Abstract
Purpose
The aim of this study was to investigate the efficacy of shear wave elastography (SWE) in the diagnosis of perforating vein insufficiency, and to determine the applicability of these measurements.
Methods
A total of 140 symptomatic patients with a total of 280 lower extremities were investigated. All patients presented with venous insufficiency (VI) symptoms, and received Doppler ultrasound assessment to determine VI and SWE measurements. The SWE values were measured in the adjacent perivenous tissue of the largest Cockett’s perforating vein (PV) of both lower extremities, at the level where they pass the fascia. The Cockett’s PV diameter and the presence of reflux in Cockett’s PV and the great saphenous vein were compared with SWE values in perivenous tissue of PVs.
Results
The SWE values of the perforating vein insufficiency group were significantly higher than those of the normal PV without insufficiency group (P < 0.001). A significant and positive relation was seen between increased PV diameter and SWE values (P < 0.001) and there was a significant relationship between the presence of perforating vein insufficiency and increase in PV diameter. A statistically significant increase was detected in SWE values for the PV for those with reflux in the great saphenous vein (P < 0.001). The best cut-off values that can be used to detect perforating vein insufficiency were found 34.600 for kPa and 3.375 for m/s.
Conclusion
SWE may be used effectively in addition to conventional Doppler ultrasound examination in diagnosing and following perforating vein insufficiency.
Keywords: Doppler ultrasound, Perforating venous insufficiency, Shear wave elastography, Venous insufficiency
Introduction
Perforating veins (PVs) receive blood from the superficial venous system and transmit it to the deep venous system by penetrating the muscle fascia. PVs contain valves that block reverse blood flow [1–3]. Perforating venous insufficiency is a pathology of valvular incompetence that affects the perforating venous system by causing increased venous hydrostatic pressure [4]. Distention of the PV because of elevated hydrostatic pressure can cause the vein to have distention and overfilling, so that the leaflets do not close as expected [4]. Perforating vein insufficiency may play a critical role in varicose veins and superficial vein insufficiency by causing venous hypertension. If the valves of PVs are damaged, the blood will be pooled in the superficial venous system due to the high pressure in the deep venous system, resulting in impaired function of the superficial veins and the occurrence of varicose veins [3]. Therefore, investigating the presence of perforating vein insufficiency is very important in the management of varicose veins and superficial vein insufficiency [3]. According to many authors, superficial vein insufficiency treatment combined with treatment of PVs with insufficiency is the best method [3]. However, there is still no clear method to determine which PVs should be included in the treatment to achieve better clinical results in patients with superficial vein insufficiency.
An increase in venous hydrostatic pressure causes fluid and blood products to leak into the perivenous soft tissue leading to an increase in perivenous soft tissue stiffness, which can be shown by elevated shear wave elastography (SWE) values [5]. SWE imaging technology is a real-time, noninvasive form of sonoelastography [6, 7]. The SWE technique allows for quantitative assessment [meters per second (m/s) and/or kilopascal (kPa)] of tissues according to their stiffness, providing data on histologic changes in tissues and assisting in differential diagnosis [8–16].
In the present research, the difference in perivenous tissue stiffness was investigated by comparing the quantitative SWE value of the lower extremities (LE) in patients who had perforating vein insufficiency and normal PV without insufficiency, and examining the applicability of the measurements. As far as we are concerned, the present study is the first one showing the feasibility of SWE for perforating vein insufficiency in the literature.
Materials and methods
After being approved by the local research ethics committee, this study was conducted at our institution between November 2018 and February 2020. All patients were informed before the US and elastography examination, and written informed consent was obtained. All patients presented with at least one venous insufficiency (VI) symptom such as edema, pain, ulcer, cramps, discoloration in ankles, tension in legs, itching, or millimetric-prominent varicose veins in LE. Patients with thrombophlebitis and deep venous thrombosis (eight patients), elderly patients who could not stand, patients who could not cooperate in the Valsalva maneuver (ten patients), and those who had endovascular treatment or surgery for VI (seven patients) were excluded. A total of 140 patients with 280 LEs were included in the current study.
