Abstract
Retinal degenerative diseases (RDDs) affecting photoreceptors (PRs) are one of the most prevalent sources of incurable blindness worldwide. Due to a lack of endogenous repair mechanisms, functional cell replacement of PRs and/or retinal pigmented epithelium (RPE) cells are among the most anticipated approaches for restoring vision in advanced RDD. Human pluripotent stem cell (hPSC) technologies have accelerated development of outer retinal cell therapies as they provide a theoretically unlimited source of donor cells. Human PSC-RPE replacement therapies have progressed rapidly, with several completed and ongoing clinical trials. Although potentially more promising, hPSC-PR replacement therapies are still in their infancy. A first-in-human trial of hPSC-derived neuroretinal transplantation has recently begun, but a number of questions regarding survival, reproducibility, functional integration, and mechanism of action remain. The discovery of biomaterial transfer between donor and PR cells has highlighted the need for rigorous safety and efficacy studies of PR replacement. In this review, we briefly discuss the history of neuroretinal and PR cell transplantation to identify remaining challenges and outline a stepwise approach to address specific pieces of the outer retinal cell replacement puzzle.
Keywords: photoreceptor, retinal organoid, transplantation, retinal degeneration, human pluripotent stem cell
Introduction
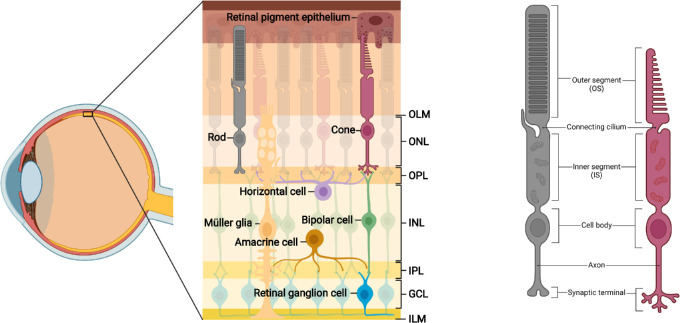
The retina is a complex tissue whose anatomy and circuitry (Fig. 1A) is predicated on the function of rod and cone photoreceptors (PRs), highly specialized neurons (Fig. 1B) that have evolved over millions of years to optimally harness light for navigating diverse environments.1,2 In healthy retinas, PRs are the initiators of visual activity; they are defined by their ability to capture light entering the eye and generate an electrical signal through a cascade of biochemical activity known as phototransduction.2 Sparking this signal is not solely enough to confer vision—PRs must also successfully relay light sensory information via synapses with retinal interneurons to begin a stepwise process of conveying visual stimuli to the brain along retinal ganglion cell (RGC) axons. The biochemical processes within PRs require extensive metabolic activity, largely mediated by their interactions with the retinal pigment epithelium (RPE).3 Together with Müller glia (MG), the RPE plays a crucial role in supporting PRs to maintain outer retinal structure, function, and homeostasis.3–5
Figure 1.
Organization and circuitry of the retina. (A) The retina contains three layers of cell bodies: the outer nuclear layer (ONL), in which rod and cone cell bodies reside; the inner nuclear layer (INL), containing horizontal cell (HC), bipolar cell (BC), amacrine cell (AC) and Müller glial (MG) cell bodies; and the ganglion cell layer (GCL) where retinal ganglion cell (RGC) somata and displaced ACs are found. PRs are supported by close apposition to the retinal pigment epithelium (RPE). The neural retina is bound apically by the outer limiting membrane (OLM) and basally by the inner limiting membrane (ILM), both formed by end-feet of the MG. PRs connect with BCs and HCs via synapses in the outer plexiform layer (OPL). The inner plexiform layer (IPL) contains signal-carrying synapses between BCs, ACs, and RGCs. (B) Rod and cone PRs display several distinct morphologic features. The outer segment (OS) contains stacked discs of photosensitive opsins for light detection. The connecting cilium facilitates trafficking between outer and inner segments (IS), the latter of which are rich in mitochondria. Extending from the cell body are axons with synaptic terminals, which interact with inner retinal neurons at triad ribbon synapses.
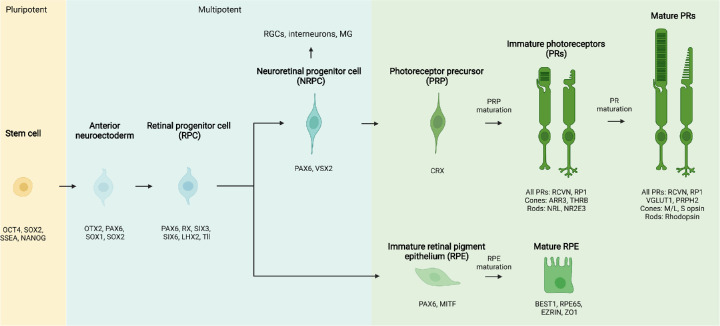
Like all retinal cells, both PRs and RPE arise from a common retinal progenitor cell (RPC) (Fig. 2); intrinsic6 and extrinsic factors work in concert to guide cells through distinct developmental stages7,8 to reach functional maturity. In outer retinal degenerative diseases (RDDs) the interdependent nature of PRs and RPE becomes a weakness; primary dysfunction in either population often causes secondary damage in the other.9–12 Regardless of the inciting cause, PR damage instigates a predictable cascade of degenerative changes within the retina,12 progressing from widespread PR malfunction to cell death, retinal remodeling, and—in the absence of successful intervention—inner retinal neurodegeneration.13 Like most neurons, human PRs are nonregenerative, and these destructive processes ultimately lead to irreversible vision loss. Retinitis pigmentosa (RP) and age-related macular degeneration (AMD) are the most common inherited and acquired outer RDDs, respectively, and collectively affect millions of individuals worldwide. Blinding outer RDDs affect an increasing proportion of the global population,14–16 and beyond being a source of visual morbidity, can cause severe emotional distress in some individuals.17,18 The estimated global economic impact of potential productivity lost due to moderate and severe visual impairment is a staggering US $411 billion annually.19 In response, the National Eye Institute launched the Audacious Goals Initiative (AGI) in 2015 to accelerate development and deployment of ocular stem cell-based therapies for incurable RDDs.20,21 Specifically, the AGI aims to restore “usable vision in humans through the regeneration of neurons and neural connections” through endogenous or exogenous replacement.20
Figure 2.
Phases of RPE and PR differentiation. Pluripotent stem cells pass through an anterior neuroectodermal stage to become multipotent retinal progenitor cells (RPCs), which are capable of producing all types of neuroretinal cells in addition to RPE. Neuroretinal progenitor cells (NRPCs) become further fate restricted over time and have the capacity to generate all neural retina cell types, including photoreceptor precursors (PRP). Over time, PRP and RPE mature to express several characteristic morphologic features. Examples of key transcription factors and defining cell markers for each stage are listed below each stage. Human PSC technologies follow these developmental pathways to reproducibly generate a variety of donor cells for replacement therapies.
Gene therapies developed for individuals across the RDD spectrum have made remarkable progress in recent years22; however, with more than 200 distinct causative genes,16 curative interventions remain out of reach for most patients. Cell therapies—the delivery of live cells to treat or cure disease—have emerged as a promising alternative (or adjunct23) to gene therapy, offering a broad-spectrum and gene-independent strategy for restoring vision. There are currently no US Food and Drug Administration (FDA)-approved cell therapy applications for retinal disease, although several approaches have reached early phases of translational research (Box 1). Cell therapies come in many varieties, but are fundamentally characterized in the context of their source, their capacity to become other types of cells,24 and their purity (Table 1). Of the many donor cell sources proposed for use in RDDs, several autologous and allogeneic cell therapies have entered clinical trials.25,26 Human pluripotent stem cells (hPSCs) have proven to be an indispensable source of cells for such therapies as they can, theoretically, self-replicate indefinitely and form virtually any type of cell. Human PSC-based therapies advancing most rapidly toward clinical translation are aimed at supporting surviving host PRs, either through neuroprotective approaches (e.g. delivery of trophic factor-secreting stem/progenitor cells) or replacement of defective RPE.25,26 Exogenous replacement of PRs has progressed comparatively slowly, as it relies upon survival and integration of a sufficient number of these complex, nonreplicative, and highly specialized sensory neurons with establishment of functional synaptic connections to host interneurons.
Box 1.
The Translational Research Continuum
| Translational research aims to maximize basic science discoveries for direct application in advancing human health (also referred to as “bench-to-bedside” research). The process of bringing a new discovery to clinical practice often takes decades, and retinal cell therapies are still in the early stages of this process. Translational research is typically classified in four phases—T1 through T4 (see Zarbin, 2020234 for further details): |
| • T1 – scientific discovery and development from preclinical studies to phase I and II clinical trials |
| • T2 – determination of efficacy in humans through phase III and IV clinical trials |
| • T3 – dissemination and implementation of therapies beyond clinical trials |
| • T4 – public health and policy-level assessment of established therapies |
| Each phase also represents a continuum of research activities. Retinal cell therapies—including RPE and PR replacement—are both currently in phase T1. RPE replacement is nearing phase T2 with several clinical trials underway, while PR replacement is largely still in preclinical development. |
Table 1.
