Abstract
Background
Vaccine-induced thrombotic thrombocytopenia (VITT) is a rare coagulation disorder reported after administration of COVID-19 adenovirus-vectored vaccines. VITT is mediated by anti-platelet factor 4 (PF4) antibodies activating platelets through the Fcγ-receptor II (FcγRII), and it is associated with strong fibrin turnover. The complement system is involved in several other immunothrombotic entities, but its impact on VITT is not established.
Objective
To assess antibodies in interaction with the activation of platelets and complement triggered by VITT.
Methods
Antibodies against adenovirus type 2 hexon protein, ChAdOx1 adenoviral vector-specific IgG and PF4 were analyzed by enzyme immunoassays from VITT patients (n = 5). The EDTA plasma samples of the patients and controls were used to measure both terminal complement complexes (TCC) by ELISA and aggregation of healthy donor platelets. We studied the effects of human immunoglobulin (IVIG) and glycoprotein IIb/IIIa inhibitor (GPIIb/IIIa) on spontaneous and collagen-induced platelet aggregation supplemented with VITT plasma.
Results
None of the patients had experienced a COVID-19 infection. Antibody analyses confirmed the immunogenicity of the adenovirus-vectored ChAdOx1 vaccine. Moreover, VITT plasma had anti-PF4 antibodies and elevated TCC levels as a sign of complement activation. In isolated healthy donor platelets, VITT patient plasma caused marked, spontaneous aggregation of platelets, which was abolished by eptifibatide and high-dose therapeutic IVIG.
Conclusions
Our findings suggest that VITT is triggered by antibodies against adenovirus vector and PF4-polyanion complexes which strongly co-activate complement and platelets. The spontaneous platelet aggregation was suppressed by IVIG or eptifibatide, indicating that besides FcγRII, also GPIIb/IIIa receptor exerts platelet procoagulant role in VITT.
Keywords: Vaccine-induced thrombotic thrombocytopenia, Anti-PF4 antibodies, Complement activation, Platelets aggregation, Fcγ-receptor II, GPIIb/IIIa receptor
Graphical abstract
1. Introduction
Several reports have described COVID-19 adenoviral vector vaccine-induced thrombotic thrombocytopenia (VITT) or thrombotic thrombocytopenic syndrome (TTS) [1], [2], [3], [4]. VITT/TTS typically involves both venous and/or arterial thrombosis and moderate thrombocytopenia (20–100 × 109/L) [4]. VITT resembles heparin-induced thrombocytopenia (HIT) without prior heparin use, characterized by antibodies against complexes of platelet factor 4 (PF4) and polyanions, such as free DNA and endogenous glycosaminoglycans [1], [5]. The symptoms develop within 4–28 days after vaccination, but persisting antibodies may induce VITT later by other triggering factors. In the most severe cases disseminated intravascular coagulation (DIC) may develop leading to a fatal outcome. This immunothrombotic syndrome is clinically associated with an extensive immune-related fibrin turnover, but the detailed mechanisms have still remained elusive [2], [4]. So far it has become clear that antibodies against the adenoviral vector and PF4-polyanions generate immune complexes, which interact with and activate platelets through their Fcγ-receptor II (FcγRII).
Several components of the complement activation pathway can enhance thrombin-induced platelet activation and aggregation [6]. Vice versa, activated platelets and certain coagulation proteins can also interact with and activate the complement [7]. The complement system is involved in other VITT-related entities, i.e. antiphospholipid antibody syndrome and immune thrombocytopenic purpura [8], but its role in the pathogeneses of VITT has not yet been established in clinical samples.
In Finland, 358,000 first and 55,000 second doses of ChAdOx1 nCoV-19 (Vaxzevria®, AstraZeneca) adenovirus-vectored vaccine had been administered by the end of March 2021 [9]. Here, we report interactions between antibodies, the complement system and platelet activation in five VITT patients´ serum or plasma after their first dose of ChAdOx1 nCoV-19 vaccine.
2. Patients and methods
2.1. Plasma and serum samples
Written informed consents were obtained from the control individuals and patients 2–5, and a close relative of the deceased patient 1. Serum for antibody assessments and ethylene diamine tetra-acetic acid (EDTA, 1.8 mg/mL) plasma for complement and platelet activation studies were collected upon VITT diagnosis before administration of intravenous immunoglobulin (IVIG). All VITT diagnoses were observed after the first vaccination dose. Follow-up samples were collected 5 weeks later from patients 2, 3 and 5. Due to the emergency setting, the availability of the samples was limited. Except for one acute phase serum sample (patient 2), and a five-week follow-up sample (patient 3), all other complement and platelet studies were conducted with EDTA-plasma. Since the complement studies were preferably analyzed in EDTA-plasma and the serum was collected for antibody studies, platelet aggregation was assessed with EDTA-plasma, the same matrix as the complement system.
Health care workers (HCWs) who received their first ChAdOx1 vaccine (n = 9) were recruited as controls from our hospital under the Helsinki-Uusimaa health district ethical permission HUS/1238/2020. Serum was collected before or on the day of the first vaccine dose and 3 to 4 weeks later. All samples were stored at −80 °C until analyzed. Samples (n = 20) negative for antibodies against the human adenovirus hexon protein were randomly selected from a child serum panel [10].
2.2. Antibodies against ChAdOx1, HAdV-2 hexon, and SARS-CoV-2 S and N proteins
Human adenovirus type 2 (HAdV-2) hexon protein and ChAdOx1 adenovirus-vectored vaccine- specific IgG antibodies were measured with an enzyme immunoassay (EIA). 96-well plates (Nunc Maxisorp, Thermo Fisher Scientific) were coated with 4 μg/mL AdV2 hexon protein and 6 μg/mL ChAdOx1 nCoV-19 vaccine (Vaxzevria®, AstraZeneca) in PBS for 16 h at +4 °C. HAdV-2 was cultivated in HeLa cells, and hexon protein was purified with anion-exchange liquid chromatography [11]. SARS-CoV-2 nucleoprotein (N) and spike protein (S1)-specific IgG antibodies were detected with an in-house EIA described previously [12]. Cut-off values for HAdV-2 hexon and ChAdOx1 were calculated as the mean EIA units plus three times standard deviations (SDs) of 20 negative serum samples. Positive control specimens included samples with high antibody levels against SARS-CoV-2 S1 and N proteins [12] and adenovirus hexon proteins [10], assigned EIA unit values of 100, respectively.
