Abstract
Pseudoaneurysm occurs when the artery wall is damaged and the blood is contained by the surrounding tissues with the eventual formation of a fibrous sac communicating with the artery. We report a case of a 74-year-old man with inferior epigastric artery (IEA) pseudoaneurysm secondary to an 8-mm port placement during a robot-assisted laparoscopic radical cystectomy with ureteroileocutaneostomy. The pseudoaneurysm was initially diagnosed by contrast-enhanced ultrasound (CEUS); subsequently, a computed tomography (CT) scan and an angiography test were performed. The pseudoaneurysm was then treated successfully with embolization of the inferior epigastric artery. Awareness of this rare complication is of clinical importance to avoid excessive morbidity of affected individuals.
Keywords: Epigastric artery, Pseudoaneurysm, Contrast-enhanced ultrasound, Embolization
Introduction
A pseudoaneurysm can be defined as a vessel-associated contained blood collection. Inferior epigastric artery (IEA) false aneurysms are recognized complications following abdominal surgery or trauma [1]. Occasional case reports have been published describing IEA pseudoaneurysms as a result of abdominal wall sutures, laparoscopic trocar insertion, surgical drain insertion, therapeutic paracentesis, peritoneal dialysis, femoral vessel catheterization, or even spontaneous occurrence [2]. Due to its rarity, the diagnosis may be missed. If not treated, potential complications include painful persistent swelling, abscess formation, or rupture [3]. Abdominal incisional hernia repair represents a potential cause of an IEA false aneurysm. We give an overview of the literature and emphasize the importance of considering the diagnosis to help avoid inappropriate interventions that could increase patient morbidity [4].
Case report
A 74-year-old man presented with anamnesis quadruple aortocoronaric bypass and three bladder resections for urothelial carcinoma; the histological examination of the last procedure was a pT2 high-grade urothelial carcinoma [5]. The preoperative computed tomography (CT) scan showed no lymphoadenomegaly and bilateral hydronephrosis and no other localization than the bladder. In March 2019, a radical cystectomy with Bricker ureteroileocutaneostomy and extended pelvic lymph node dissection was performed. The histological examination was a high-grade urothelial carcinoma pT3b, pN0 (0/36), R0 and a prostate adenocarcinoma Gleason score 6 (3 + 3), pT2b, R0. During the hospitalization, he had a Clavien–Dindo grade 3 surgical complication that required a blood transfusion. The patient was dismissed after 8 days in a good state. After 28 days, during an ambulatory visit, a palpable and compressible abdominal mass was identified on the left side. He stated that this mass appeared shortly after the procedure and had progressively increased in size and tenderness until a few days before the follow-up visit. Differential diagnosis included a hematoma and deep wound infection or pointing abscess [6]. At first, an ultrasound (US) with color Doppler modality integrated with contrast-enhanced ultrasound (CEUS), with 2.4 ml SonoVue (Bracco SpA, Milan, Italy), was performed (Fig. 1a, b), showing a fluid-corpuscular collection in the subcutaneous tissue and in the rectus muscle of 73 × 25 mm at about 17 mm from the skin compatible with hematoma. It was closely adjacent and could not be dissociated from a partially thrombosed pseudoaneurysm of 39 × 28 mm, which seemed to originate below the fascia. It appeared to be supplied by two branches of the epigastric left artery, one branch from above and one inferior branch. Subsequently, an abdominal CT was performed (Fig. 2), confirming the fluid-corpuscular collection, with a significant and early spreading of contrast media due to a high-flow phenomenon, probably originating from the ipsilateral epigastric artery about 6 cm from its origin. A selective angiography test was promptly performed. A pseudoaneurysm arising from the left IEA was seen with successive exclusion after embolization (Fig. 3) [7]. Subsequent angiograms revealed a total exclusion of the pseudoaneurysm from circulation. No complications occurred during or after the procedure. Follow-up serial color Doppler US and CEUS (Fig. 4) confirmed the complete exclusion of the pseudoaneurysm from circulation and showed gradual resorption of the residual hematoma over a 1-month period.
Fig. 1.
