Abstract
Background
Cell transplantation offers a potential therapeutic approach to the repair and regeneration of damaged vascular and cardiac tissue after acute myocardial infarction (AMI). This has resulted in multiple randomised controlled trials (RCTs) across the world.
Objectives
To determine the safety and efficacy of autologous adult bone marrow stem cells as a treatment for acute myocardial infarction (AMI), focusing on clinical outcomes.
Search methods
This Cochrane review is an update of a previous version (published in 2012). We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 2), MEDLINE (1950 to March 2015), EMBASE (1974 to March 2015), CINAHL (1982 to March 2015) and the Transfusion Evidence Library (1980 to March 2015). In addition, we searched several international and ongoing trial databases in March 2015 and handsearched relevant conference proceedings to January 2011.
Selection criteria
RCTs comparing autologous bone marrow‐derived cells with no cells in patients diagnosed with AMI were eligible.
Data collection and analysis
Two review authors independently screened all references, assessed the risk of bias of the included trials and extracted data. We conducted meta‐analyses using random‐effects models throughout. We analysed outcomes at short‐term (less than 12 months) and long‐term (12 months or more) follow‐up. Dichotomous outcomes are reported as risk ratio (RR) and continuous outcomes are reported as mean difference (MD) or standardised MD (SMD). We performed sensitivity analyses to evaluate the results in the context of the risk of selection, performance and attrition bias. Exploratory subgroup analysis investigated the effects of baseline cardiac function (left ventricular ejection fraction, LVEF) and cell dose, type and timing of administration, as well as the use of heparin in the final cell solution.
Main results
Forty‐one RCTs with a total of 2732 participants (1564 cell therapy, 1168 controls) were eligible for inclusion. Cell treatment was not associated with any changes in the risk of all‐cause mortality (34/538 versus 32/458; RR 0.93, 95% CI 0.58 to 1.50; 996 participants; 14 studies; moderate quality evidence), cardiovascular mortality (23/277 versus 18/250; RR 1.04, 95% CI 0.54 to 1.99; 527 participants; nine studies; moderate quality evidence) or a composite measure of mortality, reinfarction and re‐hospitalisation for heart failure (24/262 versus 33/235; RR 0.63, 95% CI 0.36 to 1.10; 497 participants; six studies; moderate quality evidence) at long‐term follow‐up. Statistical heterogeneity was low (I2 = 0% to 12%). Serious periprocedural adverse events were rare and were generally unlikely to be related to cell therapy. Additionally, cell therapy had no effect on morbidity, quality of life/performance or LVEF measured by magnetic resonance imaging. Meta‐analyses of LVEF measured by echocardiography, single photon emission computed tomography and left ventricular angiography showed evidence of differences in mean LVEF between treatment groups although the mean differences ranged between 2% and 5%, which are accepted not to be clinically relevant. Results were robust to the risk of selection, performance and attrition bias from individual studies.
Authors' conclusions
The results of this review suggest that there is insufficient evidence for a beneficial effect of cell therapy for AMI patients. However, most of the evidence comes from small trials that showed no difference in clinically relevant outcomes. Further adequately powered trials are needed and until then the efficacy of this intervention remains unproven.
Plain language summary
Stem cell treatment following a heart attack
Review question: Are bone marrow cells safe and effective as a treatment following a heart attack?
Background: Currently the standard treatment for people suffering a heart attack (due to a blockage in the artery supplying blood to the heart) is direct opening of the artery with a tiny balloon in a procedure called primary angioplasty and introduction of a small tube (called a stent) into the artery to keep it open. The use of primary angioplasty and stents to reopen the blocked artery can lead to a 35% reduction in the mortality (death rate) associated with this condition. In recent years, bone marrow stem/progenitor cells have been investigated as a potential treatment. They may prevent the damage to the heart muscle caused by a heart attack, when used in addition to the treatment offered by primary angioplasty and standard medical therapy.
Study characteristics: Randomised trials comparing bone marrow‐derived cells with no cells in patients diagnosed with acute myocardial infarction were eligible for this review. We searched databases to March 2015. This review was supported by the National Institute of Health Research (NIHR) through its Cochrane Incentive Award programme.
Key results: In this updated systematic review we analysed data from a total of 41 trials with over 2700 patients. Evaluation of the currently available evidence indicates that this treatment may not lead to improvement when compared to standard treatment, as measured by the frequency of deaths, heart attacks and/or heart failure requiring re‐hospitalisation following treatment, as well as tests of heart function, in the short and long term.
Quality of evidence for primary outcomes: The evidence in this review is of moderate quality due to the small number of events.
Summary of findings
Summary of findings for the main comparison. Cells compared to no cells for acute myocardial infarction (AMI).
| Cells compared to no cells for acute myocardial infarction (AMI) | ||||||
| Patient or population: patients with AMI Settings: Hospitalised patients Intervention: cells Comparison: no cells | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No cells | Cells | |||||
| All‐cause mortality ‐ short‐term follow‐up (< 12 months) | Study population | RR 0.80 (0.43 to 1.49) | 1365 (17 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Further research may change the estimate | |
| 28 per 1000 | 23 per 1000 (12 to 42) | |||||
| All‐cause mortality ‐ long‐term follow‐up (≥ 12 months) | Study population | RR 0.93 (0.58 to 1.50) | 996 (14 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Further research may change the estimate | |
| 70 per 1000 | 65 per 1000 (41 to 105) | |||||
| Cardiovascular mortality ‐ short‐term follow‐up (< 12 months) | Study population | RR 0.72 (0.28 to 1.82) | 290 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Further research may change the estimate | |
| 54 per 1000 | 39 per 1000 (15 to 99) | |||||
| Cardiovascular mortality ‐ long‐term follow‐up (≥ 12 months) | Study population | RR 1.04 (0.54 to 1.99) | 527 (9 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Further research may change the estimate | |
| 72 per 1000 | 75 per 1000 (39 to 143) | |||||
| Composite death, reinfarction and hospitalisation for heart failure ‐ short‐term follow‐up (< 12 months) | Study population | RR 0.36 (0.12 to 1.14) | 379 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Further research may change the estimate | |
| 66 per 1000 | 24 per 1000 (8 to 76) | |||||
| Composite death, reinfarction and hospitalisation for heart failure ‐ long‐term follow‐up (≥ 12 months) | Study population | RR 0.63 (0.36 to 1.10) | 497 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Further research may change the estimate | |
| 140 per 1000 | 88 per 1000 (51 to 154) | |||||
| *The assumed risk is based on the observed incidence across the pooled control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Imprecision: information size criterion not met. Small size effect.
Background
Description of the condition
Despite major advances in treatment regimes, ischaemic heart disease remains a major cause of mortality and morbidity worldwide (BHF 2014). In the UK alone there are more than 2.3 million people living with ischaemic heart disease, causing approximately 153 deaths for every 100,000 people and representing a substantial cost to our healthcare system (BHF 2014). For example, more than GBP 6.8 billion was spent on treating the disease within NHS England in 2012/2013 (BHF 2014). The main symptom of ischaemic heart disease is a heart attack or myocardial infarction. Acute myocardial infarction (AMI) most often occurs when there is rupture of an atherosclerotic plaque into a coronary artery, which may cause thrombosis and occlusion of the artery, stopping the blood supply in that region of the heart and causing necrosis of the affected area (Falk 1995). Subsequently, both infarcted and unaffected myocardium undergo adverse remodelling that can sometimes extend to the entire ventricular wall. The first changes occur almost immediately after coronary occlusion and lead to loss of contractility, followed by the growth of the necrotic areas in the following days. The infarcted region would have healed after two to three months, leaving a scar (fibrotic, non‐contracting region) in the ventricular wall (ESC/ACC 2000).
Current medical treatment can ameliorate the symptoms of the disease. First thrombolytic therapy and, most recently, primary angioplasty have become the standard treatment choice for those suffering from AMI. However, although optimal medical therapy reduces mortality (Hartwell 2005), patients continue to face risks of heart failure following heart attacks (Velagaleti 2008). Therefore, the search for treatment options that prevent this adverse ventricular remodelling following AMI has been at the forefront of clinical research in cardiology.
Description of the intervention
For more than a decade cell therapies have been developed as new treatments for patients suffering from AMI (Strauer 2002). The first non‐randomised trials demonstrated the feasibility of infusing bone marrow‐derived mononuclear cells (BMMNC) into the infarcted area of the myocardium via the infarct‐related artery (IRA) using a procedure similar to percutaneous coronary intervention or PCI (Assmus 2002; Fernandez‐Aviles 2004; Meyer 2006; Strauer 2002; Tse 2003). This was later expanded to the direct injection of cells into the ischaemic cardiac muscle during coronary artery bypass graft (CABG) (Stamm 2003). The study by Stamm in 2003 administered bone marrow‐derived CD133+ haematopoietic progenitor cells and showed that these cells could improve revascularisation of the infarcted myocardium (Stamm 2003). The success of these first trials resulted in a number of larger randomised controlled clinical trials (RCTs) world‐wide (Cao 2009; Gao 2013; Grajek 2010; Hirsch 2011; Janssens 2006; Lee 2014; Lunde 2006; Nogueira 2009; Roncalli 2010; Schachinger 2006; Sürder 2013; Tendera 2009; Traverse 2010; Traverse 2011; Traverse 2012; Wohrle 2010; Wollert 2004; Yao 2009). To date, the majority of RCTs infuse a pool of BMMNC, but recently the first placebo‐controlled study comparing enriched CD34+ haematopoietic progenitor cells with non‐selected BMMNC has been published (Tendera 2009). In addition, bone marrow‐derived mesenchymal stromal cells (BM‐MSC) have been also tested in the clinic as a treatment for AMI (Gao 2013; Lee 2014).
Bone marrow harvest, containing the mononuclear cells and a small proportion of stem/progenitor cells (e.g. CD34+ or CD133+ enriched progenitor cells), is undertaken by a haematologist, whilst a specialised technician or scientist undertakes the isolation of the mononuclear cells or the selection of stem/progenitor cells. Finally, the cardiologist undertakes the infusion or injection of the cells.
Bone marrow harvest and isolation of BMMNC is a standard procedure in bone marrow transplantation for haematological malignancies. Cell transplantation in the context of heart disease is not currently available as standard clinical practice. The treatment is only available in research‐associated facilities, whilst its safety and efficacy is tested, but it is conceivable that this procedure may be available to all myocardial infarction patients, if long‐term effectiveness, prevention of heart failure and reduced morbidity are demonstrated.
The procedure at the current time is as follows: the bone marrow is harvested under general anaesthesia from the pelvic bone of the recipient using large suction needles. Thereafter, the BMMNC, CD34+ or CD133+ haematopoietic progenitor cells (BM‐HPCs) are enriched away from other bone marrow cells in sterile conditions by a specialised technician or scientist. The bone marrow harvest and separation of stem cells may take several hours. Unlike BMMNC, BM‐MSC have to be cultured in the laboratory for two to four weeks to obtain a large enough number of cells prior to their administration. The enriched or cultured cell populations are infused directly into the recipient's heart by a cardiologist during angioplasty (e.g. PCI) with a catheter allowing the administration of cells in a stop‐flow technique via a special balloon catheter (Strauer 2002). The time interval between the removal of the cells from the participant and their reinfusion varies.
The costs of the intervention may be high depending on the procedures used, and currently relate to the costs of the cell procedure (cell harvest) and the costs of the isolation of the stem/progenitor cells (approximately a 10th of the cost of the trial) or the cost of culturing cells in a dish.
How the intervention might work
Regardless of intensive preclinical and clinical research in the field in the past decade, the mode of action of cell therapies has remained unclear or at least controversial. Although transplanted cells are thought to benefit heart function through direct mechanisms, such as homing to the site of injury and differentiating into neighbouring cardiac tissues (Leri 2009), there is growing evidence that their benefit might be indirect. There is presently a shift in the regenerative concept of cell therapies in heart disease towards the hypothesis that cell‐based therapies primarily have a paracrine effect (for review see Bartunek 2010; Behfar 2014). Paracrine signalling is that in which the target cell is a different type of cell but it is close by the signal‐releasing cell. Transplanted cells would produce stimulatory cytokines, which may increase vascularity and collateral growth, promote cardiomyocyte proliferation, limit or reduce fibrosis and/or activate endogenous resident stem cells (Bartunek 2010; Behfar 2014; Cheng 2014). This could lead to reverse remodelling of the infarcted tissue and reduction in scar size.
Why it is important to do this review
In 2004, the first RCTs administering cell therapies as a treatment for AMI were reported (Chen 2004; Wollert 2004). Two years later, the number of RCTs published had increased significantly (Ge 2006; Huang 2006; Janssens 2006; Kang 2006; Karpov 2005; Lunde 2006; Ruan 2005; Schachinger 2006; Wollert 2004; Yao 2006). The first version of this review evaluated the clinical evidence from 13 RCTs, the majority of which had short‐term follow‐up (e.g. less than six months follow‐up) (Martin‐Rendon 2008a; Martin‐Rendon 2008b). Those first‐generation clinical trials were not powered to assess the effect of cell therapies on clinical outcomes such as mortality. The main aim of those trials was to assess the safety of the intervention and the benefit of the treatment, measuring left ventricular ejection fraction (LVEF) as surrogate outcome. We defined safety as the absence of adverse events (e.g. increased mortality and morbidity, increased risk of secondary infarction, restenosis and arrhythmias, development of heart failure) and efficacy as improvement in cardiac function associated with cell therapy.
The second version of this review, Clifford 2012, evaluated 33 RCTs and long‐term follow‐up data had started to emerge (Cao 2009; Grajek 2010; Jin 2008; Meluzin 2008; Penicka 2007; Piepoli 2010; Yao 2009; Zhukova 2009). In that update of the review we included 20 new studies. Unlike other systematic reviews where a total of 50 trials were assessed (Jeevanantham 2012), our systematic review was the first to determine that there was no evidence of a difference in the risk of mortality between treated participants and controls (Clifford 2012).
There is currently a high degree of uncertainty about the beneficial effect of cell therapies as treatment for AMI. Both RCTs (Hirsch 2011; Lunde 2006; Roncalli 2010; Schachinger 2006), and previous systematic reviews and meta‐analyses (Clifford 2012; Delewi 2014; Gyöngyösi 2015; Jeevanantham 2012), have shown divergent results. Additionally, in light of recent studies suggesting that there are inconsistencies in the reporting of clinical trials and that the effect size of the treatment is correlated with the number of discrepancies (Nowbar 2014), it is even more important to review the clinical evidence thoroughly.
We have extracted and analysed data collected from the newly identified and included studies using the same methodology as described in the previous versions of the review (Clifford 2012; Martin‐Rendon 2007; Martin‐Rendon 2008a; Martin‐Rendon 2008b). We have also carried out 'Risk of bias' assessment of the new included studies following the same methods as previously. We have performed a new meta‐analysis that includes all 41 studies. In this version of the systematic review, we have reduced the number or surrogate outcomes analysed to focus on clinical outcomes, LVEF and quality of life outcomes. As it has become clear that cell therapies for AMI are safe and have no major adverse effects, the main questions to address in this systematic review are whether the intervention is efficacious and has a clinical benefit, and whether the findings from this systematic review can inform ongoing or future trials.
Objectives
To determine the safety and efficacy of autologous adult bone marrow stem cells as a treatment for acute myocardial infarction (AMI), focusing on clinical outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Any participants with a clinical diagnosis of AMI with no restriction on age.
Types of interventions
Studies involving the administration of autologous adult bone marrow‐derived cells following successful revascularisation by angioplasty or cardiac surgery.
Participants in the comparator treatment arm of the trial would have had either no intervention or placebo (e.g. medium where the stem cells are suspended, or plasma). Trials where surgery (e.g. coronary artery bypass graft (CABG)) or percutaneous angioplasty (e.g. PCI) have been administered were eligible.
In summary:
any autologous human adult bone marrow stem cells;
any method of stem/progenitor cell isolation or enrichment;
any route of administration;
any co‐intervention (e.g. surgery or angioplasty); and
any single dose or multiple doses of intervention.
Types of outcome measures
Primary outcomes
All‐cause mortality
Cardiovascular mortality
Composite measures of major adverse cardiac events (MACE)
Periprocedural adverse events
Secondary outcomes
Morbidity including reinfarction, incidence of arrhythmias, incidence of restenosis, target vessel revascularisation and re‐hospitalisation for heart failure
Quality of life and performance status (if measured separately from a quality of life measurement)
Left ventricular ejection fraction (LVEF)
We assessed all outcomes at short‐term (less than 12 months) and long‐term (12 months or more) follow‐up.
In this version of the review, we have focused on clinical outcomes. However, the surrogate endpoint of LVEF is a standard, widely reported surrogate for cardiac function and has been retained as a reference point with other trials and systematic reviews in AMI. Surrogate outcomes other than LVEF reported in previous versions of this review, namely engraftment and survival of the infused stem cells, left ventricular end‐systolic volume, left ventricular end‐diastolic volume, wall motion score, stroke volume index and infarct size, are no longer included as outcomes.
Search methods for identification of studies
Electronic searches
We updated the searches, originally run in August 2007 (Appendix 1), in January 2011 (Appendix 2) and then again in March 2015 (Appendix 3). We identified relevant studies from searching the following:
Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 2);
MEDLINE (OvidSP, 1946 to 11 March 2015);
EMBASE (OvidSP, 1974 to 11 March 2015);
CINAHL (EBSCOhost, 1982 to 11 March 2015);
PubMed (for e‐publications only, 11 March 2015);
LILACS (1982 to 11 March 2015);
KoreaMed (1997 to 11 March 2015);
IndMed (1986 to 11 March 2015);
PakMediNet (1995 to 11 March 2015);
Web of Science: Conference Proceedings Citation Index ‐ Science (CPCI‐S) (1990 to 11 March 2015).
Searching other resources
In addition, we carried out the following.
Handsearching of conference abstracts from relevant heart and/or stem cell conferences, e.g. the American Heart Association, International Society of Stem Cell Research (from 2005 to January 2011). Handsearching was not continued post‐January 2011, as these conference abstracts are now included within EMBASE.
Searches of three databases of ongoing trials, all performed on 11 March 2015:
ClinicalTrials.gov (https://clinicaltrials.gov/);
ISRCTN Register (http://www.isrctn.com/);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://apps,who.int/trialsearch/).
Searches of the reference lists of all identified eligible papers and relevant systematic and/or narrative reviews.
We applied no language or date restrictions.
Data collection and analysis
Selection of studies
The information specialist (CD) conducted the electronic search for potentially relevant papers and removed references that were duplicates, clearly irrelevant and/or included in previous search results. Two review authors (SF, EMR for this update) independently screened all titles and abstracts of references identified by the review search strategy for relevancy to the review question. We exclude studies that clearly did not meet the eligibility criteria at this stage. Two review authors (SF, EMR) independently assessed all other studies on the basis of their full text for inclusion/exclusion using the criteria indicated above (type of studies, participants, interventions and outcome measures). We resolved disagreements through discussion.
Data extraction and management
Two review authors (SF, HZ for this update) extracted data onto customised data extraction forms, which we created and piloted specifically for this review, and undertook data extraction for all eligible studies independently. Aside from details relating to the quality of included studies, we extracted the following two groups of data:
Trial characteristics: place of publication, date of publication, population characteristics, setting, detailed nature of intervention, detailed nature of comparator, detailed nature of outcomes. A key purpose of these data was to explain clinical heterogeneity between included studies independently from analysis of the results.
Results of included studies for each of the main outcomes indicated in the review question. For dichotomous outcomes, we recorded the numbers of outcomes in the treatment and control groups. For continuous outcomes, we recorded the mean and standard deviation. Where standard deviations of mean change from baseline values were not explicitly reported, where possible we calculated the standard deviation based on reported confidence intervals or P values as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we used these values in the analysis. In the writing of this version of the review we identified a systematic error in the previous versions of the review in the calculation of standard deviations for mean change from baseline values. This issue has now been corrected; the discrepancies between the correct and previously reported values were small in all cases. In some studies it was not possible to calculate the value of the standard deviation and imputation techniques were deemed unsuitable due to the relatively high proportion of studies with missing standard deviations in some analyses (Higgins 2011). These studies, previously analysed as mean change from baseline values, are now incorporated in combined analyses using the mean endpoint value.
We resolved data extraction disagreements by consensus between the review authors. When disagreements regarding any of the above could not be resolved through discussion, we attempted to contact authors of the original trials to provide further details (see Dealing with missing data below). We then transcribed the data into the systematic review computer software Review Manager 5.3 (Review Manager 2014).
In light of the number of studies included in the previous version of this review that have had additional publications since, we checked all previous data included in the review. This resulted in a number of minor data errors being identified; these are corrected in the current version of the review. These errors made a negligible difference to the previous results and did not affect the conclusions.
Assessment of risk of bias in included studies
Two review authors (SF, HZ for this update), undertaking the data extraction independently, assessed the risk of bias for each trial using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the design, conduct and analysis of the trial using a three‐point scale: low, high or unclear risk of bias. To assess risks of bias, the authors included the following questions in the 'Risk of bias' table for each included trial:
Was the allocation sequence adequately generated?
Was allocation adequately concealed?
Was knowledge of the allocated intervention adequately prevented (i.e. blinded) throughout the trial?
Were incomplete outcome data adequately addressed for every outcome?
Were reports of the trial free of selective outcome reporting?
Was the trial apparently free of other problems that could put it at risk of bias?
For trials included in the previous version of this review, we re‐evaluated the risk of bias in the context of the revised outcomes and updated this accordingly. We resolved disagreements through discussion with a third review author.
A study of trials published in Chinese medical journals that were described as randomised found that a high proportion of these trials did not adhere to accepted methodology for randomisation and hence could not be deemed authentic RCTs (Wu 2009). It is now widely accepted that trials carried out in China may lack appropriate randomisation, therefore we deemed any Chinese studies for which methods of randomisation were not described and could not be clarified with trial authors to have a high risk of selection bias; we evaluated sensitivity to these trials through sensitivity analyses (see Sensitivity analysis section below).
Unit of analysis issues
In the analysis of quality of life outcomes, we converted Minnesota Living with Heart Failure (MLHF) scores to negative values in order to include these in a meta‐analysis with other measures on different scales using the standardised mean difference.
Dealing with missing data
We sought clarification of the extent of possible participant overlap between potentially related studies from nine trial authors by email contact. Eight authors responded and we reached the following conclusions through email correspondence:
Twenty treatment arm participants and 10 control arm participants were included in two trials published separately (Plewka 2009). Due to the extensive participant overlap and the shared protocol design of these two studies, we extracted and combined data as a single trial.
In a large trial of 200 participants (Tendera 2009), 12 patients were also included in a separate trial (Grajek 2010). In view of the small degree of overlap, we have extracted data from these trials separately and included as them independent studies in this review.
A 2014 publication by Ryabov et al was a long‐term follow‐up of an earlier trial already included in an early version of this review (Karpov 2005).
A 2012 conference abstract published by Turan et al described long‐term follow‐up of an earlier trial reported in full (Turan 2012).
The following issues are awaiting resolution:
The extent of possible participant overlap between two conference abstracts (Huang 2007b; Huang 2008), and four separate studies from the same research group (Ge 2006; Huang 2006; Huang 2007; Yao 2006), could not be confirmed as email contact with the authors was unsuccessful. As a result, we have listed both Huang 2007b and Huang 2008 as studies awaiting classification.
We contacted a further four authors of trials published in abstract form only at the time of study selection to establish whether these trials were expected to be published in full. Two of these trials have now been published in full (Hirsch 2011; Roncalli 2010), and we have since excluded one trial (Perez‐Oteyza 2006). No further publications have been identified for the fourth trial (Fernandez‐Pereira 2006); this trial is therefore included in studies awaiting classification. We contacted one trial author to clarify the publication of further follow‐up data (Roncalli 2010).
We made attempts to contact the authors of 20 included studies by email requesting additional information on the trial design and methodology, clarification regarding data discrepancies, further detail about patient demographics and/or additional data (Cao 2009; Colombo 2011; Chen 2004; Huang 2006; Huang 2007; Janssens 2006; Jazi 2012; Jin 2008; Lunde 2006; Nogueira 2009; Piepoli 2010; Ruan 2005; Schachinger 2006; Sürder 2013; Tendera 2009; Turan 2012; Wang 2014; Wohrle 2010; Xiao 2012; Yao 2006). Authors of five trials kindly responded as follows; key data provided by authors included the following:
Lunde 2006: mean change from baseline echocardiography, MRI and SPECT data were confirmed.
Piepoli 2010: the number of participants included in the analyses and details of withdrawals and exclusions were clarified; mean and standard deviation values for echocardiography data were provided.
Schachinger 2006: surrogate endpoint data from MRI at 24‐month follow‐up were provided.
Tendera 2009: mean and standard deviation values for MRI data were provided.
Turan 2012: details of the number of withdrawals and exclusions with reasons were provided, together with clarification of patient demographics.
Assessment of reporting biases
Although we believe that we made every effort to identify unpublished studies, we assessed publication bias for the primary outcome of mortality using a funnel plot and with a formal test for publication bias using Egger's test for asymmetry (Egger 1997), implemented with the statistical software programme R v2.14.1 (R Core Team 2013).
Data synthesis
We undertook meta‐analyses using Review Manager 5.3 (Review Manager 2014), using random‐effects models throughout due to the anticipated heterogeneity arising from differences in participant characteristics, interventions and duration of follow‐up. This differs from the previous version of the review in which fixed‐effect models were used for meta‐analyses in the first instance. Although quantitative synthesis was the main method of analysis, we incorporated insights from a qualitative evaluation of studies for an overall interpretation of the data. We based conclusions on patterns of results identified across clearly tabulated results of included studies as well as summary measures, taking both direction and magnitude of any mean effect sizes from random‐effects models into account. We included all studies in the main analyses irrespective of risk of bias; we performed sensitivity analyses for risk of selection, performance and attrition bias as described in the Sensitivity analysis subsection below. We summarised periprocedural adverse events for each trial in tabular form and evaluated them descriptively.
Within each included trial, all participants were analysed in the treatment groups to which they had been randomised. We have undertaken an available case analysis, including all participants who were randomised to treatment and were included in the analysis, irrespective of whether or not they received their randomised treatment.
We carried out separate analyses according to the duration of follow‐up after treatment: short‐term (less than 12 months) and long‐term (12 months or more). We expressed dichotomous data for each arm in a particular trial as a proportion or risk and the treatment effect as a risk ratio (RR) with 95% confidence intervals (CIs). We expressed continuous data for each arm in a particular trial as a mean and standard deviation, and the mean treatment effect as the mean difference (MD) if outcomes were measured in the same way across trials. For outcomes measured using different scales (physical capacity and quality of life measures), we combined the treatment effect data and analysed them using the standardised mean difference (SMD).
Although we intended to analyse continuous outcomes as mean change from baseline, several studies only reported baseline and endpoint data. Where possible, we calculated the standard deviation of the mean change from baseline based on reported confidence intervals or P values as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we used these values in the analysis. However, for several studies, insufficient information was reported to calculate the standard deviation. The mean difference based on the change from baseline can be assumed to address the same underlying intervention effects as an analysis based on final measures (i.e. the differences in mean final values will on average be the same as the differences in mean change scores). Therefore we combined studies reporting mean change from baseline values with those reporting endpoint values (using preferentially mean change values where both were reported), but presented mean change and endpoint values separately as well as in combined analyses for clarity, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not conduct this pooling of studies by method of reporting of continuous measures for analyses of quality of life or physical capacity, since the assumption of consistent underlying effects does not hold for standardised mean differences.
Six trials reported multiple intervention groups. In order to avoid double‐counting of controls, in the main analyses we pooled data from active intervention arms across different doses (high dose/low dose (Meluzin 2008) or high/medium/low dose (Quyyumi 2011)), delivery routes (arterial or venous) (Nogueira 2009), timing of cell delivery (early or late) (Sürder 2013), type of cells (selected or unselected (Tendera 2009)) or number of cell doses (Yao 2009).
We produced a 'Summary of findings' table for the primary outcomes of all‐cause mortality, cardiovascular mortality and the composite measure of major adverse clinical cardiac events at both short‐term and long‐term follow‐up, using the GRADEpro GDT software (GRADEpro GDT 2014). We calculated risk ratios excluding trials with a high risk of randomisation sequence selection bias, assuming an underlying control risk from the observed data from included trials.
Trial sequential analysis
Cumulative meta‐analyses may result in type I errors due to an increased risk of random error arising from repeated testing of accumulating data (Borm 2009; Hu 2007; Lan 2003). Trial sequential analysis provides a method of adjusting the thresholds for statistical significance while maintaining the overall desired type I error rate (Wettersley 2008). These adjusted thresholds are known as trial sequential monitoring boundaries (TSMBs). If the cumulative Z‐curve crosses the TSMB, then statistical significance has been reached whilst maintaining the overall type I error rate. Futility boundaries may also be produced such that if the cumulative Z‐curve crosses the futility threshold, there is evidence that the two treatments do not differ more than the anticipated effect size. Trial sequential analysis also provides a required information size, the meta‐analysis information size needed to detect a statistically significant effect given a defined underlying model. We applied trial sequential analysis to the primary outcomes of all‐cause mortality, cardiovascular mortality and composite MACE, assuming a long‐term mortality incidence rate of 6.1% in the control group (as observed in our control data); we estimated control group incidence rates for cardiovascular mortality and composite MACE from the observed control data similarly. For each outcome we calculated the information size required for a relative risk reduction of 35% (equivalent to the reduce risk of mortality associated with PCI (Hartwell 2005). Using the TSA program (TSA 2011), we calculated two‐sided TSMBs using the O'Brien‐Fleming β‐spending function for an overall 5% type I error rate and 80% power. We made a model variance based heterogeneity correction to incorporate the minimal heterogeneity observed for the outcomes of cardiovascular mortality and composite major adverse clinical events. We made no adjustment for heterogeneity for the outcome of mortality, consistent with the lack of heterogeneity observed in the meta‐analysis. We produced no futility boundaries as the information fraction was too small to produce an inner wedge futility area from the trial sequential analysis program. We included studies that had reported outcomes at more than one long‐term follow‐up time point in the trial sequential analysis according to the time at which they first reported long‐term follow‐up (and hence were included in meta‐analyses).
Subgroup analysis and investigation of heterogeneity
A range of different methods were used to measure LVEF across studies (magnetic resonance imaging (MRI), left ventricular angiography (LVA), single photon emission computed tomography (SPECT), echocardiography and radionuclide ventriculography (RNV)), with several studies reporting LVEF as an outcome using more than one method of measurement. The limitations of some of these methods are well known (Arnesen 2007). Consistent with the previous version of this review, we subgrouped analyses of LVEF according to the measurement method used.
We grouped trials according to baseline cardiac function (defined by mean baseline LVEF < 45% or ≥ 45%), mean cell dose (≤ 108, > 108 and ≤ 109, > 109), timing of stem cell administration (within 10 days or more than 10 days after AMI) and use of heparinised cell solution. Planned subgroup analysis of the type/route of cell delivery was not possible as all but one trial, Nogueira 2009, administered cells into the coronary artery.
We performed a priori subgroup analyses for the primary outcome of mortality. For other outcomes with substantial observed heterogeneity (I2 ≥ 50%) (Higgins 2003), and a minimum of two studies in each subgroup, we investigated potential sources of heterogeneity by performing the subgroup analyses described above as exploratory analyses, and by visual inspection of forest plots with consideration of individual trial characteristics.
For trials with multiple active intervention arms, in subgroup analyses where the intervention arms were stratified across the subgrouping strata, we used the single control group as the comparator in each subgroup.
Sensitivity analysis
We assessed the robustness of results for the primary outcomes of all‐cause mortality, cardiovascular mortality and composite measures of MACE for sensitivity to risk of selection bias (excluding studies with a high risk of bias from random sequence generation) and attrition bias (excluding studies with a high or unclear risk of attrition bias). We also assessed the primary clinical outcomes for sensitivity to risk of performance bias (excluding those studies with a known lack of blinding of participants and clinicians).
We also assessed the primary outcome of mortality and any additional outcomes that showed evidence of a difference between trial arms for sensitivity to differences in the route of cell delivery, by excluding one trial that administered cells into the coronary artery (Nogueira 2009). This trial did not report the primary outcomes of cardiovascular mortality and composite measures of MACE.
Differences in methods of reporting for continuous outcomes across trials led us to combine mean change from baseline and endpoint data for LVEF (see Data synthesis above). We have presented the results separately as well as in combination for clarity and to assess the sensitivity of the results to the method of reporting.
Results
Description of studies
Given that a wide variety of products and terms have been used in the comparator arms of the included trials, for ease of reference we will use the term 'control' throughout this review to refer to the comparator treatment arm.
We identified a total of 6434 records (6293 references and 141 ongoing trial records) from electronic searches of the CENTRAL, MEDLINE, EMBASE, SRI Transfusion Evidence Library, ClinicalTrials.gov, CDSR, DARE, CINAHL and Current Controlled Trials databases to March 2015. Additionally, handsearching of the American Heart Association Scientific Sessions, European Society of Cardiology Congress and World Congress of Cardiology annual conference proceedings from 2005 to January 2011 identified an additional 96 references, and we identified four further references from reference lists of reviews identified in the database search to give a total of 6534 citations. De‐duplication and removal of all previously screened references by the SRI Information Specialist (CD) excluded 1753 references. Screening of the remaining 4781 records (4640 references and 141 ongoing trial records) by two review authors independently resulted in exclusion of 4465 records (4370 references and 95 ongoing trials), which were clearly irrelevant. Detailed assessment of the remaining 270 references and 46 ongoing trial records identified a total of 170 references (93 full papers and 77 abstracts) and 18 ongoing trial records, which described a total of 41 trials included in this review (see PRISMA study flow diagram in Figure 1).
1.
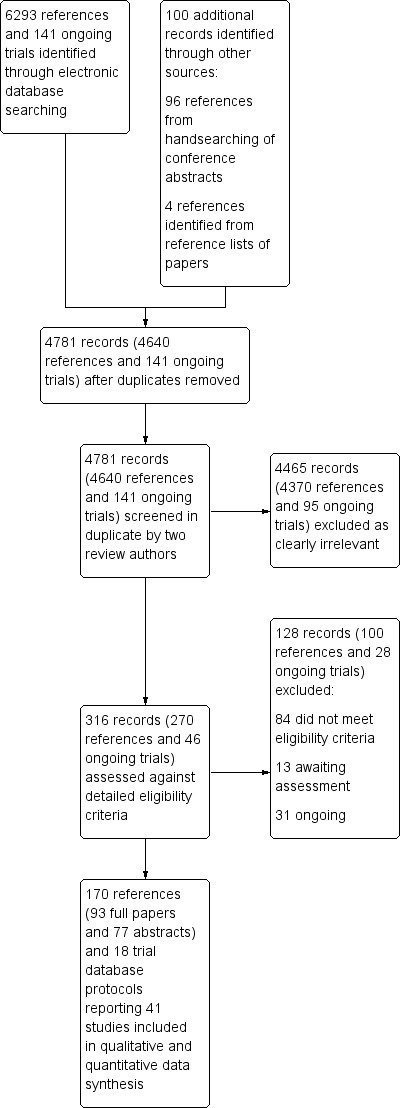
Study flow diagram.
Trials excluded from the review
We excluded 53 trials (described in 77 references and seven ongoing trial records) from the review following full‐text eligibility assessment. In summary, the reasons for exclusion were as follows: six studies were not classified as AMI, 12 studies did not include a control arm, seven studies were non‐randomised controlled trials, five studies infused G‐CSF mobilised cells but did not administer G‐CSF to the control arm, three studies mobilised cells by G‐CSF but did not administer cells, five studies did not use autologous bone marrow stem cells, two studies were systematic reviews or meta‐analyses, seven studies were commentaries or summaries, two studies were experimental, in two studies the outcomes were not relevant, one trial treated patients with acute myocardial infarction and 'old' myocardial infarction and the data were combined, and one trial had no relevant outcomes (see Characteristics of excluded studies).
Trials awaiting assessment and ongoing trials
Twelve trials described in 13 references appeared to meet the eligibility criteria for this review but reported insufficient information for the trials to be included (see Characteristics of studies awaiting classification). We await further publications on these trials. We identified 22 eligible ongoing trials described in 10 references and 21 ongoing trial database records (see Characteristics of ongoing studies). Current ongoing trials intend to recruit over 4750 participants in total and include the pan‐European Phase III trial (the BAMI trial) (NCT01569178), which is aiming to recruit 3000 participants and is expected to be completed by May 2018. These ongoing trials will be included in future updates of the review.
Trials included in the review
We translated six trials from Chinese (Mandarin) to English (Huang 2006; Huang 2007; Jin 2008; Yao 2006; You 2008; Xiao 2012), and two from Russian to English (Karpov 2005; Zhukova 2009), prior to inclusion in this review, including one report of long‐term follow‐up, which we translated using Google Translate (https://translate.google.com/) for this update. An English version of a seventh Chinese paper was identified (Ruan 2005). Following careful cross‐checking between the Chinese and English versions of the paper, which confirmed that both papers reported the same data from one trial, we used the English version of the paper within this review.
One trial included in the previous version of the review was previously referred to as Meyer 2006. This study is now referred to as Wollert 2004 in accordance with the first publication that reported results from this trial. Three trials included in the previous version of the review are now not included: two trials that used G‐CSF to mobilise stem cells in the cell therapy arm did not give G‐CSF to the control group and in view of the lack of this co‐intervention in the control arm, these studies are now excluded (Kang 2006; Li 2006), and one trial published in abstract form only has been reclassified as awaiting classification as there were insufficient data provided for inclusion in any analyses (Fernandez‐Pereira 2006).
Five trials had three‐arm comparisons (Meluzin 2008; Nogueira 2009; Sürder 2013; Tendera 2009; Yao 2009), and one trial had a four‐arm comparison (Quyyumi 2011). In Meluzin 2008, the two treatment arms compared different doses (low dose or high dose) of stem/progenitor cells administered. Likewise, in Quyyumi 2011, the three treatment arms compared low, moderate and high‐dose administrations of selected CD34+ cells. The two treatment arms in Yao 2009 compared a single dose (SD arm) of stem/progenitor cells at three to seven days post‐AMI to a repeated dose (DD arm) ‐ i.e. administration of stem/progenitor cells at both three to seven days and three months post‐AMI. The two treatment arms in Nogueira 2009 compared intracoronary artery (arterial group – AG) delivery of stem/progenitor cells against intracoronary venous (venous group – VG) delivery of stem/progenitor cells. In Tendera 2009, the two treatment arms compared selected CD34+ CXCR4+ (selected –S) stem/progenitor cell administration versus non‐selected (unselected – U) mononuclear cell administration. Sürder 2013 included two intervention groups comparing either five to seven days (early ‐ E) or three to four weeks (late ‐ L) cell administration. As stated in the Methods section, we pooled active intervention arms for the main analyses and compared this with the single control group.
We included a total of 41 trials; the number of participants included in each trial ranged from 11 to 204, and a total of 2732 participants (1564 cell therapy and 1168 controls) were included in the 41 comparisons of the review. The mean age of participants across all included trials ranged from 46.6 years (Jazi 2012) to 65.2 years (Piepoli 2010), with the mean age of participants between 50 and 60 years in all but seven trials (Table 2). All trials included predominantly male participants, with the per cent male ranging from 60.6% (Wang 2014) to 100% (Colombo 2011; Zhukova 2009); four trials reported female participants in one arm of the trial only (Gao 2013; Ge 2006; Penicka 2007;Ruan 2005) (Table 2). Ethnicity data were not available.
1. Characteristics of study participants.
| Study ID | Country of study | Patient population | Mean (SD) age of participants (years) | % Male | No. randomised participants receiving intervention | No. randomised participants receiving comparator | Mean duration of follow‐up |
| Angeli 2012 | Brazil | STEMI with LVEF < 45%; successful PCI | n/r | n/r | 11 | 11 | 12 months |
| Cao 2009 | China | STEMI; PCI within 12 hours, often with drug‐eluting stent implantation | BMMNC: 50.7 (SEM 1.1) Control: 51.1 (SEM 1.0) | BMMNC: 95.1% Control: 93.3% | 41 | 45 | 48 months |
| Chen 2004 | China | AMI; PCI within 12 hours, mostly with stent implantation | BMMNC: 58 (7.0) Control: 57 (5.0) | BMMNC: 94% Control: 97% | 34 | 35 | 6 months |
| Colombo 2011 | Italy | Large anterior STEMI; PCI with bare metal stent implantation within 12 hours | CD133+: median 54 (range 47 to 60) Control: median 56 (range 44 to 58) | CD133+: 100% Control: 100% | 5 | 5 | 12 months |
| Gao 2013 | China | Acute STEMI; PCI with stent implantation within 12 hours | BM‐MSC: 55.0 (SEM 1.6) Control: 58.6 (SEM 2.5) | BM‐MSC: 100% Control: 86.4% | 21 | 22 | 24 months |
| Ge 2006 | China | First STEMI within 24 hours; PCI with stent implantation | BMMNC: 58 (11) Control: 59 (8) | BMMNC: 80% Control: 100% | 10 | 10 | 6 months |
| Grajek 2010 | Poland | First anterior AMI; PCI within 12 hours with bare metal stent implantation | BMMNC: 49.9 (8.4) Control: 50.9 (9.3) | BMMNC: 87% Control: 86% | 31 | 14 | 12 months |
| Hirsch 2011 (HEBE) | The Netherlands | First STEMI; PCI with stent implantation within 12 hours | BMMNC: 56 (9) Control: 55 (10) | BMMNC: 84% Control: 86% | 69 | 65 | 60 months |
| Huang 2006 | China | AMI; PCI within 24 hours | BMMNC: 57.3 (10.1) Control: 56.7 (9.2) | BMMNC: 65% Control: 70% | 20 | 20 | 6 months |
| Huang 2007 | China | AMI; PCI within 24 hours with bare metal (35%) or drug‐eluting (65%) stent implantation | BMMNC: 54.8 (5.8) Control: 55.4 (7.1) | BMMNC: 85% Control: 90% | 20 | 20 | 6 months |
| Huikuri 2008 (FINCELL) | Finland | STEMI; thrombolytic drugs initiated within 12 hours | BMMNC: 60 (10) Control: 59 (10) | BMMNC: 90% Control: 85% | 40 | 40 | 6 months |
| Janssens 2006 | Belgium | STEMI; PCI with bare metal stent implantation at median 3.7 hours (IQR 2.5 to 7.6) | BMMNC: 55.8 (11) Control: 57.9 (10) | BMMNC: 82% Control: 82% | 33 | 34 | 4 months |
| Jazi 2012 | Iran | Anterior MI within 1 month with a history of anterior MI and LVEF < 35%; PCI | BMMNC: 48.0 (SEM 2.5) Control: 45.2 (SEM 3.2) | BMMNC: 66% Control: 90% | n/r | n/r | 6 months |
| Jin 2008 | China | AMI; thrombolytic drugs and PCI | BMMNC: 62.3 (7.7) Control: 60.6 (6.5) | BMMNC: 71.4% Control: 75.0% | 14 | 12 | 12 months |
| Karpov 2005 | Russia | STEMI; PCI with bare metal stent implantation within 6.6 (4.9) hours and thrombolytic drugs | BMMNC: 55.2 (8.6) Control: 52.1 (3.2) | BMMNC: 90% Control: 73% | 28 | 34 | 8.2 (0.72) years |
| Lee 2014 (SEED‐MSC) | South Korea | STEMI within 24 hours enrolled < 72 hours after revascularisation by PCI and/or thrombolytic drugs | BM‐MSC: 53.9 (10.5) Control: 54.2 (7.7) | BM‐MSC: 90.0% Control: 89.3% | 40 | 40 | 6 months |
| Lunde 2006 (ASTAMI) | Norway | Anterior STEMI; PCI within 2 to 24 hours | BMMNC: 58.1 (8.5) Control: 56.7 (9.6) | BMMNC: 84% Control: 84% | 50 | 51 | 36 months |
| Meluzin 2008 | Czech Republic | First STEMI; PCI with stent implantation within 12 hours or 3 days | BMMNC: 54 (SEM 2) Control: 55 (SEM 2) | BMMNC: 90% (HD), 95% (LD) Control: 90% | n/r (a) | n/r (a) | 12 months |
| Nogueira 2009 (EMRTCC) | Brazil | STEMI; thrombolytic drugs and PCI with stent implantation within 24 hours | BMMNC: 59.7 (14.3) (AG), 53.6 (8.3) (VG) Control: 57.2 (10.8) (AG), 57.2 (10.8) (VG) | BMMNC: 71% (AG), 70% (VG) Control: 67% | 24 (14 AG, 10 VG) | 6 | 6 months |
| Penicka 2007 | Czech Republic | First anterior STEMI and LVEF ≤ 50% | BMMNC: 61 (14) Control: 54 (10) | BMMNC: 71% Control: 100% | 17 | 10 | 24 months |
| Piepoli 2010 (CARDIAC) | Italy | Anterior STEMI; PCI with stent implantation within 2 to 6 hours | BMMNC: 63.1 (SEM 2.7) Control: 67.2 (SEM 2.4) | BMMNC: 68.4% Control: 68.4% | 19 | 19 | 24 months |
| Plewka 2009 | Poland | First anterior STEMI and LVEF < 40%; PCI within 12 hours | BMMNC: 59 (9) Control: 56 (8) | BMMNC: 68% Control: 78% | 40 | 20 | 24 months |
| Quyyumi 2011 (ARM‐1) | USA | Acute STEMI and LVEF ≤ 50% | CD34+: median 50.5 (IQR 45 ‐ 53) (HD), 63.0 (IQR 57 ‐ 66) (MD), 52.0 (IQR 51 ‐ 52) (LD) Control: median 52.0 (IQR 47 ‐ 57) | CD34+: 100% (HD), 80% (MD), 80% (LD) Control: 87% | 16 (5 LD, 5 MD, 6 HD) | 15 | 12 months |
| Roncalli 2010 (BONAMI) | France | Acute STEMI and LVEF ≤ 45%; PCI with bare metal stent implantation within 24 hours | BMMNC: 56 (12) Control: 55 (11) | BMMNC: 80.8% Control: 89.8% | 52 | 49 | 12 months |
| Ruan 2005 | China | AMI admitted within mean 12.1 (12.6) hours of onset; PCI | BMMNC: 61 (8) Control: 58 (6) | BMMNC: 88.9 Control: 100% | 9 | 11 | 6 months |
| Schachinger 2006 (REPAIR‐AMI) | Germany; Switzerland | Acute STEMI and visual estimated LVEF ≤ 45%; PCI with stent implantation at mean 7.5 (8.0) hours | BMMNC: 55 (11) Control: 57 (11) | BMMNC: 82% Control: 82% | 101 | 103 | 60 months |
| Suarez de Lezo 2007 | Spain | Anterior STEMI within 12 hours; PCI (some with stent) or thrombolytics | BMMNC: 52 (12) Control: 55 (11) | BMMNC: 80% Control: 70% | 10 | 10 | 3 months |
| Sürder 2013 (SWISS‐AMI) | Switzerland | Large STEMI with LVEF < 45%; thrombolytics and PCI with stent within 24 hours | BMMNC: median 55 (IQR 15) (E), 62 (IQR 15) (L) Control: median 56 (IQR 14.5) | BMMNC: 86.2% (E), 82.5 (L) Control: 83.6% | 133 (66 E, 67 L) | 67 | 12 months |
| Tendera 2009 (REGENT) | Poland | Anterior AMI and LVEF ≤ 40% | CD34/CXCR4+: median 58 BMMNC: median 55 Control: median 59 |
CD34/CXCR4+: 63.7% BMMNC: 70.6% Control: 75.0% |
160 (80 CD34/CXCR4+, 80 BMMNC) | 40 | 6 months |
| Traverse 2010 | USA | First anterior STEMI; PCI mostly with drug‐eluting stent implantation | BMMNC: median 52.5 (IQR 43 ‐ 64) Control: median 57.5 (IQR 54 ‐ 59) | BMMNC: 83.3% Control: 60.0% | 30 | 10 | 15 months |
| Traverse 2011 (LATE‐TIME) | USA | STEMI with LVEF ≤ 45%; PCI with stent, mostly drug‐eluting, at median 3.4 (IQR 2.3 to 14.3) hours | BMMNC: 57.6 (11) Control: 54.6 (11) | BMMNC: 79% Control: 90% | 59 | 29 | 6 months |
| Traverse 2012 (TIME) | USA | Anterior STEMI with LVEF < 45%; PCI with stent, mostly drug‐eluting | BMMNC: 55.6 (10.8) (day 3)/58.2 (11.3) day 7) Control: 57.0 (12.4) (day 3)/57.0 (8.0) (day 7) | BMMNC: 88.4% (day 3)/86.1% (day 7) Control: 87.5% (day 3)/88.3% (day 7) | 43 (day 3) 36 (day 7) | 24 (day 3) 17 (day 7) | 12 months |
| Turan 2012 | Germany | Acute STEMI; PCI with stent implantation | BMMNC: 61 (15) Control: 60 (11) | BMMNC: 67% Control: 70% | 42 | 20 | 12 months |
| Wang 2014 | China | Acute STEMI; PCI predominantly with stent implantation within 8 hours | BM‐MSC: 58 (10.2) Control: 56.1 (9.8) | BM‐MSC: 67.9% Control: 53.3% | 30 | 30 | 6 months |
| Wohrle 2010 (SCAMI) | Germany | AMI; PCI with stent, some drug eluting, within 6 to 48 hours | BMMNC: 61.0 (8.1) Control: 61.1 (9.3) | BMMNC: 90% Control: 62% | 29 | 13 | 36 months |
| Wollert 2004 (BOOST) | Germany | STEMI within 5 days; PCI with bare metal stent implantation, some with thrombolytic drugs | BMMNC: 53.4 (14.8) Control: 59.2 (13.5) | BMMNC: 67% Control: 73% | 33 | 32 | 60 months |
| Xiao 2012 | China | AMI; undergoing elective PCI within 4 weeks of AMI | BM‐MSC: 60.4 (8.9) Control: 58.6 (10.0) | BM‐MSC: 58.8% Control: 61.9% | 17 | 21 | 3 months |
| Yao 2006 | China | STEMI within 1 week; PCI | BMMNC: 58.3 (9.5) Control: 58.1 (9.0) | BMMNC: 89.1% Control: 88.0% | 92 | 92 | 30 months |
| Yao 2009 | China | First anterior STEMI; PCI within 12 hours | BMMNC: 52.1 (6.3) (SD), 51.3 (7.4) (DD) Control: 52.7 (7.8) | BMMNC: 83.3& (SD), 80.0% (DD) Control: 91.7% | 30 (15 SD, 15 DD) | 15 | 12 months |
| You 2008 | China | AMI within 24 hours; thombolytic reperfusion | BM‐MSC: 60.5 Control: 62.5 | BM‐MSC: 71.4% Control: 56.3% | 7 | 16 | 8 weeks |
| Zhukova 2009 | Russia | MI of the front wall; thrombolytic drugs and/or PCI with stent implantation | BMMNC: 48 (7) Control: 50 (10) | BMMNC: 100% Control: 100% | 8 | 3 | 36 months |
STEMI, ST‐segment elevation myocardial infarction; AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection fraction; BMMNC, bone marrow mononuclear cells; BM‐MSC, bone marrow mesenchymal stem cells; SEM, standard error of the mean; SD, standard deviation; LD, low dose; MD, moderate dose; HD, high dose; AG, arterial group; VG, venous group; E, early cells; L, late cells; S, selected cells; U, unselected cells; SD, single dose; DD, double dose
(a)Meluzin 2008: 73 participants were randomised in total ‐ the number randomised to each group was not reported.
The trials included in the review were conducted in 17 countries, which included Belgium (Janssens 2006), Brazil (Angeli 2012; Nogueira 2009), China (Cao 2009; Chen 2004; Gao 2013; Ge 2006; Huang 2006; Huang 2007; Jin 2008; Ruan 2005; Wang 2014; Xiao 2012; Yao 2006; You 2008), Czech Republic (Meluzin 2008; Penicka 2007), Finland (Huikuri 2008), France (Roncalli 2010), Germany (Turan 2012; Wohrle 2010; Wollert 2004), Iran (Jazi 2012), Italy (Colombo 2011; Piepoli 2010; Yao 2009), the Netherlands (Hirsch 2011), Norway (Lunde 2006), Poland (Grajek 2010; Plewka 2009; Tendera 2009), Russia (Karpov 2005; Zhukova 2009), South Korea (Lee 2014), Spain (Suarez de Lezo 2007), Switzerland (Sürder 2013), and the USA (Quyyumi 2011; Traverse 2010; Traverse 2011; Traverse 2012), and one trial was carried out in Germany and Switzerland (Schachinger 2006).
Twenty‐three trials compared the active intervention (autologous bone marrow stem/progenitor cells) with no intervention and 18 trials compared the active intervention with placebo (Table 3). The majority of trials used PCI as the primary treatment for AMI. Thrombolytic therapy without PCI was used as the primary treatment in all patients in two trials (Huikuri 2008; You 2008), and some patients in two trials (Lee 2014; Zhukova 2009). Five trials used PCI in combination with thrombolytic therapy either in all patients (Jin 2008; Karpov 2005; Nogueira 2009; Sürder 2013), or in some patients (Wollert 2004) (Table 2). All trials maintained the patients with a standard set of drugs, including aspirin, clopidogrel, heparin, β‐blockers, statins, angiotensin converting enzyme (ACE) inhibitors, nitrates and/or diuretics.
2. Characteristics of study interventions.
| Study ID | Time of cell administration | Intervention given by: | Route of cell administration | Intervention cell type | How are cells obtained? (*) | What were they re‐suspended in? | Dose administered? | Comparator arm (placebo or control) |
| Angeli 2012 | 5 to 9 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | n/r | n/r | 260 (160) million cells | Placebo (n/r) |
| Cao 2009 | 7 days after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 500 million cells | Placebo (heparinised saline) |
| Chen 2004 | Mean 18.4 (0.5) days after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 48,000 (60,000) million cells | Placebo (heparinised saline) |
| Colombo 2011 | Day 9 to 16 after PCI | Cardiologist | Infusion into IRCA | CD133‐positive cells | BM aspiration (**), immunomagnetic selection to isolate CD133‐positive cells | 0.9% saline solution and 10% human serum albumin | Median (range): 5.9 (4.9 to 13.5) million cells | No additional therapy (Control) |
| Gao 2013 | Mean 17.1 (0.6) hours after PCI | Cardiologist | Infusion into IRCA | BM‐MSC | BM aspiration (**), culture for 14 days to select MSC | Heparinised saline | 3.08 (0.52) million cells | No additional therapy (Control) |
| Ge 2006 | Within 15 hours of AMI | Cardiologist | Infusion into IRCA | BMMNC | n/r | n/r | 40 million cells | Placebo (n/r) |
| Grajek 2010 | 5 to 6 days after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | X‐vivo 15 medium and 2% autologous plasma | 410 (180) million cells | No additional therapy (Control) |
| Hirsch 2011 (HEBE) | 3 to 8 days after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline and 4 % human serum albumin | 296 (164) million cells | No additional therapy (Control) |
| Huang 2006 | Within 2 hours of PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 180 (420) million cells | Placebo (heparinised saline) |
| Huang 2007 | Within 2 hours of PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 120 (650) million cells | Placebo (heparinised saline) |
| Huikuri 2008 (FINCELL) | Mean 70 (36) hours after thombolysis | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline and 50% autologous serum | 402 (196) million cells | Placebo (heparinised saline and 50% autologous serum) |
| Janssens 2006 | Within 20 hours of PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline and 5% autologous serum solution | 172 (72) million cells | Placebo (heparinised saline and 5% autologous serum) |
| Jazi 2012 | Within 1 month of AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | M199 medium containing VEGF, bFGF, IGF‐1 and 10% human serum | 2460 (SEM 840) million cells | No additional therapy (Control) |
| Jin 2008 | At least 7 to 10 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 62.7 (17.5) million cells | No additional therapy (Control) |
| Karpov 2005 | 7 to 21 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | n/r | 88.5 (49.2) million cells | No additional therapy (Control) |
| Lee 2014 (SEED‐MSC) | 25 (2.4) days after BM aspiration at 3.8 (1.5) days after admission | Cardiologist | Infusion into IRCA | BM‐MSC | BM aspiration (**), culture for 2 to 3 weeks to isolate MSC | n/r | 72 (9) million cells | No additional therapy (Control) |
| Lunde 2006 (ASTAMI) | 4 to 8 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised plasma | Median (interquartile range): 68 (54 to 130) million cells | No additional therapy (Control) |
| Meluzin 2008 | 5 to 9 days (mean 7 (0.3) days) after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | n/r | LD: 10 million cells (range: 9 to 20 million) HD: 100 million cells (90 to 200 million cells) |
No additional therapy (Control) |
| Nogueira 2009 (EMRTCC) | AG: 3 to 6 days (mean 5.5 (1.28) days) after PCI VG: 3 to 6 days (mean 6.1 (1.37) days) after PCI |
Cardiologist | Infusion into IRCA (AG) or IRCV (VG) | BMMNC | BM aspiration (**) | Saline solution and 5% human serum albumin | 100 million cells | No additional therapy (Control) |
| Penicka 2007 | 4 to 11 days (median 9 days) after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | n/r | 2,640 million cells | No additional therapy (Control) |
| Piepoli 2010 (CARDIAC) | 4 to 7 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Phosphate buffered saline ‐ EDTA and 5% human serum albumin | 249 million cells | No additional therapy (Control) |
| Plewka 2009 | 3 to 11 days (mean 7 (2) days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 144 (49) million cells | No additional therapy (Control) |
| Quyyumi 2011 (ARM‐1) | LD: median 191.4 (IQR 167 to 201) hours, MD: 210.0 (IQR 194 to 210) hours, HD: 207.3 (IQR 191 to 215) hours after AMI |
Cardiologist | Infusion into IRCA | CD34‐positive cells | BM aspiration (**), immunomagnetic selection to isolate CD34‐positive cells | Heparinised phosphate buffered saline, 40% autologous serum and 1% human serum albumin | LD: 4.8 (0.4) million cells MD: 9.9 (0.7) million cells HD: 14.3 (1.6) million cells |
No additional therapy (Control) |
| Roncalli 2010 (BONAMI) | At 7 to 10 days (mean 9 (SD 1.7)) days | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | 4% human serum albumin solution | 98.3 (8.7) million cells | No additional therapy (Control) |
| Ruan 2005 | Within 2 hours of successful PTCA | Cardiologist | Infusion into IRCA | BMMNC | n/r | Diluted autologous serum | n/r | Placebo (diluted autologous serum) |
| Schachinger 2006 (REPAIR‐AMI) | Within 5 days (mean 4.3 (1.3) days) of PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | X‐VIVO medium and 20% autologous serum | 236 (174) million cells | Placebo (X‐VIVO medium and 20% autologous serum) |
| Suarez de Lezo 2007 | 5 to 12 days (mean 7 (2) days) after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 900 (300) million | Placebo (heparinised saline) |
| Sürder 2013 (SWISS‐AMI) | 5 to 7 days (E) or 3 to 4 weeks (L) after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Serum‐free medium and 20% of autologous serum | E: 159.7 (125.8) million cells L: 139.5 (120.5) million cells |
No additional therapy (Control) |
| Tendera 2009 (REGENT) | Median 7 (IQR 3 to 12) days after PCI | Cardiologist | Infusion into IRCA | Selected cells (S): CD34/CXCR4‐ positive cells Unselected cells (U): BMMNC |
BM aspiration (**). Selected cells: immunomagnetic selection to isolate CD34/CXCR4‐positive cells | Phosphate‐buffered saline | S: 1.9 million cells U: 178 million cells |
No additional therapy (Control) |
| Traverse 2010 | 3 to 10 days (median 4.5 (IQR 4 to 7) days) after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | 0.9% saline solution and 5% human serum albumin | 100 million cells | Placebo (0.9% saline solution and 5% human serum albumin) |
| Traverse 2011 (LATE‐TIME) | 2 to 3 weeks (median 17.5 (IQR 15.5 to 20.0) days) after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | 0.9% saline solution and 5% human serum albumin | 147 (17) million cells | Placebo (0.9% saline solution and 5% human serum albumin) |
| Traverse 2012 (TIME) | 3 days or 7 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | 0.9% saline solution and 5% human serum albumin | 150 million cells | Placebo (0.9% saline solution and 5% human serum albumin) |
| Turan 2012 | 7 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | n/r | n/r | No additional therapy (control) |
| Wang 2014 | 15 (1) days after PCI | Cardiologist | Infusion into IRCA | BM‐MSC | BM aspiration (**) and culture of MSC | Heparinised saline | 100 million cells | Placebo (heparinised saline) |
| Wohrle 2010 (SCAMI) | 5 to 7 days (median 6.1 (IQR 5.5 to 7.3) days) after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | 0.9% saline solution, 2% human serum albumin and 0.1% autologous erythrocytes | 381 (130) million cells | Placebo (0.9% saline solution, 2% human serum albumin and 0.1% autologous erythrocytes) |
| Wollert 2004 (BOOST) | 4.7 (1.3) days after PCI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised saline | 2460 (940) million cells | No additional therapy (Control) |
| Xiao 2012 | Within 4 weeks of AMI | Cardiologist | Infusion into IRCA | BM‐MSC | BM aspiration (**) and culture of MSC | n/r | 460 (160) million cells | Placebo (heparinised saline) |
| Yao 2006 | Within 7 days of AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Lymphocyte isolation medium | 210 (370) million cells | No additional therapy (control) |
| Yao 2009 | SD: 3 to 7 days after PCI DD 3 to 7 days after PCI; second dose at 3 months |
Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Heparinised plasma | SD: 410 million cells DD: 190 (SE 120) million cells |
Placebo (heparinised plasma) |
| You 2008 | At day 14 | Cardiologist | Infusion into IRCA | BM‐MSC | BM aspiration (**), second centrifugation and culture of MSC | n/r | 75 million cells | No additional therapy (control) |
| Zhukova 2009 | 14 to 19 days after AMI | Cardiologist | Infusion into IRCA | BMMNC | BM aspiration (**) | Autologous serum | 50 million cells | No additional therapy (control) |
AMI ‐ acute myocardial infarction, PCI ‐ percutaneous coronary intervention, BM ‐ bone marrow, PTCA ‐ percutaneous transluminal coronary angioplasty, IRCA ‐ infarct‐related coronary artery, IRCV ‐ infarct‐related coronary vein, BMMNC ‐ bone marrow mononuclear cells, BM‐MSC ‐ mesenchymal stem cells; LD ‐ low dose, MD ‐ moderate dose, HD ‐ high dose, AG ‐ arterial group, VG ‐ venous group, E ‐ early cells, L ‐ late cells, S ‐ selected cells, U ‐ unselected cells, SD ‐ single dose, DD ‐ double dose
** BM aspiration‐ bone marrow aspiration and isolation of bone marrow mononuclear cells by gradient centrifugation
All but one trial, Zhukova 2009, reported short‐term follow‐up of less than 12 months with the majority reporting follow‐up after six months; only three trials reported maximum follow‐up of three months or less (Suarez de Lezo 2007; Xiao 2012; You 2008). No trial reported short‐term follow‐up of longer than six months. Twenty‐five trials reported long‐term follow‐up, all but five of which included reporting of outcomes at 12 months. Fourteen trials reported follow‐up of longer than 12 months, including 18 months (Wollert 2004), 24 months (Gao 2013; Hirsch 2011; Penicka 2007; Piepoli 2010; Plewka 2009; Schachinger 2006; Wohrle 2010; Zhukova 2009), 30 months (Yao 2006), 36 months (Lunde 2006; Wohrle 2010; Zhukova 2009), 48 months (Cao 2009), 60 months (Hirsch 2011; Schachinger 2006; Tendera 2009; Wollert 2004; Zhukova 2009), and a mean of 8.2 years (Karpov 2005). Long‐term follow‐up included both clinical outcomes and the surrogate endpoint of LVEF in all but four trials: one trial reported long‐term follow‐up of LVEF only (Janssens 2006), and three trials only reported clinical outcomes at long‐term follow‐up (Karpov 2005; Quyyumi 2011; Tendera 2009). We have analysed outcome data separately in this review; we have incorporated the maximum short‐term or long‐term time point from each trial into the analyses.
Trial design characteristics ‐ interventions
Details of the individual trial interventions are given in the Characteristics of included studies tables and are summarised in Table 3.
Thirty‐eight trials isolated the stem/progenitor cells by bone marrow aspiration and separated the mononuclear cell fraction by gradient centrifugation. Three trials failed to report the method of cell isolation or processing (Angeli 2012; Ge 2006; Ruan 2005).
Thirty‐four trials administered unfractionated bone marrow‐derived mononuclear cells intracoronally via an inflated balloon catheter. This mononuclear cell population contains stem/progenitor cells and other blood cells (Angeli 2012; Cao 2009; Chen 2004; Ge 2006; Grajek 2010; Hirsch 2011; Huang 2006; Huang 2007; Huikuri 2008; Janssens 2006; Jazi 2012; Jin 2008; Karpov 2005; Lunde 2006; Meluzin 2008; Nogueira 2009; Penicka 2007; Piepoli 2010; Plewka 2009; Roncalli 2010; Ruan 2005; Schachinger 2006; Suarez de Lezo 2007; Sürder 2013; Tendera 2009; Traverse 2010; Traverse 2011; Traverse 2012; Turan 2012; Wohrle 2010; Wollert 2004; Yao 2006; Yao 2009; Zhukova 2009). Three trials processed the mononuclear cell fraction using two‐step immunomagnetic selection to isolate and administer a suspension containing a selected CD133+ cell population (Colombo 2011; Quyyumi 2011), or in one intervention arm of a three‐arm trial, CD34+/CXCR4+ cells (Tendera 2009). Five trials cultured cells to isolate mesenchymal stem cells (BM‐MSC) (Gao 2013; Lee 2014; Wang 2014; Xiao 2012; You 2008).
One three‐arm trial also administered unfractionated mononuclear cells intravenously to the coronary vein corresponding to the culprit coronary artery via a multipurpose guiding catheter (Nogueira 2009). Simultaneous total occlusion of the coronary vein was achieved via an inflated balloon catheter in the culprit coronary artery.
Cells were suspended in heparinised saline (Cao 2009; Chen 2004; Gao 2013; Huang 2006; Huang 2007; Jin 2008; Plewka 2009; Suarez de Lezo 2007; Wang 2014; Wollert 2004), heparinised saline with human serum albumin (Hirsch 2011), or autologous serum (Huikuri 2008; Janssens 2006), heparinised plasma (Lunde 2006; Yao 2009), saline solution and human serum albumin (Colombo 2011; Nogueira 2009; Traverse 2010; Traverse 2011; Traverse 2012), with 0.1% autologous erythrocytes (Wohrle 2010), heparinised phosphase buffered saline, autologous serum and human serum albumin (Quyyumi 2011), human serum albumin solution (Roncalli 2010), diluted autologous serum (Ruan 2005; Sürder 2013), autologous serum (Zhukova 2009), X‐vivo medium and autologous serum (Schachinger 2006), or autologous plasma (Grajek 2010), M199 medium (Jazi 2012), phosphate buffered saline (Tendera 2009) with human serum albumin (Piepoli 2010), and lymphocyte isolation medium (Yao 2006).
Nine trials did not report details of the cell suspension (Angeli 2012; Ge 2006; Karpov 2005; Lee 2014; Meluzin 2008; Penicka 2007; Turan 2012; Xiao 2012; You 2008).
Timing of stem cell administration post‐AMI
Nineteen trials delivered cells within seven days of AMI: six trials within the first 24 to 48 hours (Gao 2013; Ge 2006; Huang 2006; Huang 2007; Janssens 2006; Ruan 2005), and 13 trials at up to seven days after AMI (Cao 2009; Grajek 2010; Huikuri 2008; Nogueira 2009; Piepoli 2010; Schachinger 2006; Sürder 2013; Traverse 2012; Turan 2012; Wohrle 2010; Wollert 2004; Yao 2009; You 2008), including two trials with patients randomised to receive cells at either three days or seven days (Traverse 2012), or at five to seven days or three to four weeks (Sürder 2013) after AMI, and one trial in which some patients were randomised to receive a second dose at three months (Yao 2009).
In nine trials cells were administered within seven days in some patients although other patients received cells at up to eight days (Hirsch 2011; Lunde 2006), nine days (Angeli 2012; Meluzin 2008), 10 days (Traverse 2010), 11 days (Penicka 2007; Plewka 2009), and 12 days (Suarez de Lezo 2007; Tendera 2009) after AMI.
Fourteen trials administered cells at more than seven days after AMI (Chen 2004; Colombo 2011; Jazi 2012; Jin 2008; Karpov 2005; Lee 2014; Quyyumi 2011; Roncalli 2010; Sürder 2013; Traverse 2011; Wang 2014; Xiao 2012; You 2008; Zhukova 2009)
Comparator arm
Eighteen trials administered a placebo intervention to the control group (Angeli 2012; Cao 2009; Chen 2004; Ge 2006; Huang 2006; Huang 2007; Huikuri 2008; Janssens 2006; Ruan 2005; Schachinger 2006; Suarez de Lezo 2007; Traverse 2010; Traverse 2011; Traverse 2012; Wang 2014; Wohrle 2010; Xiao 2012; Yao 2009). In two trials the placebo medium was not reported (Angeli 2012; Ge 2006). Of the remaining 16 trials, all but one, Xiao 2012, used the same media used to re‐suspend cells in the corresponding treatment arm to patients in the comparator arm (no cells). Xiao 2012 administered heparinised saline to the control group but did not report the re‐suspension medium used in the cell therapy group.
