Abstract
The highest viral loads of severe acute respiratory syndrome coronavirus-2 are detectable in the oral cavity, so a potential reduction of infectious virus by nasal and oral sprays could reduce transmission. Therefore, the inactivation capacity of nine nasal and oral sprays was evaluated according to EN 14476. One nasal spray based on sodium hypochlorite and one oral spray containing essential oils reduced viral titres by two to three orders of magnitude. Although clinical data are still sparse, nasal and oral sprays display a more convenient application for elderly people or those who are unable to rinse/gargle.
Keywords: Nasal spray, Oral spray, Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), Oral cavity, Nasopharynx, Transmission, Inactivation, Quantitative suspension test
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), represents a major health burden by infecting millions of people worldwide. Although vaccination programmes have been implemented successfully in many countries, specific therapies have not been approved to date. Furthermore, the emergence of different variants of concern displaying increased transmissibility, more severe disease and a significant reduction in neutralization by antibodies can reduce the effectiveness of treatments or vaccines, increasing cases in the community and, more importantly, among healthcare workers. Hence, social distancing, personal hygiene measures including frequent handwashing, and wearing face masks remain essential to reduce virus transmission, which mainly occurs via respiratory secretions. As the highest viral loads are detectable in the oral cavity, a reduction of infectious virus in the nasopharynx and oropharynx could lower viral shedding and, consequently, transmission by infected individuals [1]. Recent findings demonstrated the potential of commercially available oral rinses to inactivate SARS-CoV-2 in vitro [2]. In dentistry, a pre-procedural rinse is a well-established method to reduce microbial pathogens and, although clinical data are sparse, its application has also been suggested during the ongoing SARS-CoV-2 pandemic in Germany [3]. The underlying mechanism of virus inactivation is believed to be the sensitivity of the viral envelope towards chemical agents such as ethanol, chlorhexidine, cetylpyridinium chloride, hydrogen peroxide and povidone-iodine, present in both disinfectants and oral rinses [4]. Depending on their composition, nasal sprays could display similar antiviral properties. The application of oral rinses and nasal sprays that inactivate SARS-CoV-2 could be particularly important when it comes to dental practice, as aerosol and droplet spread during dental procedures is inevitable, and keeping an appropriate distance is impossible. Thus, using oral rinses and nasal sprays could reduce the risk of exposure of healthcare workers with infectious virus and cross-contamination between patients. The aim of this study was to assess the capacity of seven nasal sprays and two oral sprays to inactivate SARS-CoV-2 in vitro.
