Abstract
The pathogenicity of the current coronavirus disease (COVID-19) shows postulates that optimal status of essential nutrients is crucial in supporting both the early viraemic and later hyperinflammatory phases of COVID-19. Micronutrients such as vitamin C, D, zinc, and selenium play roles in antioxidant, anti-inflammatory, antithrombotic, antiviral, and immuno-modulatory functions and are useful in both innate and adaptive immunity. The purpose of this review is to provide a high-level summary of evidence on clinical outcomes associated with nutritional risk of these micronutrients observed in patients with COVID-19. A literature search was performed on PubMed and Google Scholar to obtain findings of cross-sectional and experimental studies in humans. The search resulted in a total of 1212 reports including all nutrients, but only 85 were included according to the eligibility criteria. Despite the diversity of studies and the lack of randomized clinical trials and prospective cohorts, there is evidence of the potential protective and therapeutic roles of vitamin C, D, zinc, and selenium in COVID-19. The findings summarized in this review will contribute to guide interventions in clinical practice or in future clinical studies.
Keywords: COVID-19, Zinc, Selenium, Vitamin C, Vitamin D, Immunonutrition
Abbreviations:
- ACE2
angiotensin converting enzyme-2
- ARDS
acute respiratory distress syndrome
- ARI
acute respiratory infection
- CYP24A
Cytochrome P450 family 24 subfamily A
- CYP27 B1
Cytochrome P450 family 27 subfamily B member 1
- GPX
glutathione peroxidase
- HDIVC
high dose intravenous vitamin C
- HR
hazard ratio
- ICU
Intensive Care Unit
- IFN-γ
Interferon γ
- IMV
invasive mechanical ventilation
- MSRB1
methionine sulfoxide reductase B1
- NIV
non-invasive ventilation
- NF-κB
nuclear factor kappa B
- OSCI
ordinal scale for clinical improvement
- TNF-α
tumor necrosis factor alpha
- TXNRD
thioredoxin reductase
- VDR
vitamin D receptor
- SECIS
selenocysteine insertion sequence
- SELENOP
selenoprotein P
- SELENOS
selenoprotein S
- SELENOK
selenoprotein K
- SECIS
selenocysteine insertion sequence
- SEPHS2
selenophosphate synthetase 2
- 25(OH)D
25-hydroxyvitamin D
- 1,25(OH)2D
1α,25-dihidroxivitamin D
1. Introduction
The Coronavirus Disease-2019 (COVID-19) is an infection caused by a coronavirus (SARS-CoV-2), an enveloped, positive single-strand RNA virus that belongs to the β-coronavirus genus and coronaviridae family [1].
The common clinical course of this infection when symptomatic is (a) mild upper respiratory tract symptoms, which can progress to (b) non-life-threatening pneumonia and (c) severe pneumonia with acute respiratory distress syndrome (ARDS). Patients with ARDS progress to rapid deterioration in a short period of time and require advanced life support [1,2].
In this context, optimal status of essential nutrients is considered crucial in supporting the immune system, helping to avoid and overcome infections. Many of the risk factors regarding infection and death from COVID-19 have underlying associations with nutritional status [3,4]. The deficiency of most nutrients increases the individual's susceptibility to virus infection with a tendency for severe clinical presentation [5]. Moreover, severe and critical patients with COVID-19 have a high risk of malnutrition [6].
The nutrients described in this review, vitamin C, D, zinc, and selenium, have been chosen due to their potential anti-inflammatory and immunomodulatory properties. In addition, their consumption has been part of cultural practice in the face of being affected by colds or acute infections of the respiratory tract [[7], [8], [9], [10], [11], [12], [13], [14]]. There are other nutrients worthy of further investigation, including vitamin A, vitamin E, copper, etc., and some probiotics and nutraceuticals; however, there are not enough studies to establish the link between their nutritional status and the effects in patients with COVID-19 [9].
Vitamin C has physiological functions relevant to COVID-19 infection, these include antioxidant, anti-inflammatory, antithrombotic and immuno-modulatory functions [10]. Moreover, vitamin C is involved in reducing the incidence, duration and mortality of other respiratory infections, and the role of vitamin C in these other respiratory infections has been previously studied [11].
Vitamin D is involved in a wide range of immunomodulatory activities helping alleviate lung injury induced by SARS-CoV-2 by upregulating angiotensin converting enzyme-2 (ACE2), decreasing inflammatory cytokines, and increasing antimicrobial peptides [7,8].
Zinc is able to modulate antiviral and antibacterial immunity as well as regulate inflammatory response. Risk factors for COVID-19 such as aging, obesity, diabetes, atherosclerosis and immunosuppression are also factors related to zinc deficiency [13].
Selenium plays a role in anti-inflammatory, antiviral, and redox and immune-cell activity; it is useful in both innate and adaptive immunity. Selenoproteins partly reduce oxidative stress generated by viral pathogens [12,14].
Based on these backgrounds, this narrative review aimed to examine nutritional risk of inadequacy of vitamin D, vitamin C, zinc and selenium in patients with COVID-19, sharing findings from cross-sectional and experimental studies in humans.
2. Methods
2.1. Search strategies
We used as the primary source of potentially relevant literature the electronic database Pubmed (MEDLINE) from January 2020 onwards. The secondary source used Google Scholar with searches limited to 100 reports as sorted in the relevance ranking [15].
2.2. Article eligibility
In regard to the number of studies on COVID-19 and their heterogeneity, to meet the focus of this review, we prioritized studies on patients diagnosed with COVID-19 who had been evaluated for vitamin C, vitamin D, zinc and selenium status before or during the hospitalization period in hospitals, clinics, or nursing homes. Studies on supplementation with these micronutrients and randomized controlled trials have also been included. No restriction was placed on the language of publication for the included reports. Eligibility criteria included full-text articles and human studies. Narrative reviews, systematic reviews, commentaries, correspondences, editorials, opinion pieces, ecological and in vitro studies were excluded.
A search was conducted using a combination of free-text and medical subject heading (MeSH) search terms and keywords (Table 1 ). Both key search terms were used to build search equations for each micronutrient. The search including all nutrients resulted in 1212 reports, but only 85 were included, as also shown in Table 1. The authors independently reviewed the titles and abstracts for inclusion. Additional relevant articles were identified based on the citations and references of each paper.
Table 1.
Equations of search for articles used in the review and total of articles obtained.
| Nutrient | Search terms | Records identified through Pubmed Search and Google Scholar | Included records |
|---|---|---|---|
| Vitamin C | ("vitamin C" OR "ascorbic acid") AND ("COVID 19" OR "COVID- 19" OR "COVID- 19 Virus Disease" OR "Coronavirus Disease 2019" OR "SARS-CoV-2 Virus" OR "Severe Acute Respiratory Syndrome Coronavirus 2" OR "2019-nCoV") | n = 214 | n = 13 |
| Vitamin D | ("Vitamin D" OR "Cholecalciferol" OR "Hydroxycholecalciferols" OR "Ergocalciferols" OR "25-Hydroxyvitamin D2" OR "Vitamin D3" OR "Calciferols" OR "Ergocalciferol" OR "Vitamin D2") AND ("COVID 19" OR "COVID- 19" OR "COVID- 19 Virus Disease" OR "Coronavirus Disease 2019" OR "SARS-CoV-2 Virus" OR "Severe Acute Respiratory Syndrome Coronavirus 2" OR "2019-nCoV") | n = 539 | n = 51 |
| Zinc | (“Zinc”) AND (“COVID-19” OR “Coronavirus Disease 2019” OR “SARS-Cov-2 Virus” OR “Severe Acute Respiratory Syndrome Coronavirus 2”) | n = 393 | n = 16 |
| Selenium | (“selenium” OR “Compounds, Selenium” OR “Selenic Acid” OR “Selenious Acid” OR “Sodium Selenite”) AND ("COVID 19" OR "COVID- 19" OR "COVID- 19 Virus Disease" OR "Coronavirus Disease 2019" OR "SARS-CoV-2 Virus" OR "Severe Acute Respiratory Syndrome Coronavirus 2" OR "2019-nCoV") | n = 66 | n = 5 |
2.3. Data extraction
Data was carefully extracted from all eligible publications, including first authors’ last names, year of publication, origin of the study (country), study design, population, sample size, exposure measure, outcomes, and main findings.
3. Vitamin C
3.1. Vitamin C, immune system, and COVID-19
Vitamin C cannot be synthesized by humans and other primates and has to be obtained from diet. Oral ingestion of food or supplements is the primary route of administration and healthy individuals get sufficient amounts through their diet. The absorption and elimination are highly dose-dependent, and several organs have concentration-dependent mechanisms, maintaining high levels during times of inadequate supply at the expense of other organs. The homeostasis of vitamin C is influenced by several factors, including genetic polymorphisms, environmental and lifestyle factors such as smoking and diet, as well as diseases [16].
The biological role of vitamin C is related to its reduced form, ascorbate, and acts in the synthesis and metabolism of vital cell compounds, antioxidant activity and immune function [17]. In immunologic defense, vitamin C is involved in cellular functions of the innate and adaptive system. In the epithelial barrier, the first line of defense, it acts in its integrity and protects against free radicals; contributes to leukocyte functions, accumulates in phagocytic cells (e.g neutrophils), increasing chemotaxis, phagocytosis, death of microorganisms, apoptosis and elimination of neutrophils in infection sites. Moreover, vitamin C appears to be involved with lymphocyte functions, through enhancing the differentiation and proliferation of B- and T-cells. Also, vitamin C has anti-inflammatory effects such as inhibition of nuclear factor kappa B (NF-κB) and reducing pro-inflammatory mediators, as well as antioxidant activities, fighting free radicals and regenerating other antioxidants [18,19].
Supplementation with vitamin C appears to have preventive and treatment capabilities against respiratory and systemic infections, improving several functions of immunological cells. These findings are still controversial and further high quality studies need to be completed in order to confirm the results. Vitamin C deficiency has been reported in respiratory infections, leading to impaired immunity and increasing susceptibility to infections. In turn, infections can significantly impact vitamin C level due to enhanced inflammation and metabolic requirements [11,18,20,21]. The therapeutic use of vitamin C may be reasonable for pneumonia patients who have low vitamin C plasma levels [20].
Vitamin C also has pleiotropic physiological functions relevant to COVID-19 (Fig. 1 ), including prevention of microthrombi formation and capillary plugging, reduction of inflammatory markers elevation (cytokine storm), reduction of oxidative stress and has been suggested as having a role in antiviral cytokine interferon levels [10]. Some studies have examined the effects of vitamin C on primary and secondary outcomes of COVID-19 patients (Supporting information Table S1).
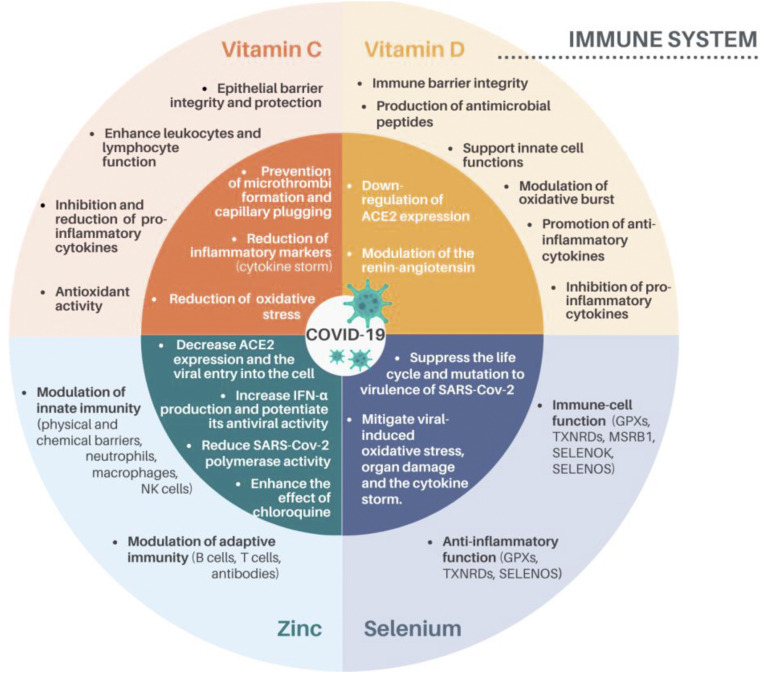
Fig. 1.
The immunomodulating actions of vitamin C, D, zinc and selenium and beneficial mechanisms proposed in the COVID-19 pathogenicity. The outer ring displays the immunomodulating properties of vitamin C, vitamin D, zinc and selenium while the internal ring displays the possible roles of these nutrients regarding COVID-19 infection. ACE2, angiotensin converting enzyme-2; IFN-α, Interferon α; NK cells, natural killer cells; GPX, glutathione peroxidase; TXNRD, thioredoxin reductase; SELENOS, selenoprotein S; MSRB1, methionine sulfoxide reductase B1; SELENOK, selenoprotein K [5,10,13,14,18,19,43,114,115].
3.2. Studies addressing previous vitamin C status and COVID-19
Studies addressing previous vitamin C status and COVID-19 were not found in our search. Despite this, low intake levels of vitamin C have adverse effects on the incidence and severity of infections [22]. Vitamin C deficiency may be associated with risk of respiratory disease incidence. A European cohort study found that high plasma vitamin C concentrations at baseline were significantly associated with the lower incidence of respiratory illness, chronic respiratory diseases and pneumonia [23].
3.3. Studies addressing vitamin C status in hospitalized patients with COVID-19
A pilot study measured serum vitamin C levels in a cohort of patients with critical COVID-19 illness in a hospital intensive care unit (ICU). The authors found low serum levels of vitamin C [22.2 μmol/L (SD = 18.3)] in most of the patients. Moreover, serum vitamin C level contributed to the significance of age being a predictor of mortality [24]. Limitation such as the sample size was related. Xing et al. (2021) [25], also found lower plasma vitamin C levels in patients with COVID-19 compared to healthy volunteers.
3.4. Studies addressing vitamin C supplementation during COVID-19
COVID-19 patients were treated with dexamethasone and vitamin C had a high recovery rate (94%) and low levels of serum ferritin. The authors suggest vitamin C and dexamethasone as key modalities for COVID-19 treatment. However, it is not safe to assert that vitamin C had a role in the recovery rate due to the absence of a comparator or a control group. Furthermore, the used dosage of vitamin C is not clear [26].
Other observational studies with hospitalized COVID-19 patients who received high-dose intravenous vitamin C (HDIVC) also reported improvements in oxygen support status, reduced mortality [27], and improvements in the inflammatory response [28,29], and immune and coagulation function [28]. Different results were found by Li et al. (2021), in which intravenous vitamin C did not decrease the incidence of mortality, vasopressor requirements, sequential organ failure assessment score (SOFA score) or ventilator settings [30].
A retrospective case series study was conducted with severe or critical COVID-19 pneumonia patients. The patients received HDIVC within 24 h after disease aggravation. The dosage of vitamin C [median (IQR)] applied to severe patients was 162.7 (71.1–328.6) mg/kg/day and 178.6 (133.3–350.6) in critical patients. Main clinical outcomes obtained from day 3 and day 7 after HDIVC were compared to previous results (day 0) HDIVC. Improvements related to inflammatory response [C-reactive protein (CRP)], immune function (lymphocyte and CD4+ T cell counts) and organ function (PaO2/FiO2 and SOFA score) were observed. The most improving effects were observed in severe patients rather than in the critical ones after HDIVC. Small sample size, no control group, duration and dosage variations, among other limitations, were reported [31].
