Abstract
Given the benefits of physical activity for breast cancer survivals, this pilot study aims to assess the feasibility of the MOTIVE program at achieving and maintaining the recommended physical activity level in women diagnosed and treated breast cancer, over 16 weeks. We conduct a pilot-controlled study of 20 women diagnosed with breast cancer stage I, II or IIIa. In this study, women of Intervention Arm (n = 10) received the MOTIVE program. This group was compared to women of Control Arm (n = 10) who received only counselling. Health-related fitness measures, and quality of life were assessed at baseline (t0) and after 4 (t1), 8 (t2) and 16 (t3) weeks. Intervention Arm women reached the recommended physical activity guidelines at t1 and t2 (eff.size = 1.9 [1.0–3.1]), and 90% continued to be active, autonomously, at t3 (eff.size = 1.12 [0.21–2.12]). Intervention Arm participants’ arm strength, fitness levels and quality of life also improved over time. No significant improvements in outcome measures were observed in Control Arm participants. These results are encouraging and suggest that the MOTIVE program may be a viable, well tolerated and effective option to help breast cancer women reaching a stable physical activity level over time, which meets prevention-related goals.
Keywords: Physical activity, Adherence, Breast cancer, Aerobic exercise, Strength training, Secondary/tertiary prevention, Lifestyle, Public health
Physical activity; Adherence; Breast cancer; Aerobic exercise; Strength training; Secondary/tertiary prevention; Lifestyle; Public health.
1. Introduction
Breast cancer (BC) patients adopting a healthy lifestyle after treatment may improve their disease-free survival rate [1, 2] and health-related outcomes [3]. Although current evidence is not strong enough to make specific recommendations for breast cancer survivors (BCSs) [4], reports are growing on the improvements and potential beneficial effects induced by change in modifiable lifestyle behaviors, such as nutrition and physical activity [5, 6, 7, 8, 9, 10, 11]. In particular, increasing physical activity (PA), due to its positive impact on body weight, physical fitness, fatigue, depressive symptoms, anxiety, inflammatory profile and quality of life [5, 6, 7, 8, 9, 10, 11, 12], may represent a feasible, safe, and effective way to help patients to manage the aftermath of BC. Indeed, evidence suggests that BCSs who meet the PA guidelines for cancer survivors [13, 14] show more benefits on the prevention of recurrences and mortality, as well as on certain side effects of the disease, compared to BCSs who do not [3]. It is concerning, therefore, that only 8.9% of BCSs [15] meet the current guidelines on aerobic and resistance exercise [15].
It is well known that the readiness and adherence of BCSs to exercise may be influenced by several factors, such as clinical complications (i.e., pain, fatigue, and cancer stage) [16], psychological conditions [17], social and environmental factors (including the availability or proximity of the training facilities) [16, 18], and personal exercise preferences. In addition, knowledge of, engagement with, and motivation to exercise play an important role in promoting PA in BCSs [19, 20, 21]. However, unfortunately, only 40% of oncologists routinely recommend PA to patients [22], and only 9% of BCSs are referred to exercise professionals [15]. This low-grade appointing to exercise professionals is particularly puzzling because he/she, in addition to act as trusted source of information and education, could manage and tailor the key components of exercise (i.e., frequency (F), intensity (I), time (T), type (T), volume (V), and progression (P) over time, namely the FITT-VP principle) [14, 23] to let BCSs safely obtain health benefits. This picture may be partially attributable to a poor understanding on how to promote, encourage and sustain people living with and beyond cancer to meet and adhere to the currently available PA recommendations [23, 24]. A recent meta-CART investigating which intervention strategy maximizes PA adherence showed that the major difference in effectiveness was attributable to supervised vs. unsupervised programs (d+ = .49 vs. .26), and greater contact time was associated with larger effects in supervised programs [25]. However, in the last fifteen years, different approaches of supervision to PA and exercise have emerged in BCSs [24, 26], and the term “supervision” has been used with different meanings and without proper specificity. In fact, it is sometimes intended as the clinical follow-up of self-managed or home-based exercise training programs and remote monitoring through wearable sensors or video recording [24, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40], while sometimes it is used to also mean the direct on-site supervision of the training sessions [24, 41, 42, 43, 44, 45]. As a consequence, studies on PA and exercise training adherence used different assessment parameters (e.g., average, median, range, number or percentage of participants completing all or a certain percentage or number of sessions; number of participants meeting PA guidelines; minutes of PA achieved per week; etc.) [24, 37, 39, 40, 42] and reported mixed results with an adherence ranging, on average, from 50% to 80% [24, 36, 37, 39, 40, 42, 46, 47, 48, 49]. Moreover, the clinical team involved in supervised training is rarely described in BCSs studies, and, to our knowledge, only a few (e.g., see Rogers Laura et al. [49]) described clearly that exercise training and supervision were provided by an exercise professional. Finally, about clinical outcomes (i.e., fitness measure, strength, and quality of life), small to moderate results in effect size with lack of precision were reported by different reviewers [24, 25]. Therefore, methods and efficacy of promoting a physically active lifestyle in BCSs have been poorly and confusedly studied. Particularly, the effectiveness of professionally guided exercise training and supervision on PA recommendations adherence has been poorly investigated in prior non-physically active BCSs. Thus, the present study aimed to compare the physical activity level, over 16 weeks, between a mixed on-site supervised exercise training group and a group receiving exercise education only. This pilot study was also designed to assess the feasibility, in the Italian clinical settings, of the MOTIVE program, which mixes exercise education and on-site supervised and tailored exercise training by an exercise professional to encourage BCSs to adapt an active lifestyle using an individually tailored exercise “dose”.
2. Materials and methods
2.1. Study design
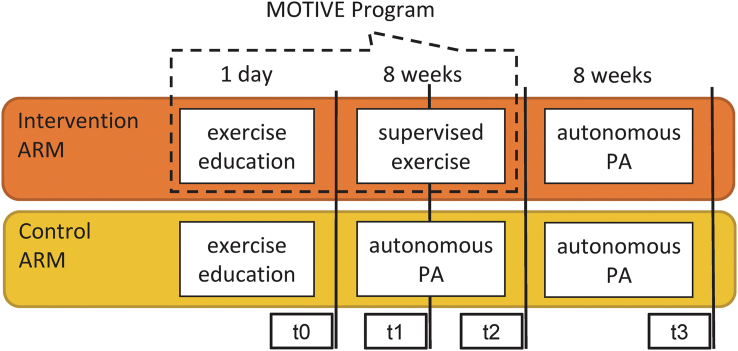
This pilot study was designed as a two-armed – Intervention and Control - controlled trial lasting 16 weeks (see Figure 1 for details). Intervention arm BCSs received the MOTIVE program, which consisted in a single exercise education session followed by a 8 weeks, 3 time-a-week supervised training program (see below for details). From week 9–16 of the trial, intervention arm participants were free to continue being physically active. Control arm BCSs received the exercise education session only and were free to adopt or not an active lifestyle throughout the 16 weeks duration of the trial. Participants of both groups were not contacted from week 9–16. Throughout the whole trial, participants had to collect data on their weekly PA habits in a diary (Figure 2), which was picked up and replaced by the research staff at each follow-up.
Figure 1.
Study design. Abbreviation: PA, physical activity.
Figure 2.
Physical Activity Diary. (a) cover; (b) recommendations; (c) instructions on physical activity programming; (d) (e) and (f) instructions for completing the diary “My Physical Activity Diary”.
Assessments of study outcomes were performed at baseline (t0) and at 4 (t1), 8 (t2), and 16 (t3) weeks from t0.
The procedures for this study were conformed to the Helsinki protocol for clinical trials and the study was approved by the local regional Ethics Committee (ANBU 03/2018).
2.2. Study population
Women diagnosed with BC Stage I, II, or IIIa, consecutively referred to the Breast Unit facilities for clinical follow-up were recruited and checked for eligibility by the clinicians (oncologist or physiatrists). BCSs were enrolled if they met the following inclusion criteria: ≤ 5 years after surgery for BC (mastectomy and/or quadrantectomy with or without axillary surgery); age from 30 to 70 years; ≥ 6 months since completion chemotherapy; not physically active (i.e., not meeting the physical activity guidelines for cancer survivors in the previous 6 months: 150 min/week of moderate intensity aerobic exercise and two days/week of muscle-strengthening exercise [14]). Exclusion criteria were: presence of medical contraindication to participation in a regular PA program (i.e., unstable angina, debilitating arthritis pain); disabling neurological or psychiatric disorders; BC recurrence; metastatic disease; moderate to severe walking disability.