Defining Characteristics of Donor Cell Populations
| Source | • Autologous: patient-derived• Allogeneic: donor-derived (potentially HLA-matched and/or genetically engineered) |
| Potential | • Pluripotent: capable of forming cells from all three germ layers (e.g. human ES or iPS cell)• Multipotent: capable of forming a limited range of cell types from a common lineage (e.g. retinal progenitor cell)• Unipotent: capable of forming one cell type or class (e.g. photoreceptor precursor) |
| Purity | • Heterogenous: the cell product consists of the target cell type intermixed with multiple off-target cell types• Enriched: the cell product is predominantly comprised of the target cell type• Purified: the cell product is exclusively comprised of the target cell type |
Exogenous PR replacement currently appears best poised to reach the National Eye Institute's (NEI's) audacious goal first, although several key hurdles remain as the field advances into clinical trials. With the initiation of a first-in-human safety study of hiPSC-derived neuroretinal sheets in advanced RP (RIKEN, JRCT ID jRCTa050200027),27 a critical appraisal of where the field currently stands with respect to PR replacement is warranted. This review summarizes relevant historic literature and highlights recent developments in exogenous hPSC-derived PR replacement therapies, identifying remaining challenges and emerging strategies. Therapies aimed at rescue of PRs have recently been reviewed elsewhere and, for the purposes of this review, are largely discussed in the context of principles relevant to PR replacement. Readers interested in PR rescue are directed to several excellent reviews of RPE replacement27,28 and other approaches including stem/progenitor trophic cell therapies.29 In addition, recent reviews of biomaterial transfer,30 outer retinal scaffolds,31,32 immune responses in subretinal cell therapies,33 PR replacement in rodents,34 and clinical trials of cell transplantation in RDDs25,26 may aid the reader in gaining a comprehensive understanding of the field.
The Rise of Photoreceptor Replacement Therapy
1950s to 1990s: Early Studies in Primary Cell Transplantation
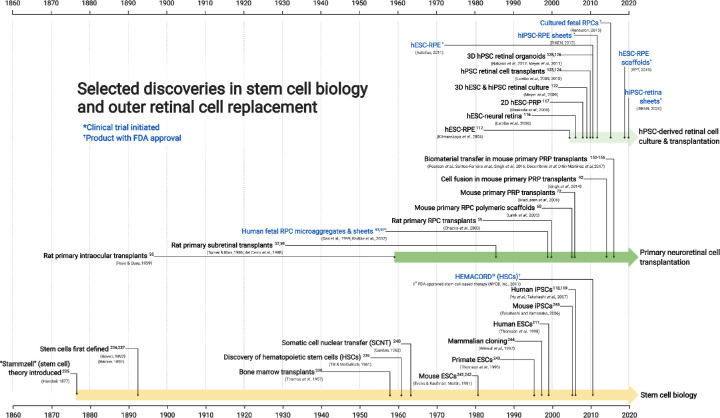
The number of published studies aimed at retinal cell replacement has increased in recent years, but the field itself is far from new. Retinal regeneration stands on the shoulders of giants in many regards, building upon more than a century of research in stem cell biology and retinal development (Fig. 3). While intraocular delivery of retinal tissue began in the late 1950s,35,36 proof-of-principle for neuroretinal cell replacement (i.e. retina-into-retina transplantation) was established by a series of landmark studies by del Cerro, Turner, and Blair in the late 1980s.37–41 Turner and Blair were the first to transplant primary retinal tissue from neonatal rats into the subretinal space (SRS) of adult rats with outer retinal lesions,37 reporting survival and differentiation of grafts at 4 weeks post-transplant. These experiments documented some of the earliest evidence of “integration” between donor cells and host retinal tissue, a phenomenon that would eventually grow to become a source of significant debate in retinal regenerative therapies (see Supplementary Note S1).
Figure 3.
An abbreviated history of stem cell biology and its applications to retinal cell replacement. Selected discoveries in stem cell biology and retinal cell transplantation that have contributed to the advancement of outer retinal cell replacement therapies.
Retinal developmental biology saw unprecedented progress throughout the 1980s and 1990s; the introduction of cell birth dating and advanced molecular biology techniques uncovered mechanisms of cell specification, organization, and neuronal maturation within the retina.6–8,42 As the developmental trajectory of PRs was defined (see Fig. 2), investigators continued to experiment with primary cell isolation to determine the optimal donor stage for cell replacement.43–45 Those studies led to the observation that donor cell suitability for transplantation decreased with age. That is, in both allografts (i.e. same-species transplants) and xenografts (i.e. cross-species transplants), embryonic and early postnatal retinal grafts incorporated into lesioned retinas far more readily than their older counterparts.45,46 Given these findings, dissociated suspensions of multipotent neural47–52 or retinal progenitor cells53–56 were initially preferred by many. However, limited survival due to reflux and cell death (often less than 0.01% of the starting dose53,57) in addition to unpredictable differentiation led to low rates of PR engraftment, making translation to human therapies difficult.51
1990s to Early 2000s: Improving on Dissociated RPC Delivery
Two main approaches emerged to address the issue of poor engraftment. Studies building on the findings of del Cerro, Turner, and Blair suggested that human fetal RPC (fRPC) microaggregates (i.e. clusters of cells) and retinal sheets offered improved survival relative to dissociated cell transplants,58 likely due to enhanced structural support and maintenance of cell-cell contacts (reviewed by Seiler and Aramant, 2012). Anoikis, the anchorage-dependent death of cells following loss of extracellular matrix (ECM) contacts, was thought to play a role in the poor survival of subretinally transplanted dissociated cells.59 Tissue-engineered scaffolds were introduced as a customizable approach for mimicking the native structure of retinal tissue to improve survival in RPC transplants.57,60–66 A variety of naturally occurring gelatinous matrices, hydrogels, and decellularized tissues were initially used; however, graft organization was limited and concerns regarding batch-to-batch variability restricted future clinical use.31,67–69 Among others, the Young laboratory developed criteria for an ideal neuroretinal scaffold: biodegradable and/or biocompatible, optically clear, porous, flexible yet strong, and thin enough for relatively easy subretinal delivery (<50 µm).57,60–66 Many synthetic biomaterials met these criteria, and a variety of polymers including poly(e-caprolactone) (PCL), poly(L-lactic acid) (PLLA), poly(lactic-co-glycolic acid) (PLGA), poly(methyl methacrylate) (PMMA), polydimethylsiloxane (PDMS), and poly(glycerol sebacate) (PGS) were found to be well-tolerated in the SRS and supportive of improved RPC delivery in pigs and rodents.60,62–65,70,71 However, RPCs were not limited to producing PRs (see Fig. 2), and despite enhanced survival, the efficiency of PR engraftment following RPC scaffold delivery remained relatively low.57,64
The second approach—enrichment of committed PR precursors (PRPs)—was introduced by MacLaren et al. in 2006 with transplantation of green fluorescent protein (GFP)-labeled rod precursors (Nrl-gfp+/+ cells) in mice.72 Characterized by a defined cell fate, PRPs offered substantial improvement in apparent PR engraftment in the retinas of wild type and rhodopsin-null mice. MacLaren and colleagues observed recovery of light sensitivity in rhodopsin-null mice, providing some of the earliest evidence of functional rescue following subretinal cell transplantation and sparking broad interest within scientific and lay communities alike. Although the study (and at least one subsequent report73) referenced fusion between donor and host cells as a potential alternative explanation for their results,72 it was not believed to occur to a significant degree in the retina at the time. Indeed, as a control, Nrl-gfp+/+ cells were transplanted into transgenic cyan fluorescent protein (CFP) reporter mice, and on the basis of qualitative data showing a lack of multinucleate or double-labeled GFP+/CFP+ cells, MacLaren et al. argued that cell fusion—at least in the classical sense—was unlikely.
Studies within the Ali, Wallace, and Ader laboratories (among others) replicated the findings of MacLaren et al. in the years that followed, primarily in rodent models with an intact or partially degenerated outer nuclear layer (ONL). Manipulation of the degenerative retinal environment by disrupting potential barriers to integration—including the outer limiting membrane (OLM),74,75 glial scarring,76,77 and chondroitin sulfate proteoglycan deposition78–80—was proposed to further enhance PRP incorporation. Fluorescence-activated cell sorting (FACS)72,81–83 and magnetic-activated cell sorting (MACS)84–86 were optimized to obtain relatively uniform batches of transplantable cells. The developmental window paradigm, which proposed that effective PRP cell replacement is best achieved through delivery of postmitotic cells at the precise peak of PRP genesis (E15 to P4 in mice), was introduced during this time.87 With mounting evidence of their success in cell replacement—including improvements in light-mediated activity76,82,88 even in advanced degeneration89—PRP became the preferred developmental stage for primary cell transplantation among most investigators.73,81–86,88–91 As in MacLaren et al.,72 integration was assumed to be the predominant mechanism in these studies; however, the MacLaren laboratory first raised concerns regarding PRs double-labeled with donor and host fluorescent reporters in 2014,92 suggesting that fusion between donor and host cells was indeed possible.
Limitations of Primary Cells
Both strategies—scaffolds and PRP enrichment—offered apparent improvements relative to dissociated RPC delivery, but primary cells presented major challenges to translation beyond animal studies. Phase I and II clinical trials of human fetal-derived retinal cells,93 microaggregates,94 and retinal sheets with RPE95–97 in advanced RP and AMD began in the late 1990s, but ultimately reported mixed effects on visual outcomes in humans. A phase II study led by Radtke and colleagues reported transient visual acuity improvement in 7 of 10 subjects with long-term stabilization in a single subject,95 but the study did not distinguish trophic effects from functional integration, and interpretations of the underlying mechanism varied.26,58,98,99 These early studies (conducted without immunosuppression) provided evidence of safety for future cell therapies, demonstrating a clear path to clinical trials through careful preclinical study planning, but the primary cell approach to replacing PRs faced a difficult road to widespread application.
