Abstract
Aims
Developmental dysplasia of the hip is an important cause of disability in children and young adult and it also has a significant socio-economic impact in our society. The main objective of our study is to evaluate, in our hospital, the effectiveness of a universal ultrasound screening protocol and to assess the general knowledge about the theme of pediatricians and neonatologists.
Methods
Retrospective study of infants born from January 2016 to April 2019, evaluated with hip ultrasound (Graf method). Risk factors assessed were female gender, breech presentation at birth, positive family history and twin birth. For the secondary objective, an anonymous and validated questionnaire was distributed to all pediatricians and neonatologists.
Results
Among the 4000 hips analyzed, on ultrasound examination, 98.8% hips resulted mature or immature but appropriate for age, while 1,2% hips were pathological. Analyzing the mature or immature hips, 2,4% were positive on clinical examination and 97,6% were negative. In relation to ultrasound pathological hips, 33,3% have positive clinical examination, while 66,7% negative. From the analysis of risk factors a significant association emerged between female sex, breech presentation and family history with the ultrasound pathological findings. The results of Survey showed that inadequate training about developmental dysplasia of the hip is done during medical school.
Conclusions
A universal ultrasound screening allowed us to identify developmental dysplasia of the hip in a number of children with normal clinical examination and no risk factors. Specific training courses should be implemented regarding Developmental Dysplasia of the Hip for neonatologists and pediatricians.
Electronic supplementary material
The online version of this article (10.1007/s40477-020-00463-w) contains supplementary material, which is available to authorized users.
Keywords: DDH, Universal screening, Ortolani-barlow, Hip ultrasound
Introduction
Developmental dysplasia of the hip (DDH) is one of the most frequent congenital abnormalities in newborns, especially in Caucasians [1, 2], with a reported incidence of 1–3% in Italy [3].
The term DDH encompasses a wide spectrum of disorders, from mild acetabular dysplasia with or without instability to a complete dislocation at birth, all of which share the potential to cause long-term complications such as gait disorders, muscular atrophy and degenerative lesions of the hip and knee. The socio-economic impact of the disease is significant since DDH is responsible for about one quarter of hip replacements performed in patients younger than 40 years of age [4].
DDH is more common among females, infant with a positive family history or newborns experiencing abnormal fetal positioning and/or limited limited fetal mobility, such as breech position [5]. Other risk factors include ethnic background, co-existing lower-limb or musculoskeletal deformities, mechanical restriction before delivery (oligohydramnios, multiple pregnancies) [6] or after birth (swaddling, with the hips in an extended and adducted position) [7].
The physical examination is the first important component of a DDH screening program. The 2000 AAP clinical practice guideline gave a detailed description of the examination, including observing for limb length discrepancy, asymmetric thigh or gluteal folds, and limited or asymmetric abduction, as well as performing Barlow and Ortolani tests [8]. However, the only clinical examination is considered not sufficient for early diagnosis of DDH because the condition can be initially clinically occult.
The introduction of hip ultrasound has completely changed the natural history of the pathology, allowing an early diagnosis in order to develop a timely and effective therapeutic approach [3]. Although several authors have confirmed the diagnostic reliability of ultrasonography in the first months of life [9–13], its use in a universal screening protocol is still controversial. In some European countries, such as Austria and Germany, clinicians perform universal ultrasound screening for DDH to all newborns, regardless of risk factors. Alternatively, many health care centers, including United Kingdom and the United States, perform selective ultrasound screening (for high-risk groups) [14]. For selective ultrasound screening programs to be successful, robust clinical screening programs must already be established. It has been suggested that medical schools and pediatric and family practice residency programs need to commit themselves more strongly to provide adequate clinical diagnosis of pediatric hip dysplasia [15]. Nevertheless, in June 2019 an International Consensus on the evaluation of DDH stated that there was a strong agreement in favor of universal US screening, that is cost-effective and, using the Graf technique, would not result in overtreatment [16].
Due to controversies in current literature, we performed this retrospective study aiming i) to evaluate the effectiveness of a universal ultrasound screening protocol and ii) to assess the general knowledge and the attitude about the theme DDH of pediatricians and neonatologists working in the same institution where the study was performed.
Patients and methods
A retrospective observational study was conducted in a single, third-level University Hospital.
From January 2016 and April 2019 all newborns who accepted to undergo Hip Ultrasound in our Institution were enrolled. Newborns with musculoskeletal and neurogenic impairments were excluded.
Routinely in our Hospital all newborns undergo a clinical screening for DDH on day 1 and day 3 of life. In the case of clinical positivity, it is recommended to perform an ultrasound examination of the hips as soon as possible.
