Abstract
Purpose
The aim of this study is to compare elasticity features between patients with plantar fasciitis (PFis) and an asymptomatic healthy control group using shear wave elastography (SWE) and to correlate SWE values with clinical scores.
Methods
Consecutive patients diagnosed with PFis and asymptomatic subjects were enrolled in the present study. Both groups underwent clinical, ultrasound (US), and SWE evaluation. A plantar fascia thickness > 4 mm was considered pathognomonic of PFis. SWE stiffness elasticity (Young’s modulus in kPa and shear wave velocity in m/s) was measured 1 cm distally from the calcaneal insertion. Correlations with VAS and the 17-Italian Foot Function Index (17-FFI) were determined.
Results
A total of 19 patients satisfied the inclusion criteria for the patient group and were enrolled in the study, and 21 healthy subjects were used as a control group. Statistically significant differences were found for shear wave velocity between the patient and the control group, with SWE findings of 3.8 (5.1; 1.5) m/s and 4.7 (4.07; 7.04) m/s, respectively (p = 0.006). Strong positive correlations were found between the SWE findings and both the pain and the functional scale (VAS: p = 0.001; FFI: p = 0.012).
Conclusion
SWE allows quantitative assessment of the stiffness of the plantar fascia and can show PFis alterations, increasing the diagnostic performance of B-mode US. In addition, SWE shows a strong correlation with clinical scores, improving patient assessment and follow-up.
Keywords: Shear wave elastography, Elastography, Elastosonography, Plantar fasciitis, Tendinopathy, Plantar fasciopathy, SWE
Introduction
Plantar fasciitis (PFis) is a common cause of inferior heel pain [1]. The typical symptom in patients with PFis is pain while taking the first steps in the morning or after a period of inactivity [2]. While standing or during other daily routine activities, patients often experience a progressive worsening of symptoms, with increased complaints of pain towards the end of the day. The pain in the plantar fascia (PF) normally originates at the plantar medial tubercle of the calcaneus, at the PF insertion [3].
In about 80% of PFis cases, the pain is self-limiting and resolves within a year. However, the effect of heel pain during daily routine activities results in patients seeking treatment earlier, especially athlete patients.
Nowadays, between 70 and 80% of patients reduce pain with conservative treatment [4, 5]. Only 5% undergo surgery for PF release after all conservative measures fail.
PFis has traditionally been considered an overuse injury, with repetitive microtrauma and damage to the PF, occurring at a rate that exceeds the body’s capacity to heal.
Despite its definition, it is known from histological research that PFis is a process of chronic degeneration [5] that occurs with or without inflammatory changes, which may include fibroblastic proliferation as a result of repetitive microtears of the fascia.
Excessive stresses lead to a chronic degenerative condition that is histologically characterized by fibroblastic hypertrophy, absence of inflammatory cells, disorganized collagen, and chaotic vascular hyperplasia with zones of avascularity [6].
PFis is frequently diagnosed clinically, although magnetic resonance imaging (MRI) and ultrasound (US) evaluation can confirm the diagnosis or rule out other causes of heel pain [7, 8].
Conventional US has been widely used to assist the diagnosis of PFis due to its easy availability and cost-effectiveness. Contemporary US findings of PFis include loss of normal architecture, a hypoechoic area within the fascia, perifascial fluid, and thickening of the PF [7]. All these US features are related to PFis histological alteration, such as microtears in collagen fibers, fibroblastic hypertrophy, and chronic degeneration caused by repetitive overstrain [9].
Some authors consider thickness values > 4 mm pathognomonic of PFis [10]. These morphological changes, however, are not always observed with conventional US in patients with PFis [11, 12]. Elastography is recently known to be a feasible diagnostic tool in case of inconclusive US findings, showing early changes in elasticity of the PF in symptomatic patients [13, 14].
Evaluation of the muscle and tendon stiffness has been studied thoroughly by different methods [15, 16].
Shear wave elastography (SWE) is a noninvasive US imaging technique that provides information on tissue elasticity and stiffness using an impulse generated by the probe [17, 18]. SWE has especially been considered to obtain user-independent quantitative measures of tissue elasticity expressed in kPa or m/s, and its clinical applications have been widely approved [18].
