Abstract
Background
COVID-19 infection is known to cause a wide array of clinical chronic sequelae, but little is known regarding the long-term cardiac complications. We aim to report echocardiographic follow-up findings and describe the changes in left (LV) and right ventricular (RV) function that occur following acute infection.
Methods
Patients enrolled in the World Alliance Societies of Echocardiography-COVID study with acute COVID-19 infection were asked to return for a follow-up transthoracic echocardiogram. Overall, 198 returned at a mean of 129 days of follow-up, of which 153 had paired baseline and follow-up images that were analyzable, including LV volumes, ejection fraction (LVEF), and longitudinal strain (LVLS). Right-sided echocardiographic parameters included RV global longitudinal strain, RV free wall strain, and RV basal diameter. Paired echocardiographic parameters at baseline and follow-up were compared for the entire cohort and for subgroups based on the baseline LV and RV function.
Results
For the entire cohort, echocardiographic markers of LV and RV function at follow-up were not significantly different from baseline (all P > .05). Patients with hyperdynamic LVEF at baseline (>70%), had a significant reduction of LVEF at follow-up (74.3% ± 3.1% vs 64.4% ± 8.1%, P < .001), while patients with reduced LVEF at baseline (<50%) had a significant increase (42.5% ± 5.9% vs 49.3% ± 13.4%, P = .02), and those with normal LVEF had no change. Patients with normal LVLS (<−18%) at baseline had a significant reduction of LVLS at follow-up (−21.6% ± 2.6% vs −20.3% ± 4.0%, P = .006), while patients with impaired LVLS at baseline had a significant improvement at follow-up (−14.5% ± 2.9% vs −16.7% ± 5.2%, P < .001). Patients with abnormal RV global longitudinal strain (>−20%) at baseline had significant improvement at follow-up (−15.2% ± 3.4% vs −17.4% ± 4.9%, P = .004). Patients with abnormal RV basal diameter (>4.5 cm) at baseline had significant improvement at follow-up (4.9 ± 0.7 cm vs 4.6 ± 0.6 cm, P = .019).
Conclusions
Overall, there were no significant changes over time in the LV and RV function of patients recovering from COVID-19 infection. However, differences were observed according to baseline LV and RV function, which may reflect recovery from the acute myocardial injury occurring in the acutely ill. Left ventricular and RV function tends to improve in those with impaired baseline function, while it tends to decrease in those with hyperdynamic LV or normal RV function.
Keywords: Echocardiography, WASE, COVID-19, Left ventricular function, Right ventricular function
Abbreviations: 2CH, Two-chamber; 4CH, Four-chamber; AI, Artificial intelligence; df, Degrees of freedom; ICU, Intensive care unit; LV, Left ventricular; LVEDV, Left ventricular end-diastolic volume; LVEF, Left ventricular ejection fraction; LVESV, Left ventricular end-systolic volume; LVLS, Left ventricular longitudinal strain; RV, Right ventricular, ventricle; RVBD, Right ventricle basal diameter; RVFWS, Right ventricular free-wall strain; RVGLS, Right ventricular global longitudinal strain; WASE, World Alliance Societies of Echocardiography
The International World Alliance Societies of Echocardiography (WASE) COVID-19 study identified echocardiographic parameters associated with in-hospital mortality in patients with acute COVID-19 infection and highlighted the differences in acute cardiac manifestations in various geographic regions around the world.1
COVID-19 infection is known to cause a wide array of clinical chronic sequelae such as fatigue and muscle weakness, sleep difficulties, sinus tachycardia, anxiety, depression, or abnormal pulmonary function tests, a phenomenon referred to as “long COVID syndrome.”2 , 3 However, little is known regarding the long-term cardiac complications of this disease, with a paucity of data regarding longitudinal echocardiographic findings.
In this follow-up substudy of the WASE-COVID-19 patient cohort, we report echocardiographic follow-up findings up to 9 months after the original infection and describe the changes in cardiac structure and function that occur following acute SARS CoV-2 infection.
Methods
Data Collection
Adult patients (≥18 years old) admitted with SARS-CoV-2 infection (confirmed by positive antigen or polymerase chain reaction test) during the first wave of the pandemic (January-September 2020) were considered for the study if a transthoracic echocardiogram was performed during the initial COVID-19-related hospitalization. Patients were consented for any prospective encounter or image acquisition, and the study was approved by the local ethics or Institutional Review Board committees. Patients were enrolled retrospectively, and follow-up was conducted prospectively. The follow-up echocardiograms were ordered and acquired based on local clinical practices at a minimum of 3 months after the initial hospitalization. Acceptable transthoracic echocardiograms included both comprehensive and limited studies, as long as at least the apical four-chamber (4CH) view was acquired. Patients were enrolled at 13 medical centers in four world regions (Asia, Europe, United States, and Latin America), 12 of which participated in this follow-up substudy.
All clinical information and DICOM cardiac ultrasound images were collected from the medical records, PACS systems, and echocardiography machines, deidentified, and transferred via a web-based system (Ultromics, Oxford, UK; Castor EDC, Hoboken, NJ) to the Core Laboratories at MedStar Health (Washington, DC) and the University of Chicago (Chicago, IL).
