Abstract
Priapism is a prolonged penile tumescence or erection unrelated to sexual stimulation that lasts more than 6 h. High-flow priapism is a rare condition usually due to a perineal trauma with the formation of an arterio-cavernosal fistula between a cavernosal artery and the lacunar spaces of the penis. High-flow priapism is usually diagnosed by clinical examination and color Doppler ultrasound, and the gold standard therapeutic management is represented by digital subtraction angiography. We report a case of a young patient with high-flow priapism after a motorcycle blunt perineal trauma, examining in detail the specific color Doppler ultrasound findings, the diagnostic workup and the therapeutic management.
Keywords: Perineal trauma, High-flow priapism, Color Doppler ultrasound, Magnetic resonance imaging, Digital subtraction angiography, Transcatheter arterial embolization
Introduction
High-flow priapism (HFP) is a rare condition usually due to a perineal trauma with the formation of an arterio-cavernosal fistula (ACF) between a cavernosal artery and the lacunar spaces of the penis [1–5]. HFP is usually diagnosed by clinical examination and color Doppler ultrasound (CDUS), and the gold standard therapeutic management is represented by digital subtraction angiography (DSA), which keeps nowadays the only role to be the elective treatment by selective transcatheter arterial embolization (TAE) of the feeding vessels [1, 4, 6, 7]. We report a case of a young patient with HFP after a motorcycle blunt perineal trauma, examining in detail the specific CDUS findings, the diagnostic workup and the therapeutic management.
Case report
A 22-year-old man was admitted to our emergency department with urethrorrhagia and genital ecchymosis after a blunt perineal trauma from a motorcycle crash. His general conditions were good as well as his laboratory values. An ultrasound (US) of the penis and scrotal area was first performed with high-frequency probe transducer (10–15 MHz) and showed normal testis findings with scrotal hematoma and a hypo-anechoic mass of both the cavernous bodies, surrounded by echogenic cavernous tissue, highly suspected for laceration and hematoma, with an uncertain interruption of the tunica albuginea (Fig. 1). A watchful waiting approach was initially planned, and the day after, a magnetic resonance imaging (MRI) examination was performed with a 1.5 T scanner to evaluate the degree of injury. MRI excluded tunica albuginea tears and urethral trauma but showed a low-signal lesion in T2-weighted images at the basis of both cavernous bodies consistent with ACF (Fig. 2). At the same time, the patient complained of the onset of prolonged tumescence of the cavernous bodies without tumescence of the corpus spongiosum. Therefore, CDUS was performed, confirming the presence of a high-flow fistula on the right cavernous body: the examination showed a markedly increased flow within right the corpus cavernosum and a “low-resistance, high-velocity” arterial waveform of the cavernosal arteries (Fig. 3). All these findings suggested the diagnosis of HFP. The first clinical approach was a watchful waiting strategy again, with application of perineal compression and ice packs. However, after 10 days, the HFP was still clinically present with evolution findings at CDUS, showing the presence of bilateral ACFs with turbulent flow at the level of cavernosal arteries with increased systolic peak (Fig. 4). According to the persistence of the clinical and worsening CDUS findings, the patient underwent a selective DSA, with super-selective catheterization of both pudendal arteries, and was treated with subsequent successful TAE of both fistulas at the basis of the cavernous bodies employing metallic microcoils. This treatment led to a remission of the clinical finding of priapism and to a normalization of the Doppler findings (Fig. 5).
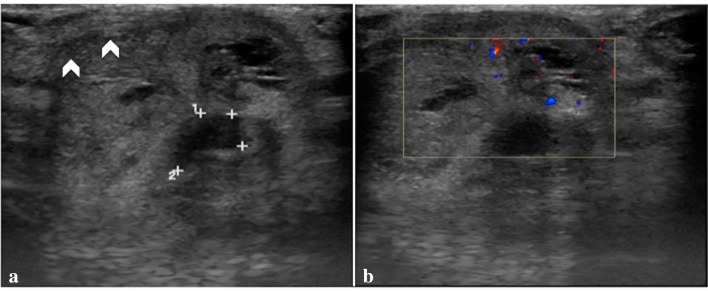
Fig. 1.