Another case series study with moderate to severe COVID-19 patients who received intravenous vitamin C, compared inflammatory markers pre and post treatment. Vitamin C was administered at a dose of 1 g every 8 h for 3 days (low dose). Significant lower D-dimer and ferritin levels post treatment with vitamin C were found. However, some limitations like the absence of a control group, low vitamin C dose, treatment duration, indeterminate extent of interaction with medications, and use of few inflammatory markers should be considered [32].
A single case of COVID-19 with ARDS was reported by Khan et al. (2020) [33]. The infusion of HDIVC started at day 6 (11 g per day) of hospitalization. The patient improved clinically and was taken off mechanical ventilation within 5 days. In this case, HDIVC treatment was associated with fewer days on mechanical ventilation, shorter ICU stay, and earlier recovery compared to other critical COVID-19 patients in the institute. However, considering the nature of the study and the information provided, there is no way of knowing whether vitamin C was in fact the differential for the result of this single case.
3.5. Randomized controlled trials of vitamin C supplementation in COVID-19
A pilot trial conducted by Zhang et al. (2021) [34] tested whether HDIVC was effective for severe COVID-19. The intervention group received 12 g of HDIVC every 12 h for 7 days. There was no difference in invasive mechanical ventilation-free days in 28 days between two groups (26.0 [9.0–28.0] in HDIVC vs 22.0 [8.50–28.0] in control, (p = 0.57)). HDIVC did not reduce 28-day mortality (p = 0.27). During the treatment period, patients in the HDIVC group had a steady rise in PaO2/FiO2, not observed in the control group. IL-6 levels in the HDIVC group were lower than that in the control group on day 7. However, some limitations were reported, among these, sample size, time for initiating vitamin C treatment, lack of viral load measurement, and the imbalance in the patient gender distribution between the groups at baseline.
In an open-label randomized controlled trial conducted in Pakistan, the intervention group received 50 mg/kg/day of intravenous vitamin C along with the standard therapy. COVID-19 patients who received intravenous vitamin C became symptom-free earlier (7.1 ± 1.8 vs. 9.6 ± 2.1 days, p < 0.0001) and spent fewer days in the hospital (8.1 ± 1.8 vs. 10.7 ± 2.2 days, p < 0.0001) compared to those who received standard therapy only. However, Vitamin C supplementation had no impact on mortality and the need for mechanical ventilation [35].
Other clinical trials have not found the same result. Siahkali et al. (2021) [36] did not observe significantly better outcomes such mortality, duration of hospitalization and need for ICU admission, with HDIVC treatment (6 g/day for 5 days). Similar work by Thomas et al. (2021) [37] also found no significant improvement in symptom reduction (fever, cough, shortness of breath, fatigue), compared with standard care, although these results have been reanalyzed and contested by Hemilä, Carr and Chalker (2021) [38].
As observed, four current clinical trials of vitamin C supplementation in COVID-19 were identified through our search. Several clinical trials have been registered on ClinicalTrials.gov using vitamin C alone or in combination with other nutrients or drugs in COVID-19 infections.
4. Vitamin D
4.1. Vitamin D, immune system, and COVID-19
Circulating 25(OH)D may also be used as substrate in many cells to locally produce 1,25(OH)2D via the CYP27B1 (1a-hydroxylase) enzyme and is inactivated by the CYP24A (24-hydroxylase) enzyme. The main role of vitamin D is to maintain serum calcium and phosphorus levels in a normal state so that they are capable of providing conditions for most metabolic functions, including bone mineralization. In addition to its essentiality for the maintenance of calcium and phosphorus homeostasis, the functions of vitamin D in the human body extend to the regulation of multiple processes in different cell types that express vitamin D receptors (VDR). Many tissues and cells in the body, such as macrophages, brain, breast, prostate, colon, and skin, have the ability to convert 25(OH)D into 1,25(OH)2D. In addition, these cells have VDR, which once activated, interacts with the nucleus, participating in metabolic processes such as DNA repair, antioxidant activity, regulation, proliferation and cell differentiation [39]. As a result, vitamin D deficiency has been extensively investigated in relation to inverse association with cardiovascular diseases, insulin resistance, respiratory diseases, cancer, tuberculosis, viral infections, and infertility, among others [40,41].
Low levels of vitamin D increase the risks, severity, morbidity, and mortality of several respiratory conditions, such as asthma, tuberculosis, chronic pulmonary disorders, viral respiratory infections, and possibly also COVID-19 [7].
Evidence for the interactions of vitamin D with the immune response shows that the final active metabolite, namely calcitriol (1,25(OH)2D3), due to being a steroid hormone, can exert immunomodulatory activities through functional cell steroid receptors [42,43]. The many levels of defense involved in the role of vitamin D in the immune system were summarized by Schwalfenberg (2011) [44]. The first level is the physical barrier of epithelial cells in the skin, gut, and respiratory system, which protects us from injury or invasion by infection. The mechanisms of this action can be explained by 1,25(OH)2D upregulating genes via the 1a-hydroxylase enzyme, which then encodes proteins required for tight junctions, gap junctions and adherens junctions [45,46]. Secondly, vitamin D stimulates the production and secretion of antimicrobial peptides by the intestinal epithelial cells, Paneth cells, and intraepithelial lymphocytes in innate immunity [47]. Thirdly, 1,25(OH)2D exerts a receptor-mediated effect on monocyte function that results in increased oxidative burst potential [48]. Moreover, vitamin D modulates T helper (Th) cell responses in inducing a shift from Th1 to Th2 and preventing cytokine storms by decreasing inflammatory cytokines and NF-κB [7]. Within the innate immune response vitamin D can promote autophagy to facilitate viral clearance indirectly. This crucial role is important for maintaining appropriate balance between autophagy and apoptosis to maximize antiviral responses to infection [49] (Fig. 1).
Several concordant studies support a protective role for vitamin D in reducing at least the risk/severity of acute respiratory tract infection (ARI) [[50], [51], [52]]. It is known that vitamin D/VDR signaling plays an important role in alleviating lipopolysaccharide-induced acute lung injury by preserving alveolar barrier integrity [53]. A study reported that children aged less than 2 years requiring hospitalization for ARI had significantly higher odds (1.7-times) of vitamin D deficiency when compared to those with mild ARI [51]. A prospective cohort study developed during 114 days of the fall and winter, which included healthy adults, showed a two-fold reduction in the risk of developing (ARI) in those with serum 25(OH)D levels of 38 ng/mL (95 nmol/L) or higher [50]. In this sense, meta-analyses of randomized controlled trials conducted including data from 46,331 participants in 42 randomized controlled trials, stratified by baseline 25(OH)D concentration, reveal protective effects of vitamin D against ARI, although these effects were of modest size [54].
After the pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), we have observed a burst of scientific publications speculating on the mechanisms of infection that determine the relationship between COVID-19 and vitamin D status [5,8,43,49,55]. In fact, this infectious disease causes ARDS and acute lung injury leading to pulmonary cytokine storm, a major cause of morbidity and mortality. Overall, vitamin D influences lung structures, size, volume, and functions. The importance of ACE2 receptors in the pathophysiology of COVID-19 has been the most discussed point in this topic. ACE2 is known to be expressed in type II pneumocytes. SARS CoV-2 activates ubiquitously expressed ACE-2 receptors on the cell surface and can subsequently ingress into the cell. Vitamin D may downregulate the ACE2 expression and prevent the viral entry into cells. Apart from the immunomodulatory and anti-viral effects, 1,25(OH)2D acts specifically as a modulator of the renin–angiotensin pathway and down-regulates ACE2 expression, which serves as the host cell receptor that mediates infection by SARS-CoV-2. This class of receptors also inhibits transcription factors important in the regulation of the genetic expression of renin, which is another mechanism for conjecture of protection in the course of the disease [43,49]. The reports selected from this topic came from prospective and retrospective cohort, case controls, and clinical trial studies described in Supporting Information Table S2.
4.2. Studies addressing previous vitamin D status and COVID-19
A retrospective cohort included 4314 patients tested for COVID-19, all of whom had a vitamin D level checked in the year before testing. Predicted COVID-19 rates in the individuals with deficient vitamin D status were 21.6% vs 12.2% in the sufficient individuals. Testing positive for COVID-19 was also associated with increasing age, up to 50 years old (relative risk, 1.06; 95% CI, 1.01–1.09; p = 0.02); non-White race (relative risk, 2.54; 95% CI, 1.26–5.12; p = 0.009), and likely deficient vitamin D status (relative risk, 1.77; 95% CI, 1.12–2.81; p = 0.02) compared with likely sufficient vitamin D status. These findings were not associated with comorbidities except for a decreased risk in persons with conditions associated with immunosuppression (Relative risk (RR), 0.39; 95% CI, 0.20–0.76; p = 0.005) [56].
Analyzing records of patients from Switzerland, 107 symptomatic individuals obtaining a SARS-CoV-2 PCR test found that PCR-positive patients had significantly lower (p = 0.004) 25(OH)D (11.1 ng/mL) when compared with test-negative subjects (24.6 ng/mL). This was also confirmed by stratifying patients according to age >70 years [57].
In a sample of 780 hospitalized patients testing positive for COVID-19 in Indonesia, the odds ratio of death was higher in older and male cases with pre-existing conditions and below normal vitamin D levels. After controlling for age, sex, and comorbidity, both insufficient (OR = 7.63) and deficient 25(OH)D levels (OR = 10.12) were significantly associated with COVID-19 mortality (p < 0.001 for each) [58]. These similar findings were also observed in a large US cohort study (n = 191,779) where SARS-CoV-2 positivity was inversely associated with circulating 25(OH)D levels after adjusting for latitudes, races/ethnicities, both sexes, and age ranges. In a multivariable analysis with adjustment for ethnicity severe vitamin D deficiency was associated with an OR of 1.275 (p < 0.001) for COVID-19 infection [59].
The mean plasma vitamin D level previously checked in health professionals from Israel was significantly lower among those who tested positive than negative for COVID-19 [19.00 ng/mL (95% confidence interval (CI) 18.41–19.59) vs. 20.55 (95% CI: 20.32–20.78)]. In multivariate analyses that controlled for demographic variables, and psychiatric and somatic disorders, the adjusted odds ratio of COVID-19 infection low plasma 25(OH)D levels appears to be an independent risk factor for COVID-19 infection and hospitalization. Age over 50 years was positively associated with the likelihood of hospitalization due to COVID-19 [60].
In 4510 UK Biobank participants tested for COVID-19 in a hospital setting, male gender, Black, Asian, and Minority ethnicity (BAME), higher BMI and greater household size were independently associated with significantly greater odds of COVID-19. In addition, greater risk of severe COVID-19 in BAME populations was not adequately explained by variations in cardiometabolic factors, 25(OH)D levels or socio-economic factors [61]. In contrast, the findings of Hastie, Pell and Sattar (2021) [62] do not support a potential link between 25(OH)D concentrations and risk of severe COVID-19 infection and mortality. The authors compared 341,484 UK Biobank participants according to vitamin D levels checked between 2006 and 2010. 25(OH)D concentration was associated with severe COVID-19 infection and mortality [per 10 nmol/L 25(OH)D Hazard ratio (HR) 0.92; 95% CI 0.86–0.98; p = 0.016], but significance was lost after adjustment for 13 confounders (mortality per 10 nmol/L 25(OH)D HR 0.98; 95% CI = 0.91–1.06; p = 0.696). Probably, the statistical adjustment model included variables that influence the status of vitamin D, decreasing the strength of association of this variable.
Two additional studies explored the same community-based UK Biobank cohort. First, researchers tested 473,550 individuals in relation to 459 COVID-19 and 2626 non-COVID-19 deaths on 21 September 2020 and suggested that age, male sex and Black ethnicity, as well as comorbidities and oral steroid use at enrollment were associated with increased risk of COVID-19 death [63]. Second, a prospective study restricted to participants (n = 8297) who had records of COVID-19 test results from 22 assessment centers (between 16 March 2020 and 29 June 2020) reported that habitual use of vitamin D supplements was associated with a lower risk of COVID-19 infection, independent of lifestyle, socio-economic status, prevalent chronic diseases, and circulating vitamin D levels [64].
Retrospectively, the association of vitamin D levels was measured within 6 months before the COVID-19 test with the incidence of COVID-19 positivity, radiological findings, and laboratory parameters. In COVID-19 (227) and non-COVID-19 patients (260) the severe vitamin D deficiency (<10 ng/mL) was considerably more common in COVID-19 patients (44%) than in non-COVID-19 patients (31%). Among COVID-19-positive patients, the group with vitamin D levels of >30 ng/mL had significantly lower D-dimer and C-reactive protein (CRP) levels and a lower number of affected lung segments and shorter hospital stays [65]. Likewise, other authors noted positive findings of vitamin D levels of >30 ng/mL in hospitalized COVID-19 patients [66,67].
Among the 287 patients, 100 (36%) were vitamin D sufficient (25[OH]D > 30 ng/mL) and 41 (14%) died during hospitalization. Multivariate analysis in patients aged ≥65 years revealed that vitamin D sufficiency (25[OH]D ≥ 30 ng/mL) was significantly associated with decreased odds of death (OR 0.33, 95% CI, 0.12–0.94), acute respiratory distress syndrome (OR 0.22, 95% CI, 0.05–0.96), and severe sepsis/septic shock (OR 0.26, 95% CI, 0.08–0.88), after adjustment for potential confounders [66]. Similar data were obtained by Angeliki et al. (2021) [67] in respect of lower in-hospital mortality and need for invasive mechanical ventilation. These results support the potential benefit of raising serum 25(OH)D level to at least 30 ng/mL to reduce the risk of morbidity and mortality of COVID-19. Contradictorily, low vitamin D level before and during the pandemic were not independently associated with SARS-CoV-2 seropositivity in a large cohort of working-age adults [68].
Exploring comorbidities, a multi-center, case–control study performed with 220 adults from the Arab Gulf found that lower levels of 25(OH)D were also observed among SARS-CoV2 positive cases when compared to negative controls, and there was increased risk of COVID-19 for old age (>60 years) [OR 6.2, 95% CI, 2–18; (p = 0.001)] as well as having type 2 diabetes [OR 6.4, (95% CI, 3–14); (p < 0.001)] and low HDL cholesterol (HDL-c) [OR 6.1 (95% CI, 3–14); (p < 0.001)]. Interestingly, the study showed a significantly higher prevalence of vitamin C and D supplement use in the SARS-CoV-2 positive group during the pandemic (p < 0.001). This probably occurred as a spontaneous preventive strategy from the individuals [69].
4.3. Studies addressing vitamin D status in hospitalized patients with COVID-19
Most studies developed with COVID-19 hospitalized patients address vitamin D as a useful prognosticator in the scenario of COVID-19 because during treatment it is possible to evaluate risk factors associated with vitamin D status and the longer duration, severity, and mortality of the disease.