The patients’ body mass indices (BMIs) were determined with their heights and weights before the Doppler examination. Each patient was examined in standard conditions. The LEs were evaluated with B-mode imaging, color, spectral Doppler evaluation, and SWE. Doppler US and SWE examinations were carried out by a radiologist who had 13 years’ experience in Doppler US and 5 years’ experience in SWE. Doppler US and SWE examinations were carried out with a high-frequency (14-MHz) linear array transducer, the Canon Aplio 500 (Canon Medical System Corporation, Tokyo, Japan).
Doppler US examination was begun in supine position to exclude thrombosis in superficial and deep veins. Then, in upright position, patients were evaluated for VI. The valvular competence of the veins was assessed at rest with the Valsalva maneuver and augmentation of the distal parts of the calf. As a criterion for significant reflux, the presence of retrograde flow with more than 0.5 s on spectral Doppler imaging was employed [3]. The diameter and reflux in the GSV were recorded. The presence of edema was investigated, especially in the crural region with B-mode examination, and those with edema were noted.
Following the Doppler US examination of LEs, SWE examinations of the PVs were carried out. In Doppler US and SWE examination, the evaluated leg was relaxed and the body weight was supported on opposite leg to eliminate increased SWE values secondary to muscle contractions. In SWE examination, pressure was not applied to the probe, which was positioned at 90° to the skin with gel to prevent erroneous pressure measurement. Each patient was also asked not to make Valsalva maneuvers and to hold their breath for 5 s, breathing spontaneously in SWE examination, to suspend venous return temporarily during capture of SWE images and eliminate changes that could occur in venous hydrostatic pressure in deep inspiration/expiration.
For standardization purposes, measurements were performed in the largest Cockett’s PV, at the level of the middle cruris. The diameter, reflux, and SWE [speed (shear velocity) mode (m/s) and elasticity mode (kPa)] measurements were performed in Cockett’s PV of both LEs, at the level where they pass the fascia. Quantitative elasticity values were measured in propagation mode, on magnified view with standard 3 mm wide round ROI, in the adjacent perivenous tissue of the PV (Fig. 1). SWE was obtained as three separate images of each Cocketts’ PV, which were analyzed quantitatively. The mean value of these three measurements was used. To evaluate VI and measure SWE, the mean time per LE examination (Doppler US and SWE) was approximately 20 min.
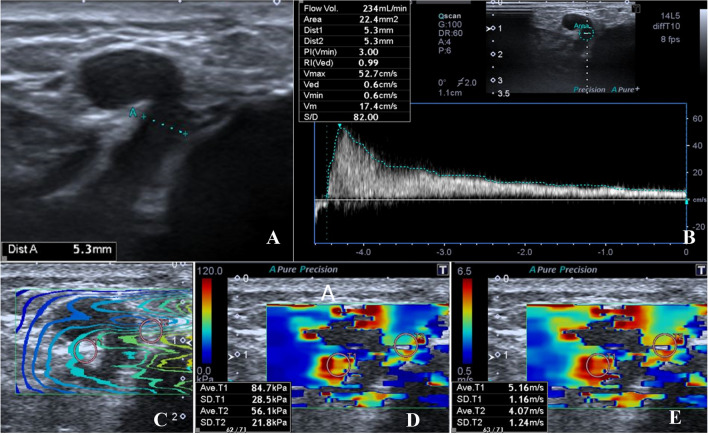
Fig. 1.
a–e 43-year-old woman with perforating vein insufficiency. The diameter, reflux and SWE measurements were performed in Cockett’s PV. The diameter of Cockett’s PV was 5.3 mm at the level where it passed the fascia (a). There was significant reflux in Cockett’s PV; the volume of reflux was 234 mL/min (b). The images were acquired and saved with three types of imaging options [propagation mode (c), elasticity mode (kPa) (d) speed mode (m/s) (e)]. Quantitative elasticity values were measured in propagation mode on magnified view with round ROI, in the adjacent perivenous tissue of the PV (c). The mean quantitative elasticity values were measured as 84.7 + 56.1/2 = 70.4 kPa (d) and 5.16 + 4.07/2 = 4.615 m/s (e)
The SWE values obtained from the PV with insufficiency and the normal PV without insufficiency groups were compared. The best cut-off values were investigated in predicting perforating vein insufficiency. The PV, GSV diameter and presence of significant reflux were compared with SWE values of Cockett’s PV. The association between the occurrence of varicose veins in the crural region and SWE values in the adjacent perivenous PV tissue was examined. Comparisons were made for increased age, BMI, and SWE.