SWE uses an acoustic radiation force impulse (ARFI) to generate shear waves. These waves are propagated perpendicularly to the US beam, causing transient displacements. The distribution of shear wave velocities at each pixel is directly related to the elastic modulus (Young’s modulus), an absolute and quantitative measure of a tissue's elastic properties [18, 19]. Shear wave velocity is a meaningful parameter, with the shear wave propagating at a greater speed in stiffer tissues. Conversely, structures with a soft consistency show less deformability when stressed by an impulse, thus showing lower velocity values [20].
SWE has been widely used in many recent studies to assess the biomechanical properties of soft tissues, which contain collagen fibers and have elastic properties similar to those of the PF [6, 18, 21]. The elastography evidence on the musculoskeletal field in the scientific literature is growing, including studies on healthy and degenerative Achilles tendons [22–24], lateral epicondylitis [25], several rheumatologic conditions [26, 27], and the PF [28–31].
The purpose of this study was to compare the SWE characteristics of the PF between patients with chronic PFis and healthy subjects.
Materials and methods
Study design and population
This study was conducted to evaluate the diagnostic role of elastography in assessing PF features between patients diagnosed with PFis and healthy subjects.
US examinations were performed on 19 patients and 20 healthy subjects. Longitudinal and short-axis B-mode imaging and longitudinal elastography imaging of the feet are included in the routine US protocol of our institute for healthy subjects and patients with unilateral or bilateral heel pain.
For patients to be included in the study, the diagnosis of PFis should have been confirmed, as follows:
Thickening of the PF at US evaluation > 4 mm;
Pain assessed through the VAS scale > 4 out of 10;
More than 3 months of heel pain in the PF insertion of the medial tubercle of the calcaneus; and
Nonresponsive pain to previous noninvasive conservative treatment with nonsteroidal anti-inflammatory medication, night splints, and stretching exercises for at least 3 months.
The exclusion criteria were as follows:
Diagnosis of a systemic inflammatory disease;
Diagnosis of connective tissue disease;
Previous local trauma;
Presence of plantar fibroma;
Previous surgery; and
Treatment with PF corticosteroids, hyaluronic acid, or PRP injections within the 3 months before screening, or oral cortisone therapy in the previous month.
Finally, 19 feet of 17 patients (2 patients with both feet) were included in the patient group. As a control, we analyzed 21 asymptomatic feet of 20 healthy subjects with no history of relevant disorders. The two groups were homogeneous regarding age, weight, and height.
All eligible patients who completed their clinical data were included in the study. Each patient underwent a clinical, US B-mode, and SWE evaluation. Furthermore, VAS [32] and the 17-Italian Foot Function Index (17-FFI) [33] were used to assess pain and function in both groups.
The study protocol was approved by the hospital’s Ethical Review Board, and the study was conducted in accordance with the principles of the Declaration of Helsinki and its amendments. The patients were fully informed of the characteristics of the study before providing consent.
Ultrasound evaluation
Both groups underwent PF US evaluation bilaterally, using a US scanner (Toshiba Aplio i800, Canon Medical System Europe BV) equipped with a multifrequency 5–18-MHz linear probe. All examinations were performed by three musculoskeletal physicians with 10 years of experience in imaging and more than 12 months of experience in US elastography. Both groups were asked to lie in a prone position with their foot hanging over the edge of the examination table.
Longitudinal and short-axis B-mode imaging and longitudinal elastography imaging of the feet were performed in both groups.
PF thickness was measured vertically from the anterior edge of the medial inferior calcaneal border to the inferior border of the PF [34], with confirmation that all symptomatic PF groups had a PF thickness > 4.0 mm.
After the B-mode US examination, US elastography was performed with the heel and probe in the same position, and three evaluations were recorded for each PF.
