Abstract
Background:
Circulating inflammation proteins may be important mediators or markers of carcinogenic mechanisms. There have been few studies with limited numbers of analytes in patients with upper gastrointestinal tract tumors. We therefore evaluated risk associations of gastric and esophageal cancers with prediagnostic levels of a wide range of these molecules.
Methods:
We performed a case-cohort analysis within the Japan Public Health Center-Based Study II, including incident cases of gastric (n=446) and esophageal (n=68) cancers and a random subcohort (n=774). Sixty-four biomarkers were measured in baseline plasma using Luminex bead-based assays. The median time between blood collection and diagnosis was 8.1 years for gastric cancer and 9.4 years for esophageal cancer. Hazard ratios for association with each marker were adjusted for potential confounders using Cox regression.
Results:
In separate models, sEGFR and TSLP were nominally associated with gastric cancer risk, and CRP, CXCL11/ITAC, and CCL15/MIP1D were associated with esophageal cancer. However, no association satisfied statistical significance after false discovery rate correction. Associations did not differ by time from blood collection to cancer (<5 vs. ≥5 years).
Conclusion:
Our study failed to identify associations of circulating inflammation markers with risk of upper gastrointestinal tract tumors.
Impact:
To date, this is the largest assessment of inflammation-related proteins with gastric and esophageal cancer risks. However, the evaluated molecules may not fully represent the complex inflammation processes preceding malignant transformation. Further investigation of other markers in prospective studies is warranted, as demonstration of associations may have important implications for prevention and treatment of these cancers.
Keywords: Gastric cancer, esophageal cancer, biomarker, inflammation, JPHC
BACKGROUND
Chronic inflammation is a recognized etiology of upper gastrointestinal tract cancers, major causes of morbidity and mortality worldwide. In carcinogenesis of the noncardia (distal) stomach, mucosal colonization by Helicobacter pylori induces chronic inflammation that may variably progress to atrophy, intestinal metaplasia, dysplasia and adenocarcinoma (1). On the other hand, the bacterial infection is inversely or not at all related to cardia (proximal stomach) and esophageal cancers in most populations. Smoking and alcohol consumption are additional risk factors for cancers of both of these organs (2).
Mucosal injury and regeneration are characterized by a complex interaction of signaling molecules, including pro- and anti-inflammatory cytokines, soluble receptors and angiogenesis and growth factors. Circulating levels of these proteins may be informative either as markers of local activity or through systemic effects. We therefore evaluated their associations with gastric and esophageal cancer risks in a prospective study.
MATERIALS AND METHODS
Study population: The Japan Public Health Center-based Prospective (JPHC) Study Cohort II is an ongoing study of middle-aged adults enrolled in 1993–94, including 23,335 who donated baseline blood samples (3). Participants were followed for cancer through December, 2010. For this case-cohort analysis, a subcohort of 774 individuals was selected by age- and sex-stratified random sampling. A total of 446 gastric cancers (ICD-O codes C16.0-C16.9) and 68 esophageal cancers (ICD-O codes C15; mainly representing esophageal squamous cell carcinomas, ESCC) were identified among the blood donors, including 27 cases among members of the subcohort. Informed written consent was obtained from all the individuals. The JPHC study was institutional review board approved and conducted in accordance with recognized ethical guidelines.
Laboratory assays: Circulating levels of 64 inflammation-related biomarkers were measured in heparinized plasma using five Luminex bead-based multiplex assay panels (Cytokine I, Cytokine II, Soluble Receptors, Cardiovascular Disease and High Sensitivity T Cell; EMD-Millipore Inc, Billerica, MA). Two biomarkers (IL3 and TNFB) detected in <10% of samples were excluded from analysis.
Statistical analyses: Biomarkers were analyzed as ordinal variables (two to four categories depending upon proportion of measurements below the lower limit of detection) based on distributions among the subcohort members. Cox proportional regression models with a baseline hazard stratified by age-group and sex were used to calculate hazard ratios and 95% confidence intervals for associations of each biomarker with cancer risks, accounting for the case-cohort design (4). Age was used as the model time metric. False discovery rate (FDR) corrected p-values were also calculated to adjust for multiple comparisons. We performed stratified analyses by latency from blood collection to cancer diagnosis (<5 vs. ≥5 years) and by gastric anatomic subsite (proximal vs. distal). We also restricted to seropositive H. pylori subcohort members as a sensitivity analysis. Tests of statistical significance were based on two-sided p-values <0.05. All analyses were performed using SAS software version 9.4 (SAS Inc, Cary, NC).
