levels of plasma phospholipid VLSFA 24:0 showed a significant 16% reduction in incident unhealthy aging events compared to those in the lowest quintile, with a similar finding for 22:0.
Meaning:
The study suggests that increasing circulating levels of VLSFA may be a novel target to promote healthy aging.
Acknowledgements
There are no conflicts of interest to report. This research was supported by R01-HL085710 and R01-HL094555 from the National Heart, Lung, and Blood Institute (NHLBI), with co-funding from the Office of Dietary Supplements. These sources funded but did not influence the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The CHS cohort was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, 75N92021D00006, and grants U01HL080295 and U01HL130114 from the NHLBI, with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Rozenn Lemaitre had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations:
- VLSFA
very-long-chain saturated fatty acid
- 20:0
arachidic acid
- 22:0
behenic acid
- 24:0
lignoceric acid
- CHS
Cardiovascular Health Study
- BMI
body mass index
- LDL
low density lipoprotein
- HR
hazard ratio
Footnotes
Clinical trial registration: This trial was registered at clinicaltrials.gov as NCT00005133.
Contributor Information
Lee B Bockus, Division of Cardiology, University of Washington, Seattle, WA.
Mary L Biggs, Cardiovascular Health Research Unit, University of Washington, Seattle, WA; Department of Biostatistics, University of Washington, Seattle, WA.
Heidi TM Lai, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA; Department of Primary Care and Public Health, Imperial College London, London, UK.
Marcia C de Olivera Otto, Department of Epidemiology, Human Genetics and Environmental Sciences, University of Texas Health Science Center at Houston, Houston, TX USA.
Amanda M. Fretts, Cardiovascular Health Research Unit, University of Washington, Seattle, WA; Department of Epidemiology, University of Washington, Seattle, WA.
Barbara McKnight, Cardiovascular Health Research Unit, University of Washington, Seattle, WA; Department of Biostatistics, University of Washington, Seattle, WA.
Nona Sotoodehnia, Cardiovascular Health Research Unit, University of Washington, Seattle, WA; Department of Medicine, University of Washington, Seattle, WA.
Irena B. King, USA Department of Internal Medicine, University of New Mexico, Albuquerque, NM.
Xiaoling Song, Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle, WA.
David S. Siscovick, New York Academy of Medicine, New York, NY..
Dariush Mozaffarian, Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA.
Rozenn N Lemaitre, Cardiovascular Health Research Unit, University of Washington, Seattle, WA; Department of Medicine, University of Washington, Seattle, WA.
REFERENCES
- 1.2010 Census Briefs: The Older Population: 2010. In: bureau UC, ed2010. [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics−−2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flock MR, Kris-Etherton PM. Diverse physiological effects of long-chain saturated fatty acids: implications for cardiovascular disease. Curr Opin Clin Nutr Metab Care. 2013;16(2):133–140. [DOI] [PubMed] [Google Scholar]
- 4.Lemaitre RN, McKnight B, Sotoodehnia N, et al. Circulating Very Long-Chain Saturated Fatty Acids and Heart Failure: The Cardiovascular Health Study. J Am Heart Assoc. 2018;7(21):e010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fretts AM, Mozaffarian D, Siscovick DS, et al. Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc. 2014;3(3):e000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ardisson Korat AV, Malik VS, Furtado JD, et al. Circulating Very-Long-Chain SFA Concentrations Are Inversely Associated with Incident Type 2 Diabetes in US Men and Women. J Nutr. 2020;150(2):340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fretts AM, Imamura F, Marklund M, et al. Associations of circulating very-long-chain saturated fatty acids and incident type 2 diabetes: a pooled analysis of prospective cohort studies. Am J Clin Nutr. 2019;109(4):1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemaitre RN, Fretts AM, Sitlani CM, et al. Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2015;101(5):1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemaitre RN, King IB, Rice K, et al. Erythrocyte very long-chain saturated fatty acids associated with lower risk of incident sudden cardiac arrest. Prostaglandins Leukot Essent Fatty Acids. 2014;91(4):149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fretts AM, Mozaffarian D, Siscovick DS, et al. Associations of Plasma Phospholipid SFAs with Total and Cause-Specific Mortality in Older Adults Differ According to SFA Chain Length. J Nutr. 2016;146(2):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. [DOI] [PubMed] [Google Scholar]