Image Analysis
Image transfer was facilitated by a two-step anonymization process to a cloud-based image analysis software. Image analysis of baseline and follow-up echocardiograms was conducted following the methodology previously reported in the WASE-COVID initial report.1 Left ventricular (LV) analyses were performed through commercially available artificial intelligence (AI) algorithms created by machine learning (EchoGo, Ultromics), which automatically traced the endocardium and, using the Simpson's method of disks,2 calculated the LV ejection fraction (LVEF), end-systolic and end-diastolic volumes (LVESV, LVEDV), and longitudinal strain (LVLS); additional details on this software have been described in our original publication and are presented in Supplemental Material S1.1 All LV measurements were repeated twice manually by board-certified echocardiographers (human reads 1 and 2) blinded to other reads and to clinical information. These echocardiographers were randomly selected from a pool of seven independent operators. For both methods (AI and human analysis), only cases with acceptable quality LV views (as determined by the expert echocardiographers) were included, which was defined as lack of apical foreshortening with adequate visualization of all segments in the apical 4CH view. Left ventricular longitudinal strain was calculated as the average of all available segments from the 4CH and two-chamber (2CH) views. The mean of the three LV reads (automated AI and human reads 1 and 2) was taken as the final value. Cutoffs for mildly, moderately, and severely reduced LVEF as well as normal and abnormal LVLS were determined by the 2015 American Society of Echocardiography/European Association of Cardiovascular Imaging Guidelines for Cardiac Chamber Quantification.2 Normal LVLS was defined as <−18% and abnormal as >−18%.2 , 3
Right ventricular (RV) analysis was performed using a semiautomated right ventricle (RV) -specific package (TOMTEC Image Arena, Build No. 494368, Unterschleissheim, Germany) and included RV global longitudinal strain (RVGLS), RV free wall strain (RVFWS), and RV basal diameter (RVBD). Only cases with acceptable quality RV views were included (among those patients with paired LV data), which was defined as presence of an RV-focused view with adequate visualization of the RV free wall. Abnormal RVFWS was defined as >−20%.4 Inter- and intraobserver reproducibility of the methodology used in this study for LV and RV analysis was very good to excellent and has been previously reported in detail.1
Statistical Analysis
Continuous variables were expressed as mean (±SD) or median (interquartile range) according to data distribution. Markers of LV (LVEF, LVLS) and RV (RVGLS, RVFWS, RVBD) function were compared between baseline and follow-up echocardiograms using paired t-tests, and the mean of differences (Δ) was calculated.
To determine accurate values and to build a homogeneous database, missing data for calculation of biplane LVEF and LVLS were determined using a multiple imputation model, following guidelines from the European Medicines Agency on confirmatory clinical trials.5 Specifically, a multiple imputation by chained equations method was used to derive the 2CH values for cases with a 4CH value but missing 2CH (n = 19 of the follow-up echocardiograms), to calculate biplane measures.
Results
Original Cohort Follow-Up
Over a 9-month period (January to September 2020), 870 patients were enrolled at 13 centers in nine countries. The baseline clinical and echocardiographic characteristics of our original cohort of 870 patients have been previously reported.1
Follow-Up Cohort
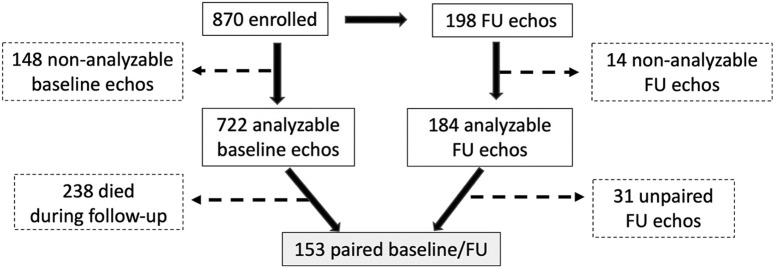
In-hospital mortality was 21.6% (188 patients) and increased to 27.4% (238 patients) through a follow-up of 230 ± 115 days. Of the original 870 patients, 198 survivors had a follow-up echocardiogram (129 ± 60 days following the initial admission), of which 184 had a successful biplane or 4CH endocardial tracing. Of the 184 with analyzable follow-up studies, 153 had paired baseline echocardiograms (Figure 1 ), including 80 with paired RV analysis. Baseline characteristics of all enrolled patients as well as those with paired follow-up echocardiograms are listed in Table 1 .
Figure 1.
Study flow chart. After excluding patients without analyzable echocardiograms at either baseline or follow-up (dashed lines and boxes), 153 patients with paired echocardiograms were included in the current substudy. These were all surviving patients that returned for follow-up, which occurred 129 ± 60 days after the initial admission for acute COVID-19 infection. FU, Follow-up.
Table 1.
