Abstract
The concept of reverse axillary mapping originated with the main purpose of reducing lymphedema. In this study, we test the advantage of reverse axillary mapping to delineate the arm-draining lymph nodes and their involvement in various stages of breast carcinoma. In this study, we also attempt to redefine the template for axillary dissection in breast cancer.
During the period of September 30, 2020, to August 30, 2021, 46 patients were recruited to undergo a procedure in which isosulfan blue dye was injected into the upper arm and the axilla was explored to isolate the lymph nodes. The lymph nodes were submitted for examination histopathologically.
The results conclusively showed that axillary lymph node metastasis was only influenced by the advanced stage of the disease (p=0.014) and the visualization of the lymphatics was independent of the stage, type of surgery, decubitus, or age.
The study conclusively shows that attempts to preserve the upper limb-draining nodes in advanced stages would be futile and the preservation of such lymph nodes should be limited to the early stages of breast cancer.
Keywords: reverse axillary mapping, india, blue dye, general surgery and breast cancer, breast cancer research
Introduction
In the management of operable breast cancer, the axillary lymph node status is the most important prognostic factor. The standard approach for axillary lymph node staging apart from imaging is surgical by either sentinel lymph node biopsy (SLNB) or axillary lymph node dissection (ALND). Among the complications associated with SLNB and ALND, upper limb lymphedema is the most distressing, occurring in 7% and 30% [1-2]. In reverse axillary mapping, the hypothesis is that the upper limb lymphatics are delineated by blue dye injected into the medial arm during SLNB or ALND, which can be preserved, thereby preventing upper limb lymphedema.
The lymph node draining the upper limb appears as a blue node called the axillary reverse mapping (ARM) node and has been reported in the early studies as not harboring metastatic disease [3-5]. Some studies like Ponzone et al. reported that approximately 10% of patients with extensive nodal diseases had ARM node involvement [6].
Hence, this preliminary analysis of ARM using blue dye is done to study the visualization, define the anatomy of upper limb lymphatics in the axilla, and identify and define the anatomical location of the ARM node in the axilla and its involvement by metastasis in breast cancer.
Materials and methods
The study included women diagnosed with breast cancer from September 30, 2020, to August 30, 2021.
Inclusion criteria were as follows: patients planned for breast conservation surgery (BCS) or modified radical mastectomy (MRM); patients aged over 18 years; patients willing to participate in the study; patients with operable breast cancer (T1-4b, N0-1, M0) upfront or post-neoadjuvant (chemo or hormone) therapy; and patients undergoing ALND post lumpectomy.
Exclusion criteria were as follows: patients aged less than 18 years; patients not willing to participate; patients who were allergic to the dye; patients with T4c, T4d, N2-3, and M1 disease undergoing palliative mastectomy.
A total of 46 patients were included in the study; their data were collected and preliminarily analyzed.
Procedure
With prior informed consent, 3-5 ml isosulfan blue dye was injected intradermally at the sulcus between the triceps and biceps of the arm before induction of anesthesia after the test dose. A gentle massage was done at the injection site and arm exercises were done for a few minutes. On entering the axillary opening, clavipectoral fascia, careful dissection was done at the superior lateral aspect 2-3 cm below the axillary vein to identify the blue lymphatics and node. The anatomical location of blue lymphatics and node is documented and the lymph nodes were sent for histological examination separately, after completion of ALND.
Pathological examination
All of the nodes harvested in ALND were sampled after being cut into 2 mm slices in the longitudinal axis and submitted to microscopic examination with hematoxylin and eosin. The ARM nodes (number and metastases) were studied and reported separately.
Statistical analysis
Data were categorized into various variables like age, TNM (Tumor Node Metastasis) stage, type of surgery performed, the location of ARM nodes, and the histological nature of ARM nodes. The correlation analysis was done using the chi-square test between the variables of age, TNM stage, histology of node, and breast surgery. Clearance was taken from the medical ethics committee (Institutional Review Board) of the institution (Kidwai Memorial Institute of Oncology) with reference identification KMIO/MEC/017/31.August.2020.
