Abstract
Background
The initial aim was to study the effects of face masks worn by recently infected individuals on the airborne spread of SARS-CoV-2, but findings motivated us to proceed with comparing the presence of SARS-CoV-2 in air samples near infected individuals at home with those near infected intensive care unit (ICU) patients.
Aim
To assess the presence of SARS-CoV-2 in the air of homes of infected individuals and in ICU rooms of critically ill patients with COVID-19 who were undergoing different forms of potential aerosol-generating medical procedures.
Methods
A high-volume air sampler method was developed that used a household vacuum cleaner with surgical face masks serving as sample filters. SARS-CoV-2 RNA was harvested from these filters and analysed by polymerase chain reaction. Fog experiments were performed to visualize the airflow around the air sampler. Air samples were acquired in close proximity of infected individuals, with or without wearing face masks, in their homes. Environmental air samples remote from these infected individuals were also obtained, plus samples near patients in the ICU undergoing potential aerosol-generating medical procedures.
Findings
Wearing a face mask resulted in a delayed and reduced flow of the fog into the air sampler. Face masks worn by infected individuals were found to contain SARS-CoV-2 RNA in 71% of cases. SARS-CoV-2 was detected in air samples regardless of mask experiments. The proportion of positive air samples was higher in the homes (29/41; 70.7%) than in the ICU (4/17; 23.5%) (P < 0.01).
Conclusion
SARS-CoV-2 RNA could be detected in air samples by using a vacuum cleaner based air sampler method. Air samples in the home environment of recently infected individuals contained SARS-CoV-2 RNA nearly three times more frequently by comparison with those obtained in ICU rooms during potential aerosol-generating medical procedures.
Keywords: SARS-CoV-2, COVID-19, Masks, Air sampling, PCR, Airborne
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has three possible transmission routes: indirect contact transmission via deposited or transmitted infectious droplets via surfaces, direct transmission of virus-carrying droplets when in close vicinity, and airborne transmission through aero-sols emitted by infected individuals [1]. By contrast with large respiratory droplets (i.e. ballistic droplets) that rapidly fall to the ground due to gravity, aerosol particles may remain suspended in air during a prolonged period of time and travel large distances, which is considered airborne transmission. Since the start of the coronavirus disease 2019 (COVID-19) pandemic, both national and international healthcare authorities have heterogeneously assessed the relative contribution and importance of each of these various transmission routes to the cumulative spread of COVID-19 [[2], [3], [4], [5]]. Subsequently, determining the most important containment measure remains challenging, as the effectiveness of each measure in combatting different transmission routes is variable. Cleaning surfaces, washing hands, and sneezing/coughing in the elbow have been adopted to reduce both indirect and direct transmission. Physical distancing of individuals and wearing face masks are primarily aimed at preventing direct transmission of large infectious droplets, whereas adequate air ventilation would be relevant in preventing airborne transmission.
Real-life measurements on the presence of SARS-CoV-2 RNA in the air surrounding infected individuals would likely provide more valuable information to assess the importance of the different transmission routes. Previous studies used several types of air samplers based on different techniques showing heterogeneous results with overall low yields due to small sampling air volumes, whereas viruses are only present at very low concentrations in the air [6].
We developed a high-volume air sampler method using a household vacuum cleaner with a surgical mask serving as a sample filter. This filter was tested on the presence of SARS-CoV-2 RNA by reverse transcriptase quantitative polymerase chain reaction (RT–qPCR). In this study, we initially aimed to estimate the efficacy of face masks worn by infected persons on the spread of SARS-CoV-2 into the surrounding air in household settings. Our hypothesis was that the use of face masks would reduce spread of SARS-CoV-2. However, based on unexpected but intriguing findings, we extended this study by assessing the presence of SARS-CoV-2 in the air of homes of infected individuals and in intensive care unit (ICU) rooms of critically ill patients with COVID-19 who were undergoing different forms of potential aerosol-generating medical procedures.
