Abstract
Chorioangiomas are generally small and associated with favorable outcomes, but large tumors can cause serious fetal complications, such as polyhydramnios, fetal anemia, intrauterine growth restriction, cardiac failure, fetal hydrops, and intrauterine fetal death. Signs of fetal cardiac failure on ultrasonography are indications for urgent in utero interventions. We report a case of a giant chorioangioma causing fetal cardiac failure at 26+3 weeks’ gestation, which was treated by embolization of the feeding vessels. We utilized a mixture of n-butyl cyanoacrylate (nBCA, Histoacryl®) and iodized oil (Lipiodol®) as an embolic agent. Fetal hydrops resolved in 4 weeks, and the cardiac size and function normalized 8 weeks after the embolization. A healthy male baby was born at the 37+5th gestational week by cesarean section.
Keywords: Chorioangioma, Embolization, Fetal heart failure, Histoacryl, n-Butyl cyanoacrylate
Introduction
A chorioangioma is the most common benign placental tumor. The incidence of large chorioangiomas is very low, ranging from 1 in 9000 to 1 in 50,000 pregnancies [1]. Most chorioangiomas are small and usually not associated with fetal or maternal complications. However, about half of the large chorioangiomas (> 5 cm) can cause fetal complications, such as polyhydramnios, fetal anemia, intrauterine growth restriction, cardiac failure, fetal hydrops, and intrauterine fetal death [2]. Maternal complications, such as hemolytic anemia, thrombocytopenia, disseminated intravascular coagulation, pregnancy-induced hypertension, hemoglobinuria, and antepartum hemorrhage, may be observed [2–6]. Although the underlying pathophysiology has not been fully elucidated, hemodynamic disturbances resulting from arteriovenous shunting and fetal-to-maternal hemorrhage through abnormal fetal tumor vessels have been postulated as the causative mechanism of chorioangiomas [2, 6].
Signs of fetal cardiac failure on ultrasonography are indications for urgent in utero interventions. The main pathophysiology underlying chorioangioma-associated complications results from functional arteriovenous shunts. Therefore, obliterating the tumor-feeding vessels is a feasible strategy for curing symptomatic chorioangioma. There are two main approaches for blocking the blood supply to the tumor: the fetoscopy-assisted approach and the ultrasound-guided approach [7]. To date, there is no consensus on which method of obliteration of chorioangioma feeding vessels is superior.
We describe successful ultrasound-guided feeding artery embolization for treating a giant chorioangioma causing fetal cardiac failure at 26+3 weeks of gestation. The procedure was performed on an outpatient basis by injecting a mixture of n-butyl cyanoacrylate (nBCA) and iodized oil. Fetal hydrops resolved in 4 weeks, and the cardiac size and function normalized 8 weeks after the embolization. A healthy male baby was born at the 37+5th gestational week by cesarean section. The reporting of this case was approved by our institution’s ethical review board (IRB number: 1911-020-16290).
Case report
A 31-year-old primigravida was referred to our hospital at the 26+3th gestational week owing to fetal cardiomegaly. Her medical and familial histories were unremarkable. The pregnancy course was uneventful. Fetal echocardiographic examination (Voluson E8 Expert, EF Healthcare Ultrasound, GE Korea) demonstrated severe cardiomegaly (cardiothoracic ratio: 0.64), thick cardiac walls, pericardial effusion (Fig. 1a), pleural effusion (Fig. 1a), ascites (Fig. 1b), and liver congestion, along with dilated hepatic veins. The myocardial performance (Tei) index was 0.58. Other fetal morphologic features were normal, and the amniotic fluid volume was increased (amniotic fluid index: 23). A large heterogeneous mass, measuring 9.4 × 8.5 × 6.5 cm, was observed in the placenta. Color Doppler ultrasonography revealed the protrusion of a highly vascularized placental tumor into the amniotic cavity (Fig. 2). The presumptive diagnosis was fetal heart failure secondary to a large chorioangioma.