Results
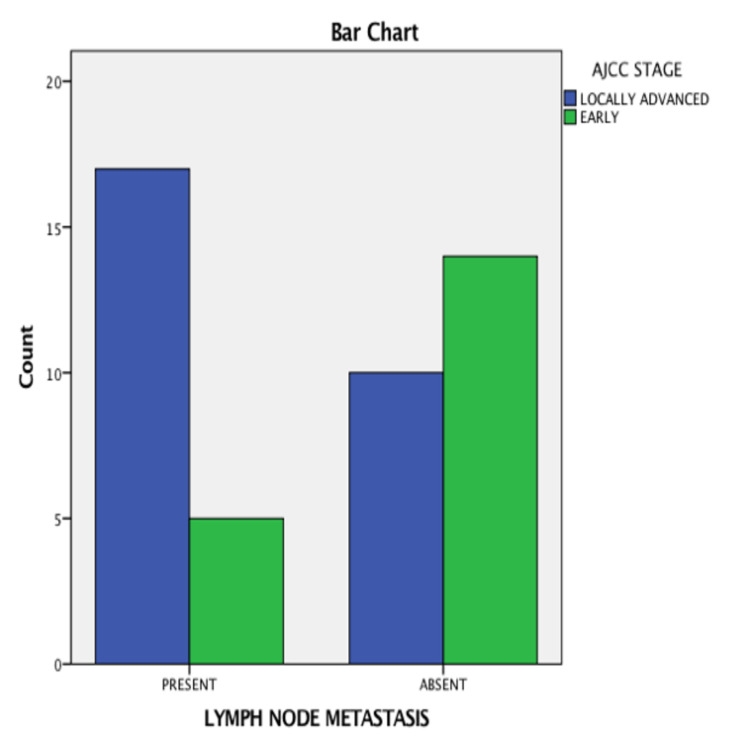
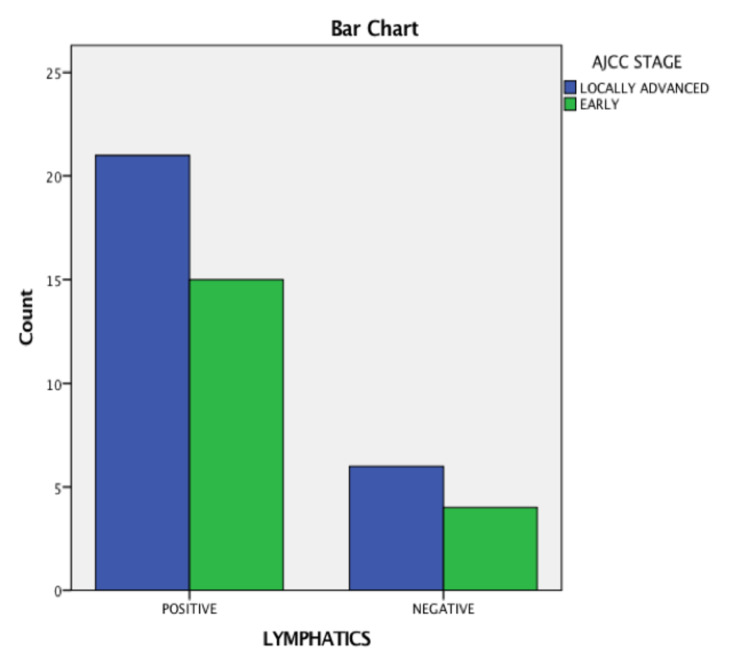
There were 46 cases selected for analysis between the period from September 2020 to August 2021. They were grouped based on tumor stage, age, visualization of lymph node, ARM node, identification, and pathological involvement. The total number of patients who underwent the study was 46 with a median age of 45.2 years. Their baseline characteristics are described in Tables 1-3. The baseline characteristics of patients with respect to the involvement of the ARM node are shown in Table 4 and with respect to the American Joint Committee on Cancer (AJCC) stage are shown in Figure 1. The baseline characteristics of patients with respect to the visualization of lymphatics are shown in Table 5 and with respect to the AJCC stage are shown in Figure 2.
Table 1. Tumor stage (T) according to the American Joint Committee on Cancer (AJCC).