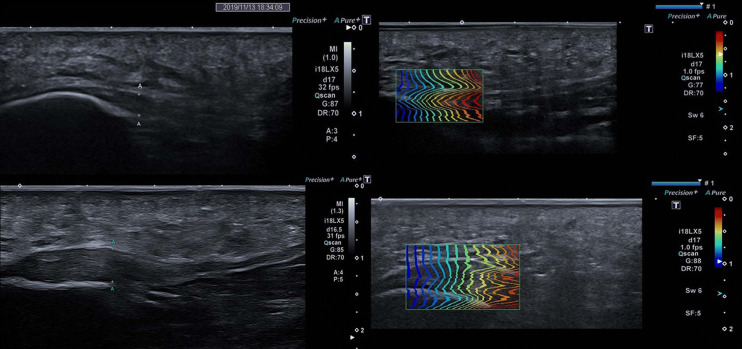
The SWE measurement was taken on the calcaneal insertion of the PF with the region of interest (ROI) 1 cm away from the insertion. The SWE measurement was taken 1 cm distally from the calcaneal insertion of the PF with a 3-mm-diameter, standardized ROI to avoid any risk of artifacts induced by other biases such as calcifications or vessels (Fig. 1).
Fig. 1.
Long-axis B-mode and Shear wave Elastography images: the top figure shows the healthy plantar fascia; the figure in the bottom shows plantar fasciopathy
Statistical analysis
Statistical analysis was performed using SPSS software, version 10 (IBM, Armonk, NY). The results are presented as the median and interval (minimum and maximum values). For all variables, the normality of the data was ascertained using the Kolmogorov–Smirnov test. Because all parameters were not normally distributed, the nonparametric Mann–Whitney U test was used to determine whether there were differences between the two groups.
Results
The two groups were homogeneous regarding age and BMI (p > 0.05). Table 1 shows the ages and anthropometric features of the patients and healthy subjects, and the values are expressed as mean and standard deviation.
Table 1.
Demographic and anthropometric characteristics
| Age (years) |
Weight (kg) |
Height (cm) |
BMI | VAS | 17-FFI | |
|---|---|---|---|---|---|---|
| Symptomatic PF | 50.5 ± 12.63037 | 67.1 ± 9.5 | 1.68 ± 0.11 | 25.01 ± 3.9 | 7.4 ± 1 | 74 ± 12.1 |
| Control group asymptomatic PF | 47.5 ± 7.4 | 66.8 ± 9.28 | 1.67 ± 0.1 | 24 ± 3.5 | 0 | 0 |
Data are showed as mean and standard deviation
A significant difference was found in the thickness of the PF. The group with symptomatic patients showed a median value of PF thickness of 5 mm (4; 6.9), whereas all asymptomatic PF subjects showed a thinner PF (< 4 mm; p < 0.001), in accordance with PF thickness being a pathognomonic sign. Statistically significant differences were found for shear wave velocity (SWV) between the two groups, with lower values in the symptomatic PF group with median values of 3.8 m/s (1.8; 4.35), whereas the asymptomatic control group showed values of 5.12 m/s (3.49; 6.9; p < 0.0001). To assess the correlation between SWV values and the clinical scores, the Spearman rank test was adopted. Strong direct correlations were found between SWV and the VAS score (r = − 0.41; p = 0.001) and between SWEv and the FFI score (r = 0.67; p = 0.012). Table 2 shows the US findings, and the values are expressed as median and range values.
Table 2.
B-mode and elastography evaluation
| Symptomatic PF | Control group | p value | |
|---|---|---|---|
| Plantar fascia thickness US (cm) | 5 (4; 6.9) | 3.1 (2; 4) |
U = 18.5 Z = − 4.83 p < 0.0001 |
| Shear wave velocity (m/s) | 3.8 (1.5; 5.1) | 5.12 (3; 6.9) |
U = 289.5 Z = 2.8 p = 0.004 |
Data are presented as median (range)
*Statistical significant differences
Discussion
This study evaluated the diagnostic potential of SWE in the diagnosis of PFis. Moreover, we evaluated the correlation between SWE findings and clinical scores.
SWE appears to be an important diagnostic tool in the early detection of PFis, showing more diagnostic accuracy than US in some cases.