Twenty‐three trials did not use a placebo intervention (Colombo 2011; Gao 2013; Grajek 2010; Hirsch 2011; Jazi 2012; Jin 2008; Karpov 2005; Lee 2014; Lunde 2006; Meluzin 2008; Nogueira 2009; Penicka 2007; Piepoli 2010; Plewka 2009; Quyyumi 2011; Roncalli 2010; Sürder 2013; Tendera 2009; Turan 2012; Wollert 2004; Yao 2006; You 2008; Zhukova 2009); no other interventions were reported other than optimal medical therapy.
Dose of stem/progenitor cells administered
The dose of cells administered varied considerably between trials; for simplicity we have grouped trials according to the mean dose: 106 cells; 107 cells; 108 cells; 109 cells and 1010 cells.
Three trials administered magnetically selected cells at a dose of 106 CD133+ cells (Colombo 2011), 106 CD34+ CXCR4+ cells (Tendera 2009), and 106 or 107 CD34+ cells (three randomised cell dose groups) (Quyyumi 2011). In five trials that administered mesenchymal stem cells, cells were administered at a dose of 106 (Gao 2013), 107 (Lee 2014; Wang 2014; You 2008), and 108 (Xiao 2012).
Bone marrow mononuclear cells were administered to patients at a dose of up to 107 (Ge 2006; Jin 2008; Karpov 2005; Lunde 2006; Nogueira 2009; Roncalli 2010; Traverse 2010; Zhukova 2009), 108 (Angeli 2012; Cao 2009; Grajek 2010; Hirsch 2011; Huang 2006; Huang 2007; Huikuri 2008; Janssens 2006; Piepoli 2010; Plewka 2009; Schachinger 2006; Suarez de Lezo 2007; Sürder 2013; Tendera 2009; Traverse 2011; Traverse 2012; Wohrle 2010; Yao 2006; Yao 2009), 109 (Jazi 2012; Penicka 2007; Wollert 2004), and 1010 (Chen 2004). One trial compared two doses of BMMNC: 106 or 108 (Meluzin 2008). Only two trials did not give details of the cell dose administered to patients (Ruan 2005; Turan 2012).
Risk of bias in included studies
A description of the risk of bias for individual studies is given in the Characteristics of included studies tables. A summary of the risk of selection bias, performance and detection bias, attrition bias, reporting bias and other potential sources of bias including baseline imbalances between trial arms, publication bias and study funding is given below.
Allocation
Twenty trials provided details as to the generation of the randomisation sequence (Cao 2009; Colombo 2011; Gao 2013; Ge 2006; Grajek 2010; Hirsch 2011; Huikuri 2008; Janssens 2006; Lunde 2006; Nogueira 2009; Piepoli 2010; Roncalli 2010; Schachinger 2006; Sürder 2013; Traverse 2010; Traverse 2011; Traverse 2012; Wollert 2004; Yao 2009; You 2008). These methods included: sequential numbers (Gao 2013; Ge 2006; Wollert 2004), "uneven vs. even numbers" (Piepoli 2010), a randomisation table (You 2008), a randomisation list generated in permuted blocks of 10, stratified according to centre (Lunde 2006), a randomisation list generated in permuted blocks of six (Grajek 2010), a randomisation list generated in permuted blocks of undefined size (Colombo 2011), a randomisation list generated in permuted blocks with variable block sizes (Huikuri 2008), a randomisation list generated according to infarct size (Nogueira 2009), a permuted‐block randomisation list stratified according to centre, diabetes status and time to PCI after the onset of AMI (Roncalli 2010), an interactive web‐based randomisation session using randomly selected block sizes of six or nine, stratified by centre (Traverse 2011), a permuted‐block randomisation list stratified according to site (Hirsch 2011), computer‐generated random lists (Cao 2009; Janssens 2006; Schachinger 2006; Yao 2009; Traverse 2012), and a randomisation algorithm developed by a biostatistician (Traverse 2010). Four trials reported using sealed envelopes (Ge 2006; Nogueira 2009; Sürder 2013; Wollert 2004), and two trials generated randomisation lists at a site external to the trial site (Schachinger 2006; Wollert 2004). We defined 19 trials as having a low risk of selection bias due to random sequence generation; we considered one trial that allocated treatment using even versus uneven numbers to have a high risk of selection bias (Piepoli 2010); we also deemed this trial to have a high risk of selection bias due to insufficient allocation concealment. We also deemed 14 trials to have used an appropriate method of allocation concealment (Cao 2009; Colombo 2011; Ge 2006; Huikuri 2008; Janssens 2006; Lunde 2006; Nogueira 2009; Roncalli 2010; Schachinger 2006; Sürder 2013; Traverse 2010; Traverse 2011; Wollert 2004; Yao 2009). One trial reported that the randomisation scheme was not blinded and we therefore considered it to have a high risk of selection bias due to lack of allocation concealment (Traverse 2012). Allocation concealment was unclear in the remaining four trials (Gao 2013; Grajek 2010; Hirsch 2011; You 2008). We defined the generation of the randomisation sequence as unclear in the 'Risk of bias' tables in 13 trials in which no description was given as to what methods were used to generate the random sequence (Angeli 2012; Jazi 2012; Karpov 2005; Lee 2014; Meluzin 2008; Penicka 2007; Plewka 2009; Quyyumi 2011; Suarez de Lezo 2007; Tendera 2009; Turan 2012; Wohrle 2010; Zhukova 2009). The method of generation of randomisation sequence was also not reported in eight Chinese trials, which we deemed to have a high risk of bias (Chen 2004; Huang 2006; Huang 2007; Jin 2008; Ruan 2005; Wang 2014; Xiao 2012; Yao 2006).
Blinding
In nine trials, the control group underwent bone marrow aspiration and were given a placebo injection. These trials also reported blinding of outcome assessors or described the trial as "double‐blind" and we therefore considered them to have a low risk of performance and detection bias (Chen 2004; Ge 2006; Huikuri 2008; Janssens 2006; Schachinger 2006; Traverse 2010; Traverse 2011; Traverse 2012; Wohrle 2010). In a further eight trials in which a placebo injection was also administered (Angeli 2012; Cao 2009; Huang 2006; Huang 2007; Ruan 2005; Suarez de Lezo 2007; Wang 2014; Xiao 2012), bone marrow aspiration in the control group was either not undertaken (Cao 2009; Suarez de Lezo 2007; Xiao 2012), or was not reported (Angeli 2012; Huang 2006; Huang 2007; Ruan 2005; Wang 2014); in these eight trials the risk of performance bias was unclear. Only four of these trials reported blinding of outcome assessors (Cao 2009; Ruan 2005; Suarez de Lezo 2007; Xiao 2012); blinding of outcome assessors was otherwise not reported (Angeli 2012; Huang 2006; Huang 2007; Wang 2014).
In one other trial, although the control group received a placebo injection, only the active intervention groups underwent bone marrow aspiration (Yao 2009). Furthermore, the active treatment groups were recalled for a second infusion of cells or placebo whereas the control group was not, and we therefore deemed these trials to have a high risk of performance bias.
Participants were not blinded to treatment in 23 trials in which no placebo infusion was administered (Colombo 2011; Gao 2013; Grajek 2010; Hirsch 2011; Jazi 2012; Jin 2008; Karpov 2005; Lee 2014; Lunde 2006; Meluzin 2008; Nogueira 2009; Penicka 2007; Piepoli 2010; Plewka 2009; Quyyumi 2011; Roncalli 2010; Sürder 2013; Tendera 2009; Turan 2012; Wollert 2004; Yao 2006; You 2008; Zhukova 2009), which we considered to have a high risk of performance bias. Outcome assessors were reported to be blinded in all trials except five: one trial stated that study processes were not blinded (Hirsch 2011), and in four trials blinding of outcome assessors was not reported (Jazi 2012; Karpov 2005; Yao 2006; You 2008).
Incomplete outcome data
Eighteen trials had a low risk of attrition bias as either all randomised participants were included in the analysis of all outcome data or all participant withdrawals were due to death or other major clinical adverse events (Angeli 2012; Cao 2009; Chen 2004; Colombo 2011; Ge 2006; Grajek 2010; Huang 2006; Huang 2007; Jin 2008; Nogueira 2009; Penicka 2007; Piepoli 2010; Ruan 2005; Suarez de Lezo 2007; Traverse 2010; Turan 2012; You 2008; Zhukova 2009). We also deemed a further 13 trials to have a low risk of attrition bias as withdrawals were low and balanced between treatment arms (Gao 2013; Hirsch 2011; Huikuri 2008; Janssens 2006; Lunde 2006; Roncalli 2010; Schachinger 2006; Traverse 2011; Traverse 2012; Wang 2014; Wohrle 2010; Wollert 2004; Yao 2009).
In two trials the risk of attrition bias was unclear as the number of participants randomised to each treatment arm was not reported (Jazi 2012; Meluzin 2008). The number of withdrawals was unbalanced in a further three trials (Quyyumi 2011; Xiao 2012; Yao 2006), although reasons for participant withdrawal were reported; these trials were considered to have an unclear risk of bias.
Five trials had a high risk of attrition bias. In three trials the number of withdrawals was high or unbalanced between treatment arms (Lee 2014; Sürder 2013; Tendera 2009), and in two trials there was incomplete participant overlap across multiple trial reports (Karpov 2005; Plewka 2009).
In the analysis of clinical outcomes, 24 trials included all randomised participants and 11 included over 90% of randomised participants. Four trials included between 80% and 90% (Grajek 2010; Meluzin 2008; Sürder 2013; Yao 2009). All four trials explained the reasons for participant withdrawal or exclusion although in one trial these did not fully account for discrepancies in the number of participants included in individual analyses (Sürder 2013). One trial only included 72.5% of randomised participants in the analysis of clinical outcomes (Lee 2014); reasons included protocol violation, loss to follow‐up and the opinion of the investigator. In one trial it was unclear how many participants were randomised to treatment (Jazi 2012).
In the analysis of LVEF, all trials that reported LVEF measured by echocardiography, SPECT, left ventricular angiography or radionuclide ventriculography included over 80% of randomised participants in the analysis of this outcome, with the exception of two trials, which analysed 72.5% (Lee 2014) and 60% (Plewka 2009) of randomised participants. A higher rate of withdrawals was observed in the analysis of LVEF measured by MRI in which five trials analysed less than 80% of randomised participants: 79.2% (Traverse 2012), 67.7% (Quyyumi 2011), 763.6% (Zhukova 2009), 58.5% (Tendera 2009) and 28.9% (Schachinger 2006), although it should be noted that not all participants are willing or able to undergo MRI leading to an expected reduction in the number of patients analysed.
One trial was terminated prematurely after enrolment of the first 27 participants (Penicka 2007). The trial was reported as being terminated early "due to the unexpected occurrence of serious complications in the BMSC group and no incremental functional effects of BMSC as compared with control patients". Fourteen of the 17 participants randomised to the BMSC arm provided scientific outcome data at four and 12‐month follow‐up assessments. All participants in the control arm were included in the final analysis in this trial.
Selective reporting
Out of 41 trials (with 2732 participants) only 18 trials (1567 participants) reported a published protocol (see Characteristics of included studies) and in this sub‐sample there was no evidence of selective reporting. However, given that the majority of trials did not report details of their protocol it is difficult to ascertain whether these trials are at low risk of selective reporting. We considered one trial to have a high risk of reporting bias as the authors failed to report quality of life and cost‐effectiveness despite these outcomes being described in their trial protocol (Nogueira 2009).
We identified no obvious asymmetry from a funnel plot for mortality (using the maximum duration of follow‐up for all trials that reported mortality) (Figure 2). In a regression test for asymmetry (Egger's test) at short‐term follow‐up the model intercept was 0.15 (P value = 0.01), suggesting that larger rather than smaller trials may be associated with a larger treatment effect. At long‐term follow‐up, the test for asymmetry was not significant (P value = 0.06) and there was no evidence of publication bias.
2.
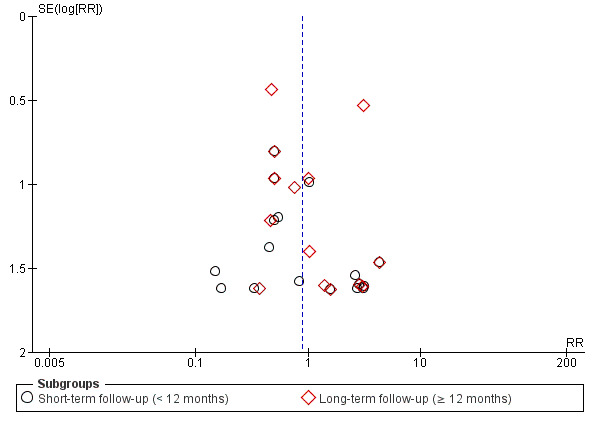
Funnel plot of comparison: 1 Cells compared to no cells, outcome: 1.1 All‐cause mortality.
Other potential sources of bias
Four trials reported statistically significant baseline differences in participant characteristics between trial arms: Sürder 2013 reported a lower percentage of smokers in the late treatment arm than controls (40.3% versus 62.7%; P value = 0.01) and a lower median baseline LVEF (median 35.6% versus 39.6%, P value = 0.03) in the cell therapy group compared with controls; Traverse 2011 reported a higher mean heart rate on initial presentation to the emergency department in the placebo group than the cell therapy group (90.3% versus 77.5%, P value = 0.01); Traverse 2012 observed high peak creatine kinase and troponin levels in the bone marrow cell (BMC) group randomised to day seven and a lack of diabetes in the placebo group randomised to day seven (P values not reported); and in Wohrle 2010 there was a significant baseline imbalance in the proportion of males (62% in the placebo group compared with 90% in the cell therapy group, P value = 0.04). These baseline differences are more likely to be a source of diversity than study bias.
Ten trials did not report the source of funding (Angeli 2012; Chen 2004; Huang 2006; Jazi 2012; Karpov 2005; Ruan 2005; Suarez de Lezo 2007; Wang 2014; Wohrle 2010; Zhukova 2009). Of 31 trials that reported funding and support, all but two trials, Lee 2014 and Schachinger 2006, received research grant funding from universities, charities or governmental agencies (see Characteristics of included studies). Schachinger 2006 received a research grant from Guidant (Guidant Corporation, part of Boston Scientific, which designs and manufactures cardiovascular medical products), as well as support from Eli Lilly (Eli Lilly is a global pharmaceutical company) and Lee 2014 was funded by PCB‐Pharmicell Company Limited, Seongnam, South Korea (a biotechnology company focusing on the development and commercialisation of stem cell therapeutics). Five trials were commercially funded in part: Huikuri 2008 received a research grant from Boston Scientific Sverige AB (a global pharmaceutical company); Grajek 2010 received a research grant from Servier Polska (a global pharmaceutical company); Hirsch 2011 received "unrestricted grants" from Biotronik (Biotronik designs and manufactures cardiovascular medical products), Boston Scientific, Guerbet (Guerbet designs and manufactures medical imaging products including contrast agents), Medtronic (Medtronic designs and manufactures cardiovascular medical products), Novartis, Pfizer and Sanofi‐Aventis (all global pharmaceutical companies); Quyyumi 2011 was funded by Amorcyte Inc (Amorcyte Inc. develops cell therapy products to treat cardiovascular disease); and in Nogueira 2009 cell preparation and characterisation was carried out by Exellion Biomedical Services S/A.
A total of 17 patients from eight trials randomised to cell therapy did not receive treatment as randomised but were included in the analysis (Hirsch 2011; Lunde 2006; Meluzin 2008; Nogueira 2009; Penicka 2007; Roncalli 2010; Traverse 2011; Yao 2009), as well as three patients randomised to a placebo arm who did not receive the placebo medium (Schachinger 2006); in all cases this was due to adverse clinical events, which precluded cell or placebo administration.
Effects of interventions
See: Table 1
An overview of results for the primary outcomes of all‐cause mortality, cardiovascular mortality and composite measures of major adverse cardiac events (MACE) are given in Table 1. A summary of outcome reporting is given in Table 4, together with the number and proportion of randomised participants from all trials included in the analysis of each outcome at short‐term and long‐term follow‐up. The number of events in each trial arm observed at the longest reported follow‐up of clinical (dichotomous) outcomes of all‐cause mortality, cardiovascular mortality, a composite measure of death, reinfarction and re‐hospitalisation for heart failure, reinfarction and target vessel revascularisation is given in Table 5.
3. Summary of outcome reporting.
| Study ID | Primary Outcomes | Secondary Outcomes | ||||||||||||||||||||||
| All‐cause mortality | Cardiovascular mortality | Composite MACE (a) | Reinfarction | Hospital readmission for HF | Target vessel revascularisation | Arrhythmias | Restenosis | NYHA class | Quality of life (QoL) | Exercise tolerance | LVEF (b) | |||||||||||||
| ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | ST | LT | |
| Angeli 2012 | PR* | PR* | PR* | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR |
| Cao 2009 | PR* | FR | NR | NR | NR | NR | PR* | PR* | NR | NR | PR* | FR | NR | NR | PR* | FR | NR | NR | NR | NR | NR | NR | FR | FR |
| Chen 2004 | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR |
| Colombo 2011 | PR* | PR* | NR | PR* | NR | NR | NR | NR | FR | PR | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | PR | FR | FR |
| Gao 2013 | FR | FR | FR | FR | NR | FR | FR | FR | NR | FR | NR | NR | PR* | PR* | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR |
| Ge 2006 | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR |
| Grajek 2010 | NR | FR | NR | NR | NR | NR | FR | NR | NR | NR | FR | NR | NR | NR | FR | NR | NR | NR | NR | NR | FR | FR | FR | FR |
| Hirsch 2011 | PR* | FR | NR | NR | FR | FR | FR | FR | FR | FR | FR | FR | FR | FR | NR | NR | NR | FR | NR | NR | NR | NR | FR | FR |
| Huang 2006 | PR* | NR | NR | NR | NR | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR |
| Huang 2007 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR |
| Huikuri 2008 | FR | NR | FR | NR | NR | NR | FR | NR | FR | NR | NR | NR | PR* | NR | PR | NR | NR | NR | NR | NR | FR | NR | FR | NR |
| Janssens 2006 | FR | NR | PR* | NR | NR | NR | NR | NR | NR | NR | PR* | NR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | FR | FR |
| Jazi 2012 | PR* | NR | PR* | NR | NR | NR | PR* | NR | NR | NR | NR | NR | PR* | NR | PR* | NR | FR | NR | NR | NR | NR | NR | FR | NR |
| Jin 2008 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | FR | FR | NR | NR | FR | FR |
| Karpov 2005 | PR* | FR | PR* | FR | NR | NR | FR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR | FR | NR | FR | NR | FR | NR |
| Lee 2014 | PR* | NR | PR* | NR | NR | NR | FR | NR | NR | NR | PR* | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR |
| Lunde 2006 | NR | FR | NR | NR | NR | NR | FR | FR | FR | FR | NR | FR | NR | FR | FR | NR | FR | NR | FR | NR | FR | NR | FR | FR |
| Meluzin 2008 | PR* | PR* | PR* | PR* | NR | NR | FR | FR | FR | FR | NR | NR | PR* | NR | FR | PR | NR | NR | NR | NR | NR | NR | FR | FR |
| Nogueira 2009 | FR | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR | NR | NR | NR | NR | NR | NR | NR | FR | NR |
| Penicka 2007 | FR | FR | FR | FR | NR | FR | FR | FR | FR | FR | NR | NR | NR | PR* | NR | FR | NR | FR | NR | PR | NR | NR | FR | FR |
| Piepoli 2010 | FR | FR | FR | FR | NR | NR | NR | NR | NR | NR | NR | NR | PR | NR | NR | FR | NR | NR | NR | NR | FR | PR | FR | FR |
| Plewka 2009 | FR | FR | FR | FR | NR | PR | FR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR |
| Quyyumi 2011 | FR | FR | FR | FR | NR | NR | NR | NR | NR | FR | NR | FR | NR | PR* | NR | FR | NR | NR | NR | NR | NR | NR | FR | NR |
| Roncalli 2010 | FR | PR | NR | NR | NR | NR | NR | NR | FR | NR | NR | NR | FR | NR | FR | NR | NR | NR | PR | PR | NR | NR | FR | PR |
| Ruan 2005 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR |
| Schachinger 2006 | FR | FR | NR | FR | FR | FR | FR | FR | FR | FR | FR | FR | FR | FR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR |
| Suarez de Lezo 2007 | PR* | NR | PR* | NR | NR | NR | PR* | NR | NR | NR | PR* | NR | PR* | NR | PR* | NR | NR | NR | NR | NR | NR | NR | FR | NR |
| Sürder 2013 | FR | PR | NR | NR | PR | PR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | FR | NR | NR | NR | NR | NR | FR | FR |
| Tendera 2009 | FR | NR | NR | NR | NR | NR | FR | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR |
| Traverse 2010 | PR* | NR | PR* | NR | NR | NR | NR | FR | NR | NR | NR | FR | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | FR | NR |
| Traverse 2011 | FR | NR | NR | NR | NR | NR | FR | NR | FR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR |
| Traverse 2012 | FR | FR | NR | NR | PR | PR | FR | FR | FR | FR | FR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR |
| Turan 2012 | PR* | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR | NR | NR | NR | NR | FR | FR |
| Wang 2014 | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR |
| Wohrle 2010 | FR | NR | NR | NR | FR | FR | PR* | NR | FR | NR | PR* | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | FR | FR |
| Wollert 2004 | PR* | FR | NR | FR | NR | FR | FR | FR | FR | FR | PR* | FR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | FR | FR |
| Xiao 2012 | NR | NR | NR | NR | PR | NR | NR | NR | NR | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR | NR |
| Yao 2006 | NR | PR* | NR | PR* | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | FR | FR | NR | NR | NR | NR | NR | NR | FR | NR |
| Yao 2009 | PR* | PR* | PR* | PR* | NR | NR | FR | FR | NR | NR | NR | NR | PR | PR | NR | NR | NR | NR | NR | NR | NR | NR | FR | FR |
| You 2008 | PR* | NR | PR* | NR | NR | NR | NR | NR | NR | NR | NR | NR | PR* | NR | NR | NR | PR | NR | PR | NR | NR | NR | FR | NR |
| Zhukova 2009 | FR | FR | FR | FR | NR | NR | NR | FR | NR | NR | NR | NR | NR | FR | NR | NR | NR | NR | NR | NR | NR | NR | NR | FR |
| Total (%) analysed (c) | 1365 (50.0) | 996 (36.5) | 290 (10.6) | 527 (19.3) | 379 (13.9) | 497 (18.2) | 1521 (55.7) | 1116 (40.8) | 1194 (43.7) | 825 (30.2) | 789 (28.9) | 758 (27.7) | 525 (19.2) | 457 (16.7) | 641 (23.5) | 395 (14.4) | 398 (14.6) | 237 (8.7) | 154 (5.6) | 26 (1.0) | 267 (9.8) | 45 (1.6) |
1135 (41.5)(d) |
727 (26.6)(d) |
ST ‐ short‐term follow‐up (< 12 months)
LT ‐ long‐term follow‐up (≥ 12 months)
FR ‐ full reporting, outcome included in analysis
PR ‐ partial reporting, insufficient information on outcome reported for inclusion in analysis
* no incidence of outcome observed
NR ‐ outcome not reported
HF ‐ heart failure; NYHA ‐ New York Heart Association; LVEF ‐ left ventricular ejection fraction
(a)Composite measure of mortality, reinfarction or rehospitalisation for heart failure.
(b)LVEF measured by any method.
(c)Total number of participants included in meta‐analysis of outcome (% of total number of participants from all included studies).
(d)Total number analysed given for LVEF measured by magnetic resonance imaging.
4. Clinical (dichotomous) outcomes.
| Study ID | Number of analysed participants | All‐cause mortality events | Cardiovascular mortality events | Reinfarction | Target vessel revascularisation | Composite MACE (death, reinfarction, rehospitalisation for HF) | |||||||||||
| Cells | No cells | Cells | No cells | Length of follow‐up | Cells | No cells | Length of follow‐up | Cells | No cells | Length of follow‐up | Cells | No cells | Length of follow‐up | Cells | No cells | Length of follow‐up | |
| Angeli 2012 | 11 | 11 | 0 | 0 | 12 months | 0 | 0 | 12 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Cao 2009 | 41 | 45 | 0 | 1 | 48 months | NR | NR | ‐ | 0 | 0 | 48 months | 0 | 1 | 48 months | NR | NR | ‐ |
| Chen 2004 | 34 | 35 | 0 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Colombo 2011 | 5 | 4 | 0 | 0 | 12 months | 0 | 0 | 12 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Gao 2013 | 21 | 21 | 1 | 0 | 24 months | 1 | 0 | 24 months | 1 | 0 | 24 months | NR | NR | ‐ | 2 | 1 | 24 months |
| Ge 2006 | 10 | 10 | 0 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Grajek 2010 | 27 | 12 | 1 | 0 | 12 months | NR | NR | ‐ | 1 (a) | 1 (a) | 6 months | 3 (a) | 4 (a) | 6 months | NR | NR | ‐ |
| Hirsch 2011 | 65 | 60 | 1 | 2 | 60 months | NR | NR | ‐ | 1 | 1 | 60 months | 20 | 14 | 60 months | 2 | 5 | 60 months |
| Huang 2006 | 20 | 20 | 0 | 0 | 6 months | 0 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | NR | NR | ‐ |
| Huang 2007 | 20 | 20 | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Huikuri 2008 | 40 | 40 | 0 | 1 | 6 months | 0 | 1 | 6 months | 0 | 2 | 6 months | NR | NR | ‐ | NR | NR | ‐ |
| Janssens 2006 | 33 | 34 | 1 | 0 | 4 months | 0 | 0 | 4 months | NR | NR | 4 months | NR | NR | ‐ | NR | NR | ‐ |
| Jazi 2012 | 16 | 16 | 0 | 0 | 6 months | 0 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | NR | NR | ‐ |
| Jin 2008 | 14 | 12 | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Karpov 2005 | 26 | 32 | 10 | 4 | 8.2 years | 8 | 2 | 8.2 years | 2 | 2 | 8.2 years | NR | NR | ‐ | NR | NR | ‐ |
| Lee 2014 | 30 | 28 | 0 | 0 | 6 months | 0 | 0 | 6 months | 2 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ |
| Lunde 2006 | 49 | 50 | 1 | 1 | 36 months | NR | NR | ‐ | 1 | 2 | 36 months | 12 | 9 | 36 months | NR | NR | ‐ |
| Meluzin 2008 | 44 | 20 | 0 | 0 | 12 months | 0 | 0 | 12 months | 2 | 0 | 12 months | NR | NR | ‐ | NR | NR | ‐ |
| Nogueira 2009 | 24 | 6 | 1 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Penicka 2007 | 17 | 10 | 3 | 0 | 24 months | 2 | 0 | 24 months | 1 | 1 | 24 months | NR | NR | ‐ | 6 | 5 | 24 months |
| Piepoli 2010 | 19 | 19 | 2 | 4 | 12 months | 2 | 3 | 12 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Plewka 2009 | 40 | 20 | 2 | 2 | 24 months | 2 | 2 | 24 months | 1 | 1 | 24 months | NR | NR | ‐ | NR (c) | NR (c) | ‐ |
| Quyyumi 2011 | 16 | 15 | 1 | 0 | 12 months | 1 | 0 | 12 months | NR | NR | ‐ | 2 | 1 | 12 months | NR | NR | ‐ |
| Roncalli 2010 | 48 | 44 | 1 | 0 | 3 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Ruan 2005 | 9 | 11 | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Schachinger 2006 | 100 (b) | 100 (b) | 7 | 15 | 60 months | 5 | 9 | 60 months | 5 (b) | 7 (b) | 24 months | 18 (b) | 28 (b) | 60 months | 4 | 15 | 24 months |
| Suarez de Lezo 2007 | 10 | 10 | 0 | 0 | 3 months | 0 | 0 | 3 months | 0 | 0 | 3 months | 0 | 0 | 3 months | NR | NR | ‐ |
| Sürder 2013 | 115 | 60 | 2 | 0 | 4 months | 0 | 0 | 4 months | 1 | 1 | 4 months | NR | NR | ‐ | NR (d) | NR (d) | ‐ |
| Tendera 2009 | 160 | 40 | 2 | 1 | 6 months | NR | NR | 3 | 2 | 6 months | 25 | 7 | 6 months | NR | NR | ‐ | |
| Traverse 2010 | 30 | 10 | 0 | 0 | 15 months | 0 | 0 | 15 months | 0 | 1 | 15 months | 0 | 1 | 15 months | NR | NR | ‐ |
| Traverse 2011 | 58 | 29 | 0 | 1 | 6 months | NR | NR | ‐ | 1 | 0 | 6 months | 1 | 2 | 6 months | NR | NR | ‐ |
| Traverse 2012 | 79 | 41 | 1 | 0 | 12 months | NR | NR | ‐ | 2 | 3 | 12 months | 4 | 4 | 12 months | NR (e) | NR (e) | ‐ |
| Turan 2012 | 42 | 20 | 0 | 0 | 6 months | 0 | 0 | 6 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Wang 2014 | 28 | 30 | 1 | 2 | 6 months | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Wohrle 2010 | 29 | 13 | 1 | 1 | 6 months | NR | NR | ‐ | 0 | 0 | 6 months | 0 | 0 | 6 months | 5 | 1 | 36 months |
| Wollert 2004 | 30 | 30 | 2 | 2 | 61 months | NR | NR | ‐ | 1 | 1 | 61 months | 6 | 4 | 61 months | 5 | 6 | 61 months |
| Xiao 2012 | 17 | 21 | NR | NR | 3 months | NR | NR | 3 months | NR | NR | 3 months | NR | NR | 3 months | NR (f) | NR (f) | 3 months |
| Yao 2006 | 90 | 84 | 0 | 0 | 30 months | 0 | 0 | 30 months | 2 | 2 | 30 months | NR | NR | ‐ | NR | NR | ‐ |
| Yao 2009 | 27 | 12 | 0 | 0 | 12 months | 0 | 0 | 12 months | 0 | 1 | 12 months | NR | NR | ‐ | NR | NR | ‐ |
| You 2008 | 7 | 16 | 0 | 0 | 8 weeks | 0 | 0 | 8 weeks | NR | NR | ‐ | NR | NR | ‐ | NR | NR | ‐ |
| Zhukova 2009 | 8 | 3 | 2 | 1 | 36 months * | 2 | 1 | 36 months * | 1 | 0 | 36 months | NR | NR | ‐ | NR | NR | ‐ |
(a)Grajek 2010: 31 BMMNC and 14 controls available for analysis at 6 months.
(b)Schachinger 2006: 100 BMMNC and 101 controls analysed at 24 months; 3 patients (2 BMMNC and 1 control) only had mortality data at 60 months.
(c)Plewka 2009: Composite death, MI, hospitalisation for HF, TVR: 9 BMMNC and 11 controls at 24 months.
(d)Sürder 2013: Composite death, MI, revascularisation, hospitalisation for HF: 9 BMMNC and 8 controls at 12 months.
(e)Traverse 2012: Composite death, MI, hospitalisation for HF, revascularisation, ICD, stroke: 18 BMMNC and 9 controls at 12 months.
(f)Xiao 2012: Composite MACE (undefined): 3 BMMNC and 2 controls at 3 months.
Primary outcomes
All‐cause mortality
Seventeen trials reported incidences of mortality in the short‐term follow‐up period of less than 12 months from cell therapy (Gao 2013; Huikuri 2008; Janssens 2006; Nogueira 2009; Penicka 2007; Piepoli 2010; Plewka 2009; Quyyumi 2011; Roncalli 2010; Schachinger 2006; Sürder 2013; Tendera 2009; Traverse 2011; Traverse 2012; Wang 2014; Wohrle 2010; Zhukova 2009). All incidences of mortality in the short‐term follow‐up period occurred within six months of cell therapy. A further 17 trials reported that no deaths occurred during short‐term follow‐up (see Table 4).
In trials that reported long‐term follow‐up, 14 reported incidences of mortality (Cao 2009; Gao 2013; Grajek 2010; Hirsch 2011; Karpov 2005; Lunde 2006; Penicka 2007; Piepoli 2010; Plewka 2009; Quyyumi 2011; Schachinger 2006; Traverse 2012; Wollert 2004; Zhukova 2009), with nine trials reporting no deaths during long‐term follow‐up. The duration of long‐term follow‐up ranged from 12 months (Grajek 2010; Piepoli 2010; Quyyumi 2011; Traverse 2012), 24 months (Gao 2013; Penicka 2007; Plewka 2009), 36 months (Lunde 2006; Zhukova 2009) and 48 months (Cao 2009), to 60 months (Hirsch 2011; Schachinger 2006; Wollert 2004), and in one trial there was a mean follow‐up of 8.2 (standard deviation (SD) 0.72) years (Karpov 2005).
The mortality incidence rate was low in all trials. Overall, there was no evidence for a difference in the risk of mortality between patients who received cell therapy and those who received no cells at short‐term (21/836 versus 15/529; risk ratio (RR) 0.80, 95% confidence interval (CI) 0.43 to 1.49; 1365 participants; 17 studies) or long‐term follow‐up (34/538 versus 32/458; RR 0.93, 95% CI 0.58 to 1.50; 996 participants; 14 studies) with no evidence of heterogeneity (I2 = 0% in both analyses) (Analysis 1.1).
1.1. Analysis.
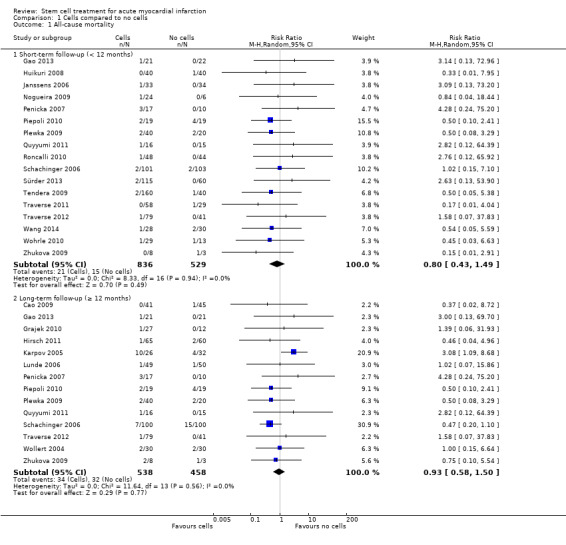
Comparison 1 Cells compared to no cells, Outcome 1 All‐cause mortality.
Sensitivity analyses did not affect the results for mortality. Exclusion of the trial that administered cells via the coronary artery, Nogueira 2009, did not affect short‐term mortality results (20/812 versus 15/523; RR 0.80, 95% CI 0.42 to 1.51; 1335 participants; 16 studies) (Analysis 2.1). Only one trial included in the analysis of short‐term follow‐up had a high risk of selection bias due to lack of appropriate randomisation sequence generation (Wang 2014); the difference in risk of mortality between groups when we excluded this trial was negligible (20/808 versus 13/499; RR 0.83, 95% CI 0.43 to 1.57; 1307 participants; 16 studies) (Analysis 3.1). No trials reporting long‐term follow‐up had a high risk of selection bias due to randomisation methods. When we excluded trials with a high or unclear risk of attrition bias, there remained no evidence for a difference in all‐cause mortality at either short‐term (14/505 versus 12/394; RR 0.78, 95% CI 0.38 to 1.61; 899 participants; 13 studies) or long‐term follow‐up (21/456 versus 26/391; RR 0.67, 95% CI 0.38 to 1.17; 847 participants; 11 studies) (Analysis 4.1; Analysis 4.2). Similarly, exclusion of trials with a high risk of performance bias due to lack of blinding revealed no evidence for differences in the risk of mortality at either short‐term (6/376 versus 8/293; RR 0.60, 95% CI 0.23 to 1.56; 669 participants; eight studies) or long‐term follow‐up (8/220 versus 16/186; RR 0.50, 95% CI 0.22 to 1.10; 406 participants; three studies) (Analysis 5.1; Analysis 5.2).
2.1. Analysis.
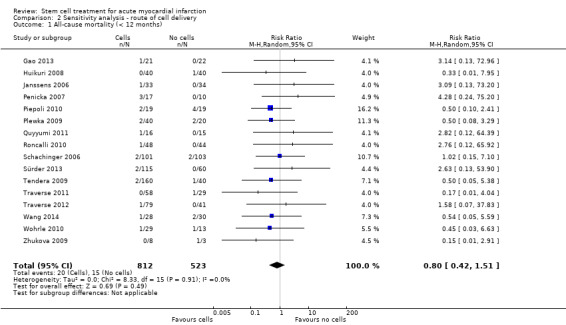
Comparison 2 Sensitivity analysis ‐ route of cell delivery, Outcome 1 All‐cause mortality (< 12 months).
3.1. Analysis.
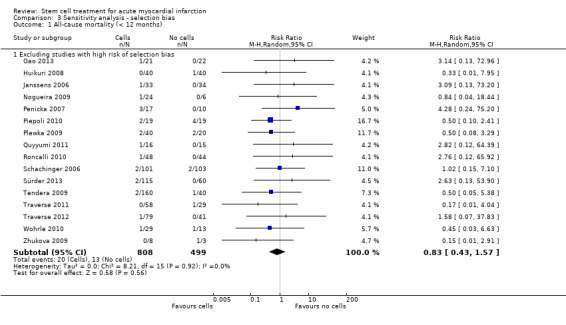
Comparison 3 Sensitivity analysis ‐ selection bias, Outcome 1 All‐cause mortality (< 12 months).
4.1. Analysis.
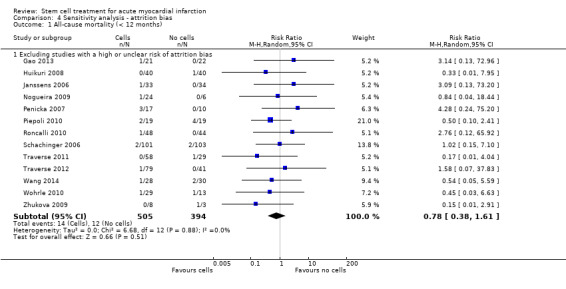
Comparison 4 Sensitivity analysis ‐ attrition bias, Outcome 1 All‐cause mortality (< 12 months).
4.2. Analysis.
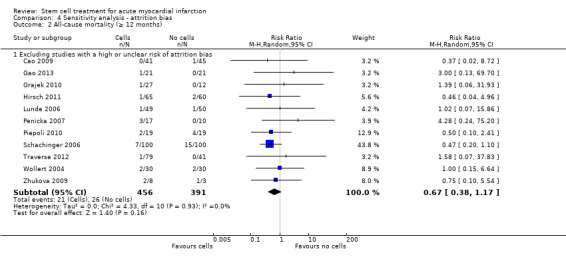
Comparison 4 Sensitivity analysis ‐ attrition bias, Outcome 2 All‐cause mortality (≥ 12 months).
5.1. Analysis.
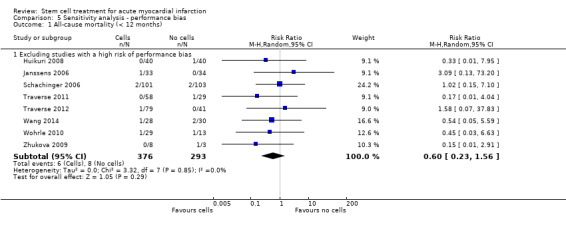
Comparison 5 Sensitivity analysis ‐ performance bias, Outcome 1 All‐cause mortality (< 12 months).
5.2. Analysis.
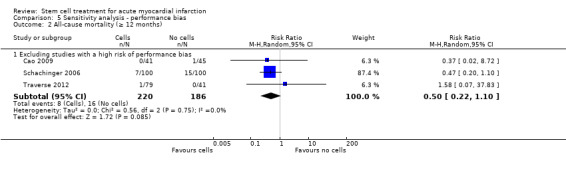
Comparison 5 Sensitivity analysis ‐ performance bias, Outcome 2 All‐cause mortality (≥ 12 months).
Subgroup analysis of mortality measured at short‐term follow‐up revealed no differences between trials grouped according to baseline left ventricular ejection fraction (LVEF) as measured by magnetic resonance imaging (MRI) (Analysis 6.1), cell type (Analysis 7.1), cell dose (Analysis 8.1), timing of cell infusion (Analysis 9.1), or use of heparinised cell solution (Analysis 10.1). However, stratification of trials by cell dose revealed a significant difference in the effect of cells on long‐term mortality (test for subgroup differences, P value = 0.02) (Analysis 8.2), with a reduced risk of mortality in patients who received > 108 and ≤ 109 cells (14/371 versus 24/297; RR 0.52, 95% CI 0.28 to 0.97; 668 participants; seven studies), whereas there was no evidence for a difference in the risk of long‐term mortality associated with a lower dose (≤ 108 cells) (15/120 versus 6/121; RR 2.20, 95% CI 0.97 to 4.95; 241 participants; five studies) (Analysis 8.2). Only two trials administered > 109 cells; there was no difference in the risk of mortality between treatment groups from meta‐analysis of these two trials (5/47 versus 2/40; RR 1.56, 95% CI 0.32 to 7.55; 87 participants; two studies). There was no difference in the risk of long‐term mortality associated with cell therapy associated with either baseline LVEF (Analysis 6.2), cell type (Analysis 7.2), timing of cell administration (Analysis 9.2), or use of heparinised cell solution (Analysis 10.2).
6.1. Analysis.
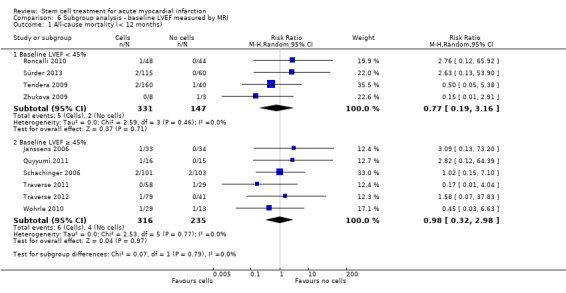
Comparison 6 Subgroup analysis ‐ baseline LVEF measured by MRI, Outcome 1 All‐cause mortality (< 12 months).
7.1. Analysis.
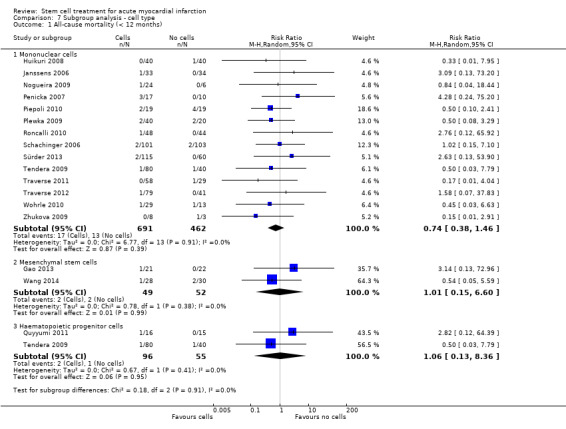
Comparison 7 Subgroup analysis ‐ cell type, Outcome 1 All‐cause mortality (< 12 months).
8.1. Analysis.
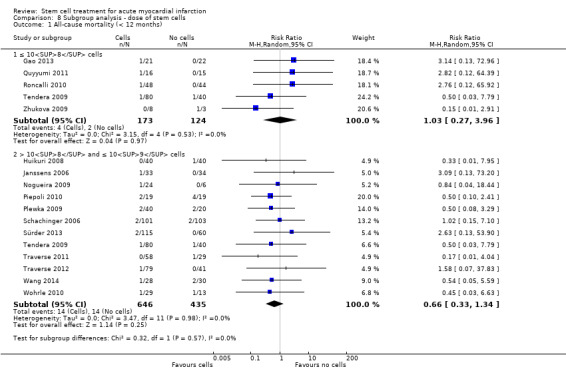
Comparison 8 Subgroup analysis ‐ dose of stem cells, Outcome 1 All‐cause mortality (< 12 months).
9.1. Analysis.
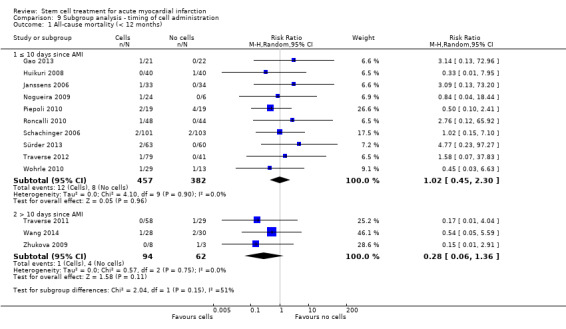
Comparison 9 Subgroup analysis ‐ timing of cell administration, Outcome 1 All‐cause mortality (< 12 months).
10.1. Analysis.
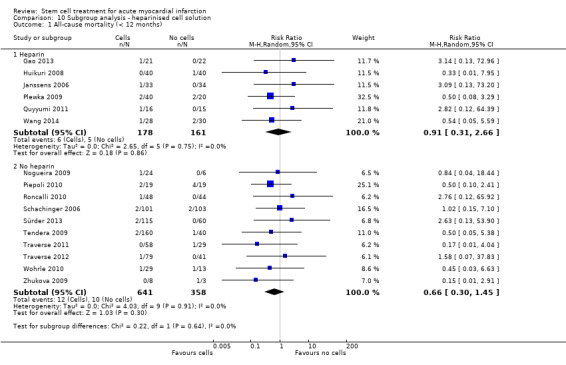
Comparison 10 Subgroup analysis ‐ heparinised cell solution, Outcome 1 All‐cause mortality (< 12 months).
8.2. Analysis.
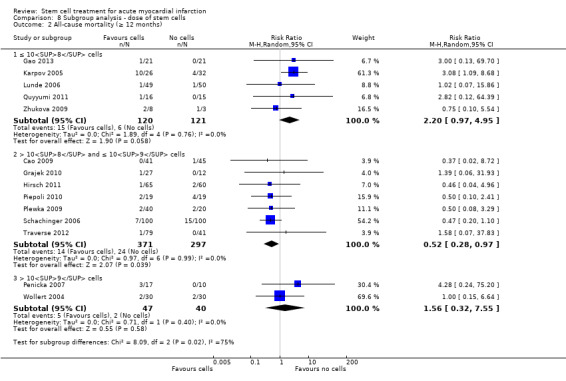
Comparison 8 Subgroup analysis ‐ dose of stem cells, Outcome 2 All‐cause mortality (≥ 12 months).
6.2. Analysis.
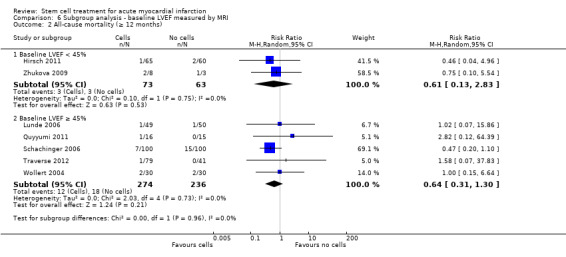
Comparison 6 Subgroup analysis ‐ baseline LVEF measured by MRI, Outcome 2 All‐cause mortality (≥ 12 months).
7.2. Analysis.
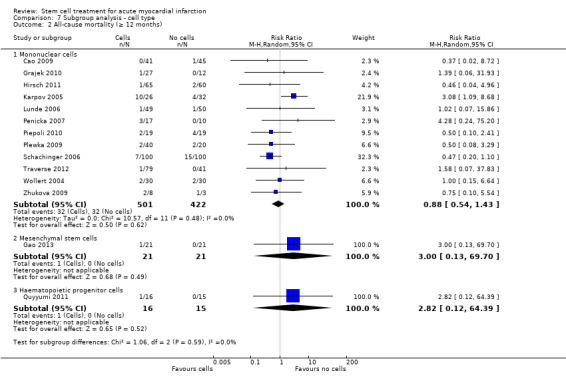
Comparison 7 Subgroup analysis ‐ cell type, Outcome 2 All‐cause mortality (≥ 12 months).
9.2. Analysis.
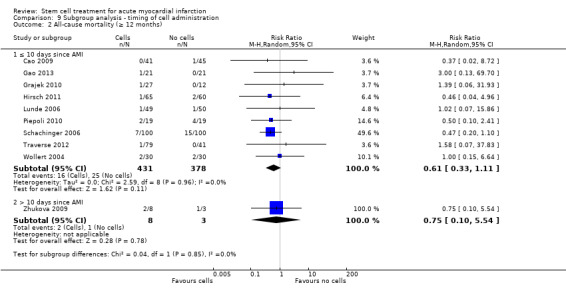
Comparison 9 Subgroup analysis ‐ timing of cell administration, Outcome 2 All‐cause mortality (≥ 12 months).
10.2. Analysis.
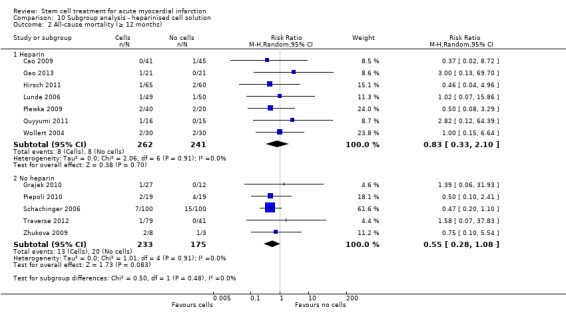
Comparison 10 Subgroup analysis ‐ heparinised cell solution, Outcome 2 All‐cause mortality (≥ 12 months).
In trial sequential analysis of all‐cause mortality at long‐term follow‐up, the cumulative Z‐curve did not cross the conventional thresholds or trial sequential monitoring boundaries for significance (see Figure 3). The required information size, based on a random‐effects model and a relative risk reduction of 35%, a mean effect size equivalent to that associated with revascularisation by percutaneous coronary intervention (PCI) (Hartwell 2005), was 3275, suggesting that the current meta‐analysis is considerably underpowered to detect a reduction in relative risk of 35% or lower. Smaller relative risks would result in a considerably greater information size.
3.
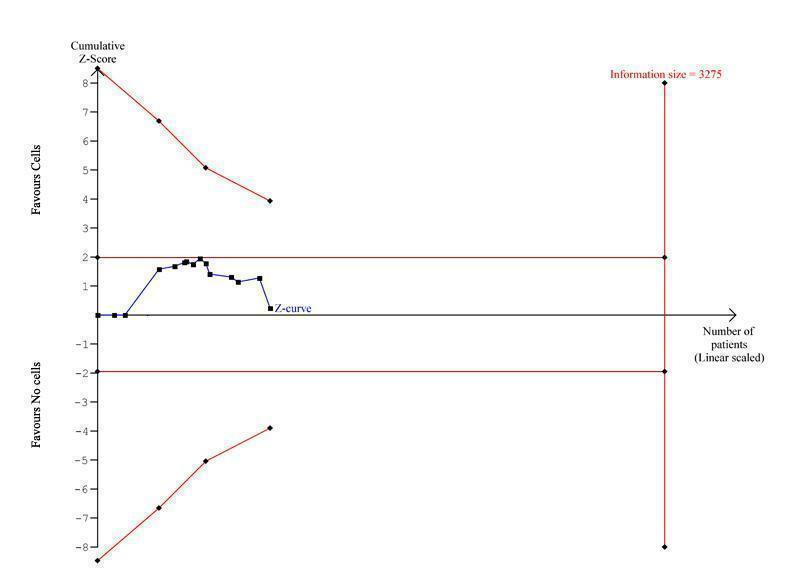
Trial sequential analysis of all‐cause mortality at long term follow‐up, assuming a long‐term mortality incidence rate of 6.1% in controls and a relative risk reduction of 35% in cell therapy patients
Cardiovascular mortality
Incidence of cardiovascular mortality was reported in seven trials at short‐term follow‐up (Gao 2013; Huikuri 2008; Penicka 2007; Piepoli 2010; Plewka 2009; Quyyumi 2011; Zhukova 2009), and nine trials at long‐term follow‐up (Gao 2013; Karpov 2005; Penicka 2007; Piepoli 2010; Plewka 2009; Quyyumi 2011; Schachinger 2006; Wollert 2004; Zhukova 2009). There was no evidence for a difference in the risk of cardiovascular mortality at either short‐term (7/161 versus 7/129; RR 0.72, 95% CI 0.28 to 1.82; 290 participants; seven studies) or at long‐term follow‐up (23/277 versus 18/250; RR 1.04, 95% CI 0.54 to 1.99; 527 participants; nine studies) (Analysis 1.2).
1.2. Analysis.
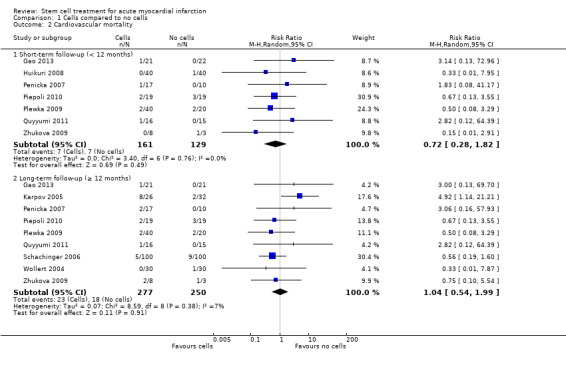
Comparison 1 Cells compared to no cells, Outcome 2 Cardiovascular mortality.
None of the trials that reported cardiovascular mortality had a high risk of selection bias. The lack of evidence for a difference in the risk of cardiovascular mortality remained when we excluded trials with a high or unclear risk of attrition bias at both short‐term (4/105 versus 5/94; RR 0.69, 95% CI 0.22 to 2.14; 199 participants; five studies) (Analysis 4.3) and long‐term follow‐up (12/195 versus 14/183; RR 0.71, 95% CI 0.34 to 1.50; 378 participants; six studies) (Analysis 4.4). The number of appropriately blinded trials precluded sensitivity analysis for performance bias.
4.3. Analysis.
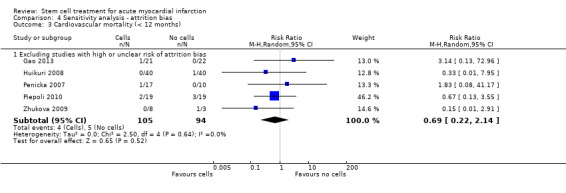
Comparison 4 Sensitivity analysis ‐ attrition bias, Outcome 3 Cardiovascular mortality (< 12 months).
4.4. Analysis.
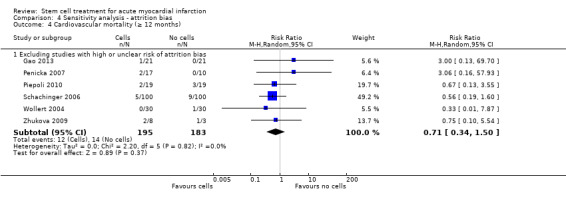
Comparison 4 Sensitivity analysis ‐ attrition bias, Outcome 4 Cardiovascular mortality (≥ 12 months).
Trial sequential analysis of cardiovascular mortality at long‐term follow‐up found an information size of 3064 participants based on a relative risk reduction of 35%, demonstrating that the current meta‐analysis is considerably underpowered to detect an effect of this magnitude.
Composite measures of major adverse cardiac events (MACE)
Composite measures of MACE were reported in 10 trials (Gao 2013; Hirsch 2011; Penicka 2007; Plewka 2009; Schachinger 2006; Sürder 2013; Traverse 2012; Wohrle 2010; Wollert 2004; Xiao 2012). Six trials defined composite MACE as death, reinfarction or re‐hospitalisation for heart failure (Gao 2013; Hirsch 2011; Penicka 2007; Schachinger 2006; Wohrle 2010; Wollert 2004). Other definitions of composite MACE were as follows: death, reinfarction or target vessel revascularisation (Hirsch 2011; Schachinger 2006), death, reinfarction, re‐hospitalisation for heart failure or revascularisation (Plewka 2009; Sürder 2013), death, reinfarction, re‐hospitalisation for heart failure, revascularisation, implantable cardioverter‐defibrillator (ICD) implantation or stroke (Traverse 2012), and death, reinfarction, re‐hospitalisation for heart failure, stroke or arrhythmia (Gao 2013). One trial did not define the composite measure of MACE (Xiao 2012). Analysis was restricted to composite death, reinfarction or re‐hospitalisation for heart failure due to the lack of data from alternative measures. Of note, one study with mortality data reported at five‐year follow‐up only reported two‐year follow‐up data for composite MACE, the incidence of which is lower than the five‐year mortality rate (Schachinger 2006).
There was no evidence for a reduction in the risk of composite death, reinfarction or re‐hospitalisation for heart failure associated with cell therapy at either short‐term (5/198 versus 12/181; RR 0.36, 95% CI 0.12 to 1.14; 379 participants; three studies) or long‐term follow‐up (24/262 versus 33/235; RR 0.63, 95% CI 0.36 to 1.10; 497 participants; six studies) with low or negligible heterogeneity in both analyses (I2 = 0%; I2 = 12% respectively) (Analysis 1.3). The limited number of trials that reported other composite measures of MACE at short‐term or long‐term follow‐up prevented formal analysis of these outcomes.
1.3. Analysis.
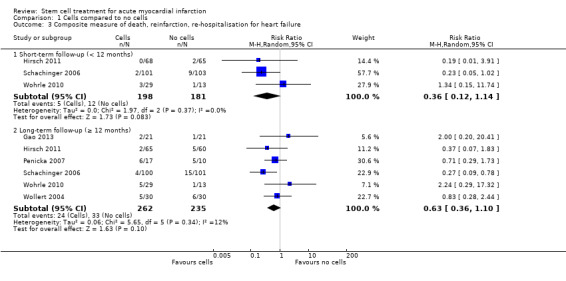
Comparison 1 Cells compared to no cells, Outcome 3 Composite measure of death, reinfarction, re‐hospitalisation for heart failure.
We did not perform sensitivity analysis as no trials that reported composite measures of MACE had a high risk of selection bias or a high or unclear risk of attrition bias, and the number of appropriately blinded trials precluded sensitivity analysis for performance bias.
Trial sequential analysis of cardiovascular mortality at long‐term follow‐up showed that based on a relative risk reduction of 35%, 1572 participants would be required, demonstrating that the current meta‐analysis is considerably underpowered to detect such a difference in the risk of composite MACE between treatment groups.
Periprocedural adverse events
Twenty‐seven trials reported periprocedural adverse events as an outcome, six of which reported no periprocedural adverse events (Colombo 2011; Ge 2006; Karpov 2005; Traverse 2010; Turan 2012; Wollert 2004) (see Table 6 for details). Adverse events associated with bone marrow aspiration were rare; only one trial reported a serious adverse event at the time of bone marrow harvest (one patient experienced a stent thrombosis with reinfarction which occurred immediately after the procedure) (Penicka 2007); a second trial reported three patients with mild self limiting vasovagal reactions during bone marrow aspiration (Huikuri 2008). No other adverse events associated with bone marrow harvest were reported. Three deaths were reported in patients randomised to cell therapy prior to cell infusion (one patient died due to subarachnoid haemorrhage (Traverse 2012) and in two patients the cause of death was not reported (Sürder 2013)), and three patients died soon after cell therapy was administered (one at three days after cell therapy due to suspected acute in‐stent thrombosis (Gao 2013), one from ventricular fibrillation attributed to recurrent myocardial infarction from stent thrombosis preceding cell infusion (Quyyumi 2011), and one with cause of death not reported (Schachinger 2006)). Other serious periprocedural adverse events observed in patients who received cell therapy included one transient acute heart failure (Cao 2009), one acute coronary occlusion during cell injection (Gao 2013), one patient with a small thrombus in the infarct‐related artery diagnosed immediately after cell transplantation (Meluzin 2008), one patient with sub‐acute stent thrombosis (Huikuri 2008), four patients with periprocedural myocardial infarction (Lee 2014; Schachinger 2006), one transient ischaemic attack (Roncalli 2010), and one post‐procedural arteriovenous fistula of the femoral artery (Tendera 2009). In summary, serious periprocedural adverse events were rare and unlikely to be associated with treatment.
5. Periprocedural adverse events.
| Study ID | Periprocedural adverse events |
| Angeli 2012 | Not reported |
| Cao 2009 | 1 x transient acute heart failure 7 days after cell transplantation |
| Chen 2004 | Not reported |
| Colombo 2011 | No adverse events were reported until the end of hospitalisation |
| Gao 2013 | 1 x death 3 days after cell transplantation due to suspected acute in‐stent thrombosis; 1 x serious complication of acute coronary occlusion during cell injection with subsequent recurrent MI |
| Ge 2006 | No bleeding complications at BM puncture site and no angina aggravation, malignant diseases or substantial arrhythmias after PCI and BM transfer during hospitalisation in either treatment group |
| Grajek 2010 | Not reported |
| Hirsch 2011 | No complications of cell harvesting. A CK or CK‐MB elevation between 1 and 2 times the ULN was detected in 4 patients and between 2 and 3 times the ULN in one patient. 1 x occluded infarct‐related artery (patient did not receive cell therapy as randomised). During cell catheterisation: 1 x coronary spasm, 1 x transient brachycardia and 1 x thrombus in the infarct related artery |
| Huang 2006 | Not reported |
| Huang 2007 | Not reported |
| Huikuri 2008 | 3 x mild self terminating vasovagal reactions during BM aspiration; no other procedural complications relating to aspiration. Subacute stent thrombosis occurred in 4 patients (1 x cell therapy and 3 x placebo); 1 x cell therapy patient had 'no reflow' phenomenon after stenting of the infarcted artery |
| Janssens 2006 | 11 x treatment‐related tachycardia (supraventricular arrhythmia: 5 in the cell therapy group and 6 in the control group); 3 patients in the control group experienced non‐sustained ventricular tachycardia |
| Jazi 2012 | Not reported |
| Jin 2008 | Not reported |
| Karpov 2005 | No complications of BM aspiration or cell infusion |
| Lee 2014 | No serious inflammatory reactions or bleeding complications from BM aspiration. No (or mild) angina during balloon inflation. No serious procedural complications related to intracoronary administration of MSCs including ventricular arrhythmia, thrombus formation or dissection. Periprocedural MI occurred in 2 patients |
| Lunde 2006 | 2 x stent thrombosis in the acute phase in the cell therapy group (no cells administered as randomised); 1 x sustained ventricular tachycardia before cell administration; 1 x ventricular fibrillation at day 6, 24 hours after injection.1 x pulseless ventricular tachycardia in control patient ‐ converted to sinus rhythm by means of a precordial thump on day 2 |
| Meluzin 2008 | 2 patients had fever and 1 patient had brachycardia, all within 20 hours prior to cells (these patients did not receive cell therapy as randomised). 3 x cell therapy‐related complications: 1 x intimal dissection during repeat balloon inflations at time of cell implantation, 1 x short‐lasting fever on day of scheduled transplantation, 1 x small thrombus in infarct‐related artery diagnosed immediately after cell transplantation. 2 x control patients had repeat MI 2 days after the hospital discharge due to in‐stent thrombosis |
| Nogueira 2009 | Ck‐MB elevation (3 x normal value) in 3 patients in the arterial group and 1 patient in venous group. 1 x tortuous anterior interventricular vein (patient did not receive cell therapy as randomised). No new pericardial effusions |
| Penicka 2007 | 2 x serious complications (1 x stent thrombosis with reinfarction immediately after BM harvest, patient died 2 weeks later due to sepsis and acute respiratory distress syndrome; 1 x ventricular septal rupture before cell injection, patient died 3 months later from severe heart failure). |
| Piepoli 2010 | All procedures well tolerated. No inflammatory reaction or abscess detected at the site of puncture after BM harvest. The invasive coronary catheterisation was associated with some mild angina during balloon inflations for cell infusions. No procedural complications during cardiac catheterisation related to cell injections (no ventricular arrhythmia, new thrombus formation or embolism after cell infusion or dissections due to balloon inflations) |
| Plewka 2009 | Not reported |
| Quyyumi 2011 | 1 high‐dose treatment group patient died soon after cell infusion from ventricular fibrillation attributed to recurrent MI from stent thrombosis preceding cell infusion. 1 x high‐dose treatment group patient with acute stent thrombosis before cell infusion (patient withdrawn from study). Cell therapy group: 1 x arrhythmia, 1 x chest pain, 3 x musculoskeletal pain, 2 x upper respiratory tract infection, 2 x rash, 3 x dyspnoea, 1 x fever. Control group: 1 x arrhythmia, 3 x musculoskeletal pain, 1 x upper respiratory tract infection, 1 x dyspnoea |
| Roncalli 2010 | Cell therapy group: 1 x transient ischaemic attack and 1 x thrombopenia induced by GP2b3a inhibitor (both excluded before BM aspiration). Control group: 1 x steroids given for angioneurotic oedema; 1 x post‐MI ventricular septal defect (both withdrawn before day 7) |
| Ruan 2005 | Not reported |
| Schachinger 2006 | No bleeding complications or haematoma formation at puncture site of BM aspiration. 1 x patient was excluded owing to fever and an increase in the level of C‐reactive protein. 1 x patient in placebo group had angiographic evidence of a thrombus in a non‐infarct‐related artery (placebo medium not infused). 2 x deaths, cause not reported (1 x cell therapy group and 1 x placebo) and 2 x reinfarction (cell therapy group) prior to discharge |
| Suarez de Lezo 2007 | Not reported |
| Sürder 2013 | 1 death in cell therapy group prior to transplantation, cause of death not reported |
| Tendera 2009 | 1 patient developed arteriovenous fistula of the femoral artery after the procedure and required surgical treatment. No complications arising from BM cell transfer |
| Traverse 2010 | BM aspiration carried out without complications. No patient experienced a rise in troponin or procedure‐related complication following infusion |
| Traverse 2011 | No complications associated with BM aspiration. 2 x patients underwent additional stenting at time of cell infusion (1 x distal stent edge dissection related to primary PCI procedure; 1 x possible dissection related to stop‐flow procedure). 1 x postpartum spontaneous coronary dissection with diffuse thrombus throughout stented region of left anterior descending artery; 1 x presence of severe left main coronary stenosis identified before transfusion (this patient did not receive cell therapy as randomised). No patients experienced postprocedural increase in cardiac enzymes |
| Traverse 2012 | No complications associated with BM harvesting or intracoronary infusion. 1 x death in the BM cell therapy group due to subarachnoid haemorrhage prior to cell delivery |
| Turan 2012 | No procedural or cell‐induced complications and no side effects in any patient |
| Wang 2014 | Not reported |
| Wohrle 2010 | Not reported |
| Wollert 2004 | No bleeding complications at BM harvest site. No increases in troponin T serum levels in any patients 24 hours after BM transfer |
| Xiao 2012 | Not reported |
| Yao 2006 | 1 x temporary hypotension, 2 x brachycardia, 7 x new hyperuricaemia |
| Yao 2009 | 1 x brachycardia with subsequent pacemaker implantation, 1 x fever (these patients did not receive cells as randomised) |
| You 2008 | Not reported |
| Zhukova 2009 | Not reported |
MI, acute myocardial infarction; PCI, percutaneous coronary intervention; BM, bone marrow; MSC, mesenchymal stem cells; ULN, upper limit of normal
Secondary outcomes
Reinfarction
Seventeen trials reported incidences of reinfarction in the short‐term follow‐up period of less than 12 months from stem cell therapy (Gao 2013; Grajek 2010; Hirsch 2011; Huikuri 2008; Karpov 2005; Lee 2014; Lunde 2006; Meluzin 2008; Penicka 2007; Plewka 2009; Sürder 2013; Tendera 2009; Traverse 2011; Traverse 2012; Wollert 2004; Yao 2006; Yao 2009). A further five trials reported that no incidences of reinfarction occurred during short‐term follow‐up (see Table 4).
Incidences of reinfarction occurred in 14 trials at long‐term follow‐up (Gao 2013; Hirsch 2011; Karpov 2005; Lunde 2006; Meluzin 2008; Penicka 2007; Plewka 2009; Schachinger 2006; Traverse 2010; Traverse 2012; Wollert 2004; Yao 2006; Yao 2009; Zhukova 2009); one further trial reported no incidences of reinfarction (Cao 2009).
There was no evidence for a difference in the risk of reinfarction between treatment groups at either short‐term (16/927 versus 16/594; RR 0.66, 95% CI 0.33 to 1.30; 1521 participants; 17 studies) or long‐term follow‐up (20/624 versus 25/492; RR 0.64, 95% CI 0.36 to 1.12; 1116 participants; 14 studies) with no evidence of heterogeneity (I2 = 0% for both analyses) (Analysis 1.4).
1.4. Analysis.
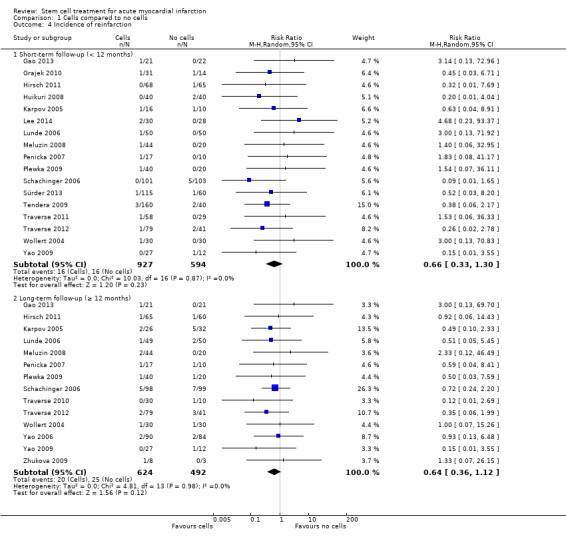
Comparison 1 Cells compared to no cells, Outcome 4 Incidence of reinfarction.
