Abstract
This mini-review aims to summarize a growing body of literature on synaptojanin 1 (Synj1), a phosphoinositide phosphatase that was initially known to have a prominent role in synaptic vesicle recycling. Synj1 is coded by the SYNJ1 gene, whose mutations and variants are associated with an increasing number of neurological disorders. To better understand the mechanistic role of Synj1 in disease pathogenesis, we review details of phosphoinositide signaling pathways and the reported involvement of Synj1 in membrane trafficking with a specific focus on Parkinson’s disease (PD). Recent studies have tremendously advanced our understanding of Synj1 protein structure and function while broadening our view of how Synj1 regulates synaptic membrane trafficking and endosomal trafficking in various organisms and cell types. A growing body of evidence points to inefficient membrane trafficking as key pathogenic mechanisms in neurodegenerative diseases associated with abnormal Synj1 expression. Despite significant progress made in the field, the mechanism by which Synj1 connects to trafficking, signaling, and pathogenesis is lacking and remains to be addressed.
Keywords: Synaptojanin1, SYNJ1, Membrane trafficking, Synaptic vesicle recycling, Autophagy, Parkinsonism, Neurodegenerative disease
1. Synj1 overview
In 1994, a then-unknown protein involved in synaptic vesicle endocytosis and recycling was found to interact with growth factor receptor-bound protein 2 (Grbp2); this unnamed protein was later labeled as the 145 kDa isoform (isoform b, NP_982271.2) of Synj1 [1]. Since then, another naturally-occurring isoform of Synj1 at 170 kDa (isoform a, NP_003886.3) has been discovered. While this isoform is widely dispersed throughout various tissues in the body, the 145 kDa Synj1 protein is predominantly localized to the brain [2]. Synj1 is coded by the SYNJ1 gene on human chromosome 21q22.2 [3]. Synj1, as a member of the synaptojanin protein family, consists of three domains: suppressor of actin 1 (SAC1), 5′-phosphatase, and a proline-rich domain (PRD) [4] (Fig. 1). Unlike most proteins, Synj1 possesses two enzymatic domains for lipid homeostasis, and these domains are crucial for Synj1-mediated molecular signaling and membrane trafficking. In Drosophila and C. elegans, there is one synaptojanin gene required for viable organisms, as opposed to mammals, which require two [5,6].
Fig. 1. The domain structures and identified mutations of Synj1 isoforms.
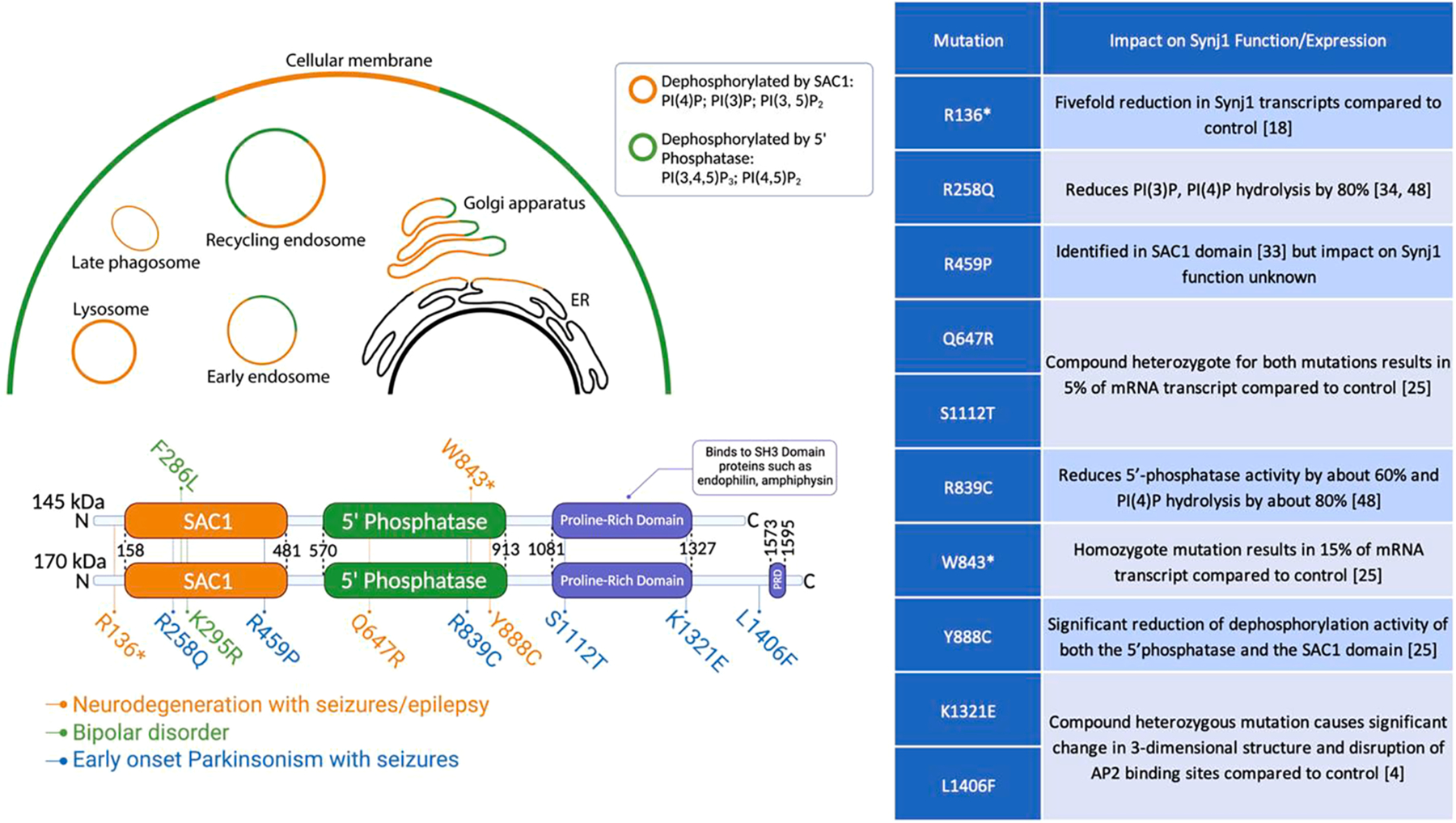
Both isoforms contain a SAC1 domain with phosphatase action on phosphatidylinositol 4-phosphate (PI(4)P), phosphatidylinositol 3-phosphate (PI(3)P), and phosphatidylinositol 3, 5- bisphosphate (PI(3,5)P2), a more selective 5′ phosphatase domain that predominantly dephosphorylates phosphatidylinositol 4, 5- bisphosphate (PI(4,5)P2) to PI(4)P, and a proline-rich domain (PRD), known to bind to multiple binding factors involved in endocytosis via SH3 domains, such as endophilin and amphiphysin. Other binding motifs with proteins like Esp15 and AP2 may vary between isoforms. SNPs in the introns [11] and postzygotic mosaic mutations [13] have also been reported for SYNJ1 associated with certain neuropsychiatric disorders but are not shown here. Created with assistance from BioRender.com.
Early research focused on unveiling the exact endocytic steps Synj1 is involved in and how each domain contributes to this process. In the past two decades, Synj1 abnormalities have been found to contribute to multiple neurological and neuropsychiatric diseases, such as PD, Alzheimer’s disease (AD), Down Syndrome (DS), autism, schizophrenia, and bipolar disorder [4,7–13] (Fig. 1). While the associations of SYNJ1 mutations or polymorphisms with many of the above disorders are still obscure or controversial, the field has seen a growing interest in investigating Synj1 irregularities in the pathogenesis of PD, which we will focus on in the latter part of this mini-review.