Those infants with unremarkable clinical findings were subjected to ultrasound examination of the hips around the 6th week of life. In the presence of risk factors for DDH, an anticipate ultrasonography is advised. Assessed risk factors included female gender, breech presentation at birth, positive family history for DDH and twin birth.
For the study analysis, only complete data with anamnesis, risk factors for DDH assessment and ultrasound data were used. All data sets were anonymized before statistical analysis. The study was approved by the Institution Review Board of our Institution.
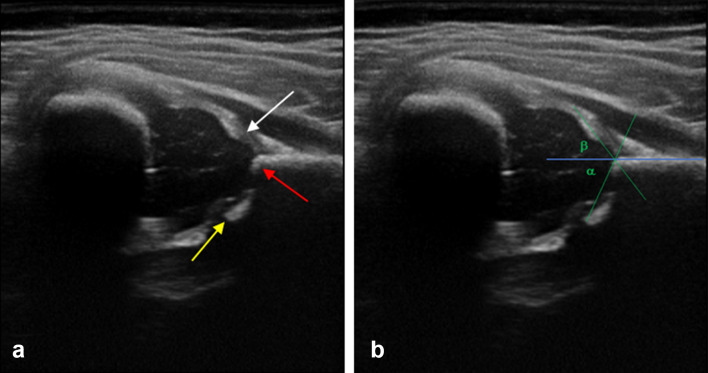
Hip ultrasounds were performed by one certified pediatrician (DB), who participated to a Hip Ultrasound Training by Graf and had several years of experience on this topic. All images were acquired using a 12 MHz linear probe (EsaoteMyLab 40). According to Graf methods, coronal scans of each hip (a minimum of 2 sonograms) were obtained with a child in the lateral decubitus position, and a significant emphasis was placed on producing the correct standard section. Firstly a morphological assessment was performed on all scans, taking into account the acetabulum conformation, the cotiloidal margin shape and the acetabolic cartilage thickness; subsequently, for the quantitative assessment, a few lines are traced upon the above scan which is essential for the Alpha and Beta angle calculus (Fig. 1a, b) [17]. Based on morphological features and on alpha and beta angles, the hips were divided into 4 groups according to a modified version of the Graf method: Graf I (normal); Graf IIa (immature); Graf II b and IIc (minor dysplasia); and Graf IId or higher dysplasia (major dysplasia which needs treatment) (Table A, supplementary material).
Fig. 1.
Ultrasound image a shows the correct standard section for the study of Hip Dysplasia: labrum (white arrow), bony rim of the acetabulum (red arrow), and lower limb of the iliac bone (yellow arrow). Ultrasound image b shows α angle generated within the intersection of the basal line and the bone roof line and β angle generated in the intersection of the lineal base with the cartilage roof line
Further follow-up examinations and therapeutic steps have been undertaken on the basis of the clinical and ultrasound findings.
Graf classification type I hips did not provide treatment or further re-examination. Graf type IIa + (physiological immature) hips needed a further assessment by ultrasound and/or orthopedic examination. In Graf type IIa-, IIb, IIc, D, III and IV the patient was immediately referred to the orthopedic specialist for treatment.
For the secondary objective of the study, an anonymous and validated questionnaire was distributed to all pediatricians and neonatologists (medical residents and specialists) of our Hospital via an online system that allowed anonymized responses. These questionnaires included 15 queries regarding doctor’s knowledge about hip dysplasia and about any training done during their course of study (Table B, supplementary material).
Statistical analysis
Summary data of continuous variables were reported as arithmetic mean ± standard deviation; for discrete variables, the number of observations and the relative percentages were reported.
For statistical analysis, two groups were built to form a yes/no variable:
Graf ultrasound types I and IIa + were considered mature or immature but appropriate for age and merged into the first group. Graf type IIa-, IIb, IIc, D, III, and IV were considered pathologic and merged into the second group.
The potential risk factors examined in the statistical analysis were female gender, breech presentation at birth, positive family history for DDH and twin birth.
In a first step, univariate analyses using Student’s t-test (continuous data) and Fisher’s exact test (qualitative data) were performed to evaluate the impact of single risk factors on the presence of an ultrasound pathological hip finding.
In addition, the differences between the groups were assessed by the Student's t-test for continuous data and Fisher's exact test for qualitative data.
Sensitivity, specificity, positive and negative predictive power were calculated for each risk factor.
In a second step, risk factors that were statistically significant with a p value < 0.05 were entered into a logistic regression analysis to evaluate the unbiased impact and predictive value of single risk factors (independent variables) on the presence of an ultrasonographic pathological hip type (dependent variable).