2.3. Group assignment
Enrolled BCSs were scheduled for a dedicated meeting with an exercise professional (the exercise education session), during which the supervised exercise training protocol was discussed in detail in order to assess participants’ exercise preferences and rule out possible barriers (i.e., socio-economic, distance and time constraints). According to Wasmann et al. [50], if the patient self-reported the preference to exercise under on-site supervision for 8 weeks (MOTIVE program), she was assigned to the Intervention arm, while patients who did not were assigned to the Control arm. The reason for acceptance or denial of the on-site supervision were collected.
2.4. Exercise education session
The exercise education session of the MOTIVE program consisted in a 30 min individual session in which an exercise professional encouraged the participants to be physically active (i.e., regularly engaged in PA), and explained the currently available exercise guidelines for cancer survivors [14]. PA goals, which were personalized, attainable, and timed, were set and discussed in detail. Participants were instructed on how to use the Talk Test [51] and Borg's rating of perceived exertion (RPE) scale [52] to self-monitor their compliance with the prescription of exercise intensity made by the exercise professional.
Participants were provided with a PA diary (Figure 2) as a tool to increase and maintain an active lifestyle [36]. The diary contains specific information on PA (i.e., explanations of the FITT principle and exercise recommendations) [14] and its benefits. BCSs were asked to record within the diary the “Start time”, “Type” and “Duration” of each exercise session performed, and any relevant “Comments or notes”. The PA diary was discussed with the patients at each follow-up to convey information on the benefits of the exercise performed, on any problem encountered during the training and, on the basis of this, to provide them with practical suggestions to start or continue to exercise regularly and autonomously.
2.5. Supervised training intervention
The training intervention of the MOTIVE program lasted 8 weeks. BCSs exercised 2 times each week (on non-consecutive days) under the supervision of an exercise professional (i.e., on-site supervision of the training sessions performed at the gym located inside the hospital) and 1 time each week autonomously, following the directions received by the exercise professional during the last on-site supervised training session. On-site supervised training sessions consisted in group-exercise designed according to the exercise guidelines for cancer survivors [14].
The exercise sessions started with 5 min of dynamic exercises (warm-up), followed by 45–50 min of training (core part) and 5 min of light exercises (cool-down). The core part of each session was structured as a circuit with eight stations: seven resistance exercises and one aerobic exercise performed on a treadmill (model EE-0720, MTR, Italy). The circuit was repeated 2 times and each round always started with the aerobic exercise. Details of exercises part of the circuit training are reported in Table 1 along with exercise intensity and progression.
Table 1.
Exercises of the circuit stations and training intensity progression.
| Parts and Exercises (Time) | Intensity/exercise mode and progression | Materials | Intervention/Instruction description |
|---|---|---|---|
| Warm-up (5 min) | n.a. | n.a. | The warm-up session included dynamic warm-up exercises. |
| Core part (from 45 to 50 min) | n.a. | n.a. | The core part consisted in circuit training exercises structured into 8 stations: 1 aerobic exercise and 7 resistance exercises. Participants always started the circuit with aerobic exercise, which was followed by the resistance training exercises (one for each station); the whole circuit was then repeated. |
| Aerobic exercise station | |||
| Walking | Ranges of the average speed of the 6MWD: L1) 80–89% L2) 90–99% L3) 100–110% |
Treadmill | One familiarization session. The walking intensity was tailored on the basis of each participant's average speed of the 6MWD. During the first week of training speed was set at 80% of the average speed of the 6MWD and progressed to 90%, 100%, and 110% in the next three weeks. Then, a second 6MWD was performed and the walking speed of the following four weeks of training was set at 80%, 90%, 100%, and 110% of the new average speed, respectively. Aerobic exercise duration progressed from a minimum of 15 min to a maximum of 20 min throughout the training intervention. Since the circuit was always repeated, each session consisted in a minimum of 30 to a maximum of 40 min of aerobic exercise, interspersed by approximately 5 min of resistance exercises. The 15-point Borg's RPE scale, which ranges from 6 (“no exertion at all”) to 20 (“maximal exertion”), was periodically assessed. |
| Resistance exercise stations | |||
| Push-up | Starting position: L1) Wall push-ups (while standing) L2) Push-ups on knees L3) Push-ups (regular) |
Mat | Resistance exercise was performed for 30s each station, followed by 20s of rest that progressively reduced to 10s throughout the training intervention. Hence, each 40–50 s, the participant moved to another station and completed the resistance exercise part of the circuit in about 5–6 min. During the 30s of exercise, the participant was asked to perform 8 to 10 repetitions of the specific resistance exercise of that station at a comfortable cadence (which resulted in an average of about 3s each repetition, i.e., ≈1s for the concentric part and ≈2s for the eccentric part). All participants began the intervention program exercising at the lowest among three levels of intensity. When the participant was able to perform more than 10 repetitions for the given time was instructed to adopt the next intensity level in the following supervised exercise session. |
| Squat | Starting position and resistance applied: L1) Wall-squats (isometric, 90° at the knees) L2) Bodyweight squats L3) Squats with MB (on the chest) |
Medicine ball (MB, 2 kg) | |
| Triceps extension | Resistance applied: L1) Low L2) Medium L3) High |
Elastic band (Therabands) | |
| Crunch | Starting position and arms position: L1) Basic with arms straight L2) Basic with arms to the chest L3) Reverse with hands behind the head |
Mat | |
| Bridge | Floor contact and resistance applied: L1) Two legs, no additional resistance L2) One leg, no additional resistance L3) Two legs with elastic band |
Mat and Elastic band (Therabands) | |
| Calf-raise | Starting position, floor contact, and knee position: L1) Wall contact (hands), with two legs, knee straight L2) Wall contact (hands), with one leg, knee straight L3) Wall contact (one hand), with one leg, knee straight |
Step | |
| Rowing | Resistance applied: L1) Low L2) Medium L3) High |
Elastic band (Therabands) | |
| Cool-down (5 min) | n.a. | n.a. | The Cool-down session included static stretching exercises. |
Note: Each participant began the intervention program exercising at the lowest level of intensity (L1) and any time she was able to perform more than 10 repetitions in 30s she progressed to a higher level of intensity (L2 or L3) starting from the next supervised exercise session. Exercise progression occurred with the passage from level 1, to level 2 up to level 3. Abbreviations: 6MWD, 6-min walk distance; RPE, Borg's rating of perceived exertion scale; n.a., not applicable.
During the not directly supervised sessions participants performed their own favorite aerobic exercise for at least 30 min. They were instructed to maintain the same level of intensity of their last on-site supervised training session by using the RPE scale and/or the talk test, and to log the main parameters of each session (type, duration, intensity, etc.) on the sheets of the PA diary.
Adherence to the prescribed exercise intensity was recorded by the exercise professional in the training logs of the patients during the on-site supervised sessions, while adherence to the directions of the exercise professional was monitored and retrieved by using the self-report sheets of a PA diary than BCSs had to fill-in.
2.6. Study outcomes
The following parameters were assessed at each time point.
Anthropometry: body mass; height; waist circumference.
Cardiovascular parameters: resting blood pressure; resting heart rate.
PA level [primary outcome]: assessed using the International Physical Activity Questionnaire – Short Form (IPAQ-SF) [53, 54].
Health-related fitness parameters [secondary outcomes]: assessed using the 6-min walk distance (6MWD) [55], the isometric hand grip strength (HGS) [56], and the distance of the sit-&reach test (SRT) [14].
Health-related quality of life questionnaire [secondary outcomes]: 36-Item Short Form Health Survey (SF-36) questionnaire [57].
The body mass index (BMI) was calculated using body mass and height (kg/m2). PA level assessed at t0 was deemed representative of participants’ actual status of non-physically active subjects when they stated that their answers would have been the same if the questionnaire was administered in the previous 6 months.
Diary completion rate was also reported to describe the process and was calculated as the percentage of given answers (about weekly physical activities) with respect to requested answers per follow-up period.