Procurement of human fetal retinal tissue for transplantation proved controversial from its introduction in the early 1980s,100 and debate around its use in biomedical research continues.101 The developmental window paradigm for PRP (E15-P4 in mice) coincided with the second trimester of pregnancy in humans, presenting an ethical minefield for translation to clinical use. Attempts to expand102,103 and immortalize104 human fetal retinal cells were largely unable to circumvent the issue as RPCs were by definition not restricted to the PR lineage (see Fig. 2) and generated few PRs. In most cases, human fRPCs lost neurogenic potential over time in culture105,106 and demonstrated poor long-term survival following transplantation.104 Improvement under low-oxygen culture conditions was reported in some cases,53,107–110 eventually resulting in the recent initiation of a phase I/IIa clinical trial of subretinal fRPC delivery in late-stage RP (ReNeuron, clinicaltrials.gov identifier NCT02464436). Although this trial is expected to yield valuable safety and efficacy data,26 results have not yet been published, and difficulty in distinguishing trophic support from functional PR replacement remains.26 Ethical constraints and ambiguous mechanisms aside, primary cells and their derivatives continued to present a yield dilemma: with millions of potential patients,14,16 reproducible manufacturing was expected to be a bottleneck for larger phase III clinical trials and beyond.26,58
Early 2000s to Late 2010s: Expanding Potential With Human Pluripotent Stem Cells
The isolation of human embryonic stem cells (hESCs) in 1998111 ushered in a new era for retinal cell replacement. The first completely in vitro differentiation of RPE was achieved in relatively short order,112 but PR differentiation proved more challenging. Building on existing mouse ESC protocols,113,114 studies by the Reubinoff and Reh laboratories showed hESCs could be guided toward a PR fate, but only when transplanted into the SRS115 or co-cultured with retinal tissue.116 Osakada et al. were the first to achieve in vitro generation of hPSC-derived PRP in the absence of mature retinal tissue117 in 2008. The earliest neuroretinal differentiation protocols yielded few PRP, however (just 12–20% of all cells116,117), and only a fraction of these expressed mature PR markers (<0.01–10% of all cells116,117). Induced pluripotent stem cells (iPSCs) were introduced shortly thereafter,118,119 and expanding on earlier approaches,106,113,117,120,121 our laboratory and the Takahashi laboratory soon reported successful differentiation of RPE, RPCs, and putative PRPs from both ESCs and iPSCs.121,122 Lamba and colleagues demonstrated that transplantation of retinal cells derived from both classes of hPSCs was feasible,123,124 reporting results strikingly similar to that of MacLaren et al., although donor cell survival and light responses were comparatively low.123,124
Protocols introduced by our laboratory and the Sasai laboratory in the early 2010s125,126 enabled hPSC-derived 3D retinal organoid production, overcoming the yield barriers of fetal-derived primary tissues and earlier differentiation protocols. Organoid cultures produced PRP far more efficiently—40% to 80% of all cells122,125—and proved to be a breakthrough technology for the field. For the first time, bulk production of PRs from a single donor source was achievable. Methods to further bias organoids toward robust PR production were refined in the years that followed,127–129 demonstrating a surprising degree of structural and functional authenticity127,129–135 (see Bell et al., 2020133 for further discussion). With growing evidence that PSC-derived retinal cells could serve as a reliable and reproducible source of donor cells, the field shifted toward rodent136–143 and human90,144,145 PSC-derived cells and tissues for PR replacement. The preference to use PRP-rich cells and retinal sheets146–149 over RPC donor material largely persisted, given the greater degree of proliferation, disorganization, and uncontrolled migration observed in transplants using the latter.150,151
Late 2010s: The Paradigm-Shifting Discovery of Material Transfer
The field effectively experienced a reset with the revelation of widespread fluorescent material transfer between conspecific donor and host PRs, independently reported by several groups between 2016 and 2017.152–156 In a subsequent transplant study by Waldron et al., nearly all GFP+ cells (99%) found in wildtype host retinas and most GFP+ cells (>75%) in degenerative Nrl−/− and Prph2rd2/rd2 retinas were estimated to result from material transfer,157 calling the results of several previous rodent studies into question.72,76,82,88,123,145,158 Although the exact mechanism and longevity of this phenomenon remains to be determined, at present, several points are clear. First, material transfer is more likely to occur in degenerating retinas with remaining host PRs152–156 than in models of end-stage retinal degeneration.89,141,146,147,149,159–163 Second, a variety of PR-specific proteins (cone arrestin, opsins, and peripherin-2 [PRPH2]; see Fig. 2) as well as cytoplasmic reporters can be passed via material transfer in mice,34 leading to a re-evaluation of how the field identifies and defines integration (see Supplementary Note S1 for further discussion). Third, PSC-derived PRP do not appear to be exempt from this phenomenon34,137,140,157; however, at least one study has suggested that the capacity for material transfer is lower in human-into-rodent xenografts than in allogeneic transplantation.144 Finally, many of the central tenets of successful cell replacement—including the developmental window paradigm, estimated donor cell survival rates, evidence of a dose response, integration, and synaptogenesis—require re-examination in the context of material transfer.
Lessons Learned From Historic Studies of PR Replacement
Collectively, early studies in retinal cell replacement identified several guiding principles for carrying PR cell therapies closer to the clinic. Multiple strategies, including the use of enriched donor cell populations and biomaterial-based scaffolds, have been shown to enhance cell survival in the face of low PR engraftment.34,70,72 The PRP stage of differentiation remains preferred for replacing PRs, although the window of transplant competence is likely not so narrow as previously estimated.34 Three-dimensional retinal organoids are the most often used source of authentic donor cells and tissue sheets, and have in some cases been associated with modest improvements in host retinal light sensitivity following transplantation.26,34 Finally, rodent PSC allografts and human-into-rodent PSC xenografts have established proof-of-concept for PR survival and anatomic engraftment following transplantation. Just as important, these studies have also identified remaining hurdles for the field to overcome. Surviving donor cells often remain disorganized within the subretinal space, and the mechanisms by which they affect host vision remain unclear. With many prior studies now known to result from material transfer rather than functional integration, there is substantial interest in the development and use of quantitative methods for assessing integration, organization, and synaptogenesis.
Current Status and Remaining Questions for Retinal Cell Therapies
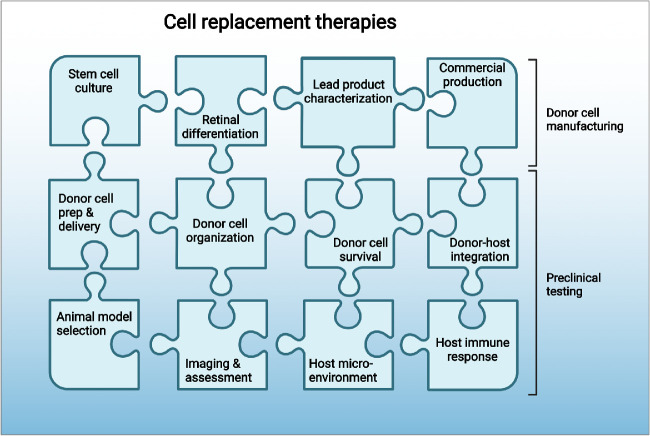
As outlined above, cell replacement therapies in the retina have been studied for decades (see Fig. 3), and hPSC-RPE and hPSC-PRP cell products are now in the T1 translational research phase (which spans preclinical studies through phase II clinical trials). Thus far, the majority of clinical trials have used hESC- or hiPSC-RPE, inserted into the SRS either as dissociated cell suspensions or as monolayer sheets with or without scaffolds (see Uyama et al., 2021 for further discussion98). Early reports suggest that these therapies are feasible, safe, and well-tolerated in individuals with advanced retinal degenerative disease.26,98 However, functional outer retinal cell replacement—and more particularly PR replacement—remains a complex puzzle of cell manufacturing and preclinical testing challenges, some of which may not be fully surmountable ahead of human trials (Fig. 4). Even so, efforts to address each piece of this puzzle in a deliberate, stepwise manner would help build confidence in the potential for success. This section discusses these pieces in detail, comparing and contrasting major strategies and identifying areas where additional research is necessary to advance outer retinal cell therapeutics.
Figure 4.
The complex puzzle of therapeutic development for outer retinal cell therapies. As cell therapies transition from phase T1 to phase T2 studies and beyond, several interconnected factors related to donor cell manufacturing and preclinical testing must be addressed.