Baseline characteristics of all enrolled patients and patients with paired follow-up echocardiograms
| All patients (N = 870) | Paired echocardiograms (n = 153) | |
|---|---|---|
| Age, years, median (Q1-Q3) | 60 (50-70) | 57 (49-66) |
| Gender, n (%): | ||
| Female | 381 (43.8) | 73 (48.0) |
| Male | 488 (56.1) | 79 (52.0) |
| Race, n (%): | ||
| White non-Hispanic | 197 (22.6) | 49 (32.0) |
| White Hispanic | 152 (17.5) | 31 (20.3) |
| Black | 136 (15.6) | 25 (16.3) |
| Asian | 271 (31.1) | 34 (22.2) |
| Mixed | 72 (8.3) | 7 (4.6) |
| Other | 34 (3.9) | 7 (4.6) |
| Geographic region, n (%): | ||
| United States | 125 (14.4) | 21 (13.7) |
| Europe | 160 (18.4) | 41 (26.8) |
| Asia | 347 (39.9) | 55 (35.9) |
| Latin America | 238 (27.5) | 36 (23.5) |
| Blood pressure, mean ± SD | ||
| Systolic, mm Hg | 123.3 ± 19.3 | 123.7 ± 16.9 |
| Diastolic, mm Hg | 74.6 ± 12.1 | 73.1 ± 10.5 |
| Heart rate, bpm, mean ± SD | 85.4 ± 15.4 | 83.29 ± 14.8 |
| Previous medical conditions, n (%): | ||
| Cardiac, all | 513 (58.9) | 79 (51.6) |
| Heart failure | 64 (7.3) | 9 (5.9) |
| Coronary artery disease | 120 (13.8) | 15 (9.8) |
| Stroke | 32 (3.6) | 6 (3.9) |
| Diabetes | 175 (20.1) | 30 (19.6) |
| Hypertension | 374 (42) | 56 (36.6) |
| Lung | 126 (14.5) | 18 (11.8) |
| Kidney | 75 (8.6) | 17 (11.1) |
| Serum biomarkers, n (%): | ||
| C-reactive protein: | ||
| Normal | 106 (12.2) | 20 (13.1) |
| Borderline | 51 (5.9) | 7 (4.6) |
| Abnormal | 635 (73.0) | 111 (72.5) |
| Unknown | 78 (9.0) | 15 (9.8) |
| Brain natriuretic peptide: | ||
| Normal | 153 (17.6) | 37 (24.2) |
| Borderline | 46 (5.3) | 7 (4.6) |
| Abnormal | 160 (18.4) | 23 (15.0) |
| Unknown | 511 (58.7) | 89 (58.2) |
| Troponin: | ||
| Normal | 18 (2.1) | 2 (13.1) |
| Borderline | 68 (7.8) | 14 (9.2) |
| Abnormal | 215 (24.7) | 38 (24.8) |
| Unknown | 569 (65.4) | 99 (64.7) |
| Lactate dehydrogenase: | ||
| Normal | 117 (13.4) | 18 (11.8) |
| Borderline | 255 (29.3) | 50 (32.7) |
| Abnormal | 152 (17.5) | 20 (13.1) |
| Unknown | 346 (39.8) | 65 (42.4) |
| D-dimer: | ||
| Normal | 85 (9.8) | 17 (11.1) |
| Borderline | 98 (11.3) | 12 (7.8) |
| Abnormal | 431 (49.5) | 73 (47.7) |
| Unknown | 256 (29.4) | 51 (33.3) |
| Baseline hospital status, n (%): | ||
| ICU | 402 (46.2) | 49 (32.0) |
| Ventilation | 236 (27.1) | 25 (16.3) |
| Hemodynamic support | 155 (17.8) | 13 (8.5) |
Echocardiographic Findings
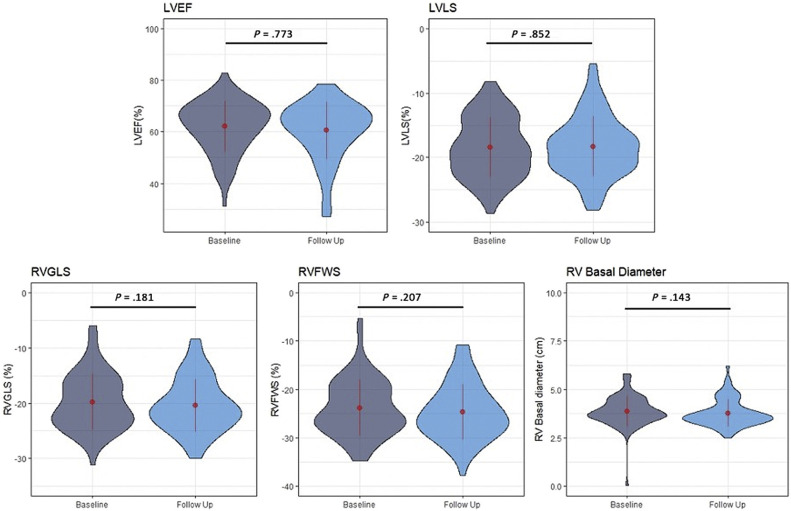
Echocardiographic findings for the patients with paired follow-up examinations are detailed in Table 2 . Using a pairwise analysis to compare baseline and follow-up echocardiograms, there were no significant differences in LV and RV function between baseline and follow-up: LVEF Δ = −0.26%, P = .77 (t = −0.290, degrees of freedom [df] = 151); LVLS Δ = −0.1%, P = .85 (t = −0.19, df = 152); RV GLS Δ = −0.6%, P = .21 (t = −1.28, df = 66); RVFWS Δ = −0.8%, P = .18 (t = −1.353, df = 66); and RVBD Δ = −0.1 cm, P = .14 (t = −1.5, df = 79; Figure 2 ). Similar findings were seen when including only patients that were in the intensive care unit (ICU) or on mechanical ventilation at the time of the initial echocardiogram (Table 2) and when considering those with longer and shorter follow-up (above and below the median—143 days; Supplemental Figure S1).
Table 2.