RESULTS
Baseline characteristics of subcohort members and cases are presented in the Table. The median time from blood collection to disease diagnosis was 8.1 years (interquartile range [IQR]: 4.2–12.7 years) for gastric cancer and 9.4 years (IQR: 5.1–13.0 years) for esophageal cancer. Compared with subcohort members, gastric cancer cases had higher frequencies of gastric cancer family history, cigarette smoking and salty foods consumption, while esophageal cancer cases had higher percentages of smoking and alcohol use.
TABLE.
Baseline characteristics of JPHC Cohort II gastric and esophageal cancer cases and subcohort members
| Subcohort n=774 |
Gastric cancer* n=446 |
Esophageal cancer* n=68 |
||||
|---|---|---|---|---|---|---|
| Age at enrollment, mean ± SD, years | 60.1 ± 7.5 | 59.9 ± 7.9 | 59.0 ± 7.5 | |||
| Male, n (%) | 413 | (53.4) | 270 | (60.5) | 58 | (85.3) |
| Family history of gastric cancer, n (%)* | 59 | (7.6) | 49 | (11.0) | ||
| Smoking, n (%) | ||||||
| Never | 452 | (58.4) | 223 | (50.0) | 13 | (19.1) |
| Former | 134 | (17.3) | 80 | (18.0) | 18 | (26.5) |
| Current ≤20 cig/day | 129 | (16.7) | 99 | (22.2) | 24 | (35.3) |
| Current >20 cig/day | 47 | (6.1) | 43 | (9.6) | 11 | (16.2) |
| Unknown | 12 | (1.5) | 1 | (0.2) | 2 | (2.9) |
| Alcohol drinking, n (%) | ||||||
| Never/occasional | 552 | (71.3) | 310 | (69.5) | 25 | (36.8) |
| 1+ per day and <300 g/week | 135 | (17.4) | 98 | (22.0) | 22 | (32.3) |
| 1+ per day and ≥300 g/week | 64 | (8.3) | 31 | (6.9) | 18 | (26.5) |
| Unknown | 23 | (3.0) | 7 | (1.6) | 3 | (4.4) |
| H. pylori status, n (%) | ||||||
| Seropositive | 501 | (64.7) | 383 | (85.9) | 49 | (72.1) |
| Seronegative | 230 | (29.7) | 37 | (8.3) | 16 | (23.5) |
| Unknown | 43 | (5.6) | 26 | (5.8) | 3 | (4.4) |
| Salty/preserved food intake, n (%) | ||||||
| 0 g/day | 363 | (46.9) | 176 | (39.5) | ||
| > 0 g/day | 411 | (53.1) | 270 | (60.5) | ||
| Gastric anatomic subsite, n (%) | ||||||
| Proximal (C160–1) | 32 | (7.2) | ||||
| Distal (C162–6) | 272 | (61.0) | ||||
| Overlapping/unspecified (C168–9) | 142 | (31.8) | ||||
| Clinical stage, n (%) | ||||||
| Early/localized | 234 | (52.5) | 29 | (42.6) | ||
| Advanced** | 152 | (34.1) | 26 | (38.2) | ||
| Unknown | 60 | (13.4) | 13 | (19.2) | ||
Abbreviations: SD, standard deviation.
Totals include 23 gastric cancer cases and 4 esophageal cancer cases in the subcohort.
Includes regional lymph node extension, adjacent organ invasion, distant metastasis.
The Figure 1 shows the 62 evaluable biomarkers and their associations with risks of gastric (A) and esophageal (B) cancer. Levels of sEGFR (Ptrend=0.017) and TSLP (Ptrend=0.048) were nominally associated with gastric cancer risk, and CRP (Ptrend=0.015), CXCL11/ITAC (Ptrend=0.017), and CCL15/MIP1D (Ptrend=0.043) with esophageal cancer. However, none of the Ptrends remained statistically significant with FDR correction. Neither stratified analysis by latency nor restriction to H. pylori seropositive cohort members revealed any significant associations. Hazard ratios for proximal and distal cancers were similar to gastric cancer overall.
1A.