Clinical-Grade Production
Clinical hPSC-derived cell therapies must be sterile and free from infectious agents, impurities, residual pluripotent cells, unidentified cell types, and genomic instabilities.28 Such criteria must be met under strict Good Manufacturing Practice (GMP)-compliant conditions28,164–166 and also be scalable far beyond the capacity of an average laboratory setting to be feasible for clinical trials and commercialization. Although detailed discussions of stem cell source (ES or iPS) and culture technique are beyond the scope of this review, proper induction and/or maintenance of PSCs is fundamental to any successful retinal differentiation program. Advantages and disadvantages of autologous and allogeneic cell replacement should be weighed early in product development to avoid the need for correction mid-program. Preclinical safety studies for autologous products emphasize the manufacturing process for producing each cell line in addition to the final clinical product, whereas safety studies for allogeneic products focus on the latter. Thus, programs are effectively “locked in” very early to an autologous or allogeneic approach.28 Autologous therapies are subject to fewer infectious disease testing requirements and are theoretically less likely to result in immune rejection,28 although cost (an estimated $800,000/cell line for clinical-grade iPSC production167) and reproducibility across patient-specific iPSC lines are often limiting factors. “Off-the-shelf” human leukocyte antigen (HLA)-matched168 or HLA knockout169 allogeneic products offer a scalable, more cost-effective production pipeline; however, immune rejection and long-term safety become a greater concern, and many dozens of HLA “super donor” cell lines would still be needed depending on the genetic diversity of the target population.167
Lessons learned from existing Investigational New Drug (IND)-enabling studies, particularly those from investigators with experience in cell therapies and Biologics License Applications with the FDA,26 should be considered early in product development to mitigate additional “known unknown” risks for scaling regenerative therapies170 (for further discussion of quality control for clinical-grade hPSC retinal cell production, see Wright et al., 2014171 and Sharma et al., 202028). Current methods for generating hPSC retinal organoids are both time and labor-intensive, limiting their utility in clinical production pipelines. The use of bioreactors,172 microfluidics,166,173 and automated culture systems27 are all promising approaches currently under investigation for scaling clinical-grade organoid-based technologies.
Donor Cell Enrichment
Robust methods for purifying or enriching PRP from stem cell-derived retinal organoids represent a critical bottleneck in the regenerative medicine pipeline for PR degenerative diseases.34 MACS and FACS-based enrichment strategies originally developed in mouse models174 have not yet translated into consistent success for enrichment of hPSC-PRP for transplantation,161 possibly due to species- or maturation stage-specific differences in PR cell surface markers. Although some groups have reported successful development of hPSC-PRP enrichment protocols, most have not been widely adopted outside individual laboratories, possibly due to low yield (<1 million cells)175–177 and/or suboptimal purity (40–70%) across various differentiation protocols.135 A fully homogenous cell product is not necessarily a prerequisite for clinical trial initiation26 because the FDA allows study sponsors to set their own release criteria for product purity, but a highly enriched PRP product (>80%) would be desirable. Some groups have proceeded with unsorted cell populations or retinal sheets in the absence of reproducible sorting methods,145,149 but residual proliferating cells (e.g. immature RPE, RPCs, etc.) often remain. Unsorted populations thus contain cells that may continue to divide, leading to PRs being outnumbered by off-target cell types,178 or to the development of disorganized, rosetted grafts following tissue sheet transplants.136,146–149,179
Human PSC reporter lines135,177,180–183 and viral labeling constructs144 have been generated as an alternative approach to enable rapid PRP enrichment via FACS. Although precedent exists for FDA allowance of biologics expressing fluorescent proteins in clinical trials184 (GenSight Biologics’ optogenetic GS030 [clinicaltrials.gov identifier NCT03326336] encodes a tdTomato-linked fusion protein), nontherapeutic inclusions add safety and regulatory hurdles to an already complex approval process. Sorting via cell surface markers162,176 (e.g. MACS) or label-free microfluidics175 offers a more favorable approach to obtaining enriched PRP for cell replacement. Given the current lack of consensus on optimal PRP sorting strategies, particularly for cones,34 further investigations of human PRP-specific cell surface markers and enrichment approaches are warranted.
Functional Validation of Donor Cells
The advancement of hPSC-RPE therapies has been accelerated in part by the relative ease with which RPE donor cell identity and function can be validated.28 A battery of biomarkers and assays, including cobblestone morphology, pigmentation, transepithelial resistance (TER), photoreceptor outer segment (POS) phagocytosis, electrophysiology, apical-basal polarization, tight junction marker expression, and microvilli formation, are all indicative of healthy, maturing hPSC-RPE.28,31,98,185,186 Standardized methods for PRP validation have proven less straightforward, partly due to cell heterogeneity (rods; short-, medium-, and long-wavelength sensitive cones) and complexity, and partly due to the range in donor cell maturation used across studies. Neuronal age is a simple and widely used metric for estimating maturity in hPSC culture systems,187 and functional maturation is associated with age in hPSC-derived retinal neurons114,117,122 regardless of the differentiation protocol used. However, the rate of maturation is often asynchronous across RO differentiation protocols, cell lines, and even differentiation batches.129 Age can serve as a rough surrogate marker of maturation, but this metric provides a somewhat false sense of assurance for consistency between lines or differentiations.129,188 A recent study by Capowski et al. demonstrated the utility of morphology for assessing maturation, introducing a light microscopic staging system for classifying ROs.129 Stage 1 ROs contain RPCs, early-born inner retinal neurons, and an outer neuroblastic layer, whereas stage 2 ROs are characterized by differentiation of an abundance of PRs and inner retinal neurons. The hallmarks of stage 3 ROs are the development of PR outer segments and increased outer neuroretinal organization along with production and maturation of Müller glia and ongoing deterioration of the innermost retinal layers.129
Because the characteristic light-sensitive component of PRs, outer segments, often appear months past the peak of PRP genesis in ROs (stage 2, approximately day 80–120 of differentiation), development of in vitro potency assays for validating hPSC-PRP remains a high priority. One intriguing approach to this conundrum is the use of optogenetically engineered hiPSC-PRP, which have recently been shown to generate modest responses to bright light in vitro and in vivo.163,189 However, such a genetic modification presumes that hPSC-derived PRs cannot innately respond to light and also introduces aforementioned regulatory hurdles. In the absence of genetic modification of hPSC-PRP, other potency assays may prove useful for authenticating batches of transplantable hPSC-PRP, including examinations of cell polarity, synaptic marker expression,190 PR marker expression, axon outgrowth, and membrane electrophysiology.135
Cell Preparation, Delivery, and Organization
A number of recent xenograft studies have demonstrated proof-of-concept for PR survival and anatomic engraftment (see Supplementary Note S1) following transplantation of dissociated hPSC-derived cell suspensions or retinal sheets in rodents146,149,161–163,179,189 and non-human primates (NHPs),147,160 using controls for biomaterial transfer. Dissociated cell injections have the advantage of being relatively simple, cost-effective, rapid, and minimally invasive, although graft organization and cell survival is often suboptimal.34 Retinal sheet delivery can afford a striking degree of self-organization relative to dissociated cells,34,98,146,147,149,179 but the surgical technique requires specialized instrumentation and is more complex and invasive than simple subretinal injections. Furthermore, rosetted PRs, formation of ectopic inner retinal laminae, and lack of apposition to host RPE remain limitations to this approach.26
PRs and RPE are both highly specialized cells for which apical-basal polarity plays a crucial role in function; there is thus substantial interest in cell delivery strategies supportive of donor cell organization. Polymeric retinal patches or scaffolds are among the most promising solutions for improving cell retention and 3D distribution191 and maintaining cell orientation.192–194 Added benefits also include a defined dose, targeted delivery to a discrete region, and potential for customization of scaffold size, shape, and material. Scaffold-based hPSC-RPE delivery has thus far been well-tolerated in clinical trials, and despite more complex surgical procedures, there is evidence to suggest that scaffold delivery may be achievable in an outpatient setting.195 Neuroretinal scaffold approaches are still in their infancy but appear to be similarly advantageous for PR replacement. Current hPSC approaches include two-photon polymerized PCL scaffolds seeded with clinical-grade RPCs191,196 and micromolded PGS scaffolds seeded with hPSC-PRPs.192,193 Both scaffolds are sterilizable, biodegradable, and have a desirable elastic and/or compressive modulus, which play a critical role in ease of surgical handling.193,196,197 Extensive in vivo safety testing has been performed for the former, although the use of RPCs rather than PRPs was a limiting factor in determining capacity for PR delivery. Micromolded scaffolds are capable of pre-organizing polarized PRPs even at high cell densities, although it remains to be seen whether such organization can be retained in vivo. Optimization of scaffold delivery requires the use of clinically relevant large animal models to simulate targeted subretinal scaffold implantation in the human macula. In addition to delivery of PRP-only scaffolds, envisaged applications include co-delivery of hPSC-RPE and PRPs, as replacement of both cell types will likely be necessary for individuals suffering from late-stage AMD198 or inherited maculopathies, such as Stargardt and Best disease.
Assessment of Donor Cell Survival
Dissociated cell survival in RPC199 or allogeneic PSC-PRP transplants in rodents143 is extremely low (1–4%), and because these studies predate the discovery of material transfer, may be overestimated. Given the widespread use of percentages rather than discrete cell counts in published datasets, it is often difficult to obtain a true approximation of cell survival relative to the starting dose. Standardized methods for counting cells or regions of interest, like the QUANTOS workflow developed for synapse identification,190 will be critical for rigorously studying such outcomes. Where possible (and with appropriate controls), unbiased stereology and automated image analysis can also provide a less subjective approach to histologic analyses. Several studies have highlighted the importance of standardized cell quantification in biological research to increase reproducibility and aid comparisons between studies or across research groups.200–202 Greater adoption of such methods for assessing donor cell survival in PR replacement would serve the field well.