The SPSS package program (Statistical Package for Social Sciences, version 15, SPSS Inc., Chicago, Illinois, USA) was used for statistical analyses. Normal distribution of continuous variables was tested with the Kolmogorov–Smirnov test. First, definitive statistics of the variables were assessed. Descriptive statistics were expressed as mean, standard deviation, frequency, and percentile. The χ2 and Student’s t test were used for statistical analyses. A P value of less than 0.05 was taken as significant. Receiver operating characteristic (ROC) curves were used for determining the best cut-off value. The sensitivity and specificity of VI in PV were calculated based on SWE measurements and diameters of PV.
Results
Among 140 participants, 280 LEs (140 right [50%] and 140 left [50%]; 150 limbs [53.6%] for females, 130 limbs [46.4%] for males) venous systems were examined. The patients were 18–66 years of age (mean, 36.49 ± 10.48 years). The mean age of the females was 35.45 ± 8.81 years, and that of the males was 37.69 ± 12.04 years.
Cockett’s perforating vein insufficiency was found in 53.6% (n = 150, 82 female, 68 male) of LEs. The mean age of the perforating vein insufficiency group was 35.66 ± 9.31, and that of the without perforating vein insufficiency group was 37.44 ± 11.65 years. The BMI of the perforating vein insufficiency group was 22.04 ± 3.86 kg/m2, and that of the without perforating vein insufficiency group was 20.88 ± 3.81 kg/m2. The difference in age between the two groups was not significant (P = 0.157), sex (P = 0.720) and BMI (P = 0.12).
The mean diameter was 4.088 ± 0.973 mm in Cocketts’ PVs in which insufficiency was observed. The PVs that did not have insufficiency had a mean diameter of 3.069 ± 0.751 mm. A significant relation was detected between the increase in PV diameter and the presence of perforating vein insufficiency (P < 0.001). In the PV with insufficiency group, the mean SWE value was 51.79 ± 17.24 kPa and 4.00 ± 0.73 m/s. In the PV without insufficiency group, the mean SWE value was 23.59 ± 12.47 kPa and 2.66 ± 0.68 m/s. The SWE values in the PV with insufficiency group were significantly higher (P < 0.001) (Table 1). The SWE images of a patient without perforating venous insufficiency and with perforating venous insufficiency are given in Figs. 2 and 3, respectively. SWE numerical values were higher in the PV with larger diameters (Fig. 4) and a significant positive correlation was observed between PV diameters and SWE values of the PV (P < 0.001, both for kPa and m/s), for m/s Pearson correlation coefficient 0.659, for kPa Pearson correlation coefficient 0.680.
Table 1.
The mean diameter and SWE values of perforating vein with and without insuffiency
| PV with insuffiency | PV without insuffiency | Statistical analysis (*) | |
|---|---|---|---|
| Mean PV diameter (mm) | 4.088 ± 0.973 | 3.069 ± 0.751 | P < 0.001 |
| Mean SWE value (m/s) | 4.00 ± 0.73 | 2.66 ± 0.68 | P < 0.001 |
| Mean SWE value (kPa) | 51.79 ± 17.24 | 23.59 ± 12.47 | P < 0.001 |
*p < 0.005 was considered to indicate a significant difference
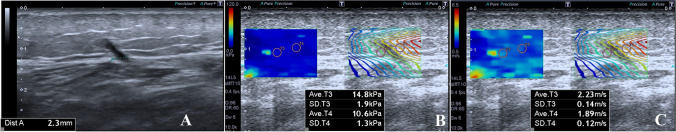
Fig. 2.