To underline the number of cases of DDH we would have diagnosed and the number of cases we would have lost if we had adopted a selective ultrasound screening policy for DDH, a selective screening protocol has been retrospectively hypothesized. This protocol would have included the execution of the hip ultrasound examination only for clinically positive patients (positive Ortolani–Barlow maneuver) and/or patients with accredited risk factors (i.e, female gender, breech presentation, family history of DDH).
In addition, the ROC curve for the model comprising female sex, presentation at birth, Ortolani/Barlow maneuver and family history for DDH with the relative value of the area under the curve was represented.
p values < 0.05 were considered significant. All statistical analyses were performed with “Stata/IC” Release 15 (StataCorp LP, College Station, TX).
The questionnaire was analyzed in “Moduli Google”, a web software for statistics that calculated percentages on the answers provided by our sample and created automatically graphs.
Results
Study population
Complete data sets were available for all 2000 universally screened newborns (4000 hips), 1007 (50.3%) males and 993 (49.6%) female. On ultrasound examination, 3952 hips resulted mature or immature but appropriate for age (Graf type Ia, Ib, IIa +), while 48 hips were pathological (Graf type IIa-, IIb, IIc, D, III and IV). The prevalence of developmental dysplasia of the hip was 1.2%, with a 95% confidence interval (range 0.89–1.59).
To ensure the validity of ultrasound interpretation, we randomly selected 10% of our subjects and all pathological hips and IIa + and IIa- hips and had an independent reviewer who reinterpreted them (AC). When we perform ultrasound, we store two images for each hip: one image with measurements and the other without measurements. The reviewer evaluated the images without measurements and performed the analyses. We had an overall concordance rate of 95.3% and a kappa value of 0.86, which confirmed that the interpretation by the first rater was valid. In particular, interobserver agreement for IA, IB and severely abnormal hips was high (98%), while was moderate for IIa + and IIa-hips (k 0.78). The following ranges were applied for the agreement: κ = 0.81 ± 1.00 excellent, 0.61 ± 0.80 good, 0.41 ± 0.60 moderate, 0.21 ± 0.40 fair, greater than 0 ± 0.20 slight, and 0 absent.
Clinical findings and DDH
Analyzing the 3952 mature or immature hips (but appropriate for age), 96 (2.4%) were positive for the Ortolani–Barlow maneuver and 3856 (97.6%) were negative. Among 75 hips with type IIa + (immature hips), 9 (12%) have a positive Ortolani-Barlow maneuver and 66 were negative (88%). In relation to 48 hips with pathological ultrasound findings, 16 (33.3%) were positive for the Ortolani-Barlow maneuver, while 32 (66.7%) were negative. Specifically, we found that the majority of the hips with type IIa-, IIb, IIc and D ultrasound findings have a negative clinical examination (74% negative Ortolani–Barlow), while those with type III and IV ultrasound findings almost always have a positive clinic (83% positive Ortolani–Barlow). If we consider clinically positive the hips with positive Ortolani–Barlow maneuver, and as positive for the ultrasound test the hips with type IIa, IIb, IIc and D findings, the sensitivity of the clinical examination is 26.2% and the specificity is 97%. Instead, If we consider only the most severe cases of DDH (type III and IV according to Graf classification), the sensitivity of the clinical examination rises to 83.3%.
Risk factors and DDH
Risk factor distribution, Ortolani–Barlow test results and detailed ultrasonographic hip types according to the Graf method are summarized in Table 1.
Table 1.
Risk factors distribution and detailed hip types according to the Graf method
| Mature or immature but appropriate for age hips (n, %) | Pathological hips (n, %) | |
|---|---|---|
| Total | 3952 (98.8) | 48 (1.2) |
| Graf type | ||
| Ia | 2854 (71.4) | 0 |
| Ib | 1023 (25.6) | 0 |
| IIa + | 75 (1.9) | 0 |
| Iia − | 0 | 29 (0.7) |
| IIb | 0 | 4 (0.1) |
| IIc | 0 | 7 (0.2) |
| D | 0 | 2 (0.05) |
| III | 0 | 3 (0.08) |
| IV | 0 | 3 (0.08) |
| Ortolani–Barlow test | ||
| Positive | 96 (2.4) | 16 (33.3) |
| Negative | 3856 (97.6) | 32 (66.7) |
| Gender | ||
| Female | 1949 (49.3) | 37 (37.1) |
| Male | 2003 (50.7) | 11 (22.9) |
| Breech presentation | ||
| Yes | 168 (4.3) | 18 (37.5) |
| No | 3784 (975) | 30 (62.5) |
| Family history of DDH | ||
| Yes | 144 (3.6) | 6 (12.5) |
| No | 3808 (96.4) | 42 (87.5) |
| Twin birth | ||
| Yes | 358 (9.1) | 2 (4.2) |
| No | 3594 (90.9) | 46 (95.8) |
From the univariate analysis, a significant association emerged between female sex, breech presentation and family history of DDH with the ultrasound findings of a pathological hip. Twin birth, on the other hand, was not found to be significantly associated with DDH ultrasound diagnosis (Table 2). Besides the risk factors "female sex", "breech presentation", "family history for DDH" and "positive Ortolani Barlow maneuver" were tested in a logistic regression model to verify the independence of association of the risk of dysplasia development of the hip. The Odds Ratios and the related confidence intervals are shown in Table 2.