2. Membrane trafficking
Membrane trafficking includes essential processes such as endocytosis and exocytosis, whereby molecular cargo is transported, in vesicles, across the cell membrane into subcellular locations for function or degradation. Synj1, which regulates membrane resident phosphatidylinositol, has prompted robust investigation regarding its integral part in membrane trafficking. Additionally, while studies have focused on the role of Synj1’s 145 kDa isoform in synaptic trafficking, recent research has shown promising insight into its significance in endosomal and autophagic trafficking.
2.1. Synaptic membrane trafficking
Synaptic membrane trafficking describes the recycling of membrane cargos in the synaptic vesicle (SV); it is an essential cellular process that regulates neurotransmission, where neurotransmitters are released from SVs and received by postsynaptic receptors. Altered synaptic transmission may contribute to Synj1-mediated neurodegeneration, and understanding how Synj1 regulates synaptic membrane trafficking will ultimately inform our understanding of pathogenic processes.
While overwhelming evidence supports the involvement of Synj1 in synaptic membrane trafficking, the exact biophysical step where Synj1 is involved is not entirely clear. Early electron microscopy (EM) analysis suggests that clathrin coat shedding is regulated by Synj1, as mouse brains without Synj1 exhibited an accumulation of clathrin-coated vesicles [3]. A study of C. elegans lacking the synaptojanin (unc 26) gene showed an accumulation of both clathrin-coated vesicles and clathrin-coated pits at the plasma membrane, suggesting an additional role of Synj1 in SV endocytosis [6], which may have been masked in mammalian synapses due to compensatory changes. Later analysis in Synj1-deficient models further supports the involvement of Synj1 in SV endocytosis [14–16]. This conclusion is not entirely surprising given the number of BAR proteins, such as endophilin and amphiphysin, which interact with the PRD of Synj1 [17,18]. A later study suggests that in addition to the PRD, mutations in the two phosphatase domains also impair SV endocytosis [15]. Such impairment may be due to PRD dysfunction through intramolecular interaction of Synj1, which has been previously demonstrated [19–22]. It is also likely that phosphatidylinositol conversion is a crucial step for membrane curvature formation and the completion of endocytosis [23–25]. Supporting this idea, flash-and-freeze EM was recently used to demonstrate that Synj1, along with endophilin, is required for the neck formation of endocytic pits [26]. Notably, the study showed that the 5′-phosphatase, but not the SAC1-like phosphatase, is involved in this process.
Alternative models have been proposed regarding Synj1′s involvement in endocytosis. For example, the endocytic function of Synj1 may be carried out by the long isoform via binding to AP-2, clathrin, and Esp15, while the short isoform is recruited in the later stage for clathrin uncoating [27]. However, this hypothesis conflicts with the finding of poor 170 kDa isoform expression in the adult rat brain [2]. It thus remains unclear if the sequential recruitment of Synj1 isoforms is the predominant endocytic mechanism at the central synapse. Interestingly, while Synj1 has long been recognized to facilitate clathrin-mediated endocytosis, recent evidence reveals its role in ultrafast endocytosis [26]. This new data expands our traditional view of Synj1-mediated synaptic trafficking and reveals further information regarding the physiological role of Synj1.