Functional Integration and Synaptogenesis
The presence of new synaptic connections following transplantation is often inferred by pre- and postsynaptic protein co-immunolabeling or electron microscopic evidence of synaptic ribbons near donor cells.136,147–149,160,161,179 However, immunocytochemical evidence of synaptic marker expression does not establish a definitive causal link to observed changes in retinal function or visual behavior. Further evidence in favor of functional synaptogenesis includes electrophysiologic, reflexive, and behavioral assessments of light responsivity, although most of these readouts measure processes several synapses downstream from presumptive donor-host contacts.23,146,147,149,159,161,163,179 High levels of donor cell disorganization26,34,136,141,146 and relatively mild degrees of light-induced response recovery observed in most hPSC-PRP transplants23,141,146,159,161,163,203 also suggest that synapse formation likely occurs at lower rates than previously predicted.34
It is currently difficult to fully distinguish bona fide synaptic connections from existing ones—however rare they may be—in the absence of direct and effective methods for studying synaptic contacts of donor cells.34,204 A recent study by Cowan et al. suggests that PRP are capable of forming functional synapses within retinal organoids as evidenced by calcium imaging.134 However, no study to date has definitively shown that hPSC-PRPs can form new functional synapses after being isolated from retinal organoids. Evidence of functional post-transplant synaptogenesis currently includes modest light responses recorded with multi-electrode array (MEA) and micro-electroretinography (mERG),146,147,161,189,205 and often does not conclusively distinguish light-induced donor cell responses from possible neuroprotective effects on residual host retinal circuitry. Reproducible, well-controlled approaches for assessing de novo synaptogenesis at the level of individual donor hPSC-PRPs (via calcium imaging or viral monosynaptic circuit tracing), particularly in the context of xenogeneic transplantation,206 will be necessary to further clarify mechanisms of functional recovery. The efficiency of synaptogenesis in xenografts is currently unknown206; however, by increasing PRP survival, alignment, and organization, it may be possible to increase the likelihood of synapse formation between donor and host cells. Strategies to directly measure hPSC-PRP synaptic contacts via trans-synaptic tracing or patch-clamp recordings have been highlighted as crucial,34 but have not yet come to fruition.
Animal Model Selection for Safety and Efficacy Studies
Rodents have historically been the preferred model system for retinal cell replacement studies due to cost, ease of genetic manipulation, and widespread availability. Several reports have shown that transplanted hPSC-PRP can survive and be associated with varying degrees of light-evoked behavior and/or electrophysiologic activity in degenerating rodent retinas,146,149,161–163,179,189 but there is not yet direct evidence of causation. The well-documented neuroprotection caused by virtually any subretinally transplanted material (including control vehicles110,207) in the Royal College of Surgeons (RCS) rat makes it highly difficult to fully control for alternate mechanisms in this model. To address the confounding variables of neuroprotection and biomaterial transfer, many investigators have instead opted for models with near-complete PR loss. Models with severe PR loss89,150,159,208 are currently considered most appropriate for studying functional integration, although even these models are not free of confounding variables, because residual cones remain in severely atrophic models like the rd1 mouse and S334ter-3 rat.26,206
To date, most available data regarding cell survival and effects on vision are skewed toward rod-dominant rodent models, although there is some evidence to suggest similar responses are possible in NHPs.147 Given notable species-specific differences in PR development and synaptic architecture,206 the degree to which these observations will directly translate to human allogeneic or autologous transplants remains to be seen. The introduction of scaffolds and more complex tissue constructs, which necessarily includes more complicated surgeries, will require a shift toward larger animal models with ocular anatomy more akin to that of humans to provide meaningful assessments of such approaches. Development of translation-enabling models that faithfully recapitulate aspects of human RDDs is an explicit aim of the NEI AGI,21 and these models will be a valuable resource for advancing retinal cell therapies. There is substantial interest in allogeneic transplantation of same-species PSC-derived retinal cells in parallel with xenogeneic studies, as this approach can potentially provide extrapolatable insight into the degree of functional restoration that may be reasonably expected in human clinical trials. Continued observance of field standards for defining integration (see Supplementary Note S1 for further discussion) and development of protocols to generate retinal organoids from additional laboratory model species will be essential to such activities.
Although a variety of reflexive and behavioral assays are available for assessing visual function, even electrophysiologic methods ultimately may not be sensitive enough to directly assay PR transplant-driven responses.161,205 Several such studies have documented light-evoked electrophysiologic responses209 and visual behavior210 in degenerating retinas even when surviving donor PRs are nearly absent. Adequately powered studies controlling for alternate explanations of restored function—including material transfer to host interneurons,206 aberrant firing of intrinsically photosensitive RGCs (ipRGCs), and neuroprotection of remaining host retinal cells—will be challenging, but necessary, for definitively establishing a causal link between anatomic integration and vision rescue.26,206
There is no single animal model that is perfect for each cell replacement application. Rather, a variety of factors, including ocular anatomy, nocturnal versus diurnal activity (i.e. rod versus cone-dominance), and genetic causation should be taken into consideration when designing preclinical IND-enabling studies for cell therapies (summarized in Table 2; also see Winkler et al. 2020211 for a discussion of RDD animal models). A recent study by Laver and Matsubara also suggests that the lack of robust responses observed in human-to-rodent xenografts147,161,163 may be due in part to synapse incompatibilities between donor PRP and host retinal interneurons.206 The degree of divergence in synaptic proteins between humans and non-human model organisms is just one of many factors to consider when selecting preclinical models for testing functional effects of hPSC-PRP therapeutics.
Table 2.
Animal RDD Models and Factors Affecting Suitability for Preclinical Retinal Cell Replacementa
| Species | Ocular Anatomy: Similarity to Human | Features | Predicted TRSb Compatibility with Humanc | Options for Immune Suppression | Selected RDD Models |
|---|---|---|---|---|---|
| Mouse | + | - Small globe with large lens | 89% | Genetically modified | Rd1, Rd10, many others |
| - Rod-dominant retina | Pharmacologic | ||||
| Rat | + | - Small globe with large lens | 88% | Genetically modified | RCS, S334ter, P23H |
| - Rod-dominant retina | Pharmacologic | ||||
| Ground squirrel | ++ | - Small globe with small lens | 44%d | Pharmacologic | Retinal detachment |
| - Cone-dominant retina | |||||
| Rabbit | ++ | - Medium-sized globe with small lens | 86% | Pharmacologic | RHO (P347L)246 |
| - Visual streak | Laser damage247 | ||||
| Cat | +++ | - Medium-sized globe with small lens | 92% | Pharmacologic | RDH5, CEP290, AIPL1 |
| - Area centralis | |||||
| Dog | +++ | - Moderately large globe with small lens - Area centralis | 81% | Pharmacologic | RHO, RPE65, PDE6A, PDE6B, SAG, ABCA4 |
| Pig | ++++ | - Large globe with small lens | 85% | Pharmacologic | RHO (P23H) |
| - Visual streak | Genetic models248 | Laser damage | |||
| Surgically-induced224 | |||||
| Macaque | +++++ | - Large globe with small lens | 98% | Pharmacologic | PDE6C, BBS7 |
| - Macula | Laser damage |
A summary of findings from: Stanzel et al., 2019249 (ocular anatomy, RDD models), Laver and Matsubara, 2017206 (xenograft compatibility), and Winkler et al., 2020211 (RDD models).
Photoreceptor triad ribbon synapse.
Based on the Pikachurin sequence similarity (percentage) between humans and the listed species.
Laver and Matsubara broadly refer to squirrels; this may not directly reflect TRS compatibility of specific models (e.g. 13-lined ground squirrels).
Noninvasive Imaging to Assess Therapeutic Efficacy
The retina is a highly organized, laminated structure that has evolved to maximally harness light entering the eye.212 Recent advances in noninvasive retinal imaging have capitalized on these features to provide increasingly detailed pictures of in vivo retinal architecture.213–216 Both the NEI AGI and the Monaciano Consortium have highlighted a relative lack of rigorous, reproducible ocular imaging as a potential bottleneck in advancing clinical trials.20,21,217 Several recent studies have demonstrated the utility of noninvasive imaging for comprehensively studying integration and therapeutic efficacy in hPSC-PRP cell therapies.160,218 The Singh laboratory at Johns Hopkins identified quantifiable biomarkers for tracking fluorescent mouse cells after transplantation, developing a scoring system for multimodal confocal scanning laser ophthalmoscopy (cSLO) imaging.218 Several properties, including fluorescence size and intensity, graft placement, lamination, and peri-retinal proliferation, were scored longitudinally, facilitating long-term tracking of individual grafts over time. Similarly, Aboualizadeh et al. recently used fluorescence adaptive optics scanning light ophthalmoscopy (FAOSLO) to follow individual PRs in vivo in a laser-damage NHP model of PR loss.160 These types of correlative studies augment histologic assessment of efficacy, although further research is necessary to determine how to translate these imaging techniques to clinical trials and commercial products and how to distinguish donor cells from host biomaterial transfer in vivo. As mentioned earlier, fluorescent reporters are not necessarily prohibited in clinical trials, but development of high-resolution, label-free, noninvasive methods for tracking migration and integration of donor cells is preferable.
