Abstract
Background
Oxidative stress may be a key player in COVID-19 pathogenesis due to its significant role in response to infections. A defective redox balance has been related to viral pathogenesis developing a massive induction of cell death provoked by oxidative stress. The aim of this study is to perform a complete oxidative stress profile evaluation regarding antioxidant enzymes, total antioxidant capacity and oxidative cell damage in order to characterize its role in diagnosis and severity of this disease.
Methods
Blood samples were obtained from 108 COVID-19 patients and 28 controls and metabolites representative of oxidative stress were assessed. The association between lipid peroxidation and 28-day intubation/death risk was evaluated by multivariable regression analysis. Probability of intubation/death to day-28 was analyzed by using Kaplan-Meier curves and tested with the log-rank test.
Results
Antioxidant enzymes (Superoxide dismutase (SOD) and Catalase) and oxidative cell damage (Carbonyl and Lipid peroxidation (LPO)) levels were significantly higher in COVID-19 patients while total antioxidant capacity (ABTS and FRAP) levels were lower in these patients. The comparison of oxidative stress molecules’ levels across COVID-19 severity revealed that only LPO was statistically different between mild and intubated/death COVID-19 patients. COX multivariate regression analysis identified LPO levels over the OOP (LPO>1948.17 μM) as an independent risk factor for 28-day intubation/death in COVID-19 patients [OR: 2.57; 95% CI: 1.10–5.99; p = 0.029]. Furthermore, Kaplan-Meier curve analysis revealed that COVID-19 patients showing LPO levels above 1948.17 μM were intubated or died 8.4 days earlier on average (mean survival time 15.4 vs 23.8 days) when assessing 28-day intubation/death risk (p < 0.001).
Conclusion
These findings deepen our knowledge of oxidative stress status in SARS-CoV-2 infection, supporting its important role in COVID-19. In fact, higher lipid peroxidation levels are independently associated to a higher risk of intubation or death at 28 days in COVID-19 patients.
Keywords: Oxidative stress, Lipid peroxidation, COVID-19, Intubation, Mortality
Graphical abstract
Highlights
-
•
Antioxidant enzymes and oxidative cell damage levels were significantly higher while total antioxidant capacity was lower in COVID-19 patients.
-
•
Only lipid peroxidation was statistically different across COVID-19 severity.
-
•
Lipid peroxidation levels over 1948.17 μM are an independent risk factor for 28-day intubation/death in COVID-19 patients.
-
•
COVID-19 patients showing lipid peroxidation levels above 1948.17 μM were intubated or died 8.4 days earlier on average.
1. Introduction
A new strain of coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was recognized to have emerged in Wuhan, China in December 2019. It is the third coronavirus that causes severe respiratory disease in humans (COVID-19) [1] along with SARS-CoV [2] and Middle East Respiratory Syndrome-coronavirus (MERS-CoV). In most cases causes a mild or no symptomatic respiratory disease but up to 20% of them present serious illness requiring hospitalization [3] with high fever and pneumonia [4], leading to acute respiratory distress syndrome (ARDS) [5]. Severe COVID-19 infection triggers imbalanced and uncontrolled cytokine response, exuberant endothelial inflammatory reactions and vascular thrombosis [6].
Oxidative stress may be a key player in COVID-19 pathogenesis due to its significant role in response to infections [7]. Several studies have reported some viruses’ ability to disrupt redox balance of a cell to ensure survival [8]. This defective redox balance has been related to viral pathogenesis developing a massive induction of cell death provoked by oxidative stress [9]. Oxidative stress is a typical phenomenon of infections produced by Respiratory Syncytial Virus (RSV) [10], which induces reactive oxygen species (ROS) production, activating pro-inflammatory cytokines and innate immunity [11]. RSV increases lipid peroxidation and decreases Glutathione (GSH) in human alveolar type II-like epithelial cells and small airway epithelial cells and inhibits Nrf2 pathway activation, decreasing gene expression of protective molecules [12]. Furthermore, an excessive amount of ROS is produced by Influenza infection in several tissues [13] such as endothelium [14] and alveolar epithelium [15]. Influenza virus induces apoptosis and cytotoxicity in alveolar epithelial cells increasing caspase 1 and 3 and IL-8 expression [16]. However, this virus facilitates the nuclear translocation of Nrf2 with subsequent expression of a protective enzyme against oxidative injury in human alveolar epithelial cells [16]. Accordingly, oxidative stress may also profoundly impact COVID-19 pathogenesis, but only few studies have been developed for this purpose [[17], [18], [19]].
In this regard, here we aimed to perform a complete oxidative stress profile evaluation regarding antioxidant enzymes, total antioxidant capacity and oxidative cell damage in plasma samples from a prospective COVID-19 patients’ cohort in order to characterize its role in diagnosis and severity of this disease.
2. Materials and methods
2.1. Patient selection
A total of 108 adult patients diagnosed with COVID-19 and admitted at the “Hospital Clínico Universitario de Valladolid” (Valladolid, Spain) were prospectively recruited between 24th of March and 11th of April 2020. Positive result in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was confirmed in all patients by polymerase chain reaction on nasopharyngeal swabs. Patients with other acute diseases, infections, or chronic terminal illness were excluded. In addition, 28 age- and gender-matched healthy volunteers were also recruited in the same time period. Those 28 control samples were collected during the preanesthetic evaluation for scheduled surgery with a negative PCR result for SARS-CoV-2 infection. The study was approved by the Hospital’s Clinical Ethics Committee (CEIm) and the informed consent was obtained from all study participants (cod: PI 20–1717). This study followed the code of ethics of the World Medical Association (Declaration of Helsinki).
