Abstract
Purpose
This study aimed to investigate the altered ultrasonographic activity of abdominal muscles during breathing in males with and without nonspecific chronic low back pain (NSCLBP).
Design
Cross-sectional study.
Methods
Twenty males with NSCLBP and 20 males without NSCLBP were recruited. Muscle thickness change was measured by ultrasonography during breathing in the end-inspiration and end-expiration phases for the transverse abdominis (TrA), internal oblique (IO), external oblique (EO), and rectus abdominis (RA) muscles. The data were normalized to the end-inspiration thickness. An independent t test was run to analyze the data at a confidence level of 95% (p < 0.05).
Results
The participants with NSCLBP had thicker IO muscles in the end-inspiration (p = 0.030) and end-expiration (p = 0.017) phases as well as greater RA (p = 0.006) and smaller EO (p = 0.003) normalized thickness changes during breathing.
Conclusion
The normalized thickness changes during breathing differed between the participants with and without NSCLBP. Reduced EO and increased RA activity may predispose the spine to further injuries. Therefore, normalizing the breathing pattern should be considered in the management of people with NSCLBP.
Keywords: Low back pain, Abdominal muscles, Ultrasonography, Respiration
Introduction
Nonspecific chronic low back pain (NSCLBP) is one of the most prevalent musculoskeletal pain disorders that impose high costs on societies and health provider services all over the world [1]. Several treatment approaches are suggested for the treatment of NSCLBP, but evidence shows that no superiority exists among these methods [2]. Therefore, the multimodal intervention approach is commonly recommended for the sake of better clinical outcomes. This approach consists of several interventions, including ergonomic advice, spinal manipulation, acupuncture, soft-tissue manipulation, psychosocial interventions, general and specific exercises [3], and the reeducation of breathing pattern disorders [4].
Breathing is a vital and automatic function that our daily lives depend on. Evidence shows a relationship between breathing and spinal stability [4]. The diaphragm muscle is considered as the upper part of the muscular cylinder to stabilize the lumbar spine. The synergistic cooperation of the diaphragm with the multifidus, transverse abdominis (TrA), and pelvic floor muscles can regulate intra-abdominal pressure, contribute to lumbopelvic stability, and facilitate ventilation [5]. A proper diaphragmatic breathing pattern has been suggested as an essential mechanism to provide lumbopelvic stability [4, 5], and its possible disorders should be addressed in designing protocols for the treatment of NSCLBP [6, 7].
Previous studies have revealed the presence of diaphragmatic and breathing disorders in people with NSCLBP. For example, people with NSCLBP demonstrate less respiratory muscle endurance and diaphragmatic mobility [7], higher positions of the diaphragm [8], decreased ventilation volumes [9], and greater diaphragm fatigability [10]. Little doubt exists that people with NSCLBP perform their daily tasks with different movement patterns in comparison with healthy people [11]. Considering the fact that the diaphragm acts as a part of synergistic muscle groups stabilizing the lumbopelvic spine [5], it appears reasonable to associate diaphragm disorders with those of other synergistic muscles. In this regard, several studies have shown that people with NSCLBP have smaller thickness changes in TrA while performing abdominal hollowing-in [12], atrophy in TrA [13], absent automatic postural contraction of the TrA [14], greater thickness changes in rectus abdominis (RA), and lower thickness changes in TrA during unstable sitting positions [15].
While several studies have been conducted to investigate possible changes in the diaphragm and abdominal muscles’ activation in people with NSCLBP [7–15], not many have focused on the assessment of abdominal muscle thickness changes during breathing movements. Considering the essential role of breathing in providing proper spinal stability in the lumbopelvic region [4, 5], this study aimed to investigate the altered ultrasonographic activity of abdominal muscles during breathing in males with and without NSCLBP.