2.2. Endosomal and autophagic trafficking
While Synj1’s role in synaptic trafficking has dominated the field since its identification, research has also indicated Synj1 expression in low levels in astrocytes [28,29] and that Synj1 substrates such as PI(3)P, PI(3,5)P2, and PI(4)P are prevalent lipids on intracellular membranes such as the autophagosome, ER and Golgi. In recent years, increasing research attention has probed the details of Synj1′s potential involvement in endosomal trafficking and autophagic function.
Among other developmental neural processes, endo-lysosomal sorting and trafficking of AMPA receptors are crucial to synaptic efficacy; an early study showed that Synj1 deficiency affects AMPA receptor recycling [30–32]. The De Camilli group found that neurotransmission was adversely affected in Synj1-deficient hippocampal neurons, where they had greater numbers of surface-exposed AMPA receptors and possessed larger miniature excitatory postsynaptic current amplitudes than wild-type (WT) mice. Whether the recycling of other plasma membrane cargo proteins requires Synj1 remains unclear. In our recent study of the Synj1-deficient cortical astrocytes, we showed reduced levels of the membrane glucose transporter, GLUT1 [29]. Similarly, the transferrin receptors were shown to exhibit intracellular retention in Synj1-deficient conditions [33]. These results suggest that Synj1 may regulate different cargo proteins via different mechanisms. While some cargos exhibit membrane retention, others may suffer from poor membrane insertion when Synj1 is deficient.
As part of intracellular trafficking, the autophagy pathway is of particular interest in neurodegenerative disorders. Macroautophagy, or autophagy, is the process whereby cells degrade unwanted molecular components to maintain proper homeostasis by forming an autophagosome. The autophagic contents are eventually degraded in the autolysosome when the autophagosome fuses with the lysosome. The multi-step autophagy pathway is complex: where Synj1 fits in remains elusive. The Verstreken group reported that the intact function of the SAC1 domain, which hydrolyzes the phosphate at the 3′ position of PI(3) P and PI(3,5)P2 [34–36], is important for autophagosome maturation [5,37]. Introducing the R258Q mutation, which nullifies SAC1 phosphatase action while leaving the 5′ phosphatase unaffected, in turn, diminished autophagosome maturation in presynaptic terminals of drosophila, likely through crowding of PI(3,5)P2 and its binding proteins [37]. A study from our lab using the Synj1+/− mouse model found enhanced LC3 immunofluorescence and increased autophagy substrate, p62, in the brains of aged mice, suggesting a defect in autolysosomal degradation [21]. Consistently, we found increased basal level autophagosome and autolysosomes in Synj1 deficient astrocytes [29]. Supporting these findings, another group showed that Synj1-deficient zebrafish exhibited enlarged acidic vesicles, abnormal late endosomes, and disrupted autophagy in the inner cone segments, suggesting a significant role of Synj1 in the endolysosomal pathway [38]. A later study from the same group demonstrated that 5′ phosphatase domain, but not SAC1 domain, activity is required to rescue the abnormalities in the endosomal pathways, suggesting that PI(4,5)P2 is crucial to autophagic clearance, at least in zebrafish [39]. These studies indicate that Synj1 may influence the autophagy pathway at various steps, from autophagosome lipidation and maturation to autolysosomal degradation. The SAC1 and the 5′-phosphatase domains may be recruited sequentially to accomplish the clearance of autophagic content. However, this hypothesis requires further research providing comprehensive molecular details downstream of the Synj1 mutations and lipid alterations, which may elucidate the connections between Synj1 and autophagy machinery.
In contrast to the above Synj1-deficient models, there has been no evidence suggesting an altered autophagy pathway in the Synj1 over-expressors, such as the Ts65Dn mouse [40]. However, enlarged early endosomes were observed in multiple Synj1 overexpressing models [8,41,42]. These studies suggest that Synj1 expression level in an intact system requires fine-tuning to maintain the proper functions of membrane trafficking.
3. Clinical pathogenesis relevance
Since 2013, SYNJ1 autosomal recessive mutations, including R258Q, R459P, R839C, and L1406F, have been identified to result in comorbidities of early-onset Parkinsonism and epilepsy [9,35,43–46]. Patients typically have juvenile-onset and exhibit fast progression. The R258Q and R839C mutations primarily impair Synj1′s function in the phosphatase domains [21,35], while the L1406F mutation impacts Synj1′s molecular interaction; these associations have not yet been fully investigated. Subsequent studies have revealed additional SYNJ1 variants, such as R136*, Y888C, W843*, Q647R, and S1112T, resulting in either protein truncation or lack of protein expression [47,48] (Fig. 1). These variants are associated with severe intellectual disabilities and early-onset aggressive neurodegeneration, suggesting an essential role of Synj1 in maintaining the proper function of the brain.