2.2. Severity and mortality
Our sample was divided into three groups: i. Controls (n = 28), ii. Mild and moderate COVID-19 patients (n = 76): admitted in ward, iii. Critical (n = 32): mechanical ventilation. Moreover, in terms of mortality there were 20 dead patients (12 of them included in critical group) and 88 alive ones. In order to focus a better approach to disease severity we joined both critical and death COVID-19 patients following another important studies [20]. So that we finally arranged two COVID-19 groups: Intubated or death patients (n = 40) and non-intubated or death (mild) patients (n = 68).
2.3. Biological samples
Plasma samples from each patient were prospectively recruited at 9 a.m. immediately after their first night of hospital admission for preventing circadian variations. Blood was collected in 3.2% sodium citrate tubes and centrifuged at 2000×g for 20 min at room temperature. The resulting plasma was aliquoted and directly frozen at −80 °C until used.
2.4. Antioxidant enzymes’ levels determination
Superoxide dismutase activity was assessed by using Superoxide Dismutase (SOD) Colorimetric Activity Kit, following the manufacturer's recommendations. In the assay, superoxide (O2-) is provided by xanthine oxidase (XO) catalyzed reaction. O2- reacts with a WST-1 dye to form a colored product. SOD scavenges the O2- thus less O2- is available for the chromogenic reaction. The color intensity at 440 nm is used to determine the SOD activity.
Catalase (CAT) activity was determined by using Catalase (CAT) Activity Assay Kit, following the manufacturer's recommendations. The reaction that CAT decomposes H2O2 can be quickly stopped by ammonium molybdate. The residual H2O2 reacts with ammonium molybdate to generate a yellowish complex. CAT activity can be calculated by production of the yellowish complex at 405 nm.
2.5. Total antioxidant capacity levels determination
The antioxidant capacity of samples was evaluated by two methods:
FRAP (Ferric Reducing Antioxidant Power): This assay is based on the ability of the sample to allow iron reduction, which is carried out as described by Benzie and Strain. Results will be quantified by absorbance at 595 nm using a standard curve of known Trolox concentrations, following our lab protocol.
ABTS (2,2-azino-bis (3-ethylbenzthioziozline-6-sulfonic acid): This technique is based on the estimation of the antioxidant capacity by performing a colorimetric test using the cationic radical (ABTS). This assay was performed following our lab protocol.
2.6. Oxidative cell damage levels determination
The DNA oxidized guanosine specie, 8-hydroxy-2′-deoxyguanosine (8-OHdG), was quantitatively measured at 450 nm wavelength by using The DetectX® DNA Damage Immunoassay Kit (Arbor Assays, Ann Arbor, MI, USA), following the manufacturer's recommendations.
Lipid peroxidation (LPO) products were analyzed by using the Bioquochem commercial kit ref KB03002 (BQCell™ MTT, Bioquochem, Oviedo, Spain), following the manufacturer's recommendations. Malondialdehyde (MDA) and 4-Hydroxynonenal (HNE) concentrations were measured as an index of lipid peroxidation. Reactions between indoles and aldehydes (MDA and HNE) gives a diindolylalkane (chromophore) whose maximal absorbance is in the 580–620 nm region.
Protein carbonyl was analyzed by using Protein Carbonyl Colorimetric Assay Kit (Tissue and Serum Samples) commercial kit ref E-BC-K117-S (Elabscience Biotechnology Inc, United States), following the manufacturer's recommendations. Protein carbonyl was indirectly calculated by measuring at 370 nm after the precipitate formed between carbonyl group and 2, 4-dinitrophenylhydrazine is dissolved.
2.7. Statistical analysis
Differences between groups were assessed as described in Tamayo-Velasco A et al. [21]. Differences in oxidative stress molecules' levels between groups were assessed using the Mann Whitney U test. Those differences across COVID-19 severity were evaluated by the Kruskal–Wallis test, with post hoc tests adjusting for multiple comparisons. The optimal operating point (OOP) of LPO was calculated, being the value for which the point on the curve had the minimum distance to the upper left corner (where sensitivity = 1, and specificity = 1). By Pythagoras’ theorem this distance is: Optimal Operating Point (OOP) = √(1−sensitivity)2 + (1−specificity) [22]. COX multivariable logistic regression analysis was employed to evaluate the association between LPO and 28-day intubation/death risk. Variables with a p-value <0.1 in the univariate regression analysis were included in the multivariate analysis as adjusting variables. Results derived from the multivariate logistic regression analysis were validated by bootstrapping method using 1000 random samples. We analyzed probability of intubation/death to day-28 in COVID-19 patients based on LPO OOP by using Kaplan-Meier curves and tested with the log-rank test (Mantel-Haenszel). We considered 2-sided p-values <0.05 to indicate statistical significance. All data were analyzed using the IBM SPSS 22.0 software (SPSS, Chicago, IL).
2.8. Role of the funding source
None.