Materials and methods
Subjects
In this cross-sectional study, 20 males with NSCLBP and 20 males without NSCLBP were recruited voluntarily from patients who had referred to public physiotherapy centers in Tehran from September 2019 to January 2020. During the familiarization session, prior to the study, all the participants received written and verbal information about the methods and aims of the study. They were asked to fill out informed consent forms, and their demographic data were collected using a researcher-made questionnaire. The participants were assured that their data would be confidential and that they could leave the study at any time. The referral time to the sonography laboratory was set up for each participant, as well. Participants with NSCLBP within the age range of 20–40 years as well as with a history of LBP for at least 3 months, local pain from the T12 to the gluteal fold, and pain intensity of less than 5 on the visual analogue scale (VAS) were included in the study. Other participants included those without NSCLBP, with no history of LBP lasting more than 1 week, and within the age range of 20–40 years. The exclusion criteria for both groups were as follows: a history of surgery in the lumbosacral region, the presence of red flags [3], a history of any fracture or dislocation in the lumbosacral region, the presence of obvious spinal scoliosis or other spinal deformities based on the New York Posture Rating chart [16], a history of respiratory diseases, and a history of digestive diseases. The participants were recruited to the study by an expert physiotherapist who had a 10-year history of practice in the musculoskeletal field. This physiotherapist was not involved in the laboratory assessment of the study participants. The methodological and ethical considerations were approved by the Committee for Ethics in Biomedical Research of the University of Social Welfare and Rehabilitation Sciences.
Data collection
In the laboratory, all the data were collected by an experienced and qualified examiner who was unaware of the medical conditions of each subject. Also, the participants were asked not to talk about their medical condition with the examiner. Muscle thickness was evaluated using a B-Mode 7.5 MHz linear array transducer (Ultrasonix-E500, made in Canada). The reliability and validity of the ultrasonographic measures in the musculoskeletal assessment were approved in the previous studies [17]. Muscle thickness was measured in two-phase breathing, the end of inspiration, and the end of expiration for the RA, TrA, internal oblique (IO), and external oblique (EO) muscles in the dominant side (the dominant hand for writing) (Fig. 1). A random order was used to assess the respiratory phases. To examine muscle thickness, the participants were asked to stay in a supine crock-lying position with a pillow under both legs for more comfort. Then, the assessment location of each selected muscle was determined as below and identified with a marker. The assessment point of the TrA, IO, and EO muscles was 2.5 cm in front of the midaxillary line, at the midpoint of the line, which connects the iliac crest to the last rib. For examining the RA muscle, assessment points 2.5 cm above the umbilicus and 2.5 cm lateral to the dominant side were marked [15, 18]. Before assessing muscle thickness, ultrasound gel was placed between the transducer and the skin. The transducer was placed perpendicular to the skin with minimal pressure. To evaluate the muscle thickness at the end of expiration/inspiration, the participants were asked to exhale/inhale normally and then to hold their breath until the examiner measured the muscle thickness. Each position was examined three times, and the mean average of measures was used for data analysis. The recorded image was frozen, and the muscle thickness was measured as a perpendicular distance from the inside edge of the bilateral muscle epimysium in millimeters (mm). All the measurements were done with the same ultrasonography machine in the biomechanics laboratory of the University of Social Welfare and Rehabilitation Sciences. Thickness change was calculated by subtracting the muscle thickness at the end-inspiration phase from that at the end-expiration phase. The data were normalized by dividing the measured thickness change by the end-inspiration thickness, multiplied by 100 to eliminate the possible effects of individual differences.
Fig. 1.
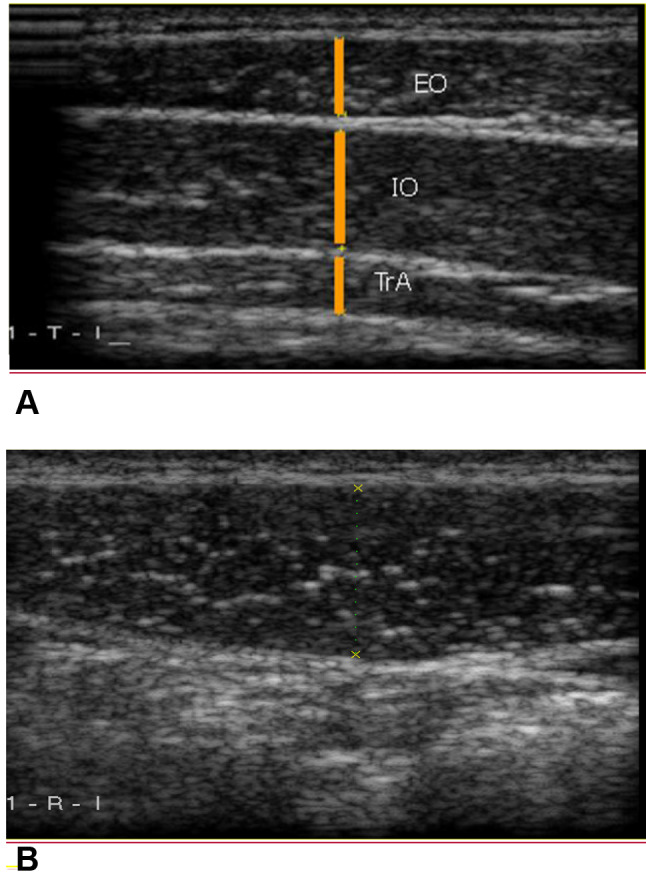
Ultrasound measurement of the abdominal muscles at end-inspiration phase. a TrA transverse abdominis, IO internal oblique, EO external oblique; b RA rectus abdominis
Statistical analyses
The data were analyzed using SPSS statistical package version 21. The Shapiro–Wilk test was run to examine the normality of data distribution. An independent t test was used to compare average data between the two groups. The statistical confidence level was considered at the 95% level (α < 0.05).
