Abstract
Purpose
Local therapy is the preferred option of treatment for most cutaneous leishmaniasis (CL); however, local therapy could be challenging, depth and size of the skin lesions are not always clinically evident and treatment response evaluation could occasionally be misleading. High frequency ultrasound is a non-invasive imaging tool which allows initial depth assessment ultrasound-guided infiltrations and ultrasound monitoring until resolution.
Methods
We present two cases of CL treated with ultrasound-guided infiltrations and ultrasound monitoring until resolution.
Results
Ultrasound imaging allowed a more accurate diagnosis of CL, defining more precisely the depth and size of the skin lesions. During follow-up, progressive decrease in dermal involvement, marked attenuation of the echogenicity of subcutaneous cellular tissue and a decrease in vascularization in the color Doppler mode was observed, which aided in evaluation of treatment response. Hypodermal inflammation observed through sonography was addressed with image-guided infiltration.
Conclusion
We would like to highlight the usefulness of skin ultrasound (both B-mode and color Doppler mode) in the diagnosis, depth assessment, imaging guided treatment, and follow-up in CL.
Keywords: Leishmaniasis, Cutaneous leishmaniasis, Dermatologic ultrasound, Skin ultrasound
Introduction
Cutaneous leishmaniasis (CL) is the most common clinical form of leishmaniasis in the world [1]. This parasitic infection causes chronic skin lesions, typically on sun-exposed areas and usually affecting the facial area and extremities. These lesions can later result in unsightly scarring, especially when the face is involved [2, 3]. Local therapies, such as intralesional injections of pentavalent antimonial drugs, are preferred for localized CL [2]. Treatment responses are evaluated weekly or monthly through clinical examination, which could occasionally be misleading [1]. High-frequency ultrasound is a noninvasive imaging tool that represents tissues with high definition and elevated clinical utility [4, 5]. We present two cases of CL treated with ultrasound-guided infiltrations and ultrasound monitoring until resolution.
Clinical cases
Case 1
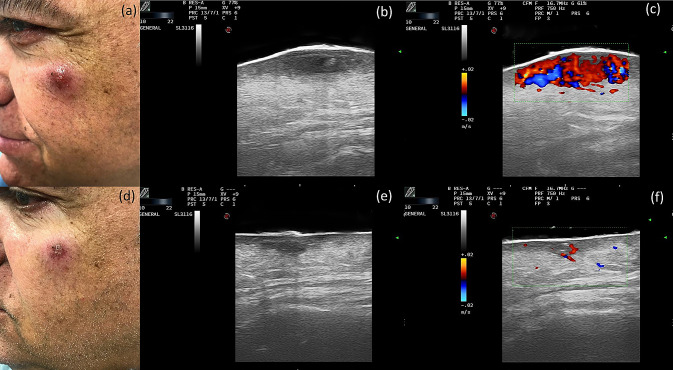
A 61-year-old male was referred to our dermatology department because of a 3-month-old lesion on the upper left cheek. The lesion was initially treated with oral cloxacillin with no improvement. On physical examination, a poorly defined erythematous–violaceous indurated papule with peripheral erythema and a crusty center was observed (Fig. 1a). We performed both B mode and color Doppler scanning imaging, using a lineal probe with a variable frequency of 10–22 MHz. The probe was attached to MyLab Class C equipment (Esaote, Genova, Italy) with a pulse repetition frequency of 1.2 kHz and a color Doppler gain immediately before a flare artifact. In the grayscale image, we could observe in the dermis a predominantly hypoechoic oval lesion with a maximum diameter of 10.8 mm and well-defined intralesional hyperechoic areas. Ill-defined hyperechoic lobular involvement of the hypodermis was also observed (Fig. 1b). Doppler mode imaging showed abundant intralesional vascularization (Fig. 1c). A histological study revealed a chronic non-necrotizing granulomatous lymphohistiocytic dermatitis, and a DNA amplification study (PCR) was positive for Leishmania infantum. Weekly intralesional ultrasound-guided infiltrations of meglumine antimoniate were initiated, both in the dermis and in the hypodermis, with the inflammation assessed by ultrasound imaging. After 4 weeks, both clinical (Fig. 1d) and ultrasound improvements were observed, with a marked reduction in diameter (from 10 to 6 mm), a lower echogenicity of the subcutaneous cellular tissue (Fig. 1e), and a decrease in Doppler flow (Fig. 1f). The lesion resolved after 8 weeks of treatment.