3. Results
3.1. Clinical characteristics
A total of 136 patients were registered in the study. Clinical characteristics of patients are shown in Discovery cohort panel of Table 1 in Tamayo-Velasco et al. [21]. In terms of age, there were no differences. Hypertension was the principal comorbidity followed by presence of diabetes, lung disease or coronary disease in both COVID-19 and Non COVID-19 patients. Referred to laboratory assessments, COVID-19 patients had significantly lower lymphocyte count as well as higher C-reactive protein and D-dimer. Both lower platelet and leukocyte count as well as higher neutrophils levels were observed. Non COVID-19 patients associated significant lower in-hospital stay. Finally, 18.5% of 28-day mortality was found in COVID-19 patients while no deaths were recorded across Non COVID-19 patients.
3.2. Oxidative stress levels in COVID-19 disease
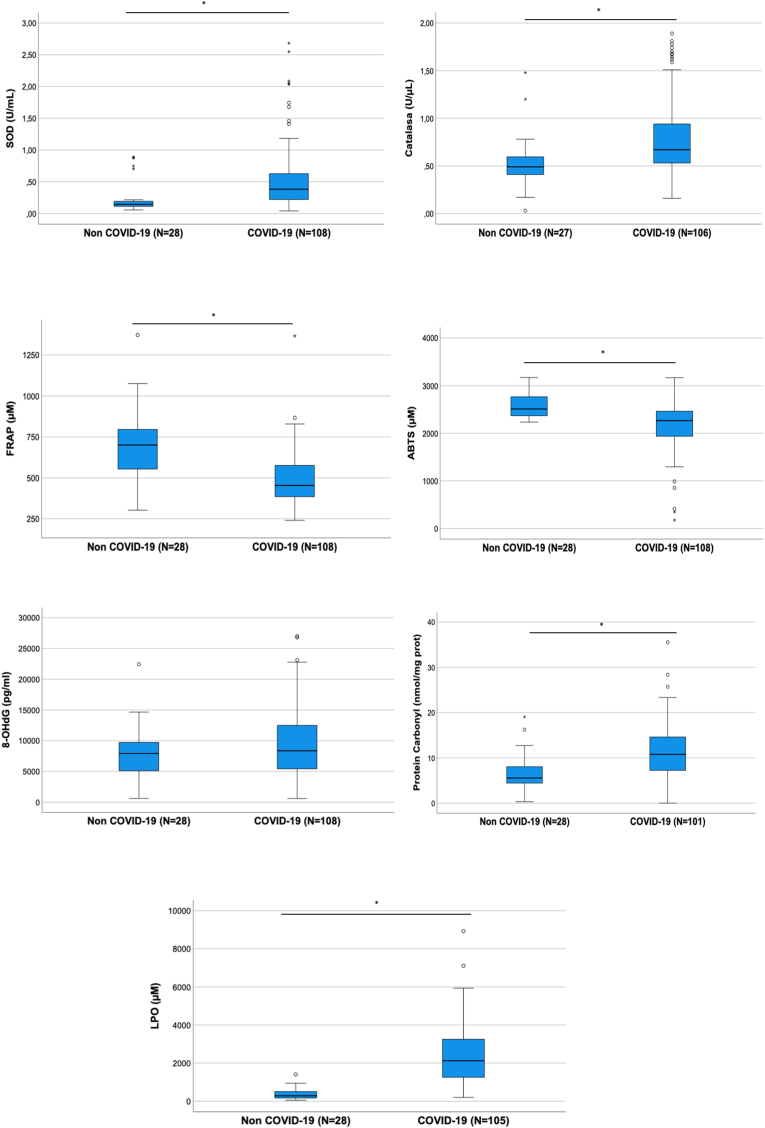
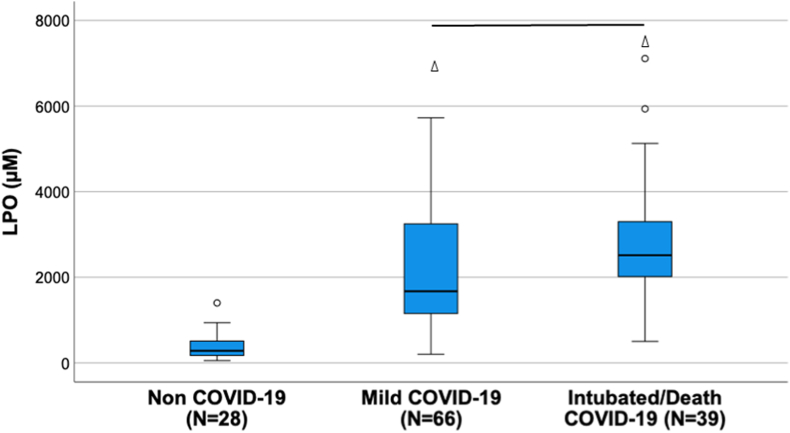
The comparison of COVID-19 patients with control group displayed statistically significant differences across all oxidative stress molecules evaluated except for 8-OHdG (Table 1). Antioxidant enzymes (Superoxide dismutase (SOD) and Catalase) and oxidative cell damage (Carbonyl and Lipid peroxidation (LPO)) levels were significantly higher in COVID-19 patients while total antioxidant capacity (ABTS and FRAP) levels were lower in these patients (Table 1 and Fig. 1). The comparison of oxidative stress molecules’ levels across COVID-19 severity revealed that only LPO was statistically different between mild and intubated/death COVID-19 patients (Supp File 1 and Fig. 2). A sub-analysis by mortality taking into account only intubated patients did not show statistically significant differences in terms of LPO values (p = 0.460).