Modulating Retinal Microenvironment and Immune Response
The ideal cell replacement toolbox will likely include approaches for priming the degenerative host retina for enhanced integration. Although there is evidence of some efficacy following hPSC-PRP delivery even in end-stage retinal degeneration—suggesting that host inner retinal circuitry remains viable for a time—the exact window of opportunity for effective cell replacement is currently unknown.34,219 Treatments being studied seek to modulate a variety of naturally occurring processes that may act as barriers to donor PR integration in the degenerate outer retina, including glial scarring,220 interneuron plasticity,221 and neurite outgrowth,222 which may in turn help create a more donor cell-receptive environment. Basic discovery research to better understand the molecular mechanisms involved in retinal circuit assembly, disassembly, and re-assembly will also be essential to address host-centered barriers to neuronal replacement.20,223
While the eye is historically considered to be immune-privileged, current evidence suggests that this privilege is relative rather than absolute, and is perhaps lost in the course of disease.33 Preclinical xenografts require immunodeficient hosts46,146,150,224 or immunosuppressive regimens,147,160,225 but allograft studies and clinical trials to date report conflicting evidence regarding the degree of immune suppression necessary for long-term donor cell survival. Recent studies suggest that the immunogenicity of hPSC-derived retinal tissues may actually be relatively low, and hPSC-derived retinal cells might even confer a degree of local immune suppression.226 As methods for assessing graft survival improve, further research regarding the role of the immune system in xenografts, allografts, and autografts will be necessary to predict best practices. Reports from hPSC-RPE clinical trials, which use a variety of immune suppressive regimens, will be highly informative for designing future clinical trials aimed at outer retinal cell replacement.26,33
Conclusions: A Shared Responsibility
Exogenous RPE and PR transplantation efforts—bolstered by decades of research in regenerative medicine and retina developmental biology—have overcome significant hurdles in recent years and are now being tested in clinical trials. Although hPSC-RPE therapies are further along, remaining challenges to clinical translation for hPSC-PRP include scaling clinical-grade cell production, creating organized grafts, addressing synapse formation and functional integration, and optimizing safety and efficacy outcomes in relevant model systems.
Singh et al. recently observed that, as these challenges are met and retinal cell therapies reach early phase clinical trials, peer-reviewed interim reports may have unintended ripple effects on patients and lay audiences.26 Eye-catching headlines rarely reflect the nuance of underlying research findings and further fuel unrestrained public desire for stem cell-based therapies. Preclinical research can often inadvertently elicit similar responses when reports of vision restoration in animal models are picked up by the media. The current landscape of milestone-oriented funding and open-source science necessitates timely publication of results, but Singh et al. stress the importance of appropriately powered, long-term follow-up to mitigate “scientifically unfounded over-optimism” within the non-scientific community. Recent case reports have underscored the grave impacts227–229 of clinics prematurely capitalizing on this enthusiasm and preying on patient hope230 with unregulated stem cell treatments.
A recent Lancet commission on regenerative medicine argues that the shift from “small-scale bespoke experimental interventions” to bona fide clinical application of hPSC-based therapies will require “substantial rethinking of the social contract that supports such research and clinical practice in the public arena.” The commission contends that improving four areas—science, funding models, governance, and public/patient engagement—can prevent erosion of public trust and bridge the gap between patient expectations and currently available therapies.231 While it is clear that tremendous scientific progress has been made toward outer retinal cell replacement, transitioning from bench to bedside will require substantial engagement from a variety of stakeholders regarding economic burden,21 international governance,27 and public/patient interaction.26
The challenges that lie ahead for outer retinal cell therapies can be overcome, and the recent advances highlighted in this review suggest that the future for retinal regenerative medicine is bright. However, translation to clinical application will require considerable investment of time and scientific effort from public and private entities alike. Moreover, the necessary focus on safety in early phase research means that efficacy in human subjects, who will necessarily be at the severe end of the disease spectrum, will likely be modest at first. In short, the reality we collectively face is that translating cell therapies to effective clinical practice will take time, and for families currently battling vision loss, it will rarely feel like progress comes fast enough. In the interim, scientists and clinicians will continue to play a crucial role in right-sizing public expectations and encouraging patients to make informed decisions regarding stem cell treatments. To this end, several organizations have developed educational materials geared toward a lay audience that are freely available to share with individuals considering stem cell therapies. Materials from the International Society for Stem Cell Research, including the Patient Handbook on Stem Cell Therapies232 (available in 12 languages) and disease-specific fact sheets,233 as well as the McPherson Eye Research Institute's similarly themed “10 Things to Know Before You Fall Victim to a Retinal Stem Cell Scam” (see Supplementary Note S2) can help patients navigate stem cell claims while researchers around the world continue to work toward solving the complex puzzle of outer retinal cell replacement.
Supplementary Material
Acknowledgments
The authors thank Kimberly Edwards, Steven Mayerl, Lindsey Jager, Praveen J. Susai Manickam, Divya Sinha, Beth Capowski, and Joe Phillips, and other members of the Gamm laboratory for providing critical feedback and helpful discussion of the contents of this review. All figures were prepared in BioRender; Figure 3 was adapted from the “Timeline of Adjuvant Used in Human Vaccines” template by BioRender.com (https://app.biorender.com/biorender-templates).
Supported in part by the Retina Research Foundation Emmett A. Humble Distinguished Directorship of the McPherson Eye Research Institute (D. M. G.), the Sandra Lemke Trout Chair in Eye Research (D. M. G.), and NIH awards no. T35 OD011078 and F30 EY031230 (A. L. L.).
Disclosure: A.L. Ludwig, None; D.M. Gamm, D.M. Gamm is an inventor on patents related to generation of 3D retinal organoids (US PTO no. 9,328,328) and production of outer retinal scaffolds (US PTO application nos. 2017/0226459 and 2020/0010799) filed by the Wisconsin Alumni Research Foundation, Madison, WI. D.M. Gamm has an ownership interest in and receives grant funding from Opsis Therapeutics, LLC, which has licensed the technology to generate 3D retinal organoids. The terms of this arrangement have been reviewed and approved by the University of Wisconsin-Madison in accordance with its conflict of interest policies
References
- 1. Baden T, Osorio D.. The Retinal Basis of Vertebrate Color Vision. Annu Rev Vis Sci. 2019; 5(1): 177–200. [DOI] [PubMed] [Google Scholar]
- 2. Lamb TD. Evolution of the genes mediating phototransduction in rod and cone photoreceptors. Prog Retin Eye Res. Published online November 29, 2019: 100823, doi: 10.1016/j.preteyeres.2019.100823 [DOI] [PubMed] [Google Scholar]
- 3. George SM, Lu F, Rao M, Leach LL, Gross JM.. The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems. Progress in Retinal and Eye Research. Published online April 23, 2021: 100969, doi: 10.1016/j.preteyeres.2021.100969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bringmann A, Iandiev I, Pannicke T, et al.. Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009; 28(6): 423–451. [DOI] [PubMed] [Google Scholar]
- 5. Reichenbach A, Bringmann A.. New functions of Müller cells. Glia. 2013; 61(5): 651–678. [DOI] [PubMed] [Google Scholar]
- 6. Cepko C. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat Rev Neuroscie. 2014; 15(9): 615–627. [DOI] [PubMed] [Google Scholar]
- 7. Swaroop A, Kim D, Forrest D.. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci. 2010; 11(8): 563–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brzezinski JA, Reh TA.. Photoreceptor cell fate specification in vertebrates. Development. 2015; 142(19): 3263–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sparrow JR, Hicks D, Hamel CP.. The Retinal Pigment Epithelium in Health and Disease. Curr Mol Med. 2010; 10(9): 802–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Marmor M, Marc RE.. Retinal remodeling in human retinitis pigmentosa. Exp Eye Res. 2016; 150: 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones BW, Marc RE, Pfeiffer RL.. Retinal Degeneration, Remodeling and Plasticity. In: Kolb H, Fernandez E, Nelson R, eds. Webvision: The Organization of the Retina and Visual System. Salt Lake City, UT: University of Utah Health Sciences Center; 1995. Accessed August 26, 2021, http://www.ncbi.nlm.nih.gov/books/NBK482309/. [Google Scholar]
- 12. Jones BW, Marc RE.. Retinal remodeling during retinal degeneration. Exp Eye Res. 2005; 81(2): 123–137. [DOI] [PubMed] [Google Scholar]
- 13. Pfeiffer RL, Marc RE, Jones BW.. Persistent remodeling and neurodegeneration in late-stage retinal degeneration. Prog Retin Eye Res. 2020; 74: 100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong WL, Su X, Li X, et al.. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014; 2(2): e106–e116. [DOI] [PubMed] [Google Scholar]
- 15. Flaxman SR, Bourne RRA, Resnikoff S, et al.. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. The Lancet Glob Health. 2017; 5(12): e1221–e1234. [DOI] [PubMed] [Google Scholar]
- 16. Verbakel SK, van Huet RAC, Boon CJF, et al.. Non-syndromic retinitis pigmentosa. Prog Retin Eye Res. 2018; 66: 157–186. [DOI] [PubMed] [Google Scholar]
- 17. van der Aa HPA, Comijs HC, Penninx BWJH, van Rens GHMB, van Nispen RMA. Major Depressive and Anxiety Disorders in Visually Impaired Older Adults. Invest Ophthalmol Vis Sci. 2015; 56(2): 849–854. [DOI] [PubMed] [Google Scholar]
- 18. Taylor DJ, Jones L, Binns AM, Crabb DP.. ‘You've got dry macular degeneration, end of story’: a qualitative study into the experience of living with non-neovascular age-related macular degeneration. Eye. 2020; 34(3): 461–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marques AP, Ramke J, Cairns J, et al.. Global economic productivity losses from vision impairment and blindness. EClinicalMedicine. 2021; 35: 100852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gamm DM, Wong R. Panelists and the AW. Report on the National Eye Institute Audacious Goals Initiative: Photoreceptor Regeneration and Integration Workshop. Trans Vis Sci Tech. 2015; 4(6): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Becker SM, Wright CB.. Update on the Status and Impact of the National Eye Institute Audacious Goals Initiative for Regenerative Medicine. J Ocul Pharmacol Therapeut. 2021; 37(3): 144–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Botto C, Rucli M, Tekinsoy MD, Pulman J, Sahel J-A, Dalkara D.. Early and late stage gene therapy interventions for inherited retinal degenerations. Prog Retin Eye Res. Published online May 29, 2021: 100975, doi: 10.1016/j.preteyeres.2021.100975 [DOI] [PubMed] [Google Scholar]
- 23. Barnea-Cramer AO, Singh M, Fischer D, et al.. Repair of Retinal Degeneration following Ex Vivo Minicircle DNA Gene Therapy and Transplantation of Corrected Photoreceptor Progenitors. Molec Ther. 2020; 28(3): 830–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gene & Cell Therapy FAQs | ASGCT - American Society of Gene & Cell Therapy | ASGCT - American Society of Gene & Cell Therapy. Accessed June 24, 2021, https://asgct.org/education/more-resources/gene-and-cell-therapy-faqs.