Methods
Quantitative suspension test and virus titration
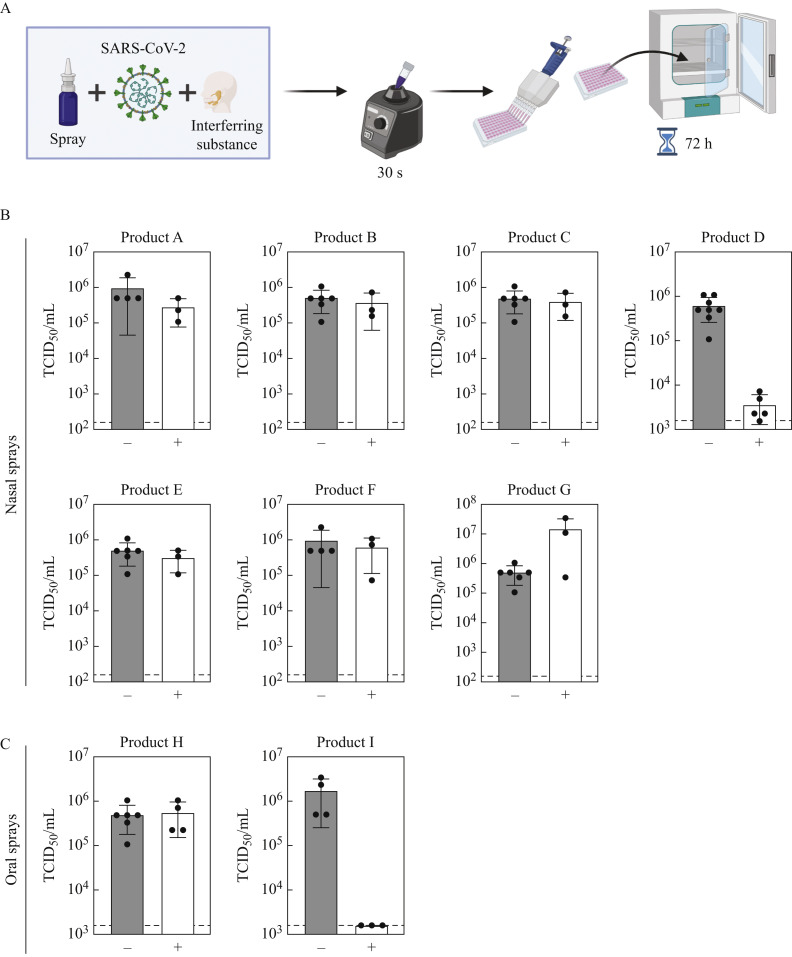
The virucidal activity of seven nasal sprays and two oral sprays was assessed as described previously [5]. Briefly, nasal or oral sprays were mixed with SARS-CoV-2 hCoV-19/Germany/BY-Bochum-1/2020 (GISAID accession ID EPI_ISL_1118929, [6]) and an interfering substance mimicking nasal secretion [5]. Medium served as a control. After 30 s of incubation, remaining viral loads were quantified by a limited dilution assay on VeroE6 cells cultured in Dulbecco's modified Eagle's medium [supplemented with 10% (v/v) fetal calf serum, 1% non-essential amino acids, 100 IU/mL penicillin, 100 μg/mL streptomycin and 2 mM L-glutamine]. Subsequently, the cells were stained with crystal violet, and the 50% tissue culture infective dose (TCID50/mL) was determined. Determination of virucidal activity was calculated by the difference between the medium control and the test product. Cytotoxicity of the different products was evaluated using non-infected cells and set as the lower limit of quantification.
Large volume plating
For Product I, large volume plating was performed to reduce cell toxicity and reach a ≥4 log10 reduction of viral loads. Thus, similar to the quantitative suspension test, eight parts of Product I were combined with one part interfering substance and one part virus. After 30 s of incubation, the mixture was diluted 1:1000 in medium and added to VeroE6 cells plated on six 96-well plates. Medium served as a control. After 72 h of incubation at 37 °C, the cells were stained with crystal violet, and the remaining infectious virus was quantified by counting cytopathic effect (CPE)-positive wells (TCID50/mL).
Results
The virucidal capacity of different commercially available nasal and oral sprays was investigated (Table I ) using a quantitative suspension test adapted to European Guideline EN 14476. One part virus was incubated with one part interfering substance mimicking nasal secretion and eight parts nasal or oral spray for 30 s (Figure 1 A). The residual infectious titre was determined by an end-point dilution assay (white bar) and compared with medium-treated virus as a control (grey bar). Only a nasal spray based on sodium hypochlorite lowered viral titres by 2.21 log10 TCID50/mL (Figure 1B, Product D), whereas all other nasal sprays, based on sodium chlorite, xylometazolin hydrochloride or homeopathic remedies, did not affect the infectivity of SARS-CoV-2. In line with this notion, an oral spray containing homeopathic remedies also had no impact on infectious viral loads, while an oral spray based on essential oils reduced viral loads to the lower limit of detection (Figure 1C, Product I). When performing large volume plating with Product I, a reduction factor of ≥4.69 log10 TCID50/mL was observed (Table I). Taken together, the majority of nasal and oral sprays did not inactivate SARS-CoV-2 within 30 s.
Table I.