Table 1.
Oxidative stress molecules’ levels in COVID-19 and Non-COVID-19 patients. Data are represented as median and interquartile range (IQR).
| Non COVID-19 | COVID-19 | p | |
|---|---|---|---|
| SOD (U/mL) | 0.15 [0.08] | 0.38 [0.42] | < 0.001 |
| Catalase (U/μL) | 0.49 [0.19] | 0.67 [0.41] | < 0.001 |
| ABTS (μM) | 2510.47 [437] | 2264.99 [525] | < 0.001 |
| FRAP (μM) | 700.67 [251.45] | 453.84 [192.30] | < 0.001 |
| 8-OHdG (pg/ml) | 7925.78 [4894] | 8373.06 [7103] | 0.246 |
| Protein Carbonyl (nmol/mg prot) | 5.56 [3.68] | 10.78 [7.41] | < 0.001 |
| MDA + HNE (μM) | 284.19 [339.84] | 2123.62 [2068] | < 0.001 |
Fig. 1.
Box plots showing oxidative stress molecules' levels across groups.
Fig. 2.
Lipid peroxidation (LPO) levels across severity. The line represents significant differences between groups. The triangle represents significant differences against the healthy control.
3.3. Evaluation of 28-day intubation/death risk depending on LPO levels
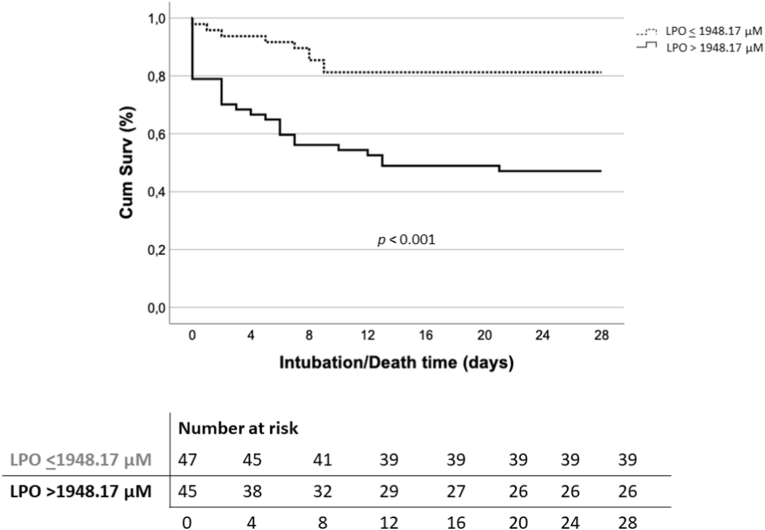
COX multivariate regression analysis identified LPO levels over the OOP (LPO>1948.17 μM) as an independent risk factor for 28-day intubation/death in COVID-19 patients [OR: 2.57; 95% CI: 1.10–5.99; p = 0.029] (Table 2). These results were validated in 1000 samples by Bootstrapping method (Table 3). Kaplan-Meier curve analysis revealed that COVID-19 patients showing LPO levels above 1948.17 μM were intubated or died 8.4 days earlier on average (mean survival time 15.4 vs 23.8 days) when assessing 28-day intubation/death risk (p < 0.001) (Fig. 3).
Table 2.
Multivariate logistic regression analysis to evaluate the independent association of LPO levels and risk of intubation or death at 28 days.
| OR | 95% CI | p | ||
|---|---|---|---|---|
| Intubated/Death COVID-19 disease | LPO >1948.17 μM | 2.57 | 1.10–5.99 | 0.029 |
| Obesity | 1.19 | 0.48–2.97 | 0.702 | |
| Diabetes mellitus | 2.81 | 1.20–6.60 | 0.017 | |
| Chronic hepatic disease | 7.44 | 1.37–40.23 | 0.020 | |
| Septic Shock | 2.61 | 1.15–5.92 | 0.022 | |
| Lymphocytes <875 cells/mm3 | 0.22 | 0.10–0.51 | <0.001 | |
| Neutrophils >5480 cells/mm3 | 2.30 | 1.04–5.09 | 0.041 | |
Table 3.
Validation of the multivariate analysis for evaluating the risk of intubation/mortality at 28 days by Bootstrapping method using 1000 random samples.
| B | 95% CI | p | ||
|---|---|---|---|---|
| Intubated/Death COVID-19 disease | LPO >1948.17 μM | 0.94 | 0.16–1.96 | 0.015 |
| Obesity | 0.18 | −0.91 – 1.72 | 0.729 | |
| Diabetes mellitus | 1.04 | −0.20 – 2.13 | 0.026 | |
| Chronic hepatic disease | 2.01 | 0.97–3.71 | 0.005 | |
| Septic Shock | 0.96 | 0.14–2.39 | 0.046 | |
| Lymphocytes <875 cells/mm3 | −1.50 | (-2.71) – (−0.64) | 0.002 | |
| Neutrophils >5480 cells/mm3 | 0.83 | 0.13–1.79 | 0.026 | |
Fig. 3.
Kaplan-Meier curve analysis showing LPO association with 28-day intubation/death risk.