Table 2.
The table shows the Univariate analyses for single risk factors associated with pathologic hip types
| p value | Odd ratio [CI 95%] | p value | |
|---|---|---|---|
| Positive Ortolani–Barlow test | < 0.001 | 15.90 [7.91–31.96] | < 0.001 |
| Female gender | < 0.001 | 2.59 [1.29–5.22] | 0.008 |
| Breech presentation | < 0.001 | 10.55 [5.35–20.79] | 0.008 |
| Positive family history of DDH | < 0.008 | 1.64 [0.56–4.84] | 0.368 |
| Twin birth | 0.315 | – | – |
The right columns shows the Odds Ratios and the related confidence intervals of every Risk Factors
CI 95% confidence interval. A p value < 0.05 was considered significant
Sensitivity, specificity and predictive values of the analyzed statistically significant factors are given in Table 3.
Table 3.
Sensitivity, specificity and predictive values of the analyzed statistically significant factors
| Factors [CI 95%] | |
|---|---|
| Positive Ortolani–Barlow test | |
| Sensitivity | 33.3% [20.4–48.4%] |
| Specificity | 97.6% [97–98%] |
| Positive predictive value | 14.3% [8.39–22.2%] |
| Negative predictive value | 99.2% [98.8–99.4%] |
| Female gender | |
| Sensitivity | 77.1% [62.7–88%] |
| Specificity | 50.7% [49.1–52.3%] |
| Positive predictive value | 1.86% [1.32–2.56%] |
| Negative predictive value | 99.5% [99–99.7%] |
| Breech presentation | |
| Sensitivity | 37.5% [24–52.6%] |
| Specificity | 95.7% [95.1–96.4%] |
| Positive predictive value | 9.68% [5.84–14.9%] |
| Negative predictive value | 99.2% [98.9–99.5%] |
| Positive family history of DDH | |
| Sensitivity | 12.5% [4.73–25.2%] |
| Specificity | 96.4% [95.7–96.9%] |
| Positive predictive value | 4% [1.48–8.5%] |
| Negative predictive value | 98.9% [98.5–99.2%] |
Among the 75 identified IIa + cases, 14 (18.67%) had no risk factors, 42 cases (56%) had only one risk factor, i.e. female (53.33%), breech presentation at birth (1.33%) or family history (1.33%). 14 cases (18.67%) had 2 risk factors simultaneously and 5 cases (6.67%) had all three risk factors.
Besides among the 29 cases IIa-, 5 (13.79%) had no risk factor, 12 (44.82%) only one risk factor, of which in 7 cases (29.14%) the risk factor was represented from female sex, breech presentation was in 3 cases (10.34%), family history in 1 (3.45%) and twinning in 1 (3.45%). 11 cases (37.93%) had two risk factors.
The positive Ortolani-Barlow maneuver was the factor that showed the strongest association with the pathological ultrasound finding. Among the risk factors a strong association emerged between the breech presentation and the diagnosis of DDH. The association between female sex and hip dysplasia is to be considered moderate. The result of the "family history for DDH-diagnosis of DDH" association was not statistically significant (p = 0.368).
The area under the ROC curve (AUC), which measured the diagnostic accuracy of selective screening was 0.8182 as showed in Fig. 2.
Fig. 2.