Four patients were reported to have died following reinfarction. One death occurred due to reinfarction as the cells were harvested; the patient died from sepsis and acute respiratory distress syndrome (ARDS) two weeks following repeat PCI and coronary artery bypass graft (CABG) (Penicka 2007). Another death occurred soon after cell infusion from ventricular fibrillation that was attributed to recurrent myocardial infarction from stent thrombosis preceding cell infusion; in this four‐armed trial it was not reported in which trial arm this patient had been randomised (Quyyumi 2011). Two other deaths due to reinfarction were reported at three‐month (Zhukova 2009) and 12‐month (Schachinger 2006) follow‐up respectively.
Arrhythmias
Twenty‐one trials reported arrhythmia as an outcome, although two trials reported summary results only (Piepoli 2010; Yao 2009), and in a further 11 trials arrhythmias were not observed during follow‐up (see Table 4). In eight trials that reported incidences of arrhythmias, arrhythmia was defined as incidences of supraventricular arrhythmia (Janssens 2006), supraventricular tachycardia (Zhukova 2009), documented ventricular arrhythmia (Schachinger 2006), ventricular fibrillation (Hirsch 2011), sustained ventricular arrhythmia (Lunde 2006), repetitive ventricular arrhythmia (Colombo 2011), malignant arrhythmia (Xiao 2012) and arrhythmia (unspecified) (Roncalli 2010).
Five trials reported incidences of arrhythmias at short‐term follow‐up (Hirsch 2011; Janssens 2006; Roncalli 2010; Schachinger 2006; Xiao 2012). There was no evidence for a difference in the risk of arrhythmias at short‐term follow‐up between patients who received cell therapy and those who did not (15/264 versus 15/261; RR 1.00, 95% CI 0.51 to 1.98; 525 participants; five studies). Similarly, in five trials that reported incidences of arrhythmia at long‐term follow‐up (Colombo 2011; Hirsch 2011; Lunde 2006; Schachinger 2006; Zhukova 2009), there was no difference in the risk of arrhythmias between treatment arms (11/231 versus 7/226; RR 1.39, 95% CI 0.58 to 3.37; 457 participants; five studies) (Analysis 1.7).
1.7. Analysis.
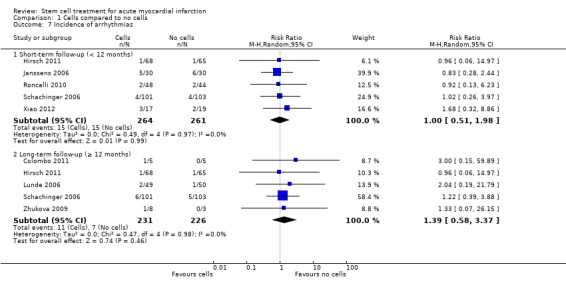
Comparison 1 Cells compared to no cells, Outcome 7 Incidence of arrhythmias.
Restenosis
Fifteen trials reported incidences of restenosis during follow‐up (Cao 2009; Grajek 2010; Huikuri 2008; Janssens 2006; Lunde 2006; Meluzin 2008; Nogueira 2009; Penicka 2007; Piepoli 2010; Quyyumi 2011; Roncalli 2010; Traverse 2010; Wohrle 2010; Wollert 2004; Yao 2006). However, one trial did not report restenosis as an outcome in the control arm of the trial (Nogueira 2009), and one trial reported results descriptively (Huikuri 2008). One trial with long‐term follow‐up data did not report individual group sample sizes (Meluzin 2008). Two trials reported no incidences of restenosis during follow‐up (Jazi 2012; Suarez de Lezo 2007).
Restenosis at short‐term follow‐up was reported in eight trials (Grajek 2010; Janssens 2006; Lunde 2006; Meluzin 2008; Roncalli 2010; Wohrle 2010; Wollert 2004; Yao 2006). The rate of restenosis at short‐term follow‐up was similar in patients who received cell therapy and in the control group (42/353 versus 34/288; RR 0.95, 95% CI 0.63 to 1.43; 641 participants; eight studies). There was also no evidence for a difference in the risk of restenosis at long‐term follow‐up in five trials (Cao 2009; Penicka 2007; Piepoli 2010; Traverse 2010; Yao 2006) (10/213 versus 14/182; RR 0.58, 95% CI 0.27 to 1.25; 395 participants; six studies) (Analysis 1.8).
1.8. Analysis.
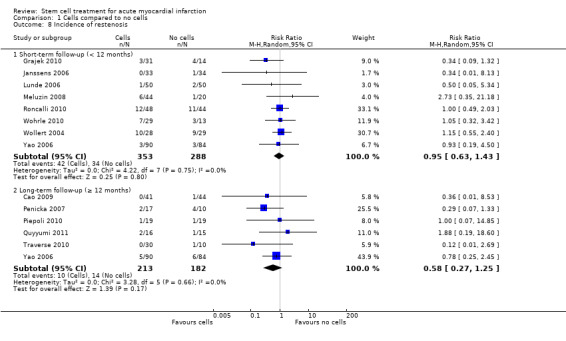
Comparison 1 Cells compared to no cells, Outcome 8 Incidence of restenosis.
Target vessel revascularisation
The requirement for percutaneous coronary intervention in the infarct‐related vessel during follow‐up and after the therapy procedure was determined as target vessel revascularisation. Eleven trials reported incidences of target vessel revascularisation in one or both trial arms (Cao 2009; Grajek 2010; Hirsch 2011; Lunde 2006; Quyyumi 2011; Schachinger 2006; Tendera 2009; Traverse 2010; Traverse 2011; Traverse 2012; Wollert 2004). Four trials reported no incidences of target vessel revascularisation during follow‐up (Janssens 2006; Lee 2014; Suarez de Lezo 2007; Wohrle 2010).
At short‐term follow‐up, there was no evidence for a difference in the risk of target vessel revascularisation between patients who received cell therapy and those who did not (50/497 versus 40/292; RR 0.70, 95% CI 0.47 to 1.06; 789 participants; six studies). There was also no difference in the risk of target vessel revascularisation between treatment arms at long‐term follow‐up (62/408 versus 62/350; RR 0.96, 95% CI 0.67 to 1.37; 758 participants; eight studies) (Analysis 1.6).
1.6. Analysis.
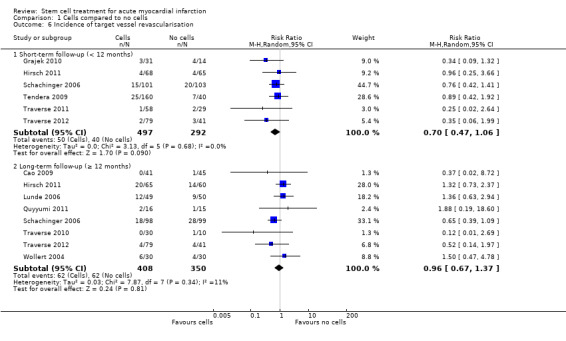
Comparison 1 Cells compared to no cells, Outcome 6 Incidence of target vessel revascularisation.
Of note, the incidence of restenosis seems to be lower than the incidence of target vessel revascularisation, and this may look like a discrepancy as the latter is a consequence of the former. However, the trials included in these two meta‐analyses differ, as not all trials reported both outcomes. Three trials reported both restenosis and target vessel revascularisation (Cao 2009; Quyyumi 2011; Traverse 2010), and the numbers were the same for both outcomes.
Re‐hospitalisation for heart failure
Incidences of hospital readmission for heart failure were reported in 13 trials at short‐term follow‐up (Colombo 2011; Hirsch 2011; Huikuri 2008; Lunde 2006; Meluzin 2008; Penicka 2007; Roncalli 2010; Schachinger 2006; Sürder 2013; Traverse 2011; Traverse 2012; Wohrle 2010; Wollert 2004), and 11 trials at long‐term follow‐up (Colombo 2011; Gao 2013; Hirsch 2011; Lunde 2006; Meluzin 2008; Penicka 2007; Plewka 2009; Quyyumi 2011; Schachinger 2006; Traverse 2012; Wollert 2004). However, in one trial reporting discrepancies between publications could not be resolved with the study authors and therefore we omitted this study from the analysis at long‐term follow‐up (Colombo 2011).
At short‐term follow‐up there was no evidence for a difference in the risk of re‐hospitalisation for heart failure between patients who received cell therapy and those who did not (17/684 versus 15/510; RR 0.81, 95% CI 0.40 to 1.62; 1194 participants; 13 studies). However, at long‐term follow‐up of 12 months or longer, there was marginally significant evidence for a difference between treatment groups in favour of cell therapy (18/459 versus 27/366; RR 0.55, 95% CI 0.30 to 1.00; 825 participants; 10 studies) (Analysis 1.5).
1.5. Analysis.
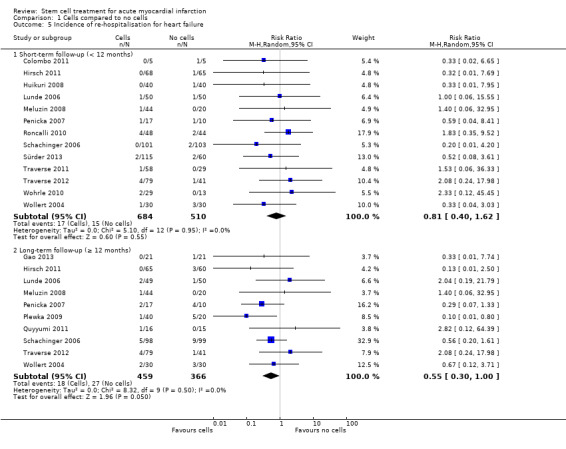
Comparison 1 Cells compared to no cells, Outcome 5 Incidence of re‐hospitalisation for heart failure.
Quality of life and performance status
Quality of life measures were reported in six trials (Jin 2008; Karpov 2005; Lunde 2006; Penicka 2007; Roncalli 2010; You 2008). Three trials used the Minnesota Living with Heart Failure Questionnaire (MLHFQ) (Jin 2008; Karpov 2005; Roncalli 2010), and two trials used the Short Form 36 Health Survey (Lunde 2006; Penicka 2007); in one trial the quality of life measure was undefined (You 2008) (see Table 7). Three trials only reported summary results and therefore could not be included in the meta‐analysis (Penicka 2007; Roncalli 2010; You 2008). At short‐term follow‐up there was no difference in quality of life score between treatment groups (standardised mean difference (SMD) 0.58, 95% CI ‐0.67 to 1.83; 154 participants; three studies). Only one trial reported quality of life at long‐term follow‐up (Jin 2008); this small trial of 26 participants found a significant difference between groups in favour of cell therapy (SMD 3.23, 95% CI 2.01 to 4.46; 26 participants; one study).
6. Quality of life and performance measures.
| Study ID | No. analysed participants | Quality of life (QoL) assessment | Reported data (EP/MC/SR) | Performance assessment | Summary measures of performance | Reported data (EP/MC/SR) | Mean follow‐up | |
| Cells | No cells | |||||||
| Colombo 2011 | 5 | 4 | n/r | n/r | Exercise stress test | Peak HR, peak MET, peak double product (SBPxHR), peak predicted HR | EP (median) | 12 months |
| Grajek 2010 | 31 | 14 | n/r | n/r | Cardiopulmonary exercise treadmill test (modified Bruce protocol) | METs, maximum VO2 , VE/VCO2 slope, RER, peak SBP, peak HR, VO2 anaerobic threshold, HR recovery | EP | 12 months |
| Hirsch 2011 | 65 | 60 | n/r | n/r | NYHA class | EP | 60 months | |
| Huikuri 2008 | 27 | 27 | n/r | n/r | Symptom‐limited maximal exercise test | METs, peak HR, T‐wave alternans | EP, MC | 6 months |
| Jazi 2012 | 16 | 16 | n/r | n/r | NYHA class | EP | 6 months | |
| Jin 2008 | 14 | 12 | MLHFQ | EP | NYHA class | EP | 12 months | |
| Karpov 2005 | 16 (a) | 28 (a) | MLHFQ | EP | Six minute walk test; functional class (undefined) | Distance (metres) | EP | 6 months |
| Lunde 2006 | 50 (b) | 50 (b) | SF‐36 | EP, MC | Electrically braked bicycle ergometer; NYHA class | Time (min), maximum VO2 , VE/VCO2 slope etc., peak HR | EP, MC | 6 months |
| Penicka 2007 | 14 | 10 | SF‐36 | SR | NYHA class | EP | 24 months | |
| Piepoli 2010 | 17 | 15 | n/r | n/r | Cardiopulmonary exercise treadmill test (modified Bruce protocol) | Exercise duration (min), maximum VO2 , VE/VCO2 slope | MC | 12 months |
| Roncalli 2010 | 52 | 49 | MLHFQ | SR | n/r | 12 months | ||
| Sürder 2013 | 117 | 61 | n/r | n/r | NYHA class | EP | 4 months | |
| Turan 2012 | 42 | 20 | n/r | n/r | NYHA class | EP | 12 months | |
| You 2008 | 7 | 16 | QoL (no details) | NYHA class | SR | 8 weeks | ||
MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association; SF‐36, Short‐Form 36 Quality of Life; MET, metabolic equivalent test (mL/kg/min); HR, heart rate (bpm); SBP, systolic blood pressure (mmHg); RER, respiratory exchange ratio; VE, minute ventilation; VO2, oxygen volume; VCO2, carbon dioxide volume; EP, endpoint; MC, mean change from baseline; SR, summary results; n/r, not reported.
(a)Karpov 2005: QoL was measured in 37 participants (cells: 18 cells, no cells: 19)
(b)Lunde 2006: QoL was measured in 46 BMMNC and 45 controls; exercise tolerance was measured in 49 BMMNC and 50 controls
Eight trials measured New York Heart Association (NYHA) class as a measure of performance status at follow‐up (Hirsch 2011; Jazi 2012; Jin 2008; Lunde 2006; Penicka 2007; Sürder 2013; Turan 2012; You 2008), although one trial reported summary results only (You 2008). Functional classification of heart failure was also measured in one further trial but it was unclear whether this was NYHA class (Karpov 2005). At short‐term follow‐up, in five trials there was no difference in NYHA class at the time of follow‐up between patients who received cell therapy and those who did not (mean difference (MD) ‐0.07, 95% CI ‐0.24 to 0.09; 398 participants; five studies). Similarly, at long‐term follow‐up in four trials there was no difference in NYHA class (MD ‐0.23, 95% CI ‐0.53 to 0.07; 237 participants; four studies) (Analysis 1.10), with considerable heterogeneity between studies (I2 = 80%).
1.10. Analysis.
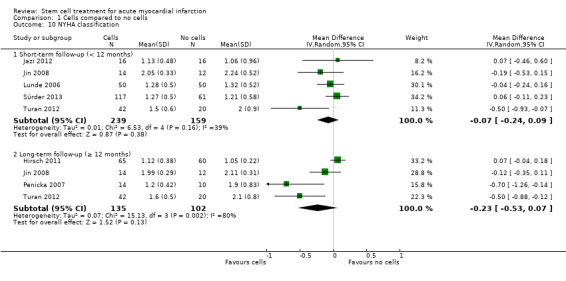
Comparison 1 Cells compared to no cells, Outcome 10 NYHA classification.
The use of exercise tests to measure performance was reported in six trials (Colombo 2011; Grajek 2010; Huikuri 2008; Karpov 2005; Lunde 2006; Piepoli 2010). Exercise performance was evaluated using a treadmill test (Grajek 2010; Piepoli 2010), a six minute walk test (Karpov 2005), an electrically braked bicycle ergometer (Lunde 2006), and a symptom‐limited maximal exercise test (Huikuri 2008). The method of measuring exercise tolerance was not reported in one trial (Colombo 2011) (see Table 7); we excluded this trial from meta‐analyses of exercise tolerance as median rather than mean values were reported. Meta‐analysis of the remaining five trials showed no difference in exercise tolerance at short‐term follow‐up between patients who received cell therapy and those who did not (SMD 0.19, 95% CI ‐0.06 to 0.43; 267 participants; five studies) (Analysis 1.11). Similarly there were no differences in maximum VO2 (MD 1.15 mL/kg/min, 95% CI ‐0.77 to 3.07; 175 participants; three studies) (Analysis 1.12), VE/VCO2 slope (MD 0.28, 95% CI ‐1.02 to 1.57; 174 participants; three studies) (Analysis 1.13) or peak heart rate (MD 0.55 bpm, 95% CI ‐6.79 to 7.89; 198 participants; three studies) (Analysis 1.14). Two trials reported exercise tolerance at long‐term follow‐up (Grajek 2010; Piepoli 2010); although the latter trial did not report endpoint values. In the remaining trial there was no difference between treatment groups (SMD ‐0.05, 95% CI ‐0.68 to 0.58; 45 participants; one study) (Analysis 1.11).
1.11. Analysis.
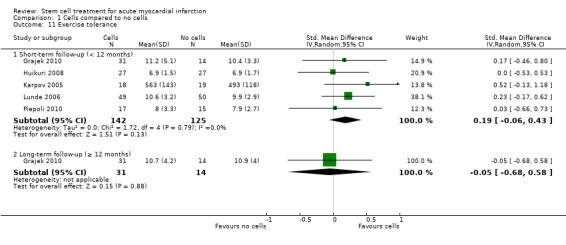
Comparison 1 Cells compared to no cells, Outcome 11 Exercise tolerance.
1.12. Analysis.
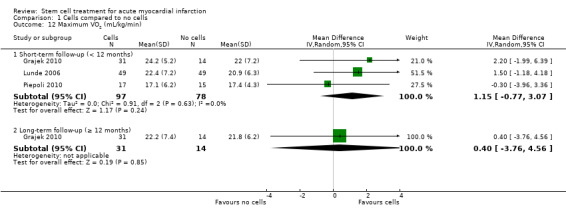
Comparison 1 Cells compared to no cells, Outcome 12 Maximum VO2 (mL/kg/min).
1.13. Analysis.
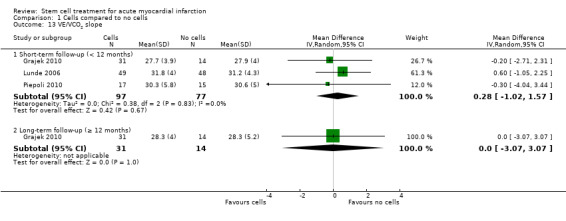
Comparison 1 Cells compared to no cells, Outcome 13 VE/VCO2 slope.
1.14. Analysis.
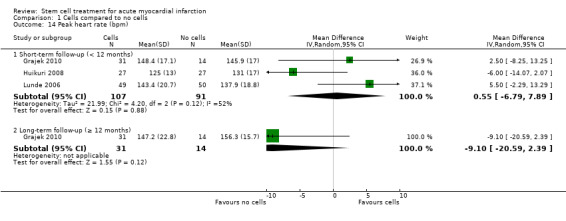
Comparison 1 Cells compared to no cells, Outcome 14 Peak heart rate (bpm).
Left ventricular ejection fraction (LVEF)
In order to limit possible heterogeneity, we have subgrouped trials reporting LVEF by the method of measurement. Results are shown in forest plots for the combined analyses of mean change from baseline and endpoint values as well as separately, as described in the Methods section.
Twelve trials used multiple methods to measure left ventricular function (Angeli 2012; Cao 2009; Grajek 2010; Huang 2006; Huikuri 2008; Lee 2014; Lunde 2006; Nogueira 2009; Piepoli 2010; Plewka 2009; Roncalli 2010; Schachinger 2006). Two trials measured these outcomes by three methods: MRI, echocardiography and single photon emission computed tomography (SPECT) (Lunde 2006), or MRI, echocardiography and radionuclide ventriculography (RNV) (Roncalli 2010). The 10 remaining trials each measured these outcomes using two methods: five used echocardiography and SPECT (Angeli 2012; Cao 2009; Lee 2014; Piepoli 2010; Plewka 2009), two used MRI and left ventricular angiography (Huang 2006; Schachinger 2006), two used echocardiography and RNV (Grajek 2010; Nogueira 2009), and one used left ventricular angiography and echocardiography (Huikuri 2008). Baseline LVEF values for each trial are given in Table 8 for each method of measurement.
7. Surrogate (continuous) outcome: LVEF.
| Study ID | No. randomised participants | No. analysed participants | Baseline LVEF | Mean follow‐up of LVEF | |||
| Cells | No cells | Cells | No cells | Cells | No cells | ||
| Measured by MRI | |||||||
| Hirsch 2011 (HEBE) | 69 | 65 | 59 | 52 | 43.7 (9.0)% | 42.4 (8.3)% | 24 months |
| Huang 2006 | 20 | 20 | 20 | 20 | 44.5 (7.1)% | 43.4 (6.7)% | 6 months |
| Janssens 2006 | 33 | 34 | 30 | 30 | 48.5 (7.2)% | 46.9 (8.2)% | 12 months |
| Lunde 2006 (ASTAMI) | 50 | 51 | 44 | 44 | 54.8 (13.6)% | 53.6 (11.6)% | 36 months |
| Quyyumi 2011 (AMR‐1) | 16 | 15 | 11 | 10 | LD: 47.0 (13)% MD: 47.3 (11)% HD: 49.9 (7)% |
53.2(10)% | 6 months |
| Roncalli 2010 (BONAMI) | 52 | 49 | 47 | 43 | 37.0 (9.8)% | 38.7 (9.2)% | 3 months |
| Schachinger 2006 (REPAIR‐AMI) | 101 | 103 | 26 | 33 | 47.8 (6.2)% | 47.7 (6.2)% | 60 months (a) |
| Sürder 2013 (SWISS‐AMI) | 133 | 67 | 107 | 60 | E: 36.5 (9.9)% L: 36.3 (8.2)% |
40.0 (9.9)% | 4 months |
| Tendera 2009(REGENT) | 160 | 40 | 97 | 20 | S: 33.9 (8.6)% U: 35.6 (6.5)% |
38.9 (5.2)% | 6 months |
| Traverse 2010 | 30 | 10 | 30 | 10 | 49 (9.5)% | 48.6 (8.5)% | 6 months |
| Traverse 2011 (LATE‐TIME) | 59 | 29 | 55 | 26 | 48.7 (12)% | 45.3 (9.9)% | 6 months |
| Traverse 2012 (TIME) | 80 | 40 | 65 | 30 | 46.2 (9.6)% | 46.3 (8.5)% | 12 months |
| Wohrle 2010 (SCAMI) | 29 | 13 | 28 | 12 | 53.5 (9.3)% | 55.7 (9.4)% | 36 months |
| Wollert 2004 (BOOST) | 33 | 32 | 30 | 30 | 50 (10)% | 51.3 (9.3)% | 60 months |
| Yao 2009 | 30 | 15 | 27 | 11 | SD: 32.5 (3.6)% DD: 33.7 (4.7)% |
32.3 (2.0)% | 12 months |
| Zhukova 2009 | 8 | 3 | 6 (b) | 1 (b) | 33.4 (3)% | 28 (4)% | 36 months (b) |
| Measured by echocardiography | |||||||
| Angeli 2012 | 11 | 11 | 11 | 11 | n/r | n/r | 12 months |
| Cao 2009 | 41 | 45 | 41 | 45 | 41.3 (2.8)% | 40.7 (3.1)% | 48 months |
| Colombo 2011 | 5 | 5 | 5 | 4 | 44.6 (8.8)% | 43.2 (9.1)% | 12 months |
| Gao 2013 | 21 | 22 | 19 | 20 | 50.8 (6.5)% | 51.4 (7.2)% | 24 months |
| Ge 2006 | 10 | 10 | 10 | 10 | 53.8 (9.2)% | 58.2 (7.5)% | 6 months |
| Grajek 2010 | 31 | 14 | 27 | 12 | 50.3 (9.8)% | 50.8 (12)% | 12 months |
| Huang 2007 | 20 | 20 | 20 | 20 | 48.5 (5.5)% | 48.2 (6.30% | 6 months |
| Huikuri 2008 (FINCELL) | 40 | 40 | 39 | 38 | 56 (10)% | 57 (10)% | 6 months |
| Jin 2008 | 14 | 12 | 14 | 12 | 54.3 (5.5)% | 55.8 (5.9)% | 12 months |
| Karpov 2005 | 22 | 22 | 16 | 10 | 49.3 (11.1)% | 47.0 (7.5)% | 6 months |
| Lee 2014 (SEED‐MSC) | 40 | 40 | 30 | 28 | 48.1 (8.0)% | 51.0 (9.2)% | 6 months |
| Lunde 2006 (ASTAMI) | 50 | 51 | 50 | 50 | 45.7 (9.4)% | 46.9 (8.6)% | 36 months |
| Nogueira 2009 (EMRTCC) | 24 | 6 | 22 | 6 | AG: 48.3 (10.4)% VG: 48.6 (7.1)% |
47.6 (14.3)% | 6 months |
| Penicka 2007 | 17 | 10 | 14 | 10 | 39.2 (9.2)% | 39.4 (5.6)% | 24 months |
| Piepoli 2010 (CARDIAC) | 19 | 19 | 17 | 15 | 38.4 (6.4)% | 38.9 (5.6)% | 24 months |
| Plewka 2009 | 40 | 20 | 38 | 18 | 35 (6)% | 33 (7)% | 24 months |
| Roncalli 2010 (BONAMI) | 52 | 49 | 47 | 43 | 38.1 (7.9)% | 39.8 (7.0)% | 12 months (c) |
| Ruan 2005 | 9 | 11 | 9 | 11 | 53.4 (8.9)% | 53.5 (5.8)% | 6 months |
| Xiao 2012 | 17 | 21 | 17 | 21 | 35.6 (3.1)% | 35.7 (3.1)% | 3 months |
| You 2008 | 7 | 16 | 7 | 16 | 37 (4.6)% | 38.6 (5.4)% | 8 weeks |
| Measured by SPECT | |||||||
| Angeli 2012 | 11 | 11 | 11 | 11 | n/r | n/r | 12 months |
| Cao 2009 | 41 | 45 | 41 | 45 | 41.2 (3.1)% | 40.8 (3.3)% | 48 months |
| Lee 2014 (SEED‐MSC) | 40 | 40 | 30 | 28 | 49.0 (11.7)% | 52.3 (9.3)% | 6 months |
| Lunde 2006 (ASTAMI) | 50 | 51 | 50 | 50 | 41.3 (10.4)% | 42.6 (11.7)% | 6 months |
| Meluzin 2008 | 44 | 22 | 40 | 20 | LD: 41 (2)% HD: 30 (2)% |
40 (2)% | 12 months |
| Piepoli 2010 (CARDIAC) | 19 | 19 | 17 | 15 | 36.6 (8.2)% | 37.5 (8.9)% | 24 months |
| Plewka 2009 | 40 | 20 | 26 | 10 | 41.2 (10.1)% | 40.0 (14.2)% | 6 months |
| Measured by LV angiography | |||||||
| Chen 2004 | 34 | 35 | 34 | 35 | 49 (9)% | 48 (10)% | 6 months |
| Huang 2006 | 20 | 20 | 20 | 20 | 56.7 (9.7)% | 57.3 (8.2)% | 6 months |
| Huikuri 2008 (FINCELL) | 40 | 40 | 36 | 36 | 59 (11)% | 62 (12)% | 6 months |
| Jazi 2012 | n/r | n/r | 16 | 16 | 33.37 (11.2)% | 29.0 (7.5)% | 6 months |
| Schachinger 2006 (REPAIR‐AMI) | 101 | 103 | 95 | 92 | 48.3 (9.2)% | 46.9 (10.4)% | 4 months |
| Suarez de Lezo 2007 | 10 | 10 | 10 | 10 | 37 (5)% | 39 (6)% | 3 months |
| Turan 2012 | 42 | 20 | 42 | 20 | 43 (10)% | 45 (10)% | 12 months |
| Wang 2014 | 30 | 30 | 27 | 28 | 37.8 (6.3)% | 20.2 (2.5)% (d) | 6 months |
| Yao 2006 | 92 | 92 | 90 | 84 | n/r | n/r | 6 months |
| Measured by RNV | |||||||
| Grajek 2010 | 31 | 14 | 27 | 12 | 45.4 (10.2)% | 42.7 (7.4)% | 12 months |
| Nogueira 2009 (EMRTCC) | 24 | 6 | 22 | 6 | AG: 41.0 (10.3)% VG: 39.9 (7.4)% |
40.1 (12.4)% | 6 months |
| Roncalli 2010 (BONAMI) | 52 | 49 | 47 | 43 | 35.6 (7.0)% | 37.0 (6.7)% | 3 months |
| Measured by gated PET | |||||||
| Colombo 2011 | 5 | 5 | 5 | 4 | 36.6 (5.4)% | 37.6 (7.0)% | 12 months |
n/r ‐ not reported
LD ‐ low dose, MD ‐ moderate dose, HD ‐ high dose, AG ‐ arterial group, VG ‐ venous group, E ‐ early cells, L ‐ late cells, S ‐ selected cells, U ‐ unselected cells, SD ‐ single dose, DD ‐ double dose
(a)Schachinger 2006: MRI was performed at five‐year follow‐up but summary results only were reported; 24‐month data are used in meta‐analysis.
(b)Zhukova 2009: 24‐month data were used in the analysis as only one control was available at 36 months.
(c)Roncalli 2010: echocardiography was performed at 12‐month follow‐up but summary results only were reported; three‐month data are used in meta‐analysis.
(d)Wang 2014: the reported baseline LVEF value in the control group is assumed to be an error since the difference between values at baseline and endpoint (49.1%) is not significant. We have been unable to clarify the correct value with the study authors.
(i) Magnetic resonance imaging (MRI)
Five trials measured baseline LVEF by MRI after cell administration, at one to three days after cells (Tendera 2009), at three to five days after cells (Janssens 2006), between four days prior to six days after cells (Schachinger 2006), after one week (Huang 2006), and after two to three weeks (Lunde 2006); these trials have been pooled alongside the outcome data for all other trials.
Fifteen trials reported LVEF measured by MRI at short‐term follow‐up (Hirsch 2011; Huang 2006; Janssens 2006; Lunde 2006; Quyyumi 2011; Roncalli 2010; Schachinger 2006; Sürder 2013; Tendera 2009; Traverse 2010; Traverse 2011; Traverse 2012; Wohrle 2010; Wollert 2004; Yao 2009), with all but two trials, Huang 2006 and Yao 2009, reporting mean change from baseline values. In the combined analysis of mean change from baseline and endpoint values, there was no evidence for a difference in mean LVEF between treatment arms (MD 1.05, 95% CI ‐0.56 to 2.67; 1135 participants; 15 studies); we observed substantial heterogeneity across studies (I2 = 64%) (Analysis 1.15).
1.15. Analysis.
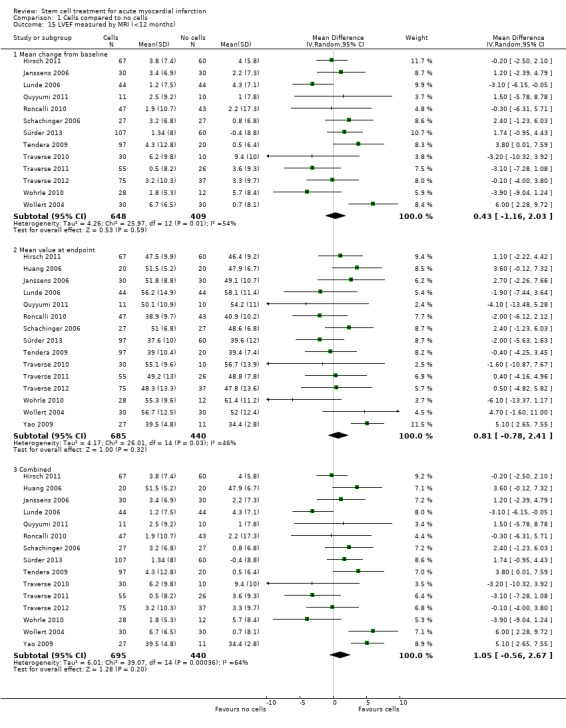
Comparison 1 Cells compared to no cells, Outcome 15 LVEF measured by MRI (<12 months).
At long‐term follow‐up, mean change from baseline values were reported in five trials (Hirsch 2011; Janssens 2006; Sürder 2013; Wohrle 2010; Wollert 2004); a further five trials reported endpoint values only (Lunde 2006; Schachinger 2006; Traverse 2012; Yao 2009; Zhukova 2009), although in one trial LVEF was only reported for two patients (Zhukova 2009); we therefore excluded this trial from the meta‐analysis. In the five trials that reported mean change from baseline values, there was no evidence for a difference in mean change in LVEF from baseline between groups (MD 0.03, 95% CI ‐1.72 to 1.78; 438 participants; five studies). Similarly, endpoint values reported in eight trials showed no difference between patients who received cell therapy and those who did not (MD 1.40, 95% CI ‐1.54 to 4.34; 551 participants; eight studies), with no difference observed in the combined analysis of mean change from baseline and endpoint values (MD 1.27, 95% CI ‐1.14 to 3.68; 718 participants; nine studies). There was evidence of substantial heterogeneity across studies (I2 = 66%) (Analysis 1.16).
1.16. Analysis.
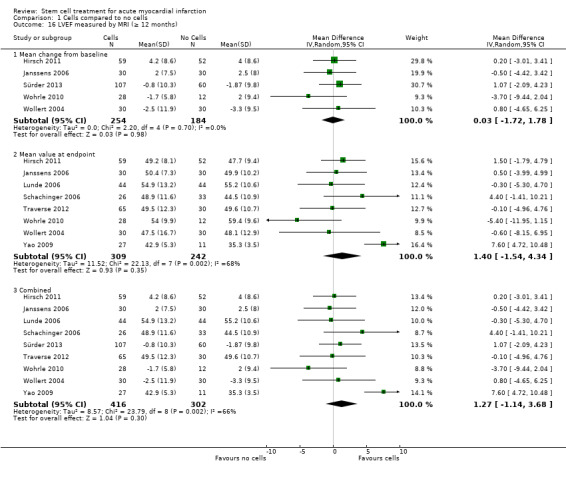
Comparison 1 Cells compared to no cells, Outcome 16 LVEF measured by MRI (≥ 12 months).
We observed substantial heterogeneity at both short‐term (I2 = 64%) and long‐term follow‐up (I2 = 66%).
We carried out exploratory subgroup analyses to investigate potential sources of heterogeneity as described in the Methods section. There was no significant evidence for subgroup differences when we stratified trials by baseline LVEF (Analysis 6.3; Analysis 6.4), cell dose (Analysis 8.3; Analysis 8.4), timing of cell administration (Analysis 9.3; Analysis 9.4) or use of heparinised cell solution (Analysis 9.3; Analysis 9.4) at either short‐term or long‐term follow‐up. There were insufficient trials using cells other than mononuclear cells to perform subgroup analysis for cell type.
6.3. Analysis.
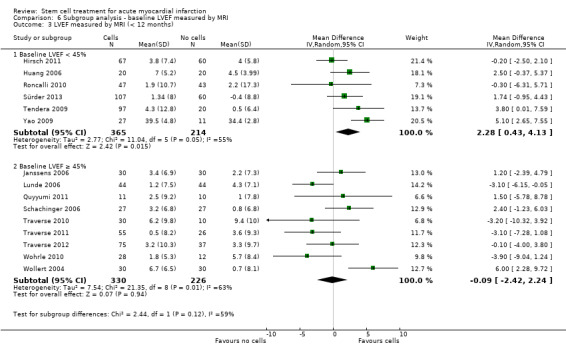
Comparison 6 Subgroup analysis ‐ baseline LVEF measured by MRI, Outcome 3 LVEF measured by MRI (< 12 months).
6.4. Analysis.
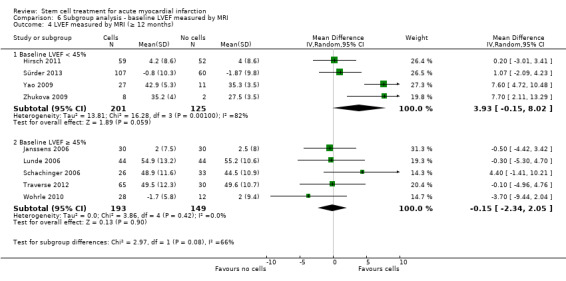
Comparison 6 Subgroup analysis ‐ baseline LVEF measured by MRI, Outcome 4 LVEF measured by MRI (≥ 12 months).
8.3. Analysis.
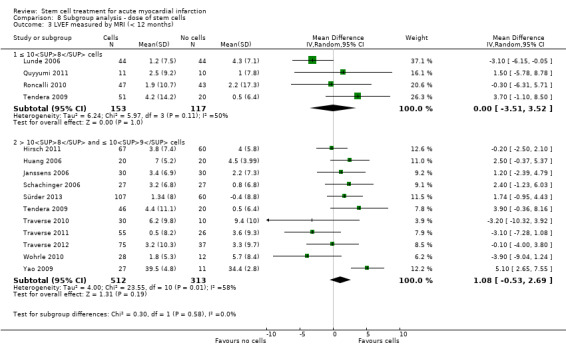
Comparison 8 Subgroup analysis ‐ dose of stem cells, Outcome 3 LVEF measured by MRI (< 12 months).
8.4. Analysis.
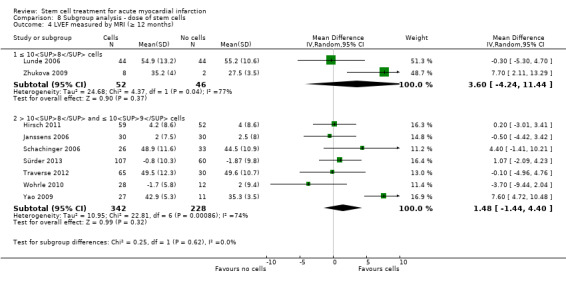
Comparison 8 Subgroup analysis ‐ dose of stem cells, Outcome 4 LVEF measured by MRI (≥ 12 months).
9.3. Analysis.
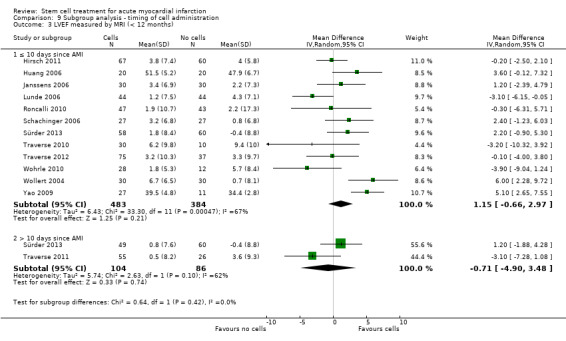
Comparison 9 Subgroup analysis ‐ timing of cell administration, Outcome 3 LVEF measured by MRI (< 12 months).
9.4. Analysis.
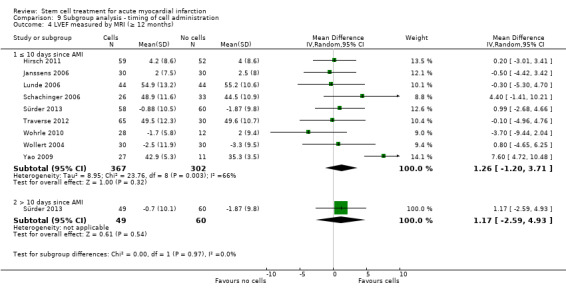
Comparison 9 Subgroup analysis ‐ timing of cell administration, Outcome 4 LVEF measured by MRI (≥ 12 months).
(ii) Echocardiography
LVEF measured by echocardiography at short‐term follow‐up was reported in 20 trials (Angeli 2012; Cao 2009; Colombo 2011; Gao 2013; Ge 2006; Grajek 2010; Huang 2007; Huikuri 2008; Jin 2008; Karpov 2005; Lee 2014; Lunde 2006; Nogueira 2009; Penicka 2007; Piepoli 2010; Plewka 2009; Roncalli 2010; Ruan 2005; Xiao 2012; You 2008). Of these 20 trials, all reported endpoint LVEF values but only six reported mean change from baseline values (Gao 2013; Huang 2007; Huikuri 2008; Lee 2014; Lunde 2006; Plewka 2009). Meta‐analysis of these six trials showed evidence for a difference in mean change from baseline LVEF in favour of cell therapy (MD 2.72, 95% CI 1.50 to 3.95; 372 participants; six studies). This improvement in LVEF associated with cell therapy was also seen in the combined analysis of all 20 trials (MD 2.31, 95% CI 1.30 to 3.33; 862 participants; 20 studies) (Analysis 1.17). The observed difference was robust to sensitivity analysis excluding the trial that administered cells via the coronary artery (Nogueira 2009).
1.17. Analysis.
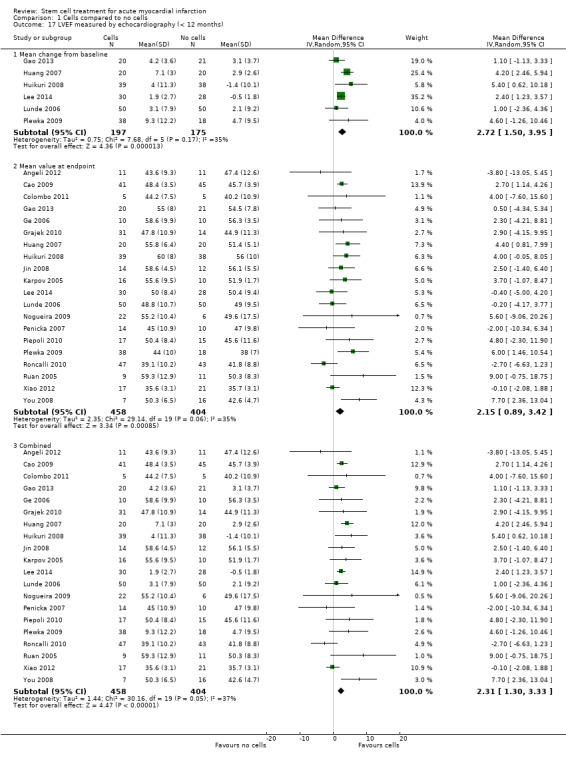
Comparison 1 Cells compared to no cells, Outcome 17 LVEF measured by echocardiography (< 12 months).
At long‐term follow‐up, only three trials reported mean change in LVEF from baseline (Gao 2013; Piepoli 2010; Plewka 2009). Meta‐analysis of these three trials showed no evidence for a difference in mean change from baseline values between trial arms (MD 1.35, 95% CI ‐2.25 to 4.96; 127 participants; three studies). However, in nine trials that reported LVEF values at the time of follow‐up (Angeli 2012; Cao 2009; Colombo 2011; Gao 2013; Grajek 2010; Jin 2008; Lunde 2006; Penicka 2007; Piepoli 2010), LVEF values at follow‐up were higher in patients who received cell therapy than those who did not (MD 2.87, 95% CI 1.42 to 4.31; 377 participants; nine studies). Evidence for an improvement in LVEF associated with cell therapy was also seen in the combined analysis (MD 2.09, 95% CI 0.74 to 3.44; 433 participants; 10 studies) (Analysis 1.18).
1.18. Analysis.
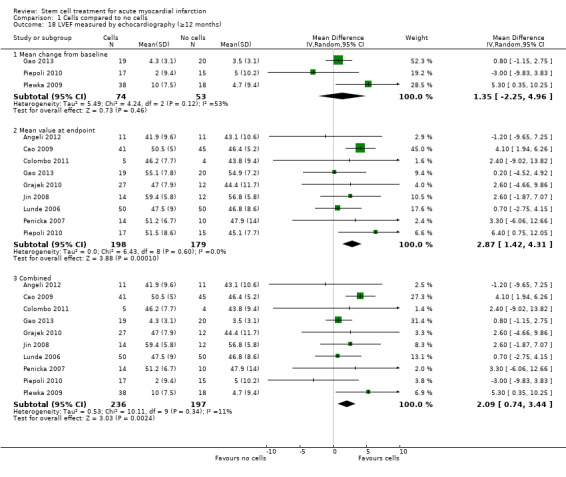
Comparison 1 Cells compared to no cells, Outcome 18 LVEF measured by echocardiography (≥12 months).
The observed heterogeneity was moderate (I2 = 37%) at short‐term follow‐up and low at long‐term follow‐up (I2 = 11%) and therefore we performed no exploratory subgroup analyses for LVEF measured by echocardiography.
(iii) SPECT
Seven trials reported LVEF measured by SPECT at short‐term follow‐up (Angeli 2012; Cao 2009; Lee 2014; Lunde 2006; Meluzin 2008; Piepoli 2010; Plewka 2009), although only five trials reported mean change from baseline values (Lee 2014; Lunde 2006; Meluzin 2008; Piepoli 2010; Plewka 2009). In one trial, endpoint values (but not mean change values) reflect an expanded cohort (Meluzin 2008). Meta‐analysis showed a greater mean change from baseline values in patients who received cell therapy compared with those who did not (MD 2.72, 95% CI 0.23 to 5.21; 286 participants; five studies). This effect was also demonstrated in six trials that reported LVEF values measured by SPECT at follow‐up (MD 2.19, 95% CI 0.58 to 3.81; 375 participants; six studies) and in the combined analysis of mean change from baseline and endpoint values (MD 2.52, 95% CI 0.59 to 4.44; 394 participants; seven studies) (Analysis 1.19).
1.19. Analysis.
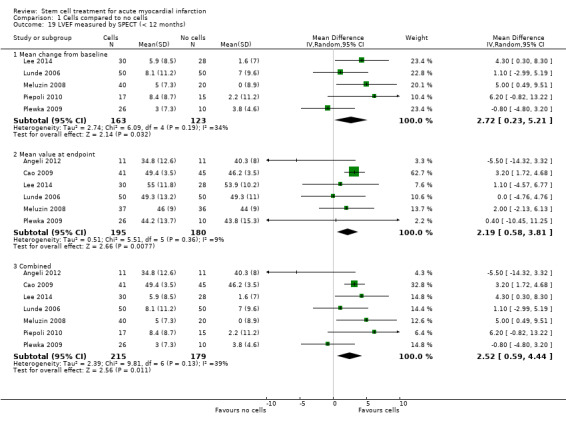
Comparison 1 Cells compared to no cells, Outcome 19 LVEF measured by SPECT (< 12 months).
An improvement in LVEF measured by SPECT associated with cell therapy was also found at long‐term follow‐up in four trials (Angeli 2012; Cao 2009; Meluzin 2008; Piepoli 2010) (MD 4.42, 95% CI 2.68 to 6.16; 200 participants; four studies); this improvement was observed in both trials that reported mean change from baseline (MD 5.63, 95% CI 1.77 to 9.49; 92 participants; two studies) and trials that only reported endpoint values (MD 3.46, 95% CI 0.82 to 6.11; 181 participants; three studies) (Analysis 1.20).
1.20. Analysis.
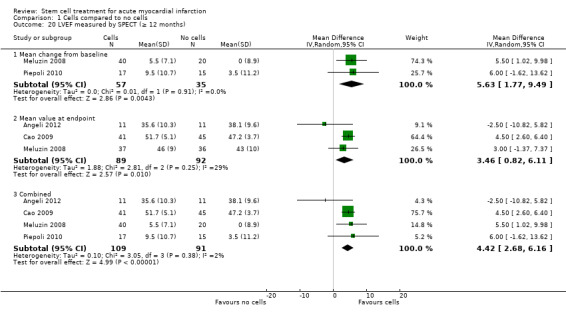
Comparison 1 Cells compared to no cells, Outcome 20 LVEF measured by SPECT (≥ 12 months).
There was no evidence for heterogeneity at long‐term follow‐up (I2 = 2%) and there was moderate heterogeneity at short‐term follow‐up (I2 = 39%) and we therefore did not perform subgroup analyses.
(iv) Left ventricular angiography
Nine trials reported LVEF measured by left ventricular angiography at short‐term follow‐up (Chen 2004; Huang 2006; Huikuri 2008; Jazi 2012; Schachinger 2006; Suarez de Lezo 2007; Turan 2012; Wang 2014; Yao 2006). All trials reported endpoint LVEF values but only three reported mean change from baseline values (Huikuri 2008; Schachinger 2006; Suarez de Lezo 2007). Meta‐analysis of these three trials showed a evidence for a difference in mean change from baseline LVEF in favour of cell therapy (MD 6.43, 95% CI 0.60 to 12.27; 279 participants; three studies). In the combined analysis of all nine trials, this effect remained (MD 5.09, 95% CI 0.95 to 9.24; 711 participants; nine studies) with considerable heterogeneity across studies (I2 = 95%) (Analysis 1.21). Only one trial reported long‐term follow‐up of LVEF measured by left ventricular angiography (Turan 2012); this trial found a significantly higher mean LVEF at follow‐up in patients who received cell therapy compared with those who did not (MD 8.00, 95% CI 4.27 to 11.73; 62 participants; one study) (Analysis 1.22). We observed considerable heterogeneity at short‐term follow‐up (I2 = 95%). Visual inspection of the forest plot revealed two potential outliers (Chen 2004; Yao 2006), although considerable heterogeneity remained when we excluded these two studies from the analysis. Exploratory subgroup analyses revealed that when trials were subgrouped according to cell dose, meta‐analysis of two trials that used > 109 cells showed a significant difference when compared to six trials that used > 108 and ≤ 109 cells (test for subgroup differences, P value = 0.0003) (Analysis 8.5), although substantial heterogeneity remained in both subgroups. We found no subgroup differences when we subgrouped trials by either timing of cell administration (P value = 0.12) (Analysis 9.5) or use of heparinised cell solution (P value = 0.26) (Analysis 10.5). The limited number of trials within groups precluded subgroup analysis by baseline LVEF or type of cells.
1.21. Analysis.
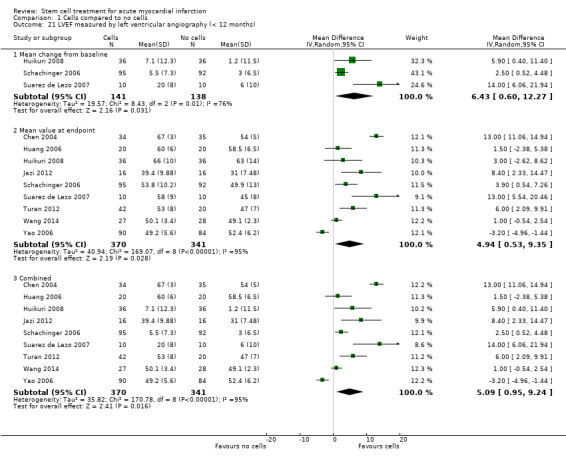
Comparison 1 Cells compared to no cells, Outcome 21 LVEF measured by left ventricular angiography (< 12 months).
1.22. Analysis.
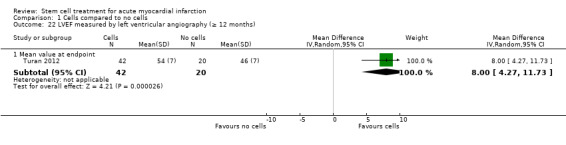
Comparison 1 Cells compared to no cells, Outcome 22 LVEF measured by left ventricular angiography (≥ 12 months).
8.5. Analysis.
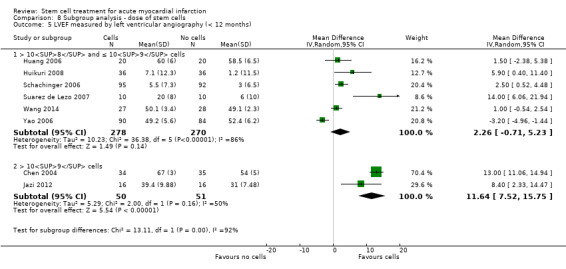
Comparison 8 Subgroup analysis ‐ dose of stem cells, Outcome 5 LVEF measured by left ventricular angiography (< 12 months).
9.5. Analysis.
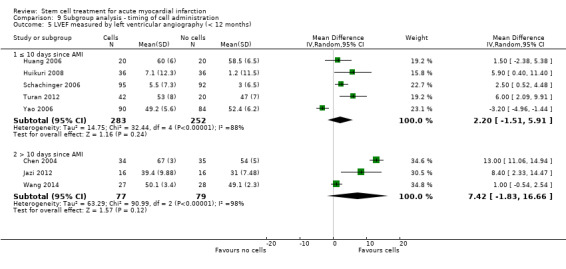
Comparison 9 Subgroup analysis ‐ timing of cell administration, Outcome 5 LVEF measured by left ventricular angiography (< 12 months).
10.5. Analysis.
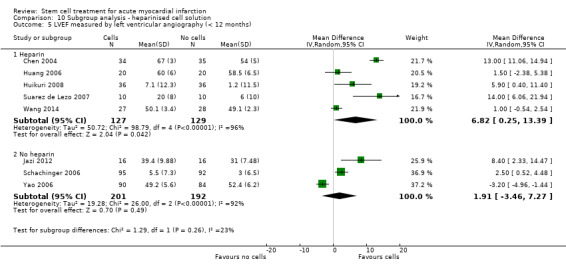
Comparison 10 Subgroup analysis ‐ heparinised cell solution, Outcome 5 LVEF measured by left ventricular angiography (< 12 months).
(v) Radionuclide ventriculography (RNV)
Three trials reported LVEF measured by radionuclide ventriculography (Grajek 2010; Nogueira 2009; Roncalli 2010). There were no differences between treatment groups in analyses of mean change in LVEF from baseline (MD 0.91, 95% CI ‐3.11 to 4.94; 118 participants; two studies), mean LVEF at endpoint (MD 1.08, 95% CI ‐4.88 to 7.04; 157 participants; three studies), or in the combined analysis (MD 1.79, 95% CI ‐1.86 to 5.43; 157 participants; three studies) (Analysis 1.23). Only one trial reported LVEF measured by radionuclide ventriculography at long‐term follow‐up (Grajek 2010); this trial found no evidence for a difference between treatment groups in LVEF measured at long‐term follow‐up (MD 6.30, 95% CI ‐1.03 to 13.63; 39 participants; one study) (Analysis 1.24).
1.23. Analysis.
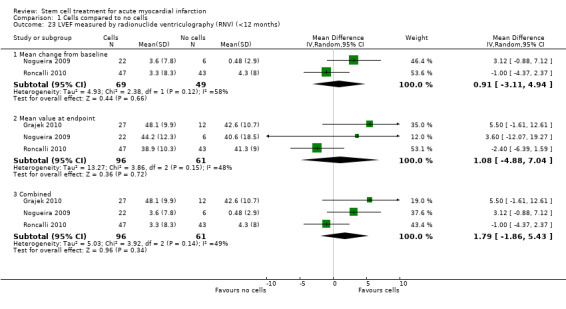
Comparison 1 Cells compared to no cells, Outcome 23 LVEF measured by radionuclide ventriculography (RNV) (<12 months).
1.24. Analysis.
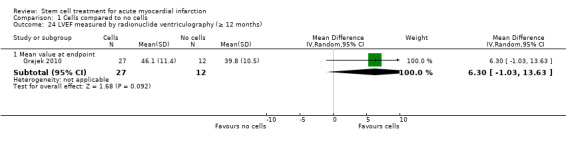
Comparison 1 Cells compared to no cells, Outcome 24 LVEF measured by radionuclide ventriculography (≥ 12 months).
Discussion
Cell transplantation has been developed clinically for over 40 years in patients with haematological malignancies (e.g. haematopoietic stem cell transplantation), but its application as a treatment for other conditions, such as heart disease, has only been possible since 2002. Over the last 13 years clinical evidence from randomised controlled trials (RCTs) has become available, allowing the robust evaluation of the safety of this alternative treatment in patients who have suffered a recent acute myocardial infarction (AMI). Meta‐analyses in cell therapy can help to show the safety of the approach and generate hypotheses, but due to the extent of the heterogeneity of the biologically active product, analysis of efficacy has to be marked with a great caveat. The present study is an update of the Cochrane systematic review published by us previously (Clifford 2012).
Nature of the intervention
Forty‐one RCTs, including 2732 participants, were eligible for inclusion in this updated Cochrane review. The characteristics of the interventions are summarised in Table 3. All included studies compared cell treatment with no cells in addition to the standard primary intervention for revascularisation (primary angioplasty and/or thrombolytic therapy) and standard medical therapy. Participants recruited to these trials have had a recent AMI and received treatment (intervention) or control (or placebo) following successful revascularisation of the infarct‐related coronary artery (IRCA). The cell‐based treatment was administered by an interventional cardiologist as a single bolus, usually by infusion into the IRCA using a balloon catheter. One trial compared two intervention groups, comparing treatment delivered by intracoronary vein infusion with arterial infusion (Nogueira 2009). However, unlike traditional drugs used in cardiology, which possess much simpler chemical and pharmacological characteristics, autologous cell therapies are experimental interventions with much more complex and individualised properties. Therefore it is not surprising that there was substantial clinical heterogeneity and diversity within and between trials: the characteristics of the participants, the type and size of infarct and the baseline outcome values (e.g. left ventricular ejection fraction (LVEF)) at admission all differed. Cell type, dose and time of administration as well as the media where cells were re‐suspended and whether the participants in the comparator arm received placebo or not also differed. There is no standard definition of an 'active' cell product at present. This is because the number of administered cells cannot be equated to active dose and the number of cells retained in the target region might be affected by disease and patient‐related factors. Having said that, all trials included in this review delivered cells of bone marrow origin, with bone marrow mononuclear cells being the starting cell population. Thirty‐eight trials isolated cells from bone marrow aspirates and enriched the mononuclear cell population by gradient centrifugation. One trial infused an enriched CD34 fraction (Quyyumi 2011), and one trial infused enriched CD133‐positive cells (Colombo 2011), whilst another trial compared the effect of unfractionated mononuclear cells with CD34+/CXCR4+ cells (Tendera 2009). Five trials cultured and administered bone marrow‐derived mesenchymal stromal cells (Gao 2013; Lee 2014; Wang 2014; Xiao 2012; You 2008). The trials also differed in their design (e.g. blinded versus open‐label), the length of follow‐up (short and long‐term) and the methodology used to measure surrogate outcome data (e.g. magnetic resonance imaging (MRI), echocardiography, single photon emission computed tomography (SPECT), etc.).
Main findings
There are 11 new trials included in this update of the Cochrane review, but the individual trials are still too small. Pooling the data together, we can conclude the following.
There was no evidence for a difference in the risk of all‐cause mortality, cardiovascular mortality, incidence of re‐hospitalisation for heart failure, re‐infarction, arrhythmias, restenosis or target vessel revascularisation in cell‐treated patients compared to controls.
Accordingly, we found no evidence for a difference in the composite measure of major adverse cardiac events (MACE) defined by death, re‐infarction and re‐hospitalisation for heart failure between treated patients and the control group.
There were no major differences in periprocedural adverse events associated with cell treatment.
The treatment was associated with no improvement in LVEF measured by MRI. We observed no differences between treatment groups in mean New York Heart Association (NYHA) class, quality of life measures and exercise/performance measures at short‐term follow‐up. There were too few trials with long‐term follow‐up that measured NYHA class, quality of life or exercise/performance to draw meaningful conclusions.
Taken together, the results of these meta‐analyses suggest that bone marrow‐derived cell therapy has no beneficial effect for patients who have suffered AMI. The quality of the evidence presented here is moderate due to imprecision: the information size criterion has not been met, meaning that this systematic review and meta‐analysis is underpowered.
Study limitations
There are a number of limitations to the strength of any conclusion that can be drawn from the evaluation of the included trials. These include sample sizes of the individual trials, statistical power, clinical heterogeneity and risk of bias of the included trials (please see below).
Sample size and statistical power
In general, the sample sizes were small in all trials included, perhaps with the exception of three trials that included at least 200 participants (Schachinger 2006; Sürder 2013; Tendera 2009). At present, results from the first large phase III randomised trials to robustly determine the efficacy of this treatment are lacking. Therefore, systematic reviews and meta‐analysis of pooled trial data can be used to generate hypotheses and to compensate for the lack of statistical power in individual trials.
Cumulative meta‐analyses may result in type I errors due to an increased risk of random error arising from repeated testing of accumulating data (Borm 2009; Hu 2007; Lan 2003). Trial sequential analysis provides a method of adjusting the thresholds for statistical significance while maintaining the overall desired type I error rate (Wettersley 2008). We applied trial sequential analysis to the primary outcome of all‐cause mortality, assuming a long‐term mortality incidence rate of 6.1% in the control group (as observed in our control data) and a relative risk reduction of 35% (equivalent to the reduce risk of mortality associated with percutaneous coronary intervention (PCI) (Hartwell 2005). In our analysis, the cumulative Z‐curve for all‐cause mortality did not cross the conventional thresholds or trial sequential monitoring boundaries (TSMB) for significance. The required information size was 3275 participants, suggesting that even the current meta‐analysis is considerably underpowered to detect a relative risk reduction of this magnitude. The required information size to detect significant effects in cardiovascular mortality and composite MACE was 3064 and 1572 participants, respectively.
The effect of intracoronary reinfusion of bone marrow‐derived mononuclear cells (BMMNC) is being assessed in a pan‐European Phase III trial (the BAMI trial) (NCT01569178). This trial is well‐powered and is planned to recruit 3000 participants who have suffered a recent myocardial infarction and have reduced LVEF (≤ 45%) following successful revascularisation. Primary and secondary outcomes include death, cardiac death, re‐hospitalisation for myocardial infarction, target vessel revascularisation (TVR), heart failure, implantation of implantable cardioverter‐defibrillator/ cardiac resynchronisation therapy (ICD/CRT) device, stroke, syncope or arrhythmias and incidence and severity of adverse events, with an estimated completion date of May 2018.
Risk of bias and heterogeneity
This systematic review is based on a comprehensive search strategy, but despite this the possibility of publication and reporting bias cannot be ruled out completely. Risk of bias is present in the included trials, as summarised in Figure 4. All trials stated that they randomised the participants, but only 49% (n = 20) and 34% (n = 14) of the included trials documented adequate methods for the generation of randomised sequences and concealment of treatment allocation, respectively. Blinding (performance and detection bias) was reported in 22% (n = 9) of the included trials, whilst the remaining 32 trials were described either as not blinded (n = 24) or blinding was unclear (n = 8). Attrition bias was low in 76% (n = 31) of the included trials, whilst it was unclear or high in the remaining trials. Finally, selective reporting bias was low in 41% (n = 17) of the included trials. Sensitivity analyses conducted for the major outcome of all‐cause mortality showed that excluding those trials with high risk of selection, attrition or performance bias had a negligible effect on all‐cause mortality.
4.
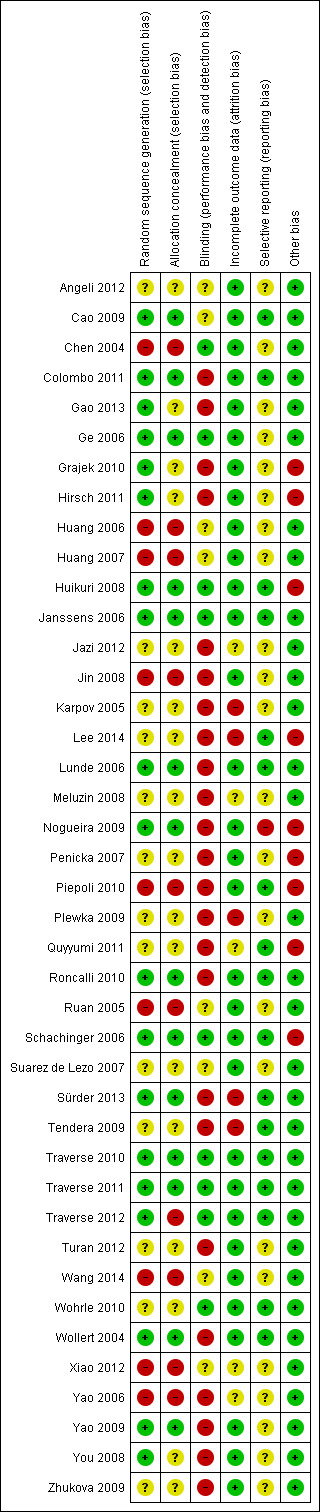
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
In summary, this review finds that the results from the meta‐analysis are of moderate quality for the primary outcomes (see Table 1) due to the information size criterion not being met (imprecision). Therefore, further research may change the estimate of the treatment effect. These results may be regarded as optimistic, however the evidence from this review, from our previous Cochrane reviews and from the recent individual patient data analysis, Gyöngyösi 2015, appears to support the conclusion that bone marrow cell therapies may not reduce the risk of clinical outcomes in patients with AMI.
Our previous versions of this Cochrane review have shown a considerable degree of heterogeneity among trials, which has been extensively explored (Clifford 2012; Fisher 2012; Martin‐Rendon 2008a; Martin‐Rendon 2008b). Interestingly, heterogeneity is negligible for the primary outcomes of this review, suggesting little variation in treatment effect. However, clinical heterogeneity is still present, which justifies using a random‐effects model in all meta‐analyses conducted. We have attempted to address some of the issues of heterogeneity by conducting exploratory subgroup analyses. One example is the timing of cell delivery. It is important to make the distinction between early and late administration of cells as remodelling of the damaged tissue is very different at seven to 10 days to four weeks. We have considered carefully the option of restricting the inclusion criteria to trials which deliver cells within 10 days. However, as there are several key trials that would be excluded from this review as a subset of patients received cells after 10 days (between three and 12 days (Penicka 2007; Plewka 2009; Tendera 2009)), we have opted to conduct subgroup analyses for timing of cell delivery. Similarly, we have stratified the length of follow‐up at less than 12 months and 12 months or more. In this case, the latter category seems to be more diverse, with one trial reporting a mean follow‐up of over eight years (Karpov 2005). Interestingly, this trial provides the most negative results in a number of clinical outcomes. One possible explanation is that the risk of mortality over longer‐term follow‐up would be increased in both treated and control patients, and therefore any observed differences between the two groups would decrease as the length of follow‐up increased.
Quality of life and exercise/performance status
Since our last update of this Cochrane review (Clifford 2012), more trials have reported patient‐centred outcomes, such as quality of life and exercise or performance status. However, quality of life and performance measures during long‐term follow‐up are still underreported. In some cases only one trial has reported these outcomes, thus precluding any further analysis. Where meta‐analysis was feasible, no differences between treated patients and controls were observed.
Left ventricular ejection fraction (LVEF)
We subgrouped LVEF data according to the method of measurement. Although each method has its limitations, it is widely accepted that MRI is the gold standard method to measure surrogate outcomes such as LVEF. A limited number of studies presented LVEF data as mean change from baseline. Many studies presented both mean change from baseline and mean value at endpoint and results are broadly similar whichever measure is used (see, for example, Analysis 1.16). We present forest plots for both mean change from baseline and endpoint values for clarity and transparency. There was evidence for an improvement in LVEF measured by MRI from baseline at both short and long‐term follow‐up. There was no improvement in LVEF measured by MRI from baseline or at endpoint, both at short and long‐term follow‐up. Although there might be an indication of an improvement of LVEF when measured by echocardiography, SPECT or left ventricular angiography, the effect sizes are within the range of 2% to 5%, which is accepted not to be clinically relevant.
Subgroup analyses
Where appropriate, exploratory subgroup analysis investigated the effects of baseline cardiac function (LVEF), cell dose, type and timing of administration, as well as the use of heparin in the final cell solution. Most of the subgroup analyses found no evidence for differences between groups, with the exception of long‐term mortality subgrouped by cell dose. The results suggest that there is no evidence for a reduction in mortality associated with a cell infusion of less than 108 cells, whilst there is a reduction in long‐term mortality in favour of cell therapy with 108 to 109 cells. There were very few trials that administered more than 109 cells to draw robust conclusions. However, in view of the low number of trials included, these results should be considered with caution.
Baseline LVEF has been previously reported to be an effect modifier (Beitnes 2009; Schachinger 2009), although we found no evidence for subgroup differences according to baseline LVEF. Ideally, subgroup analyses of baseline cardiac function would include studies where all subgroup patients have a baseline LVEF of, say, ≤ 45% or > 45%, and such an analysis could be implemented with the use of individual patient data (Gyöngyösi 2015). Unfortunately, few of the included studies used an LVEF threshold as part of their inclusion criteria. Furthermore, we used LVEF baseline measures obtained by MRI as the gold standard, which is usually done after revascularisation and so any baseline LVEF values used as eligibility criteria are unlikely to have been obtained by MRI. Subgroup analysis of studies stratified by mean LVEF using the median value as the subgroup threshold (as defined in the previous version of this review) provides a crude measure of whether baseline cardiac function is associated with efficacy, which will merely have reduced power to detect subgroup effects.
Agreements and disagreements with other studies or reviews
In this update of the Cochrane review we have focused on clinical outcomes such as death, cardiovascular death, reinfarction (MI), arrhythmias, restenosis, target vessel revascularisation and re‐hospitalisation for heart failure. We have included MACE, defined as death, reinfarction (MI) and re‐hospitalisation for heart failure.
Our results suggest that cell therapy does not appear to have a beneficial effect in patients who have experienced a recent AMI. Although this is in agreement with the previous version of this review (Clifford 2012), and with recent systematic reviews and meta‐analysis on cell therapies for patients with AMI (de Jong 2014; Delewi 2014; Gyöngyösi 2015), the present update of the Cochrane review presents long‐term data that are lacking from previous meta‐analysis (de Jong 2014; Gyöngyösi 2015). de Jong 2014 reported a meta‐analysis of 22 cell‐based therapy RCTs (2037 participants) and found that cell therapy had no effect on major adverse clinical cardiac events including all‐cause mortality for a median follow‐up of six months. In the first prospective individual patient data (IPD) meta‐analysis including 12 trials (1252 participants), Gyöngyösi 2015 confirmed no significant differences in all‐cause mortality. Like the present Cochrane review, previous meta‐analyses have shown low procedural adverse events and low incidence of clinical endpoints.