In understanding the pathogenic mechanisms underlying these disease mutations, various animal models have been generated and investigated. In a recent study by Cao et al., the authors showed that the Parkinsonism-related missense R258Q mutation in the SAC1 domain impaired cortical neuron SV endocytosis after brief or prolonged synaptic activities. The amount of exocytosis was, however, not affected at various stimulations [14]. The mild synaptic defects do not fully explain the reduced lifespan and apparent motor deficits shown in the Synj1 R258Q knock-in (KI) mice. It is possible that the R258Q mutation disrupts synaptic transmission of a yet-unknown type of synapse other than the reported cortical synapse in a more profound way. For example, in our analyses of Synj1 heterozygous midbrain neurons, we found a significant slowing of the SV endocytosis rate [21], while heterozygous deletion of Synj1 is largely tolerated in cortical neurons and hippocampal neurons [15,21]. These results suggest that midbrain synapses could be more vulnerable to the R258Q disease mutation. In another study of the Synj1 truncation mutant zebrafish, the vestibulospinal reflex was significantly defective [49], consistent with the earlier finding of poor SV turnover in the ribbon synapses of the hair cells [50]. Whether the R258Q mutation has a profound effect on the vestibular system that contributes to posture control in zebrafish and mammalian models is yet to be examined. Alternatively, it is also likely that the mutation impairs other membrane trafficking events, such as autophagy [37], which is equally essential for cellular function and survival. To understand the relevant lipid signaling pathways for Parkinsonism, a more recent study examined another PD candidate gene, Sac2/INPP5F, which specifically acts on PI(4)P; and its synergistic effect with the known SAC1 mutation on SYNJ1 [51]. While Sac2 KO mice alone demonstrated no significant defects, mice with both the Synj1 R258Q mutation and Sac2 KO exhibited an exacerbated phenotype and survived no longer than three weeks with stunted growth [51]. These results suggest an essential role of PI(4)P metabolism in neurodevelopment and dopaminergic dystrophy.
It is worth noting that different model organisms could have varying responses to Synj1 deletions/mutations. For example, unlike rodent cortical neurons, where SAC1 activity is necessary for normal SV recycling [14,15], the SYNJ1 R258Q mutation KI fly did not exhibit noticeable abnormalities in SV endocytosis compared to the WT [37,52]. Worm models then further surprise us. While they parallel the drosophila model in that the SAC1 domain’s functionality is not required for effective synaptic recycling at the neuromuscular junction, the SAC1 domain’s physical presence is involved in coordinating the Synj1 and endophilin interaction [20]. The same study found even more intriguingly that worms with truncated Synj1 without the PRD encountered no difficulties in SV recycling, contrasting results obtained in other model organisms [5,53,54]. Another example is the kinase regulation of Synj1 activity [55]. Phosphorylation driven by Cdk5 inhibits the protein’s activity in rat brains [19], yet phosphorylation mediated by a different kinase, Dyrk1A, enhances Synj1 activity at the drosophila neuromuscular junction [19,52]. Therefore, it is worthwhile to investigate each Synj1 disease mutation in multiple synaptic systems and different animal models, especially human-derived cells. Investigations along this line would likely lead to identifying specific neuronal pathways implicated in disease pathogenesis. More interestingly, a recent study has suggested possible sex-dependent homeostasis for PIP2, the primary substrate of Synj1 [56]. As PD tends to afflict males over females in the population, it would be interesting to dissect the sex-dependent synaptic regulation when addressing disease mechanisms.
4. Discussion
Our knowledge of Synj1 has seen robust growth in the past few decades. Although gaps regarding the precise mechanisms underlying Synj1-mediated membrane trafficking and Synj1-associated neurodegenerative diseases exist, there has been a growing body of evidence suggesting that the development of neurodegenerative diseases such as PD is correlated with endosomal trafficking issues, synaptic membrane trafficking issues, and sometimes both [33,57–59]. However, the mechanistic details of Synj1 function are still lacking; hence, our understanding of Synj1-mediated pathogenesis remains superficial, which calls for sustained research efforts.
One confounding factor in current Synj1 literature is the inconsistent results obtained through various model systems (summarized in Table 1). Future research, if provided cell type-specific analyses for Synj1, could bring more clarity. As we noted earlier, human cell models will be precious in elucidating disease mechanisms. Among the many disorders shown to associate with SYNJ1, PD has gained increasing credibility in recent years.
Table 1.
Summary of Synj1 models and phenotypes.