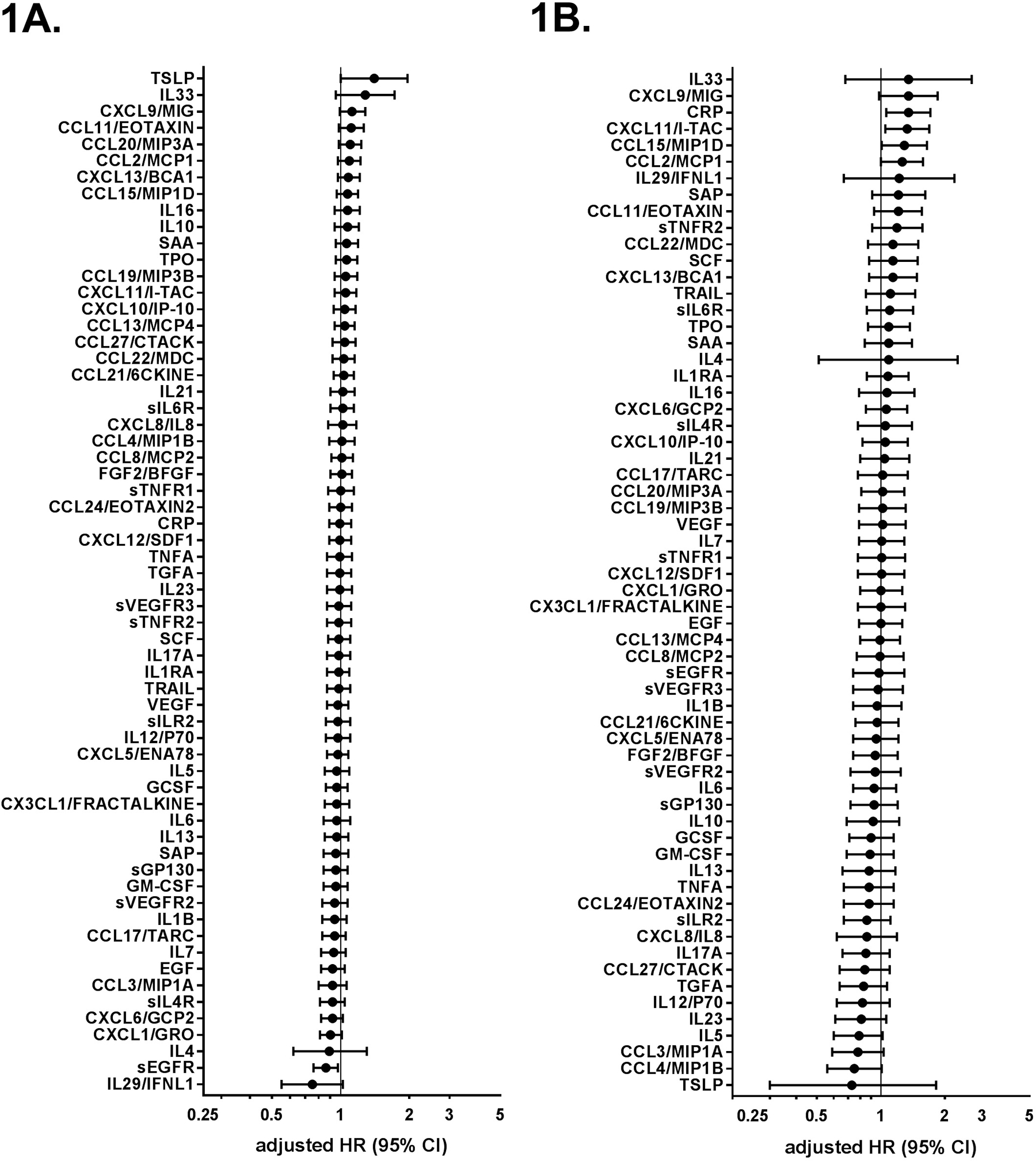
Adjusted associations of inflammatory biomarkers with incident gastric cancer in the JPHC study cohort II.
Gastric cancer hazard ratios (HR) and 95% confidence intervals (95% CIs) for gastric cancer risk per quantile increase of inflammation-related biomarkers. Estimates from Cox proportional regression models adjusted for age (in years), sex, study area (six public health centers), family history of gastric cancer (yes vs. no), smoking habits (never, former, current ≤ 20 cigarettes/day, current > 20 cigarettes/day, unknown), and salty/preserved foods intake (0 vs. > 0 g/day).
1B. Adjusted associations of inflammatory biomarkers with incident esophageal cancer in the JPHC study Cohort II.