2.3. Coagulation biomarkers, PF4 and antiphospholipid antibody assays
Standardized coagulation assays in citrated (3.2%)-plasma were as follows: prothrombin time (PT, Medirox Owren's PT, Medirox, Nyköping, Sweden), fibrinogen (Clauss method, HemosIL Q.F.A. Thrombin, Werfen, Barcelona, Spain), and D-dimer (HemosIL D-Dimer HS 500). Antibodies (classes IgG, IgM and IgA) against PF4-heparin complexes were tested both with a gel agglutination rapid assay (ID-PaGIA Heparin/PF4 Antibody Test, Bio-Rad Laboratories, USA) and an enzyme-linked immunosorbent assay (ELISA) (Asserachrom HPIA, Diagnostica Stago, France). ELISA (Asserachrom HPIA, Diagnostica Stago) results were reported as percentage of absorbance related to reference sample. The reference sample was within the manufacturer specifications (stated on package insert, dependent on reagent lot). Percentage result is calculated (sample absorbance/reference absorbance). The interpretation of positivity is as follows: < 30 % of reference; negative, 30-50%, borderline positive, >50% positive, and >100% strongly positive.These results are referred below as anti-PF4 antibodies [1]. Cardiolipin and beta-2-glycoprotein-I IgG antibodies were analyzed using Phadia 250 immuno-analyzer and reagents (Sweden).
2.4. Platelet activation studies
Fresh gel-filtered platelets were isolated from three healthy donors, essentially as previously described [13]. Briefly, blood was collected from the antecubital vein with a PTFE cannula with free flow either to EDTA containing tubes (Vacuette K2EDTA, Greiner Bio-One, Kremsmünster, Austria) to assess blood cell count (XP-300, Sysmex Corp., Kobe, Japan) and to collect plasma, or to acidic citric dextrose (HACD; ratio 1:6) anticoagulant for platelet isolation. EDTA-blood was centrifuged twice (2500 ×g, 15 min, 22 °C) to separate plasma. Platelet-rich plasma was prepared from HACD-blood by centrifugation (180 ×g, 12 min) and supplemented with apyrase (1 U/mL, Merck KGaA, Darmstadt, Germany), and prostaglandin E1 (25 ng/mL, Merck KGaA) prior to platelet pelleting (1200 ×g, 10 min) [14]. Platelets were suspended in HEPES-Tyrode buffer (137 mM NaCl, 2.7 mM KCl, 3 mM NaH2PO4 3.5 mM HEPES, 5.5 mM d-glucose, 0.35% BSA, pH 7.35) and gel-filtered with Sepharose CL-2B column (Cytiva, Uppsala, Sweden). Platelet counts were buffer-adjusted to 270–330 × 106/mL. The preanalytical conditions were assessed by comparing serum with EDTA-plasma in the aggregation experiments. Samples were supplemented with essential cations of CaCl2 and MgCl2 along with fibrinogen at their optimized concentrations. The addition of EDTA-plasma or serum (2 μL/300 μL) did not markedly influence platelet aggregation.
Immediately prior to analysis platelets were supplemented with CaCl2 (1.5 mM), MgCl2 (0.75 mM) and fibrinogen (0.1 mg/mL) after which 2 μL of plasma (or serum when available) was added. Platelets were incubated under static conditions for 5 min at 22 °C and warmed up for 1 min at 37 °C before Born turbidimetric platelet aggregation studies (AggRAM, Helena Biosciences, Gateshead, UK). Both spontaneous and collagen-induced (1.5 μg/mL; Chrono-PAR, Chronolog Corp., Havertown, PA, USA) aggregation was assessed, reporting the maximum at 6 min. Collagen induced 50–80% platelet aggregation, and was mainly used to follow the functionality of platelets during the assay. The effects of human immunoglobulin (IVIG) (5 and 10 mg/mL; Privigen® CSL Behring, Marburg, Germany, the brand for patient treatment), glycoprotein (GP) IIb/IIIa inhibitor eptifibatide (2 μg/mL; Integrelin®, GSK, Dublin, Ireland) and recombinant human PF4 (ab9562, Abcam B.V., Amsterdam, Netherlands) on platelet aggregation were assessed. Platelets were considered viable for 4 h, based on the vehicle control during the experiments. The acute phase samples from patients 1, 2, 3 and a follow-up sample from patient 3 were studied. Healthy donor samples were included as controls. Heparin-PF4 antibody positive EDTA-plasma from a patient with HIT, used as a method control, induced modest platelet aggregation. Under these experimental conditions, control plasma or serum provided similar results in platelet aggregation.
2.5. Complement activation
The parallel plasma samples were used for platelet and complement studies. The terminal complement complexes (TCC) were measured since complement activation products (C5a; membrane attack complex, MAC) activate platelets and/or endothelial cells in thrombotic microangiopathy (TMA) [15]. The concentrations of TCC were determined with an ELISA (Hycult Biotech, the Netherlands) using the kit's quality control standards. Microsoft Excel program was used for the standard curve analysis. An unpaired, two-tailed Student's t-test was used for the statistical analysis between patient and control groups.
2.6. Antibodies against gangliosides and complement factor H
There is evidence that antibodies against the polyanionic gangliosides or autoantibodies against heparin and ganglioside binding complement inhibitor factor H may cause TMA [15], [16]. Anti-ganglioside antibody reactivity (IgG or IgM) against 11 gangliosides (GM1–4, GD1a, GD1b, GD2, GD3, GT1a, GT1b, and GQ1b) of plasma or serum samples (1:100 dilution) was tested with a line blot assay according to the manufacturer's instructions (Generic Assays GmbH, Germany). All assays included lot-specific positive controls. An in-house assay was used for antibodies against the complement regulator factor H [17].