Fig. 1.
Clinical Case 1. Top row: before treatment. a Clinical photograph of a case of cutaneous leishmaniasis with a lesion in the upper cheek. b Grayscale ultrasound: a hypoechoic oval lesion in the dermis with intralesional hyperechoic areas and a lobular hyperechoic involvement of the hypodermis. c Color Doppler: increased intralesional vascularity. Bottom row: after treatment. d Clinical image after 4 weeks of treatment. e Grayscale ultrasound: a decrease in the diameter of the lesion with little involvement of the hypodermis. f Color Doppler: decreased intralesional vascularity
Case 2
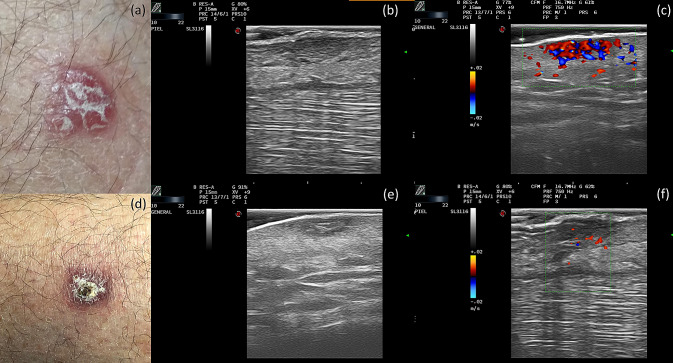
A 55-year-old male with no previous medical history was referred to our dermatology department for further investigation of a 5-month-old lesion in the pretibial area with progressive growth and no response to topical mupirocin. On physical examination, a well-defined, subtly infiltrated erythematous plaque with yellowish desquamation was found (Fig. 2a). The same exploration techniques and parameters, for both B mode and color Doppler imaging, were used as in the previous case. B mode imaging showed a dermal, hypoechogenic oval lesion, with some hyperechogenic areas and tracts inside, whereas the hypodermis showed no involvement (Fig. 2b). Color Doppler imaging showed increased intralesional vascularization (Fig. 2c). A histological study revealed a lymphohistiocytic infiltrate affecting the superficial and deep dermis. Multiple intracytoplasmic microorganisms were observed with PAS staining, all consistent with CL. Treatment was initiated with monthly intralesional ultrasound-guided infiltrations of meglumine antimoniate at the dermal level, with the inflammation assessed by ultrasound imaging. After one year, both clinical and ultrasound improvement was observed, with marked reductions in the diameter of the lesion (Fig. 2d), in dermal echogenicity (Fig. 2e), and in Doppler flow (Fig. 2f).
Fig. 2.