Overview of nasal and oral sprays used in the study with ingredients and calculated reduction factors
| Product | Ingredients | Initial viral load (log10 TCID50/mL) |
Inactivation (log10 TCID50/mL) |
Reduction factor |
|---|---|---|---|---|
| Ac | Carragelose (1.2 mg/mL), kappa-carrageenan (0.4 mg/mL), sodium chlorite | 5.98 | 5.45 | 0.53 |
| Bc | Sodium chlorite (0.9%), panthenol | 5.70 | 5.57 | 0.13 |
| Cc | Xylometazolin hydrochloride (1 mg/mL), dexpanthenol (50 mg/mL) | 5.70 | 5.61 | 0.09 |
| Dc | Sodium hypochlorite (<0.08%), lithium-magnesium-sodium-silicate | 5.78 | 3.57 | 2.21 |
| Ec | Xylometazolin hydrochloride (0.1%) | 5.70 | 5.50 | 0.20 |
| Fc | Hydroxypropyl methyl cellulose, succinic acid, disodium succinate | 5.98 | 5.80 | 0.18 |
| Gc | Galphimia, luffa operculate, sabadilla | 5.70 | 7.18 | 0 |
| Hd | Zincum aceticum, zincum gluconium | 5.70 | 5.76 | 0 |
| Id | Anise oil, eucalyptus oil, levomenthol, myrrh extract, clove oil, peppermint oil ratanhia root extract, tormentil root extract | 6.23 | ≤3.20a 1.84a,b |
≥3.03 ≥4.69b |
TCID50, 50% tissue culture infective dose.
Viral loads were reduced to lower limit of quantification.
Results from large volume plating.
Nasal spray.
Oral spray.
Figure 1.
Experimental set-up of the quantitative suspension test following virus titration to determine viral titres [50% tissue culture infective dose (TCID50/mL)] (A). Virucidal activity of nasal sprays (B, Products A–G) and oral sprays (C, Products H and I) against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (N=3; mean ± standard deviation). Virus was incubated with medium (control, grey bar) or various nasal and oral sprays (white bar) for 30 s. Hereafter, remaining viral titres (TCID50/mL) were determined upon titration on VeroE6 cells according to Spearman–Karber. Cytotoxic effects are indicated as the lower limit of quantification (dotted line).
Discussion
The application of oral rinses, and nasal or oral sprays could potentially reduce infectious viral loads in the oral cavity, thus temporarily reducing the risk of transmission. Depending on the formulation, oral rinses can impede SARS-CoV-2 infectivity in vitro [5]. In contrast to oral rinses, nasal sprays are not intended to reduce microbial contamination, but rather to promote the detumescence of the nasal mucosa. Moreover, selected nasal sprays contain different types of active ingredients compared with oral rinses, and thus need careful evaluation regarding their inactivation capacity towards SARS-CoV-2. As such, this study investigated seven nasal sprays and two oral sprays regarding their virucidal activity against SARS-CoV-2 during quantitative suspension tests. Nasal sprays based on sodium chlorite, xylometazolin hydrochloride or homeopathic remedies did not reduce virus infectivity, while a nasal spray based on sodium hypochlorite reduced viral loads by 2.21 log10 TCID50/mL (Figure 1B, Product D). In accordance, household and hand disinfectants based on sodium hypochlorite are reported to successfully inactivate a broad range of enveloped viruses [7]. The lithium-magnesium-sodium-silicate within Product D forms a gel matrix that potentially interferes with virus infection due to the formation of an aqueous film. However, further research is required to assess if sodium hypochlorite is well tolerated by individuals in clinical situations. In agreement with previous observations for oral rinses [5], an oral spray containing essential oils completely suppressed SARS-CoV-2 infectivity (Figure 1C, Product I). In the present study, a product containing carrageenan (Figure 1B, Product A) did not harbour any virucidal activity, although recent findings have reported otherwise [8,9]. This discrepancy can potentially be explained by longer incubation times, and a different experimental set-up aiming to evaluate the effect of carrageenan on the viral life cycle rather than a quantitative suspension test.