| In vivo models | In vivo phenotypes | citations | In vitro sample origin | In vitro phenotypes | citations | |
|---|---|---|---|---|---|---|
| Deficient models | KO mouse | Perinatal lethal and diminished embryonic growth rate | [3] | Rodent brain |
|
[3,15,28,29] |
| HET mouse |
|
[16,21] | Rodent brain |
|
[15,16,21] | |
| KO Drosophila eye |
Capable of detecting light and display phototaxis | [5] | Drosophila photoreceptor |
|
[5] | |
| KO Zebrafish |
|
[38,60] | Zebrafish photoreceptor |
|
[38] | |
| KO C. elegans |
|
[6] | C. elegans NMJ |
|
[6] | |
| Heterologous cells expressing Synj1 shRNA |
|
[33] | ||||
| Overexpression models | Human with DS | Human blood cells | Increased size of early endosomes | [41] | ||
| Human with DS/AD | Postmortem human brain | Reduced Synaptophysin level | [61] | |||
| Synj1 BAC transgenic Mouse |
|
[40,41,61] | Mouse brain |
|
[41,61]. | |
| Knock-in models | SYNJ1 R258Q patient-derived human induced neurons | Accumulation of WIPI2/Atg18a in neurites | [37] | |||
| R258Q KI Mouse |
|
[14] | Mouse brain |
|
[14,51] | |
| R258Q KI Drosophila |
|
[37] | Drosophila NMJ | Impaired autophagosome formation in response to synaptic activity and starvation | [37] | |
| Synjl C378S, D380N KI C. elegans | Normal EPSC from muscle wall recording | [20] | Synj1 C383S KI mouse cortical neuron |
|
[15] | |
| Synj1 ΔSAC1 KI C. elegans |
|
[20] | Synj1 R258Q KI mouse cortical neuron |
|
[14] | |
| Synj1 D716A KI C. elegans |
|
[20] | Synj1 D730A mutant KI mouse cortical neuron |
|
[15] | |
| Synjl ΔPRD KI C. elegans |
|
[20] | Synjl endophilin binding mutant (EBM) KI mouse cortical neuron |
|
[15] |
Much research is presently investigating the role of Synj1 in autophagic clearance in addition to its traditional role in synaptic trafficking. Importantly, for complex brain disorders like PD, Synj1 does not act alone. Other lipid kinases and phosphatases in the same phosphoinositide signaling pathway, as well as Synj1-associated molecules, could all contribute to defining the pathogenic course. Identifying these signaling partners through disease-based bioinformatics analyses can inform our understanding of Synj1′s roles in pathogenesis. In summary, future progress in the right direction will pave the way for us to pinpoint where Synj1 fits in membrane trafficking, signaling pathways, and ultimately pathogenesis.
Acknowledgement
The work is supported by the NINDS R01 grant (R01NS112390) to P.-Y. Pan.
Footnotes
CRediT authorship contribution statement
Choudhry H, Conceptualization, Investigation, Writing-original draft. Aggarwal M, Validation, Writing - review & editing. Pan PY, Conceptualization, Supervision, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].McPherson PS, Czernik AJ, Chilcote TJ, Onofri F, Benfenati F, Greengard P, Schlessinger J, De Camilli P, Interaction of Grb2 via its Src homology 3 domains with synaptic proteins including synapsin I, Proc. Natl. Acad. Sci. USA 91 (14) (1994) 6486–6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ramjaun AR, McPherson PS, Tissue-specific alternative splicing generates two synaptojanin isoforms with differential membrane binding properties, J. Biol. Chem 271 (40) (1996) 24856–24861. [DOI] [PubMed] [Google Scholar]
- [3].Cremona O, Di Paolo G, Wenk MR, Lüthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, McCormick DA, De Camilli P, Essential role of phosphoinositide metabolism in synaptic vesicle recycling, Cell 99 (2) (1999) 179–188. [DOI] [PubMed] [Google Scholar]
- [4].Drouet V, Lesage S, Synaptojanin 1 mutation in Parkinson’s disease brings further insight into the neuropathological mechanisms, Biomed. Res. Int 2014 (2014), 289728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Verstreken P, Koh T-W, Schulze KL, Zhai RG, Hiesinger PR, Y.i. Zhou, S. Q. Mehta, Y.u. Cao, J. Roos, H.J. Bellen, Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating, Neuron 40 (4) (2003) 733–748. [DOI] [PubMed] [Google Scholar]
- [6].Harris TW, Hartwieg E, Horvitz HR, Jorgensen EM, Mutations in synaptojanin disrupt synaptic vesicle recycling, J. Cell Biol 150 (3) (2000) 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ando K, Ndjim M, Turbant S, Fontaine G, Pregoni G, Dauphinot L, Yilmaz Z, Suain V, Mansour S, Authelet M, De Dekker R, Leroy K, Delatour B, Letournel F, Martin-Négrier M-L, Chapon F, Godfraind C, Maurage C-A, Deramecourt V, Meyronnet D, Streichenberger N, de Paula AM, Rigau V, Vandenbos-Burel F, Duyckaerts C, Seilhean D, Boluda S, Plu I, Milin S, Chiforeanu DC, Laquerrière A, Lannes B, Duyckaerts C, Potier M-C, Brion J-P, The lipid phosphatase Synaptojanin 1 undergoes a significant alteration in expression and solubility and is associated with brain lesions in Alzheimer’s disease, Acta Neuropathol. Commun 8 (1) (2020), 10.1186/s40478-020-00954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arai Y, Ijuin T, Takenawa T, Becker LE, Takashima S, Excessive expression of synaptojanin in brains with Down syndrome, Brain Dev. 24 (2) (2002) 67–72. [DOI] [PubMed] [Google Scholar]