In older patients aged ≥65 years COVID-19-positive arm demonstrated lower median serum 25(OH)D level of 27 nmol/L (IQR = 20–47 nmol/L) compared with COVID-19-negative arm, with median level of 52 nmol/L (IQR = 31.5–71.5 nmol/L) (p value = 0.0008). Among patients with vitamin D deficiency, there was higher peak D-dimer level (p = 0.034) and higher incidence of non-invasive ventilation (NIV) support and high dependency unit (HDU) admission (30.77% vs 9.68%) (p = 0.042). However, older adults with vitamin D deficiency and COVID-19 may demonstrate worse morbidity outcomes [70]. Sulli et al. (2021) [71] also found vitamin D deficiency more frequent in elderly COVID-19 hospitalized patients (78% of patients) than in sex- and age-matched controls (57% of subjects). In addition, the study reported that vitamin D deficiency was associated with more severe lung involvement, longer disease duration, and risk of death in elderly COVID-19 patients.
A pilot study on a Greek ICU Cohort showed that eighty (80%) percent of critically ill COVID-19 pneumonia patients had vitamin D deficiency. As well as this, those who died within 28 days appeared to have lower ICU admission 25(OH)D levels compared to survivors [72]. Another retrospective cohort trial in 186 cases of severe COVID-19 found that low 25(OH)D levels on hospital admission were associated with COVID-19 pneumonia radiologic stage and mortality. Vitamin D deficiency on hospital admission was associated with COVID-19 mortality with an odds ratio of 3.87, independent of age, chronic lung disease, coronary artery disease, hypertension, or diabetes [73].
Patients treated in Respiratory Intermediate Care Unit with acute respiratory failure due to COVID-19 and severe vitamin D deficiency, after 10 days of hospitalization, had 50% of mortality probability, while those with 25(OH)D levels ≥ 10 ng/mL had a 5% mortality risk (p = 0.019). This study provided preliminary evidence to demonstrate an association between vitamin D deficiency and COVID-19 disease incidence and severity [74]. Thereafter, vitamin D insufficiency (22.8%) and deficiency (69.1%) were present in patients of Turkey with severe-critical COVID-19. Serum 25(OH)D levels was an independent predictor of mortality (p = 0.010) [75]. Another study among 464 SARS-CoV-2-positive patients from the United Arab Emirates confirms the association of 25(OH)D levels <12 ng/mL with COVID-19 severity and mortality [76]. As well as this, authors described a subgroup analysis of an overall 205 COVID-19 patients with severe disease, resulting in more evidence of mortality rate in patients with very low levels of 25(OH)D (36%) [77].
Radujkovic et al. (2020) [78] tested 185 symptomatic SARS-CoV-2-positive patients treated at their center. Ninety-two (50%) patients were followed in the outpatient setting and 93 (50%) patients required hospitalization. Vitamin D-deficient patients had a higher hospitalization rate and required more (intensive) oxygen therapy and invasive mechanical ventilation (IMV). Adjusting for age, gender, and comorbidities, vitamin D deficiency was associated with higher risk of IMV/D and death (HR 6.12 and 14.73, respectively).
A case–control study found that vitamin D-deficient severe/critical COVID-19 cases (41.9%) compared to healthy controls (11.1%) were significantly older and had higher percentages of comorbidity (renal failure). Also, vitamin D deficiency was the greatest in severe/critical cases (80%), compared with mild cases (36%) these associations remaining even after controlling for demographics and comorbidities. These findings reinforce that the elderly and people with comorbidities are susceptible to severe COVID-19 infection [79]. In a cross-sectional study including 355 COVID-19 patients admitted to the Wuhan Tongji Hospital (China), the authors also reported that male sex, advanced age (>65 y) and vitamin D deficiency (<30 nmol/L) were significantly associated with COVID-19 hospitalization and severity [80].
Another case–control study demonstrated a significant negative correlation (p = 0.02) between the 25(OH)D levels and developing coronavirus infection and that the COVID-19 cases were more likely to be overweight than the controls (p = 0.023) [81].
In the study addressing several micronutrients, vitamin B1, B6, B12, vitamin D (25-hydroxyvitamin D3), folate, selenium, and zinc were analyzed in COVID-19 hospitalized patients. Vitamin D (76%) and selenium (42%) deficiencies were the most prevalent. Additionally, severe vitamin D deficiency (≤10 ng/dL) was more frequent in COVID-19 patient group than in the healthy control group. Among 12 patients with respiratory distress, 11 (91.7%) were deficient in at least one nutrient. However, it is unclear whether individual nutrient deficiencies affected immunity or whether the nutrient deficiency simply led to a decline in the patient's overall condition [82].
Later, Bennaour et al. (2021) [83] reported the high frequency of hypocalcemia and hypovitaminosis D in severe COVID-19 patients. The 28-day mortality rates, according to vitamin D status and corrected serum calcium tertiles indicated a dose–effect relationship between these two parameters and short-term mortality (p = 0.001 and 0.0001 respectively). The highest mortality rate was recorded in the group with severe vitamin D deficiency (46.9%).
COVID-19 infection can be spread by asymptomatic, presymptomatic, and symptomatic carriers. The marked variability in clinical severity provides an estimation that 40–45% patients can remain asymptomatic and 30–40% develop only mild symptoms [84]. Considering in many cases the lack of clinical notification, the nutritional status of asymptomatic patients has not been explored in the literature. In this sense, a study demonstrated that 25(OH)D levels were very low in 63 critically ill COVID-19 patients, and that the corresponding inflammatory response and fatality rates were higher compared to 91 asymptomatic participants. Serum level of inflammatory markers was found to be higher in vitamin D deficient COVID-19 patients viz. IL-6 level (in pg/mL) 19.34 ± 6.17 vs 12.18 ± 4.29: TNFα level (in pg/mL) 13.26 ± 5.64 vs 11.87 ± 3.15. The fatality rate was high in vitamin D deficient individuals (21% vs 3.1%) [85]. Similarly, Italian COVID-19 patients were reported to have low 25(OH)D levels inversely correlated with high IL-6 levels and were independent predictors of COVID-19 severity and mortality [86].
Focused on inflammatory markers associated with health-related scores, authors proposed that vitamin D levels were deficient in (80%) of hospitalized COVID-19 patients who presented elevated D-dimer values and B lymphocyte cell count and reduced CD8 + T lymphocytes with a low CD4/CD8 ratio. At the same time, compromised clinical findings measured by Lung Immune Prognosis Index (LIPI) and SOFA scores and thoracic CT scan involvement were observed. This condition reaffirms that deficient levels of vitamin D are associated with the worst prognosis in COVID-19 patients [87].
In contrast, nine analyzed studies showed no association between vitamin D status and COVID-19 clinical outcomes during hospitalization stay [[88], [89], [90], [91], [92], [93], [94], [95], [96]].
In a single-center cohort study, 129 consecutive adult COVID-19 hospitalized patients had 25(OH) vitamin D serum levels assessed 48 h since hospital admission. The prevalence of 25(OH)D insufficiency, moderate deficiency and severe deficiency was 13.2%, 22.5% and 54.3%, respectively. 25(OH)D deficiency (<20 ng/mL) was not associated with COVID-19 clinical features and outcomes [88]. Another study suggested that 25(OH)D deficiency was not associated with SARS-CoV-2 infection but may increase the risk for mortality in severely deficient cases [89]. Similar studies also reported no statistically significant evidence that insufficient vitamin D levels might play a role in adverse outcomes of COVID-19 infection [90,91].
Szeto et al. (2021) [92] examined the relationship between prehospitalization 25(OH)D levels (obtained 1–365 days prior to admission) and COVID-19 clinical outcomes in 700 COVID-19 positive hospitalized patients. There was no relationship between 25(OH)D and any outcome, even after controlling for age and pulmonary disease. In a different approach, eight weeks after the onset of COVID-19, vitamin D deficiency was frequent among COVID-19 patients, but also not associated with disease outcomes. Surprisingly, PTH concentrations were increased in patients who needed ICU treatment, while 25(OH)D levels were not significantly different between disease severity groups. However, individuals with severe coronavirus diseases displayed a disturbed parathyroid-vitamin-D axis within their recovery phase [93]. This finding helps in the understanding of endocrine disorders in vitamin D metabolism throughout the course of COVID-19.
Despite the lower values of 25(OH)D in COVID-19 patients, three additional studies did not find any significant association of vitamin D levels with mortality, the need for mechanical ventilation, ICU admission [[94], [95], [96]], and the development of thromboembolism in COVID-19 patients with a documented vitamin D level within the past 12 months [95].
4.4. Studies addressing use of vitamin D supplementation during COVID-19
A large observational study of hospital in-patients with COVID-19 was carried out on hospital in-patients with the most severe clinical manifestations of COVID-19. A total of 986 participants with COVID-19 were studied, of whom 151 (16.0%) received cholecalciferol booster therapy with a high-dose of cholecalciferol (20,000 to 50,000 weekly). This intervention, regardless of baseline serum 25(OH)D levels, appears to be associated with a reduced risk of mortality in acute in-patients admitted with COVID-19 [97]. As well as this, a descriptive study noted that COVID-19 patients who had received a high dose of vitamin D supplementation achieved normalization of vitamin D levels and improved clinical recovery evidenced by shorter lengths of stay, lower oxygen requirements, and a reduction in inflammatory marker status [98].
Recently, an interventional clinical trial tested patients with hypovitaminosis D randomised into two groups. Subjects of (vitamin D) VD group received adjunctive pulse D therapy (60,000 IUs of vitamin D) per day for 8 days for subjects with BMI of 18–25 and 10 days for subjects with BMI >25, along with the routine standard treatment for COVID-19. Subjects of the (control) NVD group received standard treatment for COVID-19 alone. The difference in the reduction of inflammatory markers [CRP, ferritin, Il-6, Lactate dehydrogenase (LDH) and Neutrophil/Lymphocyte (N/L)], between the two groups was highly significant (p < 0.01) with the reduction in VD group being markedly higher than the NVD group. Improvement of serum 25(OH)D level to 80–100 ng/mL significantly reduced the inflammatory markers without any side effects [99].
A study including patients (aged 74 ± 13 years) with COVID-19 provides supplementation (or not) with 400,000 IU (as bolus oral cholecalciferol administered on two consecutive days). Patients were followed up during their in-hospital stay for an average time of 14 ± 10 days. For analysis, a composite outcome, including the need for transfer to ICU (for invasive mechanical ventilation) and/or death from any cause, was considered. Twenty-seven (29.7%) patients were transferred to the ICU and 22 (24.2%) patients died, six cases after being transferred to the ICU (and 16 cases prior to transfer to ICU). Overall, 43 (47.3%) patients experienced the combined endpoint of transfer to ICU or death. In a crude analysis, initially including comorbidity burden as a potential confounder, vitamin D treatment was observed to be associated with a 43% and 55% reduction, respectively, in the odds ratio of the combined endpoint, but these effects did not reach statistical significance [100].
Agreeing with these findings, in a quasi-experimental study, authors noted that irrespective of all measured potential confounders, regular bolus vitamin D3 supplementation (50,000 IU vitamin D3 per month, or doses of 80,000 IU or 100,000 IU vitamin D3 every 2–3 months) was associated with less severe COVID-19 and better survival rate in frail hospitalized elderly from a French nursing-home [101].
A retrospective, multicenter, open, non-randomized cohort study reported that patients supplemented with calcifediol (0.266 mg/capsule, 2 capsules on entry and then one capsule on day 3, 7, 14, 21, and 28) had lower risk of death during hospitalization during the first 30 days (5% vs. 20%, p-value < 0.01) compared to patients not receiving such treatment [102]. In contrast, treatment with oral cholecalciferol (median dose of 60,000 IU) did not make any difference to the outcomes in hospitalized COVID-19 patients [103], and this includes the intervention of 800 IU/mo of 25(OH)D supplements used in another study [104].
4.5. Randomized controlled trials of vitamin D supplementation in COVID-19
During a pilot, randomized clinical study, patients hospitalized with COVID-19 infection were treated with oral calcifediol (0.532 mg) and continued with oral calcifediol (0.266 mg) weekly, on days 3 and 7, until discharge or ICU admission. All hospitalized patients received combined standard therapy of hydroxychloroquine and azithromycin for 5 days. Of the 50 patients treated with calcifediol, none died, and all were discharged, without complications. The 13 patients not treated with calcifediol, who were not admitted to the ICU, were discharged. Of the 13 patients admitted to the ICU, two died and the remaining 11 were discharged. In conclusion, administration of a high dose of calcifediol or 25-hydroxyvitamin D, significantly reduced the need for ICU treatment of patients requiring hospitalization due to proven COVID-19. The authors reported some limitations of the study as it is not double-blind placebo controlled, does not consider obesity as a risk factor, and does not include a comparison with cholecalciferol. Even so, they justify the choice of this intervention by commenting on the advantages of calcifediol over native vitamin D [105].
In another trial, asymptomatic or mildly symptomatic SARS-CoV-2 RNA positive vitamin D deficient [25(OH)D < 20 ng/mL] participants were randomised to receive daily 60,000 IU of cholecalciferol (oral nano-liquid droplets) for 7 days with therapeutic target 25(OH)D > 50 ng/mL (intervention group) or placebo (control group). Overall, 10 out of 16 patients achieved 25(OH)D > 50 ng/mL by day-7 and another two by day-14. A greater proportion of vitamin D-deficient individuals with SARS-CoV-2 infection became SARS-CoV-2 RNA negative with a significant decrease in fibrinogen and inflammatory markers with cholecalciferol supplementation [106]. The authors comment that the response of fibrinogen in this study may not be clinically relevant.
Sabico et al. (2021) [107] allocated 69 hospitalized patients for mild to moderate COVID-19 disease to receive once daily for 2 weeks either 5000 IU oral vitamin D3 (n = 36) or 1000 IU oral vitamin D3 (standard control) (n = 33). Vitamin D deficiency was observed in 40 cases (55%), with no difference between the groups (p = 0.1), while the rest had vitamin D insufficiency. Unadjusted Kaplan–Meier survival analysis was used to determine the differences in recovery times of symptoms and revealed that the number of days to resolve cough was significantly shorter in the 5000 IU group than the 1000 IU group (6.2 ± 0.8 versus 9.1 ± 0.8; unadjusted p = 0.007). The same shorter period was observed for ageusia (loss of taste), again in favor of the 5000 IU group (11.4 ± 1.0 versus 16.9 ± 1.7; unadjusted p = 0.035). The significance for cough decreased but persisted even after adjusting for age, sex, baseline BMI, and D-dimer (p = 0.039), while the same significance was observed for ageusia (p = 0.035). The authors suggested the use of 5000 IU vitamin D3 as an adjuvant therapy for COVID-19 patients with suboptimal vitamin D status.
A Brazilian multicenter, double-blind, parallel-group trial that was randomized and placebo-controlled was performed using a single high dose of vitamin D3 among hospitalized patients with moderate to severe COVID-19, testing as primary outcome the response on hospital length of stay (defined as the total number of days that patients remained hospitalized from the date of randomization until the date of hospital discharge). The secondary outcomes were mortality during hospitalization, admission to the ICU; need for mechanical ventilation, and serum 25(OH)D levels, among other biochemical markers. Mean serum levels of 25(OH)D significantly increased after a single dose of vitamin D3 vs placebo [44.4 ng/mL vs 19.8 ng/mL; difference, 24.1 ng/mL; (p < 0.001)]. The median (IQR) hospital length of stay was not significantly different between the vitamin D3 (p = 0.94). Moreover, a post hoc analysis showed no changes in the median length of stay between groups. There were no significant differences between the vitamin D3 and placebo groups regarding secondary outcomes [108]. In conclusion, a single high dose of vitamin D3, compared with placebo, did not significantly reduce hospital length of stay among hospitalized patients with COVID-19. Currently, there are an estimated thirty clinical trials under development ( www.clinicaltrials.gov ) which include protocols with vitamin D and COVID-19.