2.7. Sample size calculation and data analysis
The sample size was calculated using the Wilson's confidence interval method [58] with 95% confidence level, which resulted in 10 subjects per group who were needed to evaluate an adherence of 90% (i.e., 9 over 10 subjects who maintain, at t3, the PA level recommended by the exercise professional and based on current exercise guidelines for secondary prevention [13, 14] with an interval width of 0.9 (95% CI: 0.59–0.98). This number allows to obtain results characterized by an error compatible with the exploratory nature of the study.
Data were described using mean, standard deviation (SD), range, median and interquartile range (IQR) for continuous variables, and numbers and percentage rates for categorical parameters. Within-group changes were checked using the Wilcoxon rank test between each time point.
To highlight differences in measurement trends across groups, a change index (Δtn) was also computed for each outcome, at t1, t2 and t3: Δtn = ([tn score-t0 score]/t0 score)x100 for each outcome. Intergroup differences were assessed using the Mann-Whitney U test to compare data between the two groups at each time point. α was set at .05.
3. Results
Twenty women were enrolled (baseline demographic and clinical data are shown in Table 2). Ten participants who reported to have not enough time to move from their own home (n = 2) at the scheduled times or to have distance-related problems (n = 8) were assigned to the Control arm.
Table 2.
Baseline general characteristics of the study sample.
| All (n = 20) | Intervention arm (n = 10) | Control arm (n = 10) | |
|---|---|---|---|
| Age (years) | 56.0 ± 11.6 | 58.3 ± 14 | 53.8 ± 7.8 |
| Body mass (kg) | 67.9 ± 13.2 | 71.6 ± 16.7 | 64.2 ± 7.8 |
| Height (m) | 1.63 ± 0.6 | 1.65 ± 0.5 | 1.60 ± 0.5 |
| Waist circumference (m) | 0.93 ± 0.1 | 0.93 ± 0.1 | 0.94 ± 0.8 |
| BMI (kg/m2) | 25.5 ± 4.5 | 26.1 ± 5.7 | 24.9 ± 2.5 |
| Cancer stage (n) | |||
| I | 4 | 2 | 2 |
| II | 10 | 5 | 5 |
| IIIa | 6 | 3 | 3 |
| Involved body side (n) | 9L/7R/4BIL | 4L/4R/2BIL | 5L/3R/2BIL |
| Surgery type (n) | 11M + LA + R | 5M + LA + R | 6M + LA + R |
| 5M + SLB + R | 3M + SLB + R | 2M + SLB + R | |
| 4Q + SLB | 2Q + SLB | 2Q + SLB | |
| Months since surgery (n) | 31 [12–90] | 33 [12–90] | 0 [12-70] |
| Hormone therapy (n for yes) | 14 | 7 | 7 |
| Adjuvant chemotherapy (n for yes) | 12 | 6 | 6 |
| Radiotherapy (n for yes) | 20 | 10 | 10 |
| Lymphedema (n for yes) | 2 | 1 | 1 |
| PA level (MET∙min/week) | 424 ± 289 | 341 ± 294 | 507 ± 273 |
Note: Values are means ± SD or median [range] if not differently specified. Abbreviations: BMI, body mass index; PA, physical activity; R, right; L, left; BIL, bilateral; n, number; M, mastectomy; LA, lymphadenectomy; SLB, sentinel lymph-node biopsy; R, reconstruction; Q, quadrantectomy.
At baseline no differences were found in demographic and basal clinical data between the groups. All participants did not meet the exercise guidelines for cancer survivors [14] and, on average, were overweight. Control arm women were slightly (but not significantly) more active than Intervention arm women. No adverse events were recorded during the study for any of the participant. Intervention arm BCSs attendance rate for the supervised exercise sessions was 94% (one absence per participant on average) and their PA diary completion rate was 100% at t1 follow-up, 98% at t2, and 94% at t3. In the Control arm, subjects’ PA diary completion rate was 99% at t1 follow-up, 93% at t2 and 89% at t3.
3.1. Physical activity level
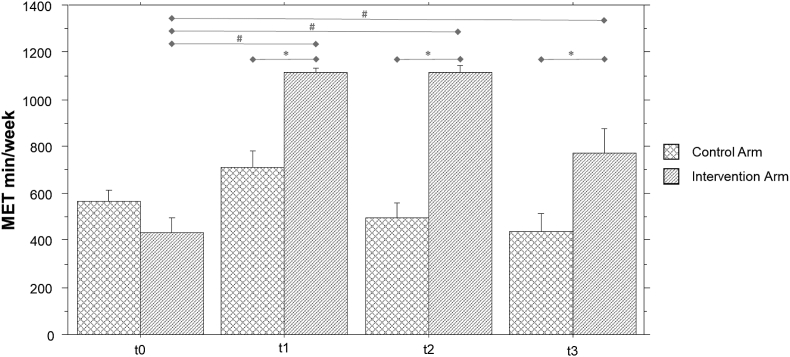
Intervention arm participants, at t1, increased their PA level more than two-fold compared to t0 (MET∙min/week t1 = 1111.8 ± 60.864 (median = 1074 [1074–1220]; Z = -2.8; p = .005). PA levels remained stable in this group both at t2 (1117 ± 84 median = 1074 [1020–1272] MET∙min/week; Z = -2.8; p = .005 versus t0) and t3 (773 ± 323 median = 735 [0–1230] MET∙min/week; Z = -2.7; p = .008 versus t0). Compared to t0, PA level increased significantly also in Control arm at t1 (682 ± 290 median = 727 [0–1050] MET∙min/week; Z = -2.2; p = .03), but returned rapidly close to the initial values at t2 (623 ± 368 median = 602 [0–834] MET∙min/week; n.s. vs t0) and remained stable at t3 (428 ± 254 median = 540 [0–735] MET∙min/week; n.s. vs t0) (see Figure 3).
Figure 3.
Bar chart of physical activity (as MET∙min/week calculated by IPAQ-SF) over time in the two groups. Note: ∗ = p<.01 in inter-group comparisons with respect to t0 value; # = p<.01 in intra-group comparisons.
At each of follow-up, the Intervention arm change index was significantly higher than Control arm. Table 3 shows details of descriptive and comparative statistical results.
Table 3.
Descriptive and comparative results.
| Control Arm |
Intervention Arm |
Inter-group comparison |
|||
|---|---|---|---|---|---|
| mean (SD) | Median [range] IQR | mean (SD) | Median [range] IQR | Z; p | |
| IPAQ-SF (International Physical Activity Questionnaire – Short Form) | |||||
| Δt1 | 35.4 (76.3) | 15 [0; +250] 29 | 224.7 (171.8) | 190 [+71; +532] 168 | −3.4; .0007 |
| Δt2 | −6.3 (43.8) | −7 [−79; +89] 14 | 225.5 (171.4) | 192 [+79; +532] 168 | −3.6; .0003 |
| Δt3 | −13.9 (56.9) | 15 [0; +250] 29 | 116.6 (138.9) | 95 [−100; +324] 223 | −2.6; .008 |
| HGS (Hand Grip Strength Test) | |||||
| Δt1 | −5.5 (19.9) | −2,5 [−38.9; +25,5] 28 | 11.3 (24.9) | 5.4 [−15; +79] 11 | −1.6; .11 |
| Δt2 | −21.9 (79.7) | −10 [−156; +102] 111 | 53.4 (76.5) | 35.8 [−50; +229] 45 | −1.9; .049 |
| Δt3 | −8.2 (17.5) | −9.5 [−34; +20] 24.3 | −24.2 (25.9) | 29 [−61.3; +30.6] 22 | −1.7; .08 |
| SRT (Sit-&-Reach Test) | |||||
| Δt1 | 52.9 (93.7) | 30 [−22; +24] 56 | −5.9 (114.8) | 9.54 [−300; +150 ] 3,6 | −.3; .76 |
| Δt2 | 52.9 (93.7) | 24 [−22; +300] 56 | −17.7 (218) | 27.4 [−600; +200] 109 | −1.8; .07 |
| Δt3 | 18.4 (83.1) | 7.7 [−100; +200] 73 | 27.8 (64) | 24.7 [−100; +140] 50 | −.77; .43 |
| 6MWD (Six-Minute Walk Distance) | |||||
| Δt1 | −2.9 (7.8) | −1.7 [−18.6; +9.4] 8 | 13.3 (18) | 4.6 [−0.7; +54.3] 22 | −2.6; .009 |
| Δt2 | −2.9 (7.8) | −1.7 [−18.6; +9.4] 7.9 | 16.6 (18) | 8.9 [0; +54.3] 33 | −2.9; .004 |
| Δt3 | −3.7 (9.9) | −4.1 [−22.6; +9.6] 14 | 11.7 (18) | 6.3 [−3,5; +54.3] 16 | −2.4; .02 |
Note: values are expressed in % and were calculated using the following formula: Δtn = ([tn score-t0 score]/t0 score)x100. Abbreviation: SD = standard deviation.