2.7. Patient characteristics
Patient 1, a 40-year-old man with a history of type 2 diabetes, arterial hypertension, hypercholesterolemia, and obesity (39 BMI kg/m2) was hospitalized (mid March 2021) nine days after vaccination due to chest pain and headache. Myocardial infarction (AMI), and intracerebral bleeding (ICH) with associated large cerebral venous sinus thrombosis (CVST), were diagnosed. Laboratory markers indicated DIC, and extremely high D-dimer level was observed (Table 1). With decreasing platelet counts, anti-PF4 platelet-activating antibodies were observed by ELISA. Despite his AMI, the low platelet count and ICH precluded heparin use, nevertheless, acetylsalicylic acid (ASA) and a low prophylactic dose of danaparoid were administered. Platelet and fibrinogen concentrates were repeatedly transfused due to progressing ICH, without recovery (Fig. 1). At day 11 his consciousness rapidly impaired, antithrombotic treatment was withdrawn, and IVIG 1 g/kg was administered. During emergency decompressive craniectomy this index patient deceased.
Table 1.
Characteristics of the patients. Anti-PF4 antibodies ELISA (Asserachrom HPIA, Diagnostica Stago) with global recognition of immunoglobulins. Interpretation of ELISA result, <30% of reference; negative, 30–50%, borderline positive, >50% positive, >100% strongly positive. IVIG; intravenous immunoglobulin (dose see p. 7–9). Reference values for hemoglobin (male 134–167 g/L and for female 117–155 g/L), platelet count (150–360 × 109/L), PT; prothrombin time (70–130% of normal), fibrinogen (2.0–4.0 g/L) and D-dimer (<0.5 mg/L).
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Sex | Male | Female | Male | Female | Female |
| Age (years) | 40 | 21 | 58 | 60 | 68 |
| Hospital admission (days after vaccination) | 9 | 12 | 9 | 19 | 16 |
| Medication on admission | Metformin, amlodipin, sertraline, pantoprazole | Insulin, pregabalin, oxycodone | Ezetimibe | Ebastine, salmeterol, fluticasone, estradiol | None |
| Initial anticoagulation (before the VITT diagnosis) | Danaparoid | Tinzaparin | Enoxaparin (ASA, ticagrelor) | Dalteparin | Danaparoid |
| Duration of initial anticoagulation (days) | Continued | 5 | 1 | 22 | Continued |
| Hemoglobin on admission | 147 | 106 | 146 | 116 | 119 |
| Platelet count - nadir | 20 | 46 | 55 | 54 | 68 |
| PT on admission | 73 | 136 | 78 | 116 | 81 |
| Fibrinogen - nadir | 1.0 | 2.3 | 2.1 | 2.7 | 1.5 |
| D-dimer - peak | >128 | 12 | 115 | 5 | 35 |
| Anti-PF antibody positivity (days after vaccination) | 10 | 17 | 10 | 41 | 17 |
| Anti-PF4 antibody level | 109 (strong positive) | 45 (borderline positive) | 96 (positive) | 96 (positive) | 36 (borderline positive) |
| IVIG administration (days after vaccination) | 11 | 22 | 10 | none | 17 |
| Danaparoid treatment started (days after vaccination) | 10 | 19 | 11 | 41 | 17 |
| Outcome | Fatal | Recovery | Recovery | Recovery | Recovery |
Fig. 1.
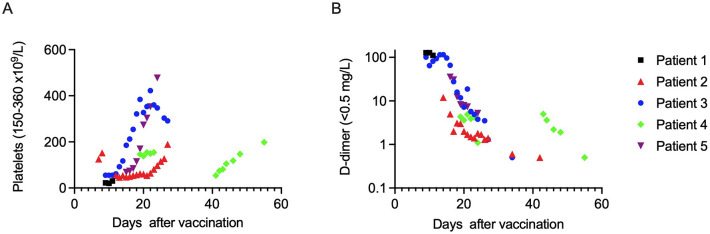
A-B. Development of platelet count (A) and D-dimer levels (B) in patients 1–5. Reference range for platelet counts 150–360 × 109/L and for D-dimer <0.5 mg/L. Due to the death of patient 1, only limited data are available. D-dimer is presented in a half-logarithmic scale.
Patient 2, a 21-year-old woman had a history of type 1 diabetes and allogeneic stem cell transplantation (SCT) for acute lymphoblastic leukemia in 2017. Three days after her first vaccine dose, she was hospitalized due to Escherichia coli sepsis, and was discharged in good condition on day 8, with a normal platelet count. On day 12 she was readmitted due to a severe headache and thrombocytopenia (Table 1). CVST was diagnosed and anticoagulation with tinzaparin was initiated. On day 19, anti-PF4 antibodies were detected with ELISA, while the rapid PaGIA test was negative (Table 1). Tinzaparin was replaced with danaparoid. Starting on day 22 she received IVIG treatment 0.4 g/kg over five consecutive days. Platelet counts normalized (Fig. 1), and she was discharged on day 27. The CVST had resolved five weeks later and laboratory values, including anti-PF4 antibodies normalized, and she continued with fondaparinux prophylaxis.
Patient 3 was a 58-year-old man with a bicuspid aortic valve disease, severe aortic insufficiency, and hypercholesterolemia. Nine days after vaccination he was hospitalized due to epigastric pain, headache, and fever. Inferior AMI was diagnosed and a stent was placed under enoxaparin, ticagrelor and ASA therapy. D-dimer level was high, while the platelet count was low (Table 1). Two cerebral microbleeds were discovered, without signs of CVST. On day 10 anti-PF4 antibodies were detected with ELISA, but the rapid PaGIA test was negative. Enoxaparin was replaced with a prophylactic dose of danaparoid, and IVIG 1 g/kg was administered for two days. Platelet counts normalized, but the D-dimer level increased again on day 13 while a long superficial thrombophlebitis in the arm was observed (Fig. 1). Epigastric pain restarted on day 14, and a portal vein thrombosis was diagnosed. Both anti-PF4 antibody ELISA and the rapid PaGIA test were positive on day 14. The patient was discharged on day 27 with ticagrelor and danaparoid; the latter was later switched to prophylactic dose of fondaparinux. After five weeks, the rapid anti-PF4 antibody test remained positive, but the ELISA was negative.