Echocardiographic characteristics of paired follow-up echocardiograms
| Characteristic | Baseline | Follow-up | P Value |
|---|---|---|---|
| Left ventricle (n = 153): | |||
| LVEDV, mL | 106.0 ± 43.9 | 105.7 ± 42.4 | .728 |
| LVESV, mL | 44.1 ± 28.4 | 44.8 ± 32.6 | .847 |
| LVEF, % | 61.6 ± 10.1 | 61.3 ± 10.6 | .773 |
| ICU | 60.8 ± 11.0 | 60.1 ± 10.3 | .604 |
| Ventilation | 61.0 ± 9.2 | 61.4 ± 8.3 | .989 |
| LVLS, % | −18.8 ± 4.4 | −18.9 ± 4.8 | .852 |
| ICU | −18.0 ± 5.0 | −18.8 ± 4.4 | .902 |
| Ventilation | −18.4 ± 5.1 | −18.3 ± 4.19 | .913 |
| RV (n = 80): | |||
| RVBD, cm | 3.9 ± 0.8 | 3.8 ± 0.7 | .143 |
| RVFWS, % | −23.8 ± 5.8 | −24.7 ± 5.7 | .181 |
| ICU | −21.5 ± 5.7 | −24.1 ± 4.7 | .3176 |
| Ventilation | −22.8 ± 4.7 | −24.8 ± 4.9 | .930 |
| RVGLS, % | −19.8 ± 5.1 | −20.4 ± 4.8 | .207 |
| ICU | −17.5 ± 4.9 | −20.4 ± 5.2 | .326 |
| Ventilation | −18.4 ± 4.1 | −21.5 ± 6.2 | .830 |
Data presented include the entire cohort and those who were in the ICU and on mechanical ventilation at time of the initial echocardiogram.
Figure 2.
Overall trends in LV and RV function from baseline to follow-up. Using a pairwise analysis to compare baseline and follow-up echocardiograms, there were no significant differences between baseline and follow-up in LV and RV function. The width of each “violin” reflects the number of cases for each echocardiographic variable in the vertical axis, a compact display of a continuous distribution of each data population. The central values displayed on these plots are the mean and SD values.
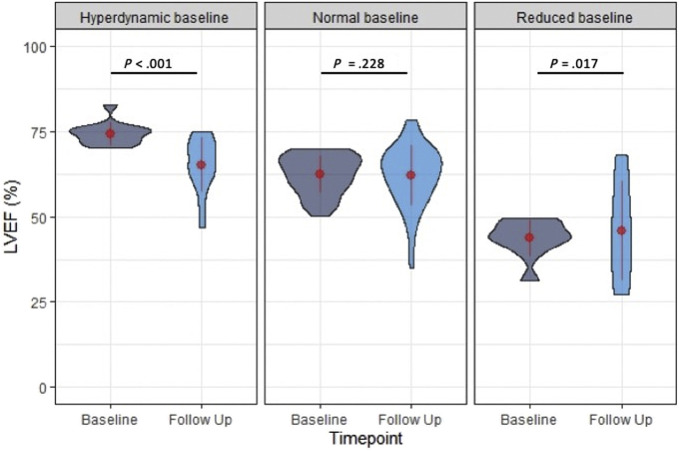
Echocardiographic findings in baseline and follow-up echocardiograms, grouped by their baseline LV and RV function categories, are detailed in Table 3 . In patients with hyperdynamic LVEF at baseline (>70%), there was a significant reduction of LVEF at the time of follow-up (Δ = −8.8%, P < .001 [t = −6.13, df = 32]) due to an increase in LVESV with no significant change in LVEDV, while in patients with normal LVEF (50%–70%) at baseline, there was no significant change in LVEF (Δ = 1.3%, P = .15 [t = −1.44, df = 131]) or LV volumes. In patients with abnormal LVEF at baseline (<50%), there was a significant increase of LVEF (Δ = 6.7%, P = .02 [t = 2.60, df = 19], Figure 3 ) with a nonsignificant decrease in LVESV and LVEDV (comparatively larger decrease in LVEDV).
Table 3.