a–c 32-year-old woman without perforating vein insufficiency. The diameter of Cockett’s PV was 2.3 mm at the level where it passed the fascia (a). The mean quantitative elasticity values were measured as 14.8 + 10.6/2 = 12.7 kPa (b) and 2.23 + 1.89/2 = 2.060 m/s (c)
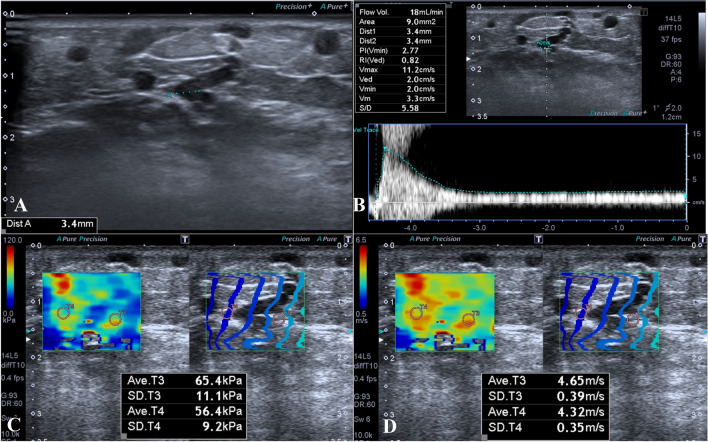
Fig. 3.
a–d 38-year-old man with perforating vein insufficiency. The diameter of PV was 3.4 mm, and there were varicose veins related with PV (a). There was significant reflux in PV; the volume of reflux was 18 mL/min. The mean quantitative elasticity values were measured as 65.4 + 56.4/2 = 60.9 kPa (c) and 4.65 + 4.32/2 = 4.485 m/s (d)
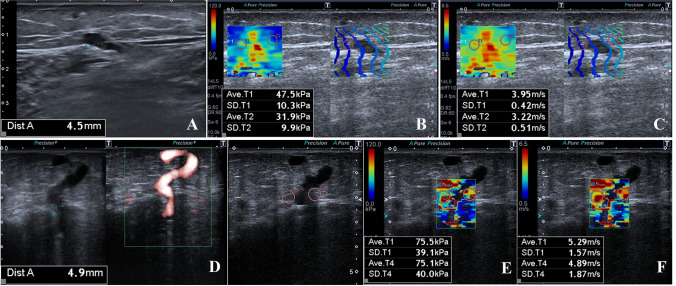
Fig. 4.
a–g 34-year-old woman with perforating vein insufficiency (a–c). The diameter of PV was 4.5 mm (a). The mean quantitative elasticity values were measured as 47.5 + 31.9/2 = 39.7 kPa (b) and 3.95 + 3.22/2 = 3.585 m/s (c). 39-year-old woman with perforating vein insufficiency (d–g). The diameter of PV was 4.9 mm (d). The mean quantitative elasticity values were measured as 75.5 + 75.1/2 = 75.3 kPa (e) and 5.29 + 4.89/2 = 5.09 m/s (f). SWE numerical values were higher in the PV with the larger diameter
In the PV with insufficiency, the mean flow volume of reflux was 31.24 mL/min. A significant increase was detected in the kPa and m/s values with increased reflux severity in PV (P < 0.001).
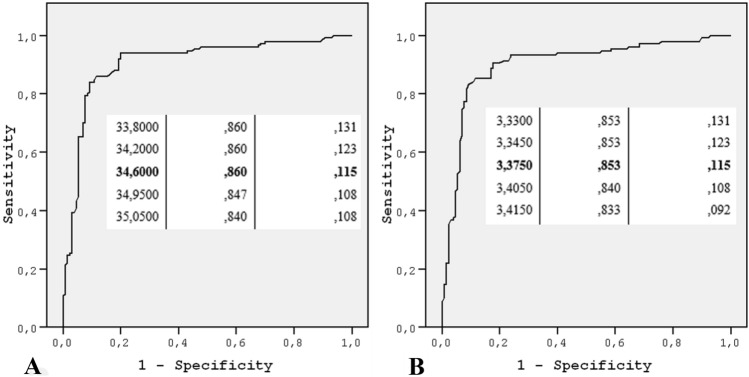
Cut-off values of SWE for insufficiency in PV with numeric values of sensitivity and specificity are given in Table 2. The ROC curve analyses of SWE values of PV and insufficiency in PV are given in Fig. 5.
Table 2.
Cut-off value of SWE for insufficiency in PV with sensitivity and specificity
| Cut-off value | Sensitivity (%) | Specificity(%) | |
|---|---|---|---|
| m/s | 3,375 | 85.3 | 88.5 |
| kPa | 34,600 | 86.0 | 88.5 |
Fig. 5.