Previous studies using strain elastography, such as those of Wu et al. [28, 29], demonstrated a softening of the PF on the affected side, whereas conventional US reported normal PF echogenicity and thickness. Sconfienza et al. [30] also found a loss of elasticity of the affected PF prior to morphological changes becoming visible on B-mode imaging using strain elastography, thus underlining the importance of elastography in the early detection of PFis. Additionally, elastography seems to have a crucial role in cases of atypical clinical presentation or in which imaging findings are undetermined [34, 35].
In our study with SWE, our aim was to obtain an interpretation that is more objective than color visual grading and more reliable in terms of interobserver reproducibility and diagnostic accuracy.
Our results show that the symptomatic PF has a significantly lower SWV than the asymptomatic PF (3.8 m/s [1.8; 4.35] vs. 5.12 m/s [3.49; 6.9]; p < 0.0001), meaning that they are “less elastic” or “softer.” The results of our study are in line with the recent literature, such as Gatz et al. [36], who demonstrated a significant reduction in Young’s moduli in the symptomatic PF [30].
Those findings could be interpreted as a lack of elasticity of the affected PF due to degenerative processes, resulting in collagen breakdown, fibroblastic hypertrophy, and matrix degradation in accordance with the hypothesis of fasciopathy patterns on the basis of PFis and PFis histological alteration [5, 9].
Our data suggest that SWE can be a valuable option in undetermined US findings. Furthermore, the results of this study showed that SWE is an important reference parameter in treatment management, as we found a strong correlation between SWEv and both pain and functional scales, with a statistical significance between SWEv and the 17-FFI score (r = 0.67; p = 0.012). As the availability of elastography increases everywhere, these results promise an effective improvement in the clinical assessment, management, and follow-up of patients.
This technique seems to be an important tool in addition to US evaluation, and in some cases, it appears to be essential for identifying early changes in PFis even in symptomatic patients with normal B-mode findings. Furthermore, SWE is useful for monitoring the efficacy of treatment and rehabilitation protocols, providing quantitative data, and showing a reliable correlation with functional and pain scores.
The limitation of this preliminary study is the small sample size, and further investigations are thus needed.
In conclusion, SWE seems to be useful in evaluating the PF and its alteration in PFis. However, further prospective evaluations in larger populations are warranted to confirm these promising results.
Funding
No funds were received.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giuseppe Schillizzi, Email: Giuseppe.schillizzi@gmail.com.
Federica Alviti, Email: federica.alviti@uniroma1.it.
Vito Cantisani, Email: Vito.cantisani@uniroma1.it.
References
- 1.Beeson P. Plantar fasciopathy: revisiting the risk factors. J Foot Ankle Surg. 2014;20:160–165. doi: 10.1016/j.fas.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum AJ, DiPreta JA, Misener D. Plantar heel pain. Med Clin N Am. 2014;98:339–352. doi: 10.1016/j.mcna.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Schneider HP, Baca JM, Carpenter BB, Dayton PD, Fleischer AE, Sachs BD. American College of Foot and Ankle Surgeons Clinical Consensus Statement: diagnosis and treatment of adult acquired infracalcaneal heel pain. J Foot Ankle Surg. 2018;57(2):370–381. doi: 10.1053/j.jfas.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Tong KB, Furia J. Economic burden of plantar fasciitis treatment in the United States. Am J Orthop (Belle Mead NJ) 2010;39(5):227–231. [PubMed] [Google Scholar]