Penile US transverse scan (a, b) shows a hypo-anechoic mass within both the cavernous bodies (a, landmarks) without vascular flow (b, color Doppler box) highly suspected for hematoma associated with uncertain albuginea tears (a, arrowheads)
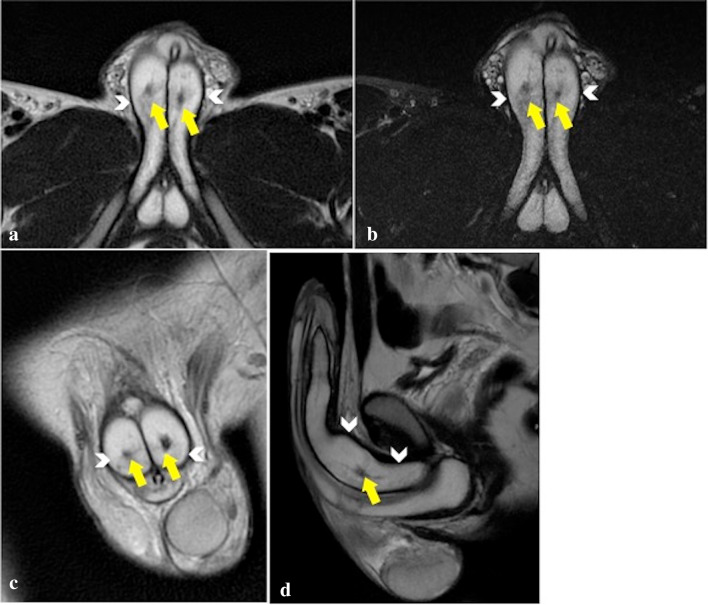
Fig. 2.
MRI axial T2-PROPELLER (a), axial T2 FAT SAT (b), coronal T2-PROPELLER (c), Sagittal T2-PROPELLER (d), images rule out tunica albuginea tears (continuous single hypointense border to the cavernosa—arrowheads) and show low-signal lesions, consistent with arterio-cavernosal fistulas (yellow arrows) not yet visible at first US examination
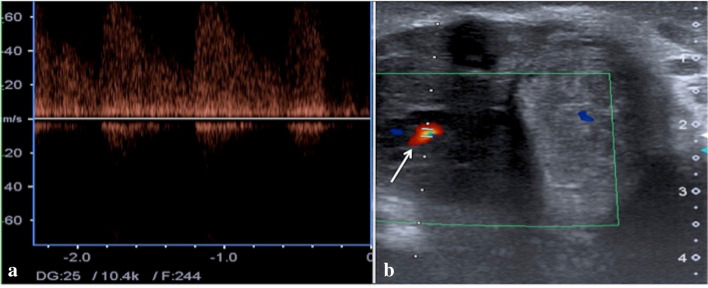
Fig. 3.
Penile US transverse scan with pulse (a) and color Doppler (b) mode shows right arterio-venous cavernous fistulas (b, arrow) with turbulent high-flow pulse pattern
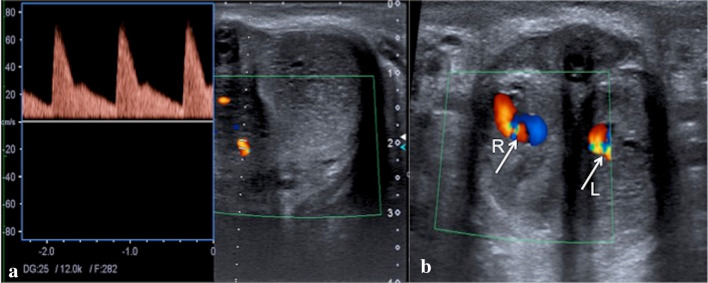
Fig. 4.
Penile US transverse scans with pulse (a) and color Doppler (b) mode of both cavernous bodies show a bilateral arterio-cavernosal fistula (b, arrows; Right—R, Left—L) with turbulent flow at the level of cavernosal arteries with increased systolic peak
Fig. 5.