Echocardiographic characteristics of paired baseline and follow-up echocardiograms, grouped by baseline LV and RV function
| Characteristic | Baseline level | N | Baseline values | Follow-up values | P Value |
|---|---|---|---|---|---|
| LVESV, mL | Hyperdynamic (EF > 70) | 33 | 24.6 ± 9.1 | 32.5 ± 9.8 | <.001 |
| Normal (EF, 50–70) | 99 | 39.0 ± 15.9 | 38.0 ± 19.8 | .826 | |
| Reduced (EF < 50) | 20 | 92.8 ± 36.0 | 81.8 ± 56.2 | .529 | |
| LVEDV, mL | Hyperdynamic (EF > 70) | 33 | 90.2 ± 29.4 | 91.8 ± 20.8 | .818 |
| Normal (EF, 50–70) | 99 | 98.9 ± 34.5 | 98.9 ± 32.1 | .606 | |
| Reduced (EF < 50) | 20 | 160.6 ± 57.5 | 140.9 ± 68.2 | .279 | |
| LVEF, % | Hyperdynamic (EF > 70) | 33 | 73.3 ± 3.1 | 64.4 ± 8.1 | <.001 |
| Normal (EF, 50–70) | 99 | 61.5 ± 5.3 | 62.7 ± 9.1 | .228 | |
| Reduced (EF < 50) | 20 | 42.5 ± 5.9 | 49.3 ± 13.4 | .017 | |
| LVLS, % | Normal (<−18) | 95 | –21.5 ± 2.6 | –20.3 ± 4.0 | .006 |
| Reduced (>−18) | 58 | –14.5 ± 2.9 | –16.7 ± 5.2 | <.001 | |
| RVFWS, % | Normal (<−20) | 48 | –26.7 ± 3.5 | –26.8 ± 4.0 | .821 |
| Reduced (>−20) | 17 | –16.3 ± 3.9 | –18.7 ± 5.5 | .055 | |
| RVGLS, % | Normal (>−20) | 37 | –23.5 ± 2.3 | –22.8 ± 3.1 | .243 |
| Reduced (<−20) | 30 | –15.2 ± 3.4 | –17.4 ± 4.9 | .004 | |
| RVBD, cm | Normal (<4.5) | 64 | 3.6 ± 0.6 | 3.6 ± 0.5 | .545 |
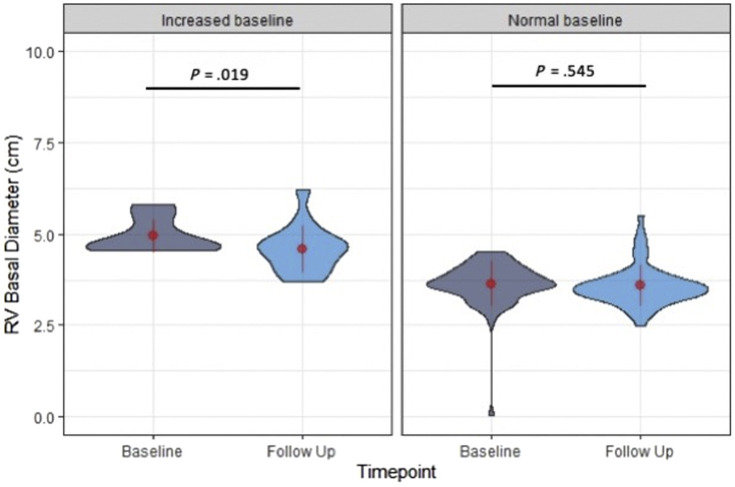
| Increased (>4.5) | 15 | 4.9 ± 0.7 | 4.6 ± 0.6 | .019 |
EF, Ejection fraction.
Figure 3.
Changes in LVEF grouped by baseline LV function. In patients with hyperdynamic EF (>70%) at baseline, there was a significant decrease in LVEF at the time of follow-up. In patients with normal but not hyperdynamic LVEF (50%–70%), there was no significant change. In patients with abnormal LVEF at baseline (<50%), there was significant improvement in LVEF at the time of follow-up. The width of each “violin” reflects the number of cases for each echocardiographic variable in the vertical axis, a compact display of a continuous distribution of each data population. The central values displayed on these plots are the mean and SD values.
Similarly, in patients with normal LVLS (<−18%) at baseline, there was a significant decrease of LVLS at the time of follow-up: Δ = 1.2%, P = .006 (t = 2.79, df = 94), while in patients with impaired LVLS at baseline, there was a significant increase: Δ = −2.2%, P < .001 (t = −3.67, df = 57; Figure 4 ). Overall, 25 of the 95 patients with normal LVLS at baseline became abnormal at follow-up, while 15 of the 58 that were initially abnormal became normal at follow-up.
Figure 4.
Changes in LVLS grouped by baseline function. In patients with normal LVLS (<−18%) at baseline, there was a significant worsening of LVLS at the time of follow-up, while in patients with reduced LVLS at baseline, there was a significant improvement. The width of each “violin” reflects the number of cases for each echocardiographic variable in the vertical axis, a compact display of a continuous distribution of each data population. The central values displayed on these plots are the mean and SD values.
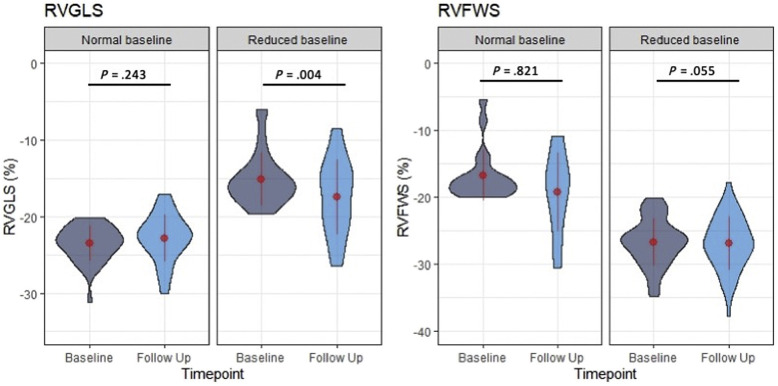
As for the evaluation of the RV, in patients with normal RVFWS at baseline (<−20%), there was no significant change in RVFWS: Δ = −0.2%, P = .82 (t = −0.23, df = 47). In patients with abnormal RVFWS (>–20%) at baseline, there was a borderline but nonsignificant improvement at the time of follow-up: Δ = 2.3%, P = .055 (t = 2.07, df = 16). In patients with normal RVGLS at baseline (<−20%), there was no significant change in RVGLS: Δ = 0.7%, P = .24 (t = 1.19, df = 36), while in patients with abnormal RVGLS at baseline, there was significant improvement in RVGLS: Δ = −2.3%, P = .004 (t = −3.11, df = 29; Figure 5 ). In patients with normal RVBD (<4.5 cm) at baseline, there was no significant change at the time of follow-up: Δ = <−0.1 cm, P = .55 (t = 0.61, df = 63), while in patients with abnormal RVBD (>4.5 cm) at baseline, there was significant improvement: Δ = −0.4 cm, P = .019 (t = −2.66, df = 14; Figure 6 ).