Although nasal sprays frequently contain potential antiviral ingredients, clinical data are sparse regarding the in vivo efficacy of oral rinses and nasal sprays, especially as many studies determine viral loads by quantitative reverse transcriptase polymerase chain reaction (RT-qPCR) and do not measure infectious virus. However, antiseptic agents presumably affect viral infectivity by damaging the viral envelope without impeding RNA integrity [4]. Therefore, clinical trials evaluating the antiviral capacity of oral rinses and nasal sprays should also assess infectious virus. In general, the application of nasal or oral sprays should be evaluated carefully as only a few sprays have demonstrated virucidal activity towards SARS-CoV-2 in vitro. Whether those findings can be transferred into clinical application, potentially contributing to reduced virus transmission, remains to be evaluated in further studies. Certainly, nasal or oral sprays do not provide a general therapeutic option as their ectopic application will not hamper viral replication. Nevertheless, nasal and oral sprays can potentially reduce the risk of transmission by temporarily reducing viral loads in the oral cavity, making dental procedures or surgery in close proximity to the nasopharynx and oropharynx safer. Recent findings have demonstrated that nasal sprays can reduce viral loads of different respiratory viruses in vivo [10]. Thus, using oral rinses and nasal sprays could reduce the risk of exposure of healthcare workers to infectious virus. Additionally, nasal and oral sprays display a more convenient application for elderly people or those who are unable to gargle due to intubation or other contraindications.
Acknowledgements
The authors wish to thank all members of the Department for Molecular and Medical Virology for helpful suggestions and discussions. The authors also wish to thank Dr. Stuart A. Gansky, Professor and Associate Dean for Research at the School of Dentistry, University of California, San Francisco for his contribution in reviewing and providing useful comments.
Conflict of interest statement
None declared.
Funding sources
ES was supported by the VIRus ALliance NRW (VIRAL) from Ministry of Culture and Science of the State of North Rhine-Westphalia (323-8.03-151826). SB was supported by an institutional NIH/NIDCR award (T32DE007306) at the University of California, San Francisco as part of her postdoctoral programme, and University of California Office of the President Emergency COVID-19 Research Seed Funding through the California Breast Cancer Research Program Grant R00RG2901. The funders played no role in determining the methods or conduct of this study.
References
- 1.Herrera D., Serrano J., Roldán S., Sanz M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin Oral Investig. 2020;24:2925–2930. doi: 10.1007/s00784-020-03413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kramer A., Eggers M., Hübner N.-O., Walger P., Steinmann E., Exner M. Virucidal gargling and virucidal nasal spray. GMS Hyg Infect Control. 2021;16 doi: 10.3205/dgkh000373. :Doc02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deutsche Gesellschaft für Zahn-, Mund- und Kieferheilkunde . S1-Leitlinie (Langversion); 2020. Umgang mit zahnmedizinischen Patienten bei Belastung mit Aerosol-übertragbaren Erregern. (083-046) [Google Scholar]
- 4.O'Donnell V.B., Thomas D., Stanton R., Maillard J.-Y., Murphy R.C., Jones S.A., et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function. 2020;1 doi: 10.1093/function/zqaa002. zqaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meister T.L., Brüggemann Y., Todt D., Conzelmann C., Müller J.A., Groß R., et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2020;222:1289–1292. doi: 10.1093/infdis/jiaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meister T.L., Fortmann J., Todt D., Heinen N., Ludwig A., Brüggemann Y., et al. Comparable environmental stability and disinfection profiles of the currently circulating SARS-CoV-2 variants of concern B.1.1.7 and B.1.351. J Infect Dis. 2021 doi: 10.1093/infdis/jiab260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanekata T., Fukuda T., Miura T., Morino H., Lee C., Maeda K., et al. Evaluation of the antiviral activity of chlorine dioxide and sodium hypochlorite against feline calicivirus, human influenza virus, measles virus, canine distemper virus, human herpesvirus, human adenovirus, canine adenovirus and canine parvovirus. Biocontrol Sci. 2010;15:45–49. doi: 10.4265/bio.15.45. [DOI] [PubMed] [Google Scholar]
- 8.Schütz D., Conzelmann C., Fois G., Groß R., Weil T., Wettstein L., et al. Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol. 2021;320:L750–L756. doi: 10.1152/ajplung.00552.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morokutti-Kurz M., Fröba M., Graf P., Große M., Grassauer A., Auth J., et al. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS One. 2021;16 doi: 10.1371/journal.pone.0237480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fazekas T., Eickhoff P., Pruckner N., Vollnhofer G., Fischmeister G., Diakos C., et al. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Complement Altern Med. 2012;12:1–8. doi: 10.1186/1472-6882-12-147. [DOI] [PMC free article] [PubMed] [Google Scholar]