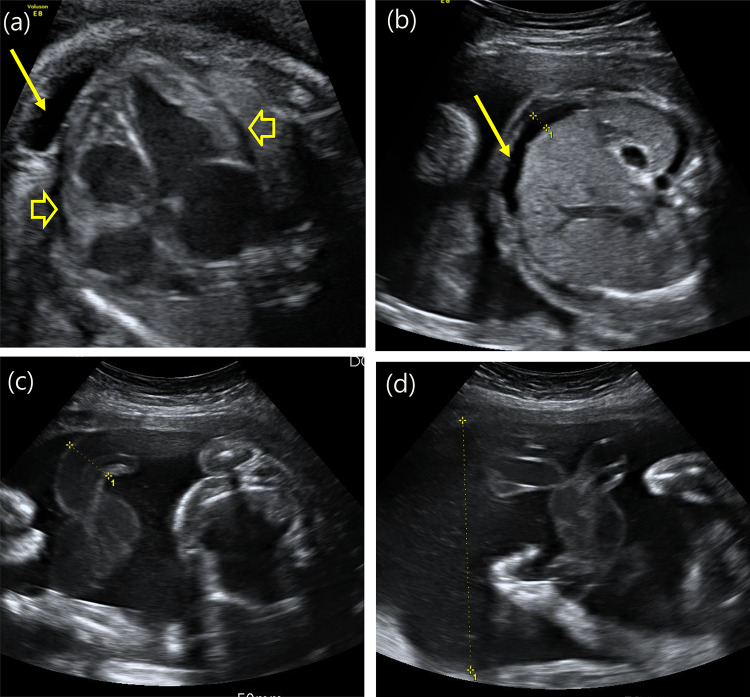
Fig. 1.
The ultrasound examination (Voluson E8 Expert, EF Healthcare Ultrasound, GE Korea) demonstrated severe cardiomegaly (cardiothoracic ratio: 0.64), thick cardiac walls, pericardial effusion (arrowhead) and pleural effusion (arrow) (a), ascites (arrow) (b), and enlargement of the umbilical vessels (c). The amniotic fluid index was elevated (AFI: 23, single-pocket depth: 10) (d)
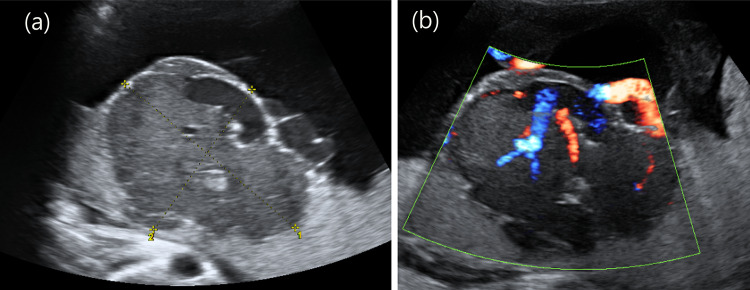
Fig. 2.
A huge mass, measuring 9.4 × 8.5 × 6.5 cm, with a heterogeneous echotexture was observed in the placenta. Color Doppler revealed a highly vascularized placental tumor protruding into the amniotic cavity. a Grayscale image of the chorioangioma. b Color Doppler image of the chorioangioma
Tumor-feeding artery embolization was planned for treating the fetal hydrops. The tumor-feeding artery was targeted using color Doppler ultrasound scans (Fig. 3a). A 22-gauge spinal needle was inserted into the target vessel within the tumor, and a mixture comprising 1.5 mL nBCA (Histoacryl®) and 10 mL iodized oil (poppyseed oil, Lipiodol®) was injected for 10 s. Immediate blood flow interruption within the tumor was observed via color Doppler imaging (Fig. 3b).
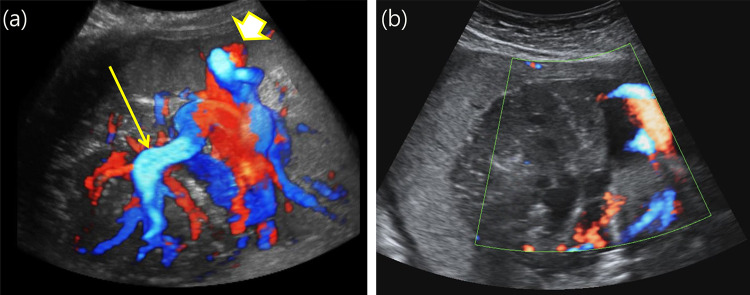
Fig. 3.