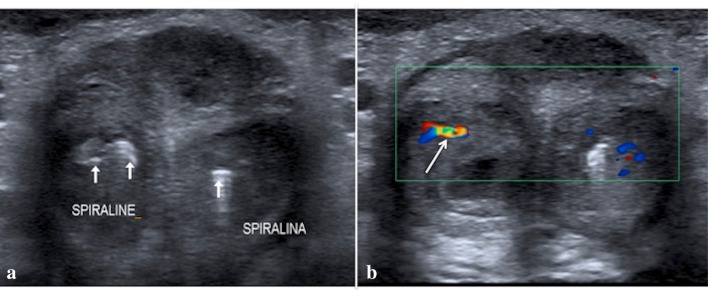
Penile US transverse B mode (a) and color Doppler mode (b) scans, performed after the embolization, show the metallic coils (a, arrows—named spiralina/spiraline) and the absence of any abnormal flow patterns, consistent with the resolution of both arterio-cavernosal fistulas with preserved flow in the cavernous artery (b, arrow)
Discussion
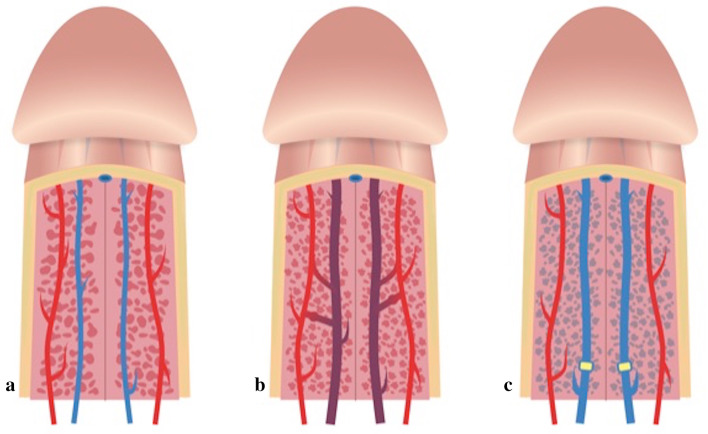
Priapism is a prolonged tumescence or erection unrelated to sexual stimulation that lasts more than 6 h [1, 6–11]. The erection is limited to the corpora cavernosa without affecting the corpus spongiosum [1, 6, 8, 11]. The two different classes of priapism are low-flow priapism (LFP) and HFP [1, 11] (Fig. 6; Table 1). LFP is characterized by a fully erect and painful penis with an ischemic component caused by venous outflow obstruction, engorging the corpora cavernosa with venous, poorly oxygenated blood at the cavernous blood gas analysis (CBGA). This condition results in hypoxia, acidosis, penile ischemia, and fibrosis, leading to erectile dysfunction and impotence if not treated immediately [1, 9–11]. LFP is a frequent side effect of commonly used intracavernous pharmacotherapies for erectile dysfunction, and it is seen more frequently than HFP [1, 11]. HFP, first described by Burt et al., in 1960 following traumatic coitus, is the less common form of priapism and is typically caused by trauma resulting in damage of the cavernosal artery, or of one of its branches, leading to formation of ACF, as in our case, and of pseudoaneurysm, circumventing the regulatory helicine arterioles [2–5].
Fig. 6.
Schematic representation of the normal corpora cavernosa vascular anatomy (a, the artery in red and the vein in blue), high-flow priapism (b, artero-cavernosal fistulas) and low-flow (c, venous outflow obstructions) priapism
Table 1.
Comparison between low- and high-flow priapism
| Low flow priapism | High flow priapism | |
|---|---|---|
| History of trauma | Uncommon | Commonly seen |
| Symptoms | Pain (for hypoxia and tissue acidosis) | Rarely pain |
| Physical examination | Corpora cavernosa completely rigid | Corpora cavernosa generally not completely rigid |
| Corpora cavernosa blood parameters | Low Po2 and acidosis | Similar to those of arterial blood |
| Pathophysiological mechanism | Out flow obstruction: | Increased inflow: |
| Idiopatic | AV fistula | |
| Drug related | No outflow obstruction | |
| Therapeutic urgency | Mandatory: | Not needed: |
| Blood stagnation leads to ischemic corpora and fibrosis | Well oxygenated corpora |
Clinical diagnosis of HFP is based on the history of perineal trauma, the physical examination showing a non-tender, non-ischemic, turgid penis, and the arterial blood at CBGA. CBGA is safe, easy to perform, and reliably distinguishes HFP from LFP [1, 11]. Usually, there is a delay of a few days between the trauma and the onset of priapism, and this can be explained by the formation of a clot that quickly seals the injured artery and which may be dislodged later during an episode of nocturnal tumescence [4]. This was, in fact, the natural evolution and modality of presentation of HFP in our case: 2 days after trauma, our patient was asymptomatic except for pain and ecchymosis to genitals. HFP treatment is not an emergency such as LFP, because venous outflow is not restricted; thus, stasis and ischemia are avoided. These patients present low risk for permanent complications, even though reduced potency has been reported in patients with untreated long-standing disease [1, 6, 10, 12, 13].