Methods
Study design
The study was performed at two sites between October 1st, 2020, and January 22nd, 2021. First, air sampling was performed in homes of SARS-CoV-2-positive healthcare workers (HCWs) while they wore different types of face masks. Second, based on observations in the first participants, we also took environmental air samples in their homes (i.e. remote from the infected person) at the end of our visit and in rooms of critically ill COVID-19 patients in the ICU.
As part of hospital policy, HCWs were tested with combined throat- and nasopharyngeal swabs in case of symptoms suggestive of SARS-CoV-2 infection. HCWs with positive SARS-CoV-2 results who had high viral loads (i.e. RT–qPCR cycle threshold (CT) values <21) were selected, and air sampling within 24 h after diagnostic testing was performed. HCWs were isolated in their homes prior to our visit, and some of the subjects were quarantining together with family members who also experienced COVID-19 symptoms. Although the ventilation rate was not measured, windows were closed as the sampling was performed during autumn and winter and mechanical room ventilation was absent. COVID-19 patients in the ICU were selected irrespective of the viral load measured in the nasopharyngeal swabs. The ICU rooms had mechanical room ventilation with an air exchange rate of six times per hour.
The Institutional Review Board approved the study protocol (IRB protocol number 2020-092) and declared that this study does not fall within the scope of the Dutch Medical Research Involving Human Subjects Act. Written informed consent was obtained from the HCWs during the home visits. As no specific instructions for wearing masks or other behaviour requirements were given to patients in the ICU and only air samples were collected, oral informed consent was obtained for this part of the study. The study was performed in accordance with the Helsinki Declaration as revised in 2013.
Performance vacuum cleaner
Air sampling was performed using a Nilfisk household vacuum cleaner (model Elite performance comfort, 2000 W), which has a high-efficiency particulate air (HEPA) filter on the air outlet. To assess the performance of our method, the volumetric airflow of the vacuum cleaner was measured with the Acin FlowFinder mk-2 in the SenseLab [7]. The air velocity of the suction was measured with the Dantec Dynamics ComfortSense air velocity meter. For the visualization experiment, aerosols with diameters ranging from 10 to 50 μm were produced with polypropylene glycol with the Ayra WSM Black 01 fogger machine that exhaled 0.4 L of air per breath [8].
Methods of air sampling
For both parts of the study, a type IIR surgical face mask (Romed Holland, Wilnis, Netherlands) was used as a sample filter and folded over the hose inlet grip of the vacuum cleaner. Two rubber bands (each wrapped around twice) made an airtight seal and prevented the mask from being suctioned into the hose. After the application of the sample filter on to the inlet of the vacuum cleaner, the air inlet circle (of ∼2.5 cm in diameter) was marked. After each measurement, the sample filter was removed from the hose inlet and carefully inserted into a plastic sampling bag without touching the sample filter. The hose inlet was cleaned with an alcohol-soaked cloth before and after starting every subsequent measurement.
Air sampling in homes was performed at ∼10 cm distance from the mouth for 2.5 min per measurement. During each measurement, HCWs were instructed to inhale and exhale deeply, and cough twice every 30 s. Each infected HCW consecutively wore no mask, a cotton non-medical mask obtained from a large international department store (HEMA, Amsterdam, Netherlands), a surgical mask without IIR and without medical classification that had poor filtration effectiveness (Khao Trang, Quoc Bao, Vietnam), and a type IIR surgical mask that had an effective particle filter (Romed Holland). A mouth-shaped area was marked in front of the mouth on each face mask that participants were wearing during the experiments.
Air samples in the ICU were collected in proximity (i.e. ∼50 cm distance) of COVID-19 patients undergoing invasive mechanical ventilation (iMV), and AGMP such as high-flow nasal canulae (HFNC) therapy and endotracheal intubation.