At t2, the effect size of training supervision, with respect to self-administering the training program, on PA levels was 1.9 [1.0–3.1] (Hedges' g [95% CI]), while at t3 follow-up was 1.12 [0.21–2.12] (Hedges’ g [95% CI]).
At t3 the number of Intervention arm participants who continued to yield autonomously an average energy expenditure attributable to PA higher than 700 MET-min/week was 9 out of 10 (90%), while in the Control arm they were 1 out of 10 (with other 2 participants who yielded an energy expenditure higher than 600 MET-min/week) (Odds ratio = 13.8 [1.4–445]; RR = 2.7 [1.04–6.9]).
3.2. Health-related fitness measures
3.2.1. Hand grip strength
Compared to t0, HGS improved significantly in Intervention arm participants at the t2 follow-up (Z = -2.66; p = .008). This score was higher than the HGS score in the Control arm at the same time point (Z = -1.9; p = .049). No other statistically significant differences were found in the intra-group and inter-groups analyses (Table 3).
3.2.2. Sit and reach test
Results of the SRT test showed no significant changes in either of the groups at the t1, t2 and t3 follow-ups. No inter-group differences were found either (Table 3).
3.2.3. Six-minute walk test
At the baseline women enrolled in the study walked 476 m on average (standard deviation = 72), without differences between Intervention and Control arm. At the follow-up assessments, subjects in the Intervention arm performed significantly better than Control arm subjects in the 6MWD (Table 3). Moreover, nine out of ten of the Intervention arm participants improved their performance in the 6MWD at t1, and all ten showed improvements at t2; five of them showed improvements in their performance that were greater than the minimal clinically important difference (MCID = 30 m according to Bohannon and Crouch) [59] at t1, and six at t2. Nine out of ten of the Intervention arm participants maintained their 6MWD performance at t3. Intervention arm participants improved their 6MWD performance by 49 m on average [range: 190, -4], while Control arm participants showed no improvements during the follow-up period, and data analysis highlighted that their 6MWD performance declined, on average, by about 15 m [range: 42, -95] at t1 to -19 m [range: 45, -105] at t3.
3.2.4. Health-related quality of life
The Intervention arm reported better Quality of Life (QoL) in several areas at the t1 follow-up assessment. Intervention arm participants had significantly higher scores (indicating better QoL) on scales measuring ‘physical functioning’ (Z = -2.8; p = .005), ‘role limitation due to physical health’ (Z = -2.0; p = .04), ‘social functioning’ (Z = -2.3; p = .02), ‘bodily pain’ (Z = -2.5; p = .01) and ‘general health perceptions’ (Z = -2.8; p = .005) (Table 4).
Table 4.
Results of descriptive and comparative statistic regarding to delta of SF-36.
| Inter-group comparison |
||||||||
|---|---|---|---|---|---|---|---|---|
| SF-36 domains | Δtn | Group | Mean (SD) | Minimum | Maximum | Median | IQR | Z; p |
| Physical functioning | Δt1 | CA | 9.9 (14.5) | −11.111 | 35.7 | 6.1 | 25 | −.98 .32 |
| Δt1 | IA | 16.9 (12.8) | 6.25 | 36.4 | 11.8 | 26.2 | ||
| Δt2 | CA | 6.6 (18.4) | −8.111 | 36.5 | 5.1 | 25 | −.81 .41 | |
| Δt2 | IA | 17.6 (12.4) | 6.25 | 36.4 | 12.1 | 26.2 | ||
| Δt3 | CA | 0.7 (2.1) | 0 | 6.25 | 0 | 0 | −2.4;.02 | |
| Δt3 | IA | 12.0 (12.2) | −6.25 | 33.3 | 9.5 | 15.5 | ||
| Role limitations due to physical problems | Δt1 | CA | 5 (59.9) | −100 | 100 | 0 | 0 | −15.; .13 |
| Δt1 | IA | 52.5 (53.3) | −25 | 100 | 75 | 100 | ||
| Δt2 | CA | 22.2 (44.1) | 0 | 100 | 0 | 25 | −1.0 .3 | |
| Δt2 | IA | 52.5 (53.3) | −25 | 100 | 75 | 100 | ||
| Δt3 | CA | 11.1 (33.3) | 0 | 100 | 0 | 0 | −1.4 .15 | |
| Δt3 | IA | 52.5 (53.3) | −25 | 100 | 75 | 100 | ||
| Social functioning | Δt1 | CA | 43.8 (72.9) | 0 | 200 | 0 | 75 | −.8 .45 |
| Δt1 | IA | 100 (113.0) | 0 | 300 | 50 | 200 | ||
| Δt2 | CA | 43.8 (72.9) | 0 | 200 | 0 | 75 | −.8 .45 | |
| Δt2 | IA | 100 (113) | 0 | 300 | 50 | 200 | ||
| Δt3 | CA | 0 (0) | 0 | 0 | 0 | 0 | −1.6 .11 | |
| Δt3 | IA | 95 (116.6) | 0 | 300 | 25 | 200 | ||
| Bodily pain | Δt1 | CA | 76.0 (186.6) | 0 | 600 | 9.8 | 27.3 | −.45 .65 |
| Δt1 | IA | 29.3 (72.6) | −21.4 | 220 | 8.3 | 18.8 | ||
| Δt2 | CA | −9.6 (19.3) | −50 | 0 | 0 | 9.1 | −2.2 .03 | |
| Δt2 | IA | 29.3 (72.6) | −21.4 | 220 | 8.3 | 18.8 | ||
| Δt3 | CA | −9.6 (19.3) | −50 | 0 | 0 | 9.1 | -1.9;.05 | |
| Δt3 | IA | 18.6 (47.1) | −21.4 | 140 | 6.5 | 13.1 | ||
| General mental health | Δt1 | CA | 20.3 (23.9) | −20 | 62.5 | 19.4 | 30.7 | −.45 .65 |
| Δt1 | IA | 106.4 (247.2) | 0 | 800 | 14.6 | 35 | ||
| Δt2 | CA | 5.2 (15.6) | 0 | 46.7 | 0 | 0 | −2.7; .007 | |
| Δt2 | IA | 110.4 (246.7) | 0 | 800 | 18.2 | 30.5 | ||
| Δt3 | CA | 5.2 (15.6) | 0 | 46.7 | 0 | 0 | −1.2 .2 | |
| Δt3 | IA | −1.1 (35.7) | −100 | 25 | 11.4 | 13.3 | ||
| Role limitations due to emotional problems | Δt1 | CA | 22.8 (56.1) | -25 | 166.7 | 7.143 | 20 | −.75 .45 |
| Δt1 | IA | 44.9 (92.2) | 0 | 300 | 14.3 | 16.7 | ||
| Δt2 | CA | 0 (0) | 0 | 0 | 0 | 0 | −2.6; .01 | |
| Δt2 | IA | 49.9 (95.5) | 0 | 300 | 14.3 | 16.7 | ||
| Δt3 | CA | 0 (0) | 0 | 0 | 0 | 0 | −2.2; .03 | |
| Δt3 | IA | 37.4 (92.7) | 0 | 300 | 14.3 | 14.3 | ||
| Vitalit | Δt1 | CA | 13.0 (32.4) | -41.9 | 72.2 | 0 | 35.2 | −1.1 .25 |
| Δt1 | IA | 28.2 (30.7) | 0 | 100 | 21.5 | 40.6 | ||
| Δt2 | CA | -2.5 (7.1) | -20 | 0 | 0 | 0 | −2.4; .02 | |
| Δt2 | IA | 35.2 (36.1) | 0 | 100 | 26.1 | 46.1 | ||
| Δt3 | CA | -2.5 (7.1) | -20 | 0 | 0 | 0 | −2.4; .02 | |
| Δt3 | IA | 25.8 (31.5) | 0 | 100 | 15.6 | 40.6 | ||
| General health perceptions | Δt1 | CA | 5.5 (10.8) | -13.0 | 17.6 | 7.2 | 15.8 | −2.4; .02 |
| Δt1 | IA | 87.1 (215.6) | 5.3 | 700 | 18.8 | 12.5 | ||
| Δt2 | CA | 8.8 (10.7) | -14.4 | 18.6 | 7.3 | 18.3 | −2.5; .02 | |
| Δt2 | IA | 75.5 (184.7) | 1.2 | 600 | 18.8 | 19.1 | ||
| Δt3 | CA | -6.2 (6.4) | -15.8 | 0 | -6.3 | 12.1 | −2.9; .003 | |
| Δt3 | IA | 12.6 (15.2) | -5.9 | 45.5 | 9.2 | 18.8 | ||
Note: Δtn = ([tn score-t0 score]/t0 score)x100. Abbreviation: SF36 = 36-item short form health survey questionnaire; CA = Control arm; IA = Interventional arm; SD = standard deviation.