4. Discussion
The findings derived from this study revealed for the very first time that COVID-19 patients showed significantly lower levels of total antioxidant capacity (ABTS and FRAP) and higher levels of antioxidant enzymes (SOD, Catalase) and oxidative cell damage (Carbonyl and Lipid peroxidation (LPO)). Indeed, LPO levels over 1948.17 μM are independently associated with higher 28-day intubation/death risk.
SARS-CoV-2 stimulates reactive oxygen species (ROS) generation [23]. In COVID-19 disease, ACE2 acts as SARS-CoV-2 cellular entry receptor in type II pneumocytes of lung alveoli. ACE2 is responsible for angiotensin II (Ang II) degradation to angiotensin- (1–7) (Ang 1–7) [24]. Ang II produces ROS by stimulating membrane-bound NADPH oxidase [25]. In consequence, Ang II degradation into Ang 1–7 by ACE2 mitigates oxidative stress as it inhibits NADPH oxidase [26]. Indeed, ACE2 bounding to the virus downregulates ACE2, leading to an increased presence of superoxide species and subsequent cell damage, which may include lipid peroxidation, protein carbonylation and DNA oxidation [27], establishing an oxidative stress cycle, and ultimately, increasing the risk of suffering severe COVID-19 illness forms [25]. Our findings suggest a reactive increase of antioxidant enzymes which may be insufficient, leading to a decreased total antioxidant capacity and biomolecules' damage. Those findings differed from previous studies in respiratory viral infections such as RSV [12], hMPV [28] and Rhinovirus [29] which documented a lower expression of antioxidant enzyme levels. However, Kosmider B et al. [16] demonstrated that Influenza virus causes an increase of antioxidant genes’ expression, in line with our results.
Lipid peroxidation is a biological free radical chain reaction. The oxidation of unsaturated fatty acids or other lipids results in peroxides of these compounds. Further reactions lead to aldehydes syntheses such as MDA or HNE. Lipid peroxidation affects all cell membranes inducing damage and loss of function [30]. MDA is commonly considered a marker of ferroptosis [31]. Ferroptosis is a form of regulated cell death characterized by iron-dependent lipid peroxidation, which induces cell death [32]. During ferroptosis, an accumulation of polyunsaturated fatty acids (PUFAs) occurs [33]. This implies a lipid peroxidation driven by PUFAs which enhances cell membrane permeability making the cell more sensitive to oxidation [34]. This phenomenon is a critical mechanism in sepsis-induced injuries in mice models. Kang R et al. [35] described that lipid peroxidation in ferroptosis induces pyroptosis, suggesting a link between ferroptosis and other forms of cell death in sepsis. Lipid peroxidation is involved in several disease conditions [36] like cardiovascular disease [37] cancer [38], Alzheimer [39] and chronic diseases such as NAFLD [40], Multiple Sclerosis [41], COPD [42] and Diabetes mellitus [43].
In fact, it has a role in both homeostasis and response to stress, such as viral infection [44]. Obesity is one of the most important medical conditions leading to an exponentially increase of SARS-CoV-2 patients' mortality risk [45]. In patients suffering from metabolic disorder and COVID-19, lipid peroxidation produces reactive lipid aldehydes which will affect its prognosis [46]. In this line, our study revealed that lipid peroxidation is related to COVID-19 severity and intubation/death risk. Potje SR et al. [47] in a preliminary study in 20 COVID-19 patients documented the presence of higher levels of lipid peroxidation in COVID-19 patients but they did not find differences across patients’ severity, which could be dued to a lower simple size in comparison with our study.
The results derived from this work highlight the importance of oxidative stress mediators in COVID-19, particularly the role of lipid peroxidation in prognosis of these patients. Taking this into account, these observations reinforce the urgent necessity of clinical trials in order to test the security and effectiveness regarding the implementation of antioxidant treatments in COVID-19 [[48], [49], [50]] for improving prognosis in this disease.
Our study has some limitations to be addressed. First, oxidative stress biomarkers were compared only at first hospital admission. Further prospective follow‐up studies with serial sampling should validate these results. Second, it was conducted in a single center and should be evaluated in a multicenter fashion design to validate the potential role of lipid peroxidation in predicting intubation/death risk in COVID-19.
5. Conclusions
In summary, our findings deepen our knowledge of oxidative stress status in SARS-CoV-2 infection, supporting its important role in COVID-19. In fact, higher lipid peroxidation levels are independently associated to a greater risk of intubation or death at 28 days in COVID-19 patients. We believe that these findings open a new avenue for designing clinical trials to evaluate the beneficial role of antioxidant treatment in patients suffering from COVID-19.
Author contribution
Conceptualization: M.M-F., R.A., E.T., Á.T.-V.; methodology: M.M-F., R.A., M.H-R, E.G-S, E.T., Á.T.-V.; formal analysis: M.M-F., Á.T.-V.; investigation: M.H-R, E.G-S, P.M − P, H.G-B, L.S-dP, O.G, I.C-F.; resources: H.G.-B., E.T.; data curation: M.H-R, E.G-S, P.M − P, H.G-B, L.S-dP, O.G, I.C-F.; writing: M.M-F., Á.T.-V., E.T., R.A.; original draft preparation: M.M-F., Á.T.-V., E.T., M.H.-R.; writing—review and editing: M.M-F., Á.T.-V., M.H.-R., E.G.-S., R.A.; visualization: M.M-F., Á.T.-V.; supervision: E.T.; project administration: E.T., Á.T.-V.; funding acquisition: E.T., R.A., E.G-S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Instituto de Salud Carlos III (Grant COV20/00491), Junta de Castilla y León (18IGOF), Consejería de Educación de Castilla y León (VA256P20) and Gerencia Regional de Salud de Castilla y León (26th April of 2021).