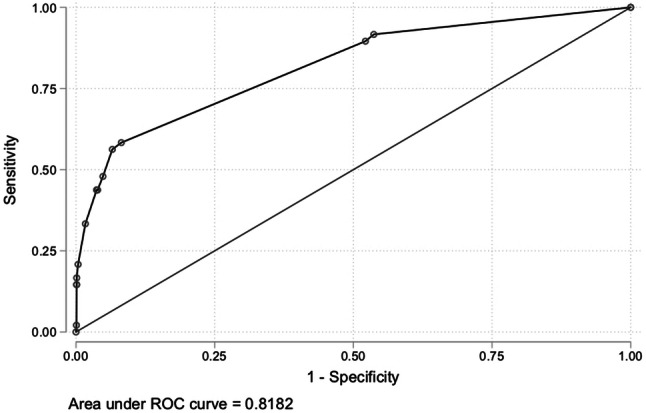
The ROC curve (AUC) shows the index of the diagnostic accuracy of selective screening
Universal vs selective screening
In order to assess the effectiveness of universal screening to identify cases of DDH, we then analyzed how many pathological cases we would have diagnosed and how many we would have lost if we had implemented a selective ultrasound screening.
A selective screening program consists of ultrasound examination of the hips only for the clinically positive patients (Positive Ortolani–Barlow maneuver) and/or those with risk factors, ie the breech presentation and the family history for DDH. It was found that among the 48 hips who were pathologic to ultrasound examination, 29 had at least one risk factor and/or clinical positivity, while 19 had neither risk factors nor clinical positivity, with a p value < 0.05 that was considered significant.
Sensitivity, specificity and predictive values of selective screening are given in Table 4.
Table 4.
Selective ultrasound hip screening
| Selective ultrasound hip screening [CI 95%] | |
|---|---|
| Sensitivity | 60.4% [45.3–74.2%] |
| Specificity | 90.3% [89.3–91.2%] |
| Positive predictive value | 7.04% [4.76–9.95%] |
| Negative predictive value | 99.5% [99.2–99.7%] |
Timing of ultrasound screening
The average age of all children at the time of the ultrasound examination was 11.08 weeks, with a 95% confidence interval between 10.93 and 11.23 and a standard deviation of 4.79. The execution time of the Ultrasound Screening is longer than the 6th week of life. This is mainly due to an incorrect indication by neonatologists to the discharge of the baby.
Among the 3952 hips mature or immature but appropriate for age, the average age at the time of ultrasonography was 11.13 weeks, with a 95% confidence interval between 10.98 and 11.28 and a standard deviation of 4.77. Considering only the 48 pathological hips, the average age at the time of ultrasonography was 6.68 weeks, with a 95% confidence interval between 5.28 and 8.08 and a standard deviation of 4.82 (Fig. 3).
Fig. 3.
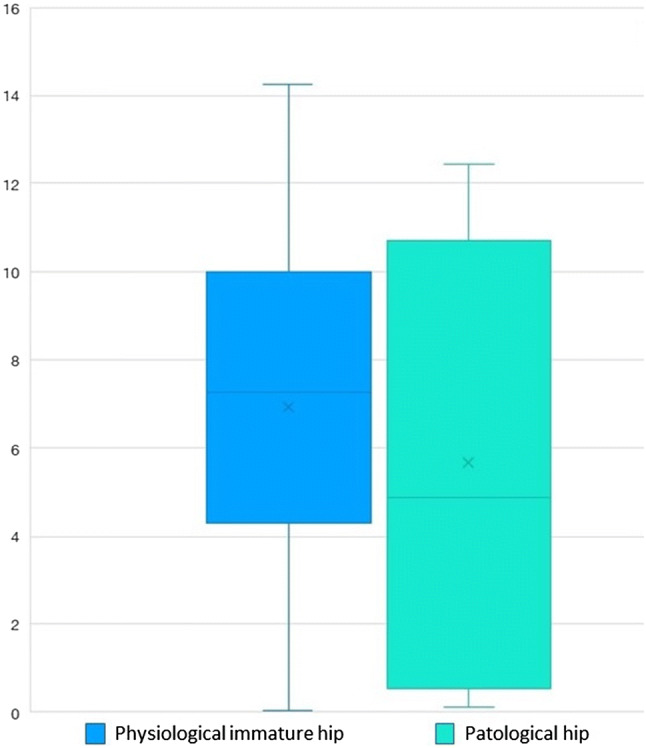
Age distribution (in weeks) at the time of ultrasound screening
The age of patients at the time of ultrasound hip screening, in reference to their risk factors, is shown in Fig. 4, showing that some children were sent for ultrasound screening at 12 weeks of age or more despite the presence of one or two risk factors; importantly, few children with positive clinical findings performed ultrasound at 6 or more weeks of life, suggesting that clinical examination was made wrong during previous routine pediatric controls.
Fig. 4.