Figure 5.
Changes in RVGLS and RVFWS grouped by baseline RV function. Patients with reduced RV function (global longitudinal strain for free wall strain) improved at the time of follow-up (significant only for RVGLS), and there was no change in those with normal baseline RV function. The width of each “violin” reflects the number of cases for each echocardiographic variable in the vertical axis, a compact display of a continuous distribution of each data population. The central values displayed on these plots are the mean and SD values.
Figure 6.
Changes in RVBD grouped by baseline RV size. In patients with abnormal RVBD (>4.5 cm) at baseline, there was significant improvement at the time of follow-up, while in patients with normal RVBD (<4.5 cm) at baseline, there was no significant change. The width of each “violin” reflects the number of cases for each echocardiographic variable in the vertical axis, a compact display of a continuous distribution of each data population. The central values displayed on these plots are the mean and SD values.
The total number of patients in each clinical category at baseline and time of follow-up is described in Table 4 .
Table 4.
Number of patients in each clinical category at baseline and time of follow-up
| Characteristic | Clinical category | No. at baseline | No. at follow-up | No. of changed groups∗ |
|---|---|---|---|---|
| LVEF, % | Hyperdynamic (EF > 70) | 33 | 29 | 23 became normal; 2 became reduced |
| Normal (EF, 50–70) | 99 | 104 | 20 became hyperdynamic; 6 became reduced | |
| Reduced (EF < 50) | 20 | 19 | 1 become hyperdynamic; 8 became normal | |
| LVLS, % | Normal (<−18) | 95 | 85 | 25 became abnormal |
| Reduced (>−18) | 58 | 68 | 15 became normal | |
| RVFWS, % | Normal (<−20) | 48 | 42 | 7 became abnormal |
| Reduced (>−20) | 17 | 23 | 1 become normal | |
| RVGLS, % | Normal (>−20) | 37 | 45 | 5 became abnormal |
| Reduced (<−20) | 30 | 22 | 13 became normal | |
| RVBD, cm | Normal (<4.5) | 64 | 64 | 5 became increased |
| Increased (>4.5) | 15 | 15 | 5 became normal |
EF, Ejection fraction.
Number of patients that moved into a different clinical category at the time of follow-up.
Discussion
In this follow-up report of surviving patients from the WASE-COVID-19 study, we have shown that while LV and RV function did not change significantly over time in our entire cohort, different patterns of change were observed according to baseline function. In patients with baseline hyperdynamic LVEF and normal LVLS, there was significant reduction at the time of follow-up. Conversely, in patients with baseline impaired LVEF, LVLS, RVGLS, and RVBD, there was significant improvement at the time of follow-up.
These findings can be explained by differences in cardiac structural and functional changes in different subgroups of patients with acute COVID-19 infection. In our cohort, 14% of patients demonstrated hyperdynamic LV function at baseline, likely an adaptive physiologic response to COVID-19 infection or acute stress response from critical illness or sepsis. In this subset of patients, follow-up echocardiograms showed a significant decrease in LVEF toward normalization, which can likely be explained by resolution of the acute infection and inflammation. This was in contrast to patients with normal EF, in whom no significant differences were seen, and those with reduced baseline LVEF, in whom LVEF improved at follow-up. This pattern of “regression to the mean” is probably the result of resolution of the stress physiological response of the acutely ill patient.
Interestingly, in patients with normal LVLS at baseline, a similar pattern was observed to that of patients with hyperdynamic LVEF, with significant reduction noted at the time of follow-up echocardiogram, perhaps due to resolution of the hyperdynamic component of the acute systemic inflammatory response. Conversely, in patients with impaired LVLS at baseline, presumably due to myocardial dysfunction, follow-up echocardiograms showed significant improvement in LVLS, possibly indicative of recovery from acute SARS-CoV-2 infection. Multiple previous studies have shown that LVLS is more sensitive at detecting subtle changes in LV function compared with LVEF.6, 7, 8, 9, 10 This may be the case in our patient cohort, with LVLS but not LVEF showing significant reduction over time in patients with normal LV function at baseline. With respect to patients with sepsis in particular, it has been postulated that LVEF could be affected by the presence of myocardial dysfunction but also be load dependent (hypovolemia, decreased preload, etc.), while longitudinal strain should reflect mostly myocardial dysfunction,11 therefore reflecting different aspects of the pathophysiology of sepsis-induced cardiac response.12
In terms of right-sided function, in patients with impaired RVGLS at baseline, there was significant improvement noted at the time of follow-up echocardiogram. Because the lungs are the main target organ of SARS-CoV-2 and given the large prevalence of acute respiratory distress syndrome in critically ill patients with COVID-19 infection, the RV is thought to be particularly susceptible to dysfunction following COVID-19 infection.13 Previous studies have demonstrated RV failure as a sequelae of acute lung injury and acute respiratory distress syndrome,14 as the RV is easily affected by changes in pulmonary vascular resistance.15 Thus, the improvement seen in RVGLS in patients with impaired RV function in our study may be indicative of improvement in lung function from the time of baseline echocardiogram to the time of the follow-up study. This was also reflected by significant improvement in RVBD in patients with increased RVBD at baseline, suggestive of RV reverse remodeling during the follow-up period possibly associated with recovery in lung function or to interim changes in the need for lung-supportive strategies (mechanical or noninvasive ventilation, etc.).