5. Zinc
5.1. Zinc, immune system, and COVID-19
Zinc deficiency is a common condition and a public health problem in almost all low- and middle-income countries [109]. Zinc is involved in numerous processes such as DNA synthesis, RNA transcription, gene expression, cell division, cell proliferation, apoptosis, immune response, intracellular signaling, growth, sense of smell and taste, and appetite [[110], [111], [112]].
Zinc affects both innate and adaptive immunity. Its role in the immune system has been reviewed elsewhere [[113], [114], [115]]. Briefly, zinc is essential for the normal development and function of innate immune cells, such as neutrophils and natural killer (NK) cells [113]. Zinc deficiency negatively influences phagocytosis and chemotaxis of neutrophils and it impairs the recognition of major histocompatibility complex class I in target cells as well as the cytotoxic and lytic activity of NK cells [114]. In addition, altered zinc homeostasis in macrophages causes impaired phagocytosis and leads to an abnormal inflammatory response [116].
Regarding the adaptive immune response, zinc is also important for the growth and function of T and B cells. As reviewed before [113], zinc deficiency is associated with thymic atrophy, lymphopenia, compromised cell- and antibody-mediated responses, and increased risk of infections. Moreover, zinc deficiency decreases the production of IFN-γ which leads to immune dysfunction mediated by the imbalance between lymphocytes Th1 and Th2. Additionally, zinc deficiency leads to increased production of inflammatory cytokines (TNF-α, IL-1b) by macrophages and activated monocytes which contribute to increased production of ROS, a byproduct of inflammation. This inflammatory and oxidative profile activates NFκB pathways aggravating this profile.
These immune responses consist of a coordinated action of specialized cells, communicating with molecules and functional responses [9]. In this context, zinc finger proteins, zinc transporters and zinc homeostasis are essential to ensure proper signaling pathways and networks of the immune response through several mechanisms which are discussed elsewhere [114,117]. However, zinc deficiency as well as zinc excess lead to malfunction of the immune system and its homeostasis is crucial to proper immune responses avoiding overreaction and cytokine storms [114].
Recently, Skalny et al. (2020) [13] reviewed possible and already proven zinc immunomodulatory mechanisms in COVID-19, including: (1) zinc cations inhibit SARS-coronavirus polymerase activity by decreasing its replication; (2) chloroquine is a zinc ionophore and the increased flux of zinc into the cell may enhance its antiviral effect; (3) zinc can bind with the structure of viral proteins (papain-like proteases) of SARS-CoV-2 and destabilize them with the use of zinc-ejector drugs; (4) zinc might decrease the expression of ACE2, an enzyme needed by SARS-CoV-2 for entry into target cells; (5) zinc is essential for respiratory epithelium, so it might ameliorate the dysfunction of mucociliary clearance; and (6) zinc increases the IFN-α production by leukocytes and potentiates its antiviral activity (Fig. 1).
5.2. Studies addressing previous zinc status and COVID-19
No cohort study addressing previous zinc status and risk of COVID-19 was identified in our search. However, zinc deficiency has been associated with the prevalence of other respiratory tract infections [13].
5.3. Studies addressing zinc status in hospitalized patients with COVID-19
In regard to our search, 10 observational studies aimed to investigate the effect of zinc status in patients with COVID-19 [[118], [119], [120], [121], [122], [123], [124], [125], [126], [127]]. All of them observed adults and elderly patients, including pregnant women in one study [123] (Supporting information Table S3).
In a case–control study, Elham et al. (2021) [118] found that COVID-19 hospitalized patients had lower serum zinc levels compared to healthy individuals. Fromonot et al. (2021) [119], found a prevalence of hypozincemia in 20% of the patients admitted to COVID-19 screening centers. What is more, hypozincemia was an independent predictor of hospitalization for respiratory complications within 10 days.
Heller et al. (2021) [120] tested the hypothesis that COVID-19 patients are characterized by zinc deficiency and that zinc status provides prognostic information. In this study most of the COVID-19 patients admitted to the hospital had low serum zinc levels (serum zinc <642.5 μg/L). What is more, non-survivors showed lower zinc levels compared to the survivors. During the hospital stay, the dynamic changes in the zinc serum concentration showed a greater increase in survivors (discharged patients). Possibly the nutritional supply of zinc contributed to maintaining a favorable zinc status and improving the antioxidative defense, energy metabolism, and immune response. These authors found that zinc associated with selenoprotein P (SELENOP) status within the reference ranges is a reliable indicator of survival in COVID-19 and a personalized supplementation of these micronutrients may support convalescence.
In another study, hypozincemia (serum zinc <70 mg/dL) was associated with COVID-19 severity in hospitalized patients. The authors suggest the use of serum zinc as a predictive clinical parameter for a critical illness of COVID-19 and to incorporate this measurement as one of the routine blood tests to focus on adequate zinc supply in ICU. They observed that an adequate enteral nutrition support for 10–12 days can recover the serum zinc levels to the normal range in critically ill patients [121].
Jothimani et al. (2020) [122] conducted a comparative analysis between COVID-19 patients and healthy volunteers and aimed to verify the correlation between serum zinc levels in patients with COVID-19 and the disease severity. COVID-19 patients were zinc deficient (serum zinc <80 mg/dL) compared to healthy adults. Moreover, low baseline zinc levels were associated with higher complication rate, increased tendency to develop ARDS, longer hospital stays, and increased mortality.
Anuk et al. (2020) [123] aimed to determine zinc status, as well as another two trace elements, in pregnant women with COVID-19. They observed that serum zinc levels decreased in pregnant women with COVID-19 in comparison to the healthy ones. Serum zinc levels had a negative relationship with acute phase markers such as interleukin 6, erythrocyte sedimentation rate, procalcitonin and c-reactive protein, demonstrating its association with infection and inflammation status.
Other studies demonstrated a correlation between serum zinc levels and COVID-19 outcome. Low serum zinc levels (50 μg/dL at admission) were correlated with worse clinical presentation, longer time to reach stability, and higher mortality of hospitalized patients [124]. In critically ill patients with COVID-19, low serum zinc levels (<70 μg/dL) were extremely prevalent at admission to the ICU and were associated with a degree of organ failure manifested with severe ARDS [125]. Skalny et al. (2021) [126] aimed to investigate the association of metal profile with markers of lung damage. These authors found that zinc and other metals (calcium, iron, and selenium) gradually decrease as the COVID-19 severity increases. These metal levels correlated positively with SpO2 and were inversely associated with fever, lung damage, and CRP concentrations.
In an investigation into multiple nutritional parameters in patients admitted with COVID-19, nutritional risk was associated with COVID-19 severity. In addition, severe patients showed lower serum zinc levels compared with non-severe patients. These authors point to the importance of developing international guidelines to advise on regular nutritional screening and nutritional support in COVID-19 [127].
5.4. Studies addressing use of zinc supplementation during COVID-19
In regard to our search, 3 retrospective observational studies aimed to investigate the effect of zinc supplementation in adults or elderly patients with COVID-19 [[128], [129], [130]] (Supporting information Table S3).
A retrospective study showed that zinc supplementation in addition to the standard care (hydroxychloroquine and azithromycin) increased the frequency of patients being discharged from the hospital and decreased the need for ventilation and admission to the ICU. Also, zinc supplementation was associated with decreased transition to hospice for patients that did not require intensive care. These authors suggest a synergistic mechanism of zinc and hydroxychloroquine in preventing the virus from progressing, however, the effectiveness of zinc may reduce in severe cases marked by intense oxidative and inflammatory profiles. In this sense, viral RNA levels in clinical specimens of patients with COVID-19 should be investigated before and after zinc supplementation [128].
Yao et al. (2021) [129] did not find significant association between zinc sulphate supplementation and risk of in-hospital mortality. This risk was higher for older patients, male sex, and clinical severity. As stated by the authors, the lack of a causal association between zinc and survival of hospitalized patients with COVID-19 does not rule out the benefit of zinc in the management of COVID-19. Future randomized clinical trials with adequate sample size are necessary to better clarify this association and substantiate the use of zinc in COVID-19 management.
Finzi and Harrington (2021) [130] aimed to evaluate the effect of zinc supplementation on COVID-19 symptoms in outpatients. They observed a positive effect of zinc supplementation in improving symptoms of COVID-19. This improvement appeared to be faster in younger patients (<40 years old). Regardless of the scenario, the symptoms of COVID-19 can impair nutritional status by limiting food intake and weakening the immune system, further aggravating the clinical condition.
5.5. Randomized controlled trials of zinc supplementation in COVID-19
According to our search, 3 randomized clinical trial studies aimed to investigate the effect of zinc supplementation in patients with COVID-19 [37,131,132]. Abd-Elsalam et al. (2020) [131] aimed to evaluate the effect of combining chloroquine/hydroxychloroquine and zinc in the treatment of COVID-19 patients. This study included 191 patients with COVID-19 from three major university hospitals in Egypt. These patients were randomized into two matched groups, one receiving both hydroxychloroquine and zinc, and the other receiving hydroxychloroquine only. The primary outcomes were recovery within 28 days, the need for mechanical ventilation, and death. Zinc supplementation was not associated with any of these outcomes. The age and the need for mechanical ventilation were the only factors associated with mortality. Some limitations pointed out by the author included possible limited intestinal absorption of zinc, non-assessment of serum zinc levels, and no investigation into viremic response.
In a multicenter randomized clinical study with ambulatory COVID-19 patients, Thomas et al. (2021) [37] aimed to investigate whether high-dose zinc and/or high-dose ascorbic acid reduce the severity or duration of symptoms compared with standard care. They observed that none of the interventions significantly decreased the duration of symptoms compared with standard care. In a pilot randomized controlled trial with hospitalized COVID-19 patients, Patel et al. (2021) [132] detected low serum zinc levels in both experimental and placebo groups (6.9 ± 1.1 and 7.7 ± 1.6 μmol/L, respectively). They also observed that a high dose of venous zinc administration (0.24 mg/kg/day of elemental zinc for a maximum of 7 days) was safe and able to increase serum zinc levels above the zinc deficiency cutoff adopted (10.7 μmol/L).
6. Selenium
6.1. Selenium, immune system, and COVID-19
Selenium (Se) is an essential trace element of high importance for human health and particularly for a well-balanced immune response. Selenium is found in foods and nutritional supplements in organic (selenomethionine, selenocysteine, selenium-methylselenocysteine) and inorganic (selenato and selenium) forms. After intestinal absorption, selenium forms are converted into hydrogen selenide (H2Se), a metabolic intermediate incorporated into selenoproteins, in the form of selenocysteine. Selenium performs its main functions in the form of selenoproteins that are encoded by the presence of a selenocysteine insertion sequence (SECIS) in the 3′-untranslated region of mRNA, the UGA codon, which normally acts as a stop codon under specific conditions. A wide range of these selenoproteins are linked to redox signaling, oxidative burst, calcium flux, and the subsequent effector functions of immune cells being grouped into families such as glutathione peroxidases (GPXs), iodothyronine deiodinases (DIOs) and thioredoxin reductases (TrxRs), methionine sulfoxide reductase B1 (MSRB1), and selenophosphate synthetase 2 (SEPHS2). In addition, the SELENOP acts as the main selenium transporter for peripheral tissues also performing extracellular antioxidant function [12,133].
Thus, selenium plays a role in antioxidant, anticarcinogenic, anti-inflammatory, redox and immune-cell function as well as in the regulation of thyroid hormone metabolism. Studies have already identified associations between selenium deficiency and increased morbidity and mortality from viral infections, cardiovascular, and thyroid diseases, as well as prostate, gastrointestinal, and breast cancers [14,133,134].
Evidently, it is well established that selenium is useful for the competency of the cellular component of both innate and adaptive immunity [12,[135], [136], [137], [138]]. As the first line of defense, innate immune cells possess immediate protective mechanisms including antimicrobial enzymes and peptides, which can target various pathogens for lysis or phagocytosis by phagocytic cells. In addition, in the cascade of reactions to infection, the inflammatory response is triggered by the production of chemical mediators, enzymes and cytokines induced by molecular mechanisms associated with pathogens [139]. On a cellular level, selenium status may influence various leukocytic functions including adherence, migration, phagocytosis, and cytokine secretion. In this context, selenoproteins also regulate or are regulated by cellular redox tone, which is a crucial modulator of immune cell signaling [12].
However, for several reasons, selenium/selenoproteins are relevant in the viral pathogenicity, notably reducing proliferation of T cells, lymphocyte-mediated toxicity and NK cell activity, all of which are crucial for antiviral immunity [12,136,137]. Keshan disease was the first endemic cardiomyopathy from a virus related to selenium deficiency which had a reduced incidence in the face of selenium supplementation. Mechanisms related to low GPx-1 activity have been associated with the incidence of Keshan disease [140]. Selenium intervention has also shown benefits in the response to viral infections according to animal models of lethal influenza infection [141]. On the other hand, in selenium-deficient mice, the levels of macrophages and CD8+ and CD4+ T cells were lower compared to mice with adequate selenium levels, suggesting that selenium deficiency affects cellular immunity [142]. Other examples of the effects of selenium on viral pathogenicity have been demonstrated based on lower plasma selenium concentrations during early stages of HIV-1 [143]; and due to adjuvant treatment of patients using high levels of selenium, there was reduced mortality rate in patients with severe sepsis or septic shock [144].
Contextualizing the impact of selenium on COVID-19, Zhang et al. (2020) [14] in a long review article emphasized how involvement of selenium, via selenoproteins functions and selenium species, might counteract infection with SARS-CoV-2. Anti-inflammatory, immune-cell function and antiviral effects of selenoproteins were described in detail showing that, particularly, GPXs, TXNRDs, SELENOS, and MSRB1 are involved in most of these functions. GPXs, TXNRDs and ER selenoproteins influence the viral pathogenicity, partly by reducing oxidative stress generated by viral pathogens [[145], [146], [147], [148]] (Fig. 1). What is more, prostacyclin (PGI2) regulation and the cyclooxygenase-2 (COX2) expression can be influenced by selenium status [139]. The authors describe in detail mechanisms linked to accumulation of macrophages, exhaustion of NK cells and the inflammatory cytokine storm identified as a predictor in clinical outcomes and lung damage particular to COVID-19 [79]. It's known that the SARS-CoV-2 infection may affect primarily T lymphocytes, particularly CD4+ and CD8+ T cells, resulting in a decrease in numbers as well as IFN-γ production by CD4+ T cells. These immunological dysfunctions may be important because of their association with outcomes that indicate disease severity in COVID-19 [45,149]. In summary, there is a mechanism proposed by Zhang et al. (2020) [14] by which selenium might suppress the life cycle and mutation to virulence of SARS-COV-2 while attenuating viral-induced oxidative stress, organ damage and the cytokine storm. Adding these findings, a current question has been posed speculating whether the disturbances induced by SARS-CoV-2 in the homeostasis of selenium contribute to the manifestation of COVID-19 coagulopathy [150].