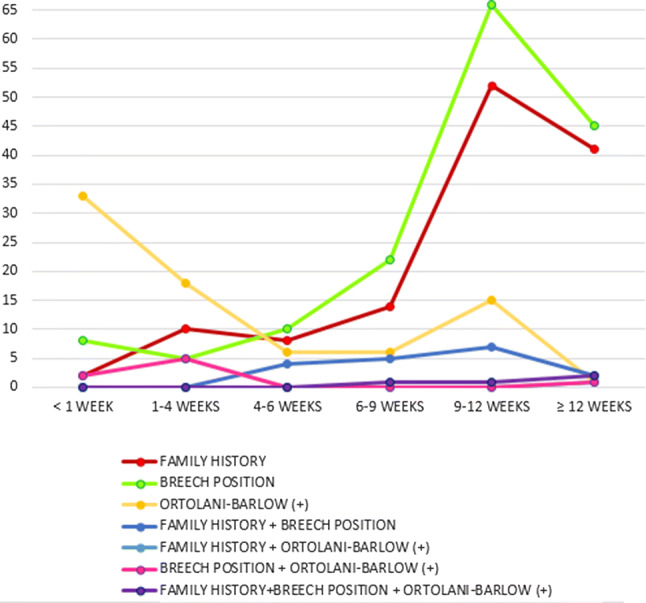
Age (in weeks) at the time of ultrasound screening in relation to risk
Survey on DDH
38 pediatricians and neonatologists (medical residents and specialists) belonging to our Institution agreed to answer the anonymous questionnaire in order to evaluate their theoretical-clinical knowledge on DDH.
The results show that pediatricians and neonatologist are aware that DDH is an important public health problem, but training that should be done about this condition during medical school or residency is scarce. 60% of them declare to adequately collect the medical history related to the risk factors for DDH. Everyone claims to perform the Ortolani-Barlow maneuver at birth (100%) and 97.5% of them think to do it correctly. However, 42.5% of pediatricians and neonatologists answer that they do not know how to distinguish a “click” from a “cluck” and 87.5% consider the click as a false positive. 86.8% recommend performing the ultrasound examination between the eighth and twelfth week of life and only 13.2% between the sixth and eighth week of life; no one within the sixth week of life. Furthermore, most pediatricians and neonatologists are oriented to prescribe the early execution of screening ultrasonography in patients with positive Ortolani-Barlow maneuver.
In a patient with one single risk factor (family history or breech presentation), 50–60% of doctor recommended to perform the ultrasound examination between the sixth and eighth week of life. In a child with two risk factors (family history and breech presentation), 50% recommend it within the sixth week. In addition, 52.6% believe it would be optimal to begin treatment for DDH between the eighth and twelfth week of life. Furthermore, 85% state that they are not able to interpret the images of an ultrasound of the hips, while 77.5% know the classification of Graf.
Discussion
In this study we retrospectively evaluated the results of an universal ultrasound screening for DDH, obtaining several interesting findings.
First of all, if we had performed a selective ultrasound screening (only for patients at high risk and/or clinically positive to the Ortolani–Barlow maneuver) a significant number of pathological cases would not have benefited from an early diagnosis and treatment, suggesting the usefulness of an universal screening in our setting. In fact, the comparison of the results of the clinical examination with the ultrasound findings has shown that not all anatomical-ultrasound abnormalities have a positive clinical examination; the ultrasound examination was more sensitive than the clinical one in identifying anomalies of the hip. Our data show that the concordance between clinical and ultrasound examination is good for severe dysplasia, ie cases in which the hip is subluxed or dislocated (stages III and IV according to Graf), but it is insufficient for those less severe, classified as stages IIa-, IIb, IIc and D according to Graf.
Secondly, our study confirmed the importance of female sex, breech presentation and positive Ortolani–Barlow maneuver as independent risk factors for DDH [2, 18, 19]. Twin birth, on the other hand, was not significantly associated with the ultrasound diagnosis of DDH. Importantly, we also showed that a negative family history and clinical examination has not excluded the presence of DDH. Therefore, a selective ultrasound screening based only on the presence of risk factors or on the positivity of the Ortolani-Barlow maneuver, as happens in several countries including the USA and the UK [6, 20], can be a source of potential late diagnosis. In accordance with the recent consensus statement [16], we believe that universal ultrasound screening allows a less invasive treatment, but also more advantageous from an economic point of view when compared to the costs of the interventions necessary in cases of late diagnosis of DDH.
More authors report a significant decrease in treatment costs both for hospitals, in relation to the reduction in the rate of surgical procedures and the days of hospitalization, and for patients' families [21–23].