A ROC curve analysis of the SWE value for kPa (a), for m/s (b) of PV and insufficiency in PV and the best cut-off values. The best cut-off values that can be used to detect perforating vein insufficiency were found to be 34.600 for kPa (a) and 3.375 for m/s (b)
GSV insufficiency was present in 61.4% (n = 172) of LEs. GSVs with reflux had a mean diameter of 6.78 ± 1.59 mm, while GSVs without reflux had a mean diameter of 4.33 ± 0.45 mm at the widest segment in the crural region. A significant relation was observed between GSV diameter and the presence of VI (P < 0.001). Perforating vein insufficiency was observed in 80.23% (n = 138) of LEs with GSV insufficiency. There was a significant relationship between GSV and perforating vein insufficiency (P < 0.001). Values of SWE in PV were higher at significant levels in LEs that had reflux on Doppler examination in the GSV (mean SWE value 49.59 ± 18.46 for kPa, 3.90 ± 0.81 for m/s) than in those that did not (mean SWE value 21.35 ± 8.95 for kPa, 2.54 ± 0.55 for m/s) (P < 0.001). A positive and significant correlation was observed between GSV diameter and SWE values of the PV (P < 0.001, for m/s Pearson correlation coefficient 0.592, for kPa Pearson correlation coefficient 0.602).
Varicose veins related to PVs in the crural region were found in 64.28% of LEs (n: 180). Perforating vein associated with crural varicose vein insufficiency was observed in 51.42% of LEs (n: 144). There was a significant relation between perforating vein insufficiency and associated varicose vein insufficiency (P < 0.001). Values of SWE in PV were higher at significant levels in LEs that had reflux on Doppler examination in varicose veins (mean SWE value 48.99 ± 17.88 for kPa, 3.88 ± 0.78 for m/s) than in the without insufficiency in varicose veins group (mean SWE value 20.17 ± 9.59 for kPa, 2.48 ± 0.56 for m/s) (P < 0.001).
Edema in the crural region was found in 30.7% (n = 86) of LEs. There was a significant relation between edema and perforating vein insufficiency (P < 0.001). SWE values of PV were higher at significant levels in LEs with edema (P < 0.001).
Discussion
In this study, we evaluated the role and importance of SWE in the evaluation of perforating vein insufficiency. Adjacent perivenous tissue of Cocketts’ PV was evaluated for differences in hydrostatic pressure with SWE. We found a statistically significant and positive relation between PV diameter and SWE value. SWE values of PV with insufficiency were significantly higher. Thus, we found that SWE can also provide important data in the evaluation of perforating vein insufficiency.
We found a significant relation between PV diameter and perforating vein insufficiency. In another study, there was also a significant relation between the increase in PV diameter and the presence of perforating vein insufficiency [3]. However, in that study, the author stated that reflux could not be monitored even though the PV diameter was increased, though reflux could be monitored in small-diameter PVs [3]. For these reasons, size alone cannot be the criteria for perforating vein insufficiency, and symptoms may occur before dilatation of veins. Diagnosis of perforating vein insufficiency is based on the presence of reflux in the lumen of the vein [3]. Reflux is seen on LE Doppler US after increased hydrostatic pressure in the vein lumen. While the vein diameter is enlarged and patients present with high venous hydrostatic pressure, reflux cannot be seen on Doppler imaging [5, 17]. An increase in venous hydrostatic pressure causes fluid and blood products to leak into the perivenous soft tissues, leading to an increase in perivenous soft tissue stiffness and edema [5]. In this symptomatic group of patients with high venous hydrostatic pressure but without enlarged vein diameter, who do not have insufficiency on Doppler US examination, but have complaints suggestive of perforating vein insufficiency, increases in perivenous tissue stiffness due to increased venous hydrostatic pressure can be shown by high SWE values, and perforating vein insufficiency can be diagnosed at an early stage. Early conservative treatment may avoid the perforating vein insufficiency and reduce perforating vein insufficiency complications. In our study, a significant relation was detected between presence of edema in the crural region and perforating vein insufficiency. We also obtained significantly higher SWE values in LEs with edema. These findings support the idea that the stiffness caused by edema, which may develop secondary to increased hydrostatic pressure, can be evaluated with SWE. When cut-off values of 3.375 m/s and 34.600 kPa were used for VI in PV, the sensitivity and specificity were more than 85%.