T1- tumor size ≤20 mm; T2- tumor size 20 mm to ≤50 mm; T3- tumor size >50 mm; T4- tumor with infiltration into the chest wall, skin, ulceration or both, inflammatory carcinoma
| Tumor stage | Number (percentage of total) |
| T1 | 3(6.5%) |
| T2 | 22(47.8%) |
| T3 | 17(36.9%) |
| T4 | 4(8.6%) |
| Total | 46 |
Table 3. American Joint Committee on Cancer (AJCC) stage of the various patients involved.
| AJCC stage | Number(percentage of total) |
| 1 | 1(2.1%) |
| 2A | 6(13.04%) |
| 2B | 12(26.08%) |
| 3A | 22(47.8%) |
| 3B | 4(8.6%) |
| 3C | 1(2.1%) |
| Total | 46 |
Table 4. Characteristics of the patient as compared to the involvement of the axillary reverse mapping (ARM) nodes.
AJCC: American Joint Committee on Cancer; BCS: breast conservation surgery; MRM: modified radical mastectomy
| Number of patients | ARM node-positive | ARM node-negative | Chi-square | P-value | Odds ratio (95%CI) | |
| AJCC stage | 6.002 | 0.014 | 4.76(17.21-1.31) | |||
| Early | 19 | 14 | 5 | |||
| Locally advanced | 27 | 10 | 17 | |||
| Surgery | 0.987 | 0.331 | 1.813(6.04-0.54) | |||
| BCS | 18 | 7 | 11 | |||
| MRM | 28 | 15 | 13 | |||
| Decubitus | 2.14 | 0.14 | 0.41(1.35-0.12) | |||
| Right | 22 | 13 | 9 | |||
| Left | 24 | 9 | 15 | |||
| Age | 0.19 | 0.65 | 0.75(2.65-0.21) | |||
| ≥50 | 14 | 6 | 8 | |||
| <50 | 32 | 16 | 16 |
Table 5. Visualization of the lymphatics as compared to the patient characteristics.
AJCC: American Joint Committee on Cancer; BCS: breast conservation surgery; MRM: modified radical mastectomy
| Number of patients | Lymphatics seen | Lymphatics not seen | Chi-square | P-value | Odds ratio (95%CI) | |
| AJCC stage | 6.002 | 0.014 | 4.76(17.21-1.31) | |||
| Early | 19 | 15 | 4 | |||
| Locally advanced | 27 | 10 | 17 | |||
| Surgery | 0.447 | 0.50 | 0.60(2.70-0.13) | |||
| BCS | 18 | 15 | 3 | |||
| MRM | 28 | 21 | 7 | |||
| Decubitus | 0.75 | 0.38 | 1.87(7.80-0.45) | |||
| Right | 22 | 16 | 6 | |||
| Left | 24 | 20 | 4 | |||
| Age | 0.552 | 0.45 | 0.57(2.48-0.13) | |||
| ≥50 | 14 | 10 | 4 | |||
| <50 | 32 | 26 | 6 |
Table 2. Nodal stage (N) according to the American Joint Committee on Cancer (AJCC).
N1 - metastasis to ipsilateral level 1, 2 axillary node; N2 - metastasis to matted ipsilateral level 1, 2 axillary node or metastasis to ipsilateral internal mammary nodes; N3 - metastasis to ipsilateral level 3 axillary node or metastasis to ipsilateral supraclavicular node or metastasis to both ipsilateral level 1, 2 axillary node and ipsilateral internal mammary nodes
| Nodal stage | Number (percentage of total) |
| N0 | 7(15.2%) |
| N1 | 23(50%) |
| N2 | 15(32.6%) |
| N3 | 1(2.1%) |
| Total | 46 |
Figure 1. Lymph node metastasis seen in the axillary reverse mapping (ARM) node as compared to the American Joint Committee on Cancer (AJCC) stage.
Figure 2. Visualization of lymphatics in relation to the American Joint Committee on Cancer (AJCC) stage.
Discussion
In the management of breast carcinoma, the axillary nodal status has been shown to have a prognostic effect in both the early and locally advanced status of the disease. Although the management of axillary nodes has drastically changed over time, including progression to a more conservative approach - from complete axillary nodal removal to the preservation of uninvolved nodes.
Axillary reverse mapping was propounded in lieu of such an approach, which includes the identification and preservation of limb-draining lymphatics with the help of a dye. Hence, in this study, we attempt to identify limb-draining lymphatics using isosulfan dye and ascertaining their involvement with the disease. Table 6 demonstrates the salient features of various studies that attempted reverse axillary mapping.
Table 6. Comparison of the ARM procedure between various studies.