The investigators used protective clothing, FFP2 masks, and eye protection glasses during the experiments (Figure 1 ). Before the start of the study, one pilot experiment was performed with an infected volunteer with a high viral load, in which a double face mask on the hose inlet grip of the vacuum cleaner was used. The outside mask tested positive, whereas the inside mask tested negative. This indicated that the air entering the vacuum cleaner did not contain the virus. As an additional precaution, a vacuum cleaner with a HEPA filter on the airflow leaving the vacuum cleaner to prevent the potential spread of the virus into the environment was used.
Figure 1.
Setting household experiment. Two arrows point towards the face mask that the infected healthcare worker was wearing (with a mouth-shaped area marked in front of the mouth) and the face mask that was used as the sample filter (with the air inlet circle marked).
Harvesting of viral RNA from the sample filters and face masks
Both sample filters and face masks (i.e. that were worn by infected HCWs during the home experiments) were analysed for the presence of SARS-CoV-2. In the medical laboratory the marked circle of the sample filters and the marked mouth shapes of the face masks worn by the participants were cut out using scissors. Subsequently, these cut-out pieces were inserted into separate tubes each with 3 mL PCR extraction buffer and incubated for 40 min at room temperature, while during this period samples were also vortexed four times for 1 min. Finally, 500 μL of the extraction was used for RNA extraction using the MagNA Pure Total Nucleic Acid Isolating Large Volume Kit (Roche, Mannheim, Germany).
RT–qPCR
Original patient samples obtained during routine clinical care were tested on our validated in-house RT–qPCR assay according to the national reference method that was established after international collaboration, the ELITe InGenius® (Elitech, Paris, France) platform, or the GeneXpert Xpress SARS-CoV-2 PCR assay (Cepheid Inc., Sunnyvale, CA, USA) according to the instructions of the manufacturer [9,10]. All study samples, i.e. sample filters and cut-out mouth-shaped parts of face masks, were assessed by our in-house RT–qPCR assay that targets SARS-CoV-2 E-gene and RdRp-gene, whereby 0.1 mL from the sample extraction was analysed on the presence of SARS-CoV-2 RNA.
Statistical analysis
All data were analysed using Microsoft Excel and R version 3.3.2 (R Foundation for Statistical Computing). Proportions were compared by using χ2-test or Fisher's exact test as appropriate. P < 0.05 was considered to be statistically significant.
Results
Air sampler characteristics
The volumetric airflow into the vacuum cleaner was 97 m3/h without sample filter, and 29 m3/h when the sample filter was applied on to the inlet (i.e. corresponding to 483 L/min).
The airflow velocity was 0.15 m/s (SD: 0.06) at 10 cm distance of the hose inlet, and 0.08 m/s (SD 0.03) at 25 cm distance. During air sampling, suction of the air into the vacuum cleaner did not cause any visible changes in the shape or position of the face masks.
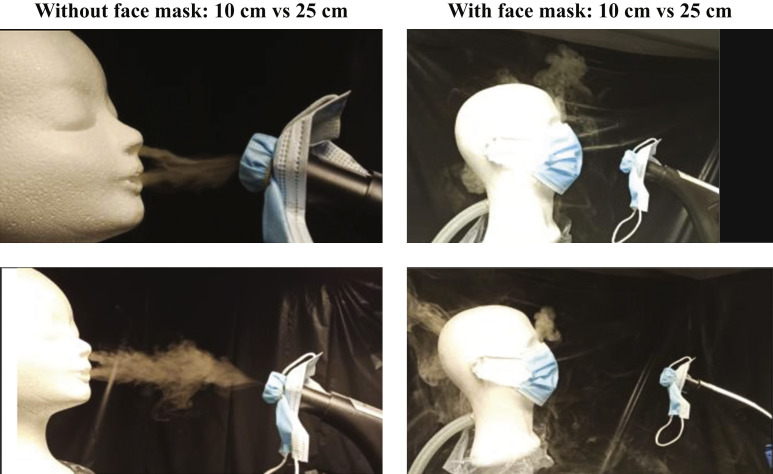
In the visualization experiment, aerosols were visibly suctioned into the vacuum cleaner at 10 cm and 25 cm distance of the breathing manikin head without face mask (Figure 2 and Supplementary Appendix). By contrast, putting a face mask on the breathing manikin head resulted in a delayed suction of a part of the aerosols that leaked around the borders of the face mask.