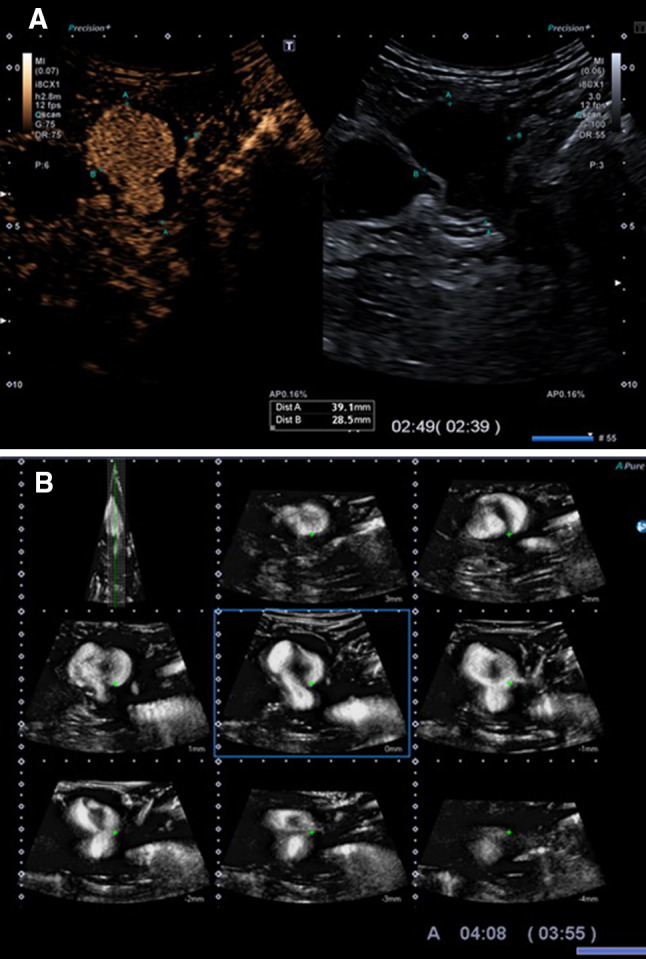
Contrast-enhanced ultrasound (CEUS) a and 3D reconstruction b show the blood pseudoaneurysm fill-in
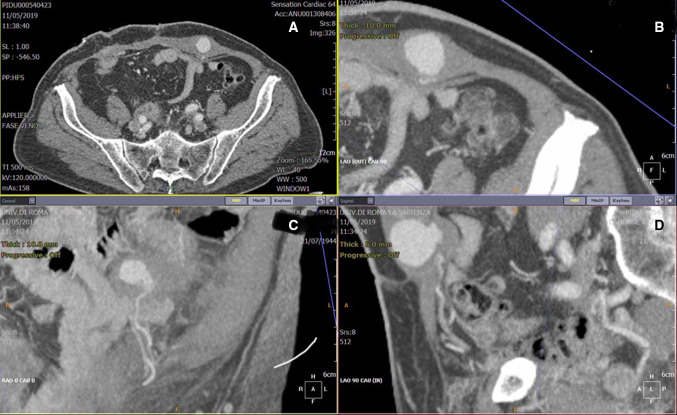
Fig. 2.
Thick maximum intensity projection computed tomography (CT) reconstructions on axial a and on multiplanar oblique reconstructions b, c, d the pseudoaneurysm, arising from the left inferior epigastric artery
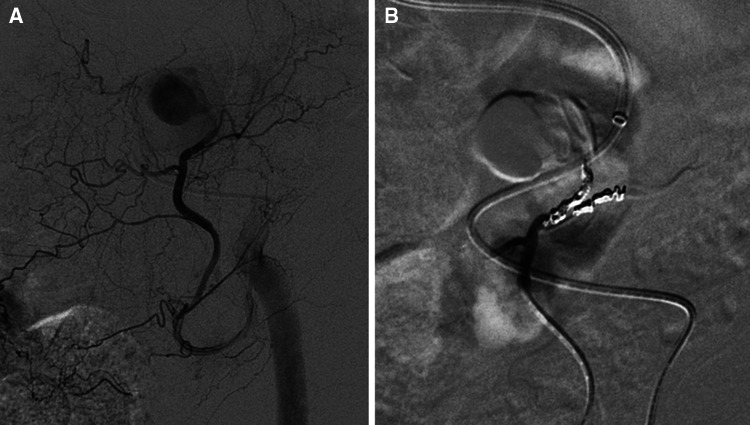
Fig. 3.
Angiography procedure (a) shows the pseudoaneurysm arising from the left inferior epigastric artery with successive total exclusion after embolization (b)
Fig. 4.
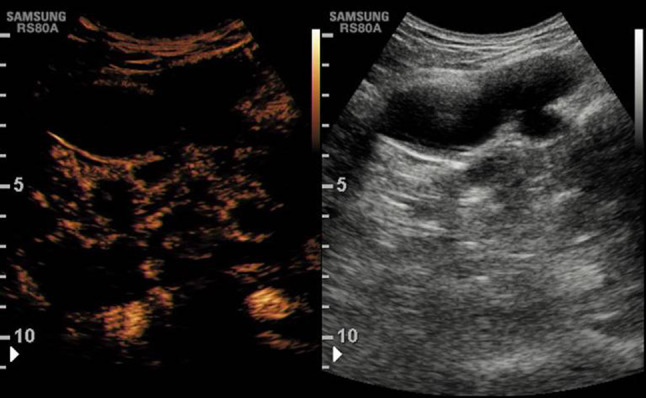
Contrast-enhanced ultrasound (CEUS) follow-up confirms the persistent pseudoaneurysm exclusion
Discussion
The IEA, which lies in the posterior wall of the rectus sheath, is susceptible to injury during abdominal wall procedures. However, pseudoaneurysms of the IEA are rare. Reported cases in the literature have been associated with trauma, removal of retention sutures, percutaneous biopsy, paracentesis, Tenckhoff catheter removal, and formation of stoma. The literature was limited to case reports [8, 9]. Due to its rarity and lack of characteristic symptoms or signs, an inferior epigastric artery aneurysm can be difficult to diagnose. It usually presents as a diffuse, tender, yet nonpulsatile mass, and no bruits may be auscultated on it. Thus, it can be similar to simple hematomas. This makes physical examination less reliable for diagnosing IEA pseudoaneurysm. In our patient, there were no bruits or pulsations in the physical examination. This shows the importance of considering IEA pseudoaneurysm as a differential diagnosis in any patient with a tender mass in the abdomen, especially after invasive procedures [9]. The CT scan is usually the imaging method of choice. CEUS and color Doppler US are also useful in detecting false aneurysms. Performance with CEUS, as in other image-guided procedures, depends on both the operator’s technical skill and the knowledge embedded in the imaging technology, available tools, and existing protocols. The purpose of this article is also to emphasize the role of CEUS in the correct evaluation of cases like this one [10–12]. Various treatment options have been reported, including open surgical ligation, ultrasonography-guided percutaneous thrombin, coil embolization, and ultrasonography-guided compression [13, 14].