- 25. Wang Y, Tang Z, Gu P.. Stem/progenitor cell-based transplantation for retinal degeneration: a review of clinical trials. Cell Death Dis. 2020; 11(9): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh MS, Park SS, Albini TA, et al.. Retinal stem cell transplantation: Balancing safety and potential. Prog Retin Eye Res. 2020; 75: 100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maeda T, Sugita S, Kurimoto Y, Takahashi M.. Trends of Stem Cell Therapies in Age-Related Macular Degeneration. J Clin Med. 2021; 10(8): 1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma R, Bose D, Maminishkis A, Bharti K.. Retinal Pigment Epithelium Replacement Therapy for Age-Related Macular Degeneration: Are We There Yet? Ann Rev Pharmacol Toxicol. 2020; 60(1): 553–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Canto-Soler V, Flores-Bellver M, Vergara MN.. Stem Cell Sources and Their Potential for the Treatment of Retinal Degenerations. Invest Ophthalmol Vis Sci. 2016; 57(5): ORSFd1–ORSFd9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nickerson PEB, Ortin-Martinez A, Wallace VA.. Material Exchange in Photoreceptor Transplantation: Updating Our Understanding of Donor/Host Communication and the Future of Cell Engraftment Science. Front Neural Circuits. 2018; 12: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hunt NC, Hallam D, Chichagova V, Steel DH, Lako M.. The Application of Biomaterials to Tissue Engineering Neural Retina and Retinal Pigment Epithelium. Advanced Healthcare Materials. 2018; 7(23): 1800226. [DOI] [PubMed] [Google Scholar]
- 32. Jemni-Damer N, Guedan-Duran A, Fuentes-Andion M, et al.. Biotechnology and Biomaterial-Based Therapeutic Strategies for Age-Related Macular Degeneration. Part II: Cell and Tissue Engineering Therapies. Front Bioeng Biotechnol. 2020; 8: 588014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petrash CC, Palestine AG, Canto-Soler MV.. Immunologic Rejection of Transplanted Retinal Pigmented Epithelium: Mechanisms and Strategies for Prevention. Front Immunol. 2021; 12: 621007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gasparini SJ, Llonch S, Borsch O, Ader M.. Transplantation of photoreceptors into the degenerative retina: Current state and future perspectives. Prog Retin Eye Res. Published online November 13, 2018, doi: 10.1016/j.preteyeres.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 35. Royo PE, Quay WB.. Retinal transplantation from fetal to maternal mammalian eye. Growth. 1959; 23: 313–336. [PubMed] [Google Scholar]
- 36. del Cerro M, Gash DM, Rao GN, Notter MF, Wiegand SJ, Gupta M. Intraocular retinal transplants. Invest Ophthalmol Vis Sci. 1985; 26(8): 1182–1185. [PubMed] [Google Scholar]
- 37. Turner JE, Blair JR.. Newborn rat retinal cells transplanted into a retinal lesion site in adult host eyes. Brain Res. 1986; 391(1): 91–104. [DOI] [PubMed] [Google Scholar]
- 38. Blair JR, Turner JE.. Optimum conditions for successful transplantation of immature rat retina to the lesioned adult retina. Devel Brain Res. 1987; 36(2): 257–270. [DOI] [PubMed] [Google Scholar]
- 39. del Cerro M, Notter MF, Grover DA, Gash DM, Jiang LQ, del Cerro C.. Chapter 16 Retinal transplants into adult eyes affected by phototoxic retinopathy. In: Gash DM, Sladek JR, eds. Progress in Brain Research. Vol. 78. New York, NY: Elsevier; 1988: 125–130. [DOI] [PubMed] [Google Scholar]
- 40. Del Cerro M, Notter M, Wiegand S, Jiang L, del Cerro C.. Replacement of rod cells into adult eyes affected by late-state phototoxic retinopathy by transplantation of developing retinal cells. J Neur Transpl. 1989; 1: 1–10. [Google Scholar]
- 41. Turner JE, Seiler M, Aramant R, Blair JR.. Chapter 17 Embryonic retinal grafts transplanted into the lesioned adult rat retina. In: Gash DM, Sladek JR, eds. Progress in Brain Research. Vol. 78. New York, NY: Elsevier; 1988: 131–139. [DOI] [PubMed] [Google Scholar]
- 42. Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D.. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA. 1996; 93(2): 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gouras P, Du J, Gelanze M, et al.. Survival and Synapse Formation of Transplanted Rat Rods. J Neur Transplant Plasticity. 1991; 2(2): 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gust J, Reh TA.. Adult Donor Rod Photoreceptors Integrate into the Mature Mouse Retina. Invest Ophthalmol Vis Sci. 2011; 52(8): 5266–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Aramant R, Seiler M, Turner JE.. Donor age influences on the success of retinal grafts to adult rat retina. Invest Ophthalmol Vis Sci. 1988; 29(3): 498–503. [PubMed] [Google Scholar]
- 46. Aramant RB, Seiler MJ.. Human Embryonic Retinal Cell Transplants in Athymic Immunodeficient Rat Hosts. Cell Transplant. 1994; 3(6): 461–474. [DOI] [PubMed] [Google Scholar]
- 47. Francis PJ, Wang S, Zhang Y, et al.. Subretinal transplantation of forebrain progenitor cells in nonhuman primates: survival and intact retinal function. Invest Ophthalmol Vis Sci. 2009; 50(7): 3425–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang S, Girman S, Lu B, et al.. Long-term vision rescue by human neural progenitors in a rat model of photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2008; 49(7): 3201–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gamm DM, Wang S, Lu B, et al.. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS One. 2007; 2(3): e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Hoffelen SJ, Young MJ, Shatos MA, Sakaguchi DS.. Incorporation of murine brain progenitor cells into the developing mammalian retina. Invest Ophthalmol Vis Sci. 2003; 44(1): 426–434. [DOI] [PubMed] [Google Scholar]
- 51. Young MJ, Ray J, Whiteley SJ, Klassen H, Gage FH.. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol Cell Neurosci. 2000; 16(3): 197–205. [DOI] [PubMed] [Google Scholar]
- 52. Takahashi M, Palmer TD, Takahashi J, Gage FH.. Widespread Integration and Survival of Adult-Derived Neural Progenitor Cells in the Developing Optic Retina. Molec Cell Neurosci. 1998; 12(6): 340–348. [DOI] [PubMed] [Google Scholar]
- 53. Klassen HJ, Ng TF, Kurimoto Y, et al.. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest Ophthalmol Vis Sci. 2004; 45(11): 4167–4173. [DOI] [PubMed] [Google Scholar]
- 54. Qiu G, Seiler MJ, Mui C, et al.. Photoreceptor differentiation and integration of retinal progenitor cells transplanted into transgenic rats. Exp Eye Res. 2005; 80(4): 515–525. [DOI] [PubMed] [Google Scholar]
- 55. Akagi T, Haruta M, Akita J, Nishida A, Honda Y, Takahashi M.. Different characteristics of rat retinal progenitor cells from different culture periods. Neurosci Lett. 2003; 341(3): 213–216. [DOI] [PubMed] [Google Scholar]
- 56. Chacko DM, Rogers JA, Turner JE, Ahmad I.. Survival and Differentiation of Cultured Retinal Progenitors Transplanted in the Subretinal Space of the Rat. Biochem Biophysic Res Commun. 2000; 268(3): 842–846. [DOI] [PubMed] [Google Scholar]
- 57. Tucker BA, Redenti SM, Jiang C, et al.. The use of progenitor cell/biodegradable MMP2-PLGA polymer constructs to enhance cellular integration and retinal repopulation. Biomaterials. 2010; 31(1): 9–19. [DOI] [PubMed] [Google Scholar]
- 58. Seiler MJ, Aramant RB.. Cell replacement and visual restoration by retinal sheet transplants. Prog Retin Eye Res. 2012; 31(6): 661–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lund RD, Ono SJ, Keegan DJ, Lawrence JM.. Retinal transplantation: progress and problems in clinical application. J Leukocyte Biol. 2003; 74(2): 151–160. [DOI] [PubMed] [Google Scholar]
- 60. Lavik EB, Klassen H, Warfvinge K, Langer R, Young MJ.. Fabrication of degradable polymer scaffolds to direct the integration and differentiation of retinal progenitors. Biomaterials. 2005; 26(16): 3187–3196. [DOI] [PubMed] [Google Scholar]
- 61. Pritchard CD, Arnér KM, Langer RS, Ghosh FK.. Retinal transplantation using surface modified poly(glycerol-co-sebacic acid) membranes. Biomaterials. 2010; 31(31): 7978–7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Neeley WL, Redenti S, Klassen H, et al.. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials. 2008; 29(4): 418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Redenti S, Neeley WL, Rompani S, et al.. Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials. 2009; 30(20): 3405–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yao J, Ko CW, Baranov PY, et al.. Enhanced differentiation and delivery of mouse retinal progenitor cells using a micropatterned biodegradable thin-film polycaprolactone scaffold. Tissue Eng Part A. 2015; 21(7-8): 1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yao J, Tucker BA, Zhang X, Checa-Casalengua P, Herrero-Vanrell R, Young MJ.. Robust cell integration from co-transplantation of biodegradable MMP2-PLGA microspheres with retinal progenitor cells. Biomaterials. 2011; 32(4): 1041–1050. [DOI] [PubMed] [Google Scholar]