The picture is somewhat more confusing when measuring surrogate outcomes such as LVEF. Mean changes scores may be less efficient for outcomes that are difficult to measure with precision (Higgins 2011), and it may be that one has to take this into consideration when describing continuous surrogate outcomes such as LVEF. The present Cochrane review and meta‐analysis shows no improvement in LVEF in favour of cell therapy when measured by MRI during either short‐term or long‐term follow‐up. Whilst de Jong 2014 observed a significant improvement in LVEF during short‐term follow‐up (in 1513 participants), Gyöngyösi 2015 observed no significant improvement (in 734 participants) when analysing individual patient data. de Jong 2014 found that the improvement in LVEF in favour of cell therapies was not sustained long‐term and explained this by a gradual increase in LV volumes during the first year after AMI in reperfused patients (Engblom 2009).
Our data are in disagreement with results obtained in systematic reviews and meta‐analysis where the cell therapies have been administered to patients with chronic ischaemic heart disease and heart failure (Afzal 2015; Fisher 2014; Fisher 2015; Wen 2012), which may indicate that heart failure patients may benefit more from cell‐based therapies than AMI patients.
Summary
The first‐generation clinical trials were designed to prove safety of the procedure but were not statistically powered to assess efficacy of the treatment and longer‐term effects on survival free of major associated cardiac events. This systematic review and meta‐analysis of pooled trials suggests that cell‐based therapies do not lead to a reduction in hard clinical outcomes such as all‐cause mortality, cardiovascular mortality, rehospitalisation for heart failure, target vessel re‐vascularisation or composite measures of MACE, or indeed an improvement in LVEF as a surrogate of heart function. Although the quality of this evidence is moderate due to imprecision (Table 1), the findings of this review are consistent with the previous version (Clifford 2012), and with the recently published individual patient data analysis (Gyöngyösi 2015). Although these results are robust to sensitivity analyses, this systematic review is most likely underpowered. There is ultimately no substitute for adequately powered phase III RCTs, such as the BAMI trial.
The findings from this systematic review provide further support to previous statements by the National Institute of Clinical Excellence (NICE) and the European Society of Cardiology (ESC) Task Force (Bartunek 2006) that stem cell therapy remains "an experimental therapy". Evidence reported to date, although almost entirely from small trials, does not support the incorporation of stem cell therapy in the management of patients with AMI. A re‐evaluation of this systematic review is warranted on completion of the BAMI trial, expected in 2018.
Authors' conclusions
Implications for practice.
Evidence from the included trials indicates that adult cell‐based therapies seem to be safe. The incidence of mortality following successful revascularisation of the culprit artery is very low and the introduction of primary angioplasty as the standard primary intervention in acute myocardial infarction (AMI) has already reduced short‐term mortality by 33% and re‐infarction by 50%. However, there seems currently to be insufficient evidence to suggest that cell therapy reduces mortality and morbidity beyond standard therapy in this group of patients. Most of the evidence comes from small trials and small numbers of events. Larger and adequately powered clinical trials, such as the BAMI trial, are required to robustly assess the efficacy of cell‐based therapies post‐AMI.
Implications for research.
This review shows that currently there is no evidence for a reduction in mortality and morbidity when bone marrow‐derived stem cell treatment is administered to patients who had standard primary intervention following AMI. Further research may be justified to address current uncertainties, such as the mechanism of action and the need for patient selection. The first phase III trials to assess hard clinical outcomes are underway. Future clinical trials should be adequately powered, consider the best surrogate outcomes to measure and the best method to measure them, and should standardise composite major adverse cardiac events (MACE). They should also reduce the risk of selection, attrition, performance and reporting bias.
What's new
| Date | Event | Description |
|---|---|---|
| 30 June 2015 | New search has been performed | The searches from 2011 were re‐run in March 2015. This is a major update, which includes 41 independent trials. Two trials that were included in the previous version of the review are now excluded since the co‐intervention of G‐CSF was only administered to the intervention arm (Kang 2006; Li 2006). One trial that was previously included is now defined as awaiting classification as this trial did not publish any data that could be incorporated into the analyses (Fernandez‐Pereira 2006). In this update we have revised the primary and secondary outcomes, which now focus on clinical outcomes as well as the surrogate endpoint of left ventricular ejection fraction. Multiple intervention arm trials are now pooled throughout the review, avoiding double counting of controls. In light of the potential sources of heterogeneity, meta‐analyses using random‐effects models are now performed throughout. |
| 30 June 2015 | New citation required and conclusions have changed | This update includes 11 new trials and the conclusions of the review have changed. We no longer find evidence of an improvement in left ventricular ejection fraction associated with stem cell therapy. Meta‐analyses of the increased number of trials in this update have failed to find any evidence of differences in clinical outcomes between treatment groups. We conclude that there is insufficient evidence of a beneficial effect of stem cell therapy for acute myocardial infarction patients. |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 16 December 2011 | New citation required and conclusions have changed | This is a major update including 33 randomised trials (formerly 13) with changes to the conclusions. Whilst in the previous version of this review there was little evidence to assess the effect of this treatment, the results of this update of the review indicate that the treatment moderately improves heart function and contractility and that this effect is sustained in the long term. However, in order to observe significant changes in mortality and morbidity larger numbers of participants would need to be enrolled in such trials and more robust surrogate outcome measures to be agreed and standardised. |
| 16 December 2011 | New search has been performed | Addition of 20 new trials identified from a search from July 2007 to January 2011. Additional secondary references with long‐term follow‐up from previously included trials were also identified in this search. Change from Meluzin 2006 (LD and HD) to Meluzin 2008 (LD and HD). Meluzin 2008, with long‐term follow‐up data, has become the main study. Meluzin 2006 is now considered a substudy. |
| 14 September 2008 | Amended | Amendment to the order of authors in the byline. |
| 2 April 2008 | Amended | Converted to new review format. |
Acknowledgements
We acknowledge with thanks the contributions of Susan Brunskill, Dr David Clifford, Professor C. Hyde, Dr Simon Stanworth and Professor Suzanne Watt, for their contribution to previous versions of this review. We thank Pat Tsang for the translation of Xiao 2012 from Mandarin to English for this update and Mrs F‐J Lu for translating papers from Mandarin to English in the original version of this review.
We are grateful to those trial investigators who kindly responded to our questions and requests for further clarification or information on published and unpublished studies in this update of the review: Dr B Assmus and Prof. V Schachinger (JW Goethe University, Frankfurt, Germany), Prof. M Tendera and Prof. W Wojakowski (Medical University of Silesia, Poland), Prof. S Grajek (Poznan University of Medical Science, Poland), Prof. L Chojnowska (Institute of Cardiology, Warsaw, Poland), Dr K Lunde (Oslo University Hospital, Norway), Prof. M Plewka and Dr P Lipiec (Medical University of Lodz, Poland), Prof. V Ryabov (FBGU Institute of Cardiology SB RAMS, Russia), Prof. A Hirsch (University of Amsterdam, The Netherlands), Prof. RG Turan (University Hospital Cologne, Germany), Prof. P Lemarchand (University of Nantes, France), Prof. J Roncalli (Hôpital Ranguei, Toulouse, France) and Dr M Piepoli (Guglielmo da Saliceto Policherurgico Hospital, Italy).
Appendices
Appendix 1. Search strategies 2007
CENTRAL (The Cochrane Library)
#1 STEM CELL TRANSPLANTATION single term (MeSH) #2 PERIPHERAL BLOOD STEM CELL TRANSPLANTATION single term (MeSH) #3 HEMATOPOIETIC STEM CELL TRANSPLANTATION single term (MeSH) #4 HEMATOPOIETIC STEM CELL MOBILIZATION single term (MeSH) #5 STEM CELLS single term (MeSH) #6 HEMATOPOIETIC STEM CELLS explode all trees (MeSH) #7 BONE MARROW CELLS single term (MeSH) #8 haematopoietic OR hematopoietic OR haematopoetic OR hematopoetic OR haemopoietic OR haemopoietic OR marrow NEAR cell* OR stem cell* OR progenitor cell* OR precursor cell* #9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 #10 MYOCARDIAL ISCHEMIA explode all trees (MeSH) #11 myocardial NEAR infarct* OR myocardium NEAR infarct* OR subendocardial NEAR infarct* OR transmural NEAR infarct* OR cardiac NEAR infarct* OR cardial NEAR infarct* OR heart NEAR infarct* OR acute NEAR infarct* #12 ischemi* NEAR myocard* OR ischemi* NEAR heart OR ischaemi* NEAR myocard* OR ischaemi* NEAR heart #13 acute NEAR coronary OR occlusion* NEAR coronary OR disease* NEAR coronary #14 unstable NEAR angina OR heart NEXT attack* OR AMI #15 heart NEAR repair* OR heart NEAR reparation OR heart NEAR improve* OR heart NEAR regenerate* OR cardiac NEAR repair* OR cardiac NEAR reparation OR cardiac NEAR improve* OR cardiac NEAR regenerat* OR myocard* NEAR repair* OR myocard* NEAR reparation OR myocard* NEAR improve* OR myocard* NEAR regenerat* #16 myoblast* NEAR transplantation OR myoblast* NEAR graft* OR myoblast* NEAR implant* #17 #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 #18 #9 AND #17 #19 cellular NEXT cardiomyoplasty or cardiomyocyte* NEAR transplantation* OR intramyocardial NEAR transplantation* OR transendocardial NEAR stem NEXT cell* OR intracoronary NEXT progenitor NEXT cell* #20 #18 OR #19
MEDLINE (Dialog DataStar)
1. STEM‐CELL‐TRANSPLANTATION.DE. 2. PERIPHERAL‐BLOOD‐STEM‐CELL‐TRANSPLANTATION.DE. 3. HEMATOPOIETIC‐STEM‐CELL‐TRANSPLANTATION.DE. 4. HEMATOPOIETIC‐STEM‐CELL‐MOBILIZATION.DE. 5. STEM‐CELLS.DE. 6. HEMATOPOIETIC‐STEM‐CELLS#.DE. 7. BONE‐MARROW‐CELLS.DE. 8. (haematopoietic OR hematopoietic OR haematopoetic OR hematopoetic OR hemopoietic OR haemopoietic OR marrow NEAR cell$1 OR stem cell$1 OR progenitor cell$1 OR precursor cell$1).TI,AB. 9. 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 10. MYOCARDIAL‐ISCHEMIA#.DE. 11. (myocardial OR myocardium OR subendocardial OR transmural OR cardiac OR cardial OR heart OR acute) NEAR infarct$3 12. (ischemi$1 OR ischaemi$1) NEAR (myocardium OR myocardial OR heart) 13. (acute OR occlusion$1 OR disease$1) NEAR coronary 14. unstable NEAR angina OR heart NEXT attack$1 OR AMI 15. (heart or cardiac OR myocardium OR myocardial) NEAR (repair$3 OR reparation OR improve$1 OR regenerat$3) 16. (myoblast$1 NEAR (transplantation OR graft$3 OR implant$3) 17. 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 18. 9 AND 17 19. cellular ADJ cardiomyoplasty or cardiomyocyte$1 NEAR transplantation OR intramyocardial NEAR transplantation OR transendocardial NEAR stem ADJ cell$1 OR intracoronary ADJ progenitor ADJ cell$1 20. 18 OR 19
EMBASE (Dialog DataStar)
1. STEM‐CELL‐TRANSPLANTATION#.DE. 2. STEM‐CELL‐MOBILIZATION.DE. 3. STEM‐CELL.DE. 4. HEMATOPOIETIC‐STEM‐CELL.DE. 5. BONE‐MARROW‐CELL.DE. 6. (haematopoietic OR hematopoietic OR haematopoetic OR hematopoetic OR hemopoietic OR haemopoietic OR marrow NEAR cell$1 OR stem cell$1 OR progenitor cell$1 OR precursor cell$1).TI,AB. 7. 1 OR 2 OR 3 OR 4 OR 5 OR 6 8. HEART‐INFARCTION#.DE. 9. (myocardial OR myocardium OR subendocardial OR transmural OR cardiac OR cardial OR heart OR acute) NEAR infarct$3 10. (ischemi$1 OR ischaemi$1) NEAR (myocardium OR myocardial OR heart) 11. (acute OR occlusion$1 OR disease$1) NEAR coronary 12. unstable NEAR angina OR heart NEXT attack$1 OR AMI 13. (heart or cardiac OR myocardium OR myocardial) NEAR (repair$3 OR reparation OR improve$1 OR regenerat$3) 14. (myoblast$1 NEAR (transplantation OR graft$3 OR implant$3) 15. 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 16. 7 AND 15 17. cellular ADJ cardiomyoplasty or cardiomyocyte$1 NEAR transplantation OR intramyocardial NEAR transplantation OR transendocardial NEAR stem ADJ cell$1 OR intracoronary ADJ progenitor ADJ cell$1 18. 16 OR 17
CINAHL (Dialog DataStar)
1. HEMATOPOIETIC‐STEM‐CELL‐TRANSPLANTATION.DE. 2. STEM‐CELLS#.DE. 3. (haematopoietic OR hematopoietic OR haematopoetic OR hematopoetic OR hemopoietic OR haemopoietic OR marrow NEAR cell$1 OR stem cell$1 OR progenitor cell$1 OR precursor cell$1).TI,AB. 4. 1 OR 2 OR 3 5. MYOCARDIAL‐ISCHEMIA#.DE. 6. (myocardial OR myocardium OR subendocardial OR transmural OR cardiac OR cardial OR heart OR acute) NEAR infarct$3 7. (ischemi$1 OR ischaemi$1) NEAR (myocardium OR myocardial OR heart) 8. (acute OR occlusion$1 OR disease$1) NEAR coronary 9. unstable NEAR angina OR heart NEXT attack$1 OR AMI 10. (heart or cardiac OR myocardium OR myocardial) NEAR (repair$3 OR reparation OR improve$1 OR regenerat$3) 11. (myoblast$1 NEAR (transplantation OR graft$3 OR implant$3) 12. 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 13. 4 AND 12 14. cellular ADJ cardiomyoplasty or cardiomyocyte$1 NEAR transplantation OR intramyocardial NEAR transplantation OR transendocardial NEAR stem ADJ cell$1 OR intracoronary ADJ progenitor ADJ cell$1 15. 13 OR 14
LILACS and INDMED
((marrow cell$ OR stem cell$ OR progenitor cell$ OR precursor cell$) AND (infarct$ OR coronar$ OR myocard$ OR heart attack$ OR heart failure OR cardiac$ OR cardiomyo$ OR intramyocardial$ OR ischemia))
KOREAMED
((marrow cell$ OR stem cell$ OR progenitor cell$ OR precursor cell$) AND (infarct$ OR coronar$ OR myocard$ OR heart attack$ OR heart failure OR cardiac$ OR cardiomyo$ OR intramyocardial$ OR ischemia))
mRCT
(("marrow cell%" OR "stem cell%" OR "progenitor cell%" or "precursor cell%") AND (infarct% OR coronar% OR myocard% OR "heart attack%" OR "heart failure" OR cardiac% OR cardiomyo% OR intramyocardial% OR ischemia))
Appendix 2. Search strategies 2011
CENTRAL (The Cochrane Library)
#1 STEM CELL TRANSPLANTATION single term (MeSH) #2 PERIPHERAL BLOOD STEM CELL TRANSPLANTATION single term (MeSH) #3 HEMATOPOIETIC STEM CELL TRANSPLANTATION single term (MeSH) #4 HEMATOPOIETIC STEM CELL MOBILIZATION single term (MeSH) #5 STEM CELLS single term (MeSH) #6 HEMATOPOIETIC STEM CELLS explode all trees (MeSH) #7 BONE MARROW CELLS single term (MeSH) #8 haematopoietic OR hematopoietic OR haematopoetic OR hematopoetic OR haemopoietic OR haemopoietic OR (marrow NEAR/3 cell*) OR stem cell* OR progenitor cell* OR precursor cell* or cell* therap* or ((mesenchymal or stromal) AND marrow) #9 (cell* NEAR/3 transplantation) OR (cell* NEAR/3 graft*) OR (cell* NEAR/3 implant*) #10 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 #11 MYOCARDIAL ISCHEMIA explode all trees (MeSH) #12 myocardial NEAR/3 infarct* OR myocardium NEAR/3 infarct* OR subendocardial NEAR/3 infarct* OR transmural NEAR/3 infarct* OR cardiac NEAR/3 infarct* OR cardial NEAR/3 infarct* OR heart NEAR/3 infarct* OR acute NEAR/3 infarct* #13 ischemi* NEAR/3 myocard* OR ischemi* NEAR/3 heart OR ischaemi* NEAR/3 myocard* OR ischaemi* NEAR/3 heart #14 acute NEAR/3 coronary OR occlusion* NEAR/3 coronary OR disease* NEAR/3 coronary #15 unstable NEAR/3 angina OR heart NEXT attack* OR AMI #16 heart NEAR/3 repair* OR heart NEAR/3 reparation OR heart NEAR/3 improve* OR heart NEAR/3 regenerate* OR cardiac NEAR/3 repair* OR cardiac NEAR/3 reparation OR cardiac NEAR/3 improve* OR cardiac NEAR/3 regenerat* OR myocard* NEAR/3 repair* OR myocard* NEAR/3 reparation OR myocard* NEAR/3 improve* OR myocard* NEAR/3 regenerat* #17 #11 OR #12 OR #13 OR #14 OR #15 OR #16 #18 #10 AND #17 #19 (cellular NEXT cardiomyoplasty) or (cardiomyocyte* NEAR/3 transplantation*) OR (intramyocardial NEAR/3 transplantation*) OR (transendocardial NEAR/3 stem NEXT cell*) #20 (intracoronary NEAR/4 cell*) or (intracoronary NEAR/3 bone NEXT marrow) or (intracoronary NEAR/3 BMC*) or (intracoronary NEAR/3 infus*) #21 #18 OR #19 OR #20
MEDLINE (Ovid)
1. exp STEM CELL TRANSPLANTATION/ 2. exp STEM CELLS/ 3. BONE MARROW TRANSPLANTATION/ 4. BONE MARROW CELLS/ 5. CELL TRANSPLANTATION/ 6. (haematopoietic OR hematopoietic OR haematopoetic OR hematopoetic OR hemopoietic OR haemopoietic OR (marrow adj3 cell*) OR stem cell* OR progenitor cell* OR precursor cell* OR cell* therap* OR ((mesenchymal OR stromal) AND marrow).ti,ab. 7. (cell* adj3 (transplant* or graft* or implant*)).ti,ab 8. cell transplantation.jn. or cell stem cell.jn. or stem cell reviews.jn. or bone marrow transplantation.jn. 9. or/1‐8 10. exp MYOCARDIAL ISCHEMIA/ 11. ((myocardial OR myocardium OR subendocardial OR transmural OR cardiac OR cardial OR heart OR acute) adj3 infarct*).ti,ab. 12. ((ischemi* OR ischaemi*) adj3 (myocardium OR myocardial OR heart)).ti,ab. 13. ((acute OR occlusion* OR disease*) adj3 coronary).ti,ab. 14. ((unstable adj3 angina) OR heart attack* OR AMI).ti,ab. 15. ((heart or cardiac OR myocardium OR myocardial) adj3 (repair* OR reparation OR improve* OR regenerat*)).ti,ab. 16. or/10‐15 17. 9 AND 16 18. (cellular cardiomyoplasty or (cardiomyocyte* adj3 transplant*) OR (intramyocardial* adj3 transplant*) OR (transendocardial* adj3 stem cell*)).ti,ab. 19. (intracoronary adj4 (cell* or BMC* or infus*)).ti,ab. 20. or/17‐19 21. RANDOMIZED CONTROLLED TRIAL.pt. 22. CONTROLLED CLINICAL TRIAL.pt. 23. exp CLINICAL TRIAL/ 24. MULTICENTER STUDY.pt. 25. CLINICAL TRIALS AS TOPIC/ 26. CLINICAL TRIALS PHASE III AS TOPIC/ 27. CLINICAL TRIALS PHASE IV AS TOPIC/ 28. exp CONTROLLED CLINICAL TRIALS AS TOPIC/ 29. RANDOM ALLOCATION/ 30. DOUBLE BLIND METHOD/ 31. SINGLE BLIND METHOD/ 32. CROSSOVER STUDIES/ 33. PLACEBOS/ 34. or/21‐33 35. (controlled adj3 (trial* or stud*)).ti,ab. 36. (blind* or mask*).ti,ab. 37. (placebo* or random* or factorial*).ti,ab. 38. (crossover or (cross adj over)).ti,ab. 39. aleatori*.ti,ab. 40. (treatment adj arm*).ti,ab. 41. ((phase adj iii) or (phase adj three) or (phase adj '3')).ti,ab. 42. (latin adj square).ti,ab. 43. or/35‐42 44. 34 or 43 45. ANIMALS/ 46. HUMANS/ 47. 45 and 46 48. 45 not 47 49. 44 not 48 50. 20 and 49
EMBASE (Ovid)
1. exp CELL THERAPY/ 2. exp STEM CELL/ 3. BONE MARROW CELL/ 4. (haematopoietic OR hematopoietic OR haematopoetic OR hematopoetic OR hemopoietic OR haemopoietic OR (marrow adj3 cell*) OR stem cell* OR progenitor cell* OR precursor cell* OR cell* therap*).ti,ab. 5. ((mesenchymal OR stromal) AND marrow).ti,ab. 6. (cell* adj3 (transplant* or graft* or implant*)).ti,ab. 7. or/1‐6 8. exp HEART INFARCTION/ 9. ((myocardial OR myocardium OR subendocardial OR transmural OR cardiac OR cardial OR heart OR acute) adj3 infarct*).ti,ab. 10. ((ischemi* OR ischaemi*) adj3 (myocardium OR myocardial OR heart)).ti,ab. 11. ((acute OR occlusion* OR disease*) adj3 coronary).ti,ab. 12. ((unstable adj3 angina) OR heart attack* OR AMI).ti,ab. 13. ((heart or cardiac OR myocardium OR myocardial) adj3 (repair* OR reparation OR improve* OR regenerat*)).ti,ab. 14. or/8‐13 15. 7 AND 14 16. (cellular cardiomyoplasty OR (cardiomyocyte* adj3 transplant*) OR (intramyocardial* adj3 transplant*) OR (transendocardial* adj3 stem cell*)).ti,ab. 17. (intracoronary adj4 (cell* OR BMC* OR infus*)).ti,ab. 18. or/15‐17 19. random*.ti,ab. 20. factorial*.ti,ab. 21. (crossover* OR cross over* OR cross‐over*).ti,ab. 22. placebo*.ti,ab. 23. (double* adj blind*).ti,ab. 24. (singl* adj blind*).ti,ab. 25. assign*.ti,ab. 26. allocat*.ti,ab. 27. volunteer*.ti,ab. 28. CROSSOVER PROCEDURE/ 29. DOUBLE BLIND PROCEDURE/ 30. RANDOMIZED CONTROLLED TRIAL/ 31. SINGLE BLIND PROCEDURE/ 32. or/19‐31 33. exp ANIMAL/ 34. NONHUMAN/ 35. exp ANIMAL EXPERIMENT/ 36. or/33‐35 37. exp HUMAN/ 38. 36 NOT 37 39. 32 NOT 38 40. 18 AND 39
CINAHL (NHS Evidence)
1. exp CELL TRANSPLANTATION/ 2. exp STEM CELLS/ 3. exp BONE MARROW TRANSPLANTATION/ 4. (haematopoietic OR hematopoietic OR haematopoetic OR hematopoetic OR hemopoietic OR haemopoietic OR (marrow adj3 cell*) OR “stem cell*” OR “progenitor cell*” OR “precursor cell*” or “cell* therap*”).ti,ab 5. ((mesenchymal OR stromal) AND marrow).ti,ab 6. ((cell* adj3 transplant*) or (cell* adj3 graft*) or (cell* adj3 implant*)).ti,ab 7. 1 OR 2 OR 3 OR 4 OR 5 OR 6 8. exp MYOCARDIAL ISCHEMIA/ 9. ((myocardial adj3 infarct*) OR (myocardium adj3 infarct*) OR (subendocardial adj3 infarct*) OR (transmural adj3 infarct*) OR (cardiac adj3 infarct*) OR (cardial adj3 infarct*) OR (heart adj3 infarct*) OR (acute adj3 infarct*).ti,ab 10. ((ischemi* adj3 myocardium) OR (ischemi* adj3 myocardial) OR (ischemi* adj3 heart)).ti,ab 11. ((ischaemi* adj3 myocardium) OR (ischaemi* adj3 myocardial) OR (ischaemi* adj3 heart)).ti,ab 12. ((acute adj3 coronary) OR (occlusion* adj3 coronary) OR (disease* adj3 coronary)).ti,ab 13. ((unstable adj3 angina) OR “heart attack*” OR AMI).ti,ab 14. ((heart adj3 repair*) or (cardiac adj3 repair*) OR (myocardium adj3 repair*) OR (myocardial* adj3 repair*)).ti,ab 15. ((heart adj3 reparation) or (cardiac adj3 reparation) OR (myocardium adj3 reparation) OR (myocardial* adj3 reparation)).ti,ab 16. ((heart adj3 improv*) or (cardiac adj3 improv*) OR (myocardium adj3 improv*) OR (myocardial* adj3 improv*)).ti,ab 17. ((heart adj3 regenerat*) or (cardiac adj3 regenerat*) OR (myocardium adj3 regenerat*) OR (myocardial* adj3 regenerat*)).ti,ab 18. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19. 7 AND 18 20. (“cellular cardiomyoplasty” or (cardiomyocyte* adj3 transplant*) OR (intramyocardial* adj3 transplant*) OR (transendocardial* adj3 stem cell*)).ti,ab 21. ((intracoronary adj4 cell*) or (intracoronary adj3 BMC*) or (intracoronary adj3 infus*)).ti,ab 22. 19 or 20 or 21 23. "CLINICAL TRIAL".pt 24. ((controlled adj trial*) OR (clinical adj trial*)).ti,ab 25. ((singl* adj blind*) OR (doubl* adj blind*) OR (trebl* adj blind*) OR (singl* adj mask*) OR (doubl* adj mask*) OR (tripl* adj mask*)).ti,ab randomi*.ti,ab 26. RANDOM ASSIGNMENT/ 27. ("phase III" OR "phase 3" OR "phase three").ti,ab 28. (random* adj1 allocat*).ti,ab 29. (random* adj1 assign*).ti,ab 30. PLACEBOS/ 31. 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 32. 19 AND 31
PubMed (for e‐publications only)
(infarct[ti] OR infarction or coronary[ti] OR myocardial[ti] OR heart attack[ti] OR heart failure[ti] OR cardiac[ti] OR cardiomyopathy[ti] OR intramyocardial[ti] OR ischemi*[ti] OR ischaemi*[ti]) AND (marrow cell[ti] OR marrow cells[ti] OR stem cell[ti] OR stem cells[ti] OR progenitor cell[ti] OR progenitor cells[ti] OR precursor cell[ti] OR precursor cells[ti] OR cell therapy[ti] OR cellular therapy[ti] OR cell‐based therapy[ti] OR intracoronary cells[ti] or mononuclear cells[ti] OR mesenchymal cells[ti]) AND (publisher[sb] NOT pubstatusnihms)
LILACS and INDMED
(marrow cell$ OR stem cell$ OR progenitor cell$ OR precursor cell$ OR cell$ therap$ or mesenchymal cell$) AND (infarct$ OR coronar$ OR intracoronary OR myocard$ OR heart attack$ OR heart failure OR cardiac$ OR cardiomyo$ OR intramyocardial$ OR ischemi$)
KoreaMed, PakMediNet and the UKBTS/SRI Transfusion Evidence Library
(marrow cell* OR stem cell* OR progenitor cell* OR precursor cell* OR cell* therap* or mesenchymal cell*) AND (infarct* OR coronar* OR intracoronary OR myocard* OR heart attack* OR heart failure OR cardiac* OR cardiomyo* OR intramyocardial* OR ischemi*)
ClinicalTrials.gov
(myocardial infarction OR cardiomyopathy OR intramyocardial OR intracoronary OR myocardial ischemia) AND ("marrow cells" OR "stem cells" OR "cell therapy" OR "cellular therapy" OR "cell‐based therapy" OR "intracoronary cells" or "mononuclear cells")
ISRCTN Register
(stem cell OR stem cells OR marrow cell OR marrow cells OR progenitor cell or progenitor cells or precursor cell or precursor cells) AND (myocardial infarction OR infarct OR heart attack OR cardiomyopathy OR intramyocardial OR intracoronary OR ischemia OR ischaemia)
WHO International Clinical Trials Registry Platform (ICTRP)
(infarct AND cell* OR infarction AND cell* OR coronary AND cell* OR myocardial AND cell* OR heart attack AND cell* OR heart failure AND cell* OR cardiac AND cell* OR cardiomyopathy AND cell* OR intramyocardial AND cell* OR ischemia AND cell* OR ischemic AND cell* OR ischaemia AND cell* OR ischaemic AND cell*)
Appendix 3. Search strategies 2015
CENTRAL (The Cochrane Library)
#1 MeSH descriptor: [Stem Cell Transplantation] explode all trees #2 MeSH descriptor: [Bone Marrow Cells] explode all trees #3 MeSH descriptor: [Stem Cells] explode all trees #4 MeSH descriptor: [Cell Transplantation] this term only #5 MeSH descriptor: [Bone Marrow Transplantation] this term only #6 MeSH descriptor: [Stromal Cells] explode all trees #7 ((stem or haematopoietic or hematopoietic or haematopoetic or hematopoetic or hemopoietic or haemopoietic or progenitor or precursor or bone marrow or mononuclear or "adipose tissue" or mesenchymal or stromal or autologous or allogeneic or allogenic or ALDH* or C‐KIT*) next/2 cell*) #8 "cell transplantation":so or "stem cell":so or "bone marrow transplantation":so #9 (autologous next/3 transplant*) or "cell* therap*" #10 ((cell* or myoblast*) near/3 (autologous or transplant* or autotransplant* or allotransplant* or graft* or implant*)) #11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor: [Heart Diseases] explode all trees #13 ((ischemi* or ischaemi* or nonischemi* or nonischaemi*) near/2 (myocardium or myocardial or cardiomyopath* or heart or coronary or cardiac or cardial or subendocardial)) #14 ((myocardial or myocardium or subendocardial or transmural or cardiac or cardial or coronary or heart) near/2 (failure* or decompensation or insufficien*)) #15 (IHD or CIHD or DCM or IDCM) #16 ((myocardial near/3 dysfunction*) or stenocardia or angina*) #17 ((end stage or endstage or dilated or idiopathic or congestive) near/2 cardiomyopath*) #18 (arter* occlusion* or arter* disease* or arterioscleros* or atheroscleros*) near/2 coronary #19 ((heart or cardiac or cardial or myocardium or myocardial) near/3 (repair* or reparation or improv* or regenerat*)) #20 (heart disease* or coronary disease* or cardiovascular disease*) #21 ((end stage or endstage or dilated or idiopathic or congestive) near/2 cardiomyopath*) #22 ((myocardial or myocardium or subendocardial or transmural or cardiac or cardial or coronary or heart or acute) near/3 (infarct* or postinfarct* or hypoxi* or anoxi*)) #23 heart attack* or coronary attack* or acute coronary syndrome* or AMI #24 #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 #25 #11 and #24 #26 cellular cardiomyoplast* or ((cardiomyocyte* or cardiac cell*) near/6 transplant*) or ((intramyocardial* or intracoronary or transendocardial* or transcoronary) near/6 (transplant* or stem or bone marrow or marrow cell* or BMC* or stromal or mesenchymal or progenitor cell* or precursor cell*)) #27 #25 or #26
MEDLINE (OvidSP)
1. exp STEM CELL TRANSPLANTATION/ 2. BONE MARROW TRANSPLANTATION/ 3. CELL TRANSPLANTATION/ 4. exp STEM CELLS/ 5. BONE MARROW CELLS/ 6. exp STROMAL CELLS/ 7. ((stem or haematopoietic or hematopoietic or haematopoetic or hematopoetic or hemopoietic or haemopoietic or progenitor or precursor or bone marrow or mononuclear or adipose tissue or mesenchymal or stromal or autologous or allogeneic or allogenic or ALDH* or C‐KIT*) adj2 cell*).ti,ab. 8. (cell transplantation or stem cell* or bone marrow transplantation).jn. 9. ((autologous adj3 transplant*) or cell* therap*).tw. 10. ((cell* or myoblast*) adj3 (autologous or transplant* or autotransplant* or allotransplant* or graft* or implant*)).ti,ab. 11. or/1‐10 12. exp HEART DISEASES/ 13. ((ischemi* or ischaemi* or nonischemi* or nonischaemi*) adj2 (myocardium or myocardial or cardiomyopath* or heart or coronary or cardiac or cardial or subendocardial)).ti,ab. 14. ((myocardial or myocardium or subendocardial or transmural or cardiac or cardial or coronary or heart) adj2 (failure* or decompensation or insufficien*)).ti,ab. 15. (IHD or CIHD or DCM or IDCM).ti,ab. 16. ((myocardial adj3 dysfunction*) or stenocardia or angina*).ti,ab. 17. ((arter* occlusion* or arter* disease* or arterioscleros* or atheroscleros*) adj2 coronary).ti,ab. 18. (heart disease* or coronary disease* or cardiovascular disease*).ti,ab. 9. ((end stage or endstage or dilated or idiopathic or congestive) adj2 cardiomyopath*).ti,ab. 20. ((heart or cardiac or cardial or myocardium or myocardial) adj3 (repair* or reparation or improv* or regenerat*)).ti,ab. 21. ((myocardial or myocardium or subendocardial or transmural or cardiac or cardial or coronary or heart or acute) adj3 (infarct* or postinfarct* or hypoxi* or anoxi*)).ti,ab. 22. (heart attack* or coronary attack* or acute coronary syndrome* or AMI).ti,ab. 23. or/12‐22 24. 11 and 23 25. (cellular cardiomyoplast* or ((cardiomyocyte* or cardiac cell*) adj6 transplant*) or ((intramyocardial* or intracoronary or transendocardial* or transcoronary) adj6 (transplant* or stem or bone marrow or marrow cell* or BMC* or stromal or mesenchymal or progenitor cell* or precursor cell*))).mp. 26. 24 or 25 27. Meta‐Analysis.pt. 28. ((meta analy* or metaanaly*) and (trials or studies)).ab. 29. (meta analy* or metaanaly* or evidence‐based).ti. 30. ((systematic* or evidence‐based) adj2 (review* or overview*)).tw. 31. (cochrane or embase or cinahl or cinhal or lilacs or citation index or psyclit or psychlit or psycinfo or psychinfo or "web of science" or scopus).ab. 32. Cochrane Database of systematic reviews.jn. 33. ((literature or systematic* or comprehensive* or electronic*) adj2 search*).ab. 34. (additional adj (papers or articles or sources)).ab. 35. (bibliograph* or handsearch* or hand search* or manual* search* or searched or reference list*).ab. 36. (relevant adj (journals or articles)).ab. 37. or/27‐36 38. Review.pt. 39. RANDOMIZED CONTROLLED TRIALS AS TOPIC/ 40. selection criteria.ab. or critical appraisal.ti. 41. (data adj (extraction or analys$)).ab. 42. RANDOMIZED CONTROLLED TRIALS/ 43. or/39‐42 44. 38 and 43 45. 37 or 44 46. randomized controlled trial.pt. 47. controlled clinical trial.pt. 48. randomi*.tw. 49. (placebo or randomly or groups).ab. 50. clinical trials as topic.sh. 51. trial.ti. 52. or/46‐51 53. 45 or 52 54. (ANIMALS/ or exp ANIMAL EXPERIMENTATION/ or exp MODELS, ANIMAL/) not HUMANS/ 55. (Comment or Editorial).pt. 56. 54 or 55 57. 53 not 56 58. 26 and 57
EMBASE (OvidSP)
1. exp STEM CELL TRANSPLANTATION/ 2. exp BONE MARROW TRANSPLANTATION/ 3. exp STEM CELL/ 4. BONE MARROW CELL/ 5. exp STROMA CELLS/ 6. ((stem or haematopoietic or hematopoietic or haematopoetic or hematopoetic or hemopoietic or haemopoietic or progenitor or precursor or bone marrow or mononuclear or adipose tissue or mesenchymal or stromal or autologous or allogeneic or allogenic or ALDH* or C‐KIT*) adj2 cell*).ti,ab. 7. (cell transplantation or stem cell* or bone marrow transplantation).jn. 8. ((autologous adj3 transplant*) or cell* therap*).tw. 9. ((cell* or myoblast*) adj3 (autologous or transplant* or autotransplant* or allotransplant* or graft* or implant*)).ti,ab. 10. or/1‐9 11. exp ISCHEMIC HEART DISEASE/ 12. exp HEART FAILURE/ 13. exp MYOCARDIAL DISEASE/ 14. ((ischemi* or ischaemi* or nonischemi* or nonischaemi*) adj2 (myocardium or myocardial or cardiomyopath* or heart or coronary or cardiac or cardial or subendocardial)).ti,ab. 15. ((myocardial or myocardium or subendocardial or transmural or cardiac or cardial or coronary or heart) adj2 (failure* or decompensation or insufficien*)).ti,ab. 16. (IHD or CIHD or DCM or IDCM).ti,ab. 17. ((myocardial adj3 dysfunction*) or stenocardia or angina*).ti,ab. 18. ((arter* occlusion* or arter* disease* or arterioscleros* or atheroscleros*) adj2 coronary).ti,ab. 19. (heart disease* or coronary disease* or cardiovascular disease*).ti,ab. 20. ((end stage or endstage or dilated or idiopathic or congestive) adj2 cardiomyopath*).ti,ab. 21. ((heart or cardiac or cardial or myocardium or myocardial) adj3 (repair* or reparation or improv* or regenerat*)).ti,ab. 22. ((myocardial or myocardium or subendocardial or transmural or cardiac or cardial or coronary or heart or acute) adj3 (infarct* or postinfarct* or hypoxi* or anoxi*)).ti,ab. 23. (heart attack* or coronary attack* or acute coronary syndrome* or AMI).ti,ab. 24. 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. 10 and 24 26. (cellular cardiomyoplast* or ((cardiomyocyte* or cardiac cell*) adj6 transplant*) or ((intramyocardial* or intracoronary or transendocardial* or transcoronary) adj6 (transplant* or stem or bone marrow or marrow cell* or BMC* or stromal or mesenchymal or progenitor cell* or precursor cell*))).mp. 27. 25 or 26 28. Meta Analysis/ 29. Systematic Review/ 30. (meta analy* or metaanalys*).tw. 31. (systematic* adj2 (review* or overview* or search*)).tw. 32. (literature adj2 (review* or overview* or search*)).tw. 33. (cochrane or embase or cinahl or cinhal or lilacs or BIDS or science citation index or psyclit or psychlit or psycinfo or psychinfo or cancerlit).ti,ab. 34. (electronic* adj (sources or resources or databases)).ab. 35. (reference lists or bibliograph* or handsearch* or hand search* or (manual* adj1 search*)).ab. 36. (additional adj (papers or articles or sources)).ab. 37. (relevant adj (journals or articles)).ab. 38. (search term* or published articles or search strateg*).ab. 39. Review.pt. and (data extraction or selection criteria).ab. 40. or/28‐39 41. Controlled Clinical Trial/ 42. Phase 3 Clinical Trial/ 43. Phase 4 Clinical Trial/ 44. Randomized Controlled Trial/ 45. Randomization/ 46. Single Blind Procedure/ 47. Double Blind Procedure/ 48. Crossover Procedure/ 49. Placebo/ 50. (randomized or randomised or RCT).tw. 51. (random* adj5 (allocat* or assign* or divid* or receiv*)).tw. 52. (single blind* or double blind* or treble blind* or triple blind*).tw. 53. (phase III or phase three or "phase 3").tw. 54. (crossover* or cross over* or cross‐over* or placebo*).tw. 55. Prospective Study/ 56. or/41‐55 57. Case Study/ 58. case report*.tw. 59. (note or editorial).pt. 60. or/57‐59 61. 56 not 60 62. 40 or 61 63. limit 62 to embase 64. 27 and 63
CINAHL (EBSCOHost)
S1 (MH "Cell Transplantation+") S2 (MH "Stem Cells+") S3 TI ( (stem or haematopoietic or hematopoietic or haematopoetic or hematopoetic or hemopoietic or haemopoietic or progenitor or precursor or bone marrow or mononuclear or adipose tissue or mesenchymal or stromal or autologous or allogeneic or allogenic or ALDH* or C‐KIT*) N2 cell* ) OR AB ( (stem or haematopoietic or hematopoietic or haematopoetic or hematopoetic or hemopoietic or haemopoietic or progenitor or precursor or bone marrow or mononuclear or adipose tissue or mesenchymal or stromal or autologous or allogeneic or allogenic or ALDH* or C‐KIT*) N2 cell) S4 TX ( (autologous N3 transplant*) or cell* therap* ) S5 TI ( (cell* or myoblast*) N3 (autologous or transplant* or autotransplant* or allotransplant* or graft* or implant*) ) OR AB ( (cell* or myoblast*) N3 (autologous or transplant* or autotransplant* or allotransplant* or graft* or implant*) ) S6 S1 OR S2 OR S3 OR S4 OR S5 S7 (MH "Heart Diseases+") S8 TI ( (myocardial or myocardium or subendocardial or transmural or cardiac or cardial or coronary or heart or acute) N3 (infarct* or postinfarct* or hypoxi* or anoxi*) ) OR AB ( (myocardial or myocardium or subendocardial or transmural or cardiac or cardial or coronary or heart or acute) N3 (infarct* or postinfarct* or hypoxi* or anoxi*) ) S9 TI ( ("heart disease*" or "coronary disease*" or IHD or CIHD or DCM or IDCM) ) AND AB ( ("heart disease*" or "coronary disease*" or IHD or CIHD or DCM or IDCM) ) S10 TI ( ((myocardial N3 dysfunction) OR angina OR stenocardia) ) OR AB ( ((myocardial N3 dysfunction) OR angina OR stenocardia) ) S11 TI ( ((ischemi* or ischaemi* or nonischemi* or nonischaemi*) N5 (myocardium or myocardial or heart or coronary or cardiac or cardial or subendocardial or cardiomyopath*)) ) OR AB ( ((ischemi* or ischaemi* or nonischemi* or nonischaemi*) N5 (myocardium or myocardial or heart or coronary or cardiac or cardial or subendocardial or cardiomyopath*)) ) S12 TI ( ((arter* occlusion* or arter* disease* or arterioscleros* or atheroscleros*) N2 coronary) ) OR AB ( ((arter* occlusion* or arter* disease* or arterioscleros* or atheroscleros*) N2 coronary) ) S13 TI ( ((myocardial or myocardium or subendocardial or transmural or cardiac or cardial or coronary or heart) N2 (failure* or decompensation or insufficien*)) ) OR AB ( ((myocardial or myocardium or subendocardial or transmural or cardiac or cardial or coronary or heart) N2 (failure* or decompensation or insufficien*)) ) S14 TI ( (end stage or endstage or dilated or idiopathic or congestive) N2 cardiomyopath* ) OR AB ( (end stage or endstage or dilated or idiopathic or congestive) N2 cardiomyopath* ) S15 TI ( (heart or cardiac or cardial or myocardium or myocardial) N3 (repair* or reparation or improv* or regenerat*) ) OR AB ( (heart or cardiac or cardial or myocardium or myocardial) N3 (repair* or reparation or improv* or regenerat*) ) S16 TI (heart attack* or coronary attack* or acute coronary syndrome* or AMI) OR AB (heart attack* or coronary attack* or acute coronary syndrome* or AMI) S17 S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 S18 S6 AND S17 S19 TI ( cellular cardiomyoplast* or ((cardiomyocyte* or cardiac cell*) N6 transplant*) or ((intramyocardial* or intracoronary or transendocardial* or transcoronary) N6 (transplant* or stem or bone marrow or marrow cell* or BMC* or stromal or mesenchymal or progenitor cell* or precursor cell*)) ) OR AB ( cellular cardiomyoplast* or ((cardiomyocyte* or cardiac cell*) N6 transplant*) or ((intramyocardial* or intracoronary or transendocardial* or transcoronary) N6 (transplant* or stem or bone marrow or marrow cell* or BMC* or stromal or mesenchymal or progenitor cell* or precursor cell*)) S20 S18 OR S19 S21 (MH CLINICAL TRIALS+) S22 PT Clinical Trial S23 TI ((controlled trial*) or (clinical trial*)) OR AB ((controlled trial*) or (clinical trial*)) S24 TI ((singl* blind*) OR (doubl* blind*) OR (trebl* blind*) OR (tripl* blind*) OR (singl* mask*) OR (doubl* mask*) OR (tripl* mask*)) OR AB ((singl* blind*) OR (doubl* blind*) OR (trebl* blind*) OR (tripl* blind*) OR (singl* mask*) OR (doubl* mask*) OR (tripl* mask*)) S25 TI randomi* OR AB randomi* S26 MH RANDOM ASSIGNMENT S27 TI ((phase three) or (phase III) or (phase three)) or AB ((phase three) or (phase III) or (phase three)) S28 ( TI (random* N2 (assign* or allocat*)) ) OR ( AB (random* N2 (assign* or allocat*)) ) S29 MH PLACEBOS S30 TI placebo* OR AB placebo* S31 MH QUANTITATIVE STUDIES S32 S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 S33 S20 and S32
PubMed (for epublications)
#1 (stem[TI] OR haematopoietic[TI] OR hematopoietic[TI] OR haematopoetic[TI] OR hematopoetic[TI] OR hemopoietic[TI] OR haemopoietic[TI] OR progenitor[TI] OR precursor[TI] OR bone marrow[TI] OR mononuclear[TI] OR "adipose tissue"[TI] OR mesenchymal[TI] OR stromal[TI] OR autologous[TI] OR allogeneic[TI] OR allogenic[TI] OR ALDH*[TI] OR C‐KIT*[TI]) AND cell*[TI] #2 cell transplantation[TA] OR stem cell*[TA] OR bone marrow transplant*[TA] #3 "autologous transplant*"[TI] OR "cell therapy"[TI] OR "cell therapies"[TI] OR "cellular therapy"[TI] #4 (cell[TI] OR cells[TI] OR cellular[TI] OR myoblast*[TI]) AND (transplant[TI] OR transplantation[TI] OR transplants[TI] OR transplanting[TI] OR transplanted[TI] OR autotransplant*[TI] or allotransplant*[TI] or graft*[TI] or implant[TI] OR implants[TI] OR implantation[TI] OR implanted[TI]) #5 #1 OR #2 OR #3 OR #4 #6 (ischemi*[TI] OR ischaemi*[TI] OR nonischemi*[TI] OR nonischaemi*) AND (myocardium[TI] OR myocardial[TI] OR cardiomyopath*[TI] OR heart[TI] OR coronary[TI] OR cardiac[TI] OR cardial[TI] OR subendocardial[TI]) #7 (myocardial[TI] OR myocardium[TI] OR subendocardial[TI] OR transmural[TI] OR cardiac[TI] OR cardial[TI] OR coronary[TI] OR heart) AND (failure*[TI] OR decompensation[TI] OR insufficien*[TI]) #8 "myocardial dysfunction*"[TI] OR stenocardia[TI] OR angina*[TI] OR IHD[TI] OR CIHD[TI] OR DCM[TI] OR IDCM[TI] OR "heart disease"[TI] OR "coronary disease"[TI] OR "coronary artery disease"[TI] OR "cardiovascular disease"[TI] #9 ("arterial occlusion*"[TI] OR "arterial disease*"[TI] OR arterioscleros*[TI] OR atheroscleros*[TI]) AND coronary[TI] #10 ("end stage"[TI] OR endstage[TI] OR dilated[TI] OR idiopathic[TI] OR congestive[TI]) AND cardiomyopath*[TI] #11 (heart[TI] OR cardiac[TI] OR cardial[TI] OR myocardium[TI] OR myocardial[TI]) AND (repair*[TI] OR reparation[TI] OR improv*[TI] OR regenerat*[TI]) #12 (myocardial[TI] OR myocardium [TI] OR subendocardial [TI] OR transmural [TI] OR cardiac [TI] OR cardial [TI] OR coronary [TI] OR heart [TI] OR acute[TI]) AND (infarct* [TI] OR postinfarct* [TI] OR hypoxi* [TI] OR anoxi*) #13 heart attack* [TI] OR coronary attack* [TI] OR acute coronary syndrome* [TI] OR AMI[TI] #14 #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 #15 #5 AND #14 #16 (cellular cardiomyoplast* OR ((cardiomyocyte* OR cardiac cell*) AND transplant*) OR ((intramyocardial* OR intracoronary OR transendocardial* OR transcoronary) AND (transplant* OR stem OR bone marrow OR marrow cell* OR BMC* OR stromal OR mesenchymal OR progenitor cell* OR precursor cell*))) #17 #15 OR #16 #18 (random* OR blind* OR control group* OR placebo OR controlled trial OR controlled study OR trials OR systematic review OR meta‐analysis OR metaanalysis OR literature search OR medline OR cochrane OR embase) AND ((publisher[sb] OR inprocess[sb]) NOT pubstatusnihms) #19 #17 AND #18
LILACS
(tw:((infarct OR infarction OR coronary OR myocardial OR heart OR cardiac OR cardiomyopathy OR myocardial OR subendocardial OR intramyocardial OR intracoronary OR ischemia OR ischemic OR nonischemic))) AND (tw:((bone marrow OR marrow cell OR marrow cells OR stem cell OR stem cells OR progenitor cells OR precursor cells OR cell therapy OR cellular therapy OR cell‐based therapy OR mononuclear cells OR mesenchymal cells OR stromal cells))) AND (instance:"regional") AND ( db:("LILACS") AND type_of_study:("clinical_trials"))
KoreaMed
Search lines were run separately, but presented this way for brevity: (stem [ALL] OR marrow [ALL] OR mesenchymal[ALL] OR stromal[ALL]) AND (myocardial [ALL] OR heart[ALL] OR cardiac[ALL] OR coronary[ALL] OR cardiomyopathy[ALL]) AND "Randomized Controlled Trial" [PT]
IndMed
(bone marrow OR marrow cell OR marrow cells OR stem cell OR stem cells OR progenitor cell OR precursor cell OR cell therapy OR cellular therapy OR mesenchymal cells OR stromal cells) AND (infarct OR infarction OR coronary OR intracoronary OR myocardial OR heart OR cardiac OR congestive OR cardiomyopathy OR intramyocardial OR intramyocardial OR intracoronary OR ischemia OR ischemic OR ischaemia OR ischaemic OR nonischemic OR nonischaemic) AND (randomised OR randomly OR randomized OR blind OR blinded OR trial OR study OR control group)
PakMediNet
Combinations of the following free text terms were used: stem cell, stem cells, bone marrow, marrow cells, progenitor cells, precursor cells, mesenchymal cells, stromal cells AND myocardial infarction, heart attack, cardiac ischemia, coronary ischemia, myocardial ischemia, cardiomyopathy, heart failure, cardiac failure, angina, coronary artery disease
Web of Science
Title: "cardiac failure" OR "heart attack" OR "heart failure" OR "coronary disease" OR "cardiovascular disease" OR "coronary artery" OR "coronary arterial" OR "myocardial infarction" OR cardiomyopathy OR "heart disease" OR "heart diseases" OR "cardiac insufficiency" OR AMI OR IHD OR CIHD OR DCM OR IDCM OR "myocardial dysfunction" OR stenocardia OR angina AND Title: "stem cell" OR "stem cells" OR "bone marrow" OR "marrow cells" OR "cellular therapy" OR "mesenchymal cells" OR "stromal cells" OR "cell transplant" OR "precursor cells" OR "progenitor cells" OR (c‐kit* NEAR/5 cells) OR HSCT OR SCT OR MSC OR MSCs OR BMT OR BMC OR BMAC OR BMCs OR HST OR HSTs AND Topic: randomised OR randomly OR randomized OR blind OR blinded OR trial OR study OR "control group" OR group
ClinicalTrials.gov
Search Terms: randomized OR randomised OR random OR randomly Study Type: Intervention Studies Condition: cardiac OR heart attack OR heart failure OR coronary OR myocardial OR cardiomyopathy OR heart disease OR angina Intervention: stem cells OR bone marrow cells OR cellular therapy OR mesenchymal cells OR stromal cells OR cell transplant OR precursor cells OR progenitor cells OR HSCT OR SCT OR MSC OR MSCs OR BMT OR BMC OR BMAC OR BMCs OR HST OR HSTs
ISRCTN Register
(("marrow cell" OR "marrow cells" OR "stem cell" OR "stem cells" OR "progenitor cells" OR "precursor cells" OR "mesenchymal cells" OR "stromal cells") AND ("myocardial infarction" OR "heart attack" OR cardiomyopathy OR intramyocardial OR intracoronary)) OR (("marrow cell" OR "marrow cells" OR "stem cell" OR "stem cells" OR "progenitor cells" OR "precursor cells" OR "mesenchymal cells" OR "stromal cells") AND ("cardiac ischemia" OR "coronary ischemia" OR "myocardial ischemia" OR "heart failure" OR "cardiac failure" OR congestive OR "coronary artery disease")) OR (("cell therapy" OR "cellular therapy") AND ("myocardial infarction" OR "heart attack" OR cardiomyopathy OR intramyocardial OR intracoronary OR "cardiac ischemia" OR "coronary ischemia" OR "myocardial ischemia" OR "heart failure" OR "cardiac failure" OR congestive OR "coronary artery disease" OR angina))
WHO ICTRP Portal
Intervention: stem cells OR bone marrow cells OR cellular therapy OR mesenchymal cells OR stromal cells OR cell transplant OR precursor cells OR progenitor cells OR HSCT OR SCT OR MSC OR MSCs OR BMT OR BMC OR BMAC OR BMCs OR HST OR HSTs Condition: cardiac OR heart OR coronary OR myocardial OR angina Recruitment Status: ALL
Data and analyses
Comparison 1. Cells compared to no cells.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term follow‐up (< 12 months) | 17 | 1365 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.43, 1.49] |
| 1.2 Long‐term follow‐up (≥ 12 months) | 14 | 996 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.58, 1.50] |
| 2 Cardiovascular mortality | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term follow‐up (< 12 months) | 7 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.28, 1.82] |
| 2.2 Long‐term follow‐up (≥ 12 months) | 9 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.54, 1.99] |
| 3 Composite measure of death, reinfarction, re‐hospitalisation for heart failure | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Short‐term follow‐up (< 12 months) | 3 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.14] |
| 3.2 Long‐term follow‐up (≥ 12 months) | 6 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.10] |
| 4 Incidence of reinfarction | 20 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Short‐term follow‐up (< 12 months) | 17 | 1521 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.33, 1.30] |
| 4.2 Long‐term follow‐up (≥ 12 months) | 14 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.36, 1.12] |
| 5 Incidence of re‐hospitalisation for heart failure | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Short‐term follow‐up (< 12 months) | 13 | 1194 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.40, 1.62] |
| 5.2 Long‐term follow‐up (≥ 12 months) | 10 | 825 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.30, 1.00] |
| 6 Incidence of target vessel revascularisation | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Short‐term follow‐up (< 12 months) | 6 | 789 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.47, 1.06] |
| 6.2 Long‐term follow‐up (≥ 12 months) | 8 | 758 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.67, 1.37] |
| 7 Incidence of arrhythmias | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Short‐term follow‐up (< 12 months) | 5 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.51, 1.98] |
| 7.2 Long‐term follow‐up (≥ 12 months) | 5 | 457 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.58, 3.37] |
| 8 Incidence of restenosis | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Short‐term follow‐up (< 12 months) | 8 | 641 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.63, 1.43] |
| 8.2 Long‐term follow‐up (≥ 12 months) | 6 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.27, 1.25] |
| 9 Quality of life measures | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Short‐term follow‐up (< 12 months) | 3 | 154 | Std. Mean Difference (IV, Random, 95% CI) | 0.58 [‐0.67, 1.83] |
| 9.2 Long‐term follow‐up (≥ 12 months) | 1 | 26 | Std. Mean Difference (IV, Random, 95% CI) | 3.23 [2.01, 4.46] |
| 10 NYHA classification | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 Short‐term follow‐up (< 12 months) | 5 | 398 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.24, 0.09] |
| 10.2 Long‐term follow‐up (≥ 12 months) | 4 | 237 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.53, 0.07] |
| 11 Exercise tolerance | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Short‐term follow‐up (< 12 months) | 5 | 267 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.06, 0.43] |
| 11.2 Long‐term follow‐up (≥ 12 months) | 1 | 45 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.68, 0.58] |
| 12 Maximum VO2 (mL/kg/min) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 Short‐term follow‐up (< 12 months) | 3 | 175 | Mean Difference (IV, Random, 95% CI) | 1.15 [‐0.77, 3.07] |
| 12.2 Long‐term follow‐up (≥ 12 months) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐3.76, 4.56] |
| 13 VE/VCO2 slope | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 13.1 Short‐term follow‐up (< 12 months) | 3 | 174 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐1.02, 1.57] |
| 13.2 Long‐term follow‐up (≥ 12 months) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐3.07, 3.07] |
| 14 Peak heart rate (bpm) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Short‐term follow‐up (< 12 months) | 3 | 198 | Mean Difference (IV, Random, 95% CI) | 0.55 [‐6.79, 7.89] |
| 14.2 Long‐term follow‐up (≥ 12 months) | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐9.10 [‐20.59, 2.39] |
| 15 LVEF measured by MRI (<12 months) | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Mean change from baseline | 13 | 1057 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐1.16, 2.03] |
| 15.2 Mean value at endpoint | 15 | 1125 | Mean Difference (IV, Random, 95% CI) | 0.81 [‐0.78, 2.41] |
| 15.3 Combined | 15 | 1135 | Mean Difference (IV, Random, 95% CI) | 1.05 [‐0.56, 2.67] |
| 16 LVEF measured by MRI (≥ 12 months) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Mean change from baseline | 5 | 438 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.72, 1.78] |
| 16.2 Mean value at endpoint | 8 | 551 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐1.54, 4.34] |
| 16.3 Combined | 9 | 718 | Mean Difference (IV, Random, 95% CI) | 1.27 [‐1.14, 3.68] |
| 17 LVEF measured by echocardiography (< 12 months) | 20 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Mean change from baseline | 6 | 372 | Mean Difference (IV, Random, 95% CI) | 2.72 [1.50, 3.95] |
| 17.2 Mean value at endpoint | 20 | 862 | Mean Difference (IV, Random, 95% CI) | 2.15 [0.89, 3.42] |
| 17.3 Combined | 20 | 862 | Mean Difference (IV, Random, 95% CI) | 2.31 [1.30, 3.33] |
| 18 LVEF measured by echocardiography (≥12 months) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Mean change from baseline | 3 | 127 | Mean Difference (IV, Random, 95% CI) | 1.35 [‐2.25, 4.96] |
| 18.2 Mean value at endpoint | 9 | 377 | Mean Difference (IV, Random, 95% CI) | 2.87 [1.42, 4.31] |
| 18.3 Combined | 10 | 433 | Mean Difference (IV, Random, 95% CI) | 2.09 [0.74, 3.44] |
| 19 LVEF measured by SPECT (< 12 months) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 19.1 Mean change from baseline | 5 | 286 | Mean Difference (IV, Random, 95% CI) | 2.72 [0.23, 5.21] |
| 19.2 Mean value at endpoint | 6 | 375 | Mean Difference (IV, Random, 95% CI) | 2.19 [0.58, 3.81] |
| 19.3 Combined | 7 | 394 | Mean Difference (IV, Random, 95% CI) | 2.52 [0.59, 4.44] |
| 20 LVEF measured by SPECT (≥ 12 months) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 20.1 Mean change from baseline | 2 | 92 | Mean Difference (IV, Random, 95% CI) | 5.63 [1.77, 9.49] |
| 20.2 Mean value at endpoint | 3 | 181 | Mean Difference (IV, Random, 95% CI) | 3.46 [0.82, 6.11] |
| 20.3 Combined | 4 | 200 | Mean Difference (IV, Random, 95% CI) | 4.42 [2.68, 6.16] |
| 21 LVEF measured by left ventricular angiography (< 12 months) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 Mean change from baseline | 3 | 279 | Mean Difference (IV, Random, 95% CI) | 6.43 [0.60, 12.27] |
| 21.2 Mean value at endpoint | 9 | 711 | Mean Difference (IV, Random, 95% CI) | 4.94 [0.53, 9.35] |
| 21.3 Combined | 9 | 711 | Mean Difference (IV, Random, 95% CI) | 5.09 [0.95, 9.24] |
| 22 LVEF measured by left ventricular angiography (≥ 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 Mean value at endpoint | 1 | 62 | Mean Difference (IV, Random, 95% CI) | 8.0 [4.27, 11.73] |
| 23 LVEF measured by radionuclide ventriculography (RNV) (<12 months) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 23.1 Mean change from baseline | 2 | 118 | Mean Difference (IV, Random, 95% CI) | 0.91 [‐3.11, 4.94] |
| 23.2 Mean value at endpoint | 3 | 157 | Mean Difference (IV, Random, 95% CI) | 1.08 [‐4.88, 7.04] |
| 23.3 Combined | 3 | 157 | Mean Difference (IV, Random, 95% CI) | 1.79 [‐1.86, 5.43] |
| 24 LVEF measured by radionuclide ventriculography (≥ 12 months) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 24.1 Mean value at endpoint | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 6.30 [‐1.03, 13.63] |
1.9. Analysis.
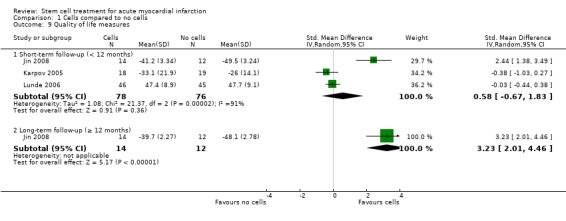
Comparison 1 Cells compared to no cells, Outcome 9 Quality of life measures.
Comparison 2. Sensitivity analysis ‐ route of cell delivery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (< 12 months) | 16 | 1335 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.42, 1.51] |
Comparison 3. Sensitivity analysis ‐ selection bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (< 12 months) | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Excluding studies with high risk of selection bias | 16 | 1307 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.43, 1.57] |
Comparison 4. Sensitivity analysis ‐ attrition bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (< 12 months) | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Excluding studies with a high or unclear risk of attrition bias | 13 | 899 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.38, 1.61] |
| 2 All‐cause mortality (≥ 12 months) | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Excluding studies with a high or unclear risk of attrition bias | 11 | 847 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.38, 1.17] |
| 3 Cardiovascular mortality (< 12 months) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Excluding studies with high or unclear risk of attrition bias | 5 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.22, 2.14] |
| 4 Cardiovascular mortality (≥ 12 months) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Excluding studies with high or unclear risk of attrition bias | 6 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.34, 1.50] |
Comparison 5. Sensitivity analysis ‐ performance bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (< 12 months) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Excluding studies with a high risk of performance bias | 8 | 669 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.23, 1.56] |
| 2 All‐cause mortality (≥ 12 months) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Excluding studies with a high risk of performance bias | 3 | 406 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.22, 1.10] |
Comparison 6. Subgroup analysis ‐ baseline LVEF measured by MRI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (< 12 months) | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Baseline LVEF < 45% | 4 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.19, 3.16] |
| 1.2 Baseline LVEF ≥ 45% | 6 | 551 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.32, 2.98] |
| 2 All‐cause mortality (≥ 12 months) | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Baseline LVEF < 45% | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.13, 2.83] |
| 2.2 Baseline LVEF ≥ 45% | 5 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.31, 1.30] |
| 3 LVEF measured by MRI (< 12 months) | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Baseline LVEF < 45% | 6 | 579 | Mean Difference (IV, Random, 95% CI) | 2.28 [0.43, 4.13] |
| 3.2 Baseline LVEF ≥ 45% | 9 | 556 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐2.42, 2.24] |
| 4 LVEF measured by MRI (≥ 12 months) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Baseline LVEF < 45% | 4 | 326 | Mean Difference (IV, Random, 95% CI) | 3.93 [‐0.15, 8.02] |
| 4.2 Baseline LVEF ≥ 45% | 5 | 342 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐2.34, 2.05] |
Comparison 7. Subgroup analysis ‐ cell type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (< 12 months) | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Mononuclear cells | 14 | 1153 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.38, 1.46] |
| 1.2 Mesenchymal stem cells | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.15, 6.60] |
| 1.3 Haematopoietic progenitor cells | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.13, 8.36] |
| 2 All‐cause mortality (≥ 12 months) | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Mononuclear cells | 12 | 923 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.54, 1.43] |
| 2.2 Mesenchymal stem cells | 1 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 69.70] |
| 2.3 Haematopoietic progenitor cells | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.12, 64.39] |
Comparison 8. Subgroup analysis ‐ dose of stem cells.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (< 12 months) | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 ≤ 108 cells | 5 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.27, 3.96] |
| 1.2 > 108 and ≤ 109 cells | 12 | 1081 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.33, 1.34] |
| 2 All‐cause mortality (≥ 12 months) | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 ≤ 108 cells | 5 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [0.97, 4.95] |
| 2.2 > 108 and ≤ 109 cells | 7 | 668 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.97] |
| 2.3 > 109 cells | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.32, 7.55] |
| 3 LVEF measured by MRI (< 12 months) | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 ≤ 108 cells | 4 | 270 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐3.51, 3.52] |
| 3.2 > 108 and ≤ 109 cells | 11 | 825 | Mean Difference (IV, Random, 95% CI) | 1.08 [‐0.53, 2.69] |
| 4 LVEF measured by MRI (≥ 12 months) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 ≤ 108 cells | 2 | 98 | Mean Difference (IV, Random, 95% CI) | 3.60 [‐4.24, 11.44] |
| 4.2 > 108 and ≤ 109 cells | 7 | 570 | Mean Difference (IV, Random, 95% CI) | 1.48 [‐1.44, 4.40] |
| 5 LVEF measured by left ventricular angiography (< 12 months) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 > 108 and ≤ 109 cells | 6 | 548 | Mean Difference (IV, Random, 95% CI) | 2.26 [‐0.71, 5.23] |
| 5.2 > 109 cells | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 11.64 [7.52, 15.75] |
Comparison 9. Subgroup analysis ‐ timing of cell administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (< 12 months) | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 ≤ 10 days since AMI | 10 | 839 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.45, 2.30] |
| 1.2 > 10 days since AMI | 3 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.06, 1.36] |
| 2 All‐cause mortality (≥ 12 months) | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 ≤ 10 days since AMI | 9 | 809 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.33, 1.11] |
| 2.2 > 10 days since AMI | 1 | 11 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.10, 5.54] |
| 3 LVEF measured by MRI (< 12 months) | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 ≤ 10 days since AMI | 12 | 867 | Mean Difference (IV, Random, 95% CI) | 1.15 [‐0.66, 2.97] |
| 3.2 > 10 days since AMI | 2 | 190 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐4.90, 3.48] |
| 4 LVEF measured by MRI (≥ 12 months) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 ≤ 10 days since AMI | 9 | 669 | Mean Difference (IV, Random, 95% CI) | 1.26 [‐1.20, 3.71] |
| 4.2 > 10 days since AMI | 1 | 109 | Mean Difference (IV, Random, 95% CI) | 1.17 [‐2.59, 4.93] |
| 5 LVEF measured by left ventricular angiography (< 12 months) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 ≤ 10 days since AMI | 5 | 535 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐1.51, 5.91] |
| 5.2 > 10 days since AMI | 3 | 156 | Mean Difference (IV, Random, 95% CI) | 7.42 [‐1.83, 16.66] |
Comparison 10. Subgroup analysis ‐ heparinised cell solution.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality (< 12 months) | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Heparin | 6 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.31, 2.66] |
| 1.2 No heparin | 10 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.30, 1.45] |
| 2 All‐cause mortality (≥ 12 months) | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Heparin | 7 | 503 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.33, 2.10] |
| 2.2 No heparin | 5 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.08] |
| 3 LVEF measured by MRI (< 12 months) | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Heparin | 7 | 434 | Mean Difference (IV, Random, 95% CI) | 1.99 [‐0.62, 4.59] |
| 3.2 No heparin | 8 | 701 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐1.67, 2.17] |
| 4 LVEF measured by MRI (≥ 12 months) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Heparin | 5 | 357 | Mean Difference (IV, Random, 95% CI) | 1.76 [‐1.93, 5.45] |
| 4.2 No heparin | 4 | 361 | Mean Difference (IV, Random, 95% CI) | 0.53 [‐2.14, 3.20] |
| 5 LVEF measured by left ventricular angiography (< 12 months) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Heparin | 5 | 256 | Mean Difference (IV, Random, 95% CI) | 6.82 [0.25, 13.39] |
| 5.2 No heparin | 3 | 393 | Mean Difference (IV, Random, 95% CI) | 1.91 [‐3.46, 7.27] |
10.3. Analysis.
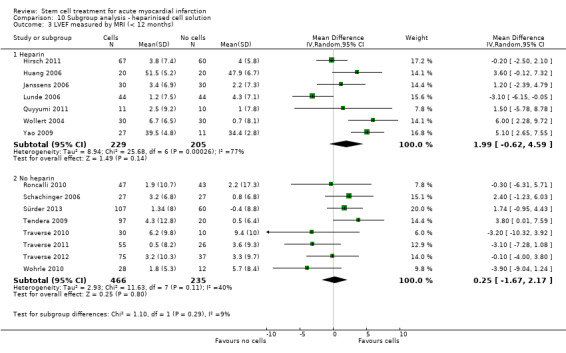
Comparison 10 Subgroup analysis ‐ heparinised cell solution, Outcome 3 LVEF measured by MRI (< 12 months).