4. Discussion
The main finding of the present study is that the MOTIVE program, which comprises an exercise education session and a mixed on-site supervised and tailored exercise training protocol of 8 weeks, produced a significantly greater increase and maintenance of BCSs PA level compared to autonomous and self-administered exercise session based on an exercise education session and clinical follow-up only.
Our results are partially consistent with previous studies on BCSs [41, 42, 43, 44, 45, 60]. In the Intervention arm, that received 8 weeks of supervised exercise, the PA levels improved compared to Control arm. Although the PA levels of the Control arm were also increased at t1, our results show that only the PA levels of the Intervention arm were maintained at t2. Over time, when Intervention arm did not receive exercise supervision, PA levels of this participants also decrease. In this context, it is interesting to note that after the MOTIVE program women reached a high level of PA differently from many previous studies which showed statistically significant effects with smaller effect size tested on a shorter follow-up [24, 25]. Our results report than 90% of subjects maintain recommended PA levels even at the end of the MOTIVE program and, in particular in the following 8 weeks unsupervised on-site by an exercise professional. Previous trials have shown that patients’ adherence to international guidelines on PA ranges from 5.4% [14] to 44% [15] after an active lifestyle counselling program. Approaches based on supervised exercise training improved adherence to PA guidelines with an effect size that ranged from 0.6 [44] to 1.02 [45]. In the present investigation, the effect size in the Intervention arm reached 1.9 after 8 weeks and 1.12 after 16 weeks of follow-up. Both effect sizes were greater than the effect size found by Rogers et al. [45] at a three-month follow-up following individual training. Indeed, we selected group training because [44] it has been shown to have a long-lasting motivational effect on BCSs, which was associated with a higher volume of PA even five years after the supervised PA intervention, with an effect size on adherence of approximately 0.6 [44].
Most of the literature reviewed to increase PA levels in BCSs women did not consider a mixed approach (i.e., MOTIVE program) or was out of the context of the Breast Unit's multidisciplinary team [24, 25]. The main differences of the MOTIVE program with respect to models described in the literature are: the presence of an exercise professional, the integration of this specialized figure in a Breast Unit and the assessment of barriers or preferences. This organization allows a personalization of the proposed exercise and the PA based on the knowledge of clinical information given by clinicians, women preferences and on the basis of FITT principle [14]. Despite this, a reduction in PA levels was observed in both groups of the present study during the second part of the trial, when women exercised autonomously according to the directions received by the exercise professional during the last supervised training session. Furthermore, at the end of the study, the PA did not reach the internationally recommended levels [13, 14]. Counselling is known to guarantee less than 50% adherence to international recommended PA levels [13, 14] and supervision is a powerful way to improve the amount of PA [25]. However, ongoing supervision is costly and difficult for the health system to sustain. In this study, specialized supervision performed for 8 weeks was helpful in achieving a high level of physical activity in BCSs, which may remain adequate for secondary and tertiary prevention even after two months of discontinuing supervised exercise.
A large body of evidence shows that low cardiorespiratory fitness in BC patients is a strong, independent, and modifiable risk factor for premature mortality and increases the risk of cardiovascular disease [61]. Therefore, physical activity becomes increasingly important for BC patients during treatment and rehabilitation. When prescribing physical activity for BCSs, all precautions should be taken into account, including the patients’ current fitness level and general health. The 6MWD is a relevant parameter for the assessment and monitoring of cardiorespiratory fitness in medical and exercise interventions also for BCSs [62]. The women enrolled in the study are representative of the general BCS population, in fact they covered 476 m in 6 min, showing performance at the lower limits of the norm for age as reported in But-Hadzic et al. [62]. The Intervention arm showed improved performance in cardiorespiratory fitness after the supervised training. Indeed, nine out of ten of the Intervention arm participants showed significant improvements in their 6MWD performance at t1, and all the Intervention arm participants showed significant improvements at t2. Notably, five out of ten of the Intervention arm participants showed improvements in their 6MWD performance that were greater than the minimal clinically important difference (MCID, i.e. 30 meters) [59] at t1 and six out of ten at t2; nine of the Intervention arm subjects maintained their fitness level at t3. On the contrary, Control arm participants showed a decline in their 6MWD performance over time.
The fitness level is known to be related with BCSs psychological state, quality of life, and body composition [62]; indeed, we found associations between PA levels and QoL. Closer examination of the SF-36 domains revealed that the improvement in the Intervention arm was greater than that which was found the Control arm; hence, this may be a clinically important improvement, which is in line with previous findings obtained with different protocols and under different conditions [63, 64, 65].
Recommendations suggest that BCS should engage in resistance exercise [14]. Anyway, according to Lahart et al. [24], less than the 40% of trials published online proposed a training protocol that combines cardio and resistance training. In this study, HGS, a measure of muscular fitness, increased at the 8-week follow-up in Intervention arm participants, who performed 75% better than Control arm participants. However, HGS gains were not maintained in the long-term, possibly indicating that more attention and insistence must be placed during the exercise education session in explaining the usefulness of muscle strengthening. The measure of flexibility (SRT) has not changed significantly in both groups and the low “dose” of flexibility exercises prescribed may explain this result.
Furthermore, the proposed training program based on the FITT principle [14] proved to be safe, considering that no side effects or complications were reported in either group. To our knowledge, these results has never been investigated in parallel studies as well as in the Italian clinical settings.
Finally, between factors influencing PA behavior, barriers and accessibility to services [66, 67] are important issues that can affect adherence to exercise, together with the age, education and social status of the subjects [19]. The present investigation showed that only half of the enrolled subjects were able to reach the hospital to undergo the supervised exercise training sessions, highlighting the problem of the accessibility of the healthcare services. This problem is particularly serious in the Italian clinical setting and in the management of BCSs, not only under normal conditions but also during major emergency (e.g., COVID-19 pandemic) that could limit access to many health services, including rehabilitation [65, 68]. For people living with and beyond cancer, is important maintaining an exercise regimen for improvement of important clinical outcomes and, to date, the challenge for clinicians caring for these patients is to provide safe and effective exercise protocols which patients may adhere to in the longer term.
Considering the impact on PA level, the MOTIVE program represents a promising tool for secondary/tertiary prevention and to help women stay active. Therefore, this approach should be scalable over a large sample size in order to prove that a spread of services dedicated to physical activity and the inclusion of the exercise professional in the multidisciplinary team for the management of the BCSs would be clinically important. There are a few experiences in Italy of effective and widespread integration between exercise professional and breast units [60, 69]. Its dissemination requires an appropriate health policy, that recognizes the key elements that have proved to influence the cancer patient's experience, including the commitment of a multidisciplinary team in care and treatment [70, 71]. Our study re-enforces the importance of mixed approaches in future studies looking at care integration and its impact on BCSs.
4.1. Limitations and future perspectives
This study has two main limitations that need to be acknowledged: a small sample size and a not-randomized controlled design.
Statistical analysis was adapted in order to reduce selection biases using analysis tests for non-parametric data. However, a larger sample size is hopeful in a scaled-up study.