PV evaluation must be a part of the LE venous system Doppler examination in VI, since perforating vein insufficiency is very common in combined VI, and incompetent PVs play a significant role in chronic VI [3, 18, 19]. A significant relationship between perforating vein insufficiency and PV associated with varicose veins and superficial VI was found [3, 20, 21]. In our study, perforating vein insufficiency was observed in about 80% of LEs with GSV insufficiency. We also found a significant relationship between GSV and PV insufficiency. SWE was significantly higher in PV in LEs with reflux in GSV and a significant positive correlation was observed between GSV diameter and SWE values of the PV. PVs associated with varicose veins especially need to be evaluated in terms of reflux [3]. We found a significant relation between perforating vein insufficiency and PV associated with varicose veins (P < 0.001). SWE was significantly higher in the PV with insufficiency group than in the without PV insufficiency group. A significant positive correlation was detected between PV diameters and SWE values. These findings show that the SWE values of the PV also provide information on the increase in diameter and the presence of insufficiency in the PV and superficial venous system, as well as the presence of insufficiency in varicose veins that are associated with PVs.
Despite the great number of studies, the mechanism of valvular dysfunction in incompetent PV is not yet fully understood [22]. One of the proposed mechanisms of the incompetence is enlargement of the diameter of the PV because of increased hydrostatic pressure in superficial VI [23]. Based on this hypothesis, elimination of superficial VI reduces hydrostatic pressure in the incompetent PV, rendering it competent. This type of incompetent PV may not require treatment if superficial VI is treated. Therefore, some authors recommend reevaluating clinical symptoms and PV after treatment of the superficial VI. If the patient still has clinical symptoms and has perforating vein insufficiency, then they should be treated for incompetent PV [23, 24]. Longer term follow-up of this population is required. The other proposed mechanism of incompetence is dysfunction due to irreversible valvular damage [22]. This type of incompetent PV might be associated with recurrence of varicose veins after treatment of the superficial VI. Routine treatment of perforating vein insufficiency is recommended at the same time as treatment of superficial VI by some authors, due to the risk of recurrence [22–25]. Therefore, preoperative evaluation of patients with a combination of superficial and perforating VI, and identification of perforating vein insufficiency as reversibly or irreversibly incompetent, would facilitate more effective treatment, but this is difficult. There is not yet a gold-standard imaging technique that indicates which incompetent PVs identified preoperatively should undergo specific treatment, and which PV with insufficiency need to be treated at the same time as the treatment of superficial VI, or which may not require treatment if superficial VI is treated. However, we think SWE is a promising imaging method for this use. The changes in perivenous hydrostatic pressure of PVs can be monitored by SWE before and after treatment of superficial VI. We found a statistically significant increase in SWE values with increased reflux volume in PV. Flow volume of reflux was taken as severity criterion for VI [5, 26]. This finding supports the idea that severity of perforating vein insufficiency and treatment response of superficial VI can be monitoring safely with SWE before and after treatment of superficial VI. Future studies with more patients are necessary to obtain cut-off SWE values that may indicate which PVs should undergo specific treatment and which should be treated at the same time with the treatment of superficial VI.
The present study had some limitations. All examinations and measurements were carried out by an experienced radiologist, but interobserver variation could not be assessed because of long examination times. The study was performed with a limited number of patients and we did not follow-up with the population postoperatively or during the conservative treatment process. The etiology of VI and varicose veins was not questioned. We did not ask about how long patients had suffered from varicose veins or VI disease. Augmentation maneuvers to investigate the presence of VI were performed by manually tightening the distal part of the kruris; we did not use tourniquets. SWE measurements were performed in the adjacent perivenous fat tissues of PV, and as the unmeasured pressure increase within the PV lumen also affects the wall and adjacent perivenous fat tissues, we think that the perivenous elastography values reflect luminal pressure. The SWE measurements were made only in the largest Cocketts’ PVs to provide standardization. The elastography values for each PV at a different level could be different. However, if there were more distal or proximal PVs, the localizations would vary, and venous hydrostatic pressure would be higher at distal level, reducing the reliability of the results. To determine the standard elastography values of PVs at other levels, studies in which large PVs are grouped according to their level should be conducted in large series.