Patient 4 was a 60-year-old woman with a history of bilateral pulmonary embolism (PE). She was hospitalized 19 days after vaccination due to dyspnea. Bilateral PE and a deep vein thrombosis (DVT) were diagnosed. Dalteparin was started (Fig. 1). On day 21 during mild thrombocytopenia the anti-PF4 antibody PaGIA test was positive, the ELISA being negative. She was discharged, but readmitted at day 41 due to dizziness and nausea. Brain CT angiography was normal, but thrombocytopenia was diagnosed (Table 1 and Fig. 1), and both anti-PF4 antibody tests were positive. Dalteparin was replaced with danaparoid. Platelet counts resolved, and she was discharged on day 44. Later, 55 days after vaccination, both anti-PF4 antibody tests remained positive.
Patient 5 was a 68-year-old previously healthy woman. 16 days after vaccination she was hospitalized due to headache, bruising and thrombocytopenia (Table 1). Massive CVST and cortical cerebral infarction were discovered, and a symptomless bilateral PE was also diagnosed. The next day anti-PF4 antibodies were positive with ELISA, but the rapid PaGIA was negative. Danaparoid was started and she received IVIG 1 g/kg for two days. She recovered and was discharged on day 24.
3. Results
3.1. Antibodies against ChAdOx1, HAdV-2 hexon, and SARS-CoV-2 S and N proteins
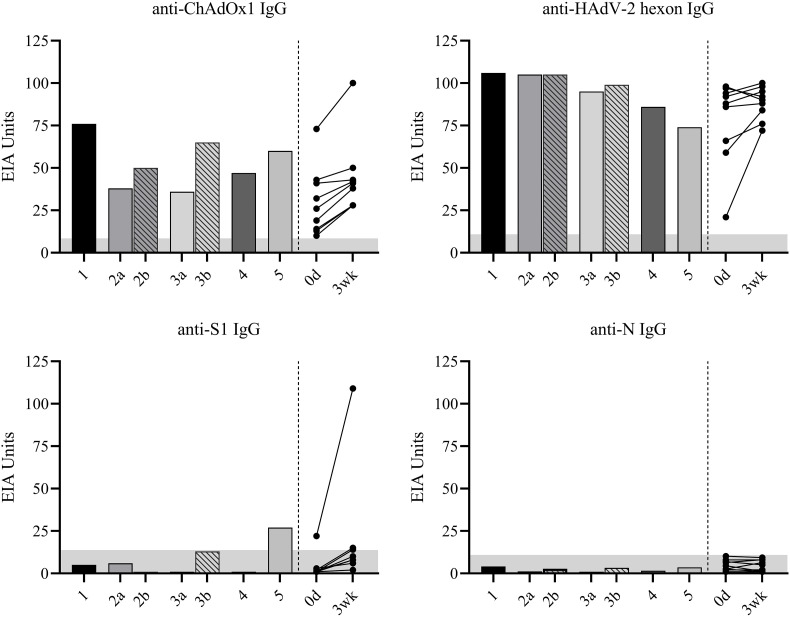
All five ChAdOx1-vaccinated VITT patients and all nine vaccinated HCWs were seronegative for SARS-CoV-2 N specific IgG antibodies (Fig. 2 ). In addition, eight of nine HCWs were seronegative for SARS-CoV-2 S1-specific IgG antibodies before the vaccination. One HCW had anti-S1 IgG antibodies already before vaccination (day 0 sample), implying a previous symptomless COVID-19 infection.
Fig. 2.
Antibody responses against ChAdOx1 nCoV19 vaccine, human AdV-2 hexon protein, and SARS-CoV-2 S1 and N proteins. IgG antibody levels in ChAdOx1 vaccinated VITT patients (n = 5) and healthy health care workers (HCWs) (n = 9) were measured with EIA. Serum samples from VITT patients were collected 10–41 days after the first vaccine dose (a) and two patients had sequential serum samples collected 5 weeks after the first sample collection (b). Serum samples from HCWs were collected before the vaccination (referred as 0d) and 3–4 weeks after the first vaccine dose (referred as 3wk). Cut-off values specific for each assay are indicated with grey area. Bars represent patients and black dots represent controls.
The first dose of ChAdOx1 vaccine induced 1) high levels of anti-S1 IgG antibodies in the HCW who was seropositive before the vaccination, and 2) moderate levels of anti-S1 IgG antibodies in three initially seronegative HCWs and in patient 5. Patients 1–4 did not have detectable anti-S1 IgG antibodies at the time of VITT diagnosis or 5 weeks after the acute phase. All serum samples from the patients and HCWs, had IgG antibodies to HAdV-2 hexon protein and ChAdOx1 vaccine at the time of VITT diagnosis or before the vaccination, respectively (Fig. 2). Antibody levels against ChAdOx1 increased both among the HCWs after the vaccination (2–27 EIA units) and during the follow-up of the VITT patients (12–29 EIA units).
3.2. Coagulation biomarkers, anti-PF4 and antiphospholipid antibodies
All patients presented with thrombocytopenia, had platelet counts in the range of 20–68 × 109/L, and peak D-dimer levels of 5–128 mg/L depending on the severity of VITT (Table 1 and Fig. 1). Only patient 1 with a fatal outcome met the ISTH DIC criteria (Table 1). None had cardiolipin or beta-2-glycoprotein-I IgG antibodies.