In the outbreak of the pandemic of COVID-19 an ecological study pointed out the cure rate from COVID-19 associated with basal hair selenium concentration in different areas of China. Cured patients were those in whom temperature returned to normal for more than 3 days, respiratory symptoms were significantly improved, lung imaging showed significant reduction of inflammation, and there was negative nucleic acid test of respiratory pathogen on two consecutive occasions with a sampling interval of at least 1 day [151]. Collectively, the available studies support the belief that selenium may be of relevance in the infection with SARS-CoV-2 and disease course of COVID-19 [82,120,[152], [153], [154]].
In the scope of this review, we found only 5 articles (all observational) aimed to evaluate the selenium status in patients with COVID-19 (Supporting information Table S4).
6.2. Studies addressing selenium status in hospitalized patients
The mortality risk was tested in 33 COVID-19 patients associated with total serum selenium total and selenoprotein P (SELENOP) compared with reference data from a European cross-sectional analysis (EPIC, n = 1915). A prominent deficiency in total serum selenium and SELENOP concentrations was found in COVID-19 patients. The stratification of the samples according to selenium status below the 2.5th percentile of the reference population i.e., [Se] < 45.7 μg/L and [SELENOP] < 2.56 mg/L showed frequency of 43.4% and 39.2%, respectively. The selenium status was significantly higher in samples from surviving COVID patients than non-survivors. Also, the recovery of selenium in survivors occurred over time, while it remained low or decreased in non-survivors [153].
In another observational study, combined zinc status and SELENOP was used to compare prognoses between hospitalized patients surviving COVID-19 and non-survivors. The prevalence of a combined deficit, i.e., serum Zn below 638.7 μg/L and serum SELENOP below 2.56 mg/L, was observed in 0.15% of samples in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort of healthy subjects, 19.7% of the samples collected from the surviving COVID-19 patients and in 50.0% of samples from the non-survivors. The combination of serum zinc and SELENOP concentrations with respect to the patients’ age tested by ROC curve analysis yielded an area under the curve (AUC) of 94.42%. This result indicates the good correlation of a novel composite biomarker that can be explored in studies on COVID-19 [120].
As previously described, Im et al. (2020) [82] measured vitamin B1, B6, B12, vitamin D (25-hydroxyvitamin D3), folate, selenium, and zinc in hospitalized patients with COVID-19 and healthy control individuals. Besides vitamin D deficiency, 42% of patients showed selenium deficiency and among these, a moderate frequency of pneumonia was observed. A recent Indian study, also case-controlled, reported significantly lower selenium levels of 69.2 ± 8.7 ng/mL in patients with COVID-19 compared to controls 79.1 ± 10.9 ng/mL (p = 0.0003). Accordingly, authors observed that 43.3% of patients with COVID-19 had lower selenium levels against 20% of the control group [152].
An innovative retrospective study investigated associations of urinary essentials and toxic trace elements with severe illness and fatal outcome of COVID-19 [154]. The authors performed analysis of the nine urinary trace elements including chromium, manganese, copper, arsenic, selenium, cadmium, mercury, thallium and lead. The patients with COVID-19 were examined according to the disease severity (non-severe or severe) and outcome (recovered or deceased), respectively. The median duration of hospital stay was 14.5 days (IQR: 8–32). The median length from disease onset to admission was 26 days (IQR: 11–41). Mostly, after creatinine adjustment, chromium, manganese, copper, selenium, cadmium, mercury and lead were higher in severe COVID-19 patients than the non-severe cases with COVID-19. Throughout the clinical course since the disease onset these urinary elements were found to be further positively correlated with serum cytokines (IL-1B, IL2R, IL6, IL8, IL10, TNFα), ferritin, and neutrophil count cell and white blood cell count. The authors considered these findings suggestive of supporting prognosis of COVID-19.
No concluded clinical trial involving selenium and COVID-19 has been published to date. Currently, there are about ten clinical trials under development, but none of them with isolated selenium intervention ( www.clinicaltrials.gov ).
7. Discussion
This review encompasses scientific studies published during the past 16 months of the COVID-19 pandemic and provides an overview focused on the impact of micronutrient status in the face of this outbreak. In addition to summarizing relevant results, we expose substantial clinical and methodological heterogeneity across these studies.
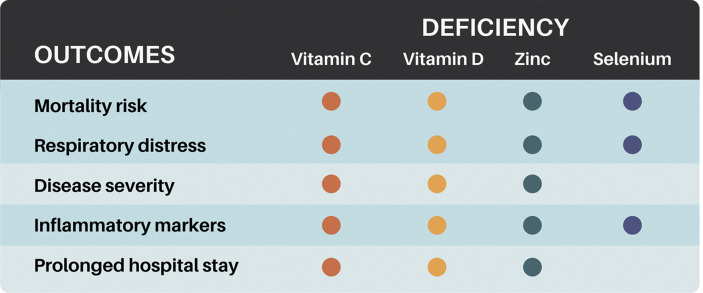
Worse clinical outcomes observed in patients with COVID-19 which have been associated with nutritional risk of vitamin C, vitamin D, zinc, and selenium deficiency are summarized in Fig. 2 . Among 85 selected studies, only 11 RCTs were completed addressing interventions with vitamin C, vitamin D, zinc, and none for selenium (Table 2 ).
Fig. 2.
Nutritional risk of vitamin C, vitamin D, zinc, and selenium deficiency related to COVID-19 clinical outcomes. Color gradient according to the number of nutrients involved in the outcome. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Clinical trials studies addressing vitamin C, vitamin D, and zinc in COVID-19 patients.
| First Author, Year | Country | Study Design | Population | Exposure Measure | Outcomes | Findings |
|---|---|---|---|---|---|---|
| Vitamin C | ||||||
| Zhang et al. (2021) [34] | China | Clinical trial | Patients with severe COVID-19 pneumonia 66.7 (12.7) years (n = 56) |
Intervention group: 24 g HDIVC per day for 7 days within 48 h after admission to the ICU | IMVFD28 period, mortality, organ functions, inflammatory parameters, patient condition improvement rate, patient condition deterioration rate, length of ICU and hospital stay, ICU and in-hospital mortality | HDIVC did not improve IMVFD28 but demonstrated a potential signal of benefit for critically ill COVID-19, with an improvement in PaO2/FiO2 ratio. |
| Placebo group: bacteriostatic water for injection | ||||||
| Kumari et al. (2020) [35] | Pakistan | Prospective clinical trial | Patients with severe COVID-19 52 (11) years (n = 150) |
Intervention group: 50 mg/kg/day of IVVC in addition to standard therapy for COVID-19 infection | Respiratory rate, oxygen saturation, CRP, LDH, number of days required for the disappearance of symptoms, number of days spent in the hospital, need for ventilation, and mortality | Vitamin C can significantly improve clinical symptoms and reduce days spent in the hospital. Vitamin C supplementation had no impact on mortality and the need for mechanical ventilation. |
| Placebo group: standard therapy | ||||||
| Siahkali et al. (2021) [36] | Iran | Randomized clinical trial | Patients with COVID-19 | Supplementation of 1.5 g IVVC every 6 h for 5 days or no supplementation | Mortality, duration of hospitalization, need for ICU admission, improvements in SpO2 and vital signs | The treatments did not significantly improve outcomes in the group who were treated with HDIVC. |
| Experimental group 37. 5 (18.3) (n = 30) | ||||||
| Control group 61.0 (15.9) years (n = 30) | ||||||
| Thomas et al. (2021) [37] | United States of America | Prospective randomized clinical trial | Ambulatory patients with COVID-19 45.2 (14.6) years (n = 214) |
Ascorbic acid only (8 g/day), zinc only (50 mg/day), both agents, or standard care. | Reduction of symptoms (fever, cough, shortness of breath, fatigue), hospitalization, death, adverse effects | The treatments did not significantly decrease the duration of symptoms, number of hospitalizations and deaths. |
| Vitamin D | ||||||
| Castillo et al. (2020) [105] | Spain | Randomized clinical trial | Hospitalized patients diagnosed with COVID-19 patients treated with calcifediol (25OHD3) 53.14 (10.77) years, (n = 50) | Oral in capsules (0.532 mg) of calcifediol (25OHD3), or not. Patients in the calcifediol treatment group continued with oral calcifediol (0.266 mg) on day 3 and 7, and then weekly until discharge or ICU admission | Effect of calcifediol treatment on Intensive Care Unit Admission and Mortality | Administration of a high dose of calcifediol significantly reduced the need for ICU treatment of patients requiring hospitalization due to proven COVID-19. |
| Non treated individuals with calcifediol 52.77(9.35) years, (n = 26) | ||||||
| Rastogi et al. (2020) [106] | India | Randomized, placebo-controlled trial | Asymptomatic or mildly symptomatic SARS-CoV-2 positive vitamin D deficient 25(OH) D individuals | Daily 60,000 IU of cholecalciferol for 7 days with therapeutic target 25(OH) D > 50 ng/mL (intervention group) or placebo (control group). Vitamin D deficient (<20 ng/mL) |
Effect of high dose, oral cholecalciferol supplementation on SARS-CoV-2 viral clearance. | Greater proportion of vitamin D-deficient individuals with SARS-CoV-2 infection became SARS-CoV-2 RNA negative with a significant decrease in fibrinogen with high-dose cholecalciferol supplementation. |
| Treated individuals 50 (36–51) years (n = 50) | ||||||
| Non treated individuals 47.5 (39.3–49.2) years (n = 24) | ||||||
| Sabico et al. (2020) [ 107 ] | Saudi Arabia | Randomized clinical trial | A total of 69 patients for mild to moderate COVID-19 were randomized into group I (n = 36) 53.5 (12.3) years and group II (n = 33) 46.3 (15.2) years |
Patients were allocated to receive once daily for 2 weeks either 5000 IU oral vitamin D3 (group I) or 1000 IU oral vitamin D3 (group II, standard control). Vitamin D sufficiency (≥75 nmol/L) Vitamin D deficiency (<50 nmol/L) |
Recovery of symptoms and other clinical parameters. | Vitamin D supplementation for 2 weeks caused a significant increase in serum 25(OH)D levels in the 5000 IU group only (adjusted p = 0.003), reducing the time of recovery for cough and gustatory sensory loss. |
| Murai et al. (2021) [108] | Brazil | Double-blind, randomized, placebo-controlled trial | Hospitalized patients with COVID-19 who were moderately to severely ill 56.2 (14.40) years n 240 |
Patients were randomly assigned to receive a single oral dose of 200,000 IU of vitamin D3 (n = 120) or placebo (n = 120). | Primary outcome was length of stay and the secondary outcomes were mortality during hospitalization, admission to the ICU; need for mechanical ventilation, and serum levels 25(OH)D, among others |
Mean serum levels of 25(OH)D significantly increased after a single dose of vitamin D3 vs placebo (44.4 ng/mL vs 19.8 ng/mL); difference, 24.1 ng/mL; (p < 0.001). A single high dose of vitamin D3 (200,000 IU), compared with placebo, did not significantly reduce hospital length of stay. There were no adverse events. |
| Zinc | ||||||
| Abd-Elsalam et al. (2020) [131] | Egypt | Randomized clinical trial | A total of 191 patients with COVID-19 were randomized into two groups: group I (n = 96) patients received both hydroxychloroquine and zinc, and group II (n = 95) received hydroxychloroquine only. Group I: 43.48 (14.62) years Group II: 43.64 (13.17) years |
Hydroxychloroquine (400 mg twice daily on the first day, then 200 mg twice daily for 5 days) and zinc sulfate (220 mg, with 50 mg of elemental zinc, twice daily) | Recovery within 28 days, need for mechanical ventilation, and death | Zinc supplements did not enhance the clinical efficacy of hydroxychloroquine |
| Thomas et al. (2021) [37] | United States | Randomized clinical trial | Ambulatory patients (n = 214) with COVID-19 and mean age of 45.2 (14.6) years | High-dose zinc gluconate (50 mg), ascorbic acid (8000 mg), both agents, or standard care. | Reduction of symptoms (fever, cough, shortness of breath, fatigue), hospitalization, death, adverse effects | None of these interventions decreased significantly the duration of symptoms compared with standard care. Despite some adverse events noted (3 deaths and 1 hospitalization), the board did not believe that any of these adverse events were caused by these interventions. |
| Patel et al. (2021) [132] | Australia | Randomized clinical trial | Hospitalized patients with COVID-19, divided into experimental (n = 15) and placebo group (n = 18), with mean age of 59.8 (16.8) and 63.8 (16.9) years, respectively | Zinc deficiency cutoff (serum zinc <10.7 μmol/L) Colorless zinc chloride (ZnCl2) with 0.24 mg/kg/day of elemental zinc concentration (experimental group) or saline solution (placebo group), for a maximum of 7 days |
Safety changes in serum zinc levels |
The venous zinc administration increased serum zinc levels above the zinc deficiency cutoff adopted. The venous zinc administration appears to be safe and feasible, with minor infusion site irritation in 3 patients |
Mean (SD); median (IQR), interquartile range; high dependency; ICU, Intensive care unit; OSCI, ordinal scale for clinical improvement; CRP, c-reactive protein; HDIVC, high-dose intravenous vitamin C; IMVFD28, invasive mechanical ventilation free days in 28-days; IVVC, intravenous vitamin C; LDH, lactate dehydrogenase.
In the set of observational studies, some confounding factors are related to the lack of knowledge on the nutritional status previous to the infection. For example, in the context of an inflammatory state, several mechanisms could be responsible for a decrease of serum 25(OH)D and plasma zinc, resulting in speculation that these events might be the cause or the consequence of inflammatory diseases [155,156]. Thus, the measurements of most micronutrient biomarkers during the acute-phase response should be evaluated with caution, taking into consideration a possible reverse causality.
Two studies found vitamin C deficient levels in COVID-19 patients [24,25]. Vitamin C levels were undetectable (detection limit 1.5 mg/L) in more than 90% of patients with COVID-19-associated ARDS in a study which was not in the scope of this review [157]. It seems reasonable to accept the prophylactic use of vitamin C in the COVID-19 context in some risk groups and regions of the world, given the favorable safety profile and low cost of vitamin C [11]. However, for the general population the current evidence for this recommendation is weak according to a previous review [20].
Overall, therapeutic doses of vitamin C used in randomized clinical trials varied on average from 50 mg/kg/day to 24 g/day (Table 2). However, the results are still controversial. While some authors found improvement in clinical outcomes with intravenous doses of vitamin C in critically ill patients [34,35], others did not find positive effects from vitamin C supplementation in hospitalized [36] and ambulatory [37] patients. The main limitations related to these polemic results were study design, sample size, and duration of treatment. Despite these limitations, the potential of vitamin C effects cannot be ignored.
Most studies explored in our review supported that vitamin D deficiency impacts on COVID-19 hospitalization, severity, and mortality. As well as this, we have also reported on studies that showed no association between vitamin D status and COVID-19 clinical outcomes. In this context, the risk factors for developing severe COVID-19 are the same as those for developing vitamin D deficiency, so it is difficult to confirm if vitamin D deficiency itself is a risk factor for severe COVID-19 [158].