Results
Both groups had no statistically significant differences in terms of age, weight, height, and BMI. The demographic data of all the participants in both groups are summarized in Table 1.
Table 1.
Baseline characteristics of participants with (N = 20) and without (N = 20) nonspecific chronic low back pain
| Group | Age (years) | Weight (kg) | Height (cm) | BMI (kg/m2) |
|---|---|---|---|---|
| With NSCLBP | 26.40 ± 3.57 | 73.55 ± 5.61 | 173.85 ± 4.96 | 24.30 ± 0.91 |
| Without NSCLBP | 26.65 ± 3.72 | 74.20 ± 5.95 | 174.20 ± 5.28 | 24.41 ± 0.65 |
| p value | 0.830 | 0.724 | 0.830 | 0.670 |
kg kilograms, cm centimeters, m meter, NSCLBP nonspecific chronic low back pain
A comparison of the data in the end-inspiration and end-expiration phases showed that the mean thickness of the IO muscle was significantly larger in the participants with NSCLBP. No statistically significant differences were found between both groups in terms of other muscle thicknesses (Table 2).
Table 2.
Comparison of the end-inspiration and end-expiration thickness of abdominal muscles in participants with (N = 20) and without (N = 20) nonspecific chronic low back pain
| Time | Group | IO (mm) | EO (mm) | RA (mm) | TrA (mm) |
|---|---|---|---|---|---|
| End inspiration | With NSCLBP | 8.97 ± 1.67 | 5.88 ± 1.12 | 12.68 ± 1.60 | 3.64 ± 0.66 |
| Without NSCLBP | 7.89 ± 1.32 | 5.50 ± 1.24 | 13.91 ± 3.09 | 3.43 ± 0.85 | |
| p value | 0.030* | 0.319 | 0.126 | 0.380 | |
| End expiration | With NSCLBP | 9.53 ± 1.35 | 6.16 ± 1.07 | 13.32 ± 1.63 | 3.91 ± 0.66 |
| Without NSCLBP | 8.35 ± 1.63 | 6.04 ± 1.22 | 14.32 ± 2.96 | 3.75 ± 0.85 | |
| p value | 0.017* | 0.748 | 0.198 | 0.530 |
IO internal oblique, EO external oblique, RA rectus abdominis, TrA transverse abdominis, mm millimeters, NSCLBP nonspecific chronic low back pain
*Statistically significant difference
The results showed statistically significant differences between normalized thickness changes in the RA and EO muscles but no significant differences between such changes in the IO and TrA muscles (Table 3).
Table 3.
Comparison of normalized thickness changes of abdominal muscles in participants with (N = 20) and without (N = 20) nonspecific chronic low back pain
| Group | IO (%) | EO (%) | RA (%) | TrA (%) |
|---|---|---|---|---|
| With NSCLBP | 6.35 ± 3.22 | 5.23 ± 4.20 | 5.11 ± 1.75 | 7.68 ± 7.25 |
| Without NSCLBP | 6.25 ± 3.66 | 10.61 ± 6.40 | 3.29 ± 2.22 | 10.04 ± 4.28 |
| p value | 0.928 | 0.003* | 0.006* | 0.218 |
IO internal oblique, EO external oblique, RA rectus abdominis, TrA transverse abdominis, NSCLBP nonspecific chronic low back pain
*Statistically significant difference
Discussion
The results demonstrated that the participants with NSCLBP had different ultrasonographic muscle activity compared with those without NSCLBP. The study also showed that the participants with NSCLBP had thicker IO muscles in the end-inspiration and end-expiration phases as well as greater RA and smaller EO normalized thickness changes during breathing.