Figure 2.
Aerosol visualization experiment. Differences in exhaled fog movements were observed when wearing masks or at different distances between the hose inlet of the vacuum cleaner and the mouth of the manikin.
Observations in HCWs
Of the 15 HCWs that were screened, 12 agreed to par-ticipate, one HCW was unable to participate because of COVID-19-related symptoms and two HCWs refused for other reasons. Nasopharyngeal samples of the HCWs had a median CT value of 17.5 (range: 13–19) (Table I ).
Table I.
SARS-CoV-2 RNA positivity in air samples of healthcare workers, with or without face mask, and critically ill intensive care unit patients
| Sample origin | CT-value of positive samples: median (range) | No. of samples |
|---|---|---|
| Early symptomatic HCW at home (N = 12) | ||
| Nasopharyngeal swab | 17.5 (13–19) | 12 |
| Air sample in front of mouth | ||
| Uncovered | 35 (32–36) | 10 |
| Covered with cotton mask | 33.5 (32–35) | 9 |
| Covered with surgical mask | 35 (32–36) | 6 |
| Covered with surgical mask RII | 34 (33–36) | 10 |
| Free air from room | 34 (32–36) | 4 |
| Sample of mask worn during experiment | ||
| Cotton mask | 33 (29–34) | 9 |
| Surgical maska | 32 (28–34) | 6 |
| Surgical mask RII | 33 (30–35) | 10 |
| Critically ill patients in ICU (N = 17) | ||
| Invasive mechanical ventilation (N = 6) | ||
| Nasopharyngeal swabb | 22.5 (16–26) | 4 |
| Air sample at 50 cm distance from mouth | 35 (35–35) | 1 |
| High-flow nasal cannula (N = 5) | ||
| Nasopharyngeal swab | 28 (17–30) | 5 |
| Air sample at 50 cm distance from mouth | 33.5 (33–34) | 1 |
| During intubation procedure (N = 6) | ||
| Nasopharyngeal swab | 28.5 (21–40) | 6 |
| Air sample at 50 cm distance from mouth | 35 (35–35) | 2 |
HCW, healthcare worker; ICU, intensive care unit.
One surgical mask (not IIR) during one experiment was missing.
Two patient nasopharyngeal swabs were taken in another healthcare institution with no available CT-values.
During air sampling, all HCWs suffered from a dry cough, but no sneezing and no productive cough. Two HCWs experienced shortness of breath following our breathing instructions during the experiment.
Air samples taken in front of the uncovered faces of HCWs were positive in 10 out of 12 (83%) subjects (Table I). No visible droplets were observed on the sample filters after each experiment. The proportion of positive air sample filters was not different between wearing or not wearing face masks: 25 out of 36 (69.4%) vs 10 out of 12 (83%) (P = 0.35), respectively. The face masks worn by the HCWs were positive in 25 out of 35 (71%) of cases.
Only small differences were observed in viral RNA load between samples taken in front of unmasked and masked faces. Therefore, we reasoned that a high viral load of the environmental air – rather than the exhaled air of infected individuals – may have resulted in the high proportion of viral RNA detection. We assessed this by additional collection of environmental air samples in the homes (i.e. remote from the infected person) at the end of the visit. Of these samples, four out of five (80%) samples were positive for SARS-CoV-2 RNA (Table I).
Observations in ICU rooms of critically ill patients
Air samples were collected near critically ill COVID-19 patients in ICU (median: 3 (range: 0–9) days after nasophangeal swab): six patients during iMV, five during HFNC (60 L/min), and six during an endotracheal intubation procedure (Table I). Air samples were positive in one out of six (16%) cases during iMV, one out of five (20%) during HFNC, and two out of six (33%) during the intubation procedure. Of note, intubations were performed using rapid sequencing induction including muscle relaxants; all procedures were without complication.