As regards the non-randomized controlled design, we chose to ask the patients about barriers to exercise according to the “partially randomized patient preference trials” design proposed by Wasmann et al. [50], which seems to be a reliable alternative for RCTs in trials comparing treatments of vastly different nature or using patient-centred outcomes in order to enroll a wide range of participants and optimize outcomes reporting. According to Wasmann et al. [50], “in case patients” preference can be assumed, a “partially randomized patient preference trial” enables “faster inclusion of a more representative population improving external validity without compromising internal validity”. Furthermore, through it, the study can provide information on facilitators or barriers in the processes of implementing health protocols.
A wider population will allow to improve heterogeneity of the sample. Population heterogeneity will be addressed both in the sample and in the exercise program: individualized programs based on disease stage, treatment, comorbidities should be considered in future research questions. In addition, demographics and composite factors that would have an effect on exercise and its outcomes, such as patients' previous exercise habits, socioeconomic status, or other potential barriers to physical access to exercise, will need to be carefully considered.
In our study, we did not evaluate hydration data. However, in a scaled-up study it would be interesting to include further outcome measures among those directly related to health and prevention, such as body composition. Indeed, since obesity is related to prognosis in breast cancer [72, 73, 74], an objective measure of fat mass, fat-free mass and hydration (e.g., by using the bioelectrical impedance analysis) may improve the understanding of the health-related effects of training in BCS. Finally, assessing the MOTIVE program on a longer follow-up will give insights on structured and unstructured PA in secondary and tertiary prevention in BCSs. In fact, at this time, literature cannot provide any conclusions regarding the relation between PA and breast cancer-related and all-cause mortality or breast cancer recurrence [24].
Promoting awareness and improving information for both patients and clinicians could be the winning key: our proposal underlines the importance of an expert multidisciplinary approach that will be improved overcoming barriers, human, social and environmental, in order to promote an effective approach on secondary and tertiary cancer prevention.
5. Conclusions
The MOTIVE program, as a pilot study, is a useful and sustainable methodology applicable in routine clinical setting based on the involvement of an exercise professional in the multidisciplinary team of the Breast Unit. It showed more efficacious than counselling or clinical follow-up alone in order to reach and maintain, over 16 weeks, an optimal adherence to the recommended PA level according to both exercise guidelines for cancer survivors and FITT principles [13, 14]. These results can confirm the efficacy of multidisciplinary clinical practices that focus on improving BCSs lifestyle behaviors as secondary and tertiary cancer care. Anyway, an adequate health policy, that brings the program closer to the subject, could optimize its results, recruiting as many subjects as possible.
Declarations
Author contribution statement
Valentina Natalucci and Marianna Capecci: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Manuela Marchegiani: Performed the experiments; Wrote the paper.
Francesco Lucertini: Analyzed and interpreted the data; Wrote the paper.
Maria Gabriella Ceravolo, Mirco Pistelli, Rossana Berardi, Luciana Vallorani, Giorgio Brandi and Elena Barbieri: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the University of Urbino Carlo Bo, Department of Biomolecular Sciences (under the grant “Progetti di Valorizzazione 2018” n. 28/2018).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank all the women who participated in the study and all the clinicians of the Breast Unit of the University Hospital of Ancona.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Karavasiloglou N., Pestoni G., Wanner M., Faeh D., Rohrmann S. Healthy lifestyle is inversely associated with mortality in cancer survivors: results from the third national health and nutrition examination survey (NHANES III) PLoS One. 2019;14 doi: 10.1371/journal.pone.0218048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wirtz P., Baumann F.T. Physical activity, exercise and breast cancer - what is the evidence for rehabilitation, aftercare, and survival? A review. Breast Care. 2018;13:93–101. doi: 10.1159/000488717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WCRF/AICR . 2018. Continuous Update Project Report: Diet, Nutrition, Physical Activity, and Breast Cancer Survivors.https://www.wcrf.org/sites/default/files/Breast-cancer-survivorsreport.pdf Available online: (accessed on 12 May) [Google Scholar]
- 5.Irwin M.L., McTiernan A., Baumgartner R.N., Baumgartner K.B., Bernstein L., Gilliland F.D., Ballard-Barbash R. Changes in body fat and weight after a breast cancer diagnosis: influence of demographic, prognostic, and lifestyle factors. J. Clin. Oncol. 2005;23:774–782. doi: 10.1200/JCO.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieli-Conwright C.M., Orozco B.Z. Exercise after breast cancer treatment: current perspectives. Breast Cancer. 2015;7:353–362. doi: 10.2147/BCTT.S82039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt M.E., Wiskemann J., Armbrust P., Schneeweiss A., Ulrich C.M., Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int. J. Cancer. 2015;137:471–480. doi: 10.1002/ijc.29383. [DOI] [PubMed] [Google Scholar]
- 8.Meneses-Echávez J.F., Correa-Bautista J.E., González-Jiménez E., Schmidt Río-Valle J., Elkins M.R., Lobelo F., Ramírez-Vélez R. The effect of exercise training on mediators of inflammation in breast cancer survivors: a systematic review with meta-analysis. Cancer Epidemiol. Biomarkers Prev. 2016;25:1009–1017. doi: 10.1158/1055-9965.EPI-15-1061. [DOI] [PubMed] [Google Scholar]
- 9.De Cicco P., Catani M.V., Gasperi V., Sibilano M., Quaglietta M., Savini I. Nutrition and breast cancer: a literature review on prevention, treatment and recurrence. Nutrients. 2019;11 doi: 10.3390/nu11071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rock C.L., Doyle C., Demark-Wahnefried W., Meyerhardt J., Courneya K.S., Schwartz A.L., Bandera E.V., Hamilton K.K., Grant B., McCullough M. Nutrition and physical activity guidelines for cancer survivors. CA A Cancer J. Clin. 2012;62:243–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz K.H., Courneya K.S., Matthews C., Demark-Wahnefried W., Galvão D.A., Pinto B.M., Irwin M.L., Wolin K.Y., Segal R.J., Lucia A. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med. Sci. Sports Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 12.Hardefeldt P.J., Penninkilampi R., Edirimanne S., Eslick G.D. Physical activity and weight loss reduce the risk of breast cancer: a meta-analysis of 139 prospective and retrospective studies. Clin. Breast Cancer. 2018;18:e601–e612. doi: 10.1016/j.clbc.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Campbell K.L., Winters-Stone K.M., Wiskemann J., May A.M., Schwartz A.L., Courneya K.S., Zucker D.S., Matthews C.E., Ligibel J.A., Gerber L.H. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Sports Medicine, Riebe D., Ehrman J.K., Liguori G., Magal M. tenth ed. xxx. Wolters Kluwer; Philadelphia: 2018. p. 472. (ACSM's Guidelines for Exercise Testing and Prescription). [Google Scholar]
- 15.Coletta A.M., Marquez G., Thomas P., Thoman W., Bevers T., Brewster A.M., Hawk E., Basen-Engquist K., Gilchrist S.C. Clinical factors associated with adherence to aerobic and resistance physical activity guidelines among cancer prevention patients and survivors. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavallée J.F., Abdin S., Faulkner J., Husted M. Barriers and facilitators to participating in physical activity for adults with breast cancer receiving adjuvant treatment: a qualitative metasynthesis. Psycho Oncol. 2019;28:468–476. doi: 10.1002/pon.4980. [DOI] [PubMed] [Google Scholar]