Thicker IO muscles in the end-inspiration and end-expiration phases may confirm the idea that participants with or without NSCLBP have different muscular thicknesses in the lumbosacral region. In the same line, several studies have proven that participants with NSCLBP have different muscle sizes [19–21]. These results contrast with those of a study that associated smaller IO, EO, and TrA with NSCLBP [22]. One explanation for this contrast may be the different baseline characteristics of the participants and the presentation of non-normalized data. A correlation has been found to exist between muscle thickness and anthropometric data, so muscle thickness data are recommended to be normalized in future studies [23]. Accordingly, normalized thickness changes were analyzed in this study to address this issue.
Furthermore, the results showed that the participants with NSCLBP had greater RA and smaller EO normalized thickness changes during breathing. To the best of our knowledge, no study has investigated abdominal muscle thickness changes during breathing. Our findings are in line with those who found that the thickness changes of abdominal muscles are different in participants with NSCLBP during tasks with standing postures [24], sitting with different stability levels [25], trunk extensions [26], changing positions [27], and sitting on unstable surfaces [15].
Respiration is a vital function with a crucial role in postural and spinal control [28]. Patients with NSCLBP have shown different patterns of breathing and postural controls [7–15]. Given this finding, altered breathing patterns may have an essential role in postural control deficits in people with NSCLBP [29]. Therefore, breathing exercises are recommended for the treatment of these patients [30, 31].
The previous studies have demonstrated that participants with NSCLBP have diaphragms with superior positions [8], more fatigability [10], less mobility [7], and reduced ventilation volumes [10]. Furthermore, this compensatory mechanism is performed because of the vital importance of human respiration. One explanation for the greater ultrasonic activity of RA may be the lesser mobility of the abdominal viscera of the diaphragm [7]. In this case, the activity of RA may increase intra-abdominal pressure and push the diaphragm cephalad for better expiration. This compensatory mechanism may apply more compressive loads on the spine and predispose the vertebral discs into further injuries [32]. Also, the smaller ultrasonographic activity of EO may predispose the spine to a higher probability of spinal instability, because the EO plays a stabilizer role in the spine [33], and hence, the importance of the consideration of respiratory patterns in the treatment of NSCLBP.
This study demonstrated that breathing patterns change in patients with CLBP. In this regard and in line with the kinesio-pathological model of developing musculoskeletal disorders, the presence of altered breathing patterns in people with CLBP [4] may place a repetitive abnormal load on the spine and may predispose them to further spinal or muscular damage [32]. This study provided more evidence to support the prescription of lumbosacral motor control exercises in the treatment of patients with CLBP.
One limitation of this study is that it was performed on 20- to 40-year-old males, so the findings may not be generalized to females or adolescents with LBP. The study was conducted on participants with NSCLBP, so muscle activity changes in specific LBP, such as LBP associated with radiculopathies, remained unclear. This study was cross-sectional, so the cause–effect relationship could not be interpreted. Furthermore, this study examined the muscle thickness only in the right body side and, as a result, ignored the possibility of different results that could be obtained from the left-side muscles.
Conclusion
This study indicates that normalized thickness changes during breathing are different between participants with or without NSCLBP. Moreover, reduced EO and increased RA thickness changes may predispose the spine to further injuries. Thus, normalizing the breathing pattern is recommended in the management and treatment of people with NSCLBP.
Author contributions
AMA, RS, and OR had contributions in the development of original idea, protocol development, data analysis, data collection, prepared the manuscript, and read and approved the final manuscript, and also, RS drafted the manuscript.
Funding
The authors received no financial support for the research.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
All research processes and methods were approved in terms of ethical considerations in the Research Ethics Committee of the University of Social Welfare and Rehabilitation Sciences coded.
Informed consent
All participants fulfilled the informed consent form.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amir Massoud Arab, Email: arabloo_masoud@hotmail.com.
Rahman Sheikhhoseini, Email: rahman.pt82@gmail.com.
Omid Rasouli, Email: omid.rasouli@ntnu.no.