Declaration of competing interest
None.
Acknowledgments
We appreciate the collaboration of the Nursing staff and the Research Unit from the Hospital Clínico Universitario de Valladolid (Spain) for the assistance during project development.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.102181.
Contributor Information
María Heredia-Rodríguez, Email: maria_her_05@hotmail.com.
Esther Gómez-Sánchez, Email: esthergzam@hotmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. China novel coronavirus investigating and Research team, A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019;382:727–733. doi: 10.1056/NEJMoa2001017. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y., Peng F., Wang R., Yange M., Guan K., Jiang T., Xu G., Sun J., Chang C. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020;109:102434. doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 Clinical Management: Living Guidance. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-clinical-2021-1 n.d.) accessed.
- 6.Jelic M.D., Mandic A.D., Maricic S.M., Srdjenovic B.U. Oxidative stress and its role in cancer. J. Cancer Res. Therapeut. 2021;17:22–28. doi: 10.4103/jcrt.JCRT_862_16. [DOI] [PubMed] [Google Scholar]
- 7.Aykac K., Ozsurekci Y., Yayla B.C.C., Gurlevik S.L., Oygar P.D., Bolu N.B., Tasar M.A., Erdinc F.S., Ertem G.T., Neselioglu S., Erel O., Cengiz A.B., Ceyhan M. Oxidant and antioxidant balance in patients with COVID-19. Pediatr. Pulmonol. 2021;56:2803–2810. doi: 10.1002/ppul.25549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cecchini R., Cecchini A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses. 2020;143:110102. doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garofalo R.P., Kolli D., Casola A. Respiratory syncytial virus infection: mechanisms of redox control and novel therapeutic opportunities. Antioxidants Redox Signal. 2013;18:186–217. doi: 10.1089/ars.2011.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez I., García-Carpizo V., Guijarro T., García-Gomez A., Navarro D., Aranda A., Zambrano A. Induction of DNA double-strand breaks and cellular senescence by human respiratory syncytial virus. Virulence. 2016;7:427–442. doi: 10.1080/21505594.2016.1144001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casola A., Burger N., Liu T., Jamaluddin M., Brasier A.R., Garofalo R.P. Oxidant tone regulates RANTES gene expression in airway epithelial cells infected with respiratory syncytial virus: role IN viral-induced interferon regulatory factor Activation. J. Biol. Chem. 2001;276 doi: 10.1074/jbc.M101526200. 19715–19722. [DOI] [PubMed] [Google Scholar]
- 12.Hosakote Y.M., Jantzi P.D., Esham D.L., Spratt H., Kurosky A., Casola A., Garofalo R.P. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 2011;183:1550–1560. doi: 10.1164/rccm.201010-1755OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buffinton G.D., Christen S., Peterhans E., Stocker R. Oxidative stress in lungs of mice infected with influenza A virus. Free Radic. Res. Commun. 1992;16:99–110. doi: 10.3109/10715769209049163. [DOI] [PubMed] [Google Scholar]
- 14.Selemidis S., Seow H.J., Broughton B.R.S., Vinh A., Bozinovski S., Sobey C.G., Drummond G.R., Vlahos R. Nox1 oxidase suppresses influenza a virus-induced lung inflammation and oxidative stress. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amatore D., Sgarbanti R., Aquilano K., Baldelli S., Limongi D., Civitelli L., Nencioni L., Garaci E., Ciriolo M.R., Palamara A.T. Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated by NOX4-derived ROS. Cell Microbiol. 2015;17:131–145. doi: 10.1111/cmi.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosmider B., Messier E.M., Janssen W.J., Nahreini P., Wang J., Hartshorn K.L., Mason R.J. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir. Res. 2012;13:43. doi: 10.1186/1465-9921-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pincemail J., Cavalier E., Charlier C., Cheramy–Bien J.-P., Brevers E., Courtois A., Fadeur M., Meziane S., Goff C.L., Misset B., Albert A., Defraigne J.-O., Rousseau A.-F. Oxidative stress status in COVID-19 patients hospitalized in intensive care unit for severe pneumonia. A Pilot Study, Antioxidants (Basel). 2021;10:257. doi: 10.3390/antiox10020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karkhanei B., Talebi Ghane E., Mehri F. Evaluation of oxidative stress level: total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19. New Microbes New Infect. 2021;42:100897. doi: 10.1016/j.nmni.2021.100897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadotti A.C., Lipinski A.L., Vasconcellos F.T., Marqueze L.F., Cunha E.B., Campos A.C., Oliveira C.F., Amaral A.N., Baena C.P., Telles J.P., Tuon F.F., Pinho R.A. Susceptibility of the patients infected with Sars-Cov2 to oxidative stress and possible interplay with severity of the disease. Free Radic. Biol. Med. 2021;165:184–190. doi: 10.1016/j.freeradbiomed.2021.