- 17.Smith-Turchyn J., Richardson J., Tozer R., McNeely M., Thabane L. Physical activity and breast cancer: a qualitative study on the barriers to and facilitators of exercise promotion from the perspective of health care professionals. Physiother. Can. 2016;68:383–390. doi: 10.3138/ptc.2015-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Browall M., Mijwel S., Rundqvist H., Wengström Y. Physical activity during and after adjuvant treatment for breast cancer: an integrative review of women's experiences. Integr. Cancer Ther. 2018;17:16–30. doi: 10.1177/1534735416683807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ormel H.L., van der Schoot G.G.F., Sluiter W.J., Jalving M., Gietema J.A., Walenkamp A.M.E. Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psycho Oncol. 2018;27:713–724. doi: 10.1002/pon.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kennedy M.A., Bayes S., Galvão D.A., Singh F., Spry N.A., Davis M., Chee R., Zissiadis Y., Hart N.H., Taaffe D.R. If you build it, will they come? Evaluation of a co-located exercise clinic and cancer treatment centre using the RE-AIM framework. Eur. J. Cancer Care. 2020;29 doi: 10.1111/ecc.13251. [DOI] [PubMed] [Google Scholar]
- 21.Stefani L., Sofi F., Magro S., Mascherini G., Petri C., Galanti G. Exercise and cancer survivors: lessons learned from a multi-faceted model for exercise prescription. J. Funct. Morphol. Kinesiol. 2018;3 doi: 10.3390/jfmk3030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardcastle S.J., Kane R., Chivers P., Hince D., Dean A., Higgs D., Cohen P.A. Knowledge, attitudes, and practice of oncologists and oncology health care providers in promoting physical activity to cancer survivors: an international survey. Support. Care Cancer. 2018;26:3711–3719. doi: 10.1007/s00520-018-4230-1. [DOI] [PubMed] [Google Scholar]
- 23.Neil-Sztramko S.E., Winters-Stone K.M., Bland K.A., Campbell K.L. Updated systematic review of exercise studies in breast cancer survivors: attention to the principles of exercise training. Br. J. Sports Med. 2019;53:504–512. doi: 10.1136/bjsports-2017-098389. [DOI] [PubMed] [Google Scholar]
- 24.Lahart I.M., Metsios G.S., Nevill A.M., Carmichael A.R. Physical activity for women with breast cancer after adjuvant therapy. Cochrane Database Syst. Rev. 2018;1:CD011292. doi: 10.1002/14651858.CD011292.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheeran P., Abraham C., Jones K., Villegas M.E., Avishai A., Symes Y.R., Ellinger H., Miles E., Gates K.M., Wright C.E. Promoting physical activity among cancer survivors: meta-analysis and meta-CART analysis of randomized controlled trials. Health Psychol. 2019;38:467–482. doi: 10.1037/hea0000712. [DOI] [PubMed] [Google Scholar]
- 26.Turner R.R., Steed L., Quirk H., Greasley R.U., Saxton J.M., Taylor S.J., Rosario D.J., Thaha M.A., Bourke L. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst. Rev. 2018;9:CD010192. doi: 10.1002/14651858.CD010192.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark B.K., Winkler E., Healy G.N., Gardiner P.G., Dunstan D.W., Owen N., Reeves M.M. Adults' past-day recall of sedentary time: reliability, validity, and responsiveness. Med. Sci. Sports Exerc. 2013;45:1198–1207. doi: 10.1249/MSS.0b013e3182837f57. [DOI] [PubMed] [Google Scholar]
- 28.Guinan E., Hussey J., Broderick J.M., Lithander F.E., O'Donnell D., Kennedy M.J., Connolly E.M. The effect of aerobic exercise on metabolic and inflammatory markers in breast cancer survivors--a pilot study. Support. Care Cancer. 2013;21:1983–1992. doi: 10.1007/s00520-013-1743-5. [DOI] [PubMed] [Google Scholar]
- 29.Irwin M.L., Cadmus L., Alvarez-Reeves M., O'Neil M., Mierzejewski E., Latka R., Yu H., Dipietro L., Jones B., Knobf M.T. Recruiting and retaining breast cancer survivors into a randomized controlled exercise trial: the Yale Exercise and Survivorship Study. Cancer. 2008;112:2593–2606. doi: 10.1002/cncr.23446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones S.B., Thomas G.A., Hesselsweet S.D., Alvarez-Reeves M., Yu H., Irwin M.L. Effect of exercise on markers of inflammation in breast cancer survivors: the Yale exercise and survivorship study. Cancer Prev. Res. 2013;6:109–118. doi: 10.1158/1940-6207.CAPR-12-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews C.E., Wilcox S., Hanby C.L., Der Ananian C., Heiney S.P., Gebretsadik T., Shintani A. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Support. Care Cancer. 2007;15:203–211. doi: 10.1007/s00520-006-0122-x. [DOI] [PubMed] [Google Scholar]
- 32.Pinto B.M., Frierson G.M., Rabin C., Trunzo J.J., Marcus B.H. Home-based physical activity intervention for breast cancer patients. J. Clin. Oncol. 2005;23:3577–3587. doi: 10.1200/JCO.2005.03.080. [DOI] [PubMed] [Google Scholar]
- 33.Pinto B.M., Stein K., Dunsiger S. Peers promoting physical activity among breast cancer survivors: a randomized controlled trial. Health Psychol. 2015;34:463–472. doi: 10.1037/hea0000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers L.Q., Courneya K.S., Anton P.M., Hopkins-Price P., Verhulst S., Vicari S.K., Robbs R.S., Mocharnuk R., McAuley E. Effects of the BEAT Cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: a multicenter randomized controlled trial. Breast Cancer Res. Treat. 2015;149:109–119. doi: 10.1007/s10549-014-3216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Short C.E., James E.L., Girgis A., D'Souza M.I., Plotnikoff R.C. Main outcomes of the Move More for Life Trial: a randomised controlled trial examining the effects of tailored-print and targeted-print materials for promoting physical activity among post-treatment breast cancer survivors. Psycho Oncol. 2015;24:771–778. doi: 10.1002/pon.3639. [DOI] [PubMed] [Google Scholar]
- 36.Vallance J.K., Courneya K.S., Plotnikoff R.C., Yasui Y., Mackey J.R. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J. Clin. Oncol. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 37.Lozano-Lozano M., Cantarero-Villanueva I., Martin-Martin L., Galiano-Castillo N., Sanchez M.J., Fernández-Lao C., Postigo-Martin P., Arroyo-Morales M. A mobile system to improve quality of life via energy balance in breast cancer survivors (BENECA mHealth): prospective test-retest quasiexperimental feasibility study. JMIR Mhealth Uhealth. 2019;7:e14136. doi: 10.2196/14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritvo P., Obadia M., Santa Mina D., Alibhai S., Sabiston C., Oh P., Campbell K., McCready D., Auger L., Jones J.M. Smartphone-enabled health coaching intervention (iMOVE) to promote long-term maintenance of physical activity in breast cancer survivors: protocol for a feasibility pilot randomized controlled trial. JMIR Res. Protoc. 2017;6:e165. doi: 10.2196/resprot.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartman S.J., Nelson S.H., Myers E., Natarajan L., Sears D.D., Palmer B.W., Weiner L.S., Parker B.A., Patterson R.E. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: the memory & motion study. Cancer. 2018;124:192–202. doi: 10.1002/cncr.30987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynch B.M., Nguyen N.H., Moore M.M., Reeves M.M., Rosenberg D.E., Boyle T., Vallance J.K., Milton S., Friedenreich C.M., English D.R. A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: the ACTIVATE Trial. Cancer. 2019;125:2846–2855. doi: 10.1002/cncr.32143. [DOI] [PubMed] [Google Scholar]
- 41.Arem H., Sorkin M., Cartmel B., Fiellin M., Capozza S., Harrigan M., Ercolano E., Zhou Y., Sanft T., Gross C. Exercise adherence in a randomized trial of exercise on aromatase inhibitor arthralgias in breast cancer survivors: the Hormones and Physical Exercise (HOPE) study. J. Cancer Surviv. 2016;10:654–662. doi: 10.1007/s11764-015-0511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leach H.J., Covington K.R., Voss C., LeBreton K.A., Harden S.M., Schuster S.R. Effect of group dynamics-based exercise versus personal training in breast cancer survivors. Oncol. Nurs. Forum. 2019;46:185–197. doi: 10.1188/19.ONF.185-197. [DOI] [PubMed] [Google Scholar]