- 66. Steedman MR, Tao SL, Klassen H, Desai TA.. Enhanced differentiation of retinal progenitor cells using microfabricated topographical cues. Biomed Microdevices. 2010; 12(3): 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ballios BG, Cooke MJ, Donaldson L, et al.. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation. Stem Cell Reports. 2015; 4(6): 1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ballios BG, Cooke MJ, van der Kooy D, Shoichet MS.. A hydrogel-based stem cell delivery system to treat retinal degenerative diseases. Biomaterials. 2010; 31(9): 2555–2564. [DOI] [PubMed] [Google Scholar]
- 69. Silverman MS, Hughes SE.. Transplantation of photoreceptors to light-damaged retina. Invest Ophthalmol Vis Sci. 1989; 30(8): 1684–1690. [PubMed] [Google Scholar]
- 70. Tomita M, Lavik E, Klassen H, Zahir T, Langer R, Young MJ.. Biodegradable polymer composite grafts promote the survival and differentiation of retinal progenitor cells. Stem cells (Dayton, Ohio). 2009; 23(10): 1579–1588. [DOI] [PubMed] [Google Scholar]
- 71. Tao S, Young C, Redenti S, et al.. Survival, migration and differentiation of retinal progenitor cells transplanted on micro-machined poly(methyl methacrylate) scaffolds to the subretinal space. Lab Chip. 2007; 7(6): 695–701. [DOI] [PubMed] [Google Scholar]
- 72. MacLaren RE, Pearson RA, MacNeil A, et al.. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006; 444(7116): 203–207. [DOI] [PubMed] [Google Scholar]
- 73. Bartsch U, Oriyakhel W, Kenna PF, et al.. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp Eye Res. 2008; 86(4): 691–700. [DOI] [PubMed] [Google Scholar]
- 74. West EL, Pearson RA, Tschernutter M, Sowden JC, MacLaren RE, Ali RR.. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res. 2008; 86(4): 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pearson RA, Barber AC, West EL, et al.. Targeted disruption of outer limiting membrane junctional proteins (Crb1 and ZO-1) increases integration of transplanted photoreceptor precursors into the adult wild-type and degenerating retina. Cell Transplant. 2010; 19(4): 487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Barber AC, Hippert C, Duran Y, et al.. Repair of the degenerate retina by photoreceptor transplantation. Proc Natl Acad Sci USA. 2013; 110(1): 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kinouchi R, Takeda M, Yang L, et al.. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat Neurosci. 2003; 6(8): 863–868. [DOI] [PubMed] [Google Scholar]
- 78. Suzuki T, Akimoto M, Imai H, et al.. Chondroitinase ABC treatment enhances synaptogenesis between transplant and host neurons in model of retinal degeneration. Cell Transplant. 2007; 16(5): 493–503. [DOI] [PubMed] [Google Scholar]
- 79. Mandai M, Homma K, Okamoto S, Yamada C, Nomori A, Takahashi M.. Adequate Time Window and Environmental Factors Supporting Retinal Graft Cell Survival in RD Mice. Cell Med. 2012; 4(1): 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ma J, Kabiel M, Tucker BA, Ge J, Young MJ.. Combining chondroitinase ABC and growth factors promotes the integration of murine retinal progenitor cells transplanted into Rho(-/-) mice. Mol Vis. 2011; 17: 1759–1770. [PMC free article] [PubMed] [Google Scholar]
- 81. Lakowski J, Baron M, Bainbridge J, et al.. Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. Human Molecular Genetics. 2010; 19(23): 4545–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pearson RA, Barber AC, Rizzi M, et al.. Restoration of vision after transplantation of photoreceptors. Nature. 2012; 485(7396): 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Smiley S, Nickerson PE, Comanita L, et al.. Establishment of a cone photoreceptor transplantation platform based on a novel cone-GFP reporter mouse line. Sci Rep. 2016; 6: 22867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Eberle D, Schubert S, Postel K, Corbeil D, Ader M.. Increased integration of transplanted CD73-positive photoreceptor precursors into adult mouse retina. Invest Ophthalmol Vis Sci. 2011; 52(9): 6462–6471. [DOI] [PubMed] [Google Scholar]
- 85. Eberle D, Santos-Ferreira T, Grahl S, Ader M.. Subretinal transplantation of MACS purified photoreceptor precursor cells into the adult mouse retina. J Vis Exp. 2014;(84): e50932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lakowski J, Han Y-T, Pearson RA, et al.. Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. Stem Cells. 2011; 29(9): 1391–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jayakody SA, Gonzalez-Cordero A, Ali RR, Pearson RA.. Cellular strategies for retinal repair by photoreceptor replacement. Prog Retin Eye Res. 2015; 46: 31–66. [DOI] [PubMed] [Google Scholar]
- 88. Santos-Ferreira T, Postel K, Stutzki H, Kurth T, Zeck G, Ader M.. Daylight vision repair by cell transplantation. Stem Cells. 2015; 33(1): 79–90. [DOI] [PubMed] [Google Scholar]
- 89. Singh MS, Charbel Issa P, Butler R, et al.. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc Natl Acad Sci USA. 2013; 110(3): 1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Neves J, Zhu J, Sousa-Victor P, et al.. Immune modulation by MANF promotes tissue repair and regenerative success in the retina. Science. 2016; 353(6294): aaf3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. West EL, Pearson RA, Barker SE, et al.. Long-Term Survival of Photoreceptors Transplanted into the Adult Murine Neural Retina Requires Immune Modulation. Stem Cells. 2010; 28(11): 1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Singh MS, Aslam SA, Duncan IL, Cramer AO, Barnard AR, MacLaren RE.. Cell fusion following photoreceptor transplantation into the non-degenerate retina. Invest Ophthalmol Vis Sci. 2014; 55(13): 3989–3989. [Google Scholar]
- 93. Das T, del Cerro M, Jalali S, et al.. The transplantation of human fetal neuroretinal cells in advanced retinitis pigmentosa patients: results of a long-term safety study. Exp Neurol. 1999; 157(1): 58–68. [DOI] [PubMed] [Google Scholar]
- 94. Humayun MS, de Juan E, del Cerro M, et al.. Human neural retinal transplantation. Invest Ophthalmol Vis Sci. 2000; 41(10): 3100–3106. [PubMed] [Google Scholar]
- 95. Radtke ND, Aramant RB, Petry HM, Green PT, Pidwell DJ, Seiler MJ.. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol. 2008; 146(2): 172–182. [DOI] [PubMed] [Google Scholar]
- 96. Radtke ND, Aramant RB, Seiler MJ, Petry HM, Pidwell D.. Vision change after sheet transplant of fetal retina with retinal pigment epithelium to a patient with retinitis pigmentosa. Arch Ophthalmol. 2004; 122(8): 1159–1165. [DOI] [PubMed] [Google Scholar]
- 97. Radtke ND, Seiler MJ, Aramant RB, Petry HM, Pidwell DJ.. Transplantation of intact sheets of fetal neural retina with its retinal pigment epithelium in retinitis pigmentosa patients. Am J Ophthalmol. 2002; 133(4): 544–550. [DOI] [PubMed] [Google Scholar]
- 98. Uyama H, Mandai M, Takahashi M.. Stem-cell-based therapies for retinal degenerative diseases: Current challenges in the establishment of new treatment strategies. Develop Growth Differentiation. 2021; 63(1): 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Stern JH, Temple S.. Stem Cells for Retinal Replacement Therapy. Neurotherapeutics. 2011; 8(4): 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Applications I of M (US) CC on FR and. Setting the Stage: Fetal Research, Fetal Tissue Research, and Historical Timeline of Regulation and Legislation. US: National Academies Press; 1994. Accessed June 6, 2021, https://www.ncbi.nlm.nih.gov/books/NBK231997/. [Google Scholar]
- 101. Stem Cell Scientists Answer the Question. Why Is It Important to You to Support Fetal Tissue Research? Stem Cell Reports. 2019; 12(2): 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schmitt S, Aftab U, Jiang C, et al.. Molecular characterization of human retinal progenitor cells. Invest Ophthalmol