- [9].Quadri M, Fang M, Picillo M, Olgiati S, Breedveld GJ, Graafland J, Wu B, Xu F, Erro R, Amboni M, Pappatà S, Quarantelli M, Annesi G, Quattrone A, Chien HF, Barbosa ER, Oostra BA, Barone P, Wang J, Bonifati V, Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism, Hum. Mutat 34 (9) (2013) 1208–1215. [DOI] [PubMed] [Google Scholar]
- [10].Rodríguez-López J, Sobrino B, Amigo J, Carrera N, Brenlla J, Agra S, Paz E, Carracedo Á, Páramo M, Arrojo M, Costas J, Identification of putative second genetic hits in schizophrenia carriers of high-risk copy number variants and resequencing in additional samples, Eur. Arch. Psychiatry Clin. Neurosci 268 (6) (2018) 585–592. [DOI] [PubMed] [Google Scholar]
- [11].Saito T, Guan F, Papolos DF, Lau S, Klein M, Fann CSJ, Lachman HM, Mutation analysis of SYNJ1: a possible candidate gene for chromosome 21q22-linked bipolar disorder, Mol. Psychiatry 6 (4) (2001) 387–395. [DOI] [PubMed] [Google Scholar]
- [12].Wang Y, Zhao X, Ju W, Flory M, Zhong J, Jiang S, Wang P, Dong X, Tao X, Chen Q, Shen C, Zhong M, Yu Y, Brown WT, Zhong N, Genome-wide differential expression of synaptic long noncoding RNAs in autism spectrum disorder, Transl. Psychiatry 5 (10) (2015) e660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lim ET, Uddin M, De Rubeis S, Chan Y, Kamumbu AS, Zhang X, D’Gama AM, Kim SN, Hill RS, Goldberg AP, Poultney C, Minshew NJ, Kushima I, Aleksic B, Ozaki N, Parellada M, Arango C, Penzol MJ, Carracedo A, Kolevzon A, Hultman CM, Weiss LA, Fromer M, Chiocchetti AG, Freitag CM, Church GM, Scherer SW, Buxbaum JD, Walsh CA, Rates, distribution and implications of postzygotic mosaic mutations in autism spectrum disorder, Nat. Neurosci 20 (9) (2017) 1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cao M, Wu Y, Ashrafi G, McCartney AJ, Wheeler H, Bushong EA, Boassa D, Ellisman MH, Ryan TA, De Camilli P, Parkinson sac domain mutation in synaptojanin 1 impairs clathrin uncoating at synapses and triggers dystrophic changes in dopaminergic axons, Neuron 93 (4) (2017) 882–896.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mani M, Lee SY, Lucast L, Cremona O, Di Paolo G, De Camilli P, Ryan TA, The dual phosphatase activity of synaptojanin1 is required for both efficient synaptic vesicle endocytosis and reavailability at nerve terminals, Neuron 56 (6) (2007) 1004–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pan P-Y, Li X, Wang J, Powell J, Wang Q, Zhang Y, Chen Z, Wicinski B, Hof P, Ryan TA, Yue Z, Parkinson’s disease-associated LRRK2 hyperactive kinase mutant disrupts synaptic vesicle trafficking in ventral midbrain neurons, J. Neurosci 37 (47) (2017) 11366–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Milosevic I, Giovedi S, Lou X, Raimondi A, Collesi C, Shen H, Paradise S, O’Toole E, Ferguson S, Cremona O, De Camilli P, Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission, Neuron 72 (4) (2011) 587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Micheva KD, Kay BK, McPherson PS, Synaptojanin forms two separate complexes in the nerve terminal. Interactions with endophilin and amphiphysin, J. Biol. Chem 272 (43) (1997) 27239–27245. [DOI] [PubMed] [Google Scholar]
- [19].Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P, Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses, Proc. Natl. Acad. Sci. USA 101 (2) (2004) 546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dong Y, Gou Y, Li Y, Liu Y, Bai J, Synaptojanin cooperates in vivo with endophilin through an unexpected mechanism, Elife 4 (2015), 10.7554/eLife.05660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pan P-Y, Sheehan P, Wang Q, Zhu X, Zhang Y, Choi I, Li X, Saenz J, Zhu J, Wang J, El Gaamouch F, Zhu L, Cai D, Yue Z, Synj1 haploinsufficiency causes dopamine neuron vulnerability and alpha-synuclein accumulation in mice, Hum. Mol. Genet 29 (14) (2020) 2300–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Paesmans J, Martin E, Deckers B, Berghmans M, Sethi R, Loeys Y, Pardon E, Steyaert J, Verstreken P, Galicia C, Versées W, A structure of substrate-bound Synaptojanin1 provides new insights in its mechanism and the effect of disease mutations, Elife 9 (2020), 10.7554/eLife.64922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Loerke D, Mettlen M, Schmid SL, Danuser G, Measuring the hierarchy of molecular events during clathrin-mediated endocytosis, Traffic 12 (7) (2011) 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Antonescu CN, Aguet F, Danuser G, Schmid SL, Gruenberg JE, Phosphatidylinositol-(4,5)-bisphosphate regulates clathrin-coated pit initiation, stabilization, and size, Mol. Biol. Cell 22 (14) (2011) 2588–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chang-Ileto B, Frere SG, Chan RB, Voronov SV, Roux A, Di Paolo G, Synaptojanin 1-mediated PI(4,5)P2 hydrolysis is modulated by membrane curvature and facilitates membrane fission, Dev. Cell 20 (2) (2011) 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Watanabe S, Mamer LE, Raychaudhuri S, Luvsanjav D, Eisen J, Trimbuch T, Söhl-Kielczynski B, Fenske P, Milosevic I, Rosenmund C, Jorgensen EM, Synaptojanin and endophilin mediate neck formation during ultrafast endocytosis, Neuron 98 (6) (2018) 1184–1197.