3.3. Aggregation of gel-filtered platelets from healthy donors supplemented with patient plasma, IVIG, GPIIb/IIIa inhibitor and PF4
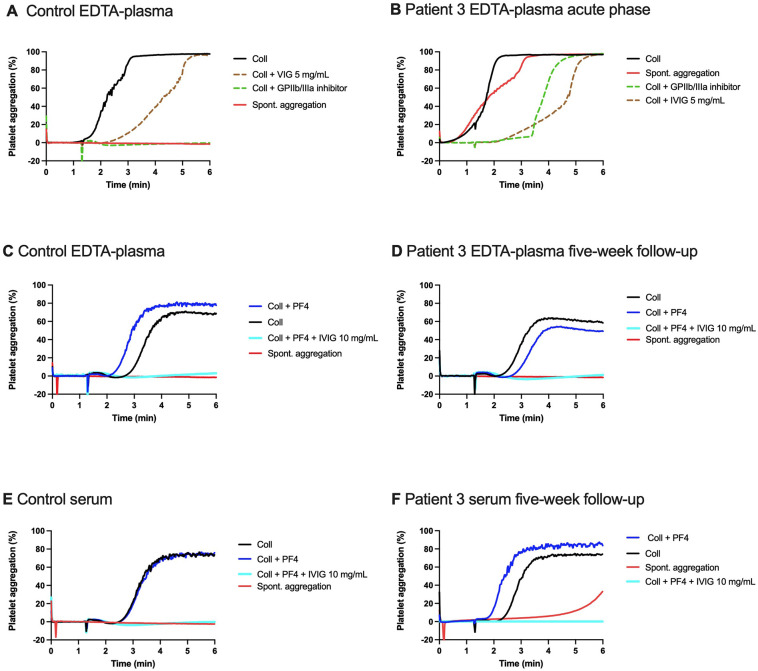
Acute phase plasma (2 μL/300 μL of platelets) from patients 1 (Fig. 3A) and 3 (Fig. 4B) triggered spontaneous aggregation of healthy platelets. Aggregation was not induced spontaneously by patient 2 serum (data not shown), nor by any control samples from healthy individuals (Figs. 3B and 4A, C, E). Supplementation of platelets with PF4 (4 μg/mL) and plasma from patient 1 triggered spontaneous aggregation which was further enhanced by collagen (Fig. 3C). After five weeks, serum but not plasma from patient 3 induced delayed spontaneous platelet aggregation (Fig. 4D and F).
Fig. 3.
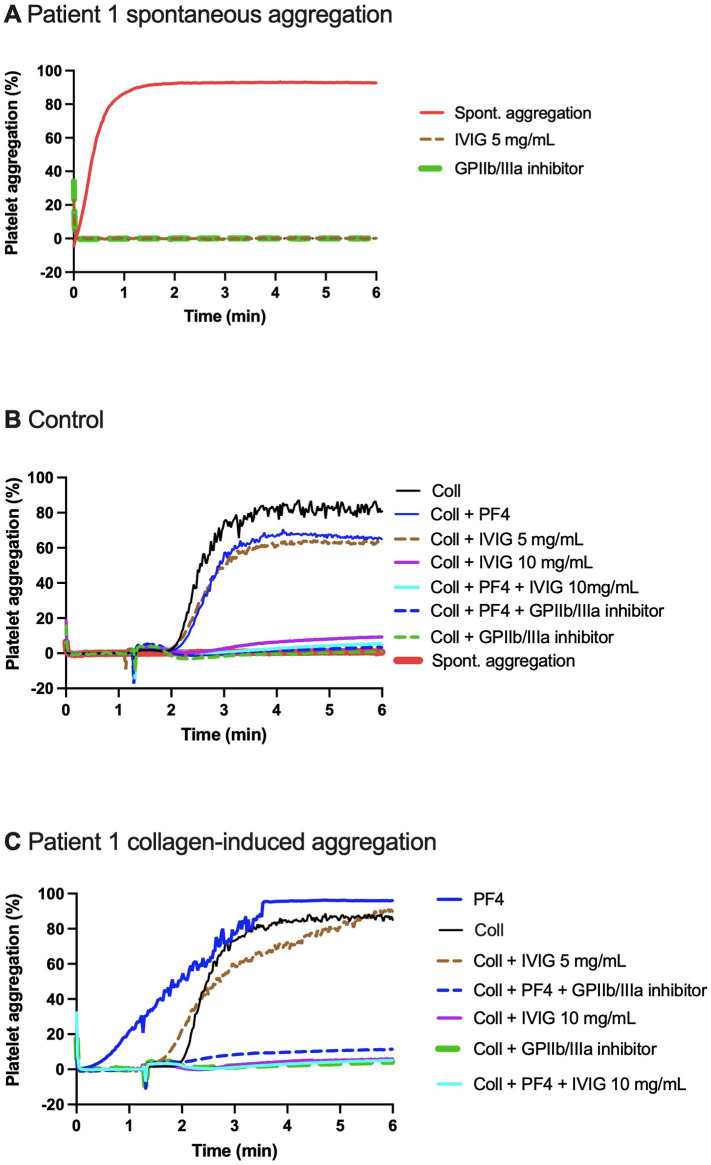
A-C. Spontaneous (A, C) and collagen-induced healthy donor platelet aggregation (B, C) triggered by the acute phase plasma from patient 1. (A) Spiking with patient EDTA-plasma: spontaneous aggregation (no agonist) of platelets from healthy donor (duplicate). Glycoprotein (GP) IIb/IIIa inhibitor (eptifibatide) (2 μg/mL) abolished the spontaneous aggregation, as did intravenous immunoglobin (IVIG) (5 mg/mL). (B) Spiking with control plasma: spontaneous aggregation (no agonist) did not occur. Collagen-induced aggregation supplemented with platelet factor 4 (PF4, 4 μg/mL) was not enhanced. (C) Spiking with patient plasma: immediate spontaneous aggregation in platelets spiked with patient plasma occurred, and continued upon adding collagen. Addition of PF4 enhanced collagen-induced aggregation. GPIIb/IIIa inhibitor and IVIG at 10 mg/mL both inhibited collagen-induced aggregation, but IVIG at 5 mg/mL had only a moderate response with control (B) or patient plasma (C). PF4 did not counteract the inhibitory effect of GPIIb/IIIa inhibitor or IVIG (10 mg/mL) in control (B) or patient plasma (C).
Fig. 4.