Few studies have reported results of follow-up echocardiograms in patients with COVID-19. In most other studies, the follow-up echocardiograms were performed during the initial hospital admission, probably in response to clinical improvement or even clinical deterioration.16, 17, 18, 19, 20 In a report of 79 patients with follow-up echocardiogram after 3 months, it was found that the proportion of cases with RV abnormalities decreased from 51% during the acute illness to 19% and that the proportion with LV systolic dysfunction decreased from 13% to 9%.21 A recent study on patients recovered from COVID-19 infection using cardiac magnetic resonance reported a high prevalence of myocardial inflammation, even in patients that were not hospitalized for their initial COVID-19 infection.22 Our findings in a larger, international cohort of surviving patients, with a longer follow-up, utilizing sensitive echocardiographic techniques (longitudinal RV and LV strain) and centralized readings, advance the knowledge of cardiovascular recovery from COVID-19 infection, a field that is still in need of further exploration.
Limitations
The limitations of our study include a relatively small number of patients with paired follow-up echocardiograms (n = 153) as well as a relatively short length of follow-up (mean 129 days). However, given the overall short time course of the global COVID-19 pandemic, our study is one of the first to report on cardiac structural and functional changes in patients with acute COVID-19 infection. We also recognize that our patient cohort is limited to those with clinical indications for serial echocardiographic follow-up and that these patients are more likely to have cardiac involvement and to be in the more severe spectrum of disease compared with the general population of patients with COVID-19 infection. On the other hand, this analysis does not include patients that died, who probably would have had the most severe abnormalities in cardiac structure and function. Nevertheless, this study provides valuable insights into longitudinal echocardiographic trends in patients with acute SARS-CoV-2 infection and demonstrates differences depending on baseline LV and RV function.
Conclusion
Overall, there were no significant changes in LV and RV function over time in patients recovering from COVID-19 infection. However, differences in cardiac functional changes were observed according to baseline LV and RV function. Left ventricular and RV function tends to improve in those with impaired baseline function, while it tends to decrease in those with hyperdynamic LV or normal RV function.
Additional WASE-COVID Investigators
Vince Ryan V. Munoz, MD, Philippine Heart Center, Quezon City, Philippines; Rafael Porto De Marchi, MD, Radiology Institute of the University of Sao Paulo Medical School, São Paulo, Brazil; Sergio M. Alday-Ramirez, PhD, and Consuelo Orihuela, MD, Instituto Nacional de Ciencias Medicas y Nutricion, Mexico City, Mexico; Anita Sadeghpour, MD FASE, Rajaie Cardiovascular Medical and Center, Echocardiography Research Center, Iran University of Medical Sciences, Tehran, Iran; Jonathan Breeze, MD, and Amy Hoare, King's College Hospital, London, United Kingdom; Carlos Ixcanparij Rosales, MD, Centro Nacional 20 de Noviembre, Instituto de Seguridad y Servicios Sociales de los Trabajadores, CDMX, Mexico; Ariel Cohen, MD, Hôpitaux de l'est parisien St. Antoine-Tenon, Universite Pierre et Marie Curie, Paris, France; Martina Milani, MD, Ilaria Trolese, RDCS, Oriana Belli, MD, and Benedetta De Chiara, MD, Ospedale Niguarda, Milan, Italy; Michele Bellino, MD, and Giuseppe Iuliano, MD, University of Salerno, Salerno, Italy; Yun Yang, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Acknowledgments
We thank Victor Mor-Avi from the University of Chicago; Katie Ions, Nancy Spagou, Alex Hudson, Jake Kenworthy, Lokken Wong, Angela Mumith, Will Hawkes, and Louise A. Tetlow from Ultromics; Andrea Van Hoever from the American Society of Echocardiography; and Patricia Marques, Gaynor Jones, Seamus Walker, Gurpreet Dhadday, Aiko Nepomuceno, Jherick Tay, Claire Mitchell, Sarah Hastings, and Emma-Jayne Robins.
Footnotes
A full list of additional WASE-COVID investigators is provided after the conclusion.
Conflicts of Interest: G.M.W., and T.D. are employees of Ultromics. R.S. is a consultant for Ultromics. M.J.M. is on the advisory board and speaker's bureau for Bracco and Philips. F.M.A. received institutional (MedStar Health) research grants from TOMTEC, Ultromics, GE, and Caption Health and is on the nonpaid scientific advisory committee for Ultromics. R.M.L. is on the advisory board and speaker's bureau for Philips and the advisory board for Caption Health. All other authors have no conflicts of interest to disclose related to this work.
This work was supported by the American Society of Echocardiography Foundation, the University of Chicago, and MedStar Health with in-kind support from Ultromics and TOMTEC.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.echo.2021.10.015.