The blood flow change within the tumor was noticed by color Doppler imaging before (a) and after the procedure (b). a Placental mass with a rich supply of blood vessels; the arrowhead indicates the cord insertion site, and the arrow indicates the target point for n-butyl cyanoacrylate injection. b Immediately after the injection with n-butyl cyanoacrylate, the blood flow was stopped; the arrow indicates the injection point
The ascites and pleural effusion were no longer evident on ultrasound scans 5 days after the embolization. The tumor decreased to half of its initial size in 4 weeks, and the hypertrophic cardiac wall and cardiothoracic index normalized after 8 weeks.
A male neonate was delivered at 37+5 weeks through cesarean section performed because of non-reassuring signs on electronic fetal monitoring. The neonate’s birth weight was 2818 g, and the Apgar scores were 5 and 7 at 1 minand 5 min, respectively.
Histopathologic examination of the placenta confirmed chorioangioma (Fig. 4a). Necrotic chorioangioma tissue with embolization material was observed on a histological slide (Fig. 4b).
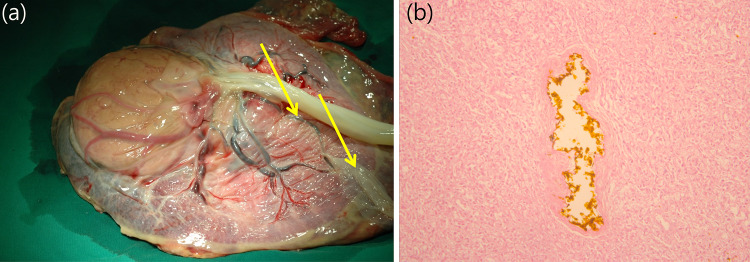
Fig. 4.
Pathological examination of the placenta confirmed the presence of a chorioangioma. a A large round tumor with necrosis and located near the cord insertion site. Surface vessels (arrows) are connected to the normal placental tissue over the tumor. b Necrotic chorioangioma tissue with embolization material observed on a histological slide. n-Butyl cyanoacrylate (dark brown color in the center) was found inside the tumor vessel on the pathological slides
The baby was last followed up when he was 4 years and 1 month old. He exhibited normal growth and development for his age.
Discussion
We reported a successful case of ultrasound-guided embolization with a mixture of nBCA and iodized oil to treat symptomatic chorioangioma on an outpatient basis. Neither expensive fetoscope equipment nor anesthesia was required in this case. On ultrasound, a chorioangioma appears as a well-circumscribed, round, heterogenous, and predominantly hypoechoic mass inside the placenta, with increased blood flow [8]. Chorioangiomas are frequently located near the cord insertion site, bulging into the amniotic cavity. Internal septation or calcification may be present. Increased blood flow detected by color Doppler ultrasonography is characteristic, and it can differentiate a chorioangioma from other placental masses, such as hematomas, partial hydatidiform moles, teratomas, tumor metastasis, and leiomyomas [9]. Color Doppler imaging is useful not only for diagnosis, but also for determining the management plan [10]. When chorioangiomas have poor vascularity, pregnancy outcomes are usually more favorable than when they are highly vascular [11–13]. Previous reports showed that the perinatal mortality of symptomatic chorioangiomas was 30–40% [7, 14]. The mean gestational age at chorioangioma diagnosis is 23–28 weeks, and the interval from diagnosis to delivery is 5–11 weeks, owing to chorioangioma-associated complications. Therefore, it is important to maintain the pregnancy until fetal maturation. Furthermore, to avoid any lethal complications and to achieve favorable outcomes during pregnancy, close surveillance and timely in utero interventions are imperative. For example, severe fetal anemia may be corrected by intrauterine blood transfusion, or polyhydramnios may be corrected via amniotic fluid drainage [15]. These interventions need to be repeated for alleviating symptoms and complications. However, a previous report described fetal demise after amnioreduction caused by increased tumor perfusion [16].
The main pathophysiology underlying chorioangioma-associated complications results from functional arteriovenous shunts. Therefore, the obliteration of tumor-feeding vessels is a feasible strategy for curing symptomatic chorioangiomas. There are two main approaches for blocking the blood supply to the tumor: fetoscopy-assisted laser ablation or suture ligation, and ultrasound-guided embolization. The decision on which vessel obliteration method to implement can be made based on the diameter or location of the target vessel, or on the distance from the umbilical cord insertion site to the tumor. Fetoscopy-assisted laser therapy has been associated with poor outcomes when the feeding vessel was located deep inside the placenta, when the tumor was large, and when the umbilical cord insertion site was close to the tumor [7, 17]. A large tumor, in combination with a placental location at the anterior wall of the uterus, has also been associated with poor outcomes with the fetoscopic approach due to limited space for manipulation [18]. Therefore, the fetoscopic approach (ligation or laser coagulation) can be implemented when the main tumor-feeding arteries are located superficially and far from the cord insertion site. Fetoscopy-assisted vessel obliteration requires an experienced operator and expensive fetoscopy equipment. Furthermore, endoscopic procedures need to be performed under epidural anesthesia with sonographic guidance in an operation room.