10.4. Analysis.
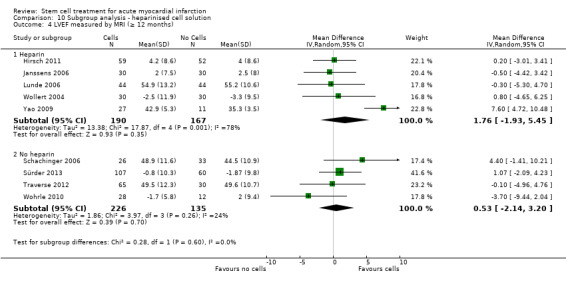
Comparison 10 Subgroup analysis ‐ heparinised cell solution, Outcome 4 LVEF measured by MRI (≥ 12 months).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Angeli 2012.
| Methods |
Type of study: parallel RCT
Type of publication: short report
Source of funding: not reported Country of origin: Brazil Number of centres: 1 Dates of trial enrolment: not reported Length of follow‐up: 12 months Number (N) of participants randomised to each arm: 11 in the treatment arm, 11 in the control arm Number (N) of participants analysed (primary outcome) in each arm: 11 in the treatment arm, 11 in the control arm |
|
| Participants |
Population: AMI successfully treated with PCI and with LVEF < 45%
Age, mean (SD) each arm: not reported
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 5 to 9 days post‐symptoms Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: methods of cell isolation not reported
Dose of stem cells: a single dose of 2.6 (± 1.6) x 108/mL mononuclear cells
Timing of stem cell procedure: cells infused 5 to 9 days following the onset of symptoms and 4 hours following harvest. Intracoronary infusion of cells in the infarct‐related artery Comparator arm: not reported |
|
| Outcomes | Primary outcomes: not reported Secondary outcomes: LVEF, LV perfusion defect, adverse events Outcome assessment points: 4 and 12 months Method(s): echocardiography, SPECT | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was described as "double blind" and a placebo was used. It was unclear whether the control group underwent bone marrow aspiration. Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes and scientific outcomes |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Cao 2009.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Shanxi Scientific and Technical Key Project, Xijing Research Boosting Program on Stem Cell Research (No. XJZT08Z04), Xijing Research Boosting Program on Cardiac Microvascular Formation Research (No. XJZT07Z05) and National Basic Research Program of China Country of origin: China Number of centres: 1 Dates of trial enrolment: 07/03 to 03/04 Length of follow‐up: 48 months Number (N) of participants randomised to each arm: 41 in treatment arm/45 in control arm Number (N) of participants analysed (primary outcome) in each arm: 41 in treatment arm/45 in control arm |
|
| Participants |
Population: AMI, within 12 hours. PCI within 12 hours
Age, mean (SD) each arm: 50.7 (SEM 1.1) years in treatment arm, 51.0 (SEM 1.0) years in control arm
Sex, % male in each arm: 95.1% in treatment arm, 93.3% in control arm Number of diseased vessels: 1 Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 6.5 (0.3) hours (mean ± SEM) before PCI in treatment arm, 6.8 (0.3) (mean ± SEM) hours before PCI in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: 40 mL bone marrow aspirated 7 days after PCI. Density gradient centrifugation (Ficoll) used to isolate BMMNC. Mononuclear cell layer harvested, washed 3 times and re‐suspended in 10 mL heparinised saline. Intracoronary infusion using PCI technique, over‐the‐wire balloon catheter advanced to the proximal part of the stented culprit lesion, inflated with 4 to 5 Atm pressure for 1 minute to occlude blood flow. At the same time MNC suspension injected into the IRA. Procedure repeated 4 times
Dose of stem cells: 4 doses of 2.5 mL cell suspension containing ˜1.25 x 108 MNC for a total of ˜ 5.00 x 108 MNC
Timing of stem cell procedure: primary PCI performed within 12 hours of onset of symptoms, cell infusion performed 7 days after primary PCI Comparator arm: patients received a 10 mL placebo intracoronary saline injection |
|
| Outcomes | Primary outcomes: ESV, EDV, LVEF, WMSI, infarct size, coronary artery restenosis Secondary outcomes: none Outcome assessment points: baseline, 1, 3, 6, 12 and 48 months Method(s): echocardiography, ECG‐gated 99m Technetium SPECT, quantitative coronary angiography | |
| Notes | Baseline values taken at day 0 (day of AMI and primary angioplasty) and at day 7 (day of BMMNC treatment or sham procedure), day 7 values entered. SPECT was also used to measure infarct size LVEF, ESV and EDV but results were not published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers between 0 and 1 were generated and a median value was calculated. Random numbers greater than the median value were allocated to the BMMNC group |
| Allocation concealment (selection bias) | Low risk | Randomisation details provided in consecutively numbered, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The control group did not undergo bone marrow aspiration although they received an injection of heparinised saline and therefore it is unclear whether participants and clinicians were sufficiently blinded to treatment. Outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient in the BMMNC group (1/41) had transient acute HF seven days after transplant. 1 patient in the control group (1/45) had in‐stent restenosis and was subjected to repeat PCI at 1‐year follow‐up. It is unclear whether these patients were included at follow‐up. One additional control had died at 1‐year follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00626145) were reported |
| Other bias | Low risk | None reported or identified |
Chen 2004.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: not reported Country of origin: China Number of centres: 1 Dates of trial enrolment: 11/02 to 05/03 Length of follow‐up: 6 months Number (N) of participants randomised to each arm: 34 in treatment arm/35 in control arm Number (N) of participants analysed (primary outcome) in each arm: 34 in treatment arm, 35 in control arm |
|
| Participants |
Population: AMI, within 12 hours
Age, mean (SD) each arm: 58 (7.0) years in treatment arm, 57 (5.0) years in control arm
Sex, % male in each arm: 94% in treatment arm, 97% in control arm Number of diseased vessels: 1.6 (0.5) in treatment arm, 1.7 (0.4) in control arm Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 8.3 (3.8) hours from AMI to PCI in treatment arm; 8.5 (3.9) hours from AMI to PCI in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: 60 mL of autologous bone marrow was aspirated under local anaesthesia from the ilea of all 69 patients in the morning 8 days after PCI and cultured for 10 days. Cells were harvested and washed 3 to 4 times with heparinised saline, and the cell suspension was mixed with heparin, filtrated and prepared for implantation 2 hours before implantation. 6 mL of the cell suspension was injected directly into the target coronary artery through an inflated over‐the‐wire balloon catheter in the central lumen with high pressure (10 atm). The balloon remained inflated for 2 or more minutes to occlude anterior blood flow just before beginning the BMMNC injection
Dose of stem cells: 6 mL containing 8 to 10 x 109 cells/mL
Timing of stem cell procedure: 18.4 (0.5) days after PCI Comparator arm: 6 mL standard saline via PCI method |
|
| Outcomes |
Primary outcomes: cardiac death Secondary outcomes: "Left ventricular haemodynamics": functional defect (%), infarcted area movement velocity, LVEF. "Cardiac functional indexes": LVESV, LVEDV, circumferential shortening, Psyst/ESV, perfusion defect by PET. Measured by echocardiography and PET Outcome assessment points: baseline, 3 and 6 months Method(s): PET, echocardiography, NOGA, left ventriculography |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The control group underwent bone marrow aspiration and received an injection of saline by the same method as the BMSC group. Blinding of clinicians was not reported. Outcome assessors were blinded to treatment allocation. 3 independent statisticians who had no knowledge of the study collected and analysed outcome data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes and scientific outcomes |
| Selective reporting (reporting bias) | Unclear risk | Incomplete data for LVEDV and LVESV were provided in the results although these outcomes are not included in this review. It would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Colombo 2011.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: supported by grants from the Italian Ministry of Health (Progetto Ricerca Finalizzata 2002 and 2005, Progetto ex art. 56 2007); the Italian Ministry of University and Research, and the 6FP EU Project ‐ THERCORD. Materials for CD133+ cell separations were kindly provided by Miltenyi Biotec Country of origin: Italy Number of centres: 2 Dates of trial enrolment: 10/03 to 10/06 Length of follow‐up: 12 months Number (N) of participants randomised to each arm: 5 in the treatment arm, 5 in the control arm Number (N) of participants analysed (primary outcome) in each arm: 5 in the treatment arm, 4 in the control arm |
|
| Participants |
Population: STEMI with PCI within 6 hours of symptom onset
Age, mean (SD) each arm: median 54 (range 47 to 60) years in treatment arm, median 56 (range 44 to 58) years in control arm
Sex, % male in each arm: 100% in both trial arms Number of diseased vessels: 1 Number of stunned hyperkinetic, etc segments: mean 4.2 (1.6) in treatment therapy arm, mean 3.8 (1.3) in control arm Time from symptom onset to initial treatment: median 265 hours from symptoms onset to PCI; cell therapy on day 9 to 16 after PCI Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: CD133+
Type of stem cells: CD133 selected bone marrow‐derived stem cells
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspiration followed by immunomagnetic selection with specific monoclonal antibody using the CliniMacs System. Re‐suspended in 10 mL (± 2) of normal saline solution (0.9% NaCl) with 10% human serum albumin. Delivery via intracoronary infusion by PCI over the wire balloon catheter technique
Dose of stem cells: median 5.9 x 106 (range 4.9 +/‐ 13.5) CD133+ cells
Timing of stem cell procedure: cell infusion was done 9 to 13 days following STEMI and successful PCI Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: 1. any adverse event during hospital stay, 2. PET‐derived changes in myocardial perfusion and infarct size at 12 months, and 3. variations in LVDV, LVEF and WMSI at 12 months by echocardiography
Secondary outcomes: all‐cause death, cardiac death, symptomatic heart failure and coronary symptoms requiring hospitalisation and target vessel revascularisation
Outcome assessment points: 3, 6, 12 months Method(s): echocardiography, gated PET |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken using a permuted block randomisation system and numbered containers |
| Allocation concealment (selection bias) | Low risk | Randomisation, patient enrolment and assignment to study group was done by a blinded co‐ordinator |
| Blinding (performance bias and detection bias) All outcomes | High risk | Controls did not undergo bone marrow aspiration; no placebo was administered to controls. After randomisation, study processes were blinded to the researchers involved in echocardiography and PET evaluation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes and scientific outcomes at 6 months. 1 patient in the control group underwent heart transplantation 6 months after STEMI and was not included in 12‐month evaluation |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00400959) were reported |
| Other bias | Low risk | None reported or identified |
Gao 2013.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: grant of the National Advanced Technology Development Plan of China Country of origin: China Number of centres: 4 Dates of trial enrolment: 05/08 to 11/09 Length of follow‐up: 24 months Number (N) of participants randomised to each arm: 21 in the treatment arm, 22 in the control arm Number (N) of participants analysed (primary outcome) in each arm: 19 in the treatment arm, 20 in the control arm |
|
| Participants |
Population: acute STEMI reperfused within 12 hours by PCI
Age, mean (SD) each arm: 55 (SEM 1.6) years in treatment arm, 58.6 (SEM 2.5) years in control arm
Sex, % male in each arm: 100% in treatment arm, 86.4% in control arm Number of diseased vessels: 1 (42.9%), 2 (19.0%), 3(38.1%) in treatment arm, 1 (50%), 2 (18.2%), 3 (31.8%) in control arm Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 17.1 (SEM 0.6) days from reperfusion to infusion of cells Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BM‐MSC
Type of stem cells: bone marrow‐derived mesenchymal stromal cells (MSC)
Summary of how stem cells were isolated and type and route of delivery: bone marrow (80 mL in 2000 IU of heparin) was harvested from each patient in the treatment group from the posterior iliac crest under local anaesthesia by a haematologist 2 to 3 days after primary PCI. The bone marrow aspirate was shipped at room temperature to the central cell‐processing laboratory. The mononuclear cell fraction was isolated using a density gradient with Lymphocyte Separation Medium (Biowhittaker) and then the low‐density cells were washed and viable cells were counted. The BM‐MCs were seeded into 75 cm² tissue culture flasks in MSCs medium consisting of Dulbecco's modified Eagle's medium containing 4.5% glucose (DMEM‐4.5, HyClone), supplemented with 10% fetal bovine serum (GIBCO) and 1% antibiotic‐antimycotic solution (Lift Technologies). The cell suspension was removed after 72 hours and the adherent cells were cultured in at 37 °C with 5% CO2. The culture medium was changed every 3 to 4 days until colonies were formed. After 14.6 ± 0.7 days of culture, passage 2 (P2) cells were harvested by trypsin treatment. Cells were washed, and viability was tested by trypan blue exclusion. Cell counts were performed, and the cells at 4 °C were delivered to the catheterisation laboratory. Cell were re‐suspended in heparinised saline
Dose of stem cells: 3.08 (± 0.52) x 106 cells
Timing of stem cell procedure: 16 to 17 days after PCI. Time from reperfusion to infusion of study therapy = 17.1 (SEM 0.6) days Comparator arm: no additional therapy (control) |
|
| Outcomes | Primary outcomes: absolute changes in myocardial viability and perfusion in the infarcted region measured by F‐18‐FDGi SPECT at 6 months, and in global LVEF measured by 2D echocardiogram at 6, 12 and 24 months after cell infusion Secondary outcomes: incidence of cardiovascular events, total mortality and adverse events at 12 and 24 months follow‐up Outcome assessment points:6, 12, 24 months Method(s): echocardiography, F‐18‐FDG SPECT | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised 1:1 to treatment or control using sequential numbers |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was described as "open label". Controls did not undergo bone marrow aspiration; no placebo was administered to controls. Echocardiography data were analysed independently by 2 experienced observers who were unaware of patients' treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant (1/22) in the control arm was lost to follow‐up at 6 months and 1 patient (1/21) in the BMSC arm had died at 6 months follow‐up; all other randomised participants were included in the analysis of clinical and scientific outcomes at 6 months. 2 further participants (1 in each treatment group) were lost to follow‐up at 12 and 24 months' follow‐up |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Ge 2006.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Shanghai Scientific Research Fund Country of origin: China Number of centres: 1 Dates of trial enrolment: not reported Length of follow‐up: 6 months Number (N) of participants randomised to each arm: 10 in treatment arm/10 in control arm Number (N) of participants analysed (primary outcome) in each arm: 10 in treatment arm/10 in control arm |
|
| Participants |
Population: AMI, within 24 hours. PCI within 24 hours. Cell transplantation after successful PCI
Age, mean (SD) each arm: 58 (11) years in treatment arm, 59 (8) years in control arm
Sex, % male in each arm: 80% in treatment arm, 100% in control arm Number of diseased vessels: 1:7, 2:2, 3:1 in treatment arm; 1:7, 2:3, 3:0 in control arm Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 7.9 (3.8) hour in treatment arm/7.1(3.1) hour in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirate (40 mL). The method of cell separation was not reported. Cells were infused after successful PCI
Dose of stem cells: a single dose of 4 x 107/mL mononuclear cells
Timing of stem cell procedure: cells infused within 15 hours of onset of AMI Comparator arm: 15 mL injection of bone marrow supernatant |
|
| Outcomes | Primary outcomes: LVEF, LVEDD, myocardial perfusion defect Secondary outcomes: not listed Outcome assessment points: baseline, 1 week and 6 months Method(s): echocardiography | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in a 1:1 ratio with the use of sequentially numbered, sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Controls underwent bone marrow aspiration and received an injection of BM supernatant. The study states that clinical data were acquired and analysed in a 'blinded fashion' by clinicians who were blinded to the groups' identities |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes and scientific outcomes |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Grajek 2010.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Polish Cardiac Society, Servier Polska and the Polish Committee for Scientific Research (Komitet Badan Naukowych) PBZ‐KBN‐099/P05/03 Country of origin: Poland Number of centres: 1 Dates of trial enrolment: 06/03 to 06/06 Length of follow‐up: 12 months Number (N) of participants randomised to each arm: 31 in treatment arm/14 in control arm Number (N) of participants analysed (primary outcome) in each arm: 31 at 3 and 6 months, 27 at 12 months in treatment arm/14 at 3 and 6 months, 12 at 12 months in control arm |
|
| Participants |
Population: AMI, within 12 hours.
Age, mean (SD) each arm: 49.9 (8.4) years in treatment arm, 50.9 (9.3) years in control arm
Sex, % male in each arm: 87% in treatment arm, 86% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 290 (234) minutes from AMI to PCI in treatment arm/190 (212) minutes from AMI to PCI in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: 80 (±30) mL (range 50 to 150 mL) bone marrow was collected from the pelvic bones into phosphate‐buffered saline (PBS) with heparin (50 U/mL) under local anaesthesia. Diluted 1:2 with PBS and centrifuged in Ficoll gradient. MNC collected, washed in PBS with heparin, re‐suspended in a few mL of X‐vivo 15 medium with 2% heat‐inactivated autologous plasma, placed in Teflon bags and overnight cultivated. Cells harvested and washed 3 times with heparinised PBS the next day. BMSC administered via IRA to the infarcted zone with a stop‐flow technique through an over‐the wire‐balloon catheter
Dose of stem cells: 0.410 ± 0.18 x 109 BMMNC (12.25 ± 2.05 mL) divided into 3 to 4 portions containing 3 to 4 mL cell suspension each
Timing of stem cell procedure: 4 to 5 days after AMI Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: left ventricle perfusion, LVEF Secondary outcomes: LVESV, LVEDV, WMSI, cardiopulmonary exercise testing results, MACE (death, AMI, and need for revascularisation) Outcome assessment points: baseline, 3, 6 and 12 months Method(s): echo, SPECT, RNV, cardiopulmonary exercise treadmill test, coronary angiography |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned to the BMSC or control group by means of restricted randomisation (permuted blocks randomisation). The block size was 6 and the number of block was chosen using a computer random number generator. Patients having numbers 1 to 4 were allocated to the treatment group, whereas patients having numbers 5 or 6 were allocated to the control group (2:1 ratio) |
| Allocation concealment (selection bias) | Unclear risk | Prepared envelopes with treatment assignment were used; it is unclear whether these were sealed or opaque |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study was "not blinded for the patients"; controls did not undergo bone marrow aspiration and no placebo was administered. Investigators assessing outcome measures were blinded to the group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical and scientific outcomes at 6 months. At 12 months, there were 4/31 withdrawals in the BMSC arm (1 sudden death at 7 months, 3 patients revascularised between 6 and 12 months) and 2/14 withdrawals in the control arm (2 patients revascularised between 6 and 12 months) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | High risk | Supported in part by commercial funding |
Hirsch 2011.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Interuniversity Cardiology Institute of The Netherlands (ICIN), the Netherlands Heart Foundation (grant 2005T101, 2003B126), Biotronik, Boston Scientific, Guerbet, Guidant, Medtronic, Novartis, Pfizer, Sanofi‐Aventis Country of origin: the Netherlands Number of centres: 8 Dates of trial enrolment: 08/05 to 04/08 Length of follow‐up: 5 years Number (N) of participants randomised to each arm: 69 in treatment arm/65 in control arm Number (N) of participants analysed (primary outcome) in each arm: 67 in treatment arm/60 in control arm |
|
| Participants |
Population: first STEMI. PCI with stent within 12 hours
Age, mean (SD) each arm: 56 (9) years in treatment arm, 55 (10) years in control arm
Sex, % male in each arm: 84% in treatment arm, 86% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: 53.3 (19.6)% dysfunctional segments in treatment arm/56.2 (24.7)% dysfunctional segments in control arm Time from symptom onset to initial treatment: median 3.5 (IQR 2.4 to 5.1) hours in treatment arm/median 3.4 (IQR 2.3 to 4.2) hours in control arm Statistically significant baseline imbalances between the groups?: none reported |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: 60 mL BM aspirated from iliac crest under local anaesthesia, collected in a sterile container with heparin, sent to 1 of 6 cell‐processing labs. MNC isolated by density gradient centrifugation using LymphoprepTM, washed twice and re‐suspended in 15 to 20 mL saline with 4% human serum albumin and 20 IU/mL sodium heparin. Cells were infused into the infarct‐related artery through the central lumen of an over‐the‐wire balloon catheter in 3 sessions of 3 minutes of coronary occlusion, interrupted by 3 minutes of coronary flow
Dose of stem cells: total 296 (164) x 106 BMMNC
Timing of stem cell procedure: cells infused 3 to 8 days after primary PCI (median 6 days) Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: "The change in regional myocardial function in dysfunctional segments at baseline defined as the percentage of dysfunctional segments with improved segmental wall thickening at 4 months" Secondary outcomes: "changes in absolute segmental wall thickening in dysfunctional segments, changes in global LVEF, volumes, mass, and infarct size, and changes in regional myocardial function stratified by transmural extent of infarction." Outcome assessment points: baseline, 4 months, 2 years, 5 years Method(s): MRI, angiogram |
|
| Notes | 3 patients did not receive cell therapy as randomised: 1 withdrew consent, 1 aspiration was unsuccessful and 1 patient experienced an occluded infarct‐related artery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation was performed with stratification according to site, with the use of a computerised voice‐response system |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Controls did not undergo bone marrow aspiration and no placebo was administered. "After randomisation, study processes were not blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes at 4 months, with the exception of 1 patient in the BMSC group who withdrew consent. In the analysis of MRI data at 4 months, 1 further patient in the BMSC group (total 2/69) and 5 patients in the control group (5/65) withdrew or were excluded due to poor quality MRI (1 BMSC patient and 3 controls), 1 control patient who received and implanted ICD, and 1 control patient who refused follow‐up. At 2 years follow‐up, a total of 10/69 BMSC patients and 13/65 control patients were withdrawn or excluded from MRI analysis; reasons were given. In the analysis of clinical outcomes at 5 years, 9 patients (BMSC: 4/69 versus controls: 5/65) were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the study design protocol are reported apart from exercise tolerance, which was included as a secondary outcome |
| Other bias | High risk | Supported in part by commercial funding |
Huang 2006.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: not reported Country of origin: China Number of centres: 1 (assumed) Dates of trial enrolment: 05/04 to 05/05 Length of follow‐up: 6 months Number (N) of participants randomised to each arm: 20 in treatment arm/20 in control arm Number (N) of participants analysed (primary outcome) in each arm: 20 in treatment arm/20 in control arm |
|
| Participants |
Population: AMI, within 24 hours. PCI within 24 hours. Cell transplantation within 2 hours of successful PCI
Age, mean (SD) each arm: 57.3 (10.1) years in treatment arm, 56.7 (9.2) years in control arm
Sex, % male in each arm: 65% in treatment arm, 70% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 6.3 (4.2) hours in treatment arm/6.3 (3.9) hours in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirate (80 to 140 mL). Cells separated by gradient centrifugation. Cells re‐suspended in heparinised saline (with 0.9% NaCl) prior to transplantation. Intracoronary infusion using a microcatheter (Judkins method)
Dose of stem cells: a single dose of 1.8 (4.2) x108/mL cells
Timing of stem cell procedure: cells infused within 2 hours of successful PCI Comparator arm: 15 mL of heparinised saline (with 0.9% NaCl) |
|
| Outcomes | Primary outcomes: not reported Secondary outcomes: LVEF, LVEDV and infarct size measured by CMR imaging and LV arteriography Outcome assessment points: baseline, 1 week and 6 months Method(s): CMR imaging | |
| Notes | Translated from Chinese (Mandarin) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The control group received a placebo but it was unclear whether they underwent bone marrow aspiration and therefore it was unclear whether they were appropriately blinded. Blinding of clinicians and outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical and scientific outcomes |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Huang 2007.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: National Technology Excellence Programme (2004BA714B05‐2) Country of origin: China Number of centres: 1 Dates of trial enrolment: 08/05 to 12/05 Length of follow‐up: 6 months Number (N) of participants randomised to each arm: 20 in treatment arm/20 in control arm Number (N) of participants analysed (primary outcome) in each arm: 20 in treatment arm/20 in control arm |
|
| Participants |
Population: AMI within 24 hours. PCI within 24 hours
Age, mean (SD) each arm: 54.8 (5.8) years in treatment arm, 55.4 (7.1) years in control arm
Sex, % male in each arm: 85% in treatment arm, 90% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: PCI within 6.9 (2.7) hours of AMI in treatment arm/PCI within 6.5 (2.4) hours of AMI in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: 80 to 140 mL of bone marrow aspirated from the hip bone under local anaesthetic. BMMNC isolated by gradient centrifugation. Intracoronary transplantation of BMMNC via a micro‐infusion catheter immediately after PCI
Dose of stem cells: single dose of (1.2 ± 6.5) x 108 BMMNC
Timing of stem cell procedure: PCI performed within 24 hours of symptom onset, BMSC transplantation performed within 2 hours of PCI Comparator arm: intracoronary transplantation of heparinised saline via a micro‐infusion catheter immediately after PCI |
|
| Outcomes |
Primary outcomes: none Secondary outcomes: LVEF, myocardial viability Outcome assessment points: baseline and 6 months Method(s): echocardiography, SPECT |
|
| Notes | Translated from Chinese (Mandarin) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The control group received an injection of heparinised saline although it is not reported whether they underwent bone marrow aspiration. It is therefore unclear whether participants and clinicians were sufficiently blinded to treatment. It was not reported whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of scientific outcomes. No clinical outcomes were reported |
| Selective reporting (reporting bias) | Unclear risk | LVESV and LVEDV were assessed but data were not provided although these outcomes are not included in this review. All other outcomes mentioned in the methods are reported in the results |
| Other bias | Low risk | None reported or identified |
Huikuri 2008.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Medical Council of the Academy of Finland, the Finnish Foundation for Cardiovascular Research & the Foundation for the Northern Health Support, Boston Scientific Sverige AB, Stockholm, Sweden Country of origin: Finland Number of centres: 2 Dates of trial enrolment: 10/04 to 02/07 Length of follow‐up: 6 months Number (N) of participants randomised to each arm: 40 in treatment arm/40 in control arm Number (N) of participants analysed (primary outcome) in each arm: 36 for LV angiography, 39 for 2‐D echocardiography, 28 for IVUS in treatment arm/36 for LV angiography, 38 for 2‐D echocardiography, 30 for IVUS in control arm |
|
| Participants |
Population: AMI, within 12 hours. Thrombolysis within 12 hours. PCI within 2 to 3 days
Age, mean (SD) each arm: 60 (10) years in treatment arm, 59 (10) years in control arm
Sex, % male in each arm: 90% in treatment arm, 85% in control arm Number of diseased vessels: 19 (48%) had 1 vessel disease, 15 (37%) had 2, 6 (15%) had 3 in treatment arm, 25 (62%) had 1 vessel disease, 13 (33%) had 2, 2 (5%) had 3 in control arm Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 2.8 (2.3) hours from AMI to thrombolysis, 48 (12) hours from thrombolysis to PCI in BMSC arm; 3.1 (3.9) hours from AMI to thrombolysis, 44 (13) hours from thrombolysis to PCI in treatment arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: 80 mL bone marrow was aspirated into heparin‐treated syringes from the posterior iliac crest under local anaesthesia. Mononuclear cells were isolated from aspirate using density gradient centrifugation on Ficoll‐Hypaque, washed twice with heparinised physiological saline and re‐suspended in 10 mL of medium containing 5 mL of the patient's own serum and heparinised physiological saline. BMC suspension then was filtered through 100 micrometre nylon mesh. Medium containing the BMCs was injected intracoronally through over the wire balloon by using intermittent balloon inflation in the stent at the time of injection
Dose of stem cells: mean 402 (196) x 106 mononuclear cells injected (median = 360 x 106) of which a mean of 2.6 (1.6) x 106
Timing of stem cell procedure: the time interval between the AMI and cell transfer was 70 (36) hours (median 60 hours) in BMMNC arm Comparator arm: placebo medium containing the same solution as cell medium without the cells |
|
| Outcomes |
Primary outcomes: (1) Absolute change in global LVEF from baseline to 6 months. (2) Absolute changes in the measures obtained by IVUS. (3) Changes in arrhythmia risk variables from baseline to 6 months Secondary outcomes: exercise stress test Outcome assessment points: baseline and 6 months Method(s): 2‐D echocardiography, LV angiography, IVUS, ECG |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes for each patient were generated by a laboratory nurse in Ouluusing using "a computer‐generated random‐permuted block design with variable block sizes and selected on the basis of whether a suspension containing BMCs or placebo medium was given to each patient". The laboratory nurse in Turku was informed by a telephone call from Oulu about the randomisation and type of treatment |
| Allocation concealment (selection bias) | Low risk | The laboratory nurse in Turku was informed by a telephone call from Oulu about the randomisation and type of treatment. The lab nurses who prepared the treatment or placebo solution according to patient allocation did not take part in any other parts of the research protocol |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All patients had bone marrow aspiration and control group patients were given an intracoronary injection of placebo medium. The treatment and control media were externally prepared by laboratory nurses. Blinded outcome assessors not involved in randomisation quantitatively analysed angiograms, echocardiograms and intravascular ultrasounds in a central core laboratory. Consecutively numbered, sealed envelopes were provided and stored in the Clinical Research Laboratory of the University of Oulu and were opened after all baseline and 6‐month data were analysed from all patients |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes. In the analysis of scientific outcomes by echocardiography at 6‐month follow‐up, the number of withdrawals was low in both trial arms (1 patient in each treatment arm due to refusal from repeat testing and 1 death in the placebo arm). Further withdrawals from LV angiography were low and balanced between treatment groups (BMSC: 4/40 versus placebo: 4:40). Analysis by IVUS incurred a higher number of withdrawals but these were balanced between treatment arms (BMSC: 28/40 versus placebo: 30/40) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00363324) were reported |
| Other bias | High risk | Supported in part by commercial funding |
Janssens 2006.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Fund of Scientific Research Flanders Country of origin: Belgium Number of centres: 1 Dates of trial enrolment: 05/03 to 11/04 Length of follow‐up: 4 months Number (N) of participants randomised to each arm: 33 in treatment arm/34 in control arm Number (N) of participants analysed (primary outcome) in each arm: 33 in treatment arm/34 in control arm |
|
| Participants |
Population: AMI, within 24 to 48 hours
Age, mean (SD) each arm: 55.8 (11) years in treatment arm, 57.9 (10) years in control arm
Sex, % male in each arm: 82% in treatment arm, 82% in control arm Number of diseased vessels: 1 in treatment arm (36% right artery/64% left artery)/1 in control arm (38% right artery/62% left artery) Number of stunned hyperkinetic, etc segments: 3 or more contiguous segments out of total 17 Time from symptom onset to initial treatment: 3.7 hours (median) before PCI in treatment arm/4.1 hours (median) before PCI in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirated, cells separated using gradient centrifugation. 4 to 6 hours after harvest, cells were washed and re‐suspended in 10 mL of saline containing 0.9% NaCl and 5% autologous serum. Intracoronary infusion using an inflated balloon catheter. 3 fractions of cells were infused over 2 to 3‐minute periods separated by 3‐minute reperfusion
Dose of stem cells: 10 mL of cell suspension, a total dose of 3.0 (1.28) x 108 nucleated cells containing 1.72 (0.72) x 108 MNC
Timing of stem cell procedure: PCI was performed about 4 hours after onset of symptoms. Cell treatment was conducted within 1 day of PCI Comparator arm: placebo consisting of 10 mL of saline containing 0.9% NaCl and 5% autologous serum |
|
| Outcomes |
Primary outcomes: changes in LVEF at 4 months
Secondary outcomes: changes in:
1. infarct size
2. LV function Outcome assessment points: baseline, 4 and 12 months. Method(s): MRI |
|
| Notes | This trial includes some patients with previous AMI, but data analysis without these patients did not significantly change the final results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised randomisation list was used |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as "double blind". All patients underwent bone marrow aspiration and control group patients were given an intracoronary injection of placebo medium. Outcome assessors were blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes. In the analysis of scientific outcomes measured by MRI at 4 and 12 months, the number of withdrawals was low and balanced between trial arms (BMSC: 3/33 versus control: 4/34). Reasons for withdrawal were 1 x technical failure, 2 x claustrophobia to MRI, 2 x patient refusal, 1 x intracochlear implant and 1 death in the BMSC arm due to haemorrhagic shock) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00264316) were reported |
| Other bias | Low risk | None reported or identified |
Jazi 2012.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: not reported Country of origin: Iran Number of centres: 1 Dates of trial enrolment: 06/02 to 01/04 Length of follow‐up: 6 months Number (N) of participants randomised to each arm: not reported Number (N) of participants analysed (primary outcome) in each arm: 16 in the treatment arm, 16 in the control arm |
|
| Participants |
Population: AMI within 1 month with a history of anterior MI and LVEF < 35%
Age, mean (SD) each arm: 48.0 (SEM 2.5) years in treatment arm, 45.2 (SEM 3.2) years in control arm
Sex, % male in each arm: 66% in treatment arm, 90% in control arm Number of diseased vessels: 1 Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: up to 1 month Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirates were obtained under local anaesthesia with a standard Jamshidi needle with heparin (50 U/mL) from posterior iliac crests. Bone marrow‐derived mononuclear cells (BMCs) were isolated by layering on a Ficoll‐Paque gradient. Cell populations included hematopoietic progenitor cells. A haemocytometer was used to estimate the number of nucleated cells in the final preparation of bone marrow cells. Nucleated cell viability was assessed by trypan blue exclusion. Nucleated cells were cultured in an M199 medium, 10% human serum supplemented with 50 ng/mL vascular endothelial growth factor (VEGF), 1 ng/mL basic fibroblast growth factor (bFGF), and 2 ng/mL insulin‐like growth factor‐1 (IGF‐1). The cells were incubated overnight at 37 ºC in a fully humidified atmosphere with 5% CO2. Then, cells were washed twice and re‐suspended in 5 mL human serum
Dose of stem cells: (24.6 ± SEM 8.4) × 108 cells Timing of stem cell procedure: within 1 month of AMI, at the time of PCI Comparator arm: no additional therapy (control) |
|
| Outcomes | Primary outcomes: not reported Secondary outcomes: perfusion defects, regional wall motion of LV and LVEF, adverse events Outcome assessment points: 6 months Method(s): SPECT, echocardiography | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Controls did not undergo bone marrow aspiration and no placebo was administered; neither participants nor patients were blinded. Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants randomised to each treatment arm was unclear; the study states that 20 participants met the inclusion criteria but the analysis includes 16 participants in each group. It is therefore unclear how many patients were randomised to each treatment group. No details of patient withdrawal were reported |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although echocardiography measurements taken at 1 month were not reported. It would be difficult to rule out other selective reporting |
| Other bias | Low risk | None reported or identified |
Jin 2008.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: the Scientific Research Program of Shanghai Health Bureau, No. 054065 Country of origin: China Number of centres: 1 Dates of trial enrolment: 05/05 to 09/06 Length of follow‐up: 12 months Number (N) of participants randomised to each arm: 14 in treatment arm/12 in control arm Number (N) of participants analysed (primary outcome) in each arm: 14 in treatment arm/12 in control arm |
|
| Participants |
Population: AMI, within 24 hours. Thrombolysis within 24 hours
Age, mean (SD) each arm: 62.3 (7.68) years in treatment arm, 60.6 (6.46) years in control arm
Sex, % male in each arm: 71.4% in treatment arm, 75% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: PCI within 7 to 10 days of AMI symptom onset Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: 40 mL BM aspirated under local anaesthesia from the left posterior superior iliac spine. Suspended in 160 mL solution of heparinised normal saline, filtered twice, centrifuged to isolate MNC, washed twice, re‐suspended in heparinised normal saline. PCI to IRA with an over‐the‐wire balloon catheter delivering BMMNC to the proximal end of the LAD in one dose within 2 to 3 minutes
Dose of stem cells: 1 dose of 15 ± 2 mL BMMNC suspension containing 6.27 ± 1.75 x 107 BMMNC and 0.36 ± 0.11% CD133+, 0.69 ± 0.13% CD34+ cells
Timing of stem cell procedure: 7 to 10 days after AMI Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: none Secondary outcomes: LVEF, parameters of cardiac geometric pattern, serum NT‐proBNP, Minnesota heart failure questionnaire before and after treatment Outcome assessment points: baseline, 6 and 12 months Method(s): echocardiography, Minnesota heart failure questionnaire, blood biochemistry tests |
|
| Notes | Translated from Chinese (Mandarin) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Controls did not undergo bone marrow aspiration and no placebo was administered; neither participants nor patients were blinded. Echocardiogram images were analysed by experienced independent echocardiographers unaware of patient allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes and scientific outcomes |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Karpov 2005.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: not reported Country of origin: Russia Number of centres: 1 (assumed) Dates of trial enrolment: not reported Length of follow‐up: mean 8.23 (0.72) years Number (N) of participants randomised to each arm: 22 in treatment arm/22 in control arm. 8‐year follow‐up: 28 in the treatment arm and 34 in the control arm Number (N) of participants analysed (primary outcome) in each arm: 22 in treatment arm/22 control arm. 8‐year follow‐up: 26 in the treatment arm and 32 in the control arm |
|
| Participants |
Population: AMI, within 7 to 21 days
Age, mean (SD) each arm: 55.2 (8.6) years in treatment arm, 52.1 (3.2) years in control arm
Sex, % male in each arm: 90% in treatment arm, 73% in control arm Number of diseased vessels: 1:1; 2:14; 3:4 in treatment arm/1:8; 2:6; 3:3 in control arm Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: PCI within 4 hours of onset of symptoms Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: BM aspirates and cells separated by density gradient centrifugation. Cells re‐suspended in heparinised solution prior to transplantation. Route of delivery not reported in the study
Dose of stem cells: a single dose of 88.5 (49.2) x 106 MNC
Timing of stem cell procedure: within 7 to 21 days after PCI Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: not reported
Secondary outcomes: not reported, but give data on mortality, morbidity, quality of life, exercise tolerance and engraftment of infused cells Outcome assessment points: baseline, 3 months and 6 months, mean 8.23 (0.72) years (clinical outcomes) Method(s): 6‐minute walking test, QoL scores, % radioactivity/no. of cells |
|
| Notes | Secondary 2006 and 2014 papers translated from Russian | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Controls did not undergo bone marrow aspiration and no placebo was administered; neither participants nor patients were blinded. Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In an early publication, 3 patients in the BMSC group (4/22) and 3 patients in the control group (3/22) were excluded due to "repeated AMI, restenosis or the infarction‐related artery, and microcoronary angiography" (no breakdown between groups was reported). However, in a subsequent study of a larger cohort reporting long‐term follow‐up, a lower number of withdrawals or exclusions was reported (BMSC: 2/28 versus controls: 2/34); reasons for withdrawals were not given. It is unclear to what extent these 2 publications overlap |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Lee 2014.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: funded by PCB‐Pharmicell Company Limited (Seongnam, Korea) Country of origin: South Korea Number of centres: 3 Dates of trial enrolment: 03/07 to 09/10 Length of follow‐up: 6 months Number (N) of participants randomised to each arm: 40 in the treatment arm, 40 in the control arm Number (N) of participants analysed (primary outcome) in each arm: 30 in the treatment arm, 28 in the control arm |
|
| Participants |
Population: AMI within 96 hours
Age, mean (SD) each arm: 53.9 (10.5) years in treatment arm, 54.2 (7.7) years in control arm
Sex, % male in each arm: 90.0% in treatment arm, 89.3% in control arm Number of diseased vessels: 1 (n = 16), 2 (n = 11), 3 (n = 3) in treatment arm, 1 (n = 16), 2 (n = 8), 3 (n = 4) in control arm Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 350.8 (325.4) minutes in treatment arm, 115.3 (35.5) minutes in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BM‐MSC
Type of stem cells: bone marrow‐derived mesenchymal stromal cells
Summary of how stem cells were isolated and type and route of delivery: 20 to 25 mL (mean ± SD: 23.1 ±1 1.5 mL) of BM aspirates were obtained under local anaesthesia from the posterior iliac crest in the treatment group on 3.8 ± 1.5 days after admission. All manufacturing and product testing procedures for the generation of clinical‐grade autologous MSCs were carried out under good manufacturing practice (FCB‐Pharmicell Company Limited, Seongnam, Korea). Mononuclear cells were separated from the BM by density gradient centrifugation (HISTOPAQUE‐1077; Sigma‐Aldrich, St. Louis, MO, USA) and washed with phosphate‐buffered saline (PBS). Cells were re‐suspended in Dulbecco's modified Eagle's medium‐low glucose (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (Gibco), 100 U/mL penicillin/100 μg/mL and streptomycin (Gibco). They were plated at 2 to 3 × 105 cells/cm2 into 75 cm2 flasks. Cultures were maintained at 37 °C in a humidified atmosphere containing 5% CO2. After 5 to 7 days, non‐adherent cells were removed by replacing the medium; adherent cells were cultured for another 2 to 3 days. When the cultures were near confluence (70% to 80%), adherent cells were detached by using trypsin containing ethylene diamine tetra‐acetic acid (EDTA; Gibco) and replated at 4 to 5 × 103 cells/cm2 in 175 cm2 flasks. Cells were serially subcultured up to passage 4 or passage 5 for infusion (mean ± SD: 4.4 ± 0.5 passages). On the day of administration, MSCs were harvested using trypsin and EDTA, washed twice with PBS and once with saline solution, and re‐suspended to a final concentration of 1 × 106 cells/kg. The criteria for the release of MSCs for clinical use included viability > 80%, absence of microbial contamination (bacteria, fungus, virus and mycoplasma) if undertaken 3 to 4 days before administration, and expression of CD73 and CD105 by > 90% of cells and absence of CD14, CD34 and CD45 by < 3% of cells as assessed by flow cytometry
Dose of stem cells: a single dose of 7.2 (± 0.90) × 107 cells
Timing of stem cell procedure: 25 (± 2.4) days following BM aspiration Comparator arm: no additional therapy (control) |
|
| Outcomes | Primary outcomes: absolute changes in global LVEF from baseline to 6 months Secondary outcomes: changes in LVEDV, LVESV, WMSI, major adverse cardiac events Outcome assessment points: 6 months Method(s): SPECT, echocardiography | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was described as "open label". Controls did not undergo bone marrow aspiration and no placebo was administered; neither participants nor patients were blinded. The analysis of SPECT images was performed by blinded independent investigators at each participating centre; off‐line assessment of all echocardiographic images was performed by one blinded independent investigator |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of withdrawals and exclusions was high (BMSC: 10/40 versus controls: 12/40). Although reasons were given, frequency differences were observed between groups including exclusions due to protocol violation, loss to follow‐up and the "opinion of the investigator" |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT01392105) were reported |
| Other bias | High risk | This is a commercially funded trial |
Lunde 2006.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: supported by research fellowships from the Norwegian Council on Cardiovascular Diseases and Medinnova and by grants from Inger and John Fredriksen’s Heart Foundation Country of origin: Norway Number of centres: 2 Dates of trial enrolment: 09/03 to 05/05 Length of follow‐up: 36 months Number (N) of participants randomised to each arm: 50 in treatment arm/51 in control arm Number (N) of participants analysed (primary outcome) in each arm: 50 in treatment arm/51 in control arm |
|
| Participants |
Population: AMI, within 2 to 12 hours
Age, mean (SD) each arm: 58.1 (8.5) years in treatment arm, 56.7 (9.6) years in control arm
Sex, % male in each arm: 84% in treatment arm, 84% in control arm Number of diseased vessels: 1:42; 2:6; 3:2 in treatment arm/1:36; 2:12; 3:2 in control arm Number of stunned hyperkinetic, etc segments: > 3 in both arms Time from symptom onset to initial treatment: median 210 minutes (range 180 to 330 minutes) in treatment arm/median 230 minutes (180 to 330 minutes) in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: BM aspirates 6 days (median, range 5 to 6 days) after PCI were separated by Ficoll gradient centrifugation and re‐suspended in heparinised plasma prior to transplantation. Intracoronary infusion using an inflated balloon catheter.
Dose of stem cells: a single dose of 0.68 x 108 MNC (median, range 0.54 to 1.3 x 108 MNC) containing 0.7 x 106 CD34+ cells (median, range 0.4 to 1.6 x 106 CD34+ cells)
Timing of stem cell procedure: 4 to 8 days after primary PCI. Median 6 days (interquartile range 5 to 6) Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: changes in LVEF (%) measured by SPECT, echocardiography and MRI
Secondary outcomes: changes in LVEDV (mL) and infarcted size. Also reported: NYHA class, quality of life, exercise tolerance Outcome assessment points: baseline, 3, 6, 12, 36 months Method(s): echocardiography, SPECT and MRI, SF‐36, electrically braked bicycle ergometer |
|
| Notes | Three patients did not receive cell therapy as randomised: 1 patient had low cell viability and 2 patients had stent thrombosis in the acute phase | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was generated by permuted blocks stratified according to centre |
| Allocation concealment (selection bias) | Low risk | Randomisation details were provided in consecutively numbered, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Controls did not undergo bone marrow aspiration and no placebo was administered; neither participants nor patients were blinded. Echocardiograms and angiograms were analysed by investigators blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the analysis of clinical outcomes and scientific outcomes measured by echocardiography and SPECT, all randomised patients were included with the exception of 1 patient in the control group who received a heart transplant at day 30. The number of withdrawals from MRI analysis was low and balanced between treatment arms (BMSC: 4/50 versus control: 4/51). Reasons were described as "contraindications or logistics" or in one case, due to incomplete MRI data |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00199823) were reported |
| Other bias | Low risk | None reported or identified |
Meluzin 2008.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Ministry of Health, Czech Republic Country of origin: Czech Republic Number of centres: 1 Dates of trial enrolment: 11/03 to 08/05 Length of follow‐up: 12 months Number (N) of participants randomised to each arm: not reported (73 in total across both intervention arms and the control group) Number (N) of participants analysed (primary outcome) in each arm: 20 treatment/20 control. Extended study of high‐dose cell therapy versus controls: 37 in the treatment group and 36 in the control group |
|
| Participants |
Population: AMI, within 24 hours
Age, mean (SD) each arm: 54 (SEM 2) years in the high cell dose group, 54 (SEM 2) years in the low cell dose group, and 55 (SEM 2) years in control
Sex, % male in each arm: 90% in the high cell dose group, 95% in the low dose group, and 90% in controls Number of diseased vessels: 1:14, 2:6, 3:0 (high dose); 1:11, 2:8, 3:1 (low dose); 1:14, 2:6, 3:0 in control Number of stunned hyperkinetic, etc segments: 0.4 (0.2) (high dose), 0.5 (0.2) (low dose), 0.4 (0.2) (controls). Irreversibly damaged segments: 6.2 (SEM 0.6) (high dose), 5.9 (SEM 0.5) (low dose), 6.1 (SEM 0.5) (controls) Time from symptom onset to initial treatment: 444 minutes (SEM 163 minutes) (high dose), 401 minutes (SEM 133 minutes) (low dose), 552 minutes (SEM 204 minutes) (controls) Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: BM aspirates after PCI. Cells were separated by density centrifugation. Cells cultivated overnight and re‐suspended in 22 mL prior to transplantation. Intracoronary infusion using an inflated balloon catheter. 7 balloon inflations for 3 minutes each, separated by 3‐minute intervals of balloon deflation. 3 mL BM cell suspension injected at each balloon deflation
Dose of stem cells: 1 x 108 MNC (range 0.9 to 2 x 108 cells) (high dose) or 1 x 107 MNC (range 0.9 to 2 x 107 cells) (low dose)
Timing of stem cell procedure: PCI within 24 hour of AMI symptoms, 3 to 7 days for randomisation, 5 to 9 days BM aspiration and infusion. Time from onset to cell transplantation: 6.8 (0.3) days (high dose) and 6.9 (0.3) days (low dose) Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: change in regional systolic function of the infarcted wall
Secondary outcomes: changes in 1. LVEF, 2. LV volumes, 3. Perfusion defect size Outcome assessment points: baseline and 3, 6 and 12 months Method(s): SPECT and Echo |
|
| Notes | Data from the 2 active intervention arms of the trial are pooled in this review. 2 patients had fever and 1 patient had brachycardia, all within 20 hours prior to cells; these 3 patients were randomised to cell therapy (unclear whether high or low dose) but they did not receive cell therapy as randomised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of participants and clinicians was not reported although controls did not undergo bone marrow aspiration and no placebo was administered. Echocardiographers were blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | From a total of 73 patients randomised to 1 of 3 treatment arms, 7 withdrew or were excluded from the analysis of all outcomes: 1 control patient was excluded because PET did not confirm the irreversibility of the myocardial damage and 2 controls underwent repeat MI 2 days after the hospital discharge due to in‐stent thrombosis. 3 patients randomised to BMSC were not transplanted because of complications within 20 hours before the procedure and a 4th patient was excluded because of an inadequate amount of implanted MBM cells; it was unclear whether these patients were randomised to high or low‐dose BMSC. 4 patients (cells: 2/22 versus no cells: 2/22) were missing from SPECT analysis at 3 and 12 months follow‐up; reasons for missing data were not reported. In separate publications, an expanded cohort of up to 73 patients (37 high dose cells and 36 controls) were included in SPECT analysis at 3, 6 and 12 months; the number of randomised patients was unclear |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Nogueira 2009.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: supported by Pro‐Cardiaco Hospital ‐ in charge of patients' care ‐ and by Exellion Biomedical Services S/A ‐ in charge of cell preparation and characterisation Country of origin: Brazil Number of centres: 2 Dates of trial enrolment: 01/05 to 01/06 Length of follow‐up: 6 months Number (N) of participants randomised to each arm: 14 in intracoronary artery route (AG) arm, 0 in intracoronary venous route (VG) arm, 6 in control arm Number (N) of participants analysed (primary outcome) in each arm: 14 in AG arm, 8 in VG arm, 6 in control arm |
|
| Participants |
Population: AMI, within 24 hours. Thrombolysis and/or PCI within 24 hours
Age, mean (SD) each arm: 59.7 (14.3) years in AG arm, 53.6 (8.3) years in VG arm, 57.2 (10.8) years in control arm
Sex, % male in each arm: 71% in AG arm, 70% in VG arm, 67% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: AG group: 29% < 12 hours, 21% > 12 hours, 50% > 6 hours and after thrombolysis (all within 24 hours) VG group: 20% < 12 hours, 20% > 12 hours, 60% > 6 hours and after thrombolysis (all within 24 hours) Control group: 50% > 12 hours, 33% > 6 hours and after thrombolysis (all within 24 hours) Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC (coronary artery route, AG or coronary venous route, VG)
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: approx. 80 mL bone marrow aspirated from the posterior iliac crest under sedation, analgesia and local anaesthesia. MNC were isolated and centrifuged in a Ficoll‐Pacque Plus and handled under aseptic conditions. The cells were washed and suspended in saline solution with 5% human serum albumin, re‐suspended and filtered to remove cell aggregates prior to transplantation. Arterial delivery via over‐the‐wire balloon catheter PCI. Venous delivery via an additional over‐the‐wire balloon catheter positioned side‐by‐side with the balloon in the artery where the stent was located
Dose of stem cells: 10 mL of solution containing 100 x 106 MNC
Timing of stem cell procedure: the time interval between the AMI and cell transfer was 5.5 (1.28) days (AG) and 6.1 (1.37) days (VG) Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: LVEF, WMSI, EDV, ESV Secondary outcomes: radiolabeled cells retention and washout in the heart tissue Outcome assessment points: baseline, 3 and 6 months Method(s): echocardiography, RNV |
|
| Notes | Data from the 2 active intervention arms of the trial are pooled in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment was made in blocks according to the AMI size (≤ 25% or < 25%), by means of sealed envelopes. Random allocation was stratified according to infarct size in 3 blocks of different size, for each stratum, with the use of sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Randomisation details were provided in sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Controls did not undergo bone marrow aspiration and no placebo was administered. Outcome assessors were blinded. Blinding of participants and clinicians not reported. The trial was described as "open‐label in relation to the clinical analysis and blind in relation to the echocardiographic analysis" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants in the control group were included in the analysis of clinical outcomes and scientific outcomes. 2 patients in the intravenous cell group were missing from echocardiographic analysis at 3 and 6 months follow‐up (1 sudden death 1 month after cell therapy, 1 tortuous anterior interventricular vein complicating BMSC transfer) |
| Selective reporting (reporting bias) | High risk | The secondary outcomes of QoL, Seattle Angina Questionnaire and cost‐effectiveness described in the trial protocol (www.clinicaltrials.gov: NCT00350766) were not reported |
| Other bias | High risk | Supported in part by commercial funding |
Penicka 2007.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Research Grant from Charles University of Prague Country of origin: Czech Republic Number of centres: not reported Dates of trial enrolment: not reported Length of follow‐up: 24 months Number (N) of participants randomised to each arm: 17 in the treatment arm/10 in the control arm Number (N) of participants analysed (primary outcome) in each arm: 14 in the treatment arm/10 in the control arm |
|
| Participants |
Population: AMI, within 24 hours
Age, mean (SD) each arm: 61 (14) in treatment arm, 54 (10) in control arm
Sex, % male in each arm: 71% in treatment arm, 100% in control arm Number of diseased vessels: not stated clearly, but assumed 1, left anterior descendent (LAD) Number of stunned hyperkinetic, etc segments: at least 3 akinetics segments in the LAD artery Time from symptom onset to initial treatment: time from onset of AMI to PCI, median 315 (range 300 to 600) days in BMSC arm, median 330 (range 300 to 630) days in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirations took place 4 to 11 (median 8) days following PCI. Cells were isolated following the protocol described by Wollert 2004. Infusion of cells in the LAD artery
Dose of stem cells: a single dose of 26.4 x 108 (median) mononuclear cells
Timing of stem cell procedure: PCI carried out 4 to 11 hours after onset of AMI, cell infusion 4 to 11 days following PCI Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: not reported
Secondary outcomes: changes in 1. LVEF, 2. LVEDV (mL), 3. LVESV (mL), 4. Infarct size. Also measured: NYHA class, QOL Outcome assessment points: baseline, 4, 12 and 24 months Method(s): echocardiography and SPECT (infarct size), SF‐36 |
|
| Notes | 2 patients originally assigned to the treatment group did not receive active treatment because of complications which occurred before the planned cell transfer. Both patients died during early follow‐up. The trial had originally intended to recruit 40 patients to the treatment arm and 20 to the control arm. Trial was prematurely stopped after 27 enrolled "because of the unexpected occurrence of serious complications in the BMSC group and no incremental functional effects of BMSCs as compared with control patients'' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of participants and clinicians was not reported although controls did not undergo bone marrow aspiration and no placebo was administered. Echocardiography specialists were blinded to patient group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes. 3 patients in the BMSC group (3/17) died prior to echocardiography follow‐up (1 in‐stent thrombosis with reinfarction immediately after BMSC harvest ‐ had a complicated PCI followed by CABG and died 2 weeks later from sepsis and ARDS, 1 ventricular rupture before BMSC injection, underwent emergency surgery and died 3 months later due to severe heart failure and 1 received BMSCs and was diagnosed with biliary carcinoma 6 weeks after BMSC infusion and died 2 months later). All patients in the control group were included in echocardiography analysis at all follow‐up time points up to 2 years |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | High risk | Trial was intending to recruit 40 participants in the intervention group and 20 in the control group. The trial was prematurely stopped after 27 participants were enrolled "because of the unexpected occurrence of serious complications in the BMSC group and no incremental functional effects of BMSC as compared with control patients" |
Piepoli 2010.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: supported by Azienda USL di Piacenza and Fondazione Piacenza & Vigevano Country of origin: Italy Number of centres: 1 Dates of trial enrolment: 07/05 to 06/07 Length of follow‐up: 24 months Number (N) of participants randomised to each arm: 19 in treatment arm/19 in control arm Number (N) of participants analysed (primary outcome) in each arm: 17 in treatment arm, 15 in control arm |
|
| Participants |
Population: AMI, within 6 hours. PCI within 2 to 6 hours of onset of symptoms
Age, mean (SD) each arm: 63.1 (SEM 2.7) years in treatment arm, 67.2 (SEM 2.4) years in control arm
Sex, % male in each arm: 68.4% in treatment arm, 68.4% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 248 (SEM 68.7) minutes from AMI to PCI in treatment arm; 265 (SEM 34.4) minutes from AMI to PCI in treatment arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: 100 mL of autologous bone marrow was aspirated under local the posterior‐superior iliac crest by multiple aspirations into heparinised syringes. The cells were suspended in 7 mL of PBS‐EDTA buffer containing 3 mL of human albumin 5% W/V. Mononuclear cell fraction was concentrated into a final volume of 25 to 30 mL. Balloon catheter was positioned at the site of the former infarct‐vessel occlusion and PCI performed 4 to 5 times, for 2 minutes each time. During this time intracoronary cell transplantation via the balloon catheter was performed, using 4 to 5 fractional high‐pressure infusions of 2 to 3 mL of the cell suspension
Dose of stem cells: mononuclear cells: mean 248.78 x 106 were infused (minimum 75.4 x 106; maximum 570.0 x 106)
Timing of stem cell procedure: 4 to 7 days after AMI Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: LVEF, LVEDV, LVESV Secondary outcomes: heart rate variability, baroreflex sensitivity, arrhythmias, exercise tolerance Outcome assessment points: baseline, 6, 12, 24 months Method(s): ECG, echocardiography, rest and stress perfusion scintigraphy G‐SPECT, cardiopulmonary exercise testing (CPET) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random assignment was made by uneven versus even numbers in a 1:1 fashion into 2 parallel groups |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of participants and clinicians was not reported although controls did not undergo bone marrow aspiration and no placebo was administered. 2 independent investigators who had no knowledge of the study collected and analysed outcome data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes. 6 patients were missing from SPECT/echocardiography analysis at follow‐up: 2/19 in the BMSC arm (1 sudden death after 2 months, 1 death due to refractory heart failure at 3 months) and 4/19 in the control arm (1 sudden death after 3 months, 2 deaths due to refractory heart failure after 1 month, 1 accidental death at 2 months) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00437710) were reported |
| Other bias | High risk | Supported in part by commercial funding |
Plewka 2009.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Polish Ministry of Science and Higher Education, Warsaw, Poland (Grant 2 P05B 178 28) Country of origin: Lodz, Poland Number of centres: 1 Dates of trial enrolment: "between 2005 ‐ 2007" Length of follow‐up: 2 years Number (N) of participants randomised to each arm: 40 in treatment arm/20 in control arm Number (N) of participants analysed (primary outcome) in each arm: 38 in treatment arm, 18 in control arm |
|
| Participants |
Population: AMI, within 12 hours. PCI within 12 hours of onset of symptoms
Age, mean (SD) each arm: 59 (9) years in treatment arm, 56 (8) years in control arm
Sex, % male in each arm: 68% in treatment arm, 78% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 7(2) hours from AMI to PCI in treatment arm; 8(3) hours from AMI to PCI in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: 100 mL bone marrow aspirated from the iliac crest using local anaesthesia. Bone marrow aspirates were diluted with 20 mL of 0.9% NaCl, filtrated, and mononuclear cells were isolated by density gradient centrifugation, washed twice with 0.9% NaCl, filtered, and subjected to quality and quantity control. Intracoronary infusion by PCI over the wire balloon catheter technique.
Dose of stem cells: 1.44 (0.49) x 108 MNC and 3.06 (2.18) x 106 CD34+ cells
Timing of stem cell procedure: bone marrow was aspirated 7 (SD = 2) days (range 3 to 11 days) after STEMI, the cell suspension was administrated within 2 hours of bone marrow harvest Comparator arm: no additional therapy (control) |
|
| Outcomes | Primary outcomes: LVEF, LVEDV, LVESV, WMSI Secondary outcomes: systolic myocardial velocity S, 2‐dimensional strain, 2‐dimensional strain in infarcted area, mitral inflow E/A, early filling propagation velocity, early diastolic myocardial velocity, transmitral flow velocity/annular velocity ratio (E/E) Outcome assessment points: baseline, 6, 12 and 24 months Method(s): echocardiography, 2‐D systolic strain, G‐SPECT | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of participants and clinicians was not reported although controls did not undergo bone marrow aspiration and no placebo was administered. Independent blinded investigators collected and analysed echocardiographic data; SPECT perfusion images were analysed quantitatively by a single investigator blinded to all other data |
| Incomplete outcome data (attrition bias) All outcomes | High risk | This study was initially reported in 2 separate publications with partial patient overlap (30 patients were included in both publications; both studies included patients which were missing from the other). In one publication, all 60 randomised participants were included in the analysis of clinical outcomes. At 6‐month follow‐up, 4 patients had died: 2/40 in the BMSC arm (1 fatal STEMI and 1 sudden cardiac death during the 6 months follow‐up) and 2/20 in the control arm (2 sudden cardiac deaths during the 6‐month follow‐up) and were not included in echocardiography analysis at 6 months and subsequent follow‐up at 1 and 2 years. In the second publication of 39 randomised patients, 3 controls (3/13) were missing from SPECT analysis at 6 months (1 death at 5 months, 2 failed to attend follow‐up visit) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Quyyumi 2011.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Amorcyte Inc., New Jersey Commission of Science and Technology (06‐2042‐014‐77) Country of origin: USA Number of centres: not reported (multicentre) Dates of trial enrolment: not reported Length of follow‐up: 12 months Number (N) of participants randomised to each arm: 6 (high dose, HD), 5 (moderate dose, MD), 5 (low dose, LD), 15 (controls) Number (N) of participants analysed (primary outcome) in each arm: 2 (high dose), 4 (moderate dose), 5 (low dose), 10 (controls) |
|
| Participants |
Population: acute STEMI. PCI with stent within 3 days
Age, mean (SD) each arm: median 50.5 (IQR 45.0 to 53.0) years (HD), 63.0 (IQR 57.0 to 66.0) years (MD), 52.0 (IQR 51.0 to 52.0) years (LD), 52.0 (IQR 47.0 to 57.0) years (controls)
Sex, % male in each arm: 100% (HD), 80% (MD), 80% (LD), 87% (controls) Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: median 3.5 (IQR 2.8 to 5.1) hours (HD), 1.3 (IQR 6.2 to 22.1) hours (MD), 21.0 (IQR 7.1 to 41.3) hours (LD), 6.7 (IQR 3.9 to 23.8) hours (controls) Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: CD34+, high dose (HD), moderate dose (MD) or low dose (LD)
Type of stem cells: bone marrow‐derived CD34+ cells
Summary of how stem cells were isolated and type and route of delivery: 320 mL (median 402 (17) mL including heparin) BM harvested under conscious sedation and local anaesthesia. CD34+ cells selected using the anti‐CD34 Mab and Dynabeads on the Isolex 300i system. CD34+ cell product re‐suspended in 6 mL of PBS, 4 mL (40%) of autologous human serum containing 1% human serum albumin and 25 USP U/mL of heparin sodium. Cell suspension infused via an over‐the‐wire balloon catheter positioned in the stented segment of the IRA
Dose of stem cells: 14.3 (1.6) x 106 CD34+ cells (HD), 9.9 (0.7) x 106 CD34+ cells (MD), 4.8(0.4) x 106 CD34+ cells (LD)
Timing of stem cell procedure: cells infused median 207.3 (IQR 191 to 215) hours (HD), 210 (IQR 194 to 210) hours (MD), 191.4 (IQR 167 to 201) hours (LD) after AMI Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: none stated Secondary outcomes: 1. Quantitative rest hypoperfusion score measured by SPECT, 2. LVEF, LVEDV, LVESV, infarct size by MRI, 3. Clinical adverse events (arrhythmia, chest pain, musculoskeletal pain, upper respiratory tract infection, rash, dyspnoea, fever, acute stent thrombosis, death MI, rehospitalisation for heart failure, cerebral infarction, ventricular arrhythmia or syncope, chronic myeloid leukaemia, revascularisation, septic thrombophlebitis) Outcome assessment points: baseline, 3, 6 and 12 months Method(s): gadolinium‐enhanced cardiac MRI, SPECT, echocardiography, ECG |
|
| Notes | Data from the 3 active intervention arms of the trial are pooled in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was described as "open label". Controls did not undergo bone marrow aspiration and no placebo was administered; neither participants nor patients were blinded. However, "all studies were analysed by operators blinded to the patient treatment designation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 patient in the high‐dose arm was excluded due to acute stent thrombosis soon after cell infusion. All other randomised patients were included in the analysis of clinical outcomes. For MRI assessment at 3 and 6 months, 1 patient had died due to ventricular fibrillation soon after cell infusion. 2 further patients in the high‐dose BMSC group (total 4/6), 1 patient in the medium‐dose BMSC arm (1/5) and 5 patients in the control group (5/15) were missing from MRI assessment. There were no withdrawals or exclusions (0/5) in the low‐dose BMSC group. The reasons for patient drop‐out were given as "death, refused, defibrillators, stent thrombosis, and poor image quality", however the number of patients falling into each category was not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00313339) were reported |
| Other bias | High risk | This is a commercially funded trial |
Roncalli 2010.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: French Department of Health ‐ Programme Hospitalier de Recherche Clinique (PHRC), the Association Francaise contre les Myopathies, the Fondation de France Country of origin: France Number of centres: 6 Dates of trial enrolment: 12/04 to 01/07 Length of follow‐up: 12 months Number (N) of participants randomised to each arm: 52 in treatment arm/49 in control arm Number (N) of participants analysed (primary outcome) in each arm: 48 in BMSC arm/44 in control arm |
|
| Participants |
Population: acute STEMI, PCI with stent within 24 hours
Age, mean (SD) each arm: 56 (12) years in treatment arm, 55 (11) years in control arm
Sex, % male in each arm: 80.8% in treatment arm, 89.8% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: "within 24h after the onset of chest pain"; < 12 hours in 75% of BMSC arm/75.5% of control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: 50 mL of bone marrow was aspirated into heparinised syringes under local anaesthesia from the iliac crest. Lymphocyte preparation medium centrifugation procedures were used to isolate and enrich progenitor cells. A heterogeneous cell suspension population was obtained that consisted of haematopoietic, endothelial and other progenitor cells, as well as mononuclear cells. A single syringe of 100 x 106 BMCs was prepared in 10 mL 4% human albumin. Intracoronary infusion using over‐the‐wire balloon catheter technique positioned within the stented segment
Dose of stem cells: 100 x 106 autologous BMMNC
Timing of stem cell procedure: infusion performed 9.3 ± 1.7 days after AMI Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: improvement of myocardial viability ‐ "a gain of at least 2/17 viable segments 3 months after STEMI, assessed by resting 4 h thallium‐201‐gated‐SPECT." Secondary outcomes: 1. changes in LVEF evaluated by RNA, MRI, and echocardiography, 2. changes in LVEDV and LVESV, 3. infarct size by MRI, 4. binary restenosis by coronary angiography, 5. segment‐by‐segment improvement of myocardial viability. Also measured: QOL Outcome assessment points: baseline, 1 month, 3 months, 12 months Method(s): radionuclide angiography (RNA), echocardiography, MRI, T201‐SPECT, MLHFQ |
|
| Notes | 1 patient did not receive BM aspirate due to thrombopenia but was included as randomised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned in a 1:1 ratio to either the control group or BMC group using permuted‐block randomisation stratified according to centre, diabetes status and time to PCI after the onset of AMI (≤ 12 or > 12 hours) |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed envelopes were provided to all participant centres |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was described as "open label"; controls did not undergo bone marrow aspiration and no placebo was administered; neither participants nor patients were blinded. 3 independent core imaging laboratories, blinded to treatment assignment, performed all cardiac imaging measurements |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the analysis of clinical outcomes, there were 9 withdrawals or exclusions: 4/52 in the BMSC arm (2 withdrawals due to adverse clinical events, 1 withdrawal due to randomisation error and 1 refusal to complete follow‐up) and 5/49 in the control arm (1 patient had steroid therapy for angioneurotic oedema, 1 had post‐MI ventricular septal defect and 3 patients refused follow‐up). In the analysis of scientific outcomes at 3 months, 1 further patient in the BMSC arm had died and 1 additional patient in the control arm was missing, the reason for which was not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00200707) were reported |
| Other bias | Low risk | None reported or identified |
Ruan 2005.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: not reported Country of origin: China Number of centres: 1 Dates of trial enrolment: 07/03 to 08/04 Length of follow‐up: 6 months Number (N) of participants randomised to each arm: 9 in the BMSC arm/11 in the control arm Number (N) of participants analysed (primary outcome) in each arm: 9 in the BMSC arm/11 in the control arm |
|
| Participants |
Population: AMI, within 24 hours
Age, mean (SD) each arm: 61 (8) years in treatment arm, 58 (6) years in control arm
Sex, % male in each arm: 88.9% in treatment arm, 100% in control arm Number of diseased vessels: range 1 to 3 but no more details stated Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 12.7 (12.6) hours in treatment arm/12.3 (13.4) hours in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: the study does not state how the cells were isolated or processed. Except that cells were suspended in diluted serum prior to transplantation. Cells were infused by percutaneous transmural coronary angioplasty (PTCA)
Dose of stem cells: not reported
Timing of stem cell procedure: within 2 hours of successful PTCA Comparator arm: diluted serum |
|
| Outcomes |
Primary outcomes: the study does not state clearly a primary outcome. The aim is to assess changes in LV segmental function by Doppler imaging
Secondary outcomes: changes in 1. LV global function and volume, 2. LVEDV (mL), 3. LVESV (mL), 4. LVEF (%) Outcome assessment points: baseline, 3 months and 6 months Method(s): Doppler imaging and echocardiography |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised; patients were selected "prospectively and consecutively" |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The control group received an injection of heparinised saline although it is not reported whether they underwent bone marrow aspiration. It is therefore unclear whether participants and clinicians were sufficiently blinded to treatment. Outcome assessors were blinded to clinical and angiographic information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical and scientific outcomes |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Schachinger 2006.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: research grant from Guidant and support from Eli Lilly Country of origin: Germany and Switzerland Number of centres: 17 (16 in Germany + 1 in Switzerland) Dates of trial enrolment: 04/04 to 04/05 Length of follow‐up: 5 years Number (N) of participants randomised to each arm: 101 in the treatment arm/103 in control arm Number (N) of participants analysed (primary outcome) in each arm: 95 in treatment arm/92 in control arm |
|
| Participants |
Population: AMI, within 5 days
Age, mean (SD) each arm: 55 (11) years in treatment arm, 57 (11) years in control arm
Sex, % male in each arm: 82% in treatment arm, 82% in control arm Number of diseased vessels: 1:61; 2:24; 3:16 in treatment arm/1:60; 2:32; 3:11 in control arm Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 7.5 (8.0) hours to PCI in treatment arm/7.0(6.5) hours to PCI in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: BM aspirates 3 to 6 days after PCI, cells were separated by Ficoll gradient centrifugation and re‐suspended in 10 mL of X‐VIVO medium containing 20% autologous serum. Intracoronary infusion using an inflated balloon catheter. 3 portions of 3.3 mL cell suspension were infused in 3‐minute occlusion time for each portion and 3‐minute intervals
Dose of stem cells: 10 mL of a single dose containing 2.36 (1.74) x 108 mononuclear cells
Timing of stem cell procedure: PCI within 12 hrs of AMI symptoms, harvest 3 to 6 days after PCI, randomisation and transport prior to infusion 3 to 6 days Comparator arm: placebo consisting of 10 mL X‐VIVO medium with 20% autologous serum |
|
| Outcomes |
Primary outcomes: changes in LVEF
Secondary outcomes: 1. Improvement of global LVEF, 2. Reduction of LVESV, 3. Improvement of regional wall motion and myocardial contractility, 4. Assessment of major adverse events, such as revascularisation, death and hospitalisation due to heart failure Outcome assessment points: baseline, 4, 12, 24 months, 5 years Method(s): LV angiography |
|
| Notes | 3 patients randomised to the placebo arm did not receive placebo medium but were included in the analysis: 1 patient in placebo group had angiographic evidence of a thrombus in a non‐infarct‐related artery, 1 patient had an air embolism during initial angiography before the guidewire could be advanced and in 1 patient the guidewire could not be advanced into the infarct‐related artery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using computer‐generated randomised lists maintained at a site external to the trial |
| Allocation concealment (selection bias) | Low risk | Bone marrow aspirates were sent to the cell processing centre (centralisation) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All patients underwent bone marrow aspiration and control group patients were given an intracoronary injection of placebo medium. Bone marrow aspirates were then sent to a central cell processing centre; participants and clinicians were therefore blinded to treatment. LV angiography was performed by an experienced investigator in a central core laboratory who was unaware of the patient's treatment assignment until after analysis of 4‐month data was complete. Study centres and investigators and those entering the data into databases remained blinded until 12‐month follow‐up was complete. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in the analysis of clinical outcomes at 4 months follow‐up; 3 and 2 patients in the control group were lost to follow‐up at 12 months and 2 years respectively. In the analysis of scientific outcomes, 6/101 in the BMSC group and 11/103 in the placebo group were missing from LV angiography analysis at 4 months (2 had poor quality results on angiography, 4 deaths before 4 months, 5 declined and 6 did not undergo angiography). A subset of 59 patients were included in a sub‐study of MRI 2 years |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00279175) were reported, with the exception of NYHA class, although all other pre‐specified morbidity outcomes were reported |
| Other bias | High risk | This is a commercially funded trial |
Suarez de Lezo 2007.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: not reported Country of origin: Spain Number of centres: 1 (assumed) Dates of trial enrolment: from 01/05, end not reported Length of follow‐up: 3 months Number (N) of participants randomised to each arm: 10 in the treatment arm/10 in control arm Number (N) of participants analysed (primary outcome) in each arm: 10 in treatment arm/10 in control arm |
|
| Participants |
Population: AMI, within 12 days
Age, mean (SD) each arm: 52 (12) years in treatment arm, 55 (11) years in control arm
Sex, % male in each arm: 80% in treatment arm, 70% in control arm Number of diseased vessels: at least 1, left anterior descendent (LAD) artery in treatment arm/at least 1 (LAD) in control arm Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: PCI was carried out 3 to 5 days post AMI, treatment intervention took place 7 (2) days after PCI Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: BM aspirates (80 to 100 mL), cells were separated by Ficoll gradient centrifugation and re‐suspended in 10 mL of 0.9% sodium chloride (saline) and 0.1% heparin. Intracoronary infusion using an inflated balloon catheter during 2 to 4 minutes
Dose of stem cells: 10 mL of a single dose containing 9 x 108 mononuclear cells, corresponding to 17 (13) x 106 CD34+ cells.