Viewed together, it is well accepted that the serum concentration of 25(OH)D is affected by a set of characteristics intrinsic to the individual in combination with environmental, socio-demographic, and lifestyle factors [159]. Furthermore, many observational studies do not perform the appropriate statistical analyses required to adjust data banks for potential confounders of low vitamin D, such as age, body mass index (BMI), and comorbidities. Well-designed observational studies are the best tools for planning clinical trials which require therapeutic evidence [160].
Apart from that, vitamin D deficiency can be plausibly linked to COVID-19. A meta-analysis conducted with observational studies found a positive association between vitamin D deficiency and the severity of the disease in a total sample of 8176 COVID-19 patients. Insufficiency of vitamin D concentration increased hospitalization (OR 1.81, 95% CI, 1.41–2.21) and mortality from COVID-19 (OR 1.82, 95% CI, 1.06 2.58) [161]. Subsequently, in a systematic review and meta-analysis of 31 peer-reviewed observational studies, despite the mean 25(OH)D levels being 5.9 ng/mL lower in COVID-19 positive compared to negative patients, the certainty of the evidence was very low for a cause–effect relationship of vitamin D status and COVID-19 outcomes [162].
Obviously, therapeutic vitamin D supplementation for treatment of COVID-19 has gained attention since studies suggested an association between vitamin D deficiency and risk or prognosis of the disease. A potential threshold of 25(OH)D (41.19 nmol/L) to protect against COVID-19 was identified by Ye et al. (2020) [79].
Four randomized placebo-controlled trials included in this review showed heterogeneity because of different supplementation strategies, formulations, vitamin D statuses of participants, and reported outcomes (Table 2). Three of these suggested positive effects from supplementation with vitamin D or derivatives in patients with COVID-19 [[105], [106], [107]]. On the other hand, Murai et al. (2021) [108] refute that a single dose of 200,000 IU of vitamin D3 is a suitable treatment for patients with this disease. Recently, a live systematic review with evidence updated to March 11, 2021, which includes the studies cited here, did not find sufficient, good-quality evidence to assert whether vitamin D is an effective or safe treatment for adults with COVID-19 [158]. Based on such precedents, the exact efficacy of vitamin D supplementation for prevention of, or as an adjunct treatment for COVID-19 remains to be determined.
The role of zinc in the immune system is well established. Due to its ubiquitous functions and influence on the immune response, zinc stands out as a promising therapeutic antiviral strategy against COVID-19. Despite the lack of clinical studies with greater samples, we found that zinc deficiency is common in patients with COVID-19 and it is related to poor outcomes.
Some authors found low levels of serum zinc in COVID-19 patients compared to healthy individuals [119,120,123]. These low zinc levels may have originated from a previous deficiency or may have been caused or aggravated by the infection itself, since there is a competition for zinc between host and pathogens and zinc redistribution during inflammatory response [156]. However, evidence shows that low baseline zinc levels are associated with higher complication rates and poor outcomes, independent of its cause.
What is more, zinc supplementation alone or in addition to standard care seems to bring positive results, especially in patients with zinc deficiency. Although authors have suggested a possible synergistic mechanism of zinc and hydroxychloroquine [129], it is important to mention that this drug still lacks efficacy in COVID-19 and it could possibly be harmful [163].
Interestingly, risk factors for severe COVID-19 are also risk factors for zinc deficiency. Even with the strong homeostatic control, zinc status should be evaluated in patients and zinc supplementation should be considered as a preventive or supportive treatment for COVID-19. It is worth mentioning that zinc supplementation has positive effects without adverse side-effects. Obviously, more attention must be given to those patients with marginal or severe zinc deficiency. Several clinical trials with zinc and/or other drugs/dietary supplements for COVID-19 have been registered in ClinicalTrials.gov [164]. Soon, there will be more evidence to guide zinc interventions in COVID-19 management.
Selenium intervention studies, although less frequent, have shown positive effects in terms of reducing the risk of totality, improving clinical parameters (e.g., OSCI score, transfer to ICU) and inflammation markers, increasing viral clearance and better survival during hospitalization in frail elderly individuals during COVID-19 [101]. The main findings of these studies indicated that low selenium and SELENOP concentrations were associated with survival and recovery rates of COVID-19 patients, and a novel composite biomarker (zinc status + SELENOP) has been tested as an indicator of survival in COVID-19 [120,152,153]. The fact that selenium and selenoprotein species have an active role in innate and adaptive immunity reinforces selenium as a nutritional tool to counteract viral infections.
Particularly relevant to COVID-19, optimal nutritional status is fundamental to modulate inflammatory and oxidative stress processes. The immune system is constantly surveilling and when threatened is highly activated, which demands more energy and substrates (nutrients) from our diet. Nutrients and bioactive compounds from one's diet play key roles in supporting the human immune system. These substrates act synergically and their joint action surpasses the effect of one single nutrient [9]. For example, micronutrients support mucosal immune function (vitamin A), epithelial tissue integrity (vitamins A, C, and D), enhance the function of certain adaptive and innate immune cells (vitamins A, C, D, and E, iron, zinc, and PUFAs), and have potential pro-oxidant effects (vitamin C) [165].
A prospective, double-blinded, randomized parallel-controlled interventional clinical trial reported that anti-inflammatory antioxidant oral dietary supplementation significantly dampens the cytokine storm and leads to partial improvements in some clinical parameters among patients with non-critical COVID-19 [166]. As is already known, it is not possible to separate the effects of individual micronutrients in treatments comprising of combinations of various antioxidants [165]. Therefore, to achieve a healthy immunity it is imperative that individuals embrace a healthy lifestyle, which includes a diversified, balanced, and healthy diet, with sufficient amounts of essential nutrients. Achieving this will benefit the nutritional status and the immunological response against all sorts of pathogens, including COVID-19.
8. Conclusion
As can be seen from the diversity of studies, deficiencies of vitamin C, vitamin D, zinc, and selenium can be considered a nutritional risk factor for patients with COVID-19. Immunological dysfunctions observed in the pathogenicity of COVID-19 may be aggravated or attenuated, depending on the nutritional status of the host [167]. Another great challenge is understanding the underlying mechanisms of these micronutrients in the host against outbreaks and the individual ability to overcome infection by SARS-Cov-2. However, there is an extreme need for well-designed RCTs investigating the effectiveness of these micronutrient supplementations in individuals diagnosed with COVID-19. Certainly, this will produce additional scientific knowledge and evidence in order to apply better evidence-based practices in clinical strategies.
Author contribution
LFCP, ANABB and LLL were responsible for conceiving the review. ANABB drew all the figures. All authors participated in analysis and interpretation of data and manuscript writing. All authors approved the final version of the manuscript before submission.
Funding statement
This research was supported by Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES), Brazil, Finance Code 001.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnesp.2021.11.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wu Y.C., Chen C.S., Chan Y.J. The outbreak of COVID-19: an overview. J Chin Med Assoc. 2020 Mar;83(3):217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heymann D.L., Shindo N. WHO scientific and technical advisory group for infectious hazards. COVID-19: what is next for public health? Lancet. 2020 Feb 22;395(10224):542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galmés S., Serra F., Palou A. Current state of evidence: influence of nutritional and nutrigenetic factors on immunity in the COVID-19 pandemic framework. Nutrients. 2020 Sep 8;12(9):2738. doi: 10.3390/nu12092738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler M.J., Barrientos R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav Immun. 2020 Jul;87:53–54. doi: 10.1016/j.bbi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gasmi A., Tippairote T., Mujawdiya P.K., Peana M., Menzel A., Dadar M., et al. Micronutrients as immunomodulatory tools for COVID-19 management. Clin Immunol. 2020 Nov;220:108545. doi: 10.1016/j.clim.2020.108545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G., Zhou C.L., Ba Y.M., Wang Y.M., Song B., Cheng X.B., et al. Nutritional risk and therapy for severe and critical COVID-19 patients: a multicenter retrospective observational study. Clin Nutr. 2021 Apr;40(4):2154–2161. doi: 10.1016/j.clnu.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae M., Kim H. Mini-review on the roles of vitamin C, vitamin D, and selenium in the immune system against COVID-19. Molecules. 2020 Nov 16;25(22):5346. doi: 10.3390/molecules25225346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benskin L.L.A. Basic review of the preliminary evidence that COVID-19 risk and severity is increased in vitamin D deficiency. Public Health Front. 2020 Sep 10;8:513. doi: 10.3389/fpubh.2020.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calder P.C. Nutrition, immunity and COVID-19. BMJ Nutr Prev Health. 2020 May 20;3(1):74–92. doi: 10.1136/bmjnph-2020-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr A.C., Rowe S. The emerging role of vitamin C in the prevention and treatment of COVID-19. Nutrients. 2020 Oct 27;12(11):3286. doi: 10.3390/nu12113286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holford P., Carr A.C., Jovic T.H., Ali S.R., Whitaker I.S., Marik P.E., et al. Vitamin C-an adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients. 2020 Dec 7;12(12):3760. doi: 10.3390/nu12123760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Z., Rose A.H., Hoffmann P.R. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxidants Redox Signal. 2012 Apr 1;16(7):705–743. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., et al. Zinc and respiratory tract infections: perspectives for COVID-19 (Review) Int J Mol Med. 2020 Jul;46(1):17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Saad R., Taylor E.W., Rayman M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020 Oct;37:101715. doi: 10.1016/j.redox.2020.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bramer W.M., Rethlefsen M.L., Kleijnen J., Franco O.H. Optimal database combinations for literature searches in systematic reviews: a prospective exploratory study. Syst Rev. 2017 Dec 6;6(1):245. doi: 10.1186/s13643-017-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lykkesfeldt J., Tveden-Nyborg P. The pharmacokinetics of vitamin C. Nutrients. 2019 Oct 9;11(10):2412. doi: 10.3390/nu11102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lykkesfeldt J., Michels A.J., Frei B. Vitamin C. Adv Nutr. 2014 Jan 1;5(1):16–18. doi: 10.3945/an.113.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017 Nov 3;9(11):1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spoelstra-de Man A.M.E., Elbers P.W.G., Straaten H.M.O. Vitamin C: should we supplement? Curr Opin Crit Care. 2018 Aug;24(4):248–255. doi: 10.1097/MCC.0000000000000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemilä H., Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013 Aug 8;(8):CD005532. doi: 10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- 21.Hemilä H., Chalker E. Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients. 2019 Mar 27;11(4):708. doi: 10.3390/nu11040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemilä H. Vitamin C and infections. Nutrients. 2017 Mar 29;9(4):339. doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myint P.K., Wilson A.M., Clark A.B., Luben R.N., Wareham N.J., Khaw K.T. Plasma vitamin C concentrations and risk of incident respiratory diseases and mortality in the European Prospective Investigation into Cancer-Norfolk population-based cohort study. Eur J Clin Nutr. 2019 Nov;73(11):1492–1500. doi: 10.1038/s41430-019-0393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arvinte C., Singh M., Marik P.E. Serum levels of vitamin C and vitamin D in a cohort of critically ill COVID-19 patients of a north American community hospital intensive care unit in may 2020: a pilot study. Med Drug Discov. 2020 Dec;8:100064. doi: 10.1016/j.medidd.2020.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing Y., Zhao B., Yin L., Guo M., Shi H., Zhu Z., et al. Vitamin C supplementation is necessary for patients with coronavirus disease: an ultra-high-performance liquid chromatography-tandem mass spectrometry finding. J Pharmaceut Biomed Anal. 2021 Mar 20;196:113927. doi: 10.1016/j.jpba.2021.113927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burugu H.R., Kandi V., Kutikuppala L.V.S., Suvvari T.K. Activities of serum ferritin and treatment outcomes among COVID-19 patients treated with vitamin C and dexamethasone: an uncontrolled single-center observational study. Cureus. 2020 Nov 11;12(11) doi: 10.7759/cureus.11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao D., Xu M., Wang G., Lv J., Ma X., Guo Y., et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: a retrospective cohort study. Aging. 2021 Feb 26;13(5):7020–7034. doi: 10.18632/aging.202557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao B., Liu M., Liu P., Peng Y., Huang J., Li M., et al. High dose intravenous vitamin C for preventing the disease aggravation of moderate COVID-19 pneumonia. A retrospective propensity matched before-after study. Front Pharmacol. 2021 Apr 22;12:638556. doi: 10.3389/fphar.2021.638556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia G., Fan D., He Y., Zhu Y., Zheng Q. High-dose intravenous vitamin C attenuates hyperinflammation in severe coronavirus disease 2019. Nutrition. 2021 Jun 26;91–92:111405. doi: 10.1016/j.nut.2021.111405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M., Ching T.H., Hipple C., Lopez R., Sahibzada A., Rahman H. Use of intravenous vitamin C in critically ill patients with COVID-19 infection. J Pharm Pract. 2021 Jun 8 doi: 10.1177/08971900211015052. 8971900211015052. [DOI] [PubMed] [Google Scholar]
- 31.Zhao B., Ling Y., Li J., Peng Y., Huang J., Wang Y., et al. Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: a retrospective case series study. Ann Palliat Med. 2021 Feb;10(2):1599–1609. doi: 10.21037/apm-20-1387. [DOI] [PubMed] [Google Scholar]