Contributor Information
WASE-COVID Investigators:
Vince Ryan V. Munoz, Rafael Porto De Marchi, Sergio M. Alday-Ramirez, Consuelo Orihuela, Anita Sadeghpour, Jonathan Breeze, Amy Hoare, Carlos Ixcanparij Rosales, Ariel Cohen, Martina Milani, Ilaria Trolese, Oriana Belli, Benedetta De Chiara, Michele Bellino, Giuseppe Iuliano, and Yun Yang
Supplementary Data
References
- 1.Karagodin I., Carvalho Singulane C., Woodward G.M., Xie M., Tucay E.S., Tude Rodrigues A.C., et al. Echocardiographic correlates of in-hospital death in patients with acute COVID-19 infection: the World Alliance Societies of Echocardiography (WASE-COVID) study. J Am Soc Echocardiogr. 2021;34:819–830. doi: 10.1016/j.echo.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Yang H., Wright L., Negishi T., Negishi K., Liu J., Marwick T.H. Research to practice: assessment of left ventricular global longitudinal strain for surveillance of cancer chemotherapeutic-related cardiac dysfunction. JACC Cardiovasc Imaging. 2018;11:1196–1201. doi: 10.1016/j.jcmg.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J.H., Park J.H. Strain analysis of the right ventricle using two-dimensional echocardiography. J Cardiovasc Imaging. 2018;26:111–124. doi: 10.4250/jcvi.2018.26.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Medicine Agency Guideline on missing data in confirmatory clinical trials. 2011:1-12. https://www.ema.europa.eu/en/missing-data-confirmatory-clinical-trials Available at:
- 6.Krishnasamy R., Isbel N.M., Hawley C.M., Pascoe E.M., Leano R., Haluska B.A., et al. The association between left ventricular global longitudinal strain, renal impairment and all-cause mortality. Nephrol Dial Transplant. 2014;29:1218–1225. doi: 10.1093/ndt/gfu004. [DOI] [PubMed] [Google Scholar]
- 7.Mignot A., Donal E., Zaroui A., Reant P., Salem A., Hamon C., et al. Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study. J Am Soc Echocardiogr. 2010;23:1019–1024. doi: 10.1016/j.echo.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Stanton T., Leano R., Marwick T.H. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 9.Tops L.F., Delgado V., Marsan N.A., Bax J.J. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur J Heart Fail. 2017;19:307–313. doi: 10.1002/ejhf.694. [DOI] [PubMed] [Google Scholar]
- 10.Zhang K.W., French B., May Khan A., Plappert T., Fang J.C., Sweitzer N.K., et al. Strain improves risk prediction beyond ejection fraction in chronic systolic heart failure. J Am Heart Assoc. 2014;3:e000550. doi: 10.1161/JAHA.113.000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmieri V., Innocenti F., Guzzo A., Guerrini E., Vignaroli D., Pini R. Left ventricular systolic longitudinal function as predictor of outcome in patients with sepsis. Circ Cardiovasc Imaging. 2015;8:e003865. doi: 10.1161/CIRCIMAGING.115.003865. discussion e003865. [DOI] [PubMed] [Google Scholar]
- 12.Hollenberg S.M., Singer M. Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol. 2021;18:424–434. doi: 10.1038/s41569-020-00492-2. [DOI] [PubMed] [Google Scholar]
- 13.Li Y., Li H., Zhu S., Xie Y., Wang B., He L., et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13:2287–2299. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osman D., Monnet X., Castelain V., Anguel N., Warszawski J., Teboul J.L., et al. Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med. 2009;35:69–76. doi: 10.1007/s00134-008-1307-1. [DOI] [PubMed] [Google Scholar]
- 15.Repessé X., Charron C., Vieillard-Baron A. Right ventricular failure in acute lung injury and acute respiratory distress syndrome. Minerva Anestesiol. 2012;78:941–948. [PubMed] [Google Scholar]
- 16.Churchill T.W., Bertrand P.B., Bernard S., Namasivayam M., Churchill J., Crousillat D., et al. Echocardiographic features of COVID-19 Illness and association with cardiac biomarkers. J Am Soc Echocardiogr. 2020;33:1053–1054. doi: 10.1016/j.echo.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazzeri C., Bonizzoli M., Batacchi S., Cianchi G., Franci A., Fulceri G.E., et al. Cardiac involvement in COVID-19-related acute respiratory distress syndrome. Am J Cardiol. 2020;132:147–149. doi: 10.1016/j.amjcard.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmoud-Elsayed H.M., Moody W.E., Bradlow W.M., Khan-Kheil A.M., Senior J., Hudsmith L.E., et al. Echocardiographic findings in patients with COVID-19 pneumonia. Can J Cardiol. 2020;36:1203–1207. doi: 10.1016/j.cjca.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothschild E., Baruch G., Szekely Y., Lichter Y., Kaplan A., Taieb P., et al. The predictive role of left and right ventricular speckle-tracking echocardiography in COVID-19. JACC Cardiovasc Imaging. 2020;13:2471–2474. doi: 10.1016/j.jcmg.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szekely Y., Lichter Y., Taieb P., Banai A., Hochstadt A., Merdler I., et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moody W.E., Liu B., Mahmoud-Elsayed H.M., Senior J., Lalla S.S., Khan-Kheil A.M., et al. Persisting adverse ventricular remodeling in COVID-19 survivors: a longitudinal echocardiographic study. J Am Soc Echocardiogr. 2021;34:562–566. doi: 10.1016/j.echo.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.