- 5.Lemont H, Ammirati KM, Usen N. Plantar fasciitis: a degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc. 2003;93:234–237. doi: 10.7547/87507315-93-3-234. [DOI] [PubMed] [Google Scholar]
- 6.Snider MP, Clancy WG, McBeath AA. Plantar fascia release for chronic plantar fasciitis in runners. Am J Sports Med. 1983;11:215–219. doi: 10.1177/036354658301100406. [DOI] [PubMed] [Google Scholar]
- 7.McNally EG, Shetty S. Plantar fascia: imaging diagnosis and guided treatment. Semin Musculoskelet Radiol. 2010;14(3):334–343. doi: 10.1055/s-0030-1254522. [DOI] [PubMed] [Google Scholar]
- 8.Theodorou DJ, Theodorou SJ, Kakitsubata Y, Lektrakul N, Gold GE, Roger B, Resnick D. Plantar fasciitis and fascial rupture: MR imaging findings in 26 patients supplemented with anatomic data in cadavers. RadioGraphics. 2000;20:S181–S197. doi: 10.1148/radiographics.20.suppl_1.g00oc01s181. [DOI] [PubMed] [Google Scholar]
- 9.Wearing SC, Smeathers JE, Urry SR, Hennig EM, Hills AP. The pathomechanics of plantar fasciitis. Sports Med. 2006;36(7):585–611. doi: 10.2165/00007256-200636070-00004. [DOI] [PubMed] [Google Scholar]
- 10.Theodorou DJ, Theodorou SJ, Resnick D. MR imaging of abnormalities of the plantar fascia. Semin Musculoskelet Radiol. 2002;6(2):105–118. doi: 10.1055/s-2002-32357. [DOI] [PubMed] [Google Scholar]
- 11.Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255–259. doi: 10.1080/030097400750041415. [DOI] [PubMed] [Google Scholar]
- 12.McMillan AM, Landorf KB, Barrett JT, Menz HB, Bird AR. Diagnostic imaging for chronic plantar heel pain: a systematic review and meta-analysis. J Foot Ankle Res. 2009;2:32. doi: 10.1186/1757-1146-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prado-Costa R, Rebelo J, Monteiro-Barroso J, Preto AS. Ultrasound elastography: compression elastography and shear-wave elastography in the assessment of tendon injury. Insights Imaging. 2018;9(5):791–814. doi: 10.1007/s13244-018-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu CH, Chen WS, Wang TG, Lew HL. Can sonoelastography detect plantar fasciitis earlier than traditional B-mode ultrasonography? Am J Phys Med Rehabil. 2012;91(2):185. doi: 10.1097/PHM.0b013e31822de9ab. [DOI] [PubMed] [Google Scholar]
- 15.Alviti F, Gurzì M, Santilli V, Paoloni M, Padua R, Bernetti A, Bernardi M, Mangone M. Achilles tendon open surgical treatment with platelet-rich fibrin matrix augmentation: biomechanical evaluation. J Foot Ankle Surg. 2017;56(3):581–585. doi: 10.1053/j.jfas.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 16.Walshe AD, Wilson GJ, Murphy AJ. The validity and reliability of a test of lower body musculotendinous stiffness. Eur J Appl Physiol Occup Physiol. 1996;73:332–339. doi: 10.1007/BF02425495. [DOI] [PubMed] [Google Scholar]
- 17.Piskin FC, Yavuz S, Kose S, et al. A comparative study of the pancreas in pediatric patients with cystic fibrosis and healthy children using two-dimensional shear wave elastography. J Ultrasound. 2020 doi: 10.1007/s40477-020-00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Săftoiu A, Gilja OH, Sidhu PS, Dietrich CF, Cantisani V, et al. The EFSUMB guidelines for the clinical practice of elastography in non-hepatic application: update 2018. Ultraschall Med. 2019;40(4):425–453. doi: 10.1055/a-0838-9937. [DOI] [PubMed] [Google Scholar]
- 19.Cocco G, Ricci V, Boccatonda A, Abate M, Guagnano MT, Schiavone C. Ultrasound follow-up of spontaneous tears of the plantar fascia treated with conservative therapies. Two cases reports. Medicine. 2019;98:52. doi: 10.1097/MD.0000000000018428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Serafino M, Severino R, Gioioso M, et al. Paediatric liver ultrasound: a pictorial essay. J Ultrasound. 2020;23:87–103. doi: 10.1007/s40477-018-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43:409–416. doi: 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- 22.Sconfienza LM, Silvestri E, Cimmino MA. Sonoelastography in the evaluation of painful Achilles tendon in amateur athletes. Clin Exp Rheumatol. 2010;28(3):373–378. [PubMed] [Google Scholar]