Clinical Case 2. Top row: before treatment. a Clinical photograph of a case of cutaneous leishmaniasis with a lesion in the pretibial area. b Grayscale ultrasound: a hypoechoic oval lesion in the dermis with intralesional hyperechoic areas and tracts. c Color Doppler: increased intralesional vascularity. Bottom row: after treatment. d Clinical image after 1 year of treatment. e Grayscale ultrasound: a decrease in the diameter of the lesion. f Color Doppler: decreased intralesional vascularity
Discussion
Several published studies support the use of skin ultrasound as a complementary technique for the diagnosis and treatment of skin lesions [4]. Regarding CL, ultrasound imaging has already been used in complex or atypical cases [6], and its potential for monitoring treatment responses has been raised [5, 6]. Rojas Mora et al. [6] published two cases of CL with ultrasound descriptions. They found diffuse thickening of the dermis in the first case, a poorly defined image of the superficial dermis in the second case, and an increase in Doppler flow in both cases. More recently, a study by Saavedra et al. [5] evaluated the diagnostic ultrasound findings in a series of 19 patients with CL. They found that all lesions affected both the dermis and the hypodermis. The most frequent dermal findings were irregular dermal thickening and decreased echogenicity. All patients also presented ultrasound signs of panniculitis, which showed a thickened, hyperechogenic, and heterogeneous hypodermis, with a predominant septal rather than lobular involvement. Doppler mode imaging showed a peripheral increase in vascularization in some cases. Our diagnostic findings showed a hypoechoic band in the dermis and hyperechoic lobular involvement of the hypodermis in the first case and a hypoechoic oval lesion in the dermis with intralesional hyperechoic areas and tracts in the second case, while both cases also presented intense intralesional vascularization on color Doppler images (Fig. 1). Ultrasound changes associated with clinical responses to the treatment were a progressive decrease in dermal involvement, a marked attenuation of the echogenicity of subcutaneous cellular tissue, and a decrease in vascularization in the color Doppler mode (Figs. 1, 2, bottom rows).
Currently, clinical management of CL is based on a hypothetical location of CL in the epidermis and dermis [5]. However, hypodermal involvement has been found in histological studies and is considered an important characteristic for differential diagnosis with other pathologies [1]. Moreover, local therapy could be challenging, as hypodermal inflammation might not be correctly addressed due to superficial infiltrations [5]. Ultrasound imaging allows a more accurate diagnosis of CL, defining more precisely the depth and size of the skin lesions. Furthermore, it allows the image-guided infiltration of drugs and improves follow-up treatments [7].
Conclusion
In two case reports, we highlight the usefulness of skin ultrasound (both B mode and color Doppler mode) in the diagnosis, depth assessment, imaging-guided treatment, and follow-up of CL.
Author contributions
All authors have contributed significantly, and all authors are in agreement with the content of this manuscript, believing that the manuscript represents honest work.
Funding
The authors declare no funding was received for this study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
We also state consent to reproduce previously published data, illustrations, report data about identifiable persons, or to acknowledging a name for their contributions.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH) Am J Trop Med Hyg. 2017;96(1):24–45. doi: 10.4269/ajtmh.16-84256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am. 2019;33(1):101–117. doi: 10.1016/j.idc.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Bennis I, De Brouwere V, Belrhiti Z, Sahibi H, Boelaert M. Psychosocial burden of localised cutaneous Leishmaniasis: a scoping review. BMC Public Health. 2018;18(1):358. doi: 10.1186/s12889-018-5260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harland CC, Bamber JC, Gusterson BA, Mortimer PS. High frequency, high resolution B-scan ultrasound in the assessment of skin tumours. Br J Dermatol. 1993;128(5):525–532. doi: 10.1111/j.1365-2133.1993.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 5.Saavedra AC, Valencia BM, Tueros P, Wortsman X, Llanos-Cuentas A, Lavarello RJ. Ultrasonographic characteristics of cutaneous leishmaniasis. J Eur Acad Dermatol Venereol. 2020;34(4):e193–e195. doi: 10.1111/jdv.16111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rojas Mora E, Garrido Ríos A, Echeverría García B, Borbujo J. An unusual presentation of cutaneous leishmaniasis: the role of skin ultrasound. Actas Dermo-Sifiliográficas Engl Ed. 2019;110(2):171–174. doi: 10.1016/j.ad.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Catalano O, Varelli C, Sbordone C, Corvino A, De Rosa D, Vallone G, et al. A bump: what to do next? Ultrasound imaging of superficial soft-tissue palpable lesions. J Ultrasound. 2020;23(3):287–300. doi: 10.1007/s40477-019-00415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]