A-F. Spontaneous and collagen-induced healthy donor platelet aggregation triggered by the acute (A, B) versus follow-up phase plasma (C, D) and serum (E, F) from patient 3. (A) Spiking with control plasma without agonist and in collagen-induced aggregation: spontaneous aggregation did not occur (no agonist) and collagen-induced aggregation was vivid. (B) Spiking with patient plasma at acute phase induced immediate spontaneous aggregation (no agonist), enhanced by collagen. Collagen-induced platelet aggregation was modestly inhibited by intravenous immunoglobin (IVIG) (5 mg/mL) when spiked with control plasma (A) or on patient plasma (B), while Glycoprotein (GP) IIb/IIIa inhibitor (eptifibatide) (2 μg/mL) abolished aggregation. The five-week follow-up patient plasma did not trigger spontaneous aggregation (D), but patient serum depicted delayed spontaneous aggregation (F). Compared with plasma (C) or serum controls (E), collagen-induced aggregation in the presence of patient plasma (D) or serum (F) was nearly normal, despite ticagrelor and fondaparinux medication. Adding platelet factor 4 (PF4) (5 μg/mL) did not markedly enhance aggregation (C-F), but IVIG 10 mg/mL abolished aggregation in the presence of PF4 (C-F).
Adding IVIG (5 mg/mL) or GPIIb/IIIa inhibitor eptifibatide (2 μg/mL) abolished spontaneous aggregation in the presence of patient 1 plasma (Fig. 3A). However, this treatment only partially inhibited aggregation (~50-%) in the case of patient 3 plasma (Fig. 4B). Of note, the response of patient 3 remained despite the antiplatelet co-treatment with ticagrelor and ASA.
Overall, the presence of serum from patient 2 or control individual led to a similar level of collagen-induced platelet aggregation, without any spontaneous aggregation (data not shown). A low concentration of IVIG (5 mg/mL), marginally inhibited platelet responses to collagen, while IVIG at a higher concentration (10 mg/mL) or GPIIb/IIIa inhibitor (2 μg/mL) abolished the collagen-induced aggregation in the presence or absence of PF4 (4–5 μg/mL) in all plasma samples (Fig. 3, Fig. 4).
3.4. Complement activation
Complement activation may activate platelets, especially via the C5a receptor type 1 or by direct membrane attack, and antibodies against PF4 and/or heparin may activate both platelets and the complement [18]. Since heparan sulphate, sialic acids and related polyanions are effective complement inhibitors on endothelial and platelet surfaces, anti-heparin antibodies could neutralize the cellular protection against complement [19]. To analyze whether complement had been activated in the VITT-blood, plasma levels of soluble TCC or SC5b-9 were compared in controls and the acute and follow-up patient samples (Fig. 5 ).
Fig. 5.

Terminal pathway complement activation in patient samples. EDTA-plasma samples from the patients (serum for the first sample from patient 2) at the acute and follow-up stages and from healthy laboratory personnel (controls) were analyzed for terminal complement complex (TCC) levels by ELISA. After excluding value from the single serum sample the difference between acute stage samples and controls was highly significant (p < 0.001; unpaired Student's t-test). Also, the difference between the follow-up samples and controls was significant (p < 0.05), while that between acute stage and follow-up samples was not. Pt = patients. The results indicate complement activation during the acute stage that continues at a lower intensity to the follow-up stage.
In the acute phase of patient 2 (only serum sample available), a high level of TCC was observed. This patient differed from the other patients by her history of SCT and Escherichia coli sepsis before the development of VITT. All other samples (Fig. 5) were plasma specimens and subjected to both complement and platelet activity studies (see above). The TCC levels were higher in acute (p < 0.001) and follow-up (p < 0.05; Student's t-test) samples of VITT patients as compared to controls, consistent with the platelet aggregation results. TCC levels in the follow-up plasma samples decreased slightly but exceeded the control levels. Factor H or ganglioside specific antibodies were not detected in VITT patient samples.
4. Discussion
Our study on healthy control and VITT patient serum samples showed that the chimpanzee adenovirus-vectored ChAdOx1 vaccine is immunogenic in humans. Importantly, EDTA-plasma samples of the VITT patients collected 9–41 days after vaccination had anti-PF4 antibodies and evidence of significant complement activation (elevated TCC levels). Acute phase plasma samples from VITT patients, unlike controls, caused marked spontaneous aggregation of platelets collected from healthy donors, when spiked to platelet suspension. Normal platelets (300 μL) aggregated upon exposure to a small volume (2 μL) of patient EDTA-plasma derived from a VITT patient, but not by control plasma. Platelet aggregation was abolished by 1) a GPIIb/IIIa inhibitor eptifibatide, possibly with a sustained activation signal mediated by the adenovirus [20], and 2) a high dose of therapeutic IVIG, compatible with immune activation of the platelet receptor FcγRII. Both of these receptors are considered as procoagulant platelet receptors [21], [22], complying with the clinical presentation of the patients. Overall, healthy platelets spontaneously aggregated by VITT EDTA-plasma, an observation in VITT patient samples that has not been reported before. Importantly, the activation of platelets and the complement system appeared to take place simultaneously.
None of the patients had a history of SARS-CoV-2 infection before the appearance of VITT. Only one patient had detectable levels of anti-S1 IgG antibodies after vaccination which may be due to a relative short time after vaccination or relatively poor immunity induced by the 1st vaccine dose. However, we detected high levels of anti-ChAdOx1 and anti-hexon IgG antibodies in all patients suffering from an acute VITT, and antibody levels against adenoviral vector increased further 5 weeks after the VITT diagnosis. These findings indicate that anti-human adenovirus antibodies cross-react with the chimpanzee adenoviruses, and the chimpanzee adenoviral vector is immunogenic in humans. It was also highly unlikely that the VITT patients had an acute or previous COVID-19 infection since none of the VITT patients were anti-SARS-CoV-2 N protein antibody that is a sign of exposure to the virus.