- 12.Folch J, Lees M, Sloane GH. A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 13.Lepage G, Roy CC. Direct transesterification of all lipids in a one-step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 14.Mozaffarian D, Lemaitre RN, King IB, et al. Circulating Long-Chain {omega}−3 Fatty Acids and Incidence of Congestive Heart Failure in Older Adults: The Cardiovascular Health Study: A Cohort Study. Ann Intern Med. 2011;155(3):160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai HT, de Oliveira Otto MC, Lemaitre RN, et al. Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: prospective cohort study. BMJ. 2018;363:k4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carleton RN, Thibodeau MA, Teale MJ, et al. The center for epidemiologic studies depression scale: a review with a theoretical and empirical examination of item content and factor structure. PLoS One. 2013;8(3):e58067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan A, Sullenbarger B, Prakash R, McDaniel JC. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: A randomized, controlled study. Prostaglandins Leukot Essent Fatty Acids. 2018;132:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panth N, Abbott KA, Dias CB, Wynne K, Garg ML. Differential effects of medium- and long-chain saturated fatty acids on blood lipid profile: a systematic review and meta-analysis. Am J Clin Nutr. 2018;108(4):675–687. [DOI] [PubMed] [Google Scholar]
- 19.Lee YS, Cho Y, Shin MJ. Dietary Very Long Chain Saturated Fatty Acids and Metabolic Factors: Findings from the Korea National Health and Nutrition Examination Survey 2013. Clin Nutr Res. 2015;4(3):182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauritzen L, Hellgren LI. Plasma phospholipid very-long-chain saturated fatty acids: a sensitive marker of metabolic dysfunction or an indicator of specific healthy dietary components? Am J Clin Nutr. 2015;101(5):901–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng JS, Sharp SJ, Imamura F, et al. Association between plasma phospholipid saturated fatty acids and metabolic markers of lipid, hepatic, inflammation and glycaemic pathways in eight European countries: a cross-sectional analysis in the EPIC-InterAct study. BMC Med. 2017;15(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgess S, Harshfield E. Mendelian randomization to assess causal effects of blood lipids on coronary heart disease: lessons from the past and applications to the future. Curr Opin Endocrinol Diabetes Obes. 2016;23(2):124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malik VS, Chiuve SE, Campos H, et al. Circulating Very-Long-Chain Saturated Fatty Acids and Incident Coronary Heart Disease in US Men and Women. Circulation. 2015;132(4):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ardisson Korat AVQ F; Imamura F; Tintle N; Chen J; Van Dam RM; Virtanen JK; Bassett JK; Bartz TM; Hirakawa Y; Chien K; Frazier-Wood A; Murphy RA; Samieri C; Sun Q; Hu F; Wu JH; Micha R; Mozaffarian D; Lemaitre R Abstract P414: Biomarkers of Very Long-chain Saturated Fatty Acids and Incident Coronary Heart Disease: Prospective Evidence From 15 Cohorts in the Fatty Acids and Outcomes Research Consortium. Circulation. 2020;141. [Google Scholar]
- 25.Quehenberger O, Armando AM, Brown AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51(11):3299–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dadsena S, Bockelmann S, Mina JGM, et al. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat Commun. 2019;10(1):1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albeituni S, Stiban J. Roles of Ceramides and Other Sphingolipids in Immune Cell Function and Inflammation. Adv Exp Med Biol. 2019;1161:169–191. [DOI] [PubMed] [Google Scholar]
- 28.Chaurasia B, Summers SA. Ceramides - Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol Metab. 2015;26(10):538–550. [DOI] [PubMed] [Google Scholar]
- 29.Sokolowska E, Blachnio-Zabielska A. The Role of Ceramides in Insulin Resistance. Front Endocrinol (Lausanne). 2019;10:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meeusen JW, Donato LJ, Bryant SC, Baudhuin LM, Berger PB, Jaffe AS. Plasma Ceramides. Arterioscler Thromb Vasc Biol. 2018;38(8):1933–1939. [DOI] [PubMed] [Google Scholar]
- 31.Grosch S, Schiffmann S, Geisslinger G. Chain length-specific properties of ceramides. Prog Lipid Res. 2012;51(1):50–62. [DOI] [PubMed] [Google Scholar]
- 32.Pewzner-Jung Y, Brenner O, Braun S, et al. A critical role for ceramide synthase 2 in liver homeostasis: II. insights into molecular changes leading to hepatopathy. J Biol Chem. 2010;285(14):10911–10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. Department of Agriculture ARS. U.S. Department of Agriculture, Agricultural Research Service. 2013. USDA National Nutrient Database for Standard Reference, Release 26. Nutrient Data Laboratory Home Page, http://www.ars.usda.gov/ba/bhnrc/ndl. 2013. [Google Scholar]
- 34.Garg ML, Blake RJ, Wills RB. Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr. 2003;133(4):1060–1063. [DOI] [PubMed] [Google Scholar]
- 35.Lam C, Wong D, Cederbaum S, Lim B, Qu Y. Peanut consumption increases levels of plasma very long chain fatty acids in humans. Mol Genet Metab. 2012;107(3):620–622. [DOI] [PubMed] [Google Scholar]
- 36.Kihara A Very long-chain fatty acids: elongation, physiology and related disorders. J Biochem. 2012;152(5):387–395. [DOI] [PubMed] [Google Scholar]
- 37.Ohno Y, Suto S, Yamanaka M, et al. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci U S A. 2010;107(43):18439–18444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemaitre RN, King IB, Kabagambe EK, et al. Genetic loci associated with circulating levels of very long-chain saturated fatty acids. J Lipid Res. 2015;56(1):176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravindrarajah R, Hazra NC, Hamada S, et al. Systolic Blood Pressure Trajectory, Frailty, and All-Cause Mortality >80 Years of Age: Cohort Study Using Electronic Health Records. Circulation. 2017;135(24):2357–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.