ARM: axillary reverse mapping
| Study | Number of ARM procedures | Rate of identification | Ratio of metastatic involvement | Technique |
| Thompson et al. [7] | 18 | 61% | 0/7 | Blue dye |
| Casabona et al. [3] | 9 | 88.9% | 0/3 | Blue dye |
| Nos et al. [4] | 21 | 71% | 0/10 | Blue dye |
| Boneti et al. [5] | 47 | 40.6% | 0/15 | Blue dye |
| Ponzone et al. [6] | 49 | 73.5% | 3/27 | Blue dye |
| Bedrosian et al. [8] | 30 | 70% | 2/ | Blue dye |
| Deng et al. [9] | 69 | NR | 6/69 | Blue dye |
| Han et al. [10] | 97 | Nr | 2.17 | Blue dye |
| Kang et al. [11] | 129 | 78.3% | 9/101 | Blue dye |
| Rubio et al. [12] | 36 | 83.3 % | 4/30 | Blue dye |
| Gobardhan et al. [13] | 93 | 90.3% | 11/93 | Blue dye |
| Connor et al. [14] | 60 | 75% | 3/19 | Blue dye |
| Schunemann et al. [15] | 45 | 88.9% | 10/45 | Blue dye |
| Ochoa et al. [16] | 127 | 75% | 5/27 | Blue dye |
| Our study | 46 | 73.2% | 24/46 | Blue dye |
As seen in this study, the rate of identification using blue dye was 73.2% %; up 40% to 90% when compared to other studies. In the case series of Gobardhan et al. [13] and Schunemann et al. [15], a high detection rate was encountered of up to 90% where a similar technique was used. Further comparison is summarized in Table 6.
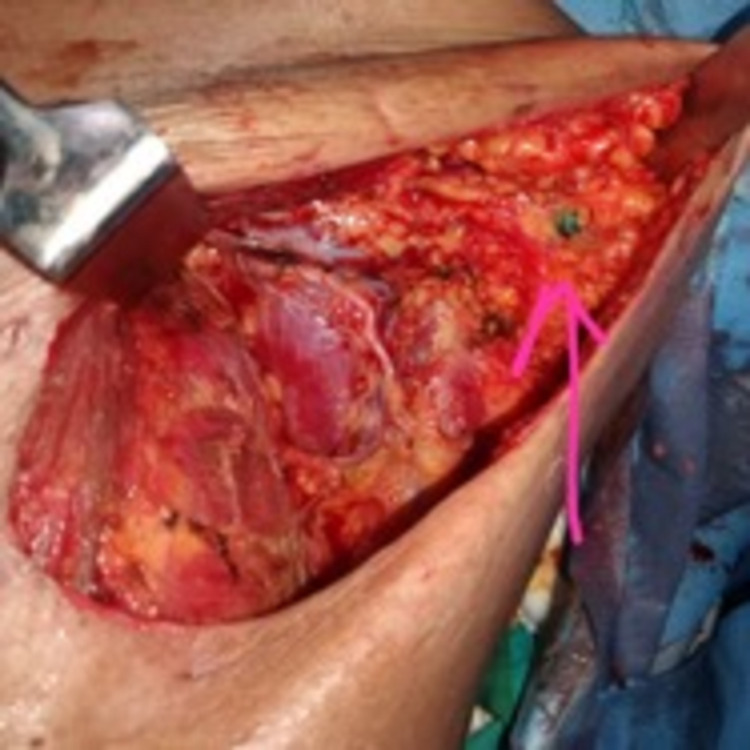
The visualization of the lymphatics remained independent of the status of the disease, showing no significant association between them, ascertaining that the visualization of the lymphatics was unimpeded in all cases, with a high success rate. The location of the lymph nodes draining the upper limb was generally found predominantly inferior to the axillary vein situated laterally to the thoracodorsal pedicle, which was similarly seen in Kumar et al. [17]; however, in a minority of cases, the draining lymph nodes were also found superior to the axillary vein and medial to the thoracodorsal pedicle. Figure 3 shows the visualized ARM node.
Figure 3. Visualized ARM node stained with blue dye (pink arrow).
ARM: axillary reverse mapping
There was no correlation found between the decubitus of the diseases or type of surgery used, breast conservation, or radical to the positivity of the limb draining lymph nodes. There was also no significant relation with the visualization of the lymphatics.