The ideal imaging modality should demonstrate the presence or absence of a clinically significant causative vascular lesion that may require intervention. CDUS is the imaging examination most commonly used to confirm HFP and is currently considered the imaging modality of choice, because it is sensitive, noninvasive, and widely available. The sensitivity and specificity of CDUS for this diagnosis have been found to be 100% and 73%, respectively [1, 4, 6, 7, 14–19]. On gray-scale US, the ACF appears as a hypoechoic lesion surrounded by echogenic cavernous tissue [1, 12]. The abnormal color Doppler finding is a markedly increased flow within the corpus cavernosum and cavernosal artery, eventually associated with the presence of pseudoaneurysm, with typically “low-resistance, high-velocity” arterial waveform [2, 4, 6, 7, 18, 19].
These were all the findings depicted at the CDUS exploration in our patient, where one of the two bilateral hypoechoic traumatic lesions on gray-scale US was clearly identifiable, surrounded by echogenic cavernous tissue and, after 1 day, the abnormal turbulent flow and alterations within cavernosal arteries, first, unilateral on the right cavernous body and, after a few days, bilateral with relief of a fistula to the left cavernous body as well. CDUS can also detect an extravasation of blood from the lacerated cavernosal artery as a characteristic color blush that extends into the erectile tissue. Besides the ACF, the penile vasculature can be explored to identify the potential feeding vessels [4, 6, 7]. A feeding cavernous artery may be identified, and draining veins with arterialized waveforms may also be detected [1, 12].
However, CDUS has many limitations: first, optimization of the color Doppler parameters to detect slow-velocity flow makes the color blush often displaying aliasing, and in this situation, it can be difficult to identify the exact site of the cavernosal artery tear; second, owing to high intracavernosal pressure, an extravasation of blood through the fistula can decrease, particularly during diastole, when the arterial pressure is lower [4, 6, 7], reducing the sensitivity in the detection; third, the location and number of accessory feeding vessels are underestimated and recognizing vessels that feed the fistula from the opposite side is difficult [1, 6, 7].
In patients with equivocal CDUS findings, DSA remains the gold standard for diagnosis; however, it is an invasive procedure with possible complications [4]. Nowadays, arteriography is no longer used for diagnostic purposes because of its invasive nature; however, during super-selective embolization, it is possible to clearly identify the arteries involved. Penile arteriography can also demonstrate the extravasation of the contrast medium into the corpora cavernosa from the arterial-sinusoidal fistula with a pooling effect [2, 14–17, 20].
In addition, in our case, the admission US revealed findings consistent with uncertain interruption of tunica albuginea due to an abundant hematoma as well as the presence of urethrorrhagia, leading us to perform an MRI for a further investigation of the injured area. The use of MRI in diagnosis and follow-up of HFP has not been studied extensively. Few reports in the literature compare CDUS and MRI [1, 4, 19, 21–23]. The authors pointed out that while CDUS can show characteristic flow patterns, MRI has better potential in tissue characterization and demonstration of a thrombus or ACF. In fact, although CDUS is valuable in diagnosing and lateralizing HFP, MRI provides improved tissue characterization and delineation of vascular anatomy with important information regarding the dominant feeders, their origin, and the lateralization facilitating angiographic planning [1].
In our case, MRI was helpful in excluding the presence of tunica albuginea interruptions, and it was valuable in showing, before clinical and color Doppler manifestations, the presence of bilateral cavernosal tissue traumatic lesion with ACFs, corresponding to low-signal tears into both cavernosal bodies at T2-weighted sequences. We just remember, in fact, that in recent years, many authors have confirmed that there is a great variability in normal penile vascular anatomy and that many different collateral pathways exist as many patients also show collateralization from left to right and vice versa, either in the common penile artery or the cavernosal branches, and perforators from the dorsal to cavernosal arteries [1, 4, 19, 23, 24]. Moreover, MRI excluded urethral injuries, and this was in our case an evident added value.