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., Horick N.K., Healy B.C., Shah R., Bensaci A.M., Woolley A.E., Nikiforow S., Lin N., Sagar M., Schrager H., Huckins D.S., Axelrod M., Pincus M.D., Fleisher J., Sacks C.A., Dougan M., North C.M., Halvorsen Y.-D., Thurber T.K., Dagher Z., Scherer A., Wallwork R.S., Kim A.Y., Schoenfeld S., Sen P., Neilan T.G., Perugino C.A., Unizony S.H., Collier D.S., Matza M.A., Yinh J.M., Bowman K.A., Meyerowitz E., Zafar A., Drobni Z.D., Bolster M.B., Kohler M., D'Silva K.M., Dau J., Lockwood M.M., Cubbison C., Weber B.N., Mansour M.K. BACC bay tocilizumab trial investigators, efficacy of tocilizumab in patients hospitalized with covid-19. N. Engl. J. Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamayo-Velasco Á., Peñarrubia-Ponce M.J., Álvarez F.J., Gonzalo-Benito H., de la Fuente I., Martín-Fernández M., Eiros J.M., Martínez-Paz P., Miramontes-González J.P., Fiz-López A., Arribas-Rodríguez E., Cal-Sabater P., Aller R., Dueñas C., Heredia-Rodríguez M., Tamayo E., Bernardo D., Gómez-Sánchez E. Evaluation of cytokines as robust diagnostic biomarkers for COVID-19 detection. J. Personalized Med. 2021;11:681. doi: 10.3390/jpm11070681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almansa R., Ortega A., Ávila-Alonso A., Heredia-Rodríguez M., Martín S., Benavides D., Martín-Fernandez M., Rico L., Aldecoa C., Rico J., López de Cenarruzabeitia I., Beltrán de Heredia J., Gomez-Sanchez E., Aragón M., Andrés C., Calvo D., Andaluz-Ojeda D., Liu P., Blanco-Antona F., Blanco L., Gómez-Herreras J.I., Tamayo E., Bermejo-Martin J.F. Quantification of immune dysregulation by next-generation polymerase chain reaction to improve sepsis diagnosis in surgical patients. Ann. Surg. 2019;269:545–553. doi: 10.1097/SLA.0000000000002406. [DOI] [PubMed] [Google Scholar]
- 23.Miripour Z.S., Sarrami-Forooshani R., Sanati H., Makarem J., Taheri M.S., Shojaeian F., Eskafi A.H., Abbasvandi F., Namdar N., Ghafari H., Aghaee P., Zandi A., Faramarzpour M., Hoseinyazdi M., Tayebi M., Abdolahad M. Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosens. Bioelectron. 2020;165:112435. doi: 10.1016/j.bios.2020.112435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suhail S., Zajac J., Fossum C., Lowater H., McCracken C., Severson N., Laatsch B., Narkiewicz-Jodko A., Johnson B., Liebau J., Bhattacharyya S., Hati S. Role of oxidative stress on SARS-CoV (sars) and SARS-CoV-2 (COVID-19) infection: a review. Protein J. 2020:1–13. doi: 10.1007/s10930-020-09935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovren F., Pan Y., Quan A., Teoh H., Wang G., Shukla P.C., Levitt K.S., Oudit G.Y., Al-Omran M., Stewart D.J., Slutsky A.S., Peterson M.D., Backx P.H., Penninger J.M., Verma S. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 27.Sena C.M., Leandro A., Azul L., Seiça R., Perry G. Vascular oxidative stress: impact and therapeutic approaches. Front. Physiol. 2018;9:1668. doi: 10.3389/fphys.2018.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao X., Sinha M., Liu T., Hong C., Luxon B.A., Garofalo R.P., Casola A. Identification of human metapneumovirus-induced gene networks in airway epithelial cells by microarray analysis. Virology. 2008;374:114–127. doi: 10.1016/j.virol.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papi A., Contoli M., Gasparini P., Bristot L., Edwards M.R., Chicca M., Leis M., Ciaccia A., Caramori G., Johnston S.L., Pinamonti S. Role of xanthine oxidase activation and reduced glutathione depletion in rhinovirus induction of inflammation in respiratory epithelial cells. J. Biol. Chem. 2008;283:28595–28606. doi: 10.1074/jbc.M805766200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tejchman K., Kotfis K., Sieńko J. Biomarkers and mechanisms of oxidative stress—last 20 Years of Research with an emphasis on kidney damage and renal transplantation. Int. J. Mol. Sci. 2021;22:8010. doi: 10.3390/ijms22158010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu M., Zhang H., Chen Z., Sun X., Zhu S., Nan K., Chen W., Miao C. The role of ferroptosis in acute respiratory distress syndrome. Front. Med. 2021;8:651552. doi: 10.3389/fmed.2021.651552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stockwell B.R., Angeli J.P.F., Bayir H., Bush A.I., Conrad M., Dixon S., Fulda S., Gascon S., Hatzios S.K., Kagan V., Noel K., Jiang X., Linkermann A., Murphy M.E., Overholtzer M., Oyagi A., Pagnussat G., Park J., Ran Q., Rosenfeld C.S., Salnikow K., Tang D., Torti F., Torti S., Toyokuni S., Woerpel K.A., Zhang D.D. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye Z., Liu W., Zhuo Q., Hu Q., Liu M., Sun Q., Zhang Z., Fan G., Xu W., Ji S., Yu X., Qin Y., Xu X. Ferroptosis: final destination for cancer? Cell Prolif. 2020;53 doi: 10.1111/cpr.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agmon E., Solon J., Bassereau P., Stockwell B.R. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci. Rep. 2018;8:5155. doi: 10.1038/s41598-018-23408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang R., Zeng L., Zhu S., Xie Y., Liu J., Wen Q., Cao L., Xie M., Ran Q., Kroemer G., Wang H., Billiar T.R., Jiang J., Tang D. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe. 2018;24:97–108. doi: 10.1016/j.chom.2018.05.009. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mas-Bargues C., Escrivá C., Dromant M., Borrás C., Viña J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021;709:108941. doi: 10.1016/j.abb.2021.108941. [DOI] [PubMed] [Google Scholar]