In a separate study [15], a correlation was found between the positivity rate of metastasis found in upper limb draining nodes and age; however, no such relation was found in this study, indicating that age had no effect on the positivity of the node
There was a significant association between a high tumor burden and ARM node involvement, which includes high tumor status, high nodal status, and advanced stage of the disease as similarly seen in Schnuemann et al. [15]. In other studies, the participants had a mild burden of disease, showing only minimal nodal involvement [2,4], hence the limb draining lymph nodes had minimal involvement.
In studies that included patients with more varied data [6,12,14,16], a similar association of limb draining nodes harboring the disease was seen.
The visualization of the lymphatics remained independent of the burden of the disease, as no significant association was found between them. Hence, it can be postulated that the lymphatics could be traced using the given dye even in the varied nature of cases.
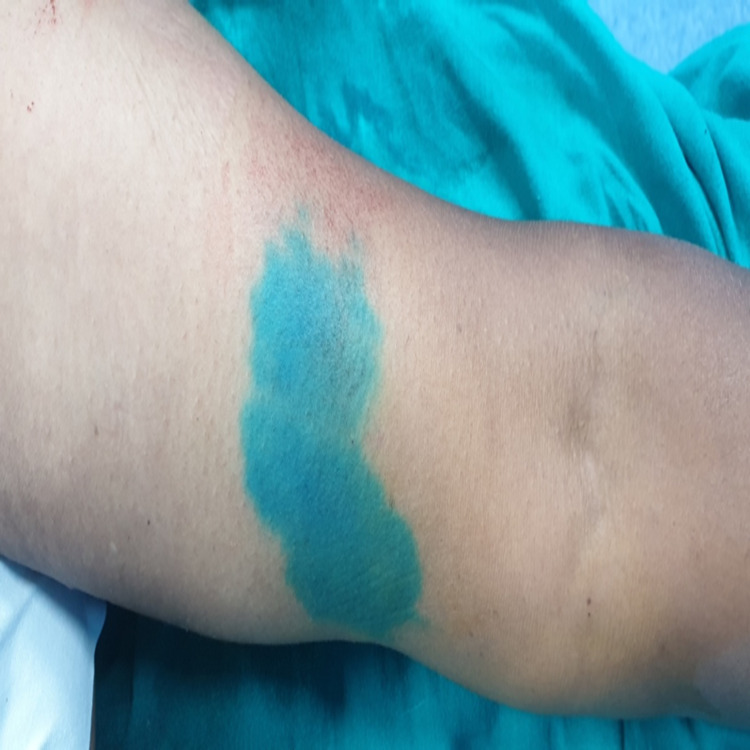
The complication present was discoloration in about 53% of the patients; there was temporary tattooing of the injection site in a minority of the cases as shown in Figure 4, which resolved itself without any intervention, and there was no permanent tattooing in any cases.
Figure 4. Discoloration leading to temporary tattooing.
Conclusions
As determined by this study, the procedure of axillary reverse mapping using isosulfan blue dye is a feasible and reliable adjunct to ALND, which can lead to the preservation of upper limb lymphatics. The study also shows that the template to be considered for axillary dissection in low-volume breast cancer disease can be limited, whereas when compared to high-volume disease, the dissection should be extended to achieve regional control of the pathology, as the axillary lymph node draining the upper limb has a significantly high rate of positivity in cases of high nodal status and locally advanced primary tumor. The procedure also presents as a cheap and reliable way to explore the lymphatic anatomy of the axilla, especially in regard to limb drainage, which can be used in a limited resource setting with minimal complications. Further studies are needed to decide on preserving the ARM node during ALND if noted within the dissection field.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. Kidwai Memorial Institute of Oncology Medical Ethics Committee issued approval KMIO/MEC/017/31.August.2020
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Wilke LG, McCall LM, Posther KE, et al. Ann Surg Oncol. 2006;13:491–500. doi: 10.1245/ASO.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Axillary reverse mapping for preventing lymphedema in axillary lymph node dissection and/or sentinel lymph node biopsy. Noguchi M. Breast Cancer. 2010;17:155–157. doi: 10.1007/s12282-009-0173-1. [DOI] [PubMed] [Google Scholar]
- 3.Feasibility of axillary reverse mapping during sentinel lymph node biopsy in breast cancer patients. Casabona F, Bogliolo S, Valenzano Menada M, Sala P, Villa G, Ferrero S. Ann Surg Oncol. 2009;16:2459–2463. doi: 10.1245/s10434-009-0554-x. [DOI] [PubMed] [Google Scholar]
- 4.Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary dissection. Nos C, Lesieur B, Clough KB, Lecuru F. Ann Surg Oncol. 2007;14:2490–2496. doi: 10.1245/s10434-007-9450-4. [DOI] [PubMed] [Google Scholar]