Regarding the therapy management, some authors suggest watchful waiting as first-line therapy in HFP as there is no tissue ischemia, and it can resolve spontaneously without hazards. The disadvantages of watchful waiting are possible structural alterations resulting from excessive arterial inflow, which may lead to impotence as well as social and psychological difficulties related to the condition and should be discouraged [4, 14]. Therapies for HFP include the use of ice packs, mechanical decompression, intracavernosal injection of alpha-agonists, surgical ligation of the cavernosal artery, and selective TAE of the feeder vessels. TAE is the preferred therapy for HFP, with success rates similar to surgical ligation and a higher rate of preservation of sexual function, and is widely accepted [1, 2, 5, 15, 16, 25]. In our case, after a watchful waiting management without resolution of priapism, a super-selective TAE of bilateral ACFs of both cavernosal arteries was necessary, which led to a clinical resolution of the HFP.
In conclusion, this case demonstrates that CDUS, in expert hands, is a valuable and powerful tool for the diagnosis and follow-up of HFP as it is highly sensitive in the detection of traumatic cavernosal lesions consistent with ACFs and in the identification of abnormal turbulent blood flow within cavernosal arteries. A MRI of the penis can add valuable supplemental information to clinical examination and CDUS findings in such patients and should be part of the workup of patients with this condition. Watchful waiting is the first management approach to the patient with HFP; however, in the absence of short-term resolution, a selective TAE of the feeder vessels is mandatory.
Acknowledgements
The authors would like to especially thank Dr. Adele Manca and Dr. Rosa Manca for drawing Fig. 6.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and its later amendments.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed consent
Additional informed consent was obtained from all the patients for which identifying information is not included in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ciro Acampora, Antonio Borzelli, Marco Di Serafino and Francesca Iacobellis contributed equally to this work.
References
- 1.White C, Gulati M, Gomes A, Rajfer J, Raman S. Pre-embolization evaluation of high-flow priapism: magnetic resonance angiography of the penis. Abdom Imaging. 2012 doi: 10.1007/s00261-012-9936-9. [DOI] [PubMed] [Google Scholar]
- 2.Prattley S, Bryant T, Rees R. Superselective embolization with microcoil and gelfoam for high-flow priapism secondary to bilateral cavernous fistulae: a case study. Case Rep Urol. 2019;3916056:4. doi: 10.1155/2019/3916056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burt FB, Schirmer HK, Scott W. A new concept in the management of priapism. J Urol. 1960;83(1):60–61. doi: 10.1016/S0022-5347(17)65655-6. [DOI] [PubMed] [Google Scholar]
- 4.El-Assmy A, Hekal IA, Abou-El-Ghar ME. Use of magnetic resonance angiography in diagnosis and decision making of post-traumatic, high-flow priapism. Sci World J. 2008;8:176–181. doi: 10.1100/tsw.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montague DK, Jarow J, Broderick GA. American urological association guideline on the management of priapism. J Urol. 2003;170:1318–1324. doi: 10.1097/01.ju.0000087608.07371.ca. [DOI] [PubMed] [Google Scholar]
- 6.De Magistris G, Pane F, Giurazza F, et al. Embolization of high-flow priapism: technical aspects and clinical outcome from a single-center experience. La Radiol Med. 2019;2019:1–8. doi: 10.1007/s11547-019-01113-w. [DOI] [PubMed] [Google Scholar]
- 7.Bertolotto M, Ouaia E, et al. Color Doppler imaging of post traumatic priapism: before and after selective embolization. Radiographics. 2003;23:495–503. doi: 10.1148/rg.232025077. [DOI] [PubMed] [Google Scholar]