Timing of stem cell procedure: PCI within 3 to 5 days of AMI symptoms, bone marrow harvest and infusion 7 (2) days post PCI Comparator arm: placebo consisting of 0.9% sodium chloride (saline) and 0.1% heparin |
|
| Outcomes |
Primary outcomes: changes in LVEF
Secondary outcomes: 1. LVEF, 2. LVESV, 3. LVEDV, 4. Wall motion Outcome assessment points: baseline, 3 months Method(s): LV angiography |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation by telephone was performed" but the sequence generation procedure was not described |
| Allocation concealment (selection bias) | Unclear risk | "Randomisation by telephone was performed" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The control group did not undergo bone marrow aspiration although they received an injection of heparinised saline and therefore it is unclear whether participants and clinicians were sufficiently blinded to treatment. 2 angiographers were unaware of patient group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical and scientific outcomes |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Sürder 2013.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: funded by Fondazione Cardiocentro Ticino, Lugano, Switzerland; Zurich Heart House‐Foundation for Cardiovascular Research, Zurich, Switzerland; Bern University Hospital, Bern, Switzerland; Cardiovascular Research Foundation, Zurich, Switzerland, and an unrestricted grant from Abbott Vascular Country of origin: Switzerland Number of centres: 4 Dates of trial enrolment: 10/06 to 01/12 Length of follow‐up: 4 months Number (N) of participants randomised to each arm: 66 in the early cell therapy arm, 67 in the late cell therapy arm, 67 in the control arm Number (N) of participants analysed (primary outcome) in each arm: 58 in the early cell therapy arm, 49 in the late cell therapy arm, 60 in the control arm |
|
| Participants |
Population: STEMI with PCI in 24 hours and EF ≤ 45%
Age, mean (SD) each arm: median 55 (IQR 15) years (early cells), 62 (IQR 15) years (late cells), 56 (IQR 14.5) years (controls)
Sex, % male in each arm: 86.2% (early cells), 82.5% (late cells), 83.6% (controls) Number of diseased vessels: 1 (54%), 2 (32%), 3 (14%) (early cells), (57%), 2 (27%), 3 (16%) (late cells), 1 (64%), 2 (21%), 3 (15%) (controls) Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 6 (2) days (early cells) or 24 (7) days (late cells) after AMI Statistically significant baseline imbalances between the groups? Higher age in the late treatment group compared with controls (median 62 years versus 56 years; P value = 0.06); lower percentage of smokers in the late treatment group compared with controls (40.3% versus 62.7%; P value = 0.01); higher baseline LVEF in the control group compared with the treatment group (median 39.6% versus 35.6%, P value = 0.03) |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspiration was performed 5 to 7 days after AMI. Between 60 and 80 mL of bone marrow was collected from the iliac crest under local anaesthesia. Then 1 mL of a solution containing 1000 IU heparin was added to each 10 mL of bone marrow aspirate to prevent clotting. Then the aspirate and 20 mL of the patient's serum were sent at room temperature by courier to the cell‐processing centre. The BM‐MNC cell suspension was shipped back to the participating hospital within 24 hours. Briefly, with the use of density gradient centrifugation, the mononuclear cell fraction was re‐suspended in 10 mL of serum‐free medium with 20% of autologous serum added without any additional heparin. An aliquot of cell suspension was utilised for fluorescence‐activated cell sorting analysis with the use of fluorochrome conjugated antibodies against anti‐human CD34 and CD133; cell viability was assessed by 7‐AAD cell uptake, and sterility was assessed by the Bact/Alert rapid method. Release criteria of the BMMNC were product sterility, a cell count between 5 × 107 and 5 × 108, and cell viability of ≥ 80%
Dose of stem cells: 1.59 (± 1.25) x 108 cells (early cells); 1.39 (± 1.20) x 108 cells (late cells)
Timing of stem cell procedure: 5 to 7 days post‐AMI (early cells); 3 to 4 weeks post‐AMI (late cells) Comparator arm: no additional therapy (control) |
|
| Outcomes | Primary outcomes: absolute change in global LVEF from baseline to 4 months Secondary outcomes: change in LVEF, LVESV, LVEDV infarct size proportion of scar mass to total LV mass, global and regional myocardial thickening, major adverse events Outcome assessment points: 4 and 12 months Method(s): MRI | |
| Notes | Data from the 2 active intervention arms of the trial are pooled in this review. There is a discrepancy between the absolute change LVEF values and baseline/endpoint values reported. The authors were contacted to request clarification on this discrepancy but none was forthcoming | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using closed envelopes in a 1:1:1 pattern |
| Allocation concealment (selection bias) | Low risk | Closed envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was described as "open label"; controls did not undergo bone marrow aspiration and no placebo was administered; neither participants nor patients were blinded. However, it is reported that "the entire analysis was performed in a CMR core laboratory, blinded to the treatment assignment of the patients enrolled." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the analysis of clinical outcomes, the number of withdrawals and exclusions was unbalanced between groups (early cells: 11/66 versus late cells: 15/67 versus control: 7/67). Although reasons for withdrawals were given (withdrawal of informed consent or death in all missing patients), these do not fully explain the sample sizes described in individual analyses. In the analysis of scientific outcomes by MRI analysis at 4 months, 8 additional patients were missing in the BMSC arm due to the lack of paired MRI data |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00355186) were reported |
| Other bias | Low risk | None reported or identified |
Tendera 2009.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Polish Ministry of Science and Higher Education (grants number PBZ‐KBN‐099/P05/2003, 0651/P01/2007/32, 2422/P01/2007/32) Country of origin: Poland Number of centres: 5 Dates of trial enrolment: 03/05 to 09/07 Length of follow‐up: 6 years Number (N) of participants randomised to each arm: 80 (selected cells), 80 (unselected cells), 40 (controls) Number (N) of participants analysed (primary outcome) in each arm: 51 (selected cells), 46 (unselected cells), 20 (controls) |
|
| Participants |
Population: AMI, within 12 hours. PCI within 12 hours
Age, mean (SD) each arm: median 58 years (selected cells), 55 years (unselected cells), 59 years (controls)
Sex, % male in each arm: 63.7% (selected cells), 70.6% (unselected cells), 75% (controls) Number of diseased vessels: 1 in all trial arms Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: from AMI to PCI: median 303 minutes (101 to 1100) (selected cells), 309 minutes (117 to 1000) (unselected cells), 300 minutes (120 to 1080) (controls) Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: CD34+CXCR4+ or BMMNC
Type of stem cells: selected cells: CD34+CXCR4+ selected bone marrow‐derived stem cells; unselected cells: bone marrow‐derived stem cells (mononuclear cells‐MNC) Summary of how stem cells were isolated and type and route of delivery: 100 to 120 mL bone marrow aspirated from the posterior superior iliac spine into heparinised syringes under general anaesthesia Selected cells: Ficoll density gradient centrifugation to isolate mononuclear cells, CD34+CXCR4+ cell population was isolated using two‐step immunomagnetic selection with monoclonal antibodies coupled with magnetic beads and MidiMACS System. Re‐suspended in phosphate‐buffered saline (final volume 10 mL). Delivery via intracoronary infusion by PCI over the wire balloon catheter technique Unselected cells: Ficoll density gradient centrifugation to isolate mononuclear cells. Delivery via intracoronary infusion by PCI over the wire balloon catheter technique Dose of stem cells: 3 infusions delivering a median of 1.9 x 106 CD34+CXCR4+ cells in total (selected cells); median of 1.78 x 108 MNCs (unselected cells) Timing of stem cell procedure: BM aspiration and BMSC infusion was done 7 (3 to 12) (median (range)) days after primary PCI. Comparator arm: no additional therapy (control) |
|
| Outcomes | Primary outcomes: LVEF by MRI Secondary outcomes: LVEF by LV angiography, LVESV, LVEDV, MACE (death, re‐infarction, stroke and target vessel revascularisation (TVR)) Outcome assessment points: baseline, 6 months, 6 years Method(s): echocardiogram, LV angiography, MRI | |
| Notes | Data from the 2 active intervention arms of the trial are pooled in this review. Table 2 footnote says values expressed as medians with quartiles, whereas text describes means and ranges ‐ unclear whether values throughout paper for medians are whole ranges or interquartile ranges | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eligible patients were randomised by centre in 2:2:1 fashion into three parallel groups" but the sequence generation procedure was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was described as "open label"; controls did not undergo bone marrow aspiration and no placebo was administered. Investigators assessing cMRI and LV angiography outcome measures were blinded to the group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All randomised participants were included in the analysis of clinical outcomes. For MRI assessment at 6 months follow‐up, there was 29/80 missing in selected BMSC arm (1 death, 28 unexplained), 34/80 missing in unselected BMSC arm (1 death, 33 unexplained), and 20/40 missing in control arm (1 death, 19 unexplained) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00316381) were reported, although LVEF and LV volumes were measured by MRI and LV angiography rather than echocardiography |
| Other bias | Low risk | None reported or identified |
Traverse 2010.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: the Jon Holden DeHaan Foundation, The Production Assistance for Cellular Therapies, N01‐HB‐37164 Country of origin: USA Number of centres: 1 Dates of trial enrolment: "beginning in 12/05" Length of follow‐up: 6 months Number (N) of participants randomised to each arm: 30 in treatment arm/10 in control arm Number (N) of participants analysed (primary outcome) in each arm: 30 in treatment arm/10 in control arm |
|
| Participants |
Population: first anterior STEMI, PCI with stent implantation
Age, mean (SD) each arm: median 52.5 years (IQR = 43, 64) in treatment arm, median 57.5 years (IQR = 54, 59) in control arm
Sex, % male in each arm: 83.33% in treatment arm, 60% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: median 4.6 hours (IQR = 2, 12 hours) in treatment arm/median 2.9 hours (IQR = 2.8, 10.6 hours) in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: patients lightly sedated, 50 to 70 mL bone marrow aspirated from posterior iliac crest. Aspirate heparinised and transported within 1 hour to cell therapy laboratory. BMMNC isolated by Ficoll density centrifugation at 450 g, cells counted with an automated cell counter and the cell suspension volume was adjusted to reach a final product of 100 million BMCs with 5% human serum albumin in 20 mL. Administered via intracoronary perfusion
Dose of stem cells: 108 BMSC
Timing of stem cell procedure: median 4.5 days (IQR = 4,7 days) after PCI, within 8 hours of BM aspiration Comparator arm: solution of 0.9% isotonic sodium chloride solution and 5% human serum albumin in an identical volume |
|
| Outcomes | Primary outcomes: "To investigate the effects of BMC administration in patients following STEMI on recovery of LV function using cardiac MRI" Secondary outcomes: LV volumes by MRI, safety as assessed by MACE (death, repeated target vessel revascularisation, recurrent MI, hospitalisation for chronic heart failure, and internal cardia defibrillator (ICD) placement) Outcome assessment points: baseline and 6 months Method(s): MRI | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on an algorithm developed by a biostatistician |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed at the cell processing facility following preparation of the bone marrow cells |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was described as "double blind"; all patients underwent bone marrow aspiration and control group patients were given an intracoronary injection of placebo medium. Blinding of clinicians was not reported. Outcome measurements were assessed by MRI readers blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical and scientific outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00268307) were reported, with the exception of infarct size which was included as a secondary outcome |
| Other bias | Low risk | None reported or identified |
Traverse 2011.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: supported by the National Heart, Lung, and Blood Institute Country of origin: USA Number of centres: 5 Dates of trial enrolment: 07/08 to 02/11 Length of follow‐up: 6 months Number (N) of participants randomised to each arm: 59 in the treatment arm, 29 in the control arm Number (N) of participants analysed (primary outcome) in each arm: 55 in the treatment arm, 26 in the control arm |
|
| Participants |
Population: AMI within 2 to 3 weeks after PCI
Age, mean (SD) each arm:57.6 (11) in the treatment arm, 54.6 (11) in the control arm
Sex, % male in each arm: 79% in the treatment arm, 90% in the control arm Number of diseased vessels: 1 or 2 or 3 Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: median 3.4 (IQR 2.3 to 14.3) hours from onset to PCI; median 17.4 (IQR 15.5 to 20.0) days from PCI to infusion Statistically significant baseline imbalances between the groups? Baseline heart rate at initial presentation was higher in the placebo group than the treatment group (90.3% versus 77.5%; P value = 0.01) |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived mononuclear cells (MNC)
Summary of how stem cells were isolated and type and route of delivery: approximately 80 to 90 mL of bone marrow was aspirated from the iliac crest using standard techniques. The aspirate was processed at all sites with a closed, automated cell processing system (Sepax, Biosafe SA) to ensure a uniform cellular product. After BMC enrichment, cells were washed 3 times and suspended in 5% human serum albumin/saline solution. The composition of CD34 and CD133 cells was determined by fluorescent activated cell sorting
Dose of stem cells: 1.47 (± 1.7) x 108 cells
Timing of stem cell procedure: median (IQR) 17.4 (15.5 to 20.0) days after PCI Comparator arm: placebo (0.9% saline and 5% human serum albumin) |
|
| Outcomes | Primary outcomes: 1. change in global LV function, 2. change in regional function by wall motion in the infarct and border zones Secondary outcomes: composite measure of major adverse clinical events, LV mass, LVEDV, LVESV, infarct size Outcome assessment points: 6 months Method(s): cardiac MRI | |
| Notes | 1 patient in the BMSC group did not receive treatment due to a new 90% stenosis in the left main artery before cell infusion but was included in the analysis as randomised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to one to the selected treatment strategies using an interactive web‐based randomisation session in a 2:1 ratio using randomly selected block sizes of 6 or 9 and stratified by centre |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by the data co‐ordinating centre. Treatment assignment was masked to all but one designated cell processing team member at each of the 5 centres who was not involved in patient care |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All patients underwent bone marrow aspiration and control group patients were given an intracoronary injection of placebo medium. Patients and research staff, including the CCTRN physicians and interventional cardiologists, were blinded to treatment assignment. The MRI core laboratory was blinded to study group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes. 6 patients (BMSC: 3/58 versus placebo: 3/29) were not included in MRI analysis at 6 months. In the placebo group, 1 patient experienced acute pancreatitis at 3 months and in 2 patients, MRI was contraindicated due to a new ICD. In the BMSC group, 1 patient did not receive cells due to severe LMS stenosis and 2 patients did not attend the 6‐month follow‐up visit |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00684060) were reported |
| Other bias | Low risk | None reported or identified |
Traverse 2012.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: National Heart, Lung, and Blood Institute under co‐operative agreement 5 UO1 HL087318‐04. Support for cell processing (Sepax) was provided by Biosafe SA Inc. Angioplasty catheters were provided by Boston Scientific Corporation Country of origin: USA Number of centres: 5 Dates of trial enrolment: 07/08 to 01/11 Length of follow‐up: 12 months Number (N) of participants randomised to each arm: 79 (day 3/day 7: 43/36) in the treatment arm, 41 (day 3/day 7: 24/17) in the control arm Number (N) of participants analysed (primary outcome) in each arm: 75 (day 3/day 7: 41/34) in the treatment arm, 37 (day 3/day 7: 22/15) in the control arm |
|
| Participants |
Population: STEMI within 7 days
Age, mean (SD) each arm: 55.6 (10.8) years (day 3) and 58.2 (11.3) years in the treatment arm, 57.0 (12.4) years (day 3) and 57.0 (8.0) years (day 7) in the control arm
Sex, % male in each arm: 88.4% (day 3) and 86.1% (day 7) in the treatment arm, 87.5% (day 3) and 88.3% (day 7) in the control arm Number of diseased vessels: 1 or 2 Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: PCI to infusion: median 3.3 (IQR 2.8 to 3.8) days or median 7.4 (IQR 7.0 to 7.9) days in BMSC arm, median 3.2 (IQR 2.5 to 4.1) days or median 7.6 (IQR 7.0 to 8.3) days in the control arm. Statistically significant baseline imbalances between the groups? Higher peak creatine kinase and troponin levels among patients randomised to day 7 treatment group and lack of diabetes among patients randomised to day 7 placebo |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived mononuclear cells (MNC)
Summary of how stem cells were isolated and type and route of delivery: patients underwent bone marrow aspiration on the morning of their treatment day, and BMCs were isolated using a closed, automated Ficoll cell processing system (Sepax, Biosafe) to ensure a uniform cellular product across centres
Dose of stem cells: 1.50 x 108 cells
Timing of stem cell procedure: 3 or 7 days post AMI Comparator arm: placebo (0.9% saline and 5% human serum albumin) |
|
| Outcomes | Primary outcomes: change in global LVEF and regional LV function (infarct and border zone) (day 7) and whether these changes were dependent on day of cell administration (day 3 versus day 7) Secondary outcomes: major adverse cardiovascular events, LV volumes, infarct size Outcome assessment points: 6 and 12 months Method(s): cardiac MRI | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated scheme randomly allocated eligible patients to an intervention time group (3 or 7 days post‐PCI), with subsequent randomisation after BM aspiration to BMC or placebo group by a computer‐generated scheme |
| Allocation concealment (selection bias) | High risk | The computer‐generated randomisation scheme was not blinded |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All patients underwent bone marrow aspiration and control group patients were given an intracoronary injection of 5% human serum albumin in an identical volume of saline with a 100 μL of blood matching the appearance of an active cell preparation and thereby blinding the identity of the infusate being delivered. Blinding of outcome assessors was not reported although the trial was described as "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes. 8 patients (BMSC: 4/79 versus placebo: 4/41) were not included in MRI analysis at 6 months. 1 patient in the BMSC group died due to subarachnoid haemorrhage after randomisation but before cell delivery, MRI was contraindicated in 2 BMSC patients and 1 control patient, and MRI was not performed (reason not reported) in 1 BMSC patient and 3 control patients |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00684021) were reported |
| Other bias | Low risk | None reported or identified |
Turan 2012.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: funded by the Division of Cardiology, Dept of Internal Medicine, University Hospital Rostock, Germany Country of origin: Germany Number of centres: not reported Dates of trial enrolment: not reported Length of follow‐up: 12 months Number (N) of participants randomised to each arm: 42 in the treatment arm, 20 in the control arm Number (N) of participants analysed (primary outcome) in each arm: 42 in the treatment arm, 20 in the control arm |
|
| Participants |
Population: acute STEMI with successful revascularisation
Age, mean (SD) each arm: 61 (15) years in the treatment arm, 60 (11) years in the control arm
Sex, % male in each arm: 67% in the treatment arm, 70% in the control arm Number of diseased vessels: 1 (n = 30), 2 (n = 12) in the treatment arm, 1 (n = 14), 2 (n = 6) in the control arm Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 7 days Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived mononuclear cells (MNC)
Summary of how stem cells were isolated and type and route of delivery: 7 days after AMI, a total of 120 mL bone marrow was taken from the iliac crest after local anaesthesia and mononuclear cells were isolated freshly by use of point of care system (with using of Harvest Technologies GmbH, Munich, Germany) and identified including CD34+ and CD133+. The cell suspension consisted of a heterogeneous cell population including haematopoietic, mesenchymal and other progenitor cells
Dose of stem cells: not reported
Timing of stem cell procedure: 7 days post‐ AMI Comparator arm: no additional therapy (control) |
|
| Outcomes | Primary outcomes: changes in global EF and infarct size Secondary outcomes: mobilisation of BM‐CPCs on days 1, 3, 5, immediately pre‐ and post day 7, 8 and 3, 6, 12 months after procedure, NYHA classification, brain natriuretic peptide level Outcome assessment points: 3 and 12 months Method(s): left ventriculography | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Controls did not undergo bone marrow aspiration and no placebo was administered; neither participants nor patients were blinded. Outcome data were "obtained by blinded expert readers unaware of patient group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Wang 2014.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: not reported Country of origin: China Number of centres: 1 Dates of trial enrolment: 07/08 to 10/09 Length of follow‐up:6 months Number (N) of participants randomised to each arm: 30 in the treatment arm, 30 in the control arm Number (N) of participants analysed (primary outcome) in each arm: 27 in the treatment arm, 28 in the control arm |
|
| Participants |
Population: acute STEMI, primary PCI within 8 hours of onset of symptoms
Age, mean (SD) each arm: 58 (10.2) years in the treatment arm, 56.1 (9.8) years in the control arm
Sex, % male in each arm: 67.9% in the treatment arm, 53.3% in the control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: 15 (1) days Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BM‐MSC
Type of stem cells: bone marrow‐derived mesenchymal stromal cells (MSC)
Summary of how stem cells were isolated and type and route of delivery: approximately 40 mL of human BM was harvested in the morning on the 8th day following PCI. Mononuclear cells were isolated by gradient centrifugation using Ficoll. Cells were then washed, counted and plated in DMEM containing FBS. Media changes every 3 to 4 days. When they were confluent they were split 1:4 and then cultured for 2 weeks before characterisation by FACS analysis. Cells were re‐suspended in heparinised saline and adjusted to 5 x 107 cells/mL 2 hours before transplantation
Dose of stem cells: 1 x 108 cells
Timing of stem cell procedure: 15 (± 1) days PCI to injection Comparator arm: identical volume of saline |
|
| Outcomes | Primary outcomes: not reported Secondary outcomes: LVEF, infarct size, left ventricular diameter, adverse events, rehospitalisation, death Outcome assessment points: 1, 3 and 6 months Method(s): left ventriculography | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The control group received an injection of saline of identical volume although it is not reported whether they underwent bone marrow aspiration. It is therefore unclear whether participants and clinicians were sufficiently blinded to treatment. All haemodynamic investigations were obtained by 2 independent observers although it was not reported whether they were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes. 5 patients (BMSC: 3/30 versus placebo: 2/30) were not included in left ventricular angiography analysis at 6 months. 1 patient in the BMSC group and 2 patients in the placebo group died during follow‐up; 1 additional patient in each group did not complete left ventricular angiography |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Wohrle 2010.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: not reported Country of origin: Germany Number of centres: not reported Dates of trial enrolment: not reported Length of follow‐up: 36 months Number (N) of participants randomised to each arm: 29 in treatment arm/13 in control arm Number (N) of participants analysed (primary outcome) in each arm: 28 in treatment arm/12 in control arm |
|
| Participants |
Population: AMI, within 48 hours. PCI within 6 to 48 hours. Treatment transplantation after successful PCI
Age, mean (SD) each arm: 61.0 (8.1) years in treatment arm, 61.1 (9.3) years in control arm
Sex, % male in each arm: 90% in treatment arm, 62% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: median delay to PCI from symptom onset 14.3 hours (BMC/placebo not distinguished). Placebo: mean 6.6 (SD 1.5), median 6.6 days from symptom onset to infusion of study therapy Statistically significant baseline imbalances between the groups? Difference in male:female ratio, 62% male in control arm versus 90% males in BMSC arm (P value = 0.04) |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: BM was aspirated from the iliac crest into 20 mL syringes containing 500 IU heparin, 0.04 mg gentamicin and 3000 IU penicillin in 3 mL 0.9% sodium chloride. Mononuclear cells were isolated with Ficoll density gradient centrifugation, washed and re‐suspended in 15 mL 0.9% sodium chloride with 2% human albumin. BM aspirated 5 to 7 days post‐AMI. PCI stop‐flow technique through an over‐the‐wire balloon catheter positioned within the stented segment
Dose of stem cells: a single dose of mean 381 x 106 (130 x 106 SD) MNC
Timing of stem cell procedure: cells infused within a median of 6.1 days (interquartile range 5.5 to 7.3) after the onset of AMI and a median of 6.1 hours after BMC aspiration Comparator arm: patients received a placebo consisting of 15 mL 0.9% sodium chloride with 2% human albumin and autologous erythrocytes with a hematocrit of 0.1% without BMC |
|
| Outcomes |
Primary outcomes: LVEF Secondary outcomes: LVEDVI, LVESVI, infarct size, major adverse cardiac events (death, myocardial infarction recurrence, and rehospitalisation for heart failure) Outcome assessment points: baseline, 1, 3, 6, 12, 24, 36 months Method(s): cardiac MRI |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper reported that randomisation was carried out by an external institute in a 2:1 ratio, but the sequence generation procedure was not described |
| Allocation concealment (selection bias) | Unclear risk | Persons involved in the randomisation had no contact with patients |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All patients underwent bone marrow aspiration and control group patients were given an intracoronary injection of a visually indistinguishable autologous erythrocyte preparation; both patients and clinicians were blinded. All personnel involved in the measurement of outcome parameters were double‐blinded throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes and in MRI analysis at 3 months follow‐up. 1 patient in each treatment arm (BMSC: 1/29 versus placebo: 1/13) was missing from MRI analysis at subsequent follow‐up due to death at 121 days and death at 158 days respectively |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00669227) were reported, although LV volumes (included as secondary outcomes) were reported as LV volume indexes |
| Other bias | Low risk | None reported or identified |
Wollert 2004.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Department of Cardiology, Hannover Medical School, Hannover Country of origin: Germany Number of centres: 1 Dates of trial enrolment: 01/02 to 05/03 Length of follow‐up: 60 months Number (N) of participants randomised to each arm: 33 in treatment arm/32 in control arm Number (N) of participants analysed (primary outcome) in each arm: 30 in treatment arm/30 in control arm |
|
| Participants |
Population: AMI, within 5 days
Age, mean (SD) each arm: 53.4 (14.8) years in treatment arm, 59.2 (13.5) years in control arm
Sex, % male in each arm: 67% in treatment arm, 73% in control arm Number of diseased vessels: 1 in both arms (23% right artery/77% left artery) Number of stunned hyperkinetic, etc segments: >2/3 LV anteroseptal, lateral or inferior wall in both arms Time from symptom onset to initial treatment: median 9.8 days (range 2 to 22 days) in treatment arm/median 8.0 days (range 3 to 12 days) in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: BM aspirate (128 +/‐ 33 mL) post baseline cardiac MRI
Separation of MNC using a 4% gelatin‐polysuccinate density gradient, under GMP regulations. Cells re‐suspended in saline with 10,000 U/L of heparin. Between 6 and 8 hours after isolation, cells were infused. Intracoronary infusion using a balloon catheter carried out as 4 to 5 coronary occlusions each lasting 2.6 to 4 minutes
Dose of stem cells: a single dose of 2.46 +/‐ 0.94 x 109 MNC, of which 9.5 +/‐ 6.3 x 106 CD34+ and 3.6 +/‐ 3.4 x 06 form colonies in CFU assays
Timing of stem cell procedure: PCI within 5 days of MI onset. 4.8 +/‐ 1.3 days after PCI the BMSC were infused
G‐CSF details: no G‐CSF Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: changes in global LVEF
Secondary outcomes: changes in: 1. LVEF (%), 2. LVEDV (mL), 3. LVESV (mL), 4. LV mass index (g/m²), 5. Wall thickening: infarct region (%), 6. wall thickening: border zone (%), 7. wall motion: infract region (mm), 8. wall motion: border zone (mm), 9. late contract enhancement volume (LE, mL) Outcome assessment points: baseline, 6, 18, 60 months Method(s): MRI |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised to treatment or control in a 1:1 ratio using sequentially numbered, sealed envelopes provided by an institute external to the trials |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes were provided by another institute |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of participants and clinicians was not reported although controls did not undergo bone marrow aspiration and no placebo was administered. Echocardiography and MRI analyses were performed by 2 investigators blinded to treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 patients (BMSC: 3/33 versus control: 2/32) were withdrawn at the start of the study as "not been able to undergo MRI because of severe obesity or claustrophobia". All other patients were included in analysis of clinical and scientific outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the trial protocol (www.clinicaltrials.gov: NCT00224536) were reported |
| Other bias | Low risk | None reported or identified |
Xiao 2012.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: funded by the Henan Provincial Public Fund Country of origin: China Number of centres: 1 Dates of trial enrolment: 03/10 to 06/11 Length of follow‐up: 3 months Number (N) of participants randomised to each arm: 17 in treatment arm/21 in control arm Number (N) of participants analysed (primary outcome) in each arm: 17 in treatment arm/19 in control arm |
|
| Participants |
Population: AMI; undergoing elective PCI within 4 weeks of AMI
Age, mean (SD) each arm: 60.4 (8.9) years in treatment arm, 58.5 (10.0) years in control arm
Sex, % male in each arm: 58.8% in treatment arm, 61.9% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: within 4 weeks of AMI Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BM‐MSC
Type of stem cells: bone marrow‐derived mesenchymal stromal cells (MSC)
Summary of how stem cells were isolated and type and route of delivery: 80 to 100 mL bone marrow was aspirated from the iliac crest. Mesenchymal stem cells were isolated from bone marrow and cultured in vitro up to 1 to 10 x 108/mL cell suspension. Cells were injected into the infarct related arteries using a guiding catheter
Dose of stem cells: 4.8 (± 1.6) x 108/mL bone marrow MSC
Timing of stem cell procedure: up to 4 weeks after AMI during elective PCI Comparator arm: saline solution |
|
| Outcomes |
Primary outcomes: not reported Secondary outcomes: death, malignant arrhythmia, and microembolitic events; LVEDD, LVEF and perfusion defect percentage Outcome assessment points: baseline, 1 and 3 months Method(s): echocardiography, SPECT |
|
| Notes | Translated from Chinese (Mandarin) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The control group received an injection of heparinised saline although they did not undergo bone marrow aspiration. It is therefore unclear whether participants and clinicians were sufficiently blinded to treatment. The outcome assessors were unaware of grouping details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 patients in the control arm (2/21) were lost to follow‐up at 1 and 3 months |
| Selective reporting (reporting bias) | Unclear risk | Mortality was not explicitly reported; the reported outcome of composite clinical events was not defined. All other outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Yao 2006.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: National Key Technologies R & D Program of China Country of origin: China Number of centres: 1 Dates of trial enrolment: 05/03 to 12/05 Length of follow‐up: 30 months Number (N) of participants randomised to each arm: 92 in treatment arm/92 in control arm Number (N) of participants analysed (primary outcome) in each arm: 90 in treatment arm/84 in control arm |
|
| Participants |
Population: AMI within 1 week, PCI within 1 week
Age, mean (SD) each arm: 58.3 (9.5) years in treatment arm, 58.1 (9.0) years in control arm
Sex, % male in each arm: 89.1% in treatment arm, 88% in control arm Number of diseased vessels: 1 Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: PCI within 1 week of AMI in both arms Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: low temperature density gradient centrifugation of heparinised bone marrow cell suspension in lymphocyte isolation medium. PCI
Dose of stem cells: single 2.1(3.7) x 108 cells
Timing of stem cell procedure: infusion performed 2 hours after revascularisation Comparator arm: no additional therapy (control) |
|
| Outcomes |
Primary outcomes: morbidity, mortality and adverse events Secondary outcomes: LVEF, LVEDD Outcome assessment points: baseline, 6 and 30 months Method(s): echocardiography, LV angiography |
|
| Notes | Translated from Chinese (Mandarin) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This Chinese trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | High risk | Treatment allocation was not concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Controls did not undergo bone marrow aspiration and no placebo was administered; neither participants nor patients were blinded. It was not reported whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10 randomised participants were withdrawn or excluded from the analysis of all outcomes, 2/92 in the BMSC group (1 emigrated to another country and one could not follow up due to economic change) and 8/92 in the control group (3 had changed address at 12 months, another 3 had changed address at 24 months, and a further 2 non‐local participants refused follow‐up) |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Yao 2009.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: Shanghai Scientific Research Fund (06DJ14001), Program for Shanghai Outstanding Medical Academic Leader (LJ06008), National Basic Research Program of China (2006CB943704), and Science Foundation for Youth of Shanghai Medical Administrative Bureau (2008Y044) Country of origin: Italy Number of centres: 1 Dates of trial enrolment: 03/04 to 02/06 Length of follow‐up: 12 months Number (N) of participants randomised to each arm: 15 in single cell transfer arm (ST), 15 in repeated cell transfer arm (RT) and 15 in control arm Number (N) of participants analysed (primary outcome) in each arm: 12 (ST), 15 (RT), 12 (controls) |
|
| Participants |
Population: AMI, within 12 hours.
Age, mean (SD) each arm: 52.1 (6.3) years in ST arm, 51.3 (7.4) years in RT arm, 52.7 (7.8) years in control arm
Sex, % male in each arm: 83.3% in ST arm, 80.0% in RT arm, 91.7% control arm Number of diseased vessels: ST arm: 1 vessel disease = 4/12 (33.33%), 2 vessel disease 5/12 (41.67%), 3 vessel disease 3/12 (25.00%) RT arm: 1 vessel disease = 5/15 (33.33%), 2 vessel disease 6/15 (40.00%), 3 vessel disease 4/15 (26.67%) Controls: 1 vessel disease = 3/12 (25.00%), 2 vessel disease 6/12 (50.00%), 3 vessel disease 3/12 (25.00%) Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: from AMI to PCI: 4.9 (2.9) hours (ST), 4.7(2.9) hours (RT), 6.0 (2.8) hours (controls) Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: single BMMNC dose (SD) or repeated BMMNC dose (DD)
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: 90 ± 18 mL bone marrow was aspirated from the posterior superior iliac spine under local anaesthesia. Bone marrow aspirates were diluted with 0.9% NaCl (1:5) and mononuclear cells were isolated by density gradient centrifugation, washed 3 times with PBS and then suspended in 16 mL heparin‐treated plasma at a density of (1.3 ± 1.0) x 107 cells/mL at room temperature. Cell transplantation via intracoronary route using an over‐the‐wire balloon catheter inserted into the stent that was implanted during primary PCI. Procedure repeated at 3 months in repeated cell dose arm
Dose of stem cells: mean 1.9 (SE 1.2) x 108 BMC (ST), 2.0 (SE 1.4) x 108 (RT, first delivery), 2.1 (SE 1.7) x 108 (RT, second delivery at 3 months)
Timing of stem cell procedure: BMC infusion 3 to 7 days after PCI, and 3 hours after BMC collection, followed by saline infusion (ST group) or second infusion (RT group) 3 months after PCI Comparator arm: saline infusion 3 to 7 days after PCI (no secondary infusion at 3 months) |
|
| Outcomes |
Primary outcomes: LVEF, LVEDV, LVESV Secondary outcomes: myocardial infarct area, myocardial perfusion defect, survival, re‐hospitalisation for congestive heart failure, serious adverse events Outcome assessment points: baseline, 6 and 12 months Method(s): MRI, SPECT, LV angiography |
|
| Notes | Data from the 2 active intervention arms of the trial are pooled in this review. 3 patients randomised to single dose BMSC were not transplanted as follows: 1 patient could not undergo MRI due to pacemaker implantation following development of bradycardia, 1 patient developed a fever 12 hours prior to the procedure, and in 1 patient an inadequate amount of cells was acquired | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken using a computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes were used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Although the control group received a placebo, only the active treatment groups (single or double dose) underwent BM aspiration. Further, the active treatment groups were recalled for the second infusion of cells or placebo whereas the control group was not recalled for further treatment. Participants were therefore not appropriately blinded. Blinding of clinicians was not reported. MRI and SPECT studies were processed and evaluated at the MRI and scintigraphy core laboratories respectively by experienced operators who were blinded to the assigned therapy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients in the repeat BMSC arm were included in the analysis of all outcomes. 3 patients in the single BMSC arm and 3 patients in the control arm (3/15) were withdrawn or excluded from the analysis of all outcomes. In the BMSC arm, 1 patient developed a fever 12 hours prior to the procedure, for one patient an inadequate amount of cells was acquired and one patient could not undergo MRI due to pacemaker implantation following development of bradycardia. In the control arm, 1 patient had a reinfarction 5 days after discharge due to in‐stent thrombosis, 1 patient was excluded due to diagnosis of liver cancer at 4 months, and 1 patient could not be contacted at 3 months follow‐up. One additional patient in the control group was missing from MRI analysis at 12 months follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
You 2008.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: the "135" Major Research Subject for Medical Talent of Jiangsu Province (No. RC2003092); the Social Technical Developing Item of Scientific Bureau of Wuxi City (No. CS040001) Country of origin: Wuxi, Jiangsu Province, China Number of centres: 1 Dates of trial enrolment: 10/03 to 06/05 Length of follow‐up: 8 weeks Number (N) of participants randomised to each arm: 7 in treatment arm/16 in control arm Number (N) of participants analysed (primary outcome) in each arm: 7 in treatment arm/16 in control arm |
|
| Participants |
Population: thrombolysis within 24 hours
Age, mean (SD) each arm: 60.5 years in treatment arm, 62.5 years in control arm
Sex, % male in each arm: 71.4% in treatment arm, 56.3% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: thrombolysis within 24 hours of AMI symptom onset Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mesenchymal stem cells)
Summary of how stem cells were isolated and type and route of delivery: 25 mL bone marrow was aspirated from the superior anterior iliac spine. Aspirate washed and centrifuged to isolate MNC layer. This was cultured in DMEM for a week and passaged 3 times. The cultured cells were harvested and suspended in solution. Infused via the femoral artery PCI route into the left and right coronary arteries
Dose of stem cells: 5 mL suspension, 1.5 x 1010 BMSC/L for a total of 7.5 x 107 cells delivered
Timing of stem cell procedure: 14 days after AMI Comparator arm: no additional therapy (control) |
|
| Outcomes | Primary outcomes: none Secondary outcomes: LVEF, CO, infarct area Outcome assessment points: baseline, 2, 4, 6 and 8 weeks Method(s): echocardiography, Sopha PET‐CT (radionuclide imaging) | |
| Notes | Translated from Chinese (Mandarin) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers were assigned via a table |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | The trial was described as a "single‐blind" evaluation. Controls did not undergo bone marrow aspiration and no placebo was administered; neither participants nor clinicians were blinded. The first author designed, carried out, collected data and assessed the results |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical and scientific outcomes |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
Zhukova 2009.
| Methods |
Type of study: parallel RCT
Type of publication: full
Source of funding: not reported Country of origin: Russia Number of centres: 1 Dates of trial enrolment: not reported Length of follow‐up: 36 months Number (N) of participants randomised to each arm: 8 in treatment arm/3 in control arm Number (N) of participants analysed (primary outcome) in each arm: 8 at 1 year, 6 at 3 years in treatment arm/2 at 1 year, 1 at 3 years in control arm |
|
| Participants |
Population: MI of the front wall and low EF (< 38%). Males with systolic dysfunction who had successful reperfusion therapy (thrombolysis and/or urgent angioplasty)
Age, mean (SD) each arm: 48 (7) years in treatment arm, 50 (10) years in control arm
Sex, % male in each arm: 100% in treatment arm/100% in control arm Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: PCI within 6.5 (3) hours of AMI in treatment arm/PCI within 6.2 (2) hours of AMI in control arm Statistically significant baseline imbalances between the groups?: none |
|
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: 50 to 80 mL bone marrow was aspirated and centrifuged to obtain the mononuclear cells. These were re‐suspended into autologous patient serum
Dose of stem cells: 2 to 5 mL portions for a total of 20 mL; 5 x 106 BMMNC
Timing of stem cell procedure: 14 to 19 days after AMI Comparator arm: no additional therapy (control) |
|
| Outcomes | Primary outcomes: none Secondary outcomes: mortality, morbidity, QOL, LVEF, LVEDV, LVESV, perfusion defect, myocardial viability Outcome assessment points: baseline, 3, 6, 12, 24 and 36 months Method(s): echocardiography, SPECT, gadolinium‐based MRI | |
| Notes | Translated from Russian | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial was described as randomised but the method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | The use of envelopes was mentioned, but insufficient detail was provided to establish whether appropriate allocation concealment was used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Controls did not undergo bone marrow aspiration and no placebo was administered; neither participants nor clinicians were blinded. Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were included in the analysis of clinical outcomes and of scientific outcomes at 3 months. In MRI and echocardiographic analysis at 12 months follow‐up, 1 control patient had died, and at 3 years follow‐up 1 further control and 2 patients in the BMSC group had died |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in the methods were reported in the results, although it would be difficult to rule out selective reporting |
| Other bias | Low risk | None reported or identified |
AE, adverse events; AMI, acute myocardial infarction; ASTAMI, Autologous Stem Cell Transplantation in Acute Myocardial Infarction; BM, bone marrow; BMMNC, bone marrow‐derived mononuclear cells; BMSC, bone marrow‐derived stem cells; CFU, colony forming units; CMR, cardiac magnetic resonance; DMEM, Dulbecco's modified Eagle's medium; DTI, Doppler tissue imaging; ECG, electrocardiogram; Echo, echocardiography; EDV, end diaslotic volume; EF, ejection fraction; ESV, end systolic volume; FACS, fluorescence‐activated cell sorting; FBS, fetal bovine serum; G‐CSF, granulocyte colony stimulating factor; GMP, good manufacturing procedures; HF, heart failure; ICD, internal cardia defibrillator; IQR, interquartile range; IRA, infarct‐related artery; IVUS, intravascular ultrasound; LAD, left anterior descending; LSM, lymphocyte separation medium; LV, left ventricle or ventricular; LVDV, left ventricular diastolic volume; LVEDD, left ventricular end diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEDVI, left ventricular end diastolic volume index; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVESVI, left ventricular end systolic volume index; MBM, creatine kinase‐MB mass; MLHFQ, Minnesota Living with Heart Failure Questionnaire; MNC, mononuclear cells; MRI, magnetic resonance imaging; MSC, mesenchymal stromal cells; NNYHA, New York Heart Association; PBS, phosphate buffered saline; PCI, percutaneous coronary intervention; PET, positron emission tomography; PTCA, percutaneous transmural coronary angioplasty; QoL, quality of life; RCT, randomised controlled trial; RNV, radionuclide ventriculography; SD, standard deviation; SEM, standard error of the mean; SPECT, single photon emission computed tomography; STEMI, ST‐elevation myocardial infarction; VMC, vasomotor centre; WMSI, wall motion score index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ang 2008 | A RCT of BMSC in patients with chronic coronary artery disease |
| Arnesen 2007 | A commentary on RCTs of cell therapy in MI |
| Atsma 2008 | An ongoing single‐arm trial investigating mesenchymal stem cell therapy after acute MI |
| Beeres 2007 | A single‐arm trial of autologous BMSC in patients with chronic MI |
| Benedek 2014 | A RCT of BMMNC versus placebo in patients with MI. This study was excluded because MI occurred up to 3 months prior to study enrollment and was therefore not classified as AMI |
| Chen 2004a | Stem cells were not removed and then reinfused, rather stem cells were mobilised following G‐CSF |
| Chen 2014 | A RCT of G‐CSF mobilised peripheral blood stem cells versus placebo in patients with AMI. The control group did not receive G‐CSF |
| Engelmann 2006 | A RCT of G‐CSF mobilised PBSC (no cells administered) compared with placebo in patients with sub‐acute MI |
| EUCTR 2010‐020497‐41‐GB | An ongoing trial of allogeneic mesenchymal precursor cells versus placebo in patients with AMI |
| Fernandez 2004 | A comparison of CD34+ cell infusion with a non‐randomised control group in patients with AMI |
| Gyongyosi 2009 | A RCT of BMMNC administration either 2 to 3 weeks or 3 to 4 months post AMI. This study did not include a control group |
| Hare 2007 | The trial used allogeneic (not autologous) mesenchymal stem cells, therefore was not eligible for inclusion in the review |
| Heeger 2012 | A non‐randomised study of BMMNC compared with a matched control group in patients with AMI |
| Hendrikx 2006 | A RCT of BMSC compared with a control group in patients with chronic ischaemic heart disease undergoing CABG |
| Holinski 2011 | A non‐randomised trial of autologous BM cells in patients with chronic heart failure scheduled for elective CABG compared with a matched control group |
| Hu 2015 | A RCT of normoxia BMMNC versus hypoxia‐preconditioned BMMNC in patients with AMI. BMMNC groups were compared with a non‐randomised control group |
| Jiang 2011 | A systematic review of RCTs of BMSC in AMI |
| Kahn 2006 | A summary of stem cell trials in MI presented at the 2nd International Conference on Cell Therapy for Cardiovascular Diseases |
| Kang 2004 | A commentary on cell therapy trials in MI |
| Kang 2006 | A RCT of infused G‐CSF mobilised peripheral blood stem cells versus placebo in patients with AMI. The control group did not receive G‐CSF |
| Kang 2007 | A RCT of BMSC infusion compared with G‐CSF compared with a control group in patients with AMI or old MI (OMI). Outcome data are not presented separately for the AMI and OMI groups |
| Kang 2008 | A commentary on results from 2 trials of mobilised PBSC in patients with AMI |
| Kang 2011 | A 3‐arm trial design protocol of intravenous darbepoetin infusion and intracoronary infusion of G‐CSF mobilised PBSC, G‐CSF mobilised PBSC alone or standard medical treatment. The control group did not receive G‐CSF |
| Li 2006 | A RCT of infused G‐CSF mobilised PBSC compared with no treatment in patients with AMI. The control group did not receive G‐CSF |
| Li 2008 | A RCT of the effect of MSC on vascular endothelial function in AMI patients. The outcomes of this study, published in full, are beyond the scope of this review |
| Lu 2012 | An experimental animal study comparing MSC and control groups in MI‐induced swine |
| Makkar 2012 | A RCT of cardiosphere‐derived cells compared with controls in patients with AMI |
| Marenzi 2007 | A comment on the conclusions of the authors of the REPAIR‐AMI trial |
| Messori 2013 | A meta‐regression analysis of 2 previously published meta‐analyses of BMMNC in AMI |
| Mills 2007 | An evaluation and commentary on the REPAIR‐AMI trial |
| Musialek 2006 | A RCT of 2 active interventions: over‐the‐wire balloon catheter for bone marrow stem cell delivery and cell infusion via a perfusion catheter with multiple side holes |
| Musialek 2010 | A RCT of 2 active interventions: over‐the‐wire balloon catheter for bone marrow stem cell delivery and cell infusion via a perfusion catheter with multiple side holes |
| Nasseri 2013 | A RCT of BMMNC versus CD133+ cells versus controls during CABG in patients enrolled 8 to 12 weeks after AMI |
| NCT00548613 | A non‐randomised trial cell therapy in patients with AMI, comparing intracoronary infusion with intramyocardial infusion of a cell mixture of BMSC and progenitor cells. This trial did not include a control group |
| NCT00874354 | An ongoing trial investigating 2 different doses of BMSC in patients with AMI. This trial does not include a control group |
| NCT00877903 | A RCT of allogeneic ex vivo cultured adult human MSCs in patients with AMI |
| Nie 2007 | A non‐randomised trial of BMMNC compared with a control group in patients with AMI |
| Obradovic 2009 | A non‐randomised trial of BMSC compared with a control group in patients with AMI |
| Osterziel 2007 | A comment on the conclusions of the authors of the REPAIR‐AMI trial |
| Ott 2013 | A RCT of G‐CSF mobilised PBSC (no cell infusion) versus placebo in patients with AMI |
| Peruga 2009 | A non‐randomised trial of BMSC compared with a control group in patients with AMI |
| Schachinger 2004 | A RCT of 2 active interventions: circulating progenitor cells and bone marrow‐derived progenitor cells with no control comparator group |
| Schueller 2007 | A non‐randomised study of BMSC versus no cells in patients with AMI |
| Shrimahachota 2011 | A RCT of BMSC compared with a control group with patients with AMI which occurred at a mean of 57.2 days and 45.3 days in the BMSC and control groups respectively |
| Taljaard 2010 | An ongoing RCT of autologous endothelial‐like culture‐modified mononuclear cell infusion (E‐CMMs) compared with both an active treatment arm receiving an infusion of autologous E‐CMMs transfected with endothelial nitric oxide synthase and a control arm receiving standard therapy. Trial excluded as the mononuclear cells collected from circulating blood are not classified as BMSC |
| Terrovitis 2011 | A RCT of intracoronarily administered G‐CSF mobilised peripheral blood stem cells versus placebo in patients with AMI. The control group did not receive G‐CSF |
| Trzos 2009 | A RCT of BMSC compared with a control group in patients with AMI. Excluded because this trial, published in full, evaluated heart rate variability which is not covered by the scope of this review |
| Vanderheyden 2007 | A RCT of enriched haematopoietic BMSC therapy in patients with MI randomised to early or late cell therapy. This trial does not include a randomised control group |
| Wang 2006 | A non‐RCT of BMSC compared with a control group in patients with AMI > 4 weeks before treatment |
| Warbington 2013 | An experimental study of allogeneic cryopreserved purified CD34+ cells to identify potential microRNAs as biomarkers for CD34+ cell SDF‐1 driven migration |
| Yang 2010 | A RCT of BMSC in patients with AMI randomised to delivery via an infarct‐related versus non‐infarct related artery. This trial does not include a randomised control group |
| Yu 2005 | A single‐arm trial of BMMNC in AMI with no control group |
| Yu 2014 | A RCT of G‐CSF mobilised peripheral blood stem cells versus no cells in patients with AMI. The control group did not receive the co‐intervention of G‐CSF |
AMI, acute myocardial infarction; BMMNC, bone marrow‐derived mononuclear cells; BMSC, bone marrow‐derived stem cell; CABG, coronary artery bypass graft; CDC, cardiosphere‐derived stem cells; E‐CMM, endothelial‐like culture modified mononuclear cells; G‐CSF, granulocyte colony stimulating factor; MI, myocardial infarction; MSC, mesenchymal stromal cells; OMI, old myocardial infarction; PBSC, peripheral blood stem cells; RCT, randomised controlled trial; SDF‐1, stromal derived factor, STEMI, ST‐segment elevation myocardial infarction
Characteristics of studies awaiting assessment [ordered by study ID]
Alves 2011.
| Methods |
Type of study: parallel RCT Type of publication: abstract Source of funding: not reported Country of origin: Brazil Number of centres: 1 Dates of trial enrolment: 12/10 to 01/11 Length of follow‐up: 5 to 8 years Number (N) of participants randomised to each arm: 10 to control; 10 to ICV and 20 to ICA Number (N) of participants analysed (primary outcome) in each arm: not reported |
| Participants |
Population: patients with ST‐elevation MI (STEMI) and LV dysfunction
Age, mean (SD) each arm: not reported
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: none |
| Interventions |
Intervention arm: BMSC
Type of stem cells: not reported
Summary of how stem cells were isolated and type and route of delivery: administration reported only; intracoronary artery (IC) or intracardiac vein (ICV) Dose of stem cells: not reported Timing of stem cell procedure: not reported Comparator arm: not reported |
| Outcomes | Primary outcomes: death and hospitalisation Secondary outcomes: not reported Outcome assessment points: baseline and 5 to 8 years Method(s): not reported |
| Notes | — |
Chang 2008.
| Methods |
Type of study: parallel RCT We have requested additional data relating to possible patient overlap with Kang 2006 |
| Participants |
Population: AMI, within 14 days, successfully treated with drug eluting stent (DES)
Age mean (SD) each arm: 56.6 (13.1) years in cell infusion arm/57.1 (11.9) in control arm
Sex % male in each arm: 85% in cell infusion arm/80% in control arm Number of diseased vessels: 11/20 (55%) had 1‐vessel disease and 9/20 (45%) had 2‐vessel disease in cell infusion arm; 11/20 (55%) had 1‐vessel disease and 9/20 (45%) had 2‐vessel disease in control arm Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: none |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: BMSC were mobilised with 10 µg/kg body weight during 3 days. At day 4, the cells were separated using a COBE® Spectra system. Intracoronary infusion using an inflated balloon catheter. SC mobilised and infused after (drug eluting stent) DES Dose of stem cells: a single dose of 1 to 2 x 109 MNC that contained a minimum of 7 x 106 CD34+ cells Timing of stem cell procedure: not reported (3 days after enrolment?) Comparator arm: no additional therapy (control) |
| Outcomes | Primary outcomes: left ventricular synchronous contraction as measured by change in time to peak positive systolic velocity (?Ts‐SD) over 6 months Secondary outcomes: LVEF, LVESV, LVEDV, LV stroke volume, Infarct volume, maximal exercise capacity (METs) Outcome assessment points: baseline and 6 months Method(s): echocardiography, cMRI, treadmill testing |
| Notes | — |
Fernandez‐Pereira 2006.
| Methods |
Type of study: parallel RCT
Type of publication: abstract
Source of funding: not reported Country of origin: Buenos Aires, Argentina Number of centres: 1 Dates of trial enrolment: 02/04 to 01/06 Length of follow‐up: 4 months Number (N) of participants randomised to each arm: not reported Number (N) of participants analysed (primary outcome) in each arm: not reported |
| Participants |
Population: AMI Age mean (SD) each arm: not reported Sex % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups? BMSC group baseline LVEF significantly lower than control group (P value = 0.005) |
| Interventions |
Intervention arm: BMSC Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC) Summary of how stem cells were isolated and type and route of delivery: not reported Dose of stem cells: not reported Timing of stem cell procedure: not reported Comparator arm: no additional therapy (control) |
| Outcomes | Primary outcomes: LVEF Secondary outcomes: cardiac events (ventricular arrhythmias, restenoses) Outcome assessment points: baseline and 4 months Method(s): angiography |
| Notes | Total sample size is 30 ‐ BMSC/control group sample sizes not reported |
Huang 2007b.
| Methods |
Type of study: parallel RCT We have requested additional information relating to possible patient overlap with Huang 2008 abstract |
| Participants |
Population: AMI, within 7 days
Age mean (SD) each arm: not reported
Sex % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: none |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: delivery "via microtubular" Dose of stem cells: not reported Timing of stem cell procedure: not reported Comparator arm: saline infusion |
| Outcomes | Primary outcomes: mortality Secondary outcomes: complications during BMSC infusion, MACE (reinfarction, restenosis, tumour) Outcome assessment points: baseline, 6 months and 12 months Method(s): not reported |
| Notes | — |
Huang 2008.
| Methods |
Type of study: parallel RCT We have requested additional information relating to possible patient overlap with Huang 2007b abstract |
| Participants |
Population: AMI, with successful PCI with stenting
Age mean (SD) each arm: not reported
Sex % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: none |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: delivery "through micro‐catheter" Dose of stem cells: not reported Timing of stem cell procedure: not reported Comparator arm: saline infusion |
| Outcomes | Primary outcomes: not reported Secondary outcomes: safety (cardiovascular events, ventricular arrhythmias, syncope), LVEF Outcome assessment points: baseline and 12 months Method(s): quantitative LV angiography, contrast‐enhanced MRI |
| Notes | — |
Lee 2005.
| Methods |
Type of study: parallel RCT Type of publication: abstract Source of funding: not reported Country of origin: China Number of centres: 1 Dates of trial enrolment: not reported Length of follow‐up: 6 months Number (N) of participants randomised to each arm: 15 control and 14 BMSC Number (N) of participants analysed (primary outcome) in each arm: not reported |
| Participants |
Population: AMI
Age mean (SD) each arm: not reported
Sex % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: none reported |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow‐derived stem cells
Summary of how stem cells were isolated and type and route of delivery: not reported except the intracoronary delivery of cells Dose of stem cells: not reported Timing of stem cell procedure: 3 hours after successful PCI Comparator arm: not reported |
| Outcomes | Primary outcomes: changes in LV function and myocardial perfusion Secondary outcomes: not reported Outcome assessment points: 6 months Method(s):echocardiography and LV angiography |
| Notes | — |
Lu 2012b.
| Methods |
Type of study: parallel RCT Type of publication: abstract Source of funding: not reported Country of origin: Beiging, China Number of centres: 1 Dates of trial enrolment: not reported Length of follow‐up: 6 months Number (N) of participants randomised to each arm: not reported Number (N) of participants analysed (primary outcome) in each arm: not reported |
| Participants |
Population: AMI
Age mean (SD) each arm: 52.18 (9.98) years
Sex % male in each arm: 72% male and 28% female Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: none |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: not reported Dose of stem cells: not reported Timing of stem cell procedure: not reported Comparator arm: not reported |
| Outcomes | Primary outcomes: feasibility and safety Secondary outcomes: LVEF, LVEDV, LVESV, cardiac output, cardiac index, cardiac mass Outcome assessment points: 6 months Method(s): MRI |
| Notes | — |
Park 2011.
| Methods |
Type of study: parallel RCT Type of publication: abstract Source of funding: not reported Country of origin: not reported Number of centres: not reported Dates of trial enrolment: not reported Length of follow‐up: 6 months Number (N) of participants randomised to each arm: 26 to control and 28 to treatment Number (N) of participants analysed (primary outcome) in each arm: not reported |
| Participants |
Population: ST elevation MI (STEMI)
Age mean (SD) each arm: not reported
Sex % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: none reported |
| Interventions |
Intervention arm: mesenchymal stem cells (MSC)
Type of stem cells: MSC
Summary of how stem cells were isolated and type and route of delivery: not reported, MSC were cultured for 4 weeks Dose of stem cells: 1 x 106 cells Timing of stem cell procedure: not reported Comparator arm: not reported |
| Outcomes | Primary outcomes: changes in Heart Rate Variability (HRV) Secondary outcomes: arrhythmias, adverse events, LVEF Outcome assessment points: baseline, 1 month and 6 months Method(s): SPECT and transthoracic echocardiography |
| Notes | — |
Perez‐Oteyza 2006.
| Methods |
Type of study: parallel RCT We are awaiting further information on number of included and followed up patients and full publication details |
| Participants |
Population: patients with AMI. BMSC transplantation after successful PCI
Age mean (SD) each arm: not reported
Sex % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups? not reported |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirate (30 to 40 mL). Cells were separated by gradient centrifugation. Cells were infused after successful PCI by intracoronary transfer Dose of stem cells: a single dose of 1.34 (0.65 to 4.0) x 108/mL mononuclear cells Timing of stem cell procedure: 1 week after PCI Comparator arm: no additional therapy (control) |
| Outcomes | Primary outcomes: LVEF, LVEDV, LVESV Secondary outcomes: not reported Outcome assessment points: baseline and 6 months Method(s): cMRI |
| Notes | — |
Sanchez‐Fernandez 2012.
| Methods |
Type of study: parallel RCT Type of publication: abstract Source of funding: not reported Country of origin: Spain Number of centres: multicentre Dates of trial enrolment: not reported Length of follow‐up: 12 months Number (N) of participants randomised to each arm: 30 control, 30 BMMNC, 30 G‐CSF, 30 BMMNC and G‐CSF Number (N) of participants analysed (primary outcome) in each arm: not reported |
| Participants |
Population: patients with AMI. BMSC transplantation after successful PCI
Age mean (SD) each arm: not reported
Sex % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported, but BMSC treatment 3 to 5 days post‐PCI Statistically significant baseline imbalances between the groups? not reported |
| Interventions |
Intervention arm: BMSC alone or BMSC and G‐CSF or G‐CSF alone
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: not reported, except for intracoronary delivery of the cells Dose of stem cells: not reported Timing of stem cell procedure: 3 to 5 days after PCI, G‐CSF given for 5 days Comparator arm: no additional therapy (control) |
| Outcomes | Primary outcomes: changes in LVEF and LVESV Secondary outcomes: not reported Outcome assessment points: baseline and 12 months Method(s): MRI |
| Notes | — |
Silva 2014.
| Methods |
Type of study: parallel RCT Type of publication: abstract Source of funding: not reported Country of origin: Portugal Number of centres: 1 Dates of trial enrolment: 01/2011 to 05/2013 Length of follow‐up: 12 months Number (N) of participants randomised to each arm: not reported Number (N) of participants analysed (primary outcome) in each arm: not reported |
| Participants |
Population: patients with AMI. BMSC transplantation after successful PCI. PCI within 12 hours of AMI
Age mean (SD) each arm: 50.9 (9.5) years
Sex % male in each arm: 91% male Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: < 12 hours Statistically significant baseline imbalances between the groups? not reported |
| Interventions |
Intervention arm: bone marrow progenitor cells
Type of stem cells: bone marrow progenitor cells
Summary of how stem cells were isolated and type and route of delivery: not reported, except for intracoronary delivery of the cells Dose of stem cells: not reported Timing of stem cell procedure: 7 days after AMI Comparator arm: no additional therapy (control) |
| Outcomes | Primary outcomes: changes in global longitudinal strain (GLS) and LVEF Secondary outcomes: not reported Outcome assessment points: baseline, 6 months and 12 months Method(s): echocardiography |
| Notes | — |
18F‐FDG, fluorodeoxyglucose; AMI, acute myocardial infarction; BMMNC, bone marrow‐derived mononuclear cells; BMSC, bone marrow stem/progenitor cell; BNP, brain natriuretic peptide; cMRI, cardiac magnetic resonance imaging; DES, drug‐eluting stent; G‐CSF, granulocyte colony stimulating factor; HF, heart failure; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; MACE, major adverse cardiac events; MBF, myocardial blood flow; MHFQ, Minnesota Heart Failure Questionnaire; MNC, mononuclear cell; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PET, positron emission tomography; RCT, randomised controlled trial; SPECT, single photon emission computed tomography
Characteristics of ongoing studies [ordered by study ID]
CTRI/2008/091/000232.
| Trial name or title | Efficacy of stem cell in improvement of left ventricular function in patients with acute myocardial infarction |
| Methods |
Type of study: parallel RCT
Source of funding: Department of Biotechnology, New Delhi Country of origin: India Number of centres: 5 Intended recruitment: 250 |
| Participants |
Population: patients with AMI
Age, mean (SD) each arm: not reported (aged 30 to 65 years)
Sex, % male in each arm: not reported Number of diseased vessels: proximal and/or mid left anterior descending artery involvement by angiography Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: > 2 hours to PCI Statistically significant baseline imbalances between the groups? not reported |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow‐derived stem cells
Summary of how stem cells were isolated and type and route of delivery: not reported
Dose of stem cells: 5 to 10 x 108 stem cells
Timing of stem cell procedure: not reported Comparator arm: no additional therapy (control) |
| Outcomes | Primary outcomes: changes in LVEF from baseline to 6 months Secondary outcomes: mortality, rehospitalisation for chest pain, heart failure or arrhythmias, and safety of the intervention to 6 months Outcome assessment points: baseline and 6 months Method (s): multi‐gated acquisition (MUGA) scan |
| Starting date | July 2007 |
| Contact information | Dept of Haematology and Bone Marrow Transplantation, R & R Army Hospital, New Delhi, India, 110 010; Lead: Dr. Velu Nair |
| Notes | — |
EUCTR 2006‐001772‐20‐ES.
| Trial name or title | Effect of intracoronary injection of autologous stem cells on left ventricular ejection fraction and volumes one year after an acute myocardial infarction |
| Methods |
Type of study: parallel RCT
Source of funding: Clinica Rotger Country of origin: Spain Number of centres: not reported Intended recruitment: 60 |
| Participants |
Population: patients with AMI
Age, mean (SD) each arm: not reported (8 to 75 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: > 2 segments Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups? not reported |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow mononuclear cells (BMMNC)
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirate and gradient centrifugation. Following the method set up by Schachinger 2006.