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Perera RM, Zoncu R, Lucast L, De Camilli P, Toomre D, Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages, Proc. Natl. Acad. Sci. USA 103 (51) (2006) 19332–19337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Herrera F, Chen Q, Fischer WH, Maher P, Schubert DR, Synaptojanin-1 plays a key role in astrogliogenesis: possible relevance for Down’s syndrome, Cell Death Differ 16 (6) (2009) 910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pan P-Y, Zhu J, Rizvi A, Zhu X, Tanaka H, Dreyfus CF, Synaptojanin1 deficiency upregulates basal autophagosome formation in astrocytes, J. Biol. Chem 297 (1) (2021) 100873, 10.1016/j.jbc.2021.100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Parkinson GT, Hanley JG, Mechanisms of AMPA receptor endosomal sorting, Front. Mol. Neurosci 11 (2018) 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hirling H, Endosomal trafficking of AMPA-type glutamate receptors, Neuroscience 158 (1) (2009) 36–44. [DOI] [PubMed] [Google Scholar]
- [32].Gong L-W, De Camilli P, Regulation of postsynaptic AMPA responses by synaptojanin 1, Proc. Natl. Acad. Sci. USA 105 (45) (2008) 17561–17566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fasano D, Parisi S, Pierantoni GM, De Rosa A, Picillo M, Amodio G, Pellecchia MT, Barone P, Moltedo O, Bonifati V, De Michele G, Nitsch L, Remondelli P, Criscuolo C, Paladino S, Alteration of endosomal trafficking is associated with early-onset parkinsonism caused by SYNJ1 mutations, Cell Death Dis. 9 (3) (2018), 10.1038/s41419-018-0410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Burman C, Ktistakis NT, Regulation of autophagy by phosphatidylinositol 3-phosphate, FEBS Lett. 584 (7) (2010) 1302–1312. [DOI] [PubMed] [Google Scholar]
- [35].Krebs CE, et al. , The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures, Hum. Mutat 34 (9) (2013) 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Noda T, Matsunaga K, Taguchi-Atarashi N, Yoshimori T, Regulation of membrane biogenesis in autophagy via PI3P dynamics, Semin. Cell Dev. Biol 21 (7) (2010) 671–676. [DOI] [PubMed] [Google Scholar]
- [37].Vanhauwaert R, et al. , The SAC1 domain in synaptojanin is required for autophagosome maturation at presynaptic terminals, EMBO J. 36 (10) (2017) 1392–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].George AA, Hayden S, Holzhausen LC, Ma EY, Suzuki SC, Brockerhoff SE, Neuhauss SCF, Synaptojanin 1 is required for endolysosomal trafficking of synaptic proteins in cone photoreceptor inner segments, PLoS One 9 (1) (2014) e84394, 10.1371/journal.pone.0084394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].George AA, Hayden S, Stanton GR, Brockerhoff SE, Arf6 and the 5’phosphatase of synaptojanin 1 regulate autophagy in cone photoreceptors, Bioessays 38 (2016) S119–S135. [DOI] [PubMed] [Google Scholar]
- [40].Voronov SV, Frere SG, Giovedi S, Pollina EA, Borel C, Zhang H, Schmidt C, Akeson EC, Wenk MR, Cimasoni L, Arancio O, Davisson MT, Antonarakis SE, Gardiner K, De Camilli P, Di Paolo G, Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down’s syndrome, Proc. Natl. Acad. Sci. USA 105 (27) (2008) 9415–9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cossec J-C, Lavaur J, Berman DE, Rivals I, Hoischen A, Stora S, Ripoll C, Mircher C, Grattau Y, OlivoMarin J-C, de Chaumont F, Lecourtois M, Antonarakis SE, Veltman JA, Delabar JM, Duyckaerts C, Di Paolo G, Potier M-C, Trisomy for synaptojanin1 in Down syndrome is functionally linked to the enlargement of early endosomes, Hum. Mol. Genet 21 (14) (2012) 3156–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cataldo AM, Petanceska S, Peterhoff CM, Terio NB, Epstein CJ, Villar A, Carlson EJ, Staufenbiel M, Nixon RA, App gene dosage modulates endosomal abnormalities of Alzheimer’s disease in a segmental trisomy 16 mouse model of down syndrome, J. Neurosci 23 (17) (2003) 6788–6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Olgiati S, De Rosa A, Quadri M, Criscuolo C, Breedveld GJ, Picillo M, Pappatà S, Quarantelli M, Barone P, De Michele G, Bonifati V, PARK20 caused by SYNJ1 homozygous Arg258Gln mutation in a new Italian family, Neurogenetics 15 (3) (2014) 183–188. [DOI] [PubMed] [Google Scholar]
- [44].Kirola L, Behari M, Shishir C, Thelma BK, Identification of a novel homozygous mutation Arg459Pro in SYNJ1 gene of an Indian family with autosomal recessive juvenile Parkinsonism, Parkinsonism Relat. Disord 31 (2016) 124–128. [DOI] [PubMed] [Google Scholar]