Third, we showed that despite our local protocol suggests performing hip ultrasound by the 6th week of life, many pediatricians and neonatologists still suggest families to do the examination between 8 and 12 weeks, also in presence of risk factors. A recent Consensus published in June 2019 suggests performing ultrasound of hips within the sixth week of life in the absence of risk factors. This, on the one hand, leads to an increase in the ultrasound findings of physiological immaturity of the hips (stage IIa according to Graf), thus causing an increase in the number of follow-up ultrasound examinations. On the other hand, it has been shown that early treatment, by the sixth week of life, is more effective [16]. Importantly, many families were suggested to perform the screening after the 9th week of life despite the presence of 1 or 2 risk factors, and in some cases a late diagnosis of DDH was performed, with potential sub-optimal treatment for the baby.
Finally, we assessed, with anonymous and validated questionnaires, the general knowledge and attitude of pediatricians and neonatologists in our Hospital regarding DDH. It emerged that the majority of neonatologists and pediatricians are oriented to prescribe the early execution of screening ultrasonography in patients with clinically positive results of the Ortolani–Barlow maneuver; the presence of risk factors, instead, did not influence the average age of performing the ultrasound examination and, in this regard, a lack of medical history collection of these risk factors was demonstrated in our study, since we performed an important number of ultrasounds after 9 weeks of life, also in children with risk factors, due to mistakes in history taking. Importantly, the survey showed knowledge gaps about the interpretation of Graf classification and images, as well as feeling that more courses should have done during the residency school in pediatrics.
In conclusion, we showed that an universal screening allowed us to identify DDH in a number of children with normal clinical examination and no risk factors, suggesting its superiority over a selective screening, at least in our setting; moreover, doctors and resident from our institution confirmed a not complete knowledge on the topic and this was reflected in clinical practice with delayed ultrasound examinations and low sensitivity of the clinical examination. In light of our results and of what is reported by different authors [16, 24, 25], we believe that currently an universal screening is still necessary and that further efforts are needed to make specialists aware of the importance of DDH, in order to appropriately refer patients for ultrasound screening. Therefore specific training courses should be implemented regarding DDH both during the pediatrician residency school and subsequently for neonatologists and pediatricians.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
DB designed the study and performed hip ultrasound. AC designed study, reviewed and interpreted images and prepared manuscript. RM and GP collected data and prepared manuscript. FC designed the study and performed the statistical analysis. IL, RM, PV and CR designed the study and reviewed all manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The study was approved by the ethical committee of the Fondazione Policlinico Universitario A. Gemelli, IRCCS, Rome, Italy.
Informed consent
The authors obtained informed consent from the patient for submission of this manuscript for publication.
Consent for publication
All patients consented to the publication of their clinical data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tschauner C, Saba Y, Berghold A, Radl R. Developmental dysplasia of the hip: impact of sonographic newborn hip screening on the outcome of early treated decentered hip joints-a single center retrospective comparative cohort study based on Graf’s method of hip ultrasonography. J Child Orthop. 2011;5:415–424. doi: 10.1007/s11832-011-0366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schams M, Labruyère R, Zuse A, Walensi M. Diagnosing developmental dysplasia of the hip using the Graf ultrasound method: risk and protective factor analysis in 11,820 universally screened newborns. Eur J Pediatr. 2017;176:1193–1200. doi: 10.1007/s00431-017-2959-z. [DOI] [PubMed] [Google Scholar]
- 3.Pavone V, Riccioli M, Borgo A. Update sulla diagnosi e trattamento precoce della displasia congenita dell’anca. Prospettive Pediatr. 2017;47:34–41. [Google Scholar]