Dose of stem cells: not reported
Timing of stem cell procedure: not reported Comparator arm: placebo (saline) |
| Outcomes | Primary outcomes: changes in LVEF, LVEDV, LVESV, perfusion, scar size Secondary outcomes: changes in LVEF at 6 months (by echocardiography and LV angiography), major adverse clinical cardiac events Outcome assessment points: baseline and 12 months Method(s): not reported |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | — |
EUCTR 2006‐005628‐17‐ES.
| Trial name or title | Open study with blind regulator on the effectiveness of autologous bone marrow mononuclear cells in patients with left ventricular dysfunction after myocardia infarction |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: Spain Number of centres: not reported Intended recruitment: 20 |
| Participants |
Population: AMI and LVEF < 35%
Age, mean (SD) each arm: not reported (18 to 75 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups? not reported |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow mononuclear cells (BMMNC)
Summary of how stem cells were isolated and type and route of delivery: intracoronary injection. Method of isolation of BMMNC not reported
Dose of stem cells: 20 to 30 x 106 cells/mL
Timing of stem cell procedure: not reported Comparator arm: no additional therapy (control) |
| Outcomes | Primary outcomes: changes in LVESV Secondary outcomes: NT‐proBNP, myocardial perfusion, MACE, hospitalisation within 24 hours Outcome assessment points: baseline and 12 months Method(s): echocardiography |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | — |
Hamshere 2014.
| Trial name or title | A randomised double‐blind control study of early intracoronary autologous bone marrow cell infusion in acute myocardial infarction (REGENERATE‐AMI) |
| Methods |
Type of study: parallel RCT
Source of funding: UK Stem Cell Foundation, Heart Cells Foundation and Barts and the London Charity Country of origin: UK, Switzerland, Denmark Number of centres: 5 Intended enrolment: total 100 (1:1 randomisation) |
| Participants |
Population: AMI
Age, mean (SD) each arm: not reported (18 to 80 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: within 24 hours Statistically significant baseline imbalances between the groups?: none |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow mononuclear cells
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirate and gradient centrifugation. Following the method set up by Schachinger 2006
Dose of stem cells: not reported
Timing of stem cell procedure: not reported Comparator arm: placebo (saline) |
| Outcomes | Primary outcomes: changes in LVEF from baseline to 12 months (by MRI) Secondary outcomes: changes in LVEF at 6 months (by echocardiography and LV angiography), major adverse clinical cardiac events Outcome assessment points: baseline, 6 and 12 months Method(s): MRI, echocardiography and LV angiography |
| Starting date | Not reported |
| Contact information | Department of Cardiology, London Chest Hospital, Barts Health NHS Trust, London, UK. Chief Investigator: Professor Anthony Mathur |
| Notes | www.clinicaltrials.gov: NCT00765453 |
ISRCTN17457407.
| Trial name or title | Bone marrow transfer to enhanced ST‐elevation infarct regeneration‐2 (BOOST‐2) |
| Methods |
Type of study: parallel RCT
Source of funding: German Research Foundation (Deutsche Forschungsgemeinschaft) Country of origin: Bulgaria, Germany, Norway Number of centres: not reported (multicentre) Intended enrolment: 200 |
| Participants |
Population: first AMI
Age, mean (SD) each arm: not reported (> 30 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: >2/3 of left ventricular anteroseptal, lateral or inferior wall Time from symptom onset to initial treatment: > 3 hours to PCI Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: high dose and low dose of non‐irradiated and irradiated BMSC
Type of stem cells: BMSC
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirate
Dose of stem cells: low and high dose
Timing of stem cell procedure: not reported Comparator arm: placebo medium |
| Outcomes | Primary outcomes: changes in LVEF from baseline to 6 months Secondary outcomes: changes in LVEF at 18 months, LVEDV, LVESV, exercise capacity, quality of life, combined endpoint mortality and heart failure Outcome assessment points: baseline, 6 and 18 months Method(s): MRI and echocardiography |
| Starting date | February 2006 |
| Contact information | Dept. of Cardiology and Angiology, Hannover Medical School, Carl‐Neuberg Str.1 Hannover, Germany. Lead: Prof. Kai Wollert |
| Notes | This trial is marked as completed but no publications have as yet been identified |
ISRCTN65630838.
| Trial name or title | Selected bone marrow cell transplantation following MI in patients undergoing coronary surgery |
| Methods |
Type of study: parallel RCT
Source of funding: Bristol Royal Infirmary Country of origin: UK Number of centres: 1 Intended enrolment: 60 |
| Participants |
Population: recent MI (> 10 days < 3 months) undergoing bypass coronary surgery
Age, mean (SD) each arm: not reported
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: > 10 days < 3 months Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: CD133+ bone marrow cells
Type of stem cells: bone marrow‐derived CD133+ cells
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirate and selection of CD133+ cells using magnetic immunoaffinity
Dose of stem cells: not reported
Timing of stem cell procedure: not reported Comparator arm: autologous plasma |
| Outcomes | Primary outcomes: quantitative assessment of myocardium at the site of injection of CD133+ cells Secondary outcomes: not reported Outcome assessment points: not reported Method(s): not reported |
| Starting date | June 2006 |
| Contact information | Research and Effectiveness Department, Level 1 Old Building, Bristol Royal Infirmary, Marlborough St., Bristol, BS2 8HW |
| Notes | This trial is marked as completed but no publications have as yet been identified |
Mansour 2011.
| Trial name or title | Comparison of intracoronary injection of CD133+ bone marrow stem cells to placebo in patients after acute myocardial infarction (COMPARE‐AMI) |
| Methods |
Type of study: parallel RCT
Source of funding: fonds de la recherche en santé du Québec, Miltenyi Biotec, Inc., and Boston Scientific in Canada Country of origin: Canada Number of centres: 1 Intended enrolment: not reported |
| Participants |
Population: AMI
Age, mean (SD) each arm: 52.2 (8.9) years
Sex, % male in each arm: 90% male Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow‐derived CD133‐positive cells
Summary of how stem cells were isolated and type and route of delivery: BM aspiration and separation of mononuclear cells using gradient centrifugation. CD133‐positive cells were immunomagnetically separated using the Clinimacs (Miltenyi)
Dose of stem cells: not reported
Timing of stem cell procedure: < 12 hours Comparator arm: saline and 10% autologous plasma |
| Outcomes | Primary outcomes: safety and efficacy and functional effect of the treatment Secondary outcomes: not reported Outcome assessment points: baseline, 4 months and 12 months Method(s): echocardiography, MRI, LV angiography |
| Starting date | — |
| Contact information | — |
| Notes | — |
Micheu 2013.
| Trial name or title | Early effect of autologous bone marrow stem cell therapy on left ventricular systolic function in acute myocardial infarction patients and low left ventricular ejection fraction ‐ a pilot study |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: Romania Number of centres: 1 Intended enrolment: not reported |
| Participants |
Population: AMI, LVEF < 40%
Age, mean (SD) each arm: not reported
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups? not reported |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow‐derived stem cells (mononuclear cells‐MNC)
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspiration and gradient centrifugation to isolate mononuclear cells. Cells were administered via intracoronary infusion
Dose of stem cells: not reported
Timing of stem cell procedure: 7 to 13 days following PCI Comparator arm: not reported |
| Outcomes | Primary outcomes: changes in LVEF Secondary outcomes: not reported Outcome assessment points: 1 month Method(s): not reported |
| Starting date | — |
| Contact information | — |
| Notes | — |
NCT00529932.
| Trial name or title | A trial using CD133 enriched bone marrow cells following primary angioplasty for acute myocardial infarction (SELECT‐AMI) |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: Belgium, France, The Netherlands, United Kingdom Number of centres: 4 Intended enrolment: 19 |
| Participants |
Population: AMI
Age, mean (SD) each arm: not reported (20 to 75 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: presence of severe hypokinesia and/or akinesia in >= 2 adjacent segments on echocardiogram at 48 to 72 hours after primary PCI Time from symptom onset to initial treatment: 2 to 24 hours after onset of chest pain Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: CD133+ cells
Type of stem cells: bone marrow‐derived selected CD133+ cells
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirated, CD133+ cells selected, intracoronary injection of autologous CD133+ cells
Dose of stem cells: not reported
Timing of stem cell procedure: not reported Comparator arm: buffered normal saline |
| Outcomes | Primary outcomes: 1) Safety ‐ progression in coronary atherosclerosis burden proximal and distal to the stented segment of the infarct‐related artery, 2) Efficacy ‐ changes in myocardial thickening in non‐viable akinetic/hypokinetic LV wall segments by cardiac magnetic resonance imaging (cMRI) Secondary outcomes: 1) Safety ‐ development of ventricular arrhythmias including failed sudden cardiac death, development of congestive heart failure 2) Efficacy ‐ LVEF, epicardial resistance and microvascular resistance, the feasibility of the CliniMACS® Reagent System to yield 5 x 106 CD133+ cells from 100 to 150 mL of autologous bone marrow Outcome assessment points: baseline and 6 months Method(s): cMRI, echocardiography |
| Starting date | September 2007 |
| Contact information | Jozef Bartunek, MD (jozef.bartunek@olvz‐aalst.be); Jonathan Hill, MD (jonathan.hill@kcl.ac.uk) |
| Notes | This study has been terminated due to insufficient recruitment |
NCT00711542.
| Trial name or title | Reinfusion of enriched progenitor cells and infarct remodeling in acute coronary syndrome (REPAIR‐ACS) |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: Germany Number of centres: 1 Intended enrolment: 31 |
| Participants |
Population: acute non‐ST segment elevation myocardial infarction, successful PCI with stent
Age, mean (SD) each arm: not reported (18‐ to 80 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: < 48 hours Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow stem cells
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirated, preparation of media, delivery via intracoronary injection
Dose of stem cells: not reported
Timing of stem cell procedure: not reported Comparator arm: placebo medium |
| Outcomes | Primary outcomes: improvement of coronary flow reserve in the infarct vessel Secondary outcomes: improvement of relative coronary flow reserve, regional and global LVEF, MACE (death, MI, rehospitalisation for heart failure, revascularisation) Outcome assessment points: baseline, 4 months and 1 year Method(s): intracoronary doppler wire |
| Starting date | September 2008 |
| Contact information | Andreas M Zeiher, MD (zeiher@em.uni‐frankfurt.de); Birgit Assmus, MD (b.assmus@em.uni‐frankfurt.de) |
| Notes | This study has been terminated due to slow recruitment |
NCT00936819.
| Trial name or title | The enhanced angiogenic cell therapy ‐ acute myocardial infarction trial (ENACT‐AMI) |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: Canada Number of centres: 5 Intended enrolment: 100 |
| Participants |
Population: AMI
Age, mean (SD) each arm: not reported (18 to 80 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: endothelial progenitor cells (EPC) or eNOS transfected EPC
Type of stem cells: endothelial progenitor cells (EPC)
Summary of how stem cells were isolated and type and route of delivery: not reported
Dose of stem cells: 20 x 106 cells in each treatment arm
Timing of stem cell procedure: after 5 to 7 days Comparator arm: plasmalyte and 25% autologous plasma |
| Outcomes |
Primary outcome: change in LVEF Secondary outcomes: changes in wall motion, clinical worsening, QoL and safety Outcome assessment points: baseline and 6 months Method(s): MRI |
| Starting date | July 2013 |
| Contact information | Contact: Dr. Duncan J. Stewart, MD FRCP C, Ottawa Hospital Research Institute |
| Notes | — |
NCT00979758.
| Trial name or title | Strengthening transplantation effects of bone marrow mononuclear cells with atorvastatin in myocardial infarction |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: China Number of centres: 1 Intended enrolment: 100 |
| Participants |
Population: STEMI
Age, mean (SD) each arm: not reported (30 to 80 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups? not reported |
| Interventions |
Intervention arm: BMSC + Artovastatin (routine or intensive dose)
Type of stem cells: BMSC (mononuclear cells)
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirated, preparation of media, delivery via intracoronary injection
Dose of stem cells: not reported
Timing of stem cell procedure: not reported Comparator arm: atorvastatin (routine or intensive dose) |
| Outcomes | Primary outcomes: LVEF Secondary outcomes: not reported Outcome assessment points: baseline and 12 months Method(s): ECG, echocardiography, MRI |
| Starting date | January 2009 |
| Contact information | Fuwai Hospital, Beijing, China, 100037; Lead: Dr Yang Yuejin |
| Notes | Estimated study completion date: January 2012. This study is enrolling participants by invitation only |
NCT00984178.
| Trial name or title | Randomised trial comparing intracoronary delivery of bone marrow‐derived stem cells versus stem cell mobilisation with G‐CSF, a combination of both therapies and conventional treatment in patients with reperfused acute myocardial infarction (TECAM2) |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: Spain Number of centres: 1 Intended enrolment: 120 |
| Participants |
Population: AMI
Age, mean (SD) each arm: not reported (18 to 75 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: < 24 hours Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: BMSC
Type of stem cells: bone marrow mononuclear cells
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspirated, mononuclear cells isolated by Ficoll technique, delivery via intracoronary injection
Dose of stem cells: not reported
Timing of stem cell procedure: not reported Comparator arm: no additional therapy (control) |
| Outcomes | Primary outcomes: change in LVEF and LVESV Secondary outcomes: change in LVEDV, segment contractility, wall thickness and intravascular ultrasound re‐endothelialisation, safety Outcome assessment points: baseline, 9 months and 12 months Method(s): MRI, ultrasound |
| Starting date | November 2005 |
| Contact information | Pedro L Sanchez, MD, PhD (pedrolsanchez@secardiologia.es); Francisco Fernández‐Aviles, MD, PhD (faviles@secardiologia.es) |
| Notes | Estimated completion date: November 2009. This trial includes 2 additional randomised groups: G‐CSF plus bone marrow mononuclear cells and progenitor cells mobilised through G‐CSF |
NCT01187654.
| Trial name or title | Bone marrow derived AC 133+ and mono‐nuclear cells (MNC) implantation in myocardial infarction (MI) patients |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: Iran Number of centres: 1 Intended enrolment: 80 |
| Participants |
Population: AMI
Age, mean (SD) each arm: not reported (18 to 75 years)
Sex, % male in each arm: not reported Number of diseased vessels: 1 Number of stunned hyperkinetic, etc segments: more than 2 Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: BMSC or CD133+
Type of stem cells: none marrow mononuclear cells (BMMNC) and CD133 cells
Summary of how stem cells were isolated and type and route of delivery: not reported
Dose of stem cells: not reported
Timing of stem cell procedure: within 3 weeks of AMI Comparator arm: no additional therapy (control) |
| Outcomes | Primary outcomes: change in LVEF Secondary outcomes: change in LVEDV, LVESV, segment contractility Outcome assessment points: baseline, 6 months Method(s): echocardiography |
| Starting date | May 2009 |
| Contact information | Principal Investigator: Masoud Ghassemi, MD; Royan Institute, Tehran, Islamic Republic of Iran |
| Notes | This trial is marked as completed but no publications have as yet been identified |
NCT01394432.
| Trial name or title | Endocardial mesenchymal stem cells implantation in patients after acute myocardial infarction (ESTIMATION Study) |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: Russia Number of centres: not reported Intended enrolment: 50 |
| Participants |
Population: AMI with successful PCI
Age, mean (SD) each arm: not reported (30 to 75 years)
Sex, % male in each arm: not reported Number of diseased vessels: 1 Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: BM‐MSC
Type of stem cells: bone marrow‐derived mesenchymal stem cells
Summary of how stem cells were isolated and type and route of delivery: not reported, except for delivery using NOGA mapping
Dose of stem cells: not reported
Timing of stem cell procedure: 7 to 10 days after PCI Comparator arm: placebo |
| Outcomes | Primary outcomes: reduction of LVESV by 15% Secondary outcomes: death, Thrombosis, hospitalisation for HF, 6 min‐walk, BNP levels Outcome assessment points: baseline, 12 months Method(s): MRI |
| Starting date | July 2011 |
| Contact information | Principal Investigator: Professor Evgeny Pokushalov, MD; State Research Institute of Circulation Pathology, Novosibirsk, Russian Federation, 630055 |
| Notes | Estimated completion date: November 2012 |
NCT01495364.
| Trial name or title | NBS10 (also known as AMR‐001) versus placebo post ST segment elevation myocardial infarction (PreSERVE‐AMI) |
| Methods |
Type of study: parallel RCT
Source of funding: NeoStem, Inc. Country of origin: USA Number of centres: not reported Intended enrolment: 160 |
| Participants |
Population: AMI
Age, mean (SD) each arm: not reported (> 18 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: CD34‐positive cells
Type of stem cells: bone marrow‐derived CD34‐positive cells
Summary of how stem cells were isolated and type and route of delivery: not reported, intracoronary delivery
Dose of stem cells: not reported
Timing of stem cell procedure: not reported Comparator arm: placebo |
| Outcomes | Primary outcomes: AE, SAE, MACE and myocardial perfusion Secondary outcomes: not reported Outcome assessment points: baseline, 6 months and 36 months Method(s): SPECT |
| Starting date | December 2011 |
| Contact information | Principal Investigator: Arshed Quyyumi, MD, Emory University |
| Notes | Estimated completion date: June 2014 |
NCT01536106.
| Trial name or title | Rapid delivery of autologous bone marrow derived stem cells in acute myocardial infarction patients (AMIRST) |
| Methods |
Type of study: parallel RCT
Source of funding: TotipotentRX Cell Therapy Pvt. Ltd. Country of origin: India Number of centres: not reported Intended enrolment: 30 |
| Participants |
Population: AMI, LVEF < 40%
Age, mean (SD) each arm: not reported (18 to 75 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: PCI within 24 hours of MI Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived mononuclear cells
Summary of how stem cells were isolated and type and route of delivery: not reported, intracoronary delivery
Dose of stem cells: not reported
Timing of stem cell procedure: 3 to 10 days after AMI Comparator arm: placebo |
| Outcomes |
Primary outcomes: AE Secondary outcomes: changes in LVEF, LVEDV, LVESV, infarct size, myocardial perfusion, MACE and QoL Outcome assessment points: baseline and 12 months Method (s): cardiac MRI |
| Starting date | December 2013 |
| Contact information | Principal Investigators: Sreenivas A Kumar, MD, DM, FACC; CARE Hospitals, Hyderabad, India; Upendra Kaul, MD,DM, FACC; Fortis Flt. Lt. Rajan Dhall Hospital and Ashok Seth, FRCP, FACC; Fortis Escorts Heart Institute and Research Centre, India |
| Notes | Estimated completion date: January 2015 |
NCT01569178.
| Trial name or title | The effect of intracoronary reinfusion of bone marrow‐derived mononuclear cells (BM‐MNC) on all cause mortality in acute myocardial infarction (BAMI) |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: Belgium, Czech Republic, Denmark, Finland, France, Germany, Italy, Poland, Spain, UK Number of centres: 24 Intended enrolment: 3000 |
| Participants |
Population: AMI, LVEF ≤ 45%
Age, mean (SD) each arm: not reported (> 18 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: thrombolytic therapy within 24 hours of MI and PCI within 24 hours of therapy Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived mononuclear cells
Summary of how stem cells were isolated and type and route of delivery: intracoronary delivery of BMMNC isolated from bone marrow aspirates and gradient centrifugation
Dose of stem cells: not reported
Timing of stem cell procedure: not reported Comparator arm: no additional therapy (control) |
| Outcomes |
Primary outcomes: time to all‐cause death Secondary outcomes: time to cardiovascular death, time to cardiovascular hospitalisation for MI, revascularisation, HF, etc., SAE and bleeding Outcome assessment points: baseline and 36 months Method(s): cardiac MRI |
| Starting date | September 2013 |
| Contact information | Principal Investigator: Professor Anthony Mathur, MB BChir, FRCP, PhD; Queen Mary University of London, UK |
| Notes | Estimated completion date: May 2017 |
NCT01625949.
| Trial name or title | Stem cell therapy in patients with myocardial infarction and persistent total occlusion of infarct related artery (COAT) |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: India Number of centres: 1 Intended enrolment: 40 |
| Participants |
Population: AMI
Age, mean (SD) each arm: not reported (18 to 80 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: time to PCI < 24 hours. Time to cell treatment > 24 hours Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived mononuclear cells
Summary of how stem cells were isolated and type and route of delivery: intracoronary delivery of BMMNC isolated from bone marrow aspirates and gradient centrifugation
Dose of stem cells: not reported
Timing of stem cell procedure: not reported Comparator arm: no additional therapy (control) |
| Outcomes |
Primary outcomes: changes in LVEF Secondary outcomes: changes in functional capacity (NYHA class), 6 minute walking distance, QoL, recurrent MI or death Outcome assessment points: baseline and 3 months Method(s): PET |
| Starting date | March 2011 |
| Contact information | Principal Investigator: Sandeep Seth, DM; All India Institute of Medical Sciences, New Delhi, India |
| Notes | Estimated completion date: June 2014 |
NCT01652209.
| Trial name or title | A randomised, open labeled, multicenter trial for safety and efficacy of intracoronary adult human mesenchymal stem cells acute myocardial infarction (RELIEF) |
| Methods |
Type of study: parallel RCT
Source of funding: Pharmicell Co., Ltd Country of origin: Korea Number of centres: not reported Intended enrolment: 135 |
| Participants |
Population: AMI, LVEF < 45%
Age, mean (SD) each arm: not reported (20 to 70 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: within 30 days of MI Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: BM‐MSC
Type of stem cells: bone marrow‐derived mesenchymal stem cells
Summary of how stem cells were isolated and type and route of delivery: intracoronary delivery of MSC, not reported how they are cultured
Dose of stem cells: not reported
Timing of stem cell procedure: after 30 days (single dose) or after 30 and 60 days (double dose) Comparator arm: no additional therapy (control) |
| Outcomes |
Primary outcomes: changes in LVEF Secondary outcomes: not reported Outcome assessment points: baseline and 13 months Method(s): MRI |
| Starting date | October 2013 |
| Contact information | Principal Investigator: Yang Soo Jang, Ph.D. M.D.; Severance Hospital, Yonsei University College of Medicine; Korea |
| Notes | Estimated completion date: December 2018 |
NCT02323620.
| Trial name or title | Impact of intracoronary injection of autologous BMMC for LV contractility and remodeling in patients with STEMI (RACE‐STEMI) |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: Poland Number of centres: not reported Intended enrolment: 200 |
| Participants |
Population: AMI, LVEF ≤ 45%
Age, mean (SD) each arm: not reported (> 18 years)
Sex, % male in each arm: not reported Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: not reported Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived mononuclear cells
Summary of how stem cells were isolated and type and route of delivery: intracoronary delivery of BMMNC isolated from BM aspirates and gradient centrifugation
Dose of stem cells: not reported
Timing of stem cell procedure: not reported Comparator arm: no additional therapy (control) |
| Outcomes |
Primary outcomes: changes in LVEF at 12 months Secondary outcomes: LVEDV, LVESV, time to cardiac death, hospitalisation for HF, SAE Outcome assessment points: baseline, 12 months and 36 months Method(s): CT |
| Starting date | March 2015 |
| Contact information | Principal Investigator: Pawel E Buszman, MD, PhD; American Heart of Poland, Poland |
| Notes | Estimated completion date: July 2018 |
Pena‐Duque 2011.
| Trial name or title | Intracoronary autologous stem cell transplantation in ST‐elevation myocardial infarction (TRACIA STUDY) |
| Methods |
Type of study: parallel RCT
Source of funding: not reported Country of origin: Mexico Number of centres: not reported Intended enrolment: not reported |
| Participants |
Population: AMI
Age, mean (SD) each arm: 53.25 (5.7) years
Sex, % male in each arm: 87.5% Number of diseased vessels: not reported Number of stunned hyperkinetic, etc segments: not reported Time from symptom onset to initial treatment: within 24 hours Statistically significant baseline imbalances between the groups?: not reported |
| Interventions |
Intervention arm: BMMNC
Type of stem cells: bone marrow‐derived mononuclear cells
Summary of how stem cells were isolated and type and route of delivery: bone marrow aspiration and separation of mononuclear cells using a Sepax machine and a gradient centrifugation
Dose of stem cells: adjusted for CD34‐positive cells 1 to 2 x 106 CD34 cells
Timing of stem cell procedure: day 5 to 6 after AMI Comparator arm: no additional therapy (control) |
| Outcomes | Primary outcomes: safety changes in LVEF from baseline to 6 months Secondary outcomes: death, re‐infection, restenosis, thrombosis, adverse events, LVEF Outcome assessment points: baseline, 6 months Method(s): MRI and SPECT |
| Starting date | — |
| Contact information | Marco Antonio Pena Duque, Juan Badiano No. 1, Col Cession XVI, Llalpan, 14080 Mexico. Email: penmar@cardiologia.org.mx |
| Notes | — |
AE, adverse effect; AMI, acute myocardial infarction; BFU‐E, burst‐forming unit ‐ erythrocyte, BM, bone marrow; BMMNC, bone marrow‐derived mononuclear cells; BMSC, bone marrow‐derived stem cells; BM‐CPC, bone marrow‐derived circulating progenitor cells; BOOST, Benefits of Oxygen Saturation Targeting; CFU‐GEMM, colony‐forming unit ‐ granulocyte erythrocyte monocyte megakaryocyte; CFU‐GM, colony‐forming unit ‐ granulocyte monocyte, CK‐MB, creatine‐kinase muscle and brain; cMRI, cardiac magnetic resonance imaging; CO, FACS, fluorescence‐activated cell sorting; G‐CSF, granulocyte colony stimulating factor; IVUS, intravascular ultrasound; LAD, left anterior descending; LV, left ventricle or ventricular; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; MACE, major adverse cardiac events; MI, myocardial infarction; MIBI, methoxyisobutylisonitrile; MNC, mononuclear cells; MRI, magnetic resonance imaging; MUGA, Multi Gated Acquisition Scan; MVO2, myocardial volume oxygen consumption; PCI, percutaneous coronary intervention; PET, positron emission tomography; QoL, quality of life; QLV, quantitative left ventriculography; SAE, serious adverse effect; SC, stem cells; SD, standard deviation; SPECT, single photon emission computed tomography; STEMI, ST‐segment elevation myocardial infarction; VEGF, vascular endothelial growth factor; WMSI, wall motion score index
Differences between protocol and review
The original outcomes of this review have been revised in this update, focusing on clinical outcomes. However, the surrogate endpoint of LVEF is a standard, widely reported marker for cardiac function and has been retained as a reference point with other trials and systematic reviews in AMI. Surrogate outcomes other than LVEF reported in previous versions of this review, namely engraftment and survival of the infused stem cells, left ventricular end‐systolic volume, left ventricular end‐diastolic volume, wall motion score, stroke volume index and infarct size are no longer included. We now define revised primary outcomes as (i) all‐cause mortality, (ii) cardiovascular mortality, (iii) composite measures of major adverse cardiac events (MACE), and (iv) periprocedural adverse events. Secondary outcomes include morbidity, LVEF and quality of life and performance measures.
In the protocol and previous versions of the review we implemented fixed‐effect models in the first instance. It is now clear that there are many potential sources of heterogeneity across trials, and in this version of the review we have performed meta‐analyses using random‐effects models throughout.
In the writing of this version of the review we identified a systematic error in the previous versions of the review in the calculation of standard deviations for mean change from baseline LVEF values. This issue has now been corrected. In some studies it was not possible to accurately calculate the value of the standard deviation. These studies, previously analysed as mean change from baseline values, are now reported as mean value at endpoint; results from combined analyses of mean change from baseline and endpoint values are reported.
Contributions of authors
Sheila Fisher: methodological expert, eligibility screening, data extraction, quality assessment, data analysis and preparation of the final report.
Huajun Zhang: eligibility screening, data extraction and comment on the final report.
Carolyn Doree: design and implementation of search strategies, initial eligibility screening, data verification and comment on the final report.
Anthony Mathur: clinical content expert, preparation of the final report.
Enca Martin‐Rendon: scientific content expert, eligibility screening, data extraction, quality assessment and preparation of the final report. Corresponding author who takes global responsibility for this review.
Sources of support
Internal sources
NHS Blood and Transplant, Research and Development (CD and SW), UK.
William Harvey Research Institute (AM), UK.
External sources
-
National Institute of Health Research (NIHR), UK.
This work was supported by NIHR under its Cochrane Incentive Award scheme (award number 14/175/34 to EMR). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Declarations of interest
Sheila Fisher: none known.
Huajun Zhang: none known.
Carolyn Doree: none known.
Anthony Mathur: none known.
Enca Martin‐Rendon: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Angeli 2012 {published data only}
- Angeli FS, Caramori PR, Costa Escobar Piccoli J, Danzmann LC, Magedanz E, Bertaso A, et al. Autologus transplantation of mononuclear bone marrow cells after acute myocardial infarction: a PILOT study. International Journal of Cardiology 2012;158(3):449‐50. [DOI] [PubMed] [Google Scholar]
Cao 2009 {published data only}
- Cao F, Sun D, Li C, Narsinh K, Zhao L, Li X, et al. Long‐term myocardial functional improvement after autologous bone marrow mononuclear cell transplantation in patients with ST‐segment elevation myocardial infarction: 4 years follow‐up. European Heart Journal 2009;30(16):1986‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Sun D, Li C, Zhao L, Wang H. Four years follow up of intracoronary delivery autologous bone marrow mononuclear cells in patients with ST‐elevation myocardial infarction [American College of Cardiology 58th Annual Scientific Session and i2 Summit: Innovation in Intervention, Orlando, FL United States, 29‐31 March 2009]. Journal of American College of Cardiology 2009;53(10):A41. [Google Scholar]
- Cao F, Sun DD, Li CX, Narsinh K, Guo W, Li X, et al. Long‐term myocardial functional improvement after autologous bone marrow mononuclear cell transplantation in patients with ST‐segment elevation myocardial infarction (STEMI): 4‐years follow‐up. [14th Annual Symposium ‐ Angioplasty Summit 2009 TCT Asia Pacific, Seoul, South Korea, 22‐24 April 2009]. American Journal of Cardiology 2009;103(9):2B‐3B. [Google Scholar]
- NCT00626145. Long‐term follow‐up of autologous bone marrow mononuclear cells therapy in STEMI. http://clinicaltrials.gov/ct2/show/NCT00626145 (accessed by 31 January 2011).
Chen 2004 {published data only}
- Chen SL, Fang WW, Qian J, Ye F, Liu YH, Shan SJ, et al. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chinese Medical Journal 2004;117(10):1443‐8. [PubMed] [Google Scholar]
- Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. American Journal of Cardiology 2004;94(1):92‐5. [DOI] [PubMed] [Google Scholar]
Colombo 2011 {published data only}
- Castellani M, Colombo A, Giordano R, Pusineri E, Canzi C, Longari V, et al. The role of PET with 13N‐ammonia and 18F‐FDG in the assessment of myocardial perfusion and metabolism in patients with recent AMI and intracoronary stem cell injection. Journal of Nuclear Medicine 2010;51(12):1908‐16. [DOI] [PubMed] [Google Scholar]
- Colombo A, Castellani M, Piccaluga E, Pusineri E, Palatresi S, Longari V, et al. Myocardial blood flow and infarct size after CD133+ cell injection in large myocardial infarction with good recanalization and poor reperfusion: results from a randomized controlled trial. Journal of Cardiovascular Medicine 2011;12(4):239‐48. [DOI] [PubMed] [Google Scholar]
- Colombo A, Piccaluga E, Castellani M, Canzi C, Palatresi S, Pusineri E. Infarct size and myocardial blood flow after CD133(+) stem cells therapy in large myocardial infarction: a randomized controlled trial. American Journal of Cardiology 2007;100(8A Suppl):2L. [Google Scholar]
- NCT00400959. CD133+ autologous cells after myocardial infarction. https://clinicaltrials.gov/ct2/show/NCT00400959 (accessed 11 March 2015).
Gao 2013 {published data only}
- Gao L. A critical challenge: Dosage‐related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. [24th Great Wall International Congress of Cardiology, Asia Pacific Heart Congress 2013; International Congress of Cardiovascular Prevention and Rehabilitation 2013, Beijing, China, 10‐13 October 2013]. Heart 2013;99:A145‐6. [DOI] [PubMed] [Google Scholar]
- Gao LR, Chen Y, Ding QA, Guo F, Li TC, Liu HL, et al. Intracoronary infusion of bone marrow mesenchymal stem cells in acute myocardial infarction ‐ a critical challenge for dosage‐related efficacy and safety. [25th Annual Symposium Transcatheter Cardiovascular Therapeutics, TCT 2013, San Francisco, CA United States, 27 October ‐ 1 November 2013]. Journal of the American College of Cardiology 2013;62(18 Suppl 1):B241. [Google Scholar]
- Gao LR, Pei XT, Ding QA, Chen Y, Zhang NK, Chen HY, et al. A critical challenge: dosage‐related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. International Journal of Cardiology 2013;168(4):3191‐9. [DOI] [PubMed] [Google Scholar]
Ge 2006 {published data only}
- Ge J, Li Y, Qian J, Shi J, Wang Q, Niu Y, et al. Efficacy of emergent transcatheter transplantation of stem cells for treatment of acute myocardial infarction (TCT‐STAMI). Heart 2006;92(12):1764‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grajek 2010 {published data only}
- Grajek S, Popiel M, Breborowicz P, Lesiak M, Araszkiewicz A, Katarzynska A, et al. Can intracoronary infusion of autologous bone marrow stem cells (BMSC) prevent remodeling of left ventricle in patients with AMI? Results of the study with 12 month follow‐up. European Heart Journal 2006;27(Suppl 1):348. [Google Scholar]
- Grajek S, Popiel M, Gil L, Breborowicz P, Lesiak M, Czepczynski R, et al. Influence of bone marrow stem cells on left ventricle perfusion and ejection fraction in patients with acute myocardial infarction of anterior wall: randomized clinical trial: Impact of bone marrow stem cell intracoronary infusion on improvement of microcirculation. European Heart Journal 2010;31(6):691‐702. [DOI] [PubMed] [Google Scholar]
- Grajek S, Popiel M, Sawinski K, Lesiak M, Breborowicz P, Czepczynski R, et al. Improvement of left ventricle function in patients with acute myocardial infarction after infusion of bone marrow stem cells. Advances in Heart Disease: Proceedings of the 12th World Congress on Heart Disease. MEDIMOND, 2005:125‐9.
- Popiel M, Grajek S, Czepczynski R, Oleksa R, Breborozicz P, Czyz A, et al. Improvement of microcirculation after bone marrow stem cells injection in patients with AMI of anterior wall. [9th International Conference of Non‐Invasive Cardiovascular Imaging, Barcelona, Spain, 10‐13 May 2009]. European Heart Journal. Supplement 2009;11:S53‐S54. [Google Scholar]
- Popiel M, Straburzynska‐Migaj E, Breborowicz P, Grajek S, Katarzynska‐Szymanska A, Lesiak M, et al. Exercise capacity, arrhythmic risk profile and pulmonary function is not influenced by bone marrow stem cells IC infusion in patients with acute myocardial infarction. [European Society of Cardiology, ESC Congress 2011, Paris, France, 27‐31 August 2011]. European Heart Journal 2011;32:819. [DOI] [PubMed] [Google Scholar]
- Straburzynska‐Migaj E, Popiel M, Grajek S, Katarzynska‐Szymanska A, Lesiak M, Breborowicz P, et al. Exercise capacity, arrhythmic risk profile, and pulmonary function is not influenced by intracoronary injection of bone marrow stem cells in patients with acute myocardial infarction. International Journal of Cardiology 2012;159(2):134‐8. [DOI] [PubMed] [Google Scholar]
Hirsch 2011 {published data only}
- Afsharzada F, Nijveldt R, Hirsch A, Vleuten PA, Beek AM, Tijssen JGP, et al. Myocardial perfusion after intracoronary infusion of mononuclear cells of bone marrow or peripheral blood after acute myocardial infarction. [American College of Cardiology's 59th Annual Scientific Session and i2 Summit: Innovation in Intervention, Atlanta, GA United States, 14‐16 March 2010]. Journal of American College of Cardiology 2010;55(10 Suppl 1):A110.e1024. [Google Scholar]
- Delewi R, Laan A, Robbers L, Hirsch A, Nijveldt R, Vleuten P, et al. Intracoronary infusion of mononuclear cells compared with standard therapy after acute myocardial infarction: 2 year magnetic resonance imaging results of the randomized controlled HEBE trial. [24th Annual Symposium of the Transcatheter Cardiovascular Therapeutics, TCT 2012, Miami, FL United States, 22‐26 October 2012]. Journal of the American College of Cardiology 2012;60:B149. [Google Scholar]
- Delewi R, Laan AM, Robbers L, Hirsch A, Nijveldt R, Tijssen JGP, et al. Long‐term follow‐up after intracoronary infusion of mononuclear cells from bone marrow or peripheral blood after acute myocardial infarction: 5 year results of the randomized controlled HEBE trial. [European Society of Cardiology, ESC Congress 2014, Barcelona, Spain. 30 August ‐ 3 September 2014]. European Heart Journal 2014;35:364. [Google Scholar]
- Delewi R, Laan AM, Robbers LF, Hirsch A, Nijveldt R, Vleuten PA, et al. Long term outcome after mononuclear bone marrow or peripheral blood cells infusion after myocardial infarction. Heart 2015;101(5):363‐8. [DOI] [PubMed] [Google Scholar]
- Hirsch A, Nijveldt R, Vleuten PA. Multicenter, randomised trial of intracoronary infusion of autologous mononuclear bone marrow cells or peripheral mononuclear blood cells after primary PCI. Netherlands Heart Journal 2005;13(10):377. [Google Scholar]
- Hirsch A, Nijveldt R, Vleuten PA, Biemond BJ, Doevendans PA, Rossum AC, et al. Intracoronary infusion of autologous mononuclear bone marrow cells or peripheral mononuclear blood cells after primary percutaneous coronary intervention: rationale and design of the HEBE trial ‐ a prospective, multicenter, randomized trial. American Heart Journal 2006;152:434‐41. [DOI] [PubMed] [Google Scholar]
- Hirsch A, Nijveldt R, Vleuten PA, Tijssen JGP, Giessen WJ, Tio RA, et al. Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE trial. European Heart Journal 2011;32(14):1736‐47. [DOI] [PubMed] [Google Scholar]
- Hirsch A, Nijveldt R, Vleuten PA, Tio RA, Giessen WJ, Marques KM, et al. Intracoronary infusion of autologous mononuclear bone marrow cells in patients with acute myocardial infarction treated with primary PCI: Pilot study of the multicenter HEBE trial. Catheterization & Cardiovascular Interventions 2008;71(3):273‐81. [DOI] [PubMed] [Google Scholar]
- Robbers LF, Nijveldt R, Beek AM, Hirsch A, Laan AM, Delewi R, et al. Cell therapy in reperfused acute myocardial infarction does not improve the recovery of perfusion in the infarcted myocardium: a cardiac MR imaging study. Radiology 2014;272(1):113‐22. [DOI] [PubMed] [Google Scholar]
- Robbers LF, Nijveldt R, Beek AM, Kemme MJ, Delewi R, Hirsch A, et al. Intracoronary infusion of mononuclear cells after PCI‐treated myocardial infarction and arrhythmogenesis: is it safe?. Netherlands Heart Journal 2012;20(3):133‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbers LFH, Delewi R, Beurden Y, Nijveldt R, Beek AM, Kemme MJB, et al. Intracoronary infusion of mononuclear stem cells after acute revascularized myocardial infarction is not associated with increased prevalence of late ventricular arrhythmias. [European Society of Cardiology, ESC Congress 2011, Paris, France, 27‐31 August 2011]. European Heart Journal 2011;32:735. [Google Scholar]
- Robbers LFHJ, Nijveldt R, Laan AM, Delewi R, Hirsch A, Vleuten PA, et al. Intracoronary infusion of bone marrow cells and peripheral mononuclear blood cells has no influence on the recovery of myocardial perfusion after acute revascularized myocardial infarction. [European Society of Cardiology, ESC Congress 2011, Paris, France, 27‐31 August 2011]. European Heart Journal 2011;32:155. [Google Scholar]
- Laan A, Hirsch A, Nijveldt R, Vleuten PA, Giessen WJ, Doevendans PA, et al. Bone marrow cell therapy after acute myocardial infarction: the HEBE trial in perspective, first results. Netherlands Heart Journal 2008;16(12):436‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan AM, Hirsch A, Haeck JD, Nijveldt R, Delewi R, Biemond BJ, et al. Recovery of microcirculation after intracoronary infusion of bone marrow mononuclear cells or peripheral blood mononuclear cells in patients treated by primary percutaneous coronary intervention the Doppler substudy of the Hebe trial. JACC: Cardiovascular interventions 2011;4(8):913‐20. [DOI] [PubMed] [Google Scholar]
- Laan AM, Vleuten PA, Hirsch A, Nijveldt R, Giessen WJ, Biemond BJ, et al. Intracoronary infusion of mononuclear cells potentially prevents post‐infarct ventricular remodeling in patients with an initial dilated left ventricle: A HEBE substudy. [European Society of Cardiology, ESC Congress 2009, Barcelona, Spain, 29 August ‐ 2 September 2009]. European Heart Journal 2009;30:505. [Google Scholar]
Huang 2006 {published data only}
- Huang RC, Yao K, Zou YZ, Ge L, Qian JY, Yang J, et al. Long term follow‐up of emergent intracoronary autologous bone marrow mononuclear cell transplantation for acute inferior‐wall myocardial infarction [Chinese]. Zhonghua Yi Xue Za Zhi [Journal of the Chinese Medical Association] 2006;86(16):1107‐11. [PubMed] [Google Scholar]
Huang 2007 {published data only}
- Huang RC, Yao K, Qian JY, Niu YH, Ge L, Chen SG, et al. Evaluation of myocardial viability with 201T1/18F‐FDG DISA SPECT technique in patients with acute myocardial infarction after emergent intracoronary autologous bone marrow mononuclear cell transplantation [Chinese]. Zhonghua Xin Xue Guan Bing Za Zhi [Chinese Journal of Cardiovascular Diseases] 2007;35(6):500‐3. [PubMed] [Google Scholar]
Huikuri 2008 {published data only}
- Huikuri HV. Efficacy and safety of intracoronary injection of mononuclear BMC after thrombolytic therapy of acute myocardial infarction: IM‐BMC Study. Clinical Research in Cardiology 2008;97(1):10. [Google Scholar]
- Huikuri HV, Kervinen K, Niemela M, Ylitalo K, Saily M, Koistinen P, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile and restenosis after thrombolytic therapy of acute myocardial infarction. European Heart Journal 2008;29(22):2723‐32. [DOI] [PubMed] [Google Scholar]
- Miettinen J, Ylitalo K, Hedberg P, Saily M, Koistinen P, Airaksinen KEJ, et al. Effects of intracoronary injection of autologous bone marrow derived stem cells on natriuretic peptides and inflammatory markers in patients with acute ST‐elevation myocardial infarction. [European Society of Cardiology, ESC Congress 2010, Stockholm, Sweden, 28 August ‐ 1 September 2010]. European Heart Journal 2010;31:307‐8. [DOI] [PubMed] [Google Scholar]
- Miettinen JA, Ylitalo K, Hedberg P, Jokelainen J, Kervinen K, Niemela M, et al. Determinants of functional recovery after myocardial infarction of patients treated with bone marrow‐derived stem cells after thrombolytic therapy. Heart 2010;96(5):362‐67. [DOI] [PubMed] [Google Scholar]
- Miettinen JA, Ylitalo K, Hedberg P, Kervinen K, Niemela M, Saily M, et al. Effects of intracoronary injection of autologous bone marrow‐derived stem cells on natriuretic peptides and inflammatory markers in patients with acute ST‐elevation myocardial infarction. Clinical Research in Cardiology 2011;100(4):317‐25. [DOI] [PubMed] [Google Scholar]
- NCT00363324. Bone marrow cells in myocardial infarction. https://clinicaltrials.gov/ct2/show/NCT00363324 (accessed 11 March 2015).
- Perkiomaki J, Luokkala J, Makikallio T, Ylitalo K, Huikuri H. Electrocardiographic risk markers in relation to intracoronary bone marrow cell therapy for acute myocardial infarction. [Venice Arrhythmias 2011, Venice, Italy, 9‐12 October 2011]. Journal of Cardiovascular Electrophysiology 2011;22:S66. [Google Scholar]
Janssens 2006 {published data only}
- Herbots L, D'hooge J, Eroglu E, Thijs D, Ganame J, Claus P, et al. Improved regional function after autologous bone marrow‐derived stem cell transfer in patients with acute myocardial infarction: a randomized, double‐blind strain rate imaging study. European Heart Journal 2009;30(6):662‐70. [DOI] [PubMed] [Google Scholar]
- Janssens S, Bogaert J, Dubois C, Cllemput P, Theunissen K, Kalantzis M, et al. Long‐term safety and efficacy of autologous bone marrow‐derived stem cell transfer after acute myocardial infarction. Circulation 2006;114(18 Suppl):II_515. [Google Scholar]
- Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow‐derived stem‐cell transfer in patients with ST‐segment elevation myocardial infarction: double‐blind, randomised controlled trial. Lancet 2006;367(9505):113‐21. [DOI] [PubMed] [Google Scholar]
- NCT00264316. Bone marrow‐derived stem cell transfer in acute myocardial infarctions. https://clinicaltrials.gov/ct2/show/NCT00264316 (accessed 11 March 2015).
- Ruwende C. Autologous bone marrow derived stem cell transfer in patients with ST‐segment elevation myocardial infarction: double blind randomised controlled trial. ACC Cardiosource Review Journal 2006;15(3):28. [DOI] [PubMed] [Google Scholar]
Jazi 2012 {published data only}
- Jazi SM, Esfahani MH, Fesharaki M, Moulavi F, Gharipour M. Initial clinical outcomes of intracoronary infusion of autologous progenitor cells in patients with acute myocardial infarction. ARYA Atherosclerosis 2012;7(4):162‐7. [PMC free article] [PubMed] [Google Scholar]
Jin 2008 {published data only}
- Jin B, Yang YG, Shi HM, Luo XP, Li Y, Sun YL, et al. Autologous intracoronary mononuclear bone marrow cell transplantation for acute anterior myocardial infarction: Outcomes after 12‐month follow‐up. Journal of Clinical Rehabilitative Tissue Engineering Research 2008;12(12):2267‐71. [Google Scholar]
Karpov 2005 {published data only}
- Karpov RS, Popov SV, Markov VA, Suslova TE, Ryabov VV, Yu S, et al. Autologous mononuclear bone marrow cells during reparative regeneration after acute myocardial infarction. Bulletin of Experimental Biology and Medicine 2005;1(4):640‐3. [DOI] [PubMed] [Google Scholar]
- Ryabov V, Kirgizova M, Suslova T, Markov V, Karpov R. Long‐term results of autologous bone marrow mononuclear cell transplantation in STEMI patients. [Heart Failure Congress 2014 and the 1st World Congress on Acute Heart Failure, Athens, Greece, 17‐20 May 2014]. European Journal of Heart Failure 2014;16:352. [Google Scholar]
- Ryabov VV, Alexandrovna KM. Long‐term results after transplantation of autologous bone marrow mononuclear cells to patients with acute myocardial infarction. Koncept 2014;20:4786‐90. [Google Scholar]
- Ryabov VV, Suslova TE, Krylov AL, Poponina YS, Vesnina ZV, Sazonove Sl, et al. Cardiomyoplasty with autological mononuclear cells of the bone marrow in patients with acute myocardial infarction. Terapevticheskii Arkhiv 2006;78(8):47‐52. [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee JW, Ahn SG, Kim JY, Yoo BS, Lee SH, Yoon J, et al. A randomized, open‐labeled, multicenter trial for safety and efficacy of intracoronary adult human mesenchymal stem cells after acute myocardial infarction. [60th Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, ACC.11, New Orleans, LA United States, 2‐5 April 2011]. Journal of the American College of Cardiology 2011;57(14 Suppl 1):E982. [Google Scholar]
- Lee JW, Lee SH, Youn YJ, Ahn MS, Kim JY, Yoo BS, et al. A randomized, open‐label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. Journal of Korean Medical Science 2014;29(1):23‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01392105. Safety and efficacy of intracoronary adult human mesenchymal stem cells after acute myocardial infarction (SEED‐MSC). https://clinicaltrials.gov/ct2/show/NCT01392105 (accessed 11 March 2015).
Lunde 2006 {published data only}
- Beitnes JO, Gjesdal O, Lunde K, Solheim S, Edvardsen T, Arnesen H, et al. Left ventricular systolic and diastolic function improve after acute myocardial infarction treated with acute percutaneous coronary intervention, but are not influenced by intracoronary injection of autologous mononuclear bone marrow cells: a 3‐year serial echocardiographic sub‐study of the randomized‐controlled ASTAMI study. European Journal of Echocardiography 2011;12(2):98‐106. [DOI] [PubMed] [Google Scholar]
- Beitnes JO, Hopp E, Lunde K, Solheim S, Arnesen H, Brinchmann JE, et al. Long‐term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: the ASTAMI randomised, controlled study. Heart 2009;95(24):1983‐9. [DOI] [PubMed] [Google Scholar]
- Beitnes JO, Hopp E, Lunde K, Solheim S, Arnesen H, Forfang K, et al. Long term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: The ASTAMI study. [European Society of Cardiology, ESC Congress 2009, Barcelona, Spain, 29 August ‐ 2 September 2009]. European Heart Journal 2009;30:912. [DOI] [PubMed] [Google Scholar]
- Beitnes JO, Lunde K, Gjesdal O, Solheim S, Edvardsen Y, Arnesen H, et al. Regional and diastolic function improves after acute myocardial infarction treated with acute PCI, but is not influenced by injection of autologous mononuclear bone marrow cells: an ASTAMI sub‐study. [European Society of Cardiology, ESC Congress 2010, Stockholm, Sweden, 28 August ‐ 1 September 2010]. European Heart Journal 2010;31:323. [Google Scholar]
- Furenes EB, Opstad TB, Solheim S, Lunde K, Arnesen H, Seljeflot I. The influence of autologous bone marrow stem cell transplantation on matric metalloproteinases in patients treated for acute ST‐elevation myocardial infarction. Mediators of Inflammation 2014;2014:285901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogaard HK, Seljeflot I, Lunde K, Solheim S, Aakhus S, Forfang K, et al. Cell treatment after acute myocardial infarction prevents early decline in circulating IGF‐1. Scandinavian Cardiovascular Journal 2010;44(5):267‐72. [DOI] [PubMed] [Google Scholar]
- Hopp E, Lunder K, Solheim S, Aakhus S, Arnesen H, Forfang K, et al. Regional myocardial function after intracoronary bone marrow cell injection in reperfused anterior wall infarction ‐ a cardiovascular magnetic resonance tagging study. Journal of Cardiovascular Magnetic Resonance 2011;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde K, Aakhus S. Intracoronary injection of mononuclear bone marrow cells after acute myocardial infarction: lessons from the ASTAMI trial. European Heart Journal Supplements 2018;10:K35‐8. [Google Scholar]
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Forfang K. Autologous stem cell transplantation in acute myocardial infarction: the ASTAMI randomized controlled trial. Intracoronary transplantation of autologous mononuclear bone marrow cells, study design and safety aspects. Scandinavian Cardiovascular Journal 2005;39:150‐8. [DOI] [PubMed] [Google Scholar]
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Forfang K, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. New England Journal of Medicine 2006;355(12):1199‐209. [DOI] [PubMed] [Google Scholar]
- Lunde K, Solheim S, Aakhus S, Arnesen H, Moum T, Abdelnoor M, et al. Exercise capacity and quality of life after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: results from the Autologous Stem cell Transplantation in Acute Myocardial Infarction (ASTAMI) randomized controlled trial. American Heart Journal 2007;154(4):710 e1‐8. [DOI] [PubMed] [Google Scholar]
- Lunde K, Solheim S, Forfang K, Arnesen H, Brinch L, Bjornerheim R, et al. Anterior myocardial infarction with acute percutaneous coronary intervention and intracoronary injection of autologous mononuclear bone marrow cells: safety. clinical outcome, and serial changes in left ventricular function during 12‐months' follow‐up. Journal of the American College of Cardiology 2008;51(6):674‐6. [DOI] [PubMed] [Google Scholar]
- NCT00199823. Autologous stem cell transplantation in acute myocardial infarction. https://clinicaltrials.gov/ct2/show/NCT00199823 (accessed 11 March 2015).
- Solheim S, Seljeflot I, Lunde K, Bratseth V, Aakhus S, Forfang K, et al. The influence of intracoronary injection of bone marrow cells on prothrombotic markers in patients with acute myocardial infarction. Thrombosis Research 2012;130(5):765‐8. [DOI] [PubMed] [Google Scholar]
Meluzin 2008 {published data only}
- Kaminek M, Meluzin J, Panovsky R, Janousek S, Mayer J, Prasek J, et al. Individual differences in the effectiveness of intracoronary bone marrow cell transplantation assessed by gatted sestamibi SPECT/FDG PET imaging. Journal of Nuclear Cardiology 2008;15(3):392‐9. [DOI] [PubMed] [Google Scholar]
- Kaminek M, Meluzin J, Panovsky R, Metelkova I, Budikova M, Richter M. Long‐term results of intracoronary bone marrow cell transplantation: the potential of gated SPECT/FDG PET imaging to select patients with maximum benefit from cell therapy. Clinical Nuclear Medicine 2010;35(10):780‐7. [DOI] [PubMed] [Google Scholar]
- Klabusay M, Navratil M, Koristek Z, Groch L, Meluzin J, Mayer J. Implantation of autologous bone marrow mononuclear cells in patients after acute myocardial infarction via coronary artery. Blood 2005;106(11):Abstract 4220. [Google Scholar]
- Meluzin J, Janousek S, Mayer J, Groch L, Hornacek I, Hlinomaz O, et al. Three‐, 6‐ and 12‐month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. International Journal of Cardiology 2008;128(2):185‐92. [DOI] [PubMed] [Google Scholar]
- Meluzin J, Mayer J, Groch L, Kanousek S, Hornacek I, Hinomaz O, et al. Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: the effect of the dose of transplanted cells on myocardial function. American Heart Journal 2006;152(5):975.e9‐15. [DOI] [PubMed] [Google Scholar]
- Panovsky R, Meluzin J, Janousek S, Mayer J, Kaminek M, Groch L, et al. Cell therapy in patients with left ventricular dysfunction due to myocardial infarction. Echocardiography 2008;25(8):888‐97. [DOI] [PubMed] [Google Scholar]
Nogueira 2009 {published data only}
- Dohmann HF, Silva SA, Sousa AL, Graga AM, Branco RV, Haddad AF, et al. Multicenter double blind trial of autologous bone marrow mononuclear cell transplantation through intracoronary injection post acute myocardium infarction‐ MiHeart/AMI study. Trials 2008;9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira RC, Haddad AF, Silva SA, Souza AL, Tuche FA, Oliveira MA, et al. Intracoronary stem‐cell injection after myocardial infarction: microcirculation sub‐study. Arquivos Brasileiros de Cardiologia 2011;97(5):420‐6. [DOI] [PubMed] [Google Scholar]
- NCT00350766. Cell therapy in myocardial infarction (EMRTCC). https://clinicaltrials.gov/ct2/show/NCT00350766 (accessed 11 March 2015).
- Nogueira FB, Silva SA, Haddad AF, Peixoto CM, Carvalho RM, Tuche FA, et al. Systolic function of patients with myocardial infarction undergoing autologous bone marrow transplantation. Arquivos Brasileiros de Cardiologia 2009;93(4):374‐9. [DOI] [PubMed] [Google Scholar]
- Silva SA, Sousa Al, Haddad AF, Azevedo JC, Soares VE, Peixoto CM, et al. Autologous bone marrow mononuclear cell transplantation after acute myocardial infarction: comparison of two delivery techniques. Cell Transplantation 2009;18(3):343‐52. [DOI] [PubMed] [Google Scholar]
Penicka 2007 {published data only}
- Horak J, Penicka M, Kobylka P, Ascheremann M, Linhart A, Skalicka H, et al. Effectivity and safety of intracoronary autologous bone marrow stem cell transplantation in large anterior myocardial infarction ‐ a randomized study. Journal of the American College of Cardiology 2007;49(9 (Suppl A)):201A. [DOI] [PubMed] [Google Scholar]
- Penicka M. Intracoronary stem cells in large myocardial infarction. www.ClinicalTrials.gov. 2006 (accessed by 31 January 2011). [NCT00389545]
- Penicka M, Horak J, Kobylka P, Pytlik R, Kozak T, Belohlavek O, et al. Intracoronary injection of autologous bone marrow‐derived mononuclear cells in patients with large anterior acute myocardial infarction. A prematurely terminated randomised study. Journal of the American College of Cardiology 2007;49(24):2373‐4. [DOI] [PubMed] [Google Scholar]
- Skalicka H, Horak J, Kobylka P, Palecek T, Linhart A, Aschermann M. Intracoronary injection of autologous bone marrow‐derived mononuclear cells in patients with large anterior acute myocardial infarction and left ventricular dysfunction: a 24‐month follow up study. Bratislavske Lekarske Listy 2012;113(4):220‐7. [DOI] [PubMed] [Google Scholar]
Piepoli 2010 {published data only}
- Malagoli A, Piepoli M, Armentano C, Vallisa D, Arbasi M, Rossi A, et al. Acute myocardial infarction: long term echocardiographic remodeling after bone marrow cell transplantation. [ESC Congress 2010, Stockholm, Sweden. 28 August ‐ 1 September 2010]. European Heart Journal 2010;31:735. [Google Scholar]
- Malagoli A, Piepoli MF, Armentano C, Vallisa D, Arbasi MC, Rossi A, et al. Bone marrow cell transplantation in patients after acute myocardial infarction and left ventricular dysfunction: long term effects on heart function and remodeling. [Heart Failure 2010 Congress, Berlin, Germany. 29 May ‐ 1 June 2010]. European Journal of Heart Failure. 2010; Vol. 9:S97‐8.
- NCT00437710. Safety and efficacy of bone marrow cell transplantation in humans myocardial infarction (CARDIAC). https://clinicaltrials.gov/ct2/show/NCT00437710 (accessed 11 March 2015).
- Piepoli MF, Vallisa D, Arbasi C, Cavanna L, Cerri L, Mori M, Passerini F, et al. 2 year follow‐up results of the CARDIAC (CARDIomyoplasty by Autologous intraCoronary bone marrow in acute myocardial infarction) randomised controlled trial. International Journal of Cardiology 2013;168(5):e132. [DOI] [PubMed] [Google Scholar]
- Piepoli MF, Vallisa D, Arbasi M, Cavanna L, Cerri L, Mori M, et al. Bone marrow cell transplantation improves cardiac, autonomic and functional indexes in acute anterior myocardial infarction patients (Cardiac study). European Journal of Heart Failure 2010;12(2):172‐80. [DOI] [PubMed] [Google Scholar]
- Vallisa D, Piepoli M, Cavanna L, Arbasi C, Moroni C, Lazzaro A, et al. Autologous bone marrow stem cell support in acute anterior myocardial infarction. [49th Annual Meeting of the American Society of Hematology, Atlanta, Georgia. 8‐11 December 2007]. Blood 2007;110(11):1078A‐9A. [Google Scholar]
Plewka 2009 {published data only}
- Lipiec P, Kreminska‐Paluka M, Plewka M, Kusmierek J, Plachcinska A, Szuminski R, et al. Impact of intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction on left ventricular perfusion and function: a 6‐month follow‐up gated 99mTc‐MiBi single‐photon emission computed tomography study. European Journal of Nuclear Medicine and Molecular Imaging 2009;36(4):587‐93. [DOI] [PubMed] [Google Scholar]
- Plewka M, Krzeminska‐Pakula M, Lipiec P, Peruga JZ, Jezewski T, Kidawa M, et al. Clinical 2‐years outcome after intracoronary injection of mononuclear bone marrow stem cells in patients with acute myocardial infarction. [European Society of Cardiology, ESC Congress 2010, Stockholm, Sweden. 28 August ‐ 1 September 2010]. European Heart Journal 2010;31:1045‐6. [Google Scholar]
- Plewka M, Krzeminska‐Pakula M, Lipiec P, Peruga JZ, KJezewski T, Kidawa M, et al. Effect of intracoronary injection of mononuclear bone marrow stem cels on left ventricular function in patients with acute myocardial infarction. American Journal of Cardiology 2009;104(10):1336‐42. [DOI] [PubMed] [Google Scholar]
- Plewka M, Krzeminska‐Pakula M, Peruga JZ, Lipiec P, Kurpesa M, Wierzbowska‐Drabik K, et al. The effects of intracoronary delivery of mononuclear bone marrow cells in patients with myocardial infarction: a two year follow‐up results. Kardiologia Polska 2011;69(12):1234‐40. [PubMed] [Google Scholar]
Quyyumi 2011 {published data only}
- Bhatti S, Hakeem A, Taylor M, Chung E, Quyyumi AA, Oshinski J, et al. MRI strain analysis as a novel modality for the assessment of myocardial function following stem cell therapy ‐ results from Amorcyte trial. [SCMR/Euro CMR Joint Scientific Sessions, Nice, France. 2‐6 February 2011]. Journal of Cardiovascular Magnetic Resonance 2011;13:2011. [Google Scholar]
- NCT00313339. Intra‐coronary infusion of bone marrow derived autologous CD34+ selected cells in patients with acute myocardial infarction (AMR‐1). http://clinicaltrials.gov/ct2/show/NCT00313339 (accessed 11 March 2015). [NCT00313339]
- Quyyumi AA, Murrow JR, Esteves F, Galt J, Oshinski J, Lerakis S, et al. CD34+ infusion after ST elevation myocardial infarction is associated with improved perfusion. Journal of American College of Cardiology 2009;53(10):A327. [DOI] [PubMed] [Google Scholar]
- Quyyumi AA, Waller EK, Murrow J, Esteves F, Galt J, Oshinski J, et al. CD34+ cell infusion after ST‐elevation myocardial infarction is associated with improved perfusion and is dose dependent. American Heart Journal 2011;161(1):98‐105. [DOI] [PubMed] [Google Scholar]
Roncalli 2010 {published data only}
- Lamirault G, Bock E, Roncalli JH, Belle E, Piot C, Corvoisier P, et al. Sustained quality of life improvement after intracoronary injection of autologous bone marrow cells in the setting of acute myocardial infarction. [European Society of Cardiology, ESC Congress 2013, Amsterdam, Netherlands. 31 August ‐ 4 September 2013]. European Heart Journal 2013;34:277‐8. [Google Scholar]
- Tourneau T, Sportouch C, Foucher C, Delasalle B, Rosso J, Neuder Y, et al. Left ventricular morphological and mechanical remodeling after acute myocardial infarction in the BONAMI trial: An isotopic and echocardiographic study. [16th Annual Meeting of the European Association of Echocardiography, EUROECHO 2012, Athens, Greece. 5‐8 December 2012]. European Heart Journal Cardiovascular Imaging 2012;13:i78‐9. [Google Scholar]
- Mouquet F, Roncalli J, Piot C, Trochu JN, Corvoisier P, Neuder V, et al. Autologous bone marrow mononucleated cell infusion for acute myocardial infarction in patients with severe left ventricular dysfunction: Results of the BONAMI Trials (a multicenter randomized controlled trial). Circulation 2008;118(Suppl 18):S764. [Google Scholar]
- NCT00200707. BONAMI (BOne Marrow in Acute Myocardial Infarction). http://clinicaltrials.gov/ct2/show/NCT00200707 (accessed 11 March 2015). [NCT00200707]
- Roncalli J, Mouquet F, Piot C, Trochu JN, Corvoisier P, Neuder Y, et al. Intracoronary autologous mononucleated bone marrow cell infusion for acute myocardial infarction: results of the randomized multicenter BONAMI trial. European Heart Journal 2011;32(14):1748‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruan 2005 {published data only}
- Ruan W, Pan CZ, Huang GQ, Li YL, Ge JB, Shu XH, et al. Assessment of left ventricular segmental function after autologous bone marrow stem cells transplantation in patients with acute myocardial infarction by tissue tracking and strain imaging. Chinese Medical Journal 2005;118(14):1175‐81. [PubMed] [Google Scholar]
Schachinger 2006 {published data only}
- Assmus B, Leistner DM, Schachinger V, Erbs S, Elsasser A, Haberbosch W, et al. Long‐term clinical outcome after intracoronary application of bone marrow‐derived mononuclear cells for acute myocardial infarction: migratory capacity of administered cells determines event‐free survival. European Heart Journal 2014;35(19):1275‐83. [DOI] [PubMed] [Google Scholar]
- Assmus B, Rolf A, Erbs A, Elsasser A, Haberbosch W, Hambrecht R, et al. Clinical outcome 2 years after intracoronary administration of bone marrow‐derived progenitor cells in acute myocardial infarction. Circulation: Heart Failure 2010;3(1):89‐96. [DOI] [PubMed] [Google Scholar]
- Assmus B, Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, et al. Cell functionality of administered autologous bone marrow‐derived mononuclear cells predict 5‐year clinical outcome in patients with acute myocardial infarction ‐ Results of the REPAIR‐AMI trial. [American Heart Association 2012 Scientific Sessions and Resuscitation Science Symposium, Los Angeles, CA United States. 3‐6 November 2012]. Circulation 2012;126(21 (Suppl 1)). [Google Scholar]
- Assmus B, Tonn T, Seeger FH, Yoo C‐H, Leistner D, Klotsche J, et al. Red blood cell contamination of the final cell product impairs the efficacy of autologous bone marrow mononuclear cell therapy. Journal of American College of Cardiology 2010;55(13):1385‐94. [DOI] [PubMed] [Google Scholar]
- Dill T, Schachinger V, Rolf A, Mollmann S, Thiele H, Tillmanns H, et al. Intracoronary administration of bone marrow‐derived progenitor cells improves left ventricular function in patients at risk of adverse remodelling after acute ST‐segment elevation myocardial infarction: Results of the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction study (REPAIR‐AMI) cardiac magnetic resonance imaging study. American Heart Journal 2009;157(3):541‐7. [DOI] [PubMed] [Google Scholar]
- Erbs S, Linke A, Assmus B, Hoffmann C, Schachinger V, Schuler G, et al. Restoration of coronary microvascular function in the infarct‐related artery by bone marrow‐derived progenitor cells after acute MI: Results from the double‐blind, placebo‐controlled REPAIR‐AMI trial. European Heart Journal 2006;27(Suppl 1):151. Meeting Abstract 923. [Google Scholar]
- Erbs S, Linke A, Assmus B, Hoffmann C, Schachinger V, Tonn T, et al. Normalization of coronary microvascular function in the infarct artery by bone marrow‐derived progenitor cells after acute myocardial infarction: results from the double‐blind, placebo‐controlled REPAIR‐AMI trial. Circulation 2006;114(18 Suppl):856‐7. [Google Scholar]
- Erbs S, Linke A, Schachinger V, Assmus B, Thiele H, Diederich KW, et al. Restoration of microvascular function in the infarct‐related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR‐AMI) trial. Circulation 2007;116(4):366‐74. [DOI] [PubMed] [Google Scholar]
- Konorza TFM. Repair AMI late: a prospective multicenter randomised placebo‐controlled trial of autologous bone marrow‐derived progenitor cells in patients with acute myocardial infarction. Herz 2007;32(2):169. [Google Scholar]
- Leistner D, Assmus B, Erbs S, Hambrecht R, Thiele H, Elsaesser A, et al. Intracoronary infusion of bone marrow‐derived mononuclear cells in acute myocardial infarction: 5 year clinical outcome and MRI data of the randomized, double‐blind, placebo‐controlled REPAIR‐AMI trial. [American Heart Association's Scientific Sessions 2011, Orlando, FL United States. 12‐16 November 2011]. Circulation 2011;124(21 (Suppl 1)). [Google Scholar]
- NCT00279175. REPAIR‐AMI: intracoronary progenitor cells in acute myocardial infarction (AMI). https://clinicaltrials.gov/ct2/show/NCT00279175 (accessed 11 March 2015).
- Rolf A, Assmus B, Schachinger V, Rixe J, Mollmann S, Mollmann H, et al. Maladaptive hypertrophy after acute myocardial infarction positive effect of bone marrow‐derived stem cell therapy on regional remodeling measured by cardiac MRI. Clinical Research in Cardiology 2011;100(11):983‐92. [DOI] [PubMed] [Google Scholar]
- Rolf A, Dill T, Moellmann S, Conradi G, Schaechinger V, Zeiher A, et al. One year follow‐up of left ventricular function and remodelling after intracoronary infusion of bone marrow‐derived progenitor cells after acute STEMI: MRI substudy of the double‐blind, randomized, placebo‐controlled multicenter REPAIR‐AMI trial. Journal of the American College of Cardiology 2007;49(9 Suppl B):22B. [Google Scholar]
- Rolf A, Mollmann S, Rixe J, Conradi G, Schachinger V, Dimmeler S, et al. Positive effect of bone marrow progenitor cell transfer on regional post infarction ventricular remodelling by magnetic resonance imaging. Insights from the REPAIR AMI trial [Circulation]. 2007 116;16 Suppl S(772):Abstract 3419. [Google Scholar]
- Schachinger V, Assmus B, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, et al. Intracoronary infusion of bone marrow‐derived mononuclear cells abrogates adverse left ventricular remodelling post‐acute myocardial infarction: insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR‐AMI) trial. European Journal of Heart Failure 2009;11(10):973‐9. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Improved clinical outcome after intracoronary administration of bone‐marrow‐derived progenitor cells in acute myocardial infarction: final 1‐year results of the REPAIR‐AMI trial. European Heart Journal 2006;27(23):2775‐83. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Intracoronary bone marrow‐derived progenitor cells in acute myocardial infarction. New England Journal of Medicine 2006;355(12):1210‐21. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Predictors of contractile recovery of left ventricular function after intracoronary infusion of bone marrow‐derived progenitor cells in patients with acute myocardial infarction: lessons from the REPAIR‐AMI trial. Circulation 2006;114(18 Suppl):788. [Google Scholar]
- Schachinger V, Tonn T, Dimmeler S, Zeiher AM. Bone‐marrow‐derived progenitor cell therapy in need of proof of concept: design of the REPAIR‐AMI trial. Nature Clinical Practice Cardiovascular Medicine 2006;3(Suppl 1):S23‐8. [DOI] [PubMed] [Google Scholar]
Suarez de Lezo 2007 {published data only}
- Suarez de Lezo J, Herrera C, Pan M, Romero M, Pavlovic D, Segura J. Regenerative therapy in patients with a revascularised acute anterior myocardial infarction and depressed ventricular function. Revista Espanola De Cardiologia 2007;60(4):357‐65. [PubMed] [Google Scholar]
Sürder 2013 {published data only}
- NCT00355186. SWiss Multicenter Intracoronary Stem Cells Study in Acute Myocardial Infarction (SWISS‐AMI). https://clinicaltrials.gov/ct2/show/NCT00355186 (accessed 11 March 2015).