- 5.Axillary reverse mapping: mapping and preserving arm lymphatics may be important in preventing lymphedema during sentinel lymph node biopsy. Boneti C, Korourian S, Bland K, Cox K, Adkins LL, Henry-Tillman RS, Klimberg VS. J Am Coll Surg. 2008;206:1038–1042. doi: 10.1016/j.jamcollsurg.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Extensive nodal disease may impair axillary reverse mapping in patients with breast cancer. Ponzone R, Cont NT, Maggiorotto F, Cassina E, Mininanni P, Biglia N, Sismondi P. J Clin Oncol. 2009;27:5547–5551. doi: 10.1200/JCO.2009.22.1846. [DOI] [PubMed] [Google Scholar]
- 7.Axillary reverse mapping (ARM): a new concept to identify and enhance lymphatic preservation. Thompson M, Korourian S, Henry-Tillman R, Adkins L, Mumford S, Westbrook KC, Klimberg VS. Ann Surg Oncol. 2007;14:1890–1895. doi: 10.1245/s10434-007-9412-x. [DOI] [PubMed] [Google Scholar]
- 8.A phase I study to assess the feasibility and oncologic safety of axillary reverse mapping in breast cancer patients. Bedrosian I, Babiera GV, Mittendorf EA, et al. Cancer. 2010;116:2543–2548. doi: 10.1002/cncr.25096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safety study of axillary reverse mapping in the surgical treatment for breast cancer patients. Deng H, Chen L, Jia W, et al. J Cancer Res Clin Oncol. 2011;137:1869. doi: 10.1007/s00432-011-1064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The efficacy of arm node preserving surgery using axillary reverse mapping for preventing lymphedema in patients with breast cancer. Han JW, Seo YJ, Choi JE, Kang SH, Bae YK, Lee SJ. J Breast Cancer. 2012;15:91–97. doi: 10.4048/jbc.2012.15.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preservation of lymphatic drainage from arm in breast cancer surgery: is it safe? Kang S, Choi J, Jeon Y, Lee S, Bae Y. Cancer Research. 2009;69:201. [Google Scholar]
- 12.Extensive nodal involvement increases the positivity of blue nodes in the axillary reverse mapping procedure in patients with breast cancer. Rubio IT, Cebrecos I, Peg V, et al. J Surg Oncol. 2012;106:89–93. doi: 10.1002/jso.23048. [DOI] [PubMed] [Google Scholar]
- 13.ARM: axillary reverse mapping - the need for selection of patients. Gobardhan PD, Wijsman JH, van Dalen T, et al. Eur J Surg Oncol. 2012;38:657–661. doi: 10.1016/j.ejso.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Axillary reverse mapping: a prospective study in women with clinically node negative and node positive breast cancer. Connor C, McGinness M, Mammen J, et al. Ann Surg Oncol. 2013;20:3303–3307. doi: 10.1245/s10434-013-3113-4. [DOI] [PubMed] [Google Scholar]
- 15.Prospective study evaluating oncological safety of axillary reverse mapping. Schunemann E Jr, Dória MT, Silvestre JB, Gasperin P Jr, Cavalcanti TC, Budel VM. Ann Surg Oncol. 2014;21:2197–2202. doi: 10.1245/s10434-014-3626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Axillary reverse mapping: five-year experience. Ochoa D, Korourian S, Boneti C, Adkins L, Badgwell B, Klimberg VS. Surgery. 2014;156:1261–1268. doi: 10.1016/j.surg.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feasibility of axillary reverse mapping and clinicopathological features predicting arm node metastasis in breast cancer—a pilot study. Kumar KS, Hemanth GN, Panjwani PK, et al. Indian J Surg Oncol. 2017;8:119–122. doi: 10.1007/s13193-016-0578-1. [DOI] [PMC free article] [PubMed] [Google Scholar]