In conclusion, our study offers important details that assist in quantifying stiffness and pressure changes in perivenous tissue due to hydrostatic pressure increase in perforating vein insufficiency, using SWE. SWE can be used effectively in addition to conventional Doppler US examination to diagnose perforating vein insufficiency and to monitor the severity of perforating vein insufficiency. PV evaluation using SWE should be part of LE venous system examination in VI. Further studies are needed to confirm the diagnosis and follow-up of SWE in perforating vein insufficiency. We hope this preliminary study will inspire future studies on the use of SWE in perforating vein insufficiency, especially to find the answer to the question of which incompetent PVs should undergo specific treatment, which PVs with insufficiency need to be treated at the same time as the treatment of superficial VI, and which may not require treatment if superficial VI is treated.
Acknowledgements
We would like to thank Funda Gökgöz Durmaz for providing help in the statistical analysis of the study.
Author contributions
MSD, UK, BÖ designed and wrote the paper. MSD performed the ultrasound assessment, MSD, HU and UK analyzed the ultrasound images, MSD performed the statistical analysis. All authors read and approved the last version of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no financial or other relations that could lead to a conflict of interest.
Ethical statements
The approval of the ethics committee (the ethics committee of Selçuk University) was obtained before the initiation of the study. All the participants were previously informed about the study, and a written informed consent was signed by each participant before the US examination. All the procedures performed in the studies on humans were in line with the ethical institutional and/or national research committee standards and with the 1964 Helsinki Declaration and its amendments or comparable ethical standards.
Consent to participate
Writen informed consent was obtained from the patients.
Consent to publish
The authors affirm that human research participants provided informed consent to publish images.
Code availability
Analyses in IBM SPSS statistics were performed without syntaxes, outputs are available by request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Neer PA, Veraart JC, Neumann HA. Venae perforantes: a clinical review. Dermatol Surg. 2003;29:931–942. doi: 10.1046/j.1524-4725.2003.29273.x. [DOI] [PubMed] [Google Scholar]
- 2.Rutherford EE, Kianifard B, Cook SJ, et al. Incompetent perforating veins are associated with recurrent varicose veins. Eur J Vasc Endovasc Surg. 2001;21:458–460. doi: 10.1053/ejvs.2001.1347. [DOI] [PubMed] [Google Scholar]
- 3.Tolu I, Durmaz MS. Frequency and significance of perforating venous insufficiency in patients with chronic venous insufficiency of lower extremity. Eurasian J Med. 2018;50:99–104. doi: 10.5152/eurasianjmed.2018.18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2014;130:333–346. doi: 10.1161/CIRCULATIONAHA.113.006898. [DOI] [PubMed] [Google Scholar]
- 5.Durmaz MS, Arslan S, Baysal AN, et al. Experience of using shear wave elastography imaging in superficial venous insufficiency of the lower extremity. Ultrasound Q. 2018;34:176–182. doi: 10.1097/RUQ.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 6.Hattapoğlu S, Göya C, Hamidi C, et al. Evaluation of parathyroid lesions with point shear wave elastography. J Ultrasound Med. 2016;35:2179–2182. doi: 10.7863/ultra.15.10074. [DOI] [PubMed] [Google Scholar]
- 7.Schillizzi G, Alviti F, D’Ercole C, et al. Evaluation of plantar fasciopathy shear wave elastography: a comparison between patients and healthy subjects. J Ultrasound. 2020 doi: 10.1007/s40477-020-00474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durmaz MS, Sivri M, Sekmenli T, et al. Experience of using shear wave elastography imaging in evaluation of undescended testes in children: feasibility, reproducibility, and clinical potential. Ultrasound Q. 2016;34:206–212. doi: 10.1097/RUQ.0000000000000344. [DOI] [PubMed] [Google Scholar]