References
- 1.da Silva T, Mills K, Brown BT, Pocovi N, de Campos T, Maher C, et al. Recurrence of low back pain is common: a prospective inception cohort study. J Physiother. 2019;65(3):159–165. doi: 10.1016/j.jphys.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Saragiotto BT, Maher CG, Yamato TP, Costa LO, Costa LC, Ostelo RW, et al. Motor control exercise for nonspecific low back pain: a cochrane review. Spine. 2016;41(16):1284–1295. doi: 10.1097/brs.0000000000001645. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin CC, Chenot JF, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–2803. doi: 10.1007/s00586-018-5673-2. [DOI] [PubMed] [Google Scholar]
- 4.Key J. 'The core': understanding it, and retraining its dysfunction. J Bodyw Mov Ther. 2013;17(4):541–559. doi: 10.1016/j.jbmt.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Koziris L. Postactivation potentiation. Strength Cond J. 2012;34(6):75–76. doi: 10.1519/SSC.0b013e31826ddc07. [DOI] [Google Scholar]
- 6.Hides JA, Donelson R, Lee D, Prather H, Sahrmann SA, Hodges PW. Convergence and divergence of exercise-based approaches that incorporate motor control for the management of low back pain. J Orthop Sports Phys Ther. 2019;49(6):437–452. doi: 10.2519/jospt.2019.8451. [DOI] [PubMed] [Google Scholar]
- 7.Mohan V, Paungmali A, Sitilerpisan P, Hashim UF, Mazlan MB, Nasuha TN. Respiratory characteristics of individuals with non-specific low back pain: a cross-sectional study. Nurs Health Sci. 2018;20(2):224–230. doi: 10.1111/nhs.12406. [DOI] [PubMed] [Google Scholar]
- 8.Kolar P, Sulc J, Kyncl M, Sanda J, Cakrt O, Andel R, et al. Postural function of the diaphragm in persons with and without chronic low back pain. J Orthop Sports Phys Ther. 2012;42(4):352–362. doi: 10.2519/jospt.2012.3830. [DOI] [PubMed] [Google Scholar]
- 9.Shah SG, Choezom T, Prabu Raja G. Comparison of respiratory parameters in participants with and without chronic low back pain. J Bodyw Mov Ther. 2019;23(4):894–900. doi: 10.1016/j.jbmt.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Janssens L, Brumagne S, McConnell AK, Hermans G, Troosters T, Gayan-Ramirez G. Greater diaphragm fatigability in individuals with recurrent low back pain. Respir Physiol Neurobiol. 2013;188(2):119–123. doi: 10.1016/j.resp.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 11.Sheikhhoseini R, O'Sullivan K, Alizadeh MH, Sadeghisani M. Altered motor control in athletes with low back pain: a literature review. Ann Appl Sport Sci. 2016;4(4):43–50. doi: 10.18869/acadpub.aassjournal.4.4.43. [DOI] [Google Scholar]
- 12.Critchley DJ, Coutts FJ. Abdominal muscle function in chronic low back pain patients: measurement with real-time ultrasound scanning. Physiotherapy. 2002;88(6):322–332. doi: 10.1016/S0031-9406(05)60745-6. [DOI] [Google Scholar]
- 13.Kim KH, Cho SH, Goo BO, Baek IH. Differences in transversus abdominis muscle function between chronic low back pain patients and healthy subjects at maximum expiration: measurement with real-time ultrasonography. J Phys Ther Sci. 2013;25(7):861–863. doi: 10.1589/jpts.25.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miura T, Yamanaka M, Ukishiro K, Tohyama H, Saito H, Samukawa M, et al. Individuals with chronic low back pain do not modulate the level of transversus abdominis muscle contraction across different postures. Man Ther. 2014;19(6):534–540. doi: 10.1016/j.math.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Arab AM, Shanbehzadeh S, Rasouli O, Amiri M, Ehsani F. Automatic activity of deep and superficial abdominal muscles during stable and unstable sitting positions in individuals with chronic low back pain. J Bodyw Mov Ther. 2018;22(3):627–631. doi: 10.1016/j.jbmt.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 16.McRoberts LB, Cloud RM, Black CM. Evaluation of the New York posture rating chart for assessing changes in postural alignment in a garment study. Cloth Text Res J. 2013;31(2):81–96. doi: 10.1177/0887302X13480558. [DOI] [Google Scholar]