All our patients met the diagnostic criteria of VITT [4]. Their anticoagulation was acutely managed with danaparoid, a drug recommended for HIT patients [23]. With the exception of patient 4, IVIG treatment was administered to all patients. Unfortunately, patient 1 succumbed to VITT, but the others recovered and thrombosis, platelet counts, and D-dimer levels resolved. Acute-phase VITT plasma induced spontaneous aggregation of platelets derived from healthy donors, indicating its strong prothrombotic activity. High-dose IVIG and platelet GPIIb/IIIa inhibitor inhibited this spontaneous aggregation (Fig. 3A). In comparison, lower doses of IVIG or GPIIb/IIIa inhibitor only partially inhibited platelet aggregation triggered by collagen in the presence of patient plasma (Fig. 4B). In the follow-up sample of patient 3 collagen-induced platelet aggregation was comparable with controls, despite concomitant medication with ticagrelor and fondaparinux. Collagen-induced aggregation of platelets was inhibited by eptifibatide and a higher dose of IVIG.
Beside their established role in coagulation, platelets also regulate innate and adaptive immunity [24]. Viruses can directly activate platelets, but host immune responses against infection can, likewise, include platelet responses, as reported also in COVID-19 [25]. Platelets express a variety of receptors including complement receptors, FcγRII, and GPIIb/IIIa [20], [22]. Adenovirus has been reported to interact with platelets via GPIIb/IIIa receptor [26]. In our study, blocking of platelet GPIIb/IIIa receptor inhibited EDTA-plasma triggered spontaneous platelet aggregation, which occurred in severe cases (patients 1 and 3).
PF4 (or CXCL4) is a neutrophil and monocyte chemokine released by activated platelets. PF4 is secreted at high local concentrations in the vicinity of blood clots to polymerize fibrin [27]. VITT is mediated by anti-PF4 antibodies activating platelets through the FcγRII receptor [1], [5]. When spiked with plasma from patient 1, PF4 triggered the aggregation of normal platelets (Fig. 3C).
The VITT patients had higher levels of TCC both in their acute phase and follow-up samples than healthy controls, a new clinical observation of our study. The plasma TCC values declined from the acute phase to the follow-up samples, but complement activation appeared to continue in VITT patients during the disease process. Complement may become activated by antibody binding to their targets, like PF4, and/or by immune complexes. The very high initial TCC level in the serum of patient 2 could be related to her recent E. coli sepsis episode. General tendency for autoantibody formation was not observed, as antibodies against gangliosides, complement factor H or phospholipids were absent.
Our study has limitations, such as the lack of platelet studies of the patient 4 and 5 plasma during the acute phase due to limited sample availability. The involvement of procoagulant microvesicles was not investigated, despite their potential role in VITT [28].
To summarise, our findings support previous studies that VITT is triggered by antibodies against adenovirus vaccine and PF4-polyanion complexes. We provide new evidence that this immunological stimulus co-activates the complement system and triggers spontaneous aggregation of healthy platelets. In addition, this spontaneous platelet aggregation was suppressed by GPIIb/IIIa inhibitor, indicating the procoagulant role of both receptors, FcγRII, and GPIIb/IIIa in VITT.
Authorship
RL, IJ, SM and AK conceptualized the study. RL, IJ, SM, HHP, AJ and PJ analyzed the data. HHP, AJ, TH, VD, JK, SM, AK, PJ, IJ, and RL were involved in the experiments. HHP, AJ, TH, VD, JK, and PJ wrote the methods. HHP wrote the original draft of the manuscript. All authors reviewed and edited the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. IJ reports research contract with Labmaster Co and speaker fees from Merck. IJ owns small number of Orion, Abbvie, and Bristol-Myers Squibb co stocks. RL reports speaker fees from Roche, Sanofi, Alexion, and Novo Nordisk. RL is a member of scientific advisory boards for Bayer, CSL Behring, Roche, Sanofi, Alexion, and Novo Nordisk. RL has issued a patent for novel antithrombotic called APAC.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the following collaborators for skillful assistance: bioanalyst Marja Lemponen from Helsinki University Hospital Research Institute and MD Heikki Valkonen from Department of Anesthesiology, Helsinki University Hospital for illustration of Graphical Abstract. We also thank for the valuable comments by Dr. Harry Magnani. This work was funded as follows: SM: Academy of Finland (1336411), Helsinki University Hospital grants for diagnostics (VTR and HUSLAB, TYH2020318), Sigrid Jusélius Foundation; AK: Academy of Finland (336439, 335527), Finnish Medical Association and Finnish Governmental Subsidy for Health Science Research (VTR, TYH2021315); IJ: was funded by Jane and Aatos Erkko Foundation (grant number 5360-cc2fc), the Academy of Finland (337530) and the Sigrid Jusélius Foundation; RL: Helsinki University Hospital grant for coagulation medicine (VTR, TYH2020318).