Conclusion
The incidence of iatrogenic IEA pseudoaneurysms appears to be increasing. Awareness of this rare complication is of clinical importance to avoid excessive morbidity of affected individuals. In addition, ultrasonography plays an important role in the diagnosis, the management, and the follow-up care of pseudoaneurysms.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and its late amendments. Additional informed consented was obtained from all patients for which identifying information is not included in this article.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rao MP, Vatsala S, et al. Study of the course of inferior epigastric artery with reference to laparoscopic portal. J Minim Access Surg. 2013;9(4):154–158. doi: 10.4103/0972-9941.118826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong C and Merkur H (2015) Inferior epigastric artery: surface anatomy, prevention and management of injury December Australian and New Zealand Journal of Obstetrics and Gynaecology 56(2):137–141. doi: 10.1111/ajo.12426 [DOI] [PubMed]
- 3.Tulsyan N, Kashyap VS, Greenberg RK, et al. The endovascular rmanagement of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2007;45:276–283. doi: 10.1016/j.jvs.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 4.Obara H, et al. Current management strategies for visceral artery aneurysms: an overview. Surg Today. 2020;50(1):38–49. doi: 10.1007/s00595-019-01898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pulli R, Dorigo W, Troisi N, Pratesi G, Innocenti AA, Pratesi C, et al. Surgical treatment of visceral artery aneurysms: a 25-year experience. J Vasc Surg. 2008;48:334–442. doi: 10.1016/j.jvs.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 6.Chiesa R, Astore D, Guzzo G, Frigerio S, Tshomba Y, Castellano R, et al. Visceral artery aneurysms. Ann Vasc Surg. 2005;19:42–48. doi: 10.1007/s10016-004-0150-2. [DOI] [PubMed] [Google Scholar]
- 7.Sessa C, Tinelli G, Porcu P, Aubert A, Thony F, Magne JL. Treatment of visceral artery aneurysms: description of a retrospective series of 42 aneurysms in 34 patients. Ann Vasc Surg. 2004;18(6):695–703. doi: 10.1007/s10016-004-0112-8. [DOI] [PubMed] [Google Scholar]
- 8.Huang YK, Hsieh HC, Tsai FC, Chang SH, Lu MS, Ko PJ, et al. Visceral artery aneurysm: risk factor analysis and therapeutic opinion. Eur J Vasc Endovasc Surg. 2007;33:293–301. doi: 10.1016/j.ejvs.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara D, Esposito F, Blasio R, et al. Role of color Doppler ultrasound in the early diagnosis of a major complication after percutaneous renal biopsy: two case reports. J Ultrasound. 2018;21(4):343–349. doi: 10.1007/s40477-018-0326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantisani V, David E, Ferrari D, Fanelli F, Di Marzo L, Catalano C, et al. Color doppler ultrasound with superb microvascular imaging compared to contrastenhanced ultrasound and computed tomography angiography to identify and classify endoleaks in patients undergoing EVAR. Ann Vasc Surg. 2017;40:136–145. doi: 10.1016/j.avsg.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 11.Cantisani V, Grazhdani H, Clevert DA, Iezzi R, Aiani L, Martegani A, et al. EVAR: Benefits of CEUS for monitoring stent-graft status. Eur J Radiol. 2015;84(9):1658–1665. doi: 10.1016/j.ejrad.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 12.David E, Cantisani V, Grazhdani H, et al. What is the role of contrast-enhanced ultrasound in the evaluation of the endoleak of aortic endoprostheses? A comparison between CEUS and CT on a widespread scale. J Ultrasound. 2016;19(4):281–287. doi: 10.1007/s40477-016-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laganà D, Carrafiello G, Mangini M, Dionigi G, Caronno R, Castelli P, et al. Multimodal approach to endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Eur J Radiol. 2006;59:104–111. doi: 10.1016/j.ejrad.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Gabelman A, Gorich J, Merkle EM. Endovascular treatment of visceral artery aneurysms. J Endovasc Ther. 2002;9:38–47. doi: 10.1177/152660280200900108. [DOI] [PubMed] [Google Scholar]