- 17.Nijholt W, Scafoglieri A, Jager-Wittenaar H, Hobbelen JSM, van der Schans CP. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle. 2017;8(5):702–712. doi: 10.1002/jcsm.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tahan N, Khademi-Kalantari K, Mohseni-Bandpei MA, Mikaili S, Baghban AA, Jaberzadeh S. Measurement of superficial and deep abdominal muscle thickness: an ultrasonography study. J Physiol Anthropol. 2016;35(1):17. doi: 10.1186/s40101-016-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gildea JE, Hides JA, Hodges PW. Morphology of the abdominal muscles in ballet dancers with and without low back pain: a magnetic resonance imaging study. J Sci Med Sport. 2014;17(5):452–456. doi: 10.1016/j.jsams.2013.09.002S1440-2440(13)00196-5[pii]. [DOI] [PubMed] [Google Scholar]
- 20.Gildea JE, Hides JA, Hodges PW. Size and symmetry of trunk muscles in ballet dancers with and without low back pain. J Orthop Sports Phys Ther. 2013;43(8):525–533. doi: 10.2519/jospt.2013.45232890[pii]. [DOI] [PubMed] [Google Scholar]
- 21.Hides J, Stanton W, Freke M, Wilson S, McMahon S, Richardson C. MRI study of the size, symmetry and function of the trunk muscles among elite cricketers with and without low back pain. Br J Sports Med. 2008;42(10):809–813. doi: 10.1136/bjsm.2007.044024. [DOI] [PubMed] [Google Scholar]
- 22.Rahmani N, Mohseni-Bandpei MA, Salavati M, Vameghi R, Abdollahi I. Comparative study of abdominal muscle thickness on ultrasonography in healthy adolescents and patients with low back pain. J Ultrasound Med. 2018;37(4):905–912. doi: 10.1002/jum.14427. [DOI] [PubMed] [Google Scholar]
- 23.Sutherlin MA, Mangum LC, Hertel J, Saliba SA, Hart JM. Correlations between anthropometric measures and muscle thickness using ultrasound imaging. Int J Athl Ther Train. 2019;24(5):207–212. doi: 10.1123/ijatt.2018-0095. [DOI] [Google Scholar]
- 24.Ehsani F, Arab AM, Jaberzadeh S, Salavati M. Ultrasound measurement of deep and superficial abdominal muscles thickness during standing postural tasks in participants with and without chronic low back pain. Man Ther. 2016;23:98–105. doi: 10.1016/j.math.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Rasouli O, Arab AM, Amiri M, Jaberzadeh S. Ultrasound measurement of deep abdominal muscle activity in sitting positions with different stability levels in subjects with and without chronic low back pain. Man Ther. 2011;16(4):388–393. doi: 10.1016/j.math.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Aboufazeli M, Akbari M, Jamshidi AA, Jafarpisheh MS. Comparison of selective local and global muscle thicknesses in females with and without chronic low back pain. Ortop Traumatol Rehabil. 2018;20(3):197–204. doi: 10.5604/01.3001.0012.1473. [DOI] [PubMed] [Google Scholar]
- 27.Sutherlin MA, Gage M, Mangum LC, Hertel J, Russell S, Saliba SA, et al. Changes in muscle thickness across positions on ultrasound imaging in participants with or without a history of low back pain. J Athl Train. 2018;53(6):553–559. doi: 10.4085/1062-6050-491-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodges PW, Gandevia SC. Changes in intra-abdominal pressure during postural and respiratory activation of the human diaphragm. J Appl Physiol. 2000;89(3):967–976. doi: 10.1152/jappl.2000.89.3.967. [DOI] [PubMed] [Google Scholar]
- 29.Hamaoui A, Do M, Poupard L, Bouisset S. Does respiration perturb body balance more in chronic low back pain subjects than in healthy subjects? Clin Biomech. 2002;17(7):548–550. doi: 10.1016/s0268-0033(02)00042-6. [DOI] [PubMed] [Google Scholar]
- 30.Oh YJ, Park SH, Lee MM. Comparison of effects of abdominal draw-in lumbar stabilization exercises with and without respiratory resistance on women with low back pain: a randomized controlled trial. Med Sci Monit. 2020;26:e921295. doi: 10.12659/msm.921295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SH, Lee MM. Effects of a progressive stabilization exercise program using respiratory resistance for patients with lumbar instability: a randomized controlled trial. Med Sci Monit. 2019;25:1740–1748. doi: 10.12659/msm.913036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahrmann S. Diagnosis and treatment of movement impairment syndromes. St. Louis: Mosby; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cramer GD, Darby SA, Cramer GD. Clinical anatomy of the spine, spinal cord, and ANS. 3. St. Louis: Elsevier; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.