- 37.Daiber A., Hahad O., Andreadou I., Steven S., Daub S., Münzel T. Redox-related biomarkers in human cardiovascular disease - classical footprints and beyond. Redox Biol. 2021;42:101875. doi: 10.1016/j.redox.2021.101875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X., Kang R., Kroemer G., Tang D. Broadening horizons: the role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021;18:280–296. doi: 10.1038/s41571-020-00462-0. [DOI] [PubMed] [Google Scholar]
- 39.Chang Y.-T., Chang W.-N., Tsai N.-W., Huang C.-C., Kung C.-T., Su Y.-J., Lin W.-C., Cheng B.-C., Su C.-M., Chiang Y.-F., Lu C.-H. The roles of biomarkers of oxidative stress and antioxidant in alzheimer's disease: a systematic review. BioMed Res. Int. 2014;2014:182303. doi: 10.1155/2014/182303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Notarnicola M., Osella A.R., Caruso M.G., Pesole P.L., Lippolis A., Tutino V., Bonfiglio C., De Nunzio V., Scavo M.P., Mirizzi A., Franco I., Lippolis T., D'Alessandro R., Refolo M.G., Messa C. Nonalcoholic fatty liver disease: focus on new biomarkers and lifestyle interventions. Int. J. Mol. Sci. 2021;22:3899. doi: 10.3390/ijms22083899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalo H., Brieva L., Tatzber F., Jové M., Cacabelos D., Cassanyé A., Lanau-Angulo L., Boada J., Serrano J.C.E., González C., Hernández L., Peralta S., Pamplona R., Portero-Otin M. Lipidome analysis in multiple sclerosis reveals protein lipoxidative damage as a potential pathogenic mechanism. J. Neurochem. 2012;123:622–634. doi: 10.1111/j.1471-4159.2012.07934.x. [DOI] [PubMed] [Google Scholar]
- 42.Paliogiannis P., Fois A.G., Sotgia S., Mangoni A.A., Zinellu E., Pirina P., Carru C., Zinellu A. Circulating malondialdehyde concentrations in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. Biomarkers Med. 2018;12:771–781. doi: 10.2217/bmm-2017-0420. [DOI] [PubMed] [Google Scholar]
- 43.Kang Q., Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799. doi: 10.1016/j.redox.2020.101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia M., Qin D., Zhao C., Chai L., Yu Z., Wang W., Tong L., Lv L., Wang Y., Rehwinkel J., Yu J., Zhao W. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat. Immunol. 2020;21:727–735. doi: 10.1038/s41590-020-0699-0. [DOI] [PubMed] [Google Scholar]
- 45.Goumenou M., Sarigiannis D., Tsatsakis A., Anesti O., Docea A.O., Petrakis D., Tsoukalas D., Kostoff R., Rakitskii V., Spandidos D.A., Aschner M., Calina D. COVID-19 in Northern Italy: an integrative overview of factors possibly influencing the sharp increase of the outbreak (Review) Mol. Med. Rep. 2020;22:20–32. doi: 10.3892/mmr.2020.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrakis D., Margină D., Tsarouhas K., Tekos F., Stan M., Nikitovic D., Kouretas D., Spandidos D.A., Tsatsakis A. Obesity - a risk factor for increased COVID-19 prevalence, severity and lethality (Review) Mol. Med. Rep. 2020;22:9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potje S.R., Costa T.J., Fraga-Silva T.F.C., Martins R.B., Benatti M.N., Almado C.E.L., de Sá K.S.G., Bonato V.L.D., Arruda E., Louzada-Junior P., Oliveira R.D.R., Zamboni D.S., Becari C., Auxiliadora-Martins M., Tostes R.C. Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci. 2021;276 doi: 10.1016/j.lfs.2021.119376. 119376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang L., Wang L., Tan J., Liu H., Ni Y. High-dose vitamin C intravenous infusion in the treatment of patients with COVID-19: a protocol for systematic review and meta-analysis. Medicine (Baltim.) 2021;100 doi: 10.1097/MD.0000000000025876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holford P., Carr A.C., Jovic T.H., Ali S.R., Whitaker I.S., Marik P.E., Smith A.D. Vitamin C-an adjunctive therapy for respiratory infection, sepsis and COVID-19. Nutrients. 2020;12:E3760. doi: 10.3390/nu12123760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu F., Zhu Y., Zhang J., Li Y., Peng Z. Intravenous high-dose vitamin C for the treatment of severe COVID-19: study protocol for a multicentre randomised controlled trial. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.