Compared to fetoscopy-assisted laser ablation, ultrasound-assisted embolization can be performed with a simple preparation in an outpatient setting and by using a small uterine puncture site. For ultrasound-guided embolization, there are several options in terms of embolic agents. Alcohol injection causes massive endothelial damage with vascular thrombosis; however, some alcohol may be distributed to the fetus as well. Therefore, injection into the tumor mass (rather than the tumor vessels) is preferred, which usually requires repetition of the procedure to achieve success [19].
Here, we used a mixture of 1.5 mL nBCA and 10 mL iodized oil. This embolization material can be injected directly into the feeding artery [20]. nBCA polymerizes within seconds upon being exposed to water or water-containing substances, such as human tissues. Rapidly injected embolic material can promptly block branching arteries within seconds. The iodized oil was used to dilute the nBCA. Iodized oil modifies the physical characteristics of the nBCA: its viscosity and density and the interfacial tension of the mixture with water. Therefore, initially, the polymerization time increases with the proportion of iodized oil. Slowing of the polymerization time helps send the embolic agent farther inside the vessel [21]. During the procedure, it is mandatory to employ color Doppler ultrasound to identify vascular shunts and to administer the nBCA injection. After the procedure, blood flow to the chorioangioma should be monitored using color Doppler ultrasonography. A previous study described nBCA embolization at 29 weeks’ gestation; the baby was delivered at 30 weeks and 2 days of gestation due to right ventricular hypertrophy and pericardial effusion. This nBCA embolization case was not successful at maintaining the pregnancy to full term, suggesting that the procedure might not fully obstruct the chorioangioma-feeding vessels [20].
Based on our experience and the literature review, ultrasound-guided embolization therapy by injection with a mixture of nBCA and iodized oil may be effective in treating chorioangioma, with several benefits over fetoscopy-assisted procedures, such as simpler preparation, less invasiveness, and cost-effectiveness.
Compliance with ethical standards
Conflicts of interest
The authors have nothing to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fox H, Sebire NJ. Pathology of the placenta. Major problems in pathology. 3. Philadelphia: Saunders; 2007. Non-trophoblastic tumors of the placenta; pp. 401–430. [Google Scholar]
- 2.Wehrens XH, Offermans JP, Snijders M, Peeters LL. Fetal cardiovascular response to large placental chorioangiomas. J Perinat Med. 2004;32(2):107–112. doi: 10.1515/JPM.2004.020. [DOI] [PubMed] [Google Scholar]
- 3.Prapas N, Liang RI, Hunter D, Copel JA, Lu LC, Pazkash V, Mari G. Color Doppler imaging of placental masses: differential diagnosis and fetal outcome. Ultrasound Obstet Gynecol. 2000;16(6):559–563. doi: 10.1046/j.1469-0705.2000.00324.x. [DOI] [PubMed] [Google Scholar]
- 4.Jauniaux E, Ogle R. Color Doppler imaging in the diagnosis and management of chorioangiomas. Ultrasound Obstet Gynecol. 2000;15(6):463–467. doi: 10.1046/j.1469-0705.2000.00127.x. [DOI] [PubMed] [Google Scholar]
- 5.Shih JC, Ko TL, Lin MC, Shyu MK, Lee CN, Hsieh FJ. Quantitative three-dimensional power Doppler ultrasound predicts the outcome of placental chorioangioma. Ultrasound Obstet Gynecol. 2004;24(2):202–206. doi: 10.1002/uog.1081. [DOI] [PubMed] [Google Scholar]