- 4.Engesæter IØ, Lehmann T, Laborie LB, Lie SA, Rosendahl K, Engesæter LB. Total hip replacement in young adults with hip dysplasia: age at diagnosis, previous treatment, quality of life, and validation of diagnoses reported to the Norwegian Arthroplasty Register between 1987 and 2007. Acta Orthop. 2011;82:149–154. doi: 10.3109/17453674.2011.566146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ortiz-Neira CL, Paolucci EO, Donnon T. A meta-analysis of common risk factors associated with the diagnosis of developmental dysplasia of the hip in newborns. Eur J Radiol. 2012;81:e344–e351. doi: 10.1016/j.ejrad.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Clarke NM, Reading IC, Corbin C, Taylor CC, Bochmann T. Twenty years experience of selective secondary ultrasound screening for congenital dislocation of the hip. Arch Dis Child. 2012;97:423–429. doi: 10.1136/archdischild-2011-301085. [DOI] [PubMed] [Google Scholar]
- 7.Shaw BA, Segal LS. Evaluation and referral for developmental dysplasia of the hip in infants. Pediatrics. 2016 doi: 10.1542/peds.2016-3107. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics Clinical practice guideline: early detection of developmental dysplasia of the hip. Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. Pediatrics. 2000;105:896–905. doi: 10.1542/peds.105.4.896. [DOI] [PubMed] [Google Scholar]
- 9.Graf R. The diagnosis of congenital hip-joint dislocation by the ultrasonic Combound treatment. Arch Orthop Trauma Surg. 1980;97:117–133. doi: 10.1007/bf00450934. [DOI] [PubMed] [Google Scholar]
- 10.Krismer M, Klestil T, Morscher M, Eggl H. The effect of ultrasonographic screening on the incidence of developmental dislocation of the hip. Int Orthop. 1996;20:80–82. doi: 10.1007/s002640050034. [DOI] [PubMed] [Google Scholar]
- 11.Tönnis D, Storch K, Ulbrich H. Results of newborn screening for CDH with and without sonography and correlation of risk factors. J Pediatr Orthop. 1990;10:145–152. doi: 10.1097/01241398-199003000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Wientroub S, Grill F. Ultrasonography in developmental dysplasia of the hip. J Bone Joint Surg Am. 2000;82-A:1004–1018. doi: 10.2106/00004623-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Biedermann R, Eastwood DM. Universal or selective ultrasound screening for developmental dysplasia of the hip? A discussion of the key issues. J Child Orthop. 2018;12:296–301. doi: 10.1302/1863-2548.12.180063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitch RD. Ultrasound for screening and management of developmental dysplasia of the hip. NC Med J. 2014;75:142–145. doi: 10.18043/ncm.75.2.142. [DOI] [PubMed] [Google Scholar]
- 15.Cady RB. Developmental dysplasia of the hip: definition, recognition, and prevention of late sequelae. Pediatr Ann. 2006;35:92–101. doi: 10.3928/0090-4481-20060201-09. [DOI] [PubMed] [Google Scholar]
- 16.O’Beirne JG, Chlapoutakis K, Alshryda S, Aydingoz U, Baumann T, Casini C, et al. International interdisciplinary consensus meeting on the evaluation of developmental dysplasia of the hip. Ultraschall in Med. 2019;40:454–464. doi: 10.1055/a-0924-5491. [DOI] [PubMed] [Google Scholar]
- 17.Barbuto L, Di Serafino M, Della Vecchia N, Rea G, Esposito F, Vezzali N, et al. Pediatric musculoskeletal ultrasound: a pictorial essay. J Ultrasound. 2019;22:491–502. doi: 10.1007/s40477-018-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal A, Gupta N. Risk factors and diagnosis of developmental dysplasia of hip in children. J Clin Orthop Trauma. 2012;3:10–14. doi: 10.1016/j.jcot.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan A, McCaul KA, Cundy PJ, Haan EA, Byron-Scott R. Perinatal risk factors for developmental dysplasia of the hip. Arch Dis Child Fetal Neonatal. 1997;76:F94–100. doi: 10.1136/fn.76.2.f94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shipman SA, Helfand M, Moyer VA, Yawn BP. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117:e557–576. doi: 10.1542/peds.2005-1597. [DOI] [PubMed] [Google Scholar]
- 21.Gray A, Elbourne D, Dezateux C, King A, Quinn A, Gardner F. Economic evaluation of ultrasonography in the diagnosis and management of developmental hip dysplasia in the UK and Ireland. J Bone Jt Surg Am. 2005;87:2472–2479. doi: 10.2106/JBJS.D.01997. [DOI] [PubMed] [Google Scholar]
- 22.Clegg J, Bache CE, Raut VV. Financial justification for routine ultrasound screening of the neonatal hip. J Bone Jt Surg Br. 1999;81:852–857. doi: 10.1302/0301-620x.81b5.9746. [DOI] [PubMed] [Google Scholar]
- 23.Thaler M, Biedermann R, Lair J, Krismer M, Landauer F. Cost-effectiveness of universal ultrasound screening compared with clinical examination alone in the diagnosis and treatment of neonatal hip dysplasia in Austria. J Bone Jt Surg Br. 2011;93:1126–1130. doi: 10.1302/0301-620X.93B8.25935. [DOI] [PubMed] [Google Scholar]
- 24.Chlapoutakis K, Kolovos S, Casini C. Ultrasonography in developmental dysplasia of the hip: a review of current clinical strategies and recommendations for revision of practice. Hell J Radiol. 2017;2:36–46. [Google Scholar]
- 25.Pellegrin MD, Boero S, Origo C, Farsetti P. La displasia congenita dell’anca (DCA). Terminologia, diagnosi precoce, screening, raccomandazioni. G Ital Ortop E Traumatol. 2019;45:1–6. doi: 10.32050/0390-0134-159. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.