The proportion of positive air samples was lower in the proximity of critically ill patients compared with HCWs: 4/17 (23.5%) vs 29/41 (70.7%) (P < 0.01); for this comparison the air samples taken in front of the uncovered mouths of the infected HCWs were excluded in order to exclude the possible contribution of large droplets instead of aerosols.
Discussion
In this study, we were able to detect SARS-CoV-2 RNA in air samples using a household vacuum cleaner and a routine RT–qPCR test. This air-sampling method uses commonly available material and techniques and is easy to perform. Air samples that were taken in the residential environment of recently infected persons with high viral loads more frequently contained SARS-CoV-2 RNA by comparison with air samples from patient rooms of critically ill COVID-19 patients during potential AGMP.
Compared with other air samplers, our approach has the advantage of including much higher air volumes in order to increase sensitivity, which could explain why many other studies were less successful in detecting SARS-CoV-2 RNA in air samples [6]. Only a few studies have shown the presence of SARS-CoV-2 RNA in air samples [11,12]. Molecular detection is more sensitive than viral cell culture, but culture remains needed to establish the presence of viable virus. Most currently available air-sampling techniques comprise ‘high-velocity’ impingers which suck airborne virus from the air into a bubbling liquid virus culture medium. These air-sampling devices create high shear forces and intense mixing at the air–liquid interface, which may damage viral surface proteins and prevent them from growing in the culture [3,13].
Other possible explanations for the high frequency of positive air samples in the home environment could be related to the specific selection of individuals with high viral loads in a very early phase of the disease and the setting with poor ventilation in which these samples were taken. In poorly ventilated spaces, exhaled aerosols may accumulate in the space, creating a higher concentration of possibly infectious aerosols [1,14]. A laboratory study reported that these aerosols can remain viable in the air for up to 3 h [15]. The apparently limited effects of face masks worn by the infected HCWs could be largely attributed to the study design in which air samples were taken in rooms where the HCWs already spent a number of hours before the test. Contaminated aerosols exhaled before the face mask test in combination with poor ventilation in homes could have contributed to the high number of positive air samples. This hypothesis is supported by the finding that most environmental air samples taken remote from the infected HCW after the mask experiments were also positive.
Furthermore, SARS-CoV-2 RNA was less frequently detected in air samples obtained in the ICU during AGMPs in comparison to those obtained in homes, which is surprising as the risk is deemed especially high during AGMPs [16,17]. These results could be related to the lower viral loads in critically ill ICU patients who are in a later phase of the disease [18,19]. Moreover, the presence of adequate ventilation in hospital rooms, in contrast to poor ventilation in private homes, likely contributed to this observation. This is also in accordance with the observation that SARS-CoV-2 infections are more frequently acquired at home and not in hospitals or ICUs [20,21].
Several reasons motivated us to postulate that circulating SARS-CoV-2 RNA due to poor ventilation around persons with high viral loads is a plausible explanation for our findings.
First, excreted large droplets are most likely effectively caught by the masks worn by an infected individual as the majority of masks were SARS-CoV-2 RNA positive. No droplets were observed on the sample filters on the hose inlet of the vacuum cleaner. Second, differences in RNA positivity of air samples between settings without a mask and various types of mask were very limited. Third, no exhaled fog appeared to go through the face masks (but only partially around the mask) in the aerosol visualization experiment. Fourth, we consider direct transmission of large infectious droplets to be an unlikely explanation for the positive environmental air samples or positive air samples taken in front of the mask-covered faces. Therefore, it is likely that both the air passing around the mask and aerosols still floating around from the period prior to the actual mask experiment contributed to the high numbers of positive air samples.