References
- 1.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021;384:2092–2101. doi: 10.1056/nejmoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scully M., Singh D., Lown R., Poles A., Solomon T., Levi M., Goldblatt D., Kotoucek P., Thomas W., Lester W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021;384:2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., Wiedmann M., Aamodt A.-H., Skattør T.H., Tjønnfjord G.E., Holme P.A. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021;384:2124–2130. doi: 10.1056/nejmoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makris M., Pavord S., Lester W., Scully M., Hunt B. Vaccine-induced immune thrombocytopenia and thrombosis (VITT) Res. Pract. Thromb. Haemost. 2021;5 doi: 10.1002/rth2.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greinacher A., Selleng K., Warkentin T.E. Autoimmune heparin-induced thrombocytopenia. J. Thromb. Haemost. 2017;15:2099–2114. doi: 10.1111/jth.13813. [DOI] [PubMed] [Google Scholar]
- 6.Polley M.J., Nachman R.L. Human complement in thrombin-mediated platelet function: uptake of the c5b–9 complex. J. Exp. Med. 1979;150:633–645. doi: 10.1084/jem.150.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peerschke E.I.B., Yin W., Grigg S.E., Ghebrehiwet B. Blood platelets activate the classical pathway of human complement. J. Thromb. Haemost. 2006;4:2035–2042. doi: 10.1111/j.1538-7836.2006.02065.x. [DOI] [PubMed] [Google Scholar]
- 8.Peerschke E.I., Yin W., Ghebrehiwet B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol. Immunol. 2010;47:2170–2175. doi: 10.1016/j.molimm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COVID-19 vaccinations in Finland - THL. https://sampo.thl.fi/pivot/prod/fi/vaccreg/cov19cov (accessed May 5, 2021)
- 10.Kazakova A., Kakkola L., Päkkilä H., Teros-Jaakkola T., Soukka T., Peltola V., Waris M., Julkunen I. Serological array-in-well multiplex assay reveals a high rate of respiratory virus infections and reinfections in young children. MSphere. 2019;4 doi: 10.1128/msphere.00447-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waris M., Halonen P. Purification of adenovirus hexon protein by high-performance liquid chromatography. J. Chromatogr. A. 1987;397:321–325. doi: 10.1016/S0021-9673(01)85015-9. [DOI] [PubMed] [Google Scholar]
- 12.Jalkanen P., Pasternack A., Maljanen S., Melén K., Kolehmainen P., Huttunen M., Lundberg R., Tripathi L., Khan H., Ritvos M.A., Naves R., Haveri A., Österlund P., Kuivanen S., Jääskeläinen A.J., Kurkela S., Lappalainen M., Rantasärkkä K., Vuorinen T., Hytönen J., Waris M., Tauriainen S., Ritvos O., Kakkola L., Julkunen I. A combination of N and S antigens with IgA and IgG measurement strengthens the accuracy of SARS-CoV-2 serodiagnostics. J. Infect. Dis. 2021;224:218–228. doi: 10.1093/infdis/jiab222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilveskero S., Siljander P., Lassila R. Procoagulant activity on platelets adhered to collagen or plasma clot. Arterioscler. Thromb. Vasc. Biol. 2001;21:628–635. doi: 10.1161/01.ATV.21.4.628. [DOI] [PubMed] [Google Scholar]
- 14.Lassila R., Lindstedt K., Kovanen P.T. Native macromolecular heparin proteoglycans exocytosed from stimulated rat serosal mast cells strongly inhibit platelet-collagen interactions. Arterioscler. Thromb. Vasc. Biol. 1997;17:3578–3587. doi: 10.1161/01.ATV.17.12.3578. [DOI] [PubMed] [Google Scholar]
- 15.Meri S. Complement activation in diseases presenting with thrombotic microangiopathy. Eur. J. Intern. Med. 2013;24:496–502. doi: 10.1016/j.ejim.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Cutillo G., Saariaho A.H., Meri S. Physiology of gangliosides and the role of antiganglioside antibodies in human diseases. Cell. Mol. Immunol. 2020;17:313–322. doi: 10.1038/s41423-020-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sénant M., Dragon-Durey M.A. Methods Mol. Biol. 2019. Anti-factor H autoantibodies assay by ELISA; pp. 191–196. [DOI] [PubMed] [Google Scholar]
- 18.Khandelwal S., Barnes A., Rauova L., Sarkar A., Rux A.H., Yarovoi S.V., Zaitsev S., Lambris J.D., Myoung S.S., Johnson A., Lee G.M., Duarte M., Poncz M., Arepally G.M., Cines D.B. Complement mediates binding and procoagulant effects of ultra-large HIT immune complexes. Blood. 2021 doi: 10.1182/blood.2020009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyvärinen S., Meri S., Jokiranta T.S. Disturbed sialic acid recognition on endothelial cells and platelets in complement attack causes atypical hemolytic uremic syndrome. Blood. 2016;127:2701–2710. doi: 10.1182/blood-2015-11-680009. [DOI] [PubMed] [Google Scholar]
- 20.Assinger A. Platelets and infection - an emerging role of platelets in viral infection. Front. Immunol. 2014;5:649. doi: 10.3389/fimmu.2014.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Béguin S., Keularts I., Al Dieri R., Bellucci S., Caen J., Hemker H.C. Fibrin polymerization is crucial for thrombin generation in platelet-rich plasma in a VWF-GPlb-dependent process, defective in Bernard-soulier syndrome. J. Thromb. Haemost. 2004;2:170–176. doi: 10.1111/J.1538-7836.2004.00558.X. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed M.U., Receveur N., Janus-Bell E., Mouriaux C., Gachet C., Jandrot-Perrus M., Hechler B., Gardiner E.E., Mangin P.H. Respective roles of glycoprotein VI and FcγRIIA in the regulation of αIIbβ3-mediated platelet activation to fibrinogen, thrombus buildup, and stability. Res. Pract. Thromb. Haemost. 2021;5 doi: 10.1002/rth2.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnani H.N. Rationale for the role of heparin and related GAG antithrombotics in COVID-19 infection. Clin. Appl. Thromb. 2021;27 doi: 10.1177/1076029620977702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vieira-De-Abreu A., Campbell R.A., Weyrich A.S., Zimmerman G.A. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin. Immunopathol. 2012;34:5–30. doi: 10.1007/s00281-011-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fard M.B., Fard S.B., Ramazi S., Atashi A., Eslamifar Z. Thrombosis in COVID-19 infection: role of platelet activation-mediated immunity. Thromb. J. 2021;19:1–11. doi: 10.1186/s12959-021-00311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupalo E., Kuk C., Qadura M., Buriachkovskaia L., Othman M. Platelet-adenovirus vs. Inert particles interaction: effect on aggregation and the role of platelet membrane receptors. Platelets. 2013;24:383–391. doi: 10.3109/09537104.2012.703792. [DOI] [PubMed] [Google Scholar]
- 27.Amelot A.A., Tagzirt M., Ducouret G., Kuen R.L., Le Bonniec B.F. Platelet factor 4 (CXCL4) seals blood clots by altering the structure of fibrin. J. Biol. Chem. 2007;282:710–720. doi: 10.1074/jbc.M606650200. [DOI] [PubMed] [Google Scholar]
- 28.Marchandot B., Carmona A., Trimaille A., Curtiaud A., Morel O. Procoagulant microparticles: a possible link between vaccine-induced immune thrombocytopenia (VITT) and cerebral sinus venous thrombosis. J. Thromb. Thrombolysis. 2021 doi: 10.1007/s11239-021-02505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]