- 6.Stiller AG, Skafish PR. Placental chorioangioma: a rare cause of fetomaternal transfusion with maternal hemolysis and fetal distress. Obstet Gynecol. 1986;67(2):296–298. doi: 10.1097/00006250-198602000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Sepulveda W, Wong AE, Herrera L, Dezerega V, Devoto JC. Endoscopic laser coagulation of feeding vessels in large placental chorioangiomas: report of three cases and review of invasive treatment options. Prenat Diagn. 2009;29(3):201–206. doi: 10.1002/pd.2197. [DOI] [PubMed] [Google Scholar]
- 8.Zalel Y, Weisz B, Gamzu R, Schiff E, Shalmon B, Achiron R. Chorioangiomas of the placenta: sonographic and Doppler flow characteristics. J Ultrasound Med. 2002;21(8):909–913. doi: 10.7863/jum.2002.21.8.909. [DOI] [PubMed] [Google Scholar]
- 9.Mary E, Norton LMS, Feldstein VA. Callen's ultrasonography in obstetrics and gynecology. 6. Amsterdam: Elsevier; 2016. [Google Scholar]
- 10.Fan M, Skupski DW. Placental chorioangioma: literature review. J Perinat Med. 2014;42(3):273–279. doi: 10.1515/jpm-2013-0170. [DOI] [PubMed] [Google Scholar]
- 11.Sepulveda W, Alcalde JL, Schnapp C, Bravo M. Perinatal outcome after prenatal diagnosis of placental chorioangioma. Obstet Gynecol. 2003;102(5 Pt 1):1028–1033. doi: 10.1016/s0029-7844(03)00859-7. [DOI] [PubMed] [Google Scholar]
- 12.Sepulveda W, Aviles G, Carstens E, Corral E, Perez N. Prenatal diagnosis of solid placental masses: the value of color flow imaging. Ultrasound Obstet Gynecol. 2000;16(6):554–558. doi: 10.1046/j.1469-0705.2000.00245.x. [DOI] [PubMed] [Google Scholar]
- 13.Zalel Y, Gamzu R, Weiss Y, Schiff E, Shalmon B, Dolizky M, Achiron R. Role of color Doppler imaging in diagnosing and managing pregnancies complicated by placental chorioangioma. J Clin Ultrasound. 2002;30(5):264–269. doi: 10.1002/jcu.10072. [DOI] [PubMed] [Google Scholar]
- 14.Zanardini C, Papageorghiou A, Bhide A, Thilaganathan B. Giant placental chorioangioma: natural history and pregnancy outcome. Ultrasound Obstet Gynecol. 2010;35(3):332–336. doi: 10.1002/uog.7451. [DOI] [PubMed] [Google Scholar]
- 15.Quintero RA, Reich H, Romero R, Johnson MP, Gonçalves L, Evans MI. In utero endoscopic devascularization of a large chorioangioma. Ultrasound Obstet Gynecol. 1996;8(1):48–52. doi: 10.1046/j.1469-0705.1996.08010048.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones K, Tierney K, Grubbs BH, Pruetz JD, Detterich J, Chmait RH. Fetoscopic laser photocoagulation of feeding vessels to a large placental chorioangioma following fetal deterioration after amnioreduction. Fetal Diagn Ther. 2012;31(3):191–195. doi: 10.1159/000331944. [DOI] [PubMed] [Google Scholar]
- 17.Quarello E, Bernard JP, Leroy B, Ville Y. Prenatal laser treatment of a placental chorioangioma. Ultrasound Obstet Gynecol. 2005;25(3):299–301. doi: 10.1002/uog.1848. [DOI] [PubMed] [Google Scholar]
- 18.Lau TK, Leung TY, Yu SC, To KF, Leung TN. Prenatal treatment of chorioangioma by microcoil embolisation. BJOG. 2003;110(1):70–73. doi: 10.1046/j.1471-0528.2003.02003.x. [DOI] [PubMed] [Google Scholar]
- 19.Wanapirak C, Tongsong T, Sirichotiyakul S, Chanprapaph P. Alcoholization: the choice of intrauterine treatment for chorioangioma. J Obstet Gynaecol Res. 2002;28(2):71–75. doi: 10.1046/j.1341-8076.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- 20.Babic I, Tulbah M, Kurdi W. Antenatal embolization of a large placental chorioangioma: a case report. J Med Case Rep. 2012;6:183. doi: 10.1186/1752-1947-6-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YJ, Barthes-Biesel D, Salsac AV. Polymerization kinetics of n-butyl cyanoacrylate glues used for vascular embolization. J Mech Behav Biomed Mater. 2017;69:307–317. doi: 10.1016/j.jmbbm.2017.01.003. [DOI] [PubMed] [Google Scholar]