- 23.De Zordo T, Chhem R, Smekal V, et al. Realtime sonoelastography: findings in patients with symptomatic Achilles tendons and comparison to healthy volunteers. Ultraschall Med. 2010;31(4):394–400. doi: 10.1055/s-0028-1109809. [DOI] [PubMed] [Google Scholar]
- 24.Drakonaki EE, Allen GM, Wilson DJ. Realtime ultrasound elastography of the normal Achilles tendon: reproducibility and pattern description. Clin Radiol. 2009;64(12):1196–1202. doi: 10.1016/j.crad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 25.De Zordo T, Lill SR, Fink C, et al. Real-time sonoelastography of lateral epicondylitis: comparison of findings between patients and healthy volunteers. Am J Roentgenol. 2009;193(1):180–185. doi: 10.2214/AJR.08.2020. [DOI] [PubMed] [Google Scholar]
- 26.Sconfienza LM, Silvestri E, Bartolini B, Garlaschi G, Cimmino MA. Sonoelastography may help in the differential diagnosis between rheumatoid nodules and tophi. Clin Exp Rheumatol. 2010;28(1):144–145. [PubMed] [Google Scholar]
- 27.Silvestri E, Garlaschi G, Bartolini B, et al. Sonoelastography can help in the localization of soft tissue damage in polymyalgia rheumatica (PMR) Clin Exp Rheumatol. 2007;25(5):796. [PubMed] [Google Scholar]
- 28.Wu CH, Chang KV, Mio S, Chen WS, Wang TG. Sonoelastography of the plantar fascia. Radiology. 2011;259(2):502–507. doi: 10.1148/radiol.11101665. [DOI] [PubMed] [Google Scholar]
- 29.Wu CH, Chen WS, Wang TG. Plantar fascia softening in plantar fasciitis with normal B-mode sonography. Skeletal Radiol. 2015;44(11):1603–1607. doi: 10.1007/s00256-015-2215-4. [DOI] [PubMed] [Google Scholar]
- 30.Sconfienza LM, Silvestri E, Orlandi D, Fabbro E, Ferrero G, Martini C, Sardanelli F, Cimmino MA. Real-time sonoelastography of the plantar fascia: comparison between patients with plantar fasciitis and healthy control subjects. Radiology. 2013;267(1):195–200. doi: 10.1148/radiol.12120969. [DOI] [PubMed] [Google Scholar]
- 31.Alviti F, D'Ercole C, Schillizzi G, Mangone M, Bernetti A, Ioppolo F, Di Sante L, Minafra P, Santilli V, Elia D, Vallone G, D'ambrosio F, Cantisani V. Elastosonographic evaluation after extracorporeal shockwave treatment in plantar fasciopathy. Med Ultrason. 2019;21(4):399–404. doi: 10.11152/mu-1976. [DOI] [PubMed] [Google Scholar]
- 32.Kersten P, Küçükdeveci AA, Tennant A. The use of the Visual Analogue Scale (VAS) in rehabilitation outcomes. J Rehabil Med. 2012;44(7):609–610. doi: 10.2340/16501977-0999. [DOI] [PubMed] [Google Scholar]
- 33.Venditto T, Tognolo L, Rizzo RS, Iannuccelli C, Di Sante L, Trevisan M, et al. 17-Italian Foot Function Index with numerical rating scale: development, reliability, and validity of a modified version of the original Foot Function Index. Foot (Edinb) 2015;25:12–18. doi: 10.1016/j.foot.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Sabir N, Demirlenk S, Yagci B, Karabulut N, Cubukcu S. Clinical utility of sonography in diagnosing plantar fasciitis. J Ultrasound Med. 2005;24:1041–1048. doi: 10.7863/jum.2005.24.8.1041. [DOI] [PubMed] [Google Scholar]
- 35.Draghi F, Gitto S, Bortolotto C, Guja Draghi A, Belometti GO. Imaging of plantar disorder: findings on plain radiolography, ultrasound and magnetic resonance imaging. Insights Imaging. 2017;8:69–78. doi: 10.1007/s13244-016-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gatz M, Bejder L, Quack V, Schrading S, Dirrichs T, Tingart M, Kuhl C, Betsch M. Shear wave elastography (SWE) for the evaluation of patients with plantar fasciitis. Acad Radiol. 2019;27:363–370. doi: 10.1016/j.acra.2019.04.009. [DOI] [PubMed] [Google Scholar]