- 32.Hiedra R., Lo K.B., Elbashabsheh M., Gul F., Wright R.M., Albano J., et al. The use of IV vitamin C for patients with COVID-19: a case series. Expert Rev Anti Infect Ther. 2020 Dec;18(12):1259–1261. doi: 10.1080/14787210.2020.1794819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan H.M.W., Parikh N., Megala S.M., Predeteanu G.S. Unusual early recovery of a critical COVID-19 patient After administration of intravenous vitamin C. Am J Case Rep. 2020 Jul 25;21 doi: 10.12659/AJCR.925521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J., Rao X., Li Y., Zhu Y., Liu F., Guo G., et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021 Jan 9;11(1):5. doi: 10.1186/s13613-020-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumari P., Dembra S., Dembra P., Bhawna F., Gul A., Ali B., et al. The role of vitamin C as adjuvant therapy in COVID-19. Cureus. 2020 Nov 30;12(11) doi: 10.7759/cureus.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siahkali S.J.M., Zarezade B., Koolaji S., Alinaghi S.S., Zendehdel A., Tabarestani M., et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res. 2021 Feb 11;26(1):20. doi: 10.1186/s40001-021-00490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021 Feb 1;4(2) doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hemilä H., Carr A., Chalker E. Vitamin C may increase the recovery rate of outpatient cases of SARS-CoV-2 infection by 70%: reanalysis of the COVID A to Z randomized clinical trial. Front Immunol. 2021 May 10;12:674681. doi: 10.3389/fimmu.2021.674681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piotrowska A., Wierzbicka J., Żmijewski M.A. Vitamin D in the skin physiology and pathology. Acta Biochim Pol. 2016;63(1):17–29. doi: 10.18388/abp.2015_1104. [DOI] [PubMed] [Google Scholar]
- 40.Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016 Jan;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee C. Controversial effects of vitamin D and related genes on viral infections, pathogenesis, and treatment outcomes. Nutrients. 2020 Mar 30;12(4):962. doi: 10.3390/nu12040962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baeke F., Takiishi T., Korf H., Gysemans C., Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010 Aug;10(4):482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Xiao D., Li X., Su X., Mu D., Qu Y. Could SARS-CoV-2-induced lung injury be attenuated by vitamin D? Int J Infect Dis. 2021 Jan;102:196–202. doi: 10.1016/j.ijid.2020.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwalfenberg G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2011 Jan;55(1):96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- 45.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020 May 1;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clairmont A., Tessman D., Stock A., Nicolai S., Stahl W., Sies H. Induction of gap junctional intercellular communication by vitamin D in human skin fibroblasts is dependent on the nuclear vitamin D receptor. Carcinogenesis. 1996 Jun;17(6):1389–1391. doi: 10.1093/carcin/17.6.1389. [DOI] [PubMed] [Google Scholar]
- 47.Fakhoury H.M.A., Kvietys P.R., AlKattan W., Anouti F.A., Elahi M.A., Karras S.N., et al. Vitamin D and intestinal homeostasis: barrier, microbiota, and immune modulation. J Steroid Biochem Mol Biol. 2020 Jun;200:105663. doi: 10.1016/j.jsbmb.2020.105663. Epub 2020 Mar 16. [DOI] [PubMed] [Google Scholar]
- 48.Cohen M.S., Mesler D.E., Snipes R.G., Gray T.K. 1,25-Dihydroxyvitamin D3 activates secretion of hydrogen peroxide by human monocytes. J Immunol. 1986 Feb 1;136(3):1049–1053. [PubMed] [Google Scholar]
- 49.Bilezikian J.P., Bikle D., Hewison M., Lazaretti-Castro M., Formenti A.M., Gupta A., et al. Mechanisms in endocrinology: vitamin D and COVID-19. Eur J Endocrinol. 2020 Nov;183(5):R133–R147. doi: 10.1530/EJE-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabetta J.R., DePetrillo P., Cipriani R.J., Smardin J., Burns L.A., Landry M.L. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010 Jun 14;5(6) doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Science M., Maguire J.L., Russell M.L., Smieja M., Walter S.D., Loeb M. Low serum 25-hydroxyvitamin D level and risk of upper respiratory tract infection in children and adolescents. Clin Infect Dis. 2013 Aug;57(3):392–397. doi: 10.1093/cid/cit289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ingham T.R., Jones B., Camargo C.A., Kirman J., Dowell A.C., Crane J., et al. Association of vitamin D deficiency with severity of acute respiratory infection: a case-control study in New Zealand children. Eur Respir J. 2014;4:439. [Google Scholar]
- 53.Shi Y.Y., Liu T.J., Fu J.H., Xu W., Wu L.L., Hou A.N., et al. Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol Med Rep. 2016 Feb;13(2):1186–1194. doi: 10.3892/mmr.2015.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jolliffe D.A., Camargo C.A., Jr., Sluyter J.D., Aglipay M., Aloia J.F., Ganmaa D., et al. Vitamin D supplementation to prevent acute respiratory infections: systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endo. 2021 Mar 30 doi: 10.1101/2020.07.14.20152728. [DOI] [PubMed] [Google Scholar]
- 55.Santaolalla A., Beckmann K., Kibaru J., Josephs D., Van Hemelrijck M., Irshad S. Association between vitamin D and novel SARS-CoV-2 respiratory dysfunction - a scoping review of current evidence and its implication for COVID-19 pandemic. Front Physiol. 2020 Nov 26;11:564387. doi: 10.3389/fphys.2020.564387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020 Sep 1;3(9) doi: 10.1001/jamanetworkopen.2020.19722. PMID: 32880651; PMCID: PMC7489852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D'Avolio A., Avataneo V., Manca A., Cusato J., De Nicolò A., Lucchini R., et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020 May 9;12(5):1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raharusun P., Priambada S., Budiarti C., Agung E., Budi C. Patterns of COVID-19 mortality and vitamin D: an Indonesian study. SSRN Electron J. 2020 doi: 10.2139/ssrn.3585561. [DOI] [Google Scholar]
- 59.Kaufman H.W., Niles J.K., Kroll M.H., Bi C., Holick M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020 Sep 17;15(9) doi: 10.1371/journal.pone.0239252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merzon E., Tworowski D., Gorohovski A., Vinker S., Golan Cohen A., Green I., et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020 Sep;287(17):3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raisi-Estabragh Z., McCracken C., Bethell M.S., Cooper J., Cooper C., Caulfield M.J., et al. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: study of 1326 cases from the UK Biobank. J Public Health. 2020 Aug 18;42(3):451–460. doi: 10.1093/pubmed/fdaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hastie C.E., Pell J.P., Sattar N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur J Nutr. 2021 Feb;60(1):545–548. doi: 10.1007/s00394-020-02372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elliott J., Bodinier B., Whitaker M., Delpierre C., Vermeulen R., Tzoulaki I., et al. COVID-19 mortality in the UK Biobank cohort: revisiting and evaluating risk factors. Eur J Epidemiol. 2021 Mar;36(3):299–309. doi: 10.1007/s10654-021-00722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma H., Zhou T., Heianza Y., Qi L. Habitual use of vitamin D supplements and risk of coronavirus disease 2019 (COVID-19) infection: a prospective study in UK Biobank. Am J Clin Nutr. 2021 May 8;113(5):1275–1281. doi: 10.1093/ajcn/nqaa381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demir M., Demir F., Aygun H. Vitamin D deficiency is associated with COVID-19 positivity and severity of the disease. J Med Virol. 2021 May;93(5):2992–2999. doi: 10.1002/jmv.26832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Charoenngam N., Shirvani A., Reddy N., Vodopivec D.M., Apovian C.M., Holick M.F. Association of vitamin D status with hospital morbidity and mortality in adult hospitalized patients with COVID-19. Endocr Pract. 2021 Apr;27(4):271–278. doi: 10.1016/j.eprac.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Angelidi A.M., Belanger M.J., Lorinsky M.K., Karamanis D., Chamorro-Pareja N., Ognibene J., et al. Vitamin D status is associated with in-hospital mortality and mechanical ventilation: a cohort of COVID-19 hospitalized patients. Mayo Clin Proc. 2021 Apr;96(4):875–886. doi: 10.1016/j.mayocp.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Y., Tong C.H., Bare L.A., Devlin J.J. Assessment of the association of vitamin D level with SARS-CoV-2 seropositivity among working-age adults. JAMA Netw Open. 2021 May 3;4(5) doi: 10.1001/jamanetworkopen.2021.11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Daghri N.M., Amer O.E., Alotaibi N.H., Aldisi D.A., Enani M.A., Sheshah E., et al. Vitamin D status of Arab Gulf residents screened for SARS-CoV-2 and its association with COVID-19 infection: a multi-centre case-control study. J Transl Med. 2021 Apr 26;19(1):166. doi: 10.1186/s12967-021-02838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baktash V., Hosack T., Patel N., Shah S., Kandiah P., Van den Abbeele K., et al. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med. 2021 Jul;97(1149):442–447. doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sulli A., Gotelli E., Casabella A., Paolino S., Pizzorni C., Alessandri E., et al. Vitamin D and lung outcomes in elderly COVID-19 patients. Nutrients. 2021 Feb 24;13(3):717. doi: 10.3390/nu13030717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vassiliou A.G., Jahaj E., Pratikaki M., Orfanos S.E., Dimopoulou I., Kotanidou A. Low 25-hydroxyvitamin D levels on admission to the intensive care unit may predispose COVID-19 pneumonia patients to a higher 28-day mortality risk: a pilot study on a Greek ICU cohort. Nutrients. 2020 Dec 9;12(12):3773. doi: 10.3390/nu12123773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Smet D., De Smet K., Herroelen P., Gryspeerdt S., Martens G.A. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am J Clin Pathol. 2021 Feb 11;155(3):381–388. doi: 10.1093/ajcp/aqaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carpagnano G.E., Di Lecce V., Quaranta V.N., Zito A., Buonamico E., Capozza E., et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest. 2021 Apr;44(4):765–771. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karahan S., Katkat F. Impact of serum 25(OH) vitamin D level on mortality in patients with COVID-19 in Turkey. J Nutr Health Aging. 2021;25(2):189–196. doi: 10.1007/s12603-020-1479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Safar H., Grant W.B., Hijazi R., Uddin M., Alkaabi N., Tay G., et al. COVID-19 disease severity and death in relation to vitamin D status among SARS-CoV-2-positive UAE residents. Nutrients. 2021 May 19;13(5):1714. doi: 10.3390/nu13051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tehrani S., Khabiri N., Moradi H., Mosavat M.S., Khabiri S.S. Evaluation of vitamin D levels in COVID-19 patients referred to Labafinejad hospital in Tehran and its relationship with disease severity and mortality. Clin Nutr ESPEN. 2021 Apr;42:313–317. doi: 10.1016/j.clnesp.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Radujkovic A., Hippchen T., Tiwari-Heckler S., Dreher S., Boxberger M., Merle U. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients. 2020 Sep 10;12(9):2757. doi: 10.3390/nu12092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ye K., Tang F., Liao X., Shaw B.A., Deng M., Huang G., et al. Does serum vitamin D level affect COVID-19 infection and its severity?-A case-control study. J Am Coll Nutr. 2020 Oct 13:1–8. doi: 10.1080/07315724.2020.1826005. [DOI] [PubMed] [Google Scholar]
- 80.Luo X., Liao Q., Shen Y., Li H., Cheng L. Vitamin D deficiency is associated with COVID-19 incidence and disease severity in Chinese people [corrected] J Nutr. 2021 Jan 4;151(1):98–103. doi: 10.1093/jn/nxaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abdollahi A., Sarvestani H.K., Rafat Z., Ghaderkhani S., Mahmoudi-Aliabadi M., Jafarzadeh B., et al. The association between the level of serum 25(OH) vitamin D, obesity, and underlying diseases with the risk of developing COVID-19 infection: a case-control study of hospitalized patients in Tehran, Iran. J Med Virol. 2021 Apr;93(4):2359–2364. doi: 10.1002/jmv.26726. [DOI] [PubMed] [Google Scholar]
- 82.Im J.H., Je Y.S., Baek J., Chung M.H., Kwon H.Y., Lee J.S. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020 Nov;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bennouar S., Cherif A.B., Kessira A., Bennouar D.E., Abdi S. Vitamin D deficiency and low serum calcium as predictors of poor prognosis in patients with severe COVID-19. J Am Coll Nutr. 2021 Feb;40(2):104–110. doi: 10.1080/07315724.2020.1856013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc. 2020 Aug 25;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 85.Jain A., Chaurasia R., Sengar N.S., Singh M., Mahor S., Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020 Nov 19;10(1):20191. doi: 10.1038/s41598-020-77093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Campi I., Gennari L., Merlotti D., Mingiano C., Frosali A., Giovanelli L., et al. Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy. BMC Infect Dis. 2021 Jun 14;21(1):566. doi: 10.1186/s12879-021-06281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ricci A., Pagliuca A., D'Ascanio M., Innammorato M., De Vitis C., Mancini R., et al. Circulating Vitamin D levels status and clinical prognostic indices in COVID-19 patients. Respir Res. 2021 Mar 3;22(1):76. doi: 10.1186/s12931-021-01666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cereda E., Bogliolo L., Klersy C., Lobascio F., Masi S., Crotti S., et al. Vitamin D 25OH deficiency in COVID-19 patients admitted to a tertiary referral hospital. Clin Nutr. 2021 Apr;40(4):2469–2472. doi: 10.1016/j.clnu.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alguwaihes A.M., Sabico S., Hasanato R., Al-Sofiani M.E., Megdad M., Albader S.S., et al. Severe vitamin D deficiency is not related to SARS-CoV-2 infection but may increase mortality risk in hospitalized adults: a retrospective case-control study in an Arab Gulf country. Aging Clin Exp Res. 2021 May;33(5):1415–1422. doi: 10.1007/s40520-021-01831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davoudi A., Najafi N., Aarabi M., Tayebi A., Nikaeen R., Izadyar H., et al. Lack of association between vitamin D insufficiency and clinical outcomes of patients with COVID-19 infection. BMC Infect Dis. 2021 May 18;21(1):450. doi: 10.1186/s12879-021-06168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gaudio A., Murabito A.R., Agodi A., Montineri A., Castellino P., D O CoV Research Vitamin D levels are reduced at the time of hospital admission in Sicilian SARS-CoV-2-positive patients. Int J Environ Res Publ Health. 2021 Mar 27;18(7):3491. doi: 10.3390/ijerph18073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szeto B., Zucker J.E., LaSota E.D., Rubin M.R., Walker M.D., Yin M.T., et al. Endocr Res. 2021 Feb-May;46(2):66–73. doi: 10.1080/07435800.2020.1867162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pizzini A., Aichner M., Sahanic S., Böhm A., Egger A., Hoermann G., et al. Impact of vitamin D deficiency on COVID-19-A prospective analysis from the CovILD registry. Nutrients. 2020 Sep 11;12(9):2775. doi: 10.3390/nu12092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orchard L., Baldry M., Nasim-Mohi M., Monck C., Saeed K., Grocott M.P.W., et al. Vitamin-D levels and intensive care unit outcomes of a cohort of critically ill COVID-19 patients. Clin Chem Lab Med. 2021 Jan 19;59(6):1155–1163. doi: 10.1515/cclm-2020-1567. [DOI] [PubMed] [Google Scholar]
- 95.Lohia P., Nguyen P., Patel N., Kapur S. Exploring the link between vitamin D and clinical outcomes in COVID-19. Am J Physiol Endocrinol Metab. 2021 Mar 1;320(3):E520–E526. doi: 10.1152/ajpendo.00517.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hernández J.L., Nan D., Fernandez-Ayala M., García-Unzueta M., Hernández-Hernández M.A., López-Hoyos M., et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab. 2021 Mar 8;106(3):e1343–e1353. doi: 10.1210/clinem/dgaa733. [DOI] [PubMed] [Google Scholar]