Several studies have shown that different face masks can allow for different levels of leakage, including a previous study that tested 14 different masks, including surgical, KN95, cotton, and homemade masks [22]. The study showed that a tight fit is important to avoid outward leakage through the per-imeter, as well as size in general. Similarly, other studies have shown that medical masks stop the forward motion of jets of both coughs and breaths by reducing the speed and redirecting backward, and that well-fitted homemade masks with several layers can also reduce the leakage [23,24].
There are several study limitations to consider. First, the initial aim was to measure the protective effects of face masks worn by SARS-CoV-2-infected persons to prevent further spread into the environment. Our approach failed to address this research question due to our sampling setting with poor ventilation resulting in many positive air samples due to the environmental presence of viral RNA. Therefore, our findings should not be interpreted as a failure of the protective effects of face masks. Importantly, SARS-CoV-2 was also detected on the masks worn by the infected persons and thus these masks limited the exposition of the virus to the environment. Also comparing the different types of face masks was not possible due to confounding by environmental SARS-CoV-2 RNA in the air and the fixed consecutive order of experiments without and with different face masks. Second, all our observations were carried out prior to the emergence of variants of concern, including the alpha, beta, gamma and delta SARS-CoV-2 variants in the Netherlands [25]. We cannot exclude that different results would have been obtained with more transmissible variants.
In conclusion, the presented vacuum cleaner-based air sampling followed by RT–qPCR method is simple to perform, does not require expensive materials, and is effective in detecting SARS-CoV-2 in air. Although AGMPs are considered high risk for airborne transmission of SARS-CoV-2 and the highest levels of personal protective equipment are thus used during these procedures, this study detected airborne viral RNA three times more often in the home environment of recently infected individuals. Airborne transmission of SARS-CoV-2 should be considered as an important transmission route, mainly in settings with poor ventilation such as private homes and during the early phase of infection.
Acknowledgements
We would like to thank our laboratory technicians, especially H. Veltman, G. Doejaaren, and D. Wille, and team managers for their assistance in performing the experiments. We would also like to thank Dr D. Verboom for her assistance during the conceptual phase of the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.10.018.
Conflict of interest statement
None declared.
Funding sources
None.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Video material of exhaled fog movements during experiments with and without wearing masks and at different distances between the hose inlet of the vacuum cleaner and the mouth of the mannequin exhaling the fog.
References
- 1.Morawska L., Tang J.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., et al. How can airborne transmission of COVID-19 indoors be minimised? Environ Int. 2020;142:105832. doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bak A., Mugglestone M.A., Ratnaraja N.V., Wilson J.A., Rivett L., Stoneham S.M., et al. SARS-CoV-2 routes of transmission and recommendations for preventing acquisition: joint British Infection Association (BIA), Healthcare Infection Society (HIS), Infection Prevention Society (IPS) and Royal College of Pathologists (RCPath) guidance. J Hosp Infect. 2021;114:79–103. doi: 10.1016/j.jhin.2021.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang J.W., Bahnfleth W.P., Bluyssen P.M., Buonanno G., Jimenez J.L., Kurnitski J., et al. Dismantling myths on the airborne transmission of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Hosp Infect. 2021;110:89–96. doi: 10.1016/j.jhin.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson N., Corbett S., Tovey E. Airborne transmission of covid-19. BMJ. 2020;370:m3206. doi: 10.1136/bmj.m3206. [DOI] [PubMed] [Google Scholar]
- 5.Greenhalgh T., Jimenez J.L., Prather K.A., Tufekci Z., Fisman D., Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021;397:1603–1605. doi: 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges J.T., Nakada L.Y.K., Maniero M.G., Guimarães J.R. SARS-CoV-2: a systematic review of indoor air sampling for virus detection. Environ Sci Pollut Res Int. 2021:1–14. doi: 10.1007/s11356-021-13001-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bluyssen P.M., Zeist van F., Kurvers S., Tenpierik M., Pont S., Wolters B., et al. The creation of Senselab: a laboratory for testing and experiencing single and combinations of indoor environmental conditions. Intell Build Int. 2018;10:5–8. [Google Scholar]