- [45].Taghavi S, Chaouni R, Tafakhori A, Azcona LJ, Firouzabadi SG, Omrani MD, Jamshidi J, Emamalizadeh B, Shahidi GA, Ahmadi M, Habibi SAH, Ahmadifard A, Fazeli A, Motallebi M, Petramfar P, Askarpour S, Askarpour S, Shahmohammadibeni HA, Shahmohammadibeni N, Eftekhari H, Shafiei Zarneh AE, Mohammadihosseinabad S, Khorrami M, Najmi S, Chitsaz A, Shokraeian P, Ehsanbakhsh H, Rezaeidian J, Ebrahimi Rad R, Madadi F, Andarva M, Alehabib E, Atakhorrami M, Mortazavi SE, Azimzadeh Z, Bayat M, Besharati AM, Harati-Ghavi MA, Omidvari S, Dehghani-Tafti Z, Mohammadi F, Mohammad Hossein Pour B, Noorollahi Moghaddam H, Esmaili Shandiz E, Habibi A, Taherian-Esfahani Z, Darvish H, Paisán-Ruiz C, ´ A clinical and molecular genetic study of 50 families with autosomal recessive parkinsonism revealed known and novel gene mutations, Mol. Neurobiol 55 (4) (2018) 3477–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ben Romdhan S, Sakka S, Farhat N, Triki S, Dammak M, Mhiri C, A novel SYNJ1 mutation in a Tunisian family with juvenile Parkinson’s disease associated with epilepsy, J. Mol. Neurosci 66 (2) (2018) 273–278. [DOI] [PubMed] [Google Scholar]
- [47].Dyment DA, et al. , Homozygous nonsense mutation in SYNJ1 associated with intractable epilepsy and tau pathology, Neurobiol. Aging, 36(2) 2015. 1222 e1–5. [DOI] [PubMed] [Google Scholar]
- [48].Hardies K, Cai Y, Jardel C, Jansen AC, Cao M, May P, Djémíe T, Hachon Le Camus C, Keymolen K, Deconinck T, Bhambhani V, Long C, Sajan SA, Helbig KL, Suls A, Balling R, Helbig I, De Jonghe P, Depienne C, De Camilli P, Weckhuysen S, Loss of SYNJ1 dual phosphatase activity leads to early onset refractory seizures and progressive neurological decline, Brain 139 (9) (2016) 2420–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gao Y, Nicolson T, Temporal Vestibular Deficits in synaptojanin 1 (synj1) mutants, Front. Mol. Neurosci 13 (2020), 604189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Trapani JG, Obholzer N, Mo W, Brockerhoff SE, Nicolson T, Moser T, Synaptojanin1 is required for temporal fidelity of synaptic transmission in hair cells, PLoS Genet. 5 (5) (2009) e1000480, 10.1371/journal.pgen.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Cao M, Park D, Wu Y, De Camilli P, Absence of Sac2/INPP5F enhances the phenotype of a Parkinson’s disease mutation of synaptojanin 1, Proc. Natl. Acad. Sci. USA 117 (22) (2020) 12428–12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chen CK, et al. , Activity-dependent facilitation of Synaptojanin and synaptic vesicle recycling by the Minibrain kinase, Nat. Commun 5 (2014) 4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cestra G, Castagnoli L, Dente L, Minenkova O, Petrelli A, Migone N, Hoffmüller U, Schneider-Mergener J, Cesareni G, The SH3 domains of endophilin and amphiphysin bind to the proline-rich region of synaptojanin 1 at distinct sites that display an unconventional binding specificity, J. Biol. Chem 274 (45) (1999) 32001–32007. [DOI] [PubMed] [Google Scholar]
- [54].Sundborger A, Soderblom C, Vorontsova O, Evergren E, Hinshaw JE, Shupliakov O, An endophilin-dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling, J. Cell Sci 124 (1) (2011) 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cousin MA, Tan TC, Robinson PJ, Protein phosphorylation is required for endocytosis in nerve terminals: potential role for the dephosphins dynamin I and synaptojanin, but not AP180 or amphiphysin, J. Neurochem 76 (1) (2001) 105–116. [DOI] [PubMed] [Google Scholar]
- [56].Zhu L, Zhong M, Elder GA, Sano M, Holtzman DM, Gandy S, Cardozo C, Haroutunian V, Robakis NK, Cai D, Phospholipid dysregulation contributes to ApoE4-associated cognitive deficits in Alzheimer’s disease pathogenesis, Proc. Natl. Acad. Sci. USA 112 (38) (2015) 11965–11970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Schreij AMA, Fon EA, McPherson PS, Endocytic membrane trafficking and neurodegenerative disease, Cell. Mol. Life Sci 73 (8) (2016) 1529–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kiral FR, et al. , Rab GTPases and membrane trafficking in neurodegeneration, Curr. Biol 28 (8) (2018) R471–R486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nguyen M, Wong YC, Ysselstein D, Severino A, Krainc D, Synaptic, mitochondrial, and Lysosomal dysfunction in Parkinson’s disease, Trends Neurosci. 42 (2) (2019) 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Holzhausen LC, Lewis AA, Cheong KK, Brockerhoff SE, Differential role for synaptojanin 1 in rod and cone photoreceptors, J. Comp. Neurol 517 (5) (2009) 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Miranda AM, Herman M, Cheng R, Nahmani E, Barrett G, Micevska E, Fontaine G, Potier M-C, Head E, Schmitt FA, Lott IT, Jiménez-Velázquez IZ, Antonarakis SE, Di Paolo G, Lee JH, Hussaini SA, Marquer C, Excess Synaptojanin 1 contributes to place cell dysfunction and memory deficits in the aging hippocampus in three types of Alzheimer’s disease, Cell Rep. 23 (10) (2018) 2967–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]