- 97.Ling S.F., Broad E., Murphy R., Pappachan J.M., Pardesi-Newton S., Kong M.F., et al. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-centre observational study. Nutrients. 2020 Dec 11;12(12):3799. doi: 10.3390/nu12123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ohaegbulam K.C., Swalih M., Patel P., Smith M.A., Perrin R. Vitamin D supplementation in COVID-19 patients: a clinical case series. Am J Therapeut. 2020 Sep/Oct;27(5):e485–e490. doi: 10.1097/MJT.0000000000001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lakkireddy M., Gadiga S.G., Malathi R.D., Karra M.L., Raju I.S.S.V.P.M., Ragini, et al. Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease. Sci Rep. 2021 May 20;11(1):10641. doi: 10.1038/s41598-021-90189-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Giannini S., Passeri G., Tripepi G., Sella S., Fusaro M., Arcidiacono G., et al. Effectiveness of in-hospital cholecalciferol use on clinical outcomes in comorbid COVID-19 patients: a hypothesis-generating study. Nutrients. 2021 Jan 14;13(1):219. doi: 10.3390/nu13010219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Annweiler G., Corvaisier M., Gautier J., Dubée V., Legrand E., Sacco G., et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020 Nov 2;12(11):3377. doi: 10.3390/nu12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alcala-Diaz J.F., Limia-Perez L., Gomez-Huelgas R., Martin-Escalante M.D., Cortes-Rodriguez B., Zambrana-Garcia J.L., et al. Calcifediol treatment and hospital mortality due to COVID-19: a cohort study. Nutrients. 2021 May 21;13(6):1760. doi: 10.3390/nu13061760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jevalikar G., Mithal A., Singh A., Sharma R., Farooqui K.J., Mahendru S., et al. Lack of association of baseline 25-hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID-19. Sci Rep. 2021 Mar 18;11(1):6258. doi: 10.1038/s41598-021-85809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cereda E., Bogliolo L., Lobascio F., Barichella M., Zecchinelli A.L., Pezzoli G., et al. Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy. Nutrition. 2021 Feb;82:111055. doi: 10.1016/j.nut.2020.111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Castillo M.E., Costa L.M.E., Barrios J.M.V., Díaz J.F.A., Miranda J.L., Bouillon R., et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020 Oct;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rastogi A., Bhansali A., Khare N., Suri V., Yaddanapudi N., Sachdeva N., et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Postgrad Med. 2020 doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 107.Sabico S., Enani M.A., Sheshah E., Aljohani N.J., Aldisi D.A., Alotaibi N.H., et al. Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate covid-19: a randomized clinical trial. Nutrients. 2021 Jun 24;13(7):2170. doi: 10.3390/nu13072170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S.C., et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. J Am Med Assoc. 2021 Mar 16;325(11):1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gupta S., Brazier A.K.M., Lowe N.M. Zinc deficiency in low- and middle-income countries: prevalence and approaches for mitigation. J Hum Nutr Diet. 2020;33(5):624–643. doi: 10.1111/jhn.12791. [DOI] [PubMed] [Google Scholar]
- 110.Prasad A.S. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013 Mar 1;4(2):176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv Nutr. 2019 Jul 1;10(4):696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stefanidou M., Maravelias C., Dona A., Spiliopoulou C. Zinc: a multipurpose trace element. Arch Toxicol. 2006 Jan;80(1):1–9. doi: 10.1007/s00204-005-0009-5. [DOI] [PubMed] [Google Scholar]
- 113.Prasad A.S. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008 May-Jun;14(5–6):353–357. doi: 10.2119/2008-00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wessels I., Maywald M., Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017 Nov 25;9(12):1286. doi: 10.3390/nu9121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maares M., Haase H. Zinc and immunity: an essential interrelation. Arch Biochem Biophys. 2016 Dec 1;611:58–65. doi: 10.1016/j.abb.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 116.Gao H., Dai W., Zhao L., Min J., Wang F. The role of zinc and zinc homeostasis in macrophage function. J Immunol Res. 2018 Dec 6;2018:6872621. doi: 10.1155/2018/6872621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Haase H., Rink L. Multiple impacts of zinc on immune function. Metall. 2014 Jul;6(7):1175–1180. doi: 10.1039/c3mt00353a. [DOI] [PubMed] [Google Scholar]
- 118.Elham A.S., Azam K., Azam J., Mostafa L., Nasrin B., Marzieh N. Serum vitamin D, calcium, and zinc levels in patients with COVID-19. Clin Nutr ESPEN. 2021 Jun;43:276–282. doi: 10.1016/j.clnesp.2021.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fromonot J., Gette M., Ben Lassoued A., Guéant J.L., Guéant-Rodriguez R.M., Guieu R. Hypozincemia in the early stage of COVID-19 is associated with an increased risk of severe COVID-19. Clin Nutr. 2021 May 4 doi: 10.1016/j.clnu.2021.04.042. S0261-S5614(21)00234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heller R.A., Sun Q., Hackler J., Seelig J., Seibert L., Cherkezov A., et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021 Jan;38:101764. doi: 10.1016/j.redox.2020.101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yasui Y., Yasui H., Suzuki K., Saitou T., Yamamoto Y., Ishizaka T., et al. Analysis of the predictive factors for a critical illness of COVID-19 during treatment - relationship between serum zinc level and critical illness of COVID-19. Int J Infect Dis. 2020 Nov;100:230–236. doi: 10.1016/j.ijid.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jothimani D., Kailasam E., Danielraj S., Nallathambi B., Ramachandran H., Sekar P., et al. COVID-19: poor outcomes in patients with zinc deficiency. Int J Infect Dis. 2020 Nov;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anuk A.T., Polat N., Akdas S., Erol S.A., Tanacan A., Biriken D., et al. The relation between trace element status (zinc, copper, magnesium) and clinical outcomes in COVID-19 infection during pregnancy. Biol Trace Elem Res. 2020 Nov 24:1–10. doi: 10.1007/s12011-020-02496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vogel-González M., Talló-Parra M., Herrera-Fernández V., Pérez-Vilaró G., Chillón M., Nogués X., et al. Low zinc levels at admission associates with poor clinical outcomes in SARS-CoV-2 infection. Nutrients. 2021 Feb 9;13(2):562. doi: 10.3390/nu13020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gonçalves T.J.M., Gonçalves S.E.A.B., Guarnieri A., Risegato R.C., Guimarães M.P., Freitas D.C., et al. Association between low zinc levels and severity of acute respiratory distress syndrome by new coronavirus SARS-CoV-2. Nutr Clin Pract. 2021 Feb;36(1):186–191. doi: 10.1002/ncp.10612. [DOI] [PubMed] [Google Scholar]
- 126.Skalny A.V., Timashev P.S., Aschner M., Aaseth J., Chernova L.N., Belyaev V.E., et al. Serum zinc, copper, and other biometals are associated with COVID-19 severity markers. Metabolites. 2021 Apr 15;11(4):244. doi: 10.3390/metabo11040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Allard L., Ouedraogo E., Molleville J., Bihan H., Giroux-Leprieur B., Sutton A., et al. Malnutrition: percentage and association with prognosis in patients hospitalized for coronavirus disease 2019. Nutrients. 2020 Nov 28;12(12):3679. doi: 10.3390/nu12123679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carlucci P.M., Ahuja T., Petrilli C., Rajagopalan H., Jones S., Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J Med Microbiol. 2020 Oct;69(10):1228–1234. doi: 10.1099/jmm.0.001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yao J.S., Paguio J.A., Dee E.C., Tan H.C., Moulick A., Milazzo C., et al. The minimal effect of zinc on the survival of hospitalized patients with COVID-19: an observational study. Chest. 2021 Jan;159(1):108–111. doi: 10.1016/j.chest.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Finzi E., Harrington A. Zinc treatment of outpatient COVID-19: a retrospective review of 28 consecutive patients. J Med Virol. 2021 May;93(5):2588–2590. doi: 10.1002/jmv.26812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abd-Elsalam S., Soliman S., Esmail E.S., Khalaf M., Mostafa E.F., Medhat M.A., et al. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine?: a randomized, multicenter trial. Biol Trace Elem Res. 2020 Nov;27:1–5. doi: 10.1007/s12011-020-02512-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 132.Patel O., Chinni V., El-Khoury J., Perera M., Neto A.S., McDonald C., et al. A pilot double-blind safety and feasibility randomized controlled trial of high-dose intravenous zinc in hospitalized COVID-19 patients. J Med Virol. 2021 May;93(5):3261–3267. doi: 10.1002/jmv.26895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rayman M.P. Selenium and human health. Lancet. 2012 Mar 31;379(9822):1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 134.Kobayashi R., Hasegawa M., Kawaguchi C., Ishikawa N., Tomiwa K., Shima M., et al. Thyroid function in patients with selenium deficiency exhibits high free T4 to T3 ratio. Clin Pediatr Endocrinol. 2021;30(1):19–26. doi: 10.1297/cpe.30.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carlson B.A., Yoo M.H., Shrimali R.K., Irons R., Gladyshev V.N., Hatfield D.L., et al. Role of selenium-containing proteins in T-cell and macrophage function. Proc Nutr Soc. 2010 Aug;69(3):300–310. doi: 10.1017/S002966511000176X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gill H., Walker G. Selenium, immune function, and resistance to viral infections. Nutr Diet. 2008;6:S41–S47. doi: 10.1111/j.1747-0080.2008.00260.x. [DOI] [Google Scholar]
- 137.Hoffmann P.R., Berry M.J. The influence of selenium on immune responses. Mol Nutr Food Res. 2008 Nov;52(11):1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoffmann F.W., Hashimoto A.C., Shafer L.A., Dow S., Berry M.J., Hoffmann P.R. Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. J Nutr. 2010 Jun;140(6):1155–1161. doi: 10.3945/jn.109.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qian F., Misra S., Prabhu K.S. Selenium and selenoproteins in prostanoid metabolism and immunity. Crit Rev Biochem Mol Biol. 2019 Dec;54(6):484–516. doi: 10.1080/10409238.2020.1717430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lei C., Niu X., Wei J., Zhu J., Zhu Y. Interaction of glutathione peroxidase-1 and selenium in endemic dilated cardiomyopathy. Clin Chim Acta. 2009 Jan;399(1–2):102–108. doi: 10.1016/j.cca.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 141.Yu L., Sun L., Nan Y., Zhu L.Y. Protection from H1N1 influenza virus infections in mice by supplementation with selenium: a comparison with selenium-deficient mice. Biol Trace Elem Res. 2011 Jun;141(1–3):254–261. doi: 10.1007/s12011-010-8726-x. [DOI] [PubMed] [Google Scholar]
- 142.Beck M.A., Nelson H.K., Shi Q., Van Dael P., Schiffrin E.J., Blum S., et al. Selenium deficiency increases the pathology of an influenza virus infection. Faseb J. 2001 Jun;15(8):1481–1483. [PubMed] [Google Scholar]
- 143.Mantero-Atienza E., Beach R.S., Gavancho M.C., Morgan R., Shor-Posner G., Fordyce-Baum M.K. Selenium status of HIV-1 infected individuals. JPEN - J Parenter Enter Nutr. 1991 Nov-Dec;15(6):693–694. doi: 10.1177/0148607191015006693. [DOI] [PubMed] [Google Scholar]
- 144.Angstwurm M.W., Engelmann L., Zimmermann T., Lehmann C., Spes C.H., Abel P., et al. Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med. 2007 Jan;35(1):118–126. doi: 10.1097/01.CCM.0000251124.83436.0E. [DOI] [PubMed] [Google Scholar]
- 145.Curran J.E., Jowett J.B., Elliott K.S., Gao Y., Gluschenko K., Wang J., et al. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet. 2005 Nov;37(11):1234–1241. doi: 10.1038/ng1655. [DOI] [PubMed] [Google Scholar]
- 146.Guillin O.M., Vindry C., Ohlmann T., Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019 Sep 4;11(9):2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Muri J., Heer S., Matsushita M., Pohlmeier L., Tortola L., Fuhrer T., et al. The thioredoxin-1 system is essential for fueling DNA synthesis during T-cell metabolic reprogramming and proliferation. Nat Commun. 2018 May 10;9(1):1851. doi: 10.1038/s41467-018-04274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pitts M.W., Hoffmann P.R. Endoplasmic reticulum-resident selenoproteins as regulators of calcium signaling and homeostasis. Cell Calcium. 2018 Mar;70:76–86. doi: 10.1016/j.ceca.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020 Jun;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vavougios G.D., Ntoskas K.T., Doskas T.K. Impairment in selenocysteine synthesis as a candidate mechanism of inducible coagulopathy in COVID-19 patients. Med Hypotheses. 2021 Feb;147:110475. doi: 10.1016/j.mehy.2020.110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang J., Taylor E.W., Bennett K., Saad R., Rayman M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020 Jun 1;111(6):1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Majeed M., Nagabhushanam K., Gowda S., Mundkur L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: the case for adequate selenium status. Nutrition. 2021 Feb;82:111053. doi: 10.1016/j.nut.2020.111053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moghaddam A., Heller R.A., Sun Q., Seelig J., Cherkezov A., Seibert L., et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020 Jul 16;12(7):2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zeng H.L., Zhang B., Wang X., Yang Q., Cheng L. Urinary trace elements in association with disease severity and outcome in patients with COVID-19. Environ Res. 2021 Mar;194:110670. doi: 10.1016/j.envres.2020.110670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Silva M.C., Furlanetto T.W. Does serum 25-hydroxyvitamin D decrease during acute-phase response? A systematic review. Nutr Res. 2015 Feb;35(2):91–96. doi: 10.1016/j.nutres.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 156.Asl S.H., Nikfarjam S., Majidi Zolbanin N., Nassiri R., Jafari R. Immunopharmacological perspective on zinc in SARS-CoV-2 infection. Int Immunopharm. 2021;96:107630. doi: 10.1016/j.intimp.2021.107630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chiscano-Camón L., Ruiz-Rodriguez J.C., Ruiz-Sanmartin A., Roca O., Ferrer R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit Care. 2020 Aug 26;24(1):522. doi: 10.1186/s13054-020-03249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stroehlein J.K., Wallqvist J., Iannizzi C., Mikolajewska A., Metzendorf M.I., Benstoem C., et al. Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021 May 24;5:CD015043. doi: 10.1002/14651858.CD015043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Holick M.F. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016 Mar;36(3):1345–1356. [PubMed] [Google Scholar]
- 160.Polis M.A. Reassessing the association of vitamin D level with SARS-CoV-2 seropositivity. JAMA Netw Open. 2021 May 3;4(5) doi: 10.1001/jamanetworkopen.2021.11750. [DOI] [PubMed] [Google Scholar]
- 161.Pereira M., Damascena A.D., Azevedo L.M.G., Oliveira T.A., Santana J.M. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020 Nov;4:1–9. doi: 10.1080/10408398.2020.1841090. [DOI] [PubMed] [Google Scholar]
- 162.Bassatne A., Basbous M., Chakhtoura M., El Zein O., Rahme M., El-Hajj Fuleihan G. The link between COVID-19 and VItamin D (VIVID): a systematic review and meta-analysis. Metabolism. 2021 Jun;119:154753. doi: 10.1016/j.metabol.2021.154753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lewis K., Chaudhuri D., Alshamsi F., Carayannopoulos L., Dearness K., Chagla Z., et al. GUIDE Group The efficacy and safety of hydroxychloroquine for COVID-19 prophylaxis: a systematic review and meta-analysis of randomized trials. PLoS One. 2021;16(1) doi: 10.1371/journal.pone.0244778. eCollection 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pal A., Squitti R., Picozza M., Pawar A., Rongioletti M., Dutta A.K., et al. Zinc and COVID-19: basis of current clinical trials. Biol Trace Elem Res. 2021 Aug;199(8):2882–2892. doi: 10.1007/s12011-020-02437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.James P.T., Ali Z., Armitage A.E., Bonell A., Cerami C., Drakesmith H., et al. The role of nutrition in COVID-19 susceptibility and severity of disease: a systematic review. J Nutr. 2021 Jul 1;151(7):1854–1878. doi: 10.1093/jn/nxab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Abulmeaty M.M.A., Aljuraiban G.S., Shaikh S.M., ALEid N.E., Mazrou L.R.A., Turjoman A.A., et al. The efficacy of antioxidant oral supplements on the progression of COVID-19 in non-critically ill patients: a randomized controlled trial. Antioxidants. 2021;10:804. doi: 10.3390/antiox10050804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Beck M.A., Handy J., Levander O.A. Host nutritional status: the neglected virulence factor. Trends Microbiol. 2004 Sep;12(9):417–423. doi: 10.1016/j.tim.2004.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.