- 43.Pinto B.M., Rabin C., Dunsiger S. Home-based exercise among cancer survivors: adherence and its predictors. Psycho Oncol. 2009;18:369–376. doi: 10.1002/pon.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trinh L., Mutrie N., Campbell A.M., Crawford J.J., Courneya K.S. Effects of supervised exercise on motivational outcomes in breast cancer survivors at 5-year follow-up. Eur. J. Oncol. Nurs. 2014;18:557–563. doi: 10.1016/j.ejon.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Rogers L.Q., Hopkins-Price P., Vicari S., Pamenter R., Courneya K.S., Markwell S., Verhulst S., Hoelzer K., Naritoku C., Jones L. A randomized trial to increase physical activity in breast cancer survivors. Med. Sci. Sports Exerc. 2009;41:935–946. doi: 10.1249/MSS.0b013e31818e0e1b. [DOI] [PubMed] [Google Scholar]
- 46.Basen-Engquist K., Taylor C.L., Rosenblum C., Smith M.A., Shinn E.H., Greisinger A., Gregg X., Massey P., Valero V., Rivera E. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ. Counsel. 2006;64:225–234. doi: 10.1016/j.pec.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Herrero F., San Juan A.F., Fleck S.J., Balmer J., Pérez M., Cañete S., Earnest C.P., Foster C., Lucía A. Combined aerobic and resistance training in breast cancer survivors: a randomized, controlled pilot trial. Int. J. Sports Med. 2006;27:573–580. doi: 10.1055/s-2005-865848. [DOI] [PubMed] [Google Scholar]
- 48.Ligibel J.A., Campbell N., Partridge A., Chen W.Y., Salinardi T., Chen H., Adloff K., Keshaviah A., Winer E.P. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J. Clin. Oncol. 2008;26:907–912. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- 49.Rogers L.Q., Fogleman A., Trammell R., Hopkins-Price P., Vicari S., Rao K., Edson B., Verhulst S., Courneya K.S., Hoelzer K. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr. Cancer Ther. 2013;12:323–335. doi: 10.1177/1534735412449687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wasmann K.A., Wijsman P., van Dieren S., Bemelman W., Buskens C. Partially randomised patient preference trials as an alternative design to randomised controlled trials: systematic review and meta-analyses. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-031151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reed J.L., Pipe A.L. The talk test: a useful tool for prescribing and monitoring exercise intensity. Curr. Opin. Cardiol. 2014;29:475–480. doi: 10.1097/HCO.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 52.Scherr J., Wolfarth B., Christle J.W., Pressler A., Wagenpfeil S., Halle M. Associations between Borg's rating of perceived exertion and physiological measures of exercise intensity. Eur. J. Appl. Physiol. 2013;113:147–155. doi: 10.1007/s00421-012-2421-x. [DOI] [PubMed] [Google Scholar]
- 53.Lee P.H., Macfarlane D.J., Lam T.H., Stewart S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int. J. Behav. Nutr. Phys. Activ. 2011;8:115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Craig C.L., Marshall A.L., Sjöström M., Bauman A.E., Booth M.L., Ainsworth B.E., Pratt M., Ekelund U., Yngve A., Sallis J.F. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt K., Vogt L., Thiel C., Jäger E., Banzer W. Validity of the six-minute walk test in cancer patients. Int. J. Sports Med. 2013;34:631–636. doi: 10.1055/s-0032-1323746. [DOI] [PubMed] [Google Scholar]
- 56.Bohannon R.W. Reference values for extremity muscle strength obtained by hand-held dynamometry from adults aged 20 to 79 years. Arch. Phys. Med. Rehabil. 1997;78:26–32. doi: 10.1016/s0003-9993(97)90005-8. [DOI] [PubMed] [Google Scholar]
- 57.Ware J.E., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 58.Lawrence D., Brown T.T.C., DasGupta Anirban. Vol. 16. 2001. Interval Estimation for a proportion. Statistical Science; pp. 101–133. [Google Scholar]
- 59.Bohannon R.W., Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J. Eval. Clin. Pract. 2017;23:377–381. doi: 10.1111/jep.12629. [DOI] [PubMed] [Google Scholar]
- 60.Natalucci V., Marini C.F., Flori M., Pietropaolo F., Lucertini F., Annibalini G., Vallorani L., Sisti D., Saltarelli R., Villarini A. Effects of a home-based lifestyle intervention program on cardiometabolic health in breast cancer survivors during the COVID-19 lockdown. J. Clin. Med. 2021:10. doi: 10.3390/jcm10122678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peel A.B., Thomas S.M., Dittus K., Jones L.W., Lakoski S.G. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.But-Hadzic J., Dervisevic M., Karpljuk D., Videmsek M., Dervisevic E., Paravlic A., Hadzic V., Tomazin K. Six-minute walk distance in breast cancer survivors-A systematic review with meta-analysis. Int. J. Environ. Res. Publ. Health. 2021:18. doi: 10.3390/ijerph18052591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peterson L.L., Ligibel J.A. Physical activity and breast cancer: an opportunity to improve outcomes. Curr. Oncol. Rep. 2018;20:50. doi: 10.1007/s11912-018-0702-1. [DOI] [PubMed] [Google Scholar]
- 64.De Luca V., Minganti C., Borrione P., Grazioli E., Cerulli C., Guerra E., Bonifacino A., Parisi A. Effects of concurrent aerobic and strength training on breast cancer survivors: a pilot study. Publ. Health. 2016;136:126–132. doi: 10.1016/j.puhe.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 65.Natalucci V., Villarini M., Emili R., Acito M., Vallorani L., Barbieri E., Villarini A. Special attention to physical activity in breast cancer patients during the first wave of COVID-19 pandemic in Italy: the DianaWeb cohort. J. Personalized Med. 2021 doi: 10.3390/jpm11050381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Avancini A., Tregnago D., Rigatti L., Sartori G., Yang L., Trestini I., Bonaiuto C., Milella M., Pilotto S., Lanza M. Factors influencing physical activity in cancer patients during oncological treatments: a qualitative study. Integr. Cancer Ther. 2020;19 doi: 10.1177/1534735420971365. 1534735420971365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Groen W.G., van Harten W.H., Vallance J.K. Systematic review and meta-analysis of distance-based physical activity interventions for cancer survivors (2013-2018): we still haven't found what we're looking for. Cancer Treat Rev. 2018;69:188–203. doi: 10.1016/j.ctrv.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 68.Boldrini P., Garcea M., Brichetto G., Reale N., Tonolo S., Falabella V., Fedeli F., Cnops A.A., Kiekens C. Living with a disability during the pandemic. "Instant paper from the field" on rehabilitation answers to the COVID-19 emergency. Eur. J. Phys. Rehabil. Med. 2020;56:331–334. doi: 10.23736/S1973-9087.20.06373-X. [DOI] [PubMed] [Google Scholar]
- 69.Mirandola D., Miccinesi G., Muraca M.G., Belardi S., Giuggioli R., Sgambati E., Manetti M., Monaci M., Marini M. Longitudinal assessment of the impact of adapted physical activity on upper limb disability and quality of life in breast cancer survivors from an Italian cohort. Support. Care Cancer. 2018;26:329–332. doi: 10.1007/s00520-017-3930-2. [DOI] [PubMed] [Google Scholar]
- 70.Barr V.J., Robinson S., Marin-Link B., Underhill L., Dotts A., Ravensdale D., Salivaras S. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp. Q. 2003;7:73–82. doi: 10.12927/hcq.2003.16763. [DOI] [PubMed] [Google Scholar]
- 71.Foglino S., Bravi F., Carretta E., Fantini M.P., Dobrow M.J., Brown A.D. The relationship between integrated care and cancer patient experience: a scoping review of the evidence. Health Pol. 2016;120:55–63. doi: 10.1016/j.healthpol.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 72.He Q., Xia B., Liu A., Li M., Zhou Z., Cheung E.C., Kuo Z.C., Wang B., Li F., Tang Y., Zheng Z., Sun R., Hu Y.J., Meng W., He Y., Yuan J., Zhang C. Association of body composition with risk of overall and site-specific cancers: a population-based prospective cohort study. Int. J. Cancer. 2021;149:1435–1447. doi: 10.1002/ijc.33697. [DOI] [PubMed] [Google Scholar]
- 73.Ewertz M., Jensen M.B., Gunnarsdóttir K.Á., Højris I., Jakobsen E.H., Nielsen D., Stenbygaard L.E., Tange U.B., Cold S. Effect of obesity on prognosis after early-stage breast cancer. J. Clin. Oncol. 2011;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 74.Wilczyński J., Sobolewski P., Zieliński R., Kabała M. Body composition in women after radical mastectomy. Int. J. Environ. Res. Publ. Health. 2020;17:8991. doi: 10.3390/ijerph17238991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.