Esophageal cancer hazard ratios (HR) and 95% confidence intervals (95% CIs) per quantile increase of inflammation-related biomarkers. Estimates from Cox proportional regression models adjusted for age (in years), sex, study area (six public health centers), smoking habits (never, former, current ≤ 20 cigarettes/day, current > 20 cigarettes/day, unknown), and alcohol drinking (never/occasional, current < 300 g/week, current ≥ 300 g/week, unknown).
DISCUSSION
Despite the well-known role of local inflammation in gastroesophageal cancers, systemic levels of inflammatory proteins were not associated with incidence of these tumors during almost two decades of follow-up. Even within 5 years of cancer diagnosis, when effects of early disease may be manifest, circulating levels were unremarkable. Our multiplex method to assess the biomarkers is extensively validated. Other study strengths include the population-based design, large numbers of cases, and adjustment for relevant risk factors.
Our findings for gastric cancer did not replicate previously reported modest associations based on plasma samples from the first JPHC Cohort for CRP and SAA (494 case-control pairs) (5), and in the Shanghai Health Cohorts for IL8 among men (180 cases vs. 358 controls) (6), and IL6 among women (141 cases vs. 282 controls) (7). For esophageal cancer, our findings differ from the results of the Iranian Golestan Cohort study, which found strong associations in a relative small sample set (36 ESCC cases vs. 81 controls) with 15 of the markers we evaluated (8).
In conclusion, our study did not identify systemic markers for gastroesophageal cancers. Given the complexity of the immune response, these findings do not preclude potential associations with other inflammation-related molecules. Further study is warranted to identify underlying mechanisms of digestive tract carcinogenesis that could eventually yield to targeted prevention and treatment approaches.
ACKNOWLEDGEMENTS
This study was supported by the Japanese National Cancer Center Research and Development Fund (Grant No. 29-A-4, Principal Investigator, Shoichiro Tsugane) and the Intramural Research Program of the U.S. National Cancer Institute (ZIA CP010212–10101, Principal Investigator, Charles Rabkin).
We acknowledge statistical assistance from Michael Curry, Information Management Services, Inc. The authors are also grateful to the Ibaraki, Niigata, Osaka, Kochi, Nagasaki, and Okinawa Cancer Registries for providing their incidence data. Members of the JPHC Study Group (Principal Investigator, Shoichiro Tsugane) are listed at https://epi.ncc.go.jp/en/jphc/781/7951.html
Footnotes
Disclosure of conflicts of interest: The authors declare no potential conflicts of interest
REFERENCES
- 1.Correa P, Piazuelo MB, Camargo MC. Etiopathogenesis of gastric cancer. Scandinavian journal of surgery : SJS : official organ for the Finnish Surgical Society and the Scandinavian Surgical Society 2006;95:218–24. [DOI] [PubMed] [Google Scholar]
- 2.Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal cancer. Nat Rev Dis Primers 2017;3:17048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsugane S, Sobue T. Baseline survey of JPHC study--design and participation rate. Japan Public Health Center-based Prospective Study on Cancer and Cardiovascular Diseases. Journal of epidemiology / Japan Epidemiological Association 2001;11(6 Suppl):S24–9. [DOI] [PubMed] [Google Scholar]
- 4.Langholz B, Jiao J. Computational methods for case-cohort studies. Comput Stat Data An 2007;51:3737–48. [Google Scholar]
- 5.Sasazuki S, Inoue M, Sawada N, Iwasaki M, Shimazu T, Yamaji T, et al. Plasma levels of C-reactive protein and serum amyloid A and gastric cancer in a nested case-control study: Japan Public Health Center-based prospective study. Carcinogenesis 2010;31:712–8. [DOI] [PubMed] [Google Scholar]
- 6.Epplein M, Xiang YB, Cai Q, Peek RM Jr., Li H, Correa P, et al. Circulating cytokines and gastric cancer risk. Cancer causes & control: CCC 2013;24:2245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong HL, Rabkin CS, Shu XO, Pfeiffer RM, Cai Q, Ji BT, et al. Systemic cytokine levels and subsequent risk of gastric cancer in Chinese Women. Cancer science 2011;102:1911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keeley BR, Islami F, Pourshams A, Poustchi H, Pak JS, Brennan P, et al. Prediagnostic serum levels of inflammatory biomarkers are correlated with future development of lung and esophageal cancer. Cancer science 2014;105:1205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]