- Sürder D, Manka R, Cicero V, Moccetti T, Rufibach K, Soncin S, et al. Intracoronary injection of bone marrow‐derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation 2013;127(19):1968‐79. [DOI] [PubMed] [Google Scholar]
- Sürder D, Manka R, Moccetti T, Rufibach K, Astori G, Cicero VL, et al. Results of the Swiss multicentre intracoronary stem cells study in acute myocardial infarction (Swiss AMI) trial. [American Heart Association 2012 Scientific Sessions and Rescitation Science Symposium, Los Angeles, CA United States, 3‐6 November 2012]. Circulation 2012;126(23):2783. [Google Scholar]
- Sürder D, Manka R, Turchetto L, Moccetti T, Erne P, Zuber M, et al. Intracoronary injection of bone marrow derived mononuclear cells, early or late after acute myocardial infarction: long‐term effects on global left ventricular function ‐ twelve months MRI and long‐term clinical results of the SWISS‐AMI trial. [26th Annual Symposium Transcatheter Cardiovascular Therapeutics, TCT2014, Washington, DC United States. 13‐17 September 2014]. Journal of the American College of Cardiology 2014;64(11 Suppl 1):B45. [Google Scholar]
- Sürder D, Moccetti T, Astori G, Soldati G, Schwitter J, Erne P, et al. From SWISS‐AMI to CARDIASTIM ‐ translation of a clinical progenitor‐cell based protocol into GMP. [European Society of Cardiology, ESC Congress 2009, Barcelona, Spain, 29 August ‐ 2 September 2009]. European Heart Journal 2009;30:503. [Google Scholar]
- Sürder D, Schwitter J, Moccetti T, Astori G, Rufibach K, Plein S, et al. Cell‐based therapy for myocardial repair in patients with acute myocardial infarction: Rationale and study design of the SWiss multicenter Intracoronary Stem cells Study in Acute Myocardial Infraction (SWISS‐AMI). American Heart Journal 2010;160(1):58‐64. [DOI] [PubMed] [Google Scholar]
Tendera 2009 {published data only}
- Bavry AA. Myocardial regeneration by intracoronary infusion of selected population of stem cells in acute myocardial infarction (REGENT). ACC Cardiosource Review Journal 2008;17(10):30. [Google Scholar]
- Chojnowska L, Kepka C, Demkow M, Chmielak Z, Kukula K, Dabrowski M, et al. Intracoronary administration of mononuclear bone marrow cells in acute STEMI: long‐term follow‐up. European Heart Journal 2007;28(Suppl 1):443. [Google Scholar]
- Kazmierski M, Wojakowski W, Michalewska‐Wludarczyk A, Ciosek J, Rychlik W, et al. Improvement of flow‐mediated dilatation in patients with acute myocardial infarction treated with intracoronary infusion of CD34+CXCR4+ cells. [European Society of Cardiology, ESC Congress 2009, Barcelona, Spain. 29 August ‐ 2 September 2009]. European Heart Journal 2009;30:502. [Google Scholar]
- NCT00316381. Stem cell therapy to improve myocardial function in patients with acute myocardial infarction. https://clinicaltrials.gov/ct2/show/NCT00316381 (accessed 11 March 2015).
- Tendera M. Myocardial regeneration by intracoronary infusion of selected population of stem cells in acute myocardial infarction (REGENT) randomized multicenter trial. Clinical Research in Cardiology 2008;97(12):853. [Google Scholar]
- Tendera M, Wojakowski W and REGENT Investigators. Intracoronary infusion of bone marrow‐derived selected CD34+CXCR4+ cells and non‐selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction. 5‐year follow‐up of randomized, multicenter myocardial regeneration by intracoronary infusion of selected population of stem cells in acute myocardial infarction (REGENT) trial. Circulation 2013;128(22 Suppl):Meeting Abstract: 17138. [DOI] [PubMed] [Google Scholar]
- Tendera M, Wojakowski W, Ruzylllo W, Chojnowska L, Kepka C, Tracz W, et al. Intracoronary infusion of bone marrow‐derived selected CD34+CXCR4+ cells and non‐selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration Infarction (REGENT) Trial. European Heart Journal 2009;30(11):1313‐21. [DOI] [PubMed] [Google Scholar]
- Wojakowski W. Intracoronary infusion of bone marrow‐derived selected CD34+CXCR4+ cells and non‐selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: Extended 6‐year follow‐up of randomized multicenter REGENT trial. [19th Cardiovascular Summit: Transcatheter Cardivascular Therapiutics Asia Pacific, TCTAP 2014, Coex, Seoul, South Korea. 22‐25 April 2014]. Journal of the American College of Cardiology 2014;63(12 Suppl 2):S44. [Google Scholar]
- Wojakowski W, Kazmierski M, Kucia M, Zuba‐Surma E, Buszman P, Ochala A, et al. Number of circulating progenitor cells is a predictor of left ventricular ejection fraction improvement in patients with acute myocardial infarction treated with intracoronary infusion of bone marrow‐derived CD34+ CXCR4+ cells. [15th Annual Interventional Vascular Therapeutics Angioplasty Summit ‐ Transcatheter Cardiovascular Therapeutics Asia Pacific Symposium, TCTAP 2010, Seoul, South Korea. 28‐30 April 2010]. American Journal of Cardiology 2010;105(9 Suppl 1):34B. [Google Scholar]
- Wojakowski W, Kucia M, Ochala Am Biszman P, Krol M, Ryderka R, Maslankiewicz K, et al. Mobilization of CXCR4+ stem cells in acute myocardial infarction is correlated with left ventricular ejection fraction and myocardial perfusion assessed by MRI in 1 year follow‐up (REGENT trial). American Journal of Cardiology 2006;98(8A Suppl):46M. [Google Scholar]
- Wojakowski W, Kucia M, Wyderka R, Maslankiewicz K, Zebzda A, Ochala A, et al. Mobilization of CXCR4+ stem cells in acute myocardial infarction is correlated with left ventricular ejection fraction and myocardial perfusion assessed by MRI in 1 year follow‐up (REGENT trial). Circulation 2006;114(18 Suppl):669. [Google Scholar]
- Wojakowski W, Michalowska A, Majka M, Kucia M, Maslankievvicz K, Wyderka R, et al. The mobilization of tissue‐committed (CD34(+),CD117(+),CXCR4(+), C‐Met(+)) stem cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in acute myocardial infarction: REGENT study. Circulation 2004;110(17 Suppl):238‐9. Meeting Abstract 1143. [DOI] [PubMed] [Google Scholar]
Traverse 2010 {published data only}
- NCT00268307. Bone marrow stem cell infusion following a heart attack. http://clinicaltrials.gov/ct2/show/NCT00268307 (accessed 11 March 2015). [NCT00268307]
- Traverse JH, McKenna DH, Harvey K, Jorgenso BC, Olson RE, Brostom N, et al. Results of a phase 1, randomized, double‐blind, placebo‐controlled trial of bone marrow mononuclear stem cell administration in patients following ST‐elevation myocardial infarction. American Heart Journal 2010;160(3):428‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Traverse 2011 {published data only}
- Bhatnagar A, Johnston BH, Traverse JH, Henry TD, Pepine CJ, Willerson JT, et al. Patient and cell characteristics associated with clinical outcomes in the CCTRN LateTime trial. [American Heart Association's Scientific Sessions and Resuscitation Science Symposium, Chicago, IL United States. 15‐18 November 2014]. Circulation 2014;130. [Google Scholar]
- Henry TD, Traverse JH, Pepine CJ, Willerson JT, Ellis S, Zhao DX, et al. Results from lateTIME: a randomized, placebo controlled trial of intracoronary stem cell delivery two to three weeks following acute myocardial infarction from the cardiovascular cell therapy research network. [25th Annual Symposium Transcatheter Cardiovascular Therapeutics, TCT 2013, San Francisco, CA United States, 27 October ‐ 1 November 2013]. Journal of the American College of Cardiology 2013;62(18 Suppl 1):B249. [Google Scholar]
- NCT00684060. Use of adult autologous stem cells in treating people 2 to 3 weeks after having a heart attack (The Late TIME Study). https://clinicaltrials.gov/ct2/show/NCT00684060 (accessed 11 March 2015).
- Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA 2011;306(19):2110‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, et al. Results from LateTIME: a randomized, placebo controlled trial of intracoronary stem cell delivery two to three weeks following acute myocardial infarction. [American Heart Association's Scientific Sessions 2011, Orlando, FL United States, 12‐16 November 2011]. Circulation 2011;124(21):2372. [Google Scholar]
- Traverse JH, Henry TD, Vaughan DE, Ellis SG, Pepine CJ, Willerson JT, et al. LateTIME: a phase‐II, randomized, double‐blinded, placebo‐controlled, pilot trial evaluating the safety and effect of administration of bone marrow mononuclear cells 2 to 3 weeks after acute myocardial infarction. Texas Heart Institute Journal 2010;37(4):412‐20. [PMC free article] [PubMed] [Google Scholar]
Traverse 2012 {published data only}
- NCT00684021. Use of adult autologous stem cells in treating people who have had a heart attack (The TIME Study). https://clinicaltrials.gov/ct2/show/NCT00684021 (accessed 11 March 2015).
- Schutt RC, Trachtenberg BH, Cooke JP, Traverse JH, Henry TD, Pepine CJ, et al. Bone marrow characteristics associated with changes in infarct size after STEMI: a biorepository evaluation from the CCTRN TIME trial. Circulation Research 2015;116(1):99‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse JH, Henry TD, Pepine CJ, Willerson JT, Ellis SG. One‐year follow‐up of intracoronary stem cell delivery on left ventricular function following ST‐elevation myocardial infarction. JAMA 2014;311(3):301‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. JAMA ‐ Journal of the American Medical Association 2012;308(22):2380‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial [Erratum]. JAMA ‐ Journal of the American Medical Association 2013;309(4):343. [Google Scholar]
- Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, et al. The NHLBI TIME trial: One‐year results. Circulation 2013;128(24):2714. American Heart Association's Scientific Sessions 2013, Dallax, TX United States, 16‐20 November 2013. [Google Scholar]
- Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, et al. The NHLBI and cardiovascular cell therapy research network (CCTRN) TIME trial: two‐year results. [American Heart Association's Scientific Sessions and Resuscitation Science Symposium, Chicago, IL United States. 15‐18 November 2014]. Circulation 2014;130. [Google Scholar]
- Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, et al. The effect of timing of stem cell delivery following acute myocardial infarction: the NHLBI and CCTRM TIME trial. Circulation 2012;126(23):2783‐4. [Google Scholar]
- Traverse JH, Henry TD, Vaughan DE, Ellis SG, Pepine CJ, Willerson JT, et al. Rationale and design for TIME: a phase II, randomized, double‐blind, placebo‐controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. American Heart Journal 2009;158(3):356‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse LH, Moye L, Henry TD. Identification of clinical factors in cell therapy trials that determine recovery versus deterioration of LV function following STEMI. [American Heart Association's Scientific Sessions and Resuscitation Science Symposium, Chicago, IL United States. 15‐18 November 2014]. Circulation 2014;130. [Google Scholar]
Turan 2012 {published data only}
- Turan RG, Bozdag TI, Turan CH, Ortak J, Akin I, Kische S, et al. Enhanced mobilization of the bone marrow‐derived circulating progenitor cells by intracoronary freshly isolated bone marrow cells transplantation in patients with acute myocardial infarction. Journal of Cellular and Molecular Medicine 2012;16(4):852‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan RG, Ilkay BT, Akin I, Kische S, Schneider H, Turan CH, et al. Enhanced cardiac chemoreflex sensitivity after intra coronary freshly isolated bone marrow cells transplantation in patients with acute myocardial infarction. [27th National Congress of Cardiology, Istanbul, Turkey, 27‐30 October 2011] [Akut miyokart enfarktuslu hastalarda taze isole edilmis intrakoroner kemik iliti kaynakli kok hucre transplantasyonu sonrasi artmis kardiyak kemorefleks duyarliliti]. Tűrk Kardiyoloji Derneği Arşivi 2011;39:83. [Google Scholar]
- Turan RG, Turan IB, Turan CH, Akin I, Kische S, Paranskaya L, et al. Long term improvement of cardiac function by intracoronary freshly isolated bone marrow cells transplantation in patients with acute myocardial infarction. [28th National Congress of Cardiology, Antalya, Turkey. 11‐14 October 2012] [Akut miyokard infarktusu hastalarinda intra koroner taze izole edilmis kemik iligi kok hucre tranplantasyonu sonrasi kardiyak fonksiyonlarinda meydana gelen uzun sureli duzelme]. Turk Kardiyoloji Dernegi Arsivi 2012;40:62. [Google Scholar]
Wang 2014 {published data only}
- Wang X, Xi WC, Wang F. The beneficial effects of intracoronary autologous bone marrow stem cell transfer as an adjunct to percutaneous coronary intervention in patients with acute myocardial infarction. Biotechnology Letters 2014;36(11):2163‐8. [DOI] [PubMed] [Google Scholar]
Wohrle 2010 {published data only}
- Mailander V, Wohrle J, Schauwecker P, Merkle N, Nusser T, Bommer M, et al. Intracoronary stem cell therapy in patients with acute myocardial infarction ‐ a randomized, double‐blind, placebo‐controlled trial (SCAMI). Transfusion Medicine and Hemotherapy 2009;36(Suppl 1):19‐20. [Google Scholar]
- NCT00669227. Intracoronary stem cell therapy in patients with acute myocardial infarction (SCAMI). https://clinicaltrials.gov/ct2/show/NCT00669227 (accessed 11 March 2015).
- Woehrle J, Scheidt F, Markovic S, Schauwecker P, Schwarz K, Wiesneth M, et al. Intracoronary stem cell therapy in patients with acute myocardial infarction ‐ 36 months results of a randomized, double‐blind, placebo controlled trials with serial MRI follow‐ups. [61st Annual Scientific Session of the American College of Cardiology and i2 Summit: Innovation in Intervention, ACC.12, Chicago, IL United States. 24‐27 March 2012]. Journal of the American College of Cardiology 2012;59(13 Suppl 1):E346. [Google Scholar]
- Wohrle J, Merkle N, Mailander V, Nusser T, Schauwecker P, Scheidt F, et al. Intracoronary stem cell therapy after myocardial infarction ‐ twelve months follow‐up of a randomized, rigorous double‐blind, placebo controlled trial. Journal of American College of Cardiology. 2010; Vol. 55 (10 Suppl 1):A100.e938.
- Wohrle J, Merkle N, Mailander V, Nusser T, Schauwecker P, Scheidt F, et al. Results of the intracoronary stem cell therapy after acute myocardial infarction. American Journal of Cardiology 2010;105(6):804‐12. [DOI] [PubMed] [Google Scholar]
- Wohrle J, Scheidt F, Schauwecker P, Wiesneth M, Markovic S, Schrezenmeier H, et al. Impact of cell number and microvascular obstruction in patients with bone‐marrow derived cell therapy: final results from the randomized, double‐blind, placebo controlled intracoronary Stem Cell therapy in patients with Acute Myocardial Infarction (SCAMI) trial. Clinical Research in Cardiology 2013;102(10):765‐70. [DOI] [PubMed] [Google Scholar]
Wollert 2004 {published data only}
- Gerd P, Schaefer A, Wollert KC, Lippolt P, Fuchs M, Kaplan M, et al. Can bone marrow transfer in patients after myocardial infarction prevent the development of diastolic dysfunction? Results from the BOOST trial. Circulation 2004;110(17 Suppl):239‐40. Abstract 1148. [Google Scholar]
- Hertenstein B, Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, et al. Bone marrow cell transfer for treatment of acute myocardial infarction: 18 months follow‐up data from the randomised‐controlled BOOST trial. Bone Marrow Transplantation 2006;27(Suppl 1):S62. [Google Scholar]
- Hertenstein B, Wollert KC, Meyer GP, Lotz J, Ringes‐Lichtenberg S, Breidenbach C, et al. Randomised controlled clinical trial of intracoronary autologous bone marrow cell transfer post‐myocardial infarction. Bone Marrow Transplantation 2004;33(Suppl 1):S2. [DOI] [PubMed] [Google Scholar]
- Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Loppolt P, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5‐year follow‐up from the randomized‐controlled BOOST trial. European Heart Journal 2009;30:2978‐84. [DOI] [PubMed] [Google Scholar]
- Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months follow‐up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST‐elevation infarct regeneration) trial. Circulation 2006;113:1287‐94. [DOI] [PubMed] [Google Scholar]
- NCT00224536. Bone marrow transfer to enhance ST‐elevation infarct regeneration. https://www.clinicaltrials.gov/ct2/show/NCT00224536 (accessed 11 March 2015).
- Schaefer A, Meyer GP, Fuchs M, Klein G, Kaplan M, Wollert KC, et al. Impact of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: results from the BOOST trial. European Heart Journal 2006;27:929‐35. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Zwadlo C, Fuchs M, Meyer GP, Lippolt P, Wollert KC, et al. Long‐term effects of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: 5‐year results from the randomized‐controlled BOOST trial‐an echocardiographic study. European Journal of Echocardiography 2010;11:165‐71. [DOI] [PubMed] [Google Scholar]
- Wollert KC, Meyer GP, Lotz J, Ringes‐Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone‐marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet 2004;364(9429):141‐8. [DOI] [PubMed] [Google Scholar]
Xiao 2012 {published data only}
- Xiao WT, Gao CY, Dai GY, Li MW, Wang XP, Liu HZ, et al. Autologous bone marrow mesenchymal stem cells for myocardial renewal and repair. Chinese Journal of Tissue Engineering Research 2012;16(27):5081‐6. [Google Scholar]
Yao 2006 {published data only}
- Yao K, Huang RC, Ge L, Qian JY, Li YL, Xu SK, et al. Observation on the safety: clinical trial on intracoronary autologous bone marrow mononuclear cells transplantation for acute myocardial infarction. Chinese Journal of Cardiovascular Diseases 2006;34(7):577‐81. [PubMed] [Google Scholar]
Yao 2009 {published data only}
- Yao K, Huang R, Sun A, Qian J, Liu X, Ge L, et al. Repeated autologous bone marrow mononuclear cell therapy in patients with large myocardial infarction. European Journal Heart Failure 2009;11(7):691‐8. [DOI] [PubMed] [Google Scholar]
You 2008 {published data only}
- You QJ, Shen ZY, Xiao MD, Jiang XC. Influence of autologous bone marrow stem cell transplantation on short‐term heart function in cardiac failure patients. Journal of Clinical Rehabilitative Tissue Engineering Research 2008;12(8):1467‐71. [Google Scholar]
Zhukova 2009 {published data only}
- Zhukova NS, Staroverov II, Stukalova OV, Samoilenko LE, Romanov YA, Sinitsin VE, et al. An experience of the use of stem cells in the treatment of patients with myocardial infarction and low ejection fraction. Kardiologia 2009;7(8):19‐24. [PubMed] [Google Scholar]
References to studies excluded from this review
Ang 2008 {published data only}
- Ang KL. Intramuscular or intracoronary administration of autologous BMC fails to improve contractility of scarred myocardium: IC/IM‐BMC study. Clinical Research in Cardiology 2008;97(1):10. [Google Scholar]
Arnesen 2007 {published data only}
- Arnesen H, Lunde K, Aakhus S, Forfang K. Cell therapy in myocardial infarction. Lancet 2007;369(9580):2142‐3. [DOI] [PubMed] [Google Scholar]
Atsma 2008 {unpublished data only}
- Atsma DE. Autologous bone marrow‐derived in vitro expanded mesenchymal stem cell transplantation in patients with an acute myocardial infarction treated by successful primary percutaneous transluminal coronary angioplasty ‐ a safety and feasibility study. http://www.trialregister.nl (accessed by 31 January 2011).
Beeres 2007 {published data only}
- Beeres SL, Bax JJ, Dibbets‐Schneider P, Stokkel MP, Fibbe WE, Wall EE, et al. Intramyocardial injection of autologous bone marrow mononuclear cells in patients with chronic myocardial infarction and severe left ventricular dysfunction. American Journal of Cardiology 2007;100(7):1094‐8. [DOI] [PubMed] [Google Scholar]
Benedek 2014 {published data only}
- Benedek I, Bucur O, Benedek T. Intracoronary infusion of mononuclear bone marrow‐derived stem cells is associated with a lower plaque burden after four years. Journal of Atherosclerosis and Thrombosis 2014;21(3):217‐29. [DOI] [PubMed] [Google Scholar]
- Benedek IS, Chitu M, Kovacs I, Benedek IJR, Suciu ZS, Benedek T. Intracoronary infusion of stem cells reduces local artherosclerosis progression on long term follow‐up ‐ an Angio CT multislice 64 study. European Heart Journal 2013;34:842‐53. [Google Scholar]
Chen 2004a {published data only}
- Chen YX, Ou RM, Zhang XY, Xu X, Zhao HY, Guan HH, et al. Effect of self‐marrow stem cell transplantation in situ on the size of infarct area and cardiac functions after acute myocardium infarction. Chinese Journal of Clinical Rehabilitation 2004;8(3):568‐9. [Google Scholar]
Chen 2014 {published data only}
- Chen XM, Cui DY, Zhang M. Clinical observation of stem cell transplantation in patients with acute myocardial infarction complicated with heart failure. Journal of Dalian Medical University 2014;36(2):157‐9. [Google Scholar]
Engelmann 2006 {published data only}
- Engelmann MG, Theiss HD, Hennig‐Theiss C, Huber A, Wintersperger BJ, Werle‐Ruedinger AE, et al. Autologous bone marrow stem cell mobilization induced by granulocyte colony stimulating factor after subacute ST‐segment elevation myocardial infarction undergoing late revascularization. Journal of the American College of Cardiology 2006;48(8):1712‐21. [DOI] [PubMed] [Google Scholar]
EUCTR 2010‐020497‐41‐GB {unpublished data only}
- EUCTR 2010‐020497‐41‐GB. A prospective, double blind, randomized, placebo‐controlled clinical trial of intracoronary infusion of immunoselected, bone marrow‐derived Stro3 mesenchymal precursor cells (MPC) in the treatment of patients with ST‐elevation myocardial infarction ‐ the AMICI trial. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2010‐020497‐41/GB (accessed 11 March 2015).
Fernandez 2004 {published data only}
- Fernandez RJ, Saslavsky J, Andrin O, Vrsalovic F, Geffner L, Camozzi L, et al. First‐reported data from Argentina of the implantation and cellular therapy in myocardial infarction (Tescelcor) study. American Journal of Cardiology 2004;94(6A Suppl):187E‐8E. [Google Scholar]
Gyongyosi 2009 {published data only}
- Gieseking E, Syeda B, Sochor H, Charwat S, Bergler‐Klein J, Maurer G, et al. Long‐term follow‐up of patients treated with combined delivery of intracoronary and intramyocardial bone‐marrow mononuclear cells. [Osterreichische Kardiologische Gesellschaft Jahrestagung 2012, Salzburg, Austria. 30 May ‐ 2 June 2012]. Journal fur Kardiologie 2012;19(5‐6):162. [Google Scholar]
- Gyongyosi M, Charwat S, Lang IM, Dettke M, Glogar D, Maurer G. 5 years clinical follow‐up of patients treated with combined delivery of intracoronary and intramyocardial bone‐marrow mononuclear cells. [Osterreichische Kardiologische Gesellschaft Jahrestagung 2011, Salzburg, Austria. 25‐28 May 2011]. Journal fur Kardiologie 2011;18(5‐6):185. [Google Scholar]
- Gyongyosi M, Lang I, Dettke M, Beran G, Graf S, Sochor H, et al. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: the MYSTAR prospective, randomized study. Nature Clinical Practice Cardiovascular Medicine 2009;6(1):70‐81. [DOI] [PubMed] [Google Scholar]
- Gyongyosi M, Lang I, Dettke M, Charwat S, Nyolczas N, Sochor H, et al. Results from the multicenter, prospective, randomized single‐blind trial on intracoronary or combined (percutaneous intramyocardial and intracoronary) administration of non‐selected autologous bone marrow cells to patients after acute myocardial infarction. American Journal of Cardiology 2007;100(8A Suppl):23L. [DOI] [PubMed] [Google Scholar]
- Nyolczas N, Gyongyosi M, Beran G, Dettke M, Graf S, Sochor H, et al. Design and rationale for the myocardial stem cell administration after acute myocardial infarction (MYSTAR) study: a multicenter, prospective, randomised, single‐blind trial comparing early and late intracoronary or combined (percutaneous intramyocardial and intracoronary) administration of nonselected autologous bone marrow cells to patients after acute myocardial infarction. American Heart Journal 2007;153(2):212.e1‐7. [DOI] [PubMed] [Google Scholar]
Hare 2007 {published data only}
- Hare J. A randomized, double‐blind, placebo‐controlled, dose‐escalation study of intravenous adult human mesenchymal stem cells (Provacel) following acute myocardial infarction (AMI). Clinical Cardiology 2007;30(7):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double‐blind, placebo controlled, dose‐escalation study of intravenous adult human mesenchymal stem cells (PROCHYMAL) after acute myocardial infarction. Journal of the American College of Cardiology 2009;54(24):2277‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richartz BB. A randomized, double blind placebo controlled, dose escalation study of intravenous human adult mesenchymal stem cells (PROVACEL (trademark)) following acute myocardial infarction. Herz 2007;32(4):338. [Google Scholar]
Heeger 2012 {published data only}
- Heeger CH, Jaquet K, Thiele H, Zulkarnaen Y, Cuneo A, Haller D, et al. Percutaneous, transendocardial injection of bone marrow‐derived mononuclear cells in heart failure patients following acute ST‐elevation myocardial infarction: ALSTER‐Stem Cell trial. Eurointervention 2012;8(6):732‐42. [DOI] [PubMed] [Google Scholar]
Hendrikx 2006 {published data only}
- Hendrikx M, Hensen K, Clijsters C, Jongen H, Koninckx R, Bijnens E, et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation. Results from a randomised controlled clinical trial. Circulation 2006;114(Suppl I):I101‐7. [DOI] [PubMed] [Google Scholar]
Holinski 2011 {published data only}
- Holinski S, Schmeck B, Claus B, Radtke H, Elgeti T, Holzhausen M, et al. Encouraging experience with intracardiac transplantation of unselected autologous bone marrow cells concomitant with coronary artery bypass surgery after myocardial infarction. Annals of Thoracic and Cardiovascular Surgery 2011;17(4):383‐9. [DOI] [PubMed] [Google Scholar]
Hu 2015 {published data only}
- Hu X, Huang X, Yang Q, Wang L, Sun J, Zhan H, et al. Safety and efficacy of intracoronary hypoxia‐preconditioned bone marrow mononuclear cell administration for acute myocardial infarction patients: the CHINA‐AMI randomized controlled trial. International Journal of Cardiology 2015;184i:446‐51. [DOI] [PubMed] [Google Scholar]
- Hu XY, Huang X, Yang Q, Wang L, Sun J, Zhan H, et al. Safety and efficacy of intracoronary hypoxia‐preconditioned bone marrow mononuclear cells administration for acute myocardial infarction patients. [American Heart Association's Scientific Sessions and Resuscitation Science Symposium, Chicago, IL United States. 15‐18 November 2014]. Circulation 2014;130. [Google Scholar]
Jiang 2011 {published data only}
- Jiang M, Pu J, He B. The short to mid‐term effect of bone marrow derived cell transfer on diastolic function after acute myocardial infarction. International Journal of Cardiology 2011;153(1):87‐8. [DOI] [PubMed] [Google Scholar]
Kahn 2006 {published data only}
- Kahn J. Stem cells show mixed results in MI patients. Journal of Interventional Cardiology 2006;19(4):297‐301. [DOI] [PubMed] [Google Scholar]
Kang 2004 {published data only}
- Kang HJ, Kim HS, Park YB. Stem cell therapy for myocardial infarction. Canadian Medical Association Journal 2004;171(5):442‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2006 {published data only}
- Kang HJ, Kim MK, Kim MG, Choi DJ, Yoon JH, Park YB, et al. A multicenter, prospective, randomized, controlled trial evaluating the safety and efficacy of intracoronary cell infusion mobilized with granulocyte colony‐stimulating factor and darbepoetin after acute myocardial infarction: study design and rationale of the 'MAGIC cell‐5‐combination cytokine trial'. Trials 2011;12(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Kim MK, Lee HY, Park KW, Lee W, Cho YS, et al. Five‐year results of intracoronary infusion of the mobilized peripheral blood stem cells by granulocyte colony‐stimulating factor in patients with myocardial infarction. European Heart Journal 2012;33(24):3062‐9. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim YS, Koo BK, Park KW, Lee HY, Sohn DW, et al. Effects of stem cell therapy with G‐CSF on coronary artery after drug‐eluting stent implantation in patients with acute myocardial infarction. Heart 2008;94(5):604‐9. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Lee HY, Na SH, Chang SA, Park KW, Kim HK, et al. Differential effect of intracoronary infusion of mobilized peripheral blood stem cells by granulocyte colony‐stimulating factor on left ventricular function and remodeling in patients with acute myocardial infarction versus old myocardial infarction: the MAGIC Cell‐3‐DES randomized, controlled trial. Circulation 2006;114(1 Suppl):I145‐51. [DOI] [PubMed] [Google Scholar]
- Kim HS. Myocardial regeneration and angiogenesis in myocardial infarction with G‐CSF and intracoronary stem cell infusion‐3‐DES. www.ClinicalTrials.gov 2006; Vol. (accessed by 31 January 2011). [NCT00291629]
- Lee HY, Kang HJ, Na SH, Chang SA, Hahn JY, Kim YS, et al. Differential effect of intracoronary infusion of the mobilized peripheral blood stem cell by G‐CSF on angionnyogenesis in patients AMI versus OMI: interim result of 'MAGIC Cell‐DES' trial. Circulation 2005;112(17 Suppl):U644. Meeting Abstract 2758. [Google Scholar]
Kang 2007 {published data only}
- Kang HJ, Kim HS, Koo BK, Kim YJ, Lee DE, Sohn DW, et al. Intracoronary infusion of the mobilized peripheral blood stem cell by G‐CSF is better than mobilization alone by G‐CSF for improvement of cardiac function and remodeling: 2‐year follow‐up results of the Myocardial Regeneration and Angiogenesis in Myocardial Infarction with G‐CSF and Intra‐Coronary Stem Cell Infusion (MAGIC Cell) 1 trial. American Heart Journal 2007;153(2):237.e1‐8. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim HS, Na SH, Zhang SY, Kang WJ, Youn TJ, et al. Six months follow‐up results of "granulocyte stimulating factor" based stem cell therapy in patients with myocardial infarction. Korean Circulation Journal 2006;36:99‐107. [Google Scholar]
- Kang HJ, Kim HS, Zhang SY, Park KW, Choo HJ, Koo BK, et al. Effects of intracoronary infusion of peripheral blood stem‐cells mobilised with granulocyte‐colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet 2004;363:751‐6. [DOI] [PubMed] [Google Scholar]
Kang 2008 {published data only}
- Kang HJ, Kim HS. Safety and efficacy of intracoronary infusion of mibilized peripheral blood stem cell in patients with myocardial infarction: Magic Cell‐1 and Magic Cell‐3‐DES trials. European Heart Journal Supplements 2008;10(K):K39‐K43. [Google Scholar]
Kang 2011 {published data only}
- Kang HJ, Kim MK, Kim MG, Choi DJ, Yoon JH, Park YB, et al. A multicenter, prospective, randomized, controlled trial evaluating the safety and efficacy of intracoronary cell infusion mobilised with granulocyte colony‐stimulating factor and darbepoetin after acute myocardial infarction: study design and rationale of the 'MAGIC cell‐5‐combination cytokine trial'. Trials 2011;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00501917. MAGIC Cell‐5‐Combicytokine Trial. https://clinicaltrials.gov/ct2/results?term=NCT00501917&Search=Search (accessed 11 March 2015).
Li 2006 {published data only}
- Li Z, Zhang M, Jin Y, Zhang W, Liu L, Cui L, et al. The clinical randomized study of autologous peripheral blood progenitor cell transplantation by intracoronary infusion in patients with acute myocardial infarction. Circulation 2006;114(Suppl 18):II514‐5. [Google Scholar]
- Li ZQ, Zhang M, Jin YZ, Zhang WW, Liu Y, Yuan L, et al. Safety and efficacy of intracoronary transplantation of G‐CSF mobilised autologous peripheral blood stem cells in patients with acute myocardial infarction. Chinese Journal of Cardiovascular Diseases 2006;34(2):99‐102. [PubMed] [Google Scholar]
- Li ZQ, Zhang M, Jing YZ, Zhang WW, Liu Y, Cui JL, et al. The clinical study of autologous peripheral blood stem cell transplantation by intracoronary infusion in patients with acute myocardial infarction (AMI). International Journal of Cardiology 2007;115(1):52‐6. [DOI] [PubMed] [Google Scholar]
- Zhang M, Li ZQ, Jun YZ, Zhang WW, Liu Y, Cui LJ, et al. Autologous peripheral blood stem cell transplantation via coronary artery for acute myocardial infarction: a follow‐up of short‐term curative effect. Journal of Clinical Rehabilitative Tissue Engineering Research 2007;11(20):3944‐7. [Google Scholar]
Li 2008 {published data only}
- Li XJ, Shang XM, Xu D, Liu CQ. Effect of bone marrow mesenchymal stem cell transplantation on vascular endothelial function in acute myocardial infarction patients. Journal of Clinical Rehabilitative Tissue Engineering Research 2008;12(38):7443‐6. [Google Scholar]
Lu 2012 {published data only}
- Lu M, Zhao S, Liu Q, Jiang S, Song P, Qian H, et al. Transplantation with autologous mesenchymal stem cells after acute myocardial infarction evaluated by magnetic resonance imaging: an experimental study. Journal of Thoracic Imaging 2012;27(2):125‐35. [DOI] [PubMed] [Google Scholar]
Makkar 2012 {published data only}
- Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, et al. Intracoronary cardiosphere‐derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase I trial. Lancet 2012;379(9819):895‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliaras K, Cheng K, Smith R, Mendizabal A, Gerstenblith G, Marban L, et al. Intracoronary cardiosphere‐derived cells for heart regeneration after myocardial infarction: determinants of regenerative efficacy in the final 1‐year results of the CADUCEUS trial. [American Heart Association 2012 Scientific Sessions and Resuscitation Science Symposium, Los Angeles, CA United States. 3‐6 November 2012]. Circulation 2012;126(21 Suppl 1). [Google Scholar]
- Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, et al. Intracoronary cardiosphere‐derived cells after myocardial infarction: Evidence of therapeutic regeneration in the final 1‐year results of the CADUCEUS trials (CArdiosphere‐Derived aUtologous stem CElls to reverse ventricUlar dySfunction). Journal of the American College of Cardiology 2014;63(2):110‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00893360. A phase I randomized, dose escalation study of the safety and efficacy of intracoronary delivery of cardiosphere‐derived stem cells in patients with ischemic left ventricular dysfunction and a recent myocardial infarction. https://clinicaltrials.gov/ct2/show/NCT00893360 (accessed 11 March 2015).
Marenzi 2007 {published data only}
- Marenzi G, Bartorelli AL. Improved clinical outcome after intracoronary administration of bone marrow‐derived progenitor cells in acute myocardial infarction: final 1‐year results of the REPAIR‐AMI trial. European Heart Journal 2007;28(17):2172‐3. [DOI] [PubMed] [Google Scholar]
Messori 2013 {published data only}
- Messori A, Fadd V, Maratea D, Trippoli S. Intracoronary infusion of bone‐marrow derived mononuclear cells in acute myocardial infarction: are outcomes influenced by the number of infused cells?. Heart, Lung and Circulation 2013;22(9):786‐7. [DOI] [PubMed] [Google Scholar]
Mills 2007 {published data only}
- Mill JS, Rao SV. REPAIR‐AMI: stem cells for acute myocardial infarction. Future Cardiology 2007;3(2):137‐40. [DOI] [PubMed] [Google Scholar]
Musialek 2006 {published data only}
- Musiatek P, Tracz W, Skotnicki AB, Zmudka K, Pienlazek P, Walter Z, et al. Transcoronary stem cell delivery using physiological endothelium‐targeting perfusion technique: the rationale and a pilot study involving a comparison with conventional over the‐wire balloon coronary occlusions in patients with recent myocardial infarction. Kardiologia Polska 2006;64:489‐98. [PubMed] [Google Scholar]
Musialek 2010 {published data only}
- Musialek P, Tekieli L, Kostkiewicz M, Majka M, Szot W, Walter Z, et al. Randomized transcoronary delivery of CD34(+) cells with perfusion versus stop‐flow method in patients with recent myocardial infarction: early cardiac retention of 99(m)Tc‐labeled cells activity. Journal of Nuclear Cardiology 2011;18(1):104‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nasseri 2013 {published data only}
- Nasseri MH, Aghdami N, Ahmadi H, Moshkani FM, Farahani M, Madani H, et al. Phase iii randomized clinical trial comparing the effects of autologous bone marrow derived MNC and CD133 cells transplantation in AMI patients during CABG. [19th Annual Meeting of the International Society for Cellular Therapy, ISCT 2013, Auckland, New Zealand. 22‐25 April 2013]. Cytotherapy 2013;15(4 Suppl 1):S12. [Google Scholar]
NCT00548613 {unpublished data only}
- NCT00548613. Combination stem cell (MESENDO) therapy for utilization and rescue of infarcted myocardium. https://clinicaltrials.gov/ct2/show/NCT00548613 (accessed 11 March 2015).
NCT00874354 {unpublished data only}
- NCT00874354. REVITALIZE: randomized evaluation of intracoronary transplantation of bone marrow stem cells in myocardial infarction. https://clinicaltrials.gov/ct2/show/NCT00874354 (accessed 11 March 2015).
NCT00877903 {unpublished data only}
- NCT00877903. A phase II, multi‐center, randomized, double‐blind, placebo‐controlled study to evaluate the safety and efficacy of Prochymal® (ex vivo cultured adult human mesenchymal stem cells) intravenous infusion following acute myocardial infarction. https://clinicaltrials.gov/ct2/show/NCT00877903 (accessed 11 March 2015).
Nie 2007 {published data only}
- Nie Y, Guo YH, Guo LJ. Intracoronary transfer autologous bone marrow stem cells can improve cardiac function in patients with left ventricular dysfunction after myocardial infarction [Chinese]. Beijing da Xue Xue Bao. Yi Xue Ban: Journal of Peking University. Health Sciences 2007;39(6):634‐7. [PubMed] [Google Scholar]
Obradovic 2009 {published data only}
- Obradovic S, Balint B, Romanovic R, Trifunovic Z, Rusovic S, Baskot B, et al. Influence of intracoronary injections of bone‐marrow‐derived mononuclear cells on large myocardial infarction outcome: quantum of initial necrosis is the key. Vojnosaniteski Pregled 2009;66(12):998‐1004. [DOI] [PubMed] [Google Scholar]
Osterziel 2007 {published data only}
- Osterziel KJ. Improved clinical outcome after intracoronary administration of bone‐marrow‐derived progenitor cells in acute myocardial infarction: final 1 year results of the REPAIR‐AMI trial. European Heart Journal 2007;28(5):638. [DOI] [PubMed] [Google Scholar]
Ott 2013 {published data only}
- Ott I, Schulz S, Cassese S, Byrne R, Kastrati A. Stem cell mobilization by granulocyte‐colony stimulating factor in patients with acute myocardial infarction: five‐year results of the REVIVAL‐2 trial. Journal of the American College of Cardiology 2013;62(18 Suppl 1):B248. [Google Scholar]
- Ott I, Schulz S, Fusaro M, Cassese S, Byrne R, Joner M, et al. Stem cell mobilization by granulocyte‐colony stimulating factor in patients with acute myocardial infarction: five‐year results of the REVIVAL‐2 trial. European Heart Journal 2013;34(Suppl 1):74. [Google Scholar]
Peruga 2009 {published data only}
- Peruga J, Plewka M, Kasprzak J, Jezewski T, Wierzbicka A, Robak T, et al. Intracoronary administration of stem cells in patients with acute myocardial infarction ‐ angiographic follow‐up. Kardiologia Polska 2009;67(5):477‐84. [PubMed] [Google Scholar]
Schachinger 2004 {published data only}
- Assmus B, Honold J, Schachinger V, Britten MB, Fischer‐Rasokat U, Lehmann R, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. New England Journal of Medicine 2006;355(12):1222‐32. [DOI] [PubMed] [Google Scholar]
- Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE‐AMI). Circulation 2002;106:3009‐17. [DOI] [PubMed] [Google Scholar]
- Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Scmitt J, et al. Infarct remodelling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE‐AMI). Mechanistic insights from serial contrast‐enhanced magnetic resonance imaging. Circulation 2003;108:2212‐8. [DOI] [PubMed] [Google Scholar]
- Leistner DM, Fischer‐Rasokat U, Honold J, Seeger FH, Schachinger V, Lehmann R, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE‐AMI): final 5‐year results suggest long‐term safety and efficacy. Clinical Research in Cardiology 2011;100(10):925‐34. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction. Final one‐year results of the TOPCARE‐AMI trial. Journal of the American College of Cardiology 2004;44:1690‐9. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Assmus B, Honold J, Lehmann R, Hofmann WK, Martin H, et al. Normalization of coronary blood flow in the infarct related artery after intracoronary progenitor cell therapy: intracoronary Doppler substudy of the TOPCARE‐AMI trial. Clinical Research in Cardiology 2006;95:13‐22. [DOI] [PubMed] [Google Scholar]
Schueller 2007 {published data only}
- Schueller PO, Meyer C, Brehm M, Wernet P, Schannwell CM, Strauer BE. Intracoronary autologous bone marrow cell transplantation beneficially modulates heart rate variability. International Journal of Cardiology 2007;119(3):398‐9. [DOI] [PubMed] [Google Scholar]
Shrimahachota 2011 {published data only}
- Srimahachota S, Boonyaratavej S, Rerkpattanapipat P, Wangsupachart S, Tumkosit M, Bunworasate U, et al. Intra‐coronary bone marrow mononuclear cell transplantation in patients with ST‐elevation myocardial infarction: a randomized controlled study. Journal of the Medical Association of Thailand 2011;94(6):657‐63. [PubMed] [Google Scholar]
Taljaard 2010 {published data only}
- NCT00936819. Enhanced Angiogenic Cell Therapy in Acute Myocardial Infarction: a multicentre, phase IIb randomised placebo‐controlled trial. https://clinicaltrials.gov/ct2/show/NCT00936819 (accessed 11 March 2015).
- Taljaard M, Ward MR, Kutryk MJB, Courtman DW, Camack N, Goodman SG, et al. Rationale and design of Enhanced Angiogenic Cell Therapy in Acute Myocardial Infarction (ENACT‐AMI): the first randomized placebo‐controlled trial of enhanced progenitor cell therapy for acute myocardial infarction. American Heart Journal 2010;159(3):354‐60. [DOI] [PubMed] [Google Scholar]
Terrovitis 2011 {published data only}
- Pantsios C, Ntalianis A, Kanakakis J, Papadimitriou C, Kapelios C, Repasos E, et al. Functional and structural benefits after therapy with autologous GCSF‐mobilized peripheral blood progenitor cells in subacute myocardial infarction: a prospective, randomized, controlled study. [Heart Failure Congress 2014 and the 1st World Congress on Acute Heart Failure, Athens, Greece. 17‐20 May 2014]. European Journal of Heart Failure 2014;16:349. [Google Scholar]
- Terrovitis J, Ntalianis A, Kanakakis J, Papadimitriou C, Pantsios C, Eleftherakis E, et al. The effect of intracoronary infusion of mobilized peripheral blood stem cells on left ventricular function after myocardial infarction. [American Heart Association's Scientific Sessions 2011, Orlando, FL United States. 12‐16 November 2011]. Circulation 2011;124(21 Suppl 1). [Google Scholar]
Trzos 2009 {published data only}
- Trzos E, Krzeminska‐Pakula M, Rechcinski T, Bugala M, Kasprzak JD, Plewka M, et al. The influence of intracoronary autologous mononuclear bone marrow cell transplantation. [European Society of Cardiology, ESC Congress 2009, Barcelona, Spain. 29 August ‐ 2 September 2009]. European Heart Journal 2009;30:496. [Google Scholar]
- Trzos E, Krzeminska‐Pakula M, Rechcinski T, Plewka M, Kasprzak J, Peruga JZ, et al. The effects of intracoronary autologous mononuclear bone marrow cell transplantation on cardiac arrhythmia and heart rate variability. Kadiologia Polska 2009;67(7):713‐21. [PubMed] [Google Scholar]
Vanderheyden 2007 {published data only}
- Vanderheyden M, Vercauteren S, Mansour S, Delrue L, Vandekerckhove B, Heyndrickx GR, et al. Time‐dependent effects on coronary remodelling and epicardial conductance after intracoronary injection of enriched hematopoietic bone marrow stem cells in patients with previous myocardial infarction. Cell Transplantation 2007;16(9):919‐25. [DOI] [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang WM, Sun NL, Liu J, Zhang P, Liu KY, Wang Q, et al. Effects of intracoronary autologous bone marrow mononuclear cells transplantation in patients with anterior myocardial infarction. Chinese Journal of Cardiology 2006;34(2):103‐6. [PubMed] [Google Scholar]
Warbington 2013 {published data only}
- Warbington B, Weinstein D, Mallinson D, Olijnyk D, Paterson S, Ridha S, et al. Characterization on bone marrow derived CD34+ cells with different mobility potentials by micro RNA fingerprinting. [55th Annual Meeting of the American Society of Hematology, ASH2013, New Orleans, LA United States. 7‐10 December 2013]. Blood 2013;122(21). [Google Scholar]
Yang 2010 {published data only}
- Jang Z, Zhang F, Ma W, Chen B, Zhou F, Xu Z, et al. A novel approach to transplanting bone marrow stem cells to repair human myocardial infarction: delivery via a noninfarct‐related artery. Cardiovascular Therapeutics 2010;28(6):380‐5. [DOI] [PubMed] [Google Scholar]
Yu 2005 {published data only}
- Yu LF, Lu B, Yu LY, Li TF, Chen YJ, Liu WH, et al. Effect of intracoronary transplantation of bone marrow mononuclear cells on cardiac function and regional myocardial movement of patients with acute myocardial infarction. Chinese Journal of Clinical Rehabilitation 2005;9(26):71‐4. [Google Scholar]
Yu 2014 {published data only}
- Yu L, Zhang M. Intracoronary transplantation of autologous peripheral blood stem cells in old patients with acute myocardial infarction: five‐year postoperative evaluation of cardiac function. Chinese Journal of Tissue Engineering Research 2014;18(1):125‐30. [Google Scholar]
References to studies awaiting assessment
Alves 2011 {published data only}
- Alves SA, Pereira SB, Frajtag R, Moreira RC, Souza ALS, Haddad A, et al. Long‐term follow‐up of stem cell therapy after acute myocardial infarction. [15th Annual Scientific Meeting, Heart Failure Society of America, Boston, MA United States. 18‐21 September 2011]. Journal of Cardiac Failure 2011;17(8 Suppl 1):S87. [Google Scholar]
Chang 2008 {published data only}
- Chang SA, Kim HK, Lee HY, Choi SY, Koo BK, Kim YJ, et al. Restoration of left ventricular synchronous contraction after acute myocardial infarction by stem cell therapy: new insights into the therapeutic implication of stem cell therapy for acute myocardial infarction. Heart 2008;94(8):995‐1001. [DOI] [PubMed] [Google Scholar]
- Chang SA, Kim HK, Lee HY, Kang HJ, Kim YJ, Zo JH. Restoration of synchronicity of the left ventricular myocardial contraction with stem cell therapy: New insights into the therapeutic implication of stem cell therapy in myocardial infarction. [American Heart Association Scientific Sessions 2006, Chicago, IL United States, 12‐15 November 2006]. Circulation 2006;114(18 Suppl):Abstract 2718. [Google Scholar]
Fernandez‐Pereira 2006 {published data only}
- Fernandez‐Pereira C, Vigo CF, Bataglia S, Perez de la Hoz R, Vetulli H, Koziner B, et al. Autologous bone marrow stem cell transplant after myocardial infarction. In‐hospital and long‐term follow‐up results of the randomized Argentina trial (STAR AMI). European Heart Journal 2006;27(Suppl 1):279. [Google Scholar]
Huang 2007b {published data only}
- Huang R, Yao K, Ge JB, Zhou YZ, Qian YJY, Hge LEI, et al. Timing and therapeutic response of intracoronary bone marrow mononuclear cells administration in acute ST‐elevation myocardial infarction patients. European Heart Journal 2007;28(Suppl 1):229. [Google Scholar]
Huang 2008 {published data only}
- Huang RC, Yao K, Qian J, Sun A, Ge L, Li YL, et al. Transcatheter transplantation of stem cells for treatment of acute myocardial infarction (TCT‐STAMI‐2). European Heart Journal 2008;28(Suppl 1):366‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2005 {published data only}
- Lee YL, Ge JB, Qian JY, Shi JH, Wang QB, Niu YH, et al. Clinical trial on emergent intracoronary autologous bone marrow mononuclear cells transfer in patients with acute anterior myocardial infarction. American Journal of Cardiology 2005;96(7A Suppl):25H. [Google Scholar]
Lu 2012b {published data only}
- Lu M, Song L, Jiang S, Yang Y, Zhang Y, Yin G, et al. Effects of autologous bone marrow mononuclear cells transplantation via coronary artery in patients with acute myocardial infarction assessed by MRI. [23rd Great Wall International Congress of Cardiology, Asia Pacific Heart Congress 2012, Beijing, China. 11‐14 October 2012]. Heart 2012;98:E172. [Google Scholar]
Park 2011 {published data only}
- Park SD, Shin SH, Woo SI, Park KS, Kim DH, Kwan J. Effect of heart rate variability of autologous bone marrow mesenchymal stem cells in patients with ST segment elevation myocardial infarction. [EHRA Europace 2011, Madrid, Spain. 26‐29 June 2011]. Europace 2011;13(Suppl 3). [Google Scholar]
Perez‐Oteyza 2006 {published data only}
- Catalan‐Sanz M, Vaticon C, Asin E, Munoz M, Perez de Oteyza J, Cuevas P, et al. Randomised trial of intracoronary autologous bone‐marrow cell transfer after myocardial infarction. Preliminary results by cardiac magnetic resonance imaging. European Heart Journal 2005;26(Suppl 1):627. [Google Scholar]
- Perez‐Oteyza J, Ramos P, Catalan SM, Perez‐Abad M, Blanchard MJ, Heras C, et al. Intracoronary autologous bone‐marrow stem cell transfer after myocardial infarction. 11th Congress of the European Haematology Association, Amsterdam, The Netherlands. Amsterdam, The Netherlands: European Haematology Association, 2006.
Sanchez‐Fernandez 2012 {published data only}
- Sanchez Fernandez PL, San Roman JA, Villa A, Gimeno F, Arnold R, Sanz‐Ruiz R, et al. A multicenter, prospective, randomized, open‐labeled, trial comparing different bone‐marrow‐derived stem cell approaches in patients with reperfused ST‐elevation myocardial infarction. [European Society of Cardiology, ESC Congress 2012, Munich, Germany. 25‐29 August 2012]. European Heart Journal 2012;33:516. [Google Scholar]
Silva 2014 {published data only}
- Silva T, Fiarresga A, Abreu J, Reffeira A, Branco L, Timoteo A, et al. Effect of intracoronary infusion of autologous bone marrow derived progenitor cells on the global longitudinal strain in patients with ST‐elevation myocardial infarction. [European Society of Cardiology, ESC Congress 2014, Barcelona, Spain. 30 August‐3 September 2014]. European Heart Journal 2014;35:306. [Google Scholar]
References to ongoing studies
CTRI/2008/091/000232 {unpublished data only}
- CTRI/2008/091/000232. Efficacy of stem cell in improvement of left ventricular function in patients with acute myocardial infarction. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=224 (accessed 11 March 2015).
EUCTR 2006‐001772‐20‐ES {unpublished data only}
- EUCTR 2006‐001772‐20‐ES. Effect of intracoronary injection of autologous stem cells on left ventricular ejection fraction and volumes one year after an acute myocardial infarction. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2006‐001772‐20 (accessed 11 March 2015).
EUCTR 2006‐005628‐17‐ES {unpublished data only}
- EUCTR 2006‐005628‐17‐ES. Open study with blind regulator on the effectiveness of autologous bone marrow mononuclear cells in patients with left ventricular dysfunction after myocardial infarction. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2006‐005628‐17 (accessed 11 March 2015).
Hamshere 2014 {published data only}
- Hamshere S, Choudhury T, Jones DA, Locca D, Mills P, Rothman M, et al. A randomised double‐blind control study of early intracoronary autologous bone marrow cell infusion in acute myocardial infarction (REGENERATE‐AMI). BMJ Open 2014;4(2):e004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locca D, Burcliell T, Flett A, Yeo C, Palma R, Knight C, et al. Randomised controlled clinical trial of the use of autologous bone marrow derived progenitor cells to salvage myocardium in patients with acute anterior myocardial infarction. [EuroPCR 2011, Paris, France. 17‐20 May 2011]. EuroIntervention 2011;7:M87. [Google Scholar]
- NCT00765453. Bone marrow derived adult stem cells for acute anterior myocardial infarction (REGEN‐AMI). https://clinicaltrials.gov/ct2/show/NCT00765453 (accessed 11 March 2015).
ISRCTN17457407 {unpublished data only}
- ISRCTN17457407. BOne marrOw transfer to enhance ST‐elevation infarct regeneration‐2 (BOOST‐2). http://www.controlled‐trials.com/ISRCTN17457407 (accessed on 11 March 2015).
ISRCTN65630838 {unpublished data only}
- ISRCTN65630838. Selected bone marrow cell transplant following myocardial infarction in patients undergoing coronary surgery: a prospective double blind controlled trial. http://www.isrctn.com/ISRCTN65630838 (accessed 11 March 2015).
Mansour 2011 {published data only}
- Mansour S, Noiseux N, Denis‐Claude R, Francois G, Rivard A, Leclerc G. COMPARE‐AMI trial: Comparison of intracoronary injection of CD133+ bone marrow stem cells to placebo in patients after acute myocardial infarction: safety and feasibility analysis. Circulation 2009;120(18 Suppl 2):S774. [Google Scholar]
- Mansour S, Roy D, Stevens LM, Leclerc G, Gobeil F, Revard A, et al. Comparison of intracoronary injection of CD133+ bone marrow stem cells to placebo in patients after acute myocardial infarction and left ventricular dysfunction: One year safety analysis of the COMPARE‐AMI trial. [2010 Canadian Council of Cardiovascular Nurses Annual Scientific Sessions, Montreal, QC Canada. 23‐26 October 2010]. Canadian Journal of Cardiology 2010;26:116D. [Google Scholar]
- Mansour S, Roy DC, Bouchard V, Nguyen BK, Stevens LM, Gobeil F, et al. COMPARE‐AMI trial: comparison of intracoronary injection of CD133+ bone marrow stem cells to placebo in patients after acute myocardial infarction and left ventricular dysfunction: study rationale and design. Journal of Cardiovascular Translational Research 2010;3(2):153‐9. [DOI] [PubMed] [Google Scholar]
- Mansour S, Roy DC, Bouchard V, Stevens LM, Gobeil F, Rivard A, et al. One‐year safety analysis of the COMPARE‐AMI trial: comparison of intracoronary injection of CD133+ bone marrow stem cells to placebo in patients after acute myocardial infarction and left ventricular dysfunction. Bone Marrow Research 2011;2011:Article Number 385124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S, Roy DC, Lemieux B, Ouellet C, Stevens LM, Noiseux N. Stem cell therapy for the broken heart: mini‐organ transplantation. Transplantation Proceedings 2009;41(8):3353‐7. [DOI] [PubMed] [Google Scholar]
- Qiu F, Maehara A, Khoury R, Genereux P, LaSalle L, Mintz GS, et al. Impact of intracoronary injection of CD133+ bone marrow stem cells on coronary atherosclerotic progression in patients with STEMI: A COMPARE‐AMI IVUS Substudy. [26th Annual Symposium Transcatheter Cardiovascular Therapeutics, TCT 2014, Washington, DC United States. 13‐17 September 2014]. Journal of the American College of Cardiology 2014;64(11 Suppl 1):B46. [Google Scholar]
Micheu 2013 {published data only}
- Micheu MM, Oprescu N, Calmac L, Pitic D, Dorobantu M. Early effect of autologous bone marrow stem cell therapy on left ventricular systolic function in acute myocardial infarction patients and low left ventricular ejection fraction ‐ a pilot study. [21st World Congress on the International Society for Heart Research, San Diego, CA United States. 30 June ‐ 4 July 2013]. Journal of Molecular and Cellular Cardiology 2013;65:S156. [Google Scholar]
NCT00529932 {unpublished data only}
- EUCTR 2007‐001448‐33‐NL. A multi‐centre, double‐blind, randomised, placebo‐controlled trial using CD133 enriched bone marrow cells following primary angioplasty for acute myocardial infarction with central core laboratory analysis. ‐ SELECT‐AMI. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2007‐001448‐33 (accessed 11 March 2015).
- NCT00529932. A trial using CD133 enriched bone marrow cells following primary angioplasty for acute myocardial infarction. http://clinicaltrials.gov/ct2/show/NCT00529932 (accessed on 11 March 2015).
NCT00711542 {unpublished data only}
- NCT00711542. Effects of intracoronary progenitor cell therapy on coronary flow reverse after acute MI. http://clinicaltrials.gov/ct2/show/NCT00711542 (accessed 11 March 2015).
NCT00936819 {unpublished data only}
- NCT00936819. The Enhanced Angiogenic Cell Therapy ‐ Acute Myocardial Infarction Trial (ENACT‐AMI). https://clinicaltrials.gov/ct2/show/NCT00936819 (accessed 11 March 2015).
NCT00979758 {unpublished data only}
- NCT00979758. Strengthening transplantation effects of bone marrow mononuclear cells with atorvastatin in myocardial infarction. http://clinicaltrials.gov/ct2/show/NCT00979758 (accessed 11 March 2015).
NCT00984178 {unpublished data only}
- NCT00984178. Trial of hematopoietic stem cells in acute myocardial infarction. http://clinicaltrials.gov/ct2/show/NCT00984178 (accessed 11 March 2015).
NCT01187654 {unpublished data only}
- NCT01187654. Bone marrow derived AC 133+ and mono‐nuclear cells (MNC) implantation in myocardial infarction (MI) patient. https://clinicaltrials.gov/ct2/show/NCT01187654 (accessed 11 March 2015).
NCT01394432 {unpublished data only}
- NCT01394432. "ESTIMATION Study" for endocardial mesenchymal stem cells implantation in patients after acute myocardial infarction. https://clinicaltrials.gov/ct2/show/NCT01394432 (accessed 11 March 2015).
NCT01495364 {unpublished data only}
- NCT01495364. NBS10 (also known as AMR‐001) versus placebo post ST segment elevation myocardial infarction (PreSERVE‐AMI). https://clinicaltrials.gov/ct2/show/NCT01495364 (accessed 11 March 2015).
NCT01536106 {unpublished data only}
- NCT01536106. Rapid delivery of autologous bone marrow derived stem cells in acute myocardial infarction patients (AMIRST). https://clinicaltrials.gov/ct2/show/NCT01536106 (accessed 11 March 2015).
NCT01569178 {unpublished data only}
- NCT01569178. BAMI. The effect of intracoronary reinfusion of bone marrow‐derived mononuclear cells (BM‐MNC) on all cause mortality in acute myocardial infarction. https://clinicaltrials.gov/ct2/show/NCT01569178 (accessed 11 March 2015).
NCT01625949 {unpublished data only}
- NCT01625949. Stem cell therapy in patients with myocardial infarction and persistent total occlusion of infarct related artery (COAT). https://clinicaltrials.gov/ct2/show/NCT01625949 (accessed 11 March 2015).
NCT01652209 {unpublished data only}
- NCT01652209. RELIEF (A Randomized, Open labEled, muLticenter Trial for Safety and Efficacy of Intracoronary Adult Human Mesenchymal stEm Cells Acute Myocardial inFarction). https://clinicaltrials.gov/ct2/show/NCT01652209 (accessed 11 March 2015).
NCT02323620 {unpublished data only}
- NCT02323620. Impact of intracoronary injection of autologous BMMC for LV contractility and remodeling in patients with STEMI (RACE‐STEMI). https://www.clinicaltrials.gov/ct2/show/NCT02323620 (accessed March 2015).
Pena‐Duque 2011 {published data only}
- NCT00725738. Intracoronary autologous stem cell transplantation in ST‐elevation myocardial infarction: TRACIA STUDY. http://clinicaltrials.gov/ct2/show/NCT00725738 (accessed 11 March 2015).
- Pena‐Duque MA, Martinez‐Rios MA, Calderon GE, Mejia AM, Gomez E, Martinez‐Sanchez C, et al. Design and implementation of the TRACIA: intracoronary autologous transplant of bone marrow‐derived stem cells for acute ST elevation myocardial infarction. Archivos de Cardiologia de Mexico 2011;81(3):183‐7. [PubMed] [Google Scholar]
Additional references
Afzal 2015
- Afzal MR, Samanta A, Shah ZI, Jeevanantham V, Abdel‐Latif A, Zuba‐Surma EK, et al. Adult bone marrow cell therapy for ischemic heart disease: evidence and insights from randomized controlled trials. Circulation Research 2015 Jul 9 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
Assmus 2002
- Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE‐AMI). Circulation 2002;106(24):3009‐17. [DOI] [PubMed] [Google Scholar]
Bartunek 2006
- Bartunek J, Dimmeler S, Drexler H, Frenandez‐Aviles F, Gallinares M, Janssens S, et al. The consensus of the task force of the European Society of Cardiology concerning the clinical investigation of the use of autologous adult stem cells for repair of the heart. European Heart Journal 2006;27(11):1338‐40. [DOI] [PubMed] [Google Scholar]
Bartunek 2010
- Bartunek J, Vanderheyden M, Hill J, Terzic A. Cells as biologics for cardiac repair in ischaemic heart failure. Heart 2010;96:792‐800. [DOI] [PubMed] [Google Scholar]
Behfar 2014
- Behfar A, Crespo‐Diaz R, Terzic A, Gersh BJ. Cell therapy for cardiac repair‐‐lessons from clinical trials. Nature Reviews Cardiology 2014;11(4):232‐46. [DOI] [PubMed] [Google Scholar]
Beitnes 2009
- Beitnes JO, Hopp E, Lunde K, Solheim S, Arnesen H, Brinchmann JE, et al. Long‐term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: the ASTAMI randomised, controlled study. Heart 2009;95(24):1983‐9. [DOI] [PubMed] [Google Scholar]
BHF 2014
- Townsend N, Williams J, Bhatnagar P, Wickramasinghe K, Rayner M. Cardiovascular disease statistics, 2014 (https://www.bhf.org.uk/˜/media/files/publications/research/bhf_cvd‐statistics‐2014_web_2.pdf). London: British Heart Foundation, 2014 (accessed 8 May 2014). [Google Scholar]
Borm 2009
- Borm GF, Donders AR. Updating meta‐analyses leads to larger type I errors than publication bias. Journal of Clinical Epidemiology 2009;62(8):825‐30. [DOI] [PubMed] [Google Scholar]
Cheng 2014
- Cheng K, Ibrahim A, Hensley MT, Shen D, Sun B, Middleton R, et al. Relative roles of CD90 and c‐kit to the regenerative efficacy of cardiosphere‐derived cells in humans and in a mouse model of myocardial infarction. Journal of the American Heart Association 2014;3(5):e001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Jong 2014
- Jong R, Houtgraaf JH, Samiei S, Boersma E, Duckers HJ. Intracoronary stem cell infusion after acute myocardial infarction: a meta‐analysis and update on clinical trials. Circulation. Cardiovascular Interventions 2014;7(2):156‐67. [DOI] [PubMed] [Google Scholar]
Delewi 2014
- Delewi R, Hirsch A, Tijssen JG, Schächinger V, Wojakowski W, Roncalli J, et al. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST‐segment elevation myocardial infarction: a collaborative meta‐analysis. European Heart Journal 2014;35(15):989‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Engblom 2009
- Engblom H, Hedström E, Heiberg E, Wagner GS, Pahlm O, Arheden H. Rapid initial reduction of hyperenhanced myocardium after reperfused first myocardial infarction suggests recovery of the peri‐infarction zone: one‐year follow‐up by MRI. Circulation. Cardiovascular Imaging 2009;2(1):47‐55. [DOI] [PubMed] [Google Scholar]
ESC/ACC 2000
- The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined ‐ a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition if myocardial infarction. European Heart Journal 2000;21(18):1502‐13. [DOI] [PubMed] [Google Scholar]
Falk 1995
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92(3):657‐71. [DOI] [PubMed] [Google Scholar]
Fernandez‐Aviles 2004
- Fernandez‐Aviles F, San Roman JA, Garcia‐Frade J, Fernandez ME, Penarrubia MJ, Fuente L, et al. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circulation Research 2004;95(7):742‐8. [DOI] [PubMed] [Google Scholar]
Fisher 2012
- Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Clarke MJ, et al. Long‐term effects of autologous bone marrow stem cell treatment in acute myocardial infarction: factors that may influence outcomes. PLoS One 2012;7(5):e37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2014
- Fisher SA, Brunskill SJ, Doree C, Mathur A, Taggart DP, Martin‐Rendon E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD007888.pub2] [DOI] [PubMed] [Google Scholar]
Fisher 2015
- Fisher SA, Doree C, Mathur A, Martin‐Rendon E. Meta‐analysis of cell therapy trials for patients with heart failure. Circulation Research 2015;116(8):1361‐77. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- McMaster University. GRADEpro GDT [www.gradepro.org]. McMaster University, 2014 (accessed 19 May 2015).
Gyöngyösi 2015
- Gyöngyösi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, et al. Meta‐Analysis of Cell‐based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circulation Research 2015;116(8):1346‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartwell 2005
- Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al. Clinical effectiveness and cost‐effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation. Health Technology Assessment 2005;9(17):1‐99. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hu 2007
- Hu M, Cappeleri J, Lan KK. Applying the law of the iterated logarithm to control type I error in cumulative meta‐analysis of binary outcomes. Clinical Trials 2007;4(4):329‐40. [DOI] [PubMed] [Google Scholar]
Jeevanantham 2012
- Jeevanantham V, Butler M, Saad A, Abdel‐Latif A, Zuba‐Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long‐term improvement in cardiac parameters: a systematic review and meta‐analysis. Circulation 2012;126(5):551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lan 2003
- Lan KK, Hu M, Cappelieri J. Applying the law of the iterated logarithm to cumulative meta‐analysis of a continuous endpoint. Statistica Sinica 2003;13:1135‐45. [Google Scholar]
Leri 2009
- Leri A, Kajstura J, Anversa P, Frishman WH. Myocardial regeneration and stem cell repair. Current Problem in Cardiology 2009;33:91‐153. [DOI] [PubMed] [Google Scholar]
Meyer 2006
- Meyer GP, Wollert KC, Drexler H. Stem cell therapy: a new perspective in the treatment of patients with acute myocardial infarction. European Journal of Medical Research 2006;11(10):439‐46. [PubMed] [Google Scholar]
Nowbar 2014
- Nowbar AN, Mielewczik M, Karavassilis M, Dehbi HM, Shun‐Shin MJ, Jones S, et al. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta‐analysis. BMJ 2014;348:g2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
R Core Team 2013 [Computer program]
- R Core Team. R: A language and environment for statistical computing (http://www.R‐project.org/). Vienna, Austria: R Foundation for Statistical Computing, 2013.
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schachinger 2009
- Schachinger V, Assmus B, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, et al. Intracoronary infusion of bone marrow‐derived mononuclear cells abrogates adverse left ventricular remodelling post‐acute myocardial infarction: insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR‐AMI) trial. European Journal of Heart Failure 2009;11(10):973‐9. [DOI] [PubMed] [Google Scholar]
Stamm 2003
- Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone‐marrow stem‐cell transplantation for myocardial regeneration. Lancet 2003;361(9351):45‐6. [DOI] [PubMed] [Google Scholar]
Strauer 2002
- Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002;106(15):1913‐8. [DOI] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenhagen Trial Unit. Trial Sequential Analysis (TSA) program (www.ctu.dk/tsa). Denmark: Copenhagen Trial Unit, 2011.
Tse 2003
- Tse HF, Kwong YL, Chan JK, Lo G, Ho CL, Lau CP. Angiogenesis in ischaemic myocardium by intramyocardial autologous bone marrow mononuclear cell implantation. Lancet 2003;361:47‐9. [DOI] [PubMed] [Google Scholar]
Velagaleti 2008
- Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D'Agostino RB, et al. Long‐term trends in the incidence of heart failure after myocardial infarction. Circulation 2008;118(20):2057‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wen 2012
- Wen Y, Chen B, Wang C, Ma X, Gao Q. Bone marrow‐derived mononuclear cell therapy for patients with ischemic heart disease and ischemic heart failure. Expert Opinion on Biological Therapy 2012;12(12):1563‐73. [DOI] [PubMed] [Google Scholar]
Wettersley 2008
- Wettersley J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61:64‐75. [DOI] [PubMed] [Google Scholar]
Wu 2009
- Wu T, Li Y, Bian Z, Liu G, Moher D. Randomized trials published in some Chinese journals: how many are randomized?. Trials 2009;20:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Clifford 2012
- Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD006536.pub3] [DOI] [PubMed] [Google Scholar]
Martin‐Rendon 2007
- Martin‐Rendon E, Brunskill S, Doree C, Hyde C, Watt S, Mathur A. Stem cell treatment for acute myocardial infarction. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD006536] [DOI] [PubMed] [Google Scholar]
Martin‐Rendon 2008a
- Martin‐Rendon E, Brunskill SJ, Doree C, Hyde CJ, Mathur A, Stanworth SJ, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006536.pub2] [DOI] [PubMed] [Google Scholar]
Martin‐Rendon 2008b
- Martin‐Rendon E, Brunskill SJ, Hyde CJ, Mathur A, Stanworth S, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. European Heart Journal 2008;29(15):1807‐18. [DOI] [PubMed] [Google Scholar]