- 8.Kim KR, Shin JH, Song HY, et al. Treatment of high-flow priapism with superselective transcatheter embolization in 27 patients: a multicenter study. J Vasc Interv Radiol. 2007;18(10):1222–1226. doi: 10.1016/j.jvir.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Sadeghi-Nejad H, et al. Priapism. Radiol Clin N Am. 2004;42(2):427–443. doi: 10.1016/j.rcl.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Tonseth KA, Egge T, Kolbenstvedt A, Hedlund H. Evaluation of patients after treatment of arterial priapism with selective micro-embolization. Scand J Urol Nephrol. 2006;40:49–52. doi: 10.1080/00365590500338040. [DOI] [PubMed] [Google Scholar]
- 11.Kuefer R, et al. Changing diagnostic and therapeutic conconcepts in high-flow priapism. Int J Impot Res. 2005;17(2):109–113. doi: 10.1038/sj.ijir.3901257. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH. Post-traumatic erectile dysfunction: doppler US findings. Abdom Imaging. 2006;31(5):598–609. doi: 10.1007/s00261-005-0217-8. [DOI] [PubMed] [Google Scholar]
- 13.Liu BX, Xin ZC. High-flow priapism: superselective cavernous artery embolization with microcoils. Urology. 2008;72(3):571–573. doi: 10.1016/j.urology.2008.01.087. [DOI] [PubMed] [Google Scholar]
- 14.Hatzichristou D, Salpiggidis G, Hatzimouratides K, Apostolidis A, Tzortzis V, Bekos A, et al. Managament strategy for arterial priapism: therapeutic dilemmas. J Urol. 2002;168:2074–2077. doi: 10.1016/S0022-5347(05)64299-1. [DOI] [PubMed] [Google Scholar]
- 15.Mizutani M, et al. Treatment of post-traumatic priapismby intracavernous injection of alpha-stimulant. Urol Int. 1986;41(4):312–314. doi: 10.1159/000281226. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro RH, Berger RE. Post-traumatic priapism treated with selective cavernosal artery ligation. Urology. 1997;49(4):638–643. doi: 10.1016/S0090-4295(97)00045-9. [DOI] [PubMed] [Google Scholar]
- 17.Gudinchet F, Fournier D, Jichlinski P, et al. Traumatic priapism in a child: evaluation with color flow Doppler sonography. J Urol. 1992;148:380–381. doi: 10.1016/S0022-5347(17)36602-8. [DOI] [PubMed] [Google Scholar]
- 18.Shigehara K, Namiki M. Clinical management of priapism: a review. World J Men’s Health. 2016;34(1):1–8. doi: 10.5534/wjmh.2016.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engin G, Tunaci M, Acunas B. High-flow priapism due to cavernous artery pseudoaneurysm: color Doppler sonographyand magnetic resonance imaging findings. Eur Radiol. 1999;9(8):1698–1699. doi: 10.1007/s003300050913. [DOI] [PubMed] [Google Scholar]
- 20.Mentzel HJ, et al. High-flow priapism in acute lymphatic leukaemia. Pediatr Radiol. 2004;34(7):560–563. doi: 10.1007/s00247-003-1124-1. [DOI] [PubMed] [Google Scholar]
- 21.Ginestie JF, Romieu A. Radiologic exploration of impotence. The Hague: Martinus Nijhoff; 1978. pp. 29–62. [Google Scholar]
- 22.O’Sullivan P, et al. Treatment of ‘‘high-flow’’ priapism with super selective transcatheter embolization: a useful alternative to surgery. Cardiovasc Interv Radiol. 2006;29(2):198–201. doi: 10.1007/s00270-005-0089-x. [DOI] [PubMed] [Google Scholar]
- 23.Trinci M, Cirimele V, Ferrari R, et al. Diagnostic value of contrast-enhanced ultrasound (CEUS) and comparison with color Doppler ultrasound and magnetic resonance in a case of scrotal trauma. J Ultrasound. 2019 doi: 10.1007/s40477-019-00389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savoca G, et al. Sexual function after highly selective embolization of cavernous artery in patients with high flow priapriapism: long-term follow up. J Urol. 2004;172(2):644–647. doi: 10.1097/01.ju.0000132494.44596.33. [DOI] [PubMed] [Google Scholar]
- 25.Hakim LS, et al. Evolving concepts in the diagnosis and treatment of arterial high flow priapism. J Urol. 1996;155(2):541–548. doi: 10.1016/S0022-5347(01)66444-9. [DOI] [PubMed] [Google Scholar]