- 9.Hamidi C, Göya C, Hattapoğlu S, et al. Acoustic radiation force impulse (ARFI) imaging for the distinction between benign and malignant thyroid nodules. Radiol Med. 2015;120:579–583. doi: 10.1007/s11547-014-0495-8. [DOI] [PubMed] [Google Scholar]
- 10.Arslan S, Durmaz MS, Erdogan H, et al. Two-dimensional shear wave elastography in the assessment of salivary gland involvement in primary sjögren's syndrome. J Ultrasound Med. 2019;39:949–956. doi: 10.1002/jum.15179. [DOI] [PubMed] [Google Scholar]
- 11.Durmaz MS, Arslan S, Özbakır B, et al. Effectiveness of shear wave elastography in the diagnosis of acute pancreatitis on admission. Med Ultrason. 2018;20:278–284. doi: 10.11152/mu-1398. [DOI] [PubMed] [Google Scholar]
- 12.Erdoğan H, Durmaz MS, Özbakır B, et al. Experience of using shear wave elastography in evaluation of testicular stiffness in cases of male infertility. J Ultrasound. 2020 doi: 10.1007/s40477-020-00430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdogan H, Durmaz MS, Arslan S, et al. Shear wave elastography evaluation of testes in patients with varicocele. Ultrasound Q. 2020;36:64–68. doi: 10.1097/RUQ.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 14.Kara T, Ateş F, Durmaz MS, et al. Assessment of thyroid gland elasticity with shear-wave elastography in Hashimoto's thyroiditis patients. J Ultrasound. 2020 doi: 10.1007/s40477-020-00437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozgokce M, Batur M, Alpaslan M, et al. A comparative evaluation of cataract classifications based on shear-wave elastography and B-mode ultrasound findings. J Ultrasound. 2019;22:447–452. doi: 10.1007/s40477-019-00400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelson SM, Ruff AN, Perillat M, Kettner NW. Sonoelastography of the trunk and lower extremity muscles in a case of Duchenne muscular dystrophy. J Ultrasound. 2019 doi: 10.1007/s40477-019-00394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durmaz MS, Özbakır B, Cebeci H, et al. The cutoff value for the diameter of the saphenous vein in predicting the presence of venous insufficiency. J Turgut Ozal Med Cent. 2018;25:135–139. [Google Scholar]
- 18.Krnić A, Vucić N, Sucić Z. Correlation of perforating vein incompetence with extent of great saphenous insufficiency: cross sectional study. Croat Med J. 2005;46:245–251. [PubMed] [Google Scholar]
- 19.Al-Mulhim AS, El-Hoseiny H, Al-Mulhim FM, et al. Surgical correction of main stem reflux in the superficial venous system: does it improve the blood flow of incompetent perforating veins? World J Surg. 2003;27:793–796. doi: 10.1007/s00268-003-6751-z. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence PF, Alktaifi A, Rigberg D, et al. Endovenous ablation of incompetent perforating veins is effective treatment for recalcitrant venous ulcers. J Vasc Surg. 2011;54:737–742. doi: 10.1016/j.jvs.2011.02.068. [DOI] [PubMed] [Google Scholar]
- 21.Mendes RR, Marston WA, Farber MA, et al. Treatment of superficial and perforator venous incompetence without deep venous insufficiency: is routine perforator ligation necessary? J Vasc Surg. 2003;38:891–895. doi: 10.1016/S0741-5214(03)00933-9. [DOI] [PubMed] [Google Scholar]
- 22.De Maeseneer MG, Pichot O, Cavezzi A, et al. Duplex ultrasound investigation of the veins of the lower limbs after treatment for varicose veins-UIP consensus document. Eur J Vasc Surg. 2011;42:89–102. doi: 10.1016/j.ejvs.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Kuyumcu G, Salazar GM, Prabhakar AM, et al. Minimally invasive treatments for perforator vein insufficiency. Cardiovasc Diagn Ther. 2016;6:593–598. doi: 10.21037/cdt.2016.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klem TM, Wittens CH. Cryoperforator surgery: a new treatment of incompetent perforating veins. Vasc Endovasc Surg. 2008;42:239–242. doi: 10.1177/1538574407312655. [DOI] [PubMed] [Google Scholar]
- 25.Tenbrook JA, Lafrati MD, O’donnell TF, et al. Systematic review of outcomes after surgical management of venous disease incorporating subfascial endoscopic perforator surgery. J Vasc Surg. 2004;39:583–589. doi: 10.1016/j.jvs.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Joh JH, Park HC. The cutoff value of saphenous vein diameter to predict reflux. J Korean Surg Soc. 2013;85:169–174. doi: 10.4174/jkss.2013.85.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]