- 8.Ortiz M.A., Ghasemieshkaftaki M., Bluyssen P.M. Testing of outward leakage of different types of masks with a breathing manikin head, ultraviolet light and coloured water mist. Intell Build Int. 2021;13:1–19. [Google Scholar]
- 9.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT–PCR. Euro Surveill. 2020;25:2431. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong D.S.Y., Claas E.C.J., Breijer S., Vaessen N. Comparison of the GeneFinderTM COVID-19 Plus RealAmp Kit on the sample-to-result Platform ELITe InGenius to the national reference method: an added value of N gene target detection? J Clin Virol. 2020;132:104632. doi: 10.1016/j.jcv.2020.104632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santarpia J.L., Rivera D.N., Herrera V.L., Morwitzer M.J., Creager H.M., Santarpia G.W., et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci Rep. 2020;10:12732–12738. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lednicky J.A., Lauzardo M., Fan Z.H., Jutla A., Tilly T.B., Gangwar M., et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis. 2020;100:476–482. doi: 10.1016/j.ijid.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown J.R., Tang J.W., Pankhurst L., Klein N., Gant V., Lai K.M., et al. Influenza virus survival in aerosols and estimates of viable virus loss resulting from aerosolization and air-sampling. J Hosp Infect. 2015;91:278–281. doi: 10.1016/j.jhin.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Somsen G.A., van Rijn C., Kooij S., Bem R.A., Bonn D. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Respir Med. 2020;8:658–659. doi: 10.1016/S2213-2600(20)30245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harding H., Broom A., Broom J. Aerosol-generating procedures and infective risk to healthcare workers from SARS-CoV-2: the limits of the evidence. J Hosp Infect. 2020;105:717–725. doi: 10.1016/j.jhin.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaeckle N.T., Lee J., Park Y., Kreykes G., Evans M.D., Hogan C.J. Aerosol generation from the respiratory tract with various modes of oxygen delivery. Am J Respir Crit Care Med. 2020;202:1115–1124. doi: 10.1164/rccm.202006-2309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong D.S.Y., Fragkou P.C., Schweitzer V.A., Chemaly R.F., Moschopoulos C.D., Skevaki C. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Respiratory Viruses. How to interpret and use COVID-19 serology and immunology tests. Clin Microbiol Infect. 2021;27:981–986. doi: 10.1016/j.cmi.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss A., Jellingsø M., Sommer M.O.A. Spatial and temporal dynamics of SARS-CoV-2 in COVID-19 patients: a systematic review and meta-analysis. EBioMedicine. 2020;58:102916. doi: 10.1016/j.ebiom.2020.102916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shields A., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E., Allen J.D., et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75:1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sikkema R.S., Pas S.D., Nieuwenhuijse D.F., O’Toole Á., Verweij J., van der Linden A., et al. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. Lancet Infect Dis. 2020;20:1273–1280. doi: 10.1016/S1473-3099(20)30527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortiz M.A., Bluyssen P.M. A method to visualize and quantify aerosols of outward leakage around the perimeter of barrier masks. Healthy Build Eur. 2021:254. [Google Scholar]
- 23.Verma S., Dhanak M., Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994) 2020;32 doi: 10.1063/5.0016018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verma S., Dhanak M., Frankenfield J. Visualizing droplet dispersal for face shields and masks with exhalation valves. Phys Fluids (1994) 2020;32 doi: 10.1063/5.0022968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong D.S.Y., Koeleman J.G.M., Vaessen N., Breijer S., Paltansing S., de Man P. Rapid screening method for the detection of SARS-CoV-2 variants of concern. J Clin Virol. 2021;141:104903. doi: 10.1016/j.jcv.2021.104903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video material of exhaled fog movements during experiments with and without wearing masks and at different distances between the hose inlet of the vacuum cleaner and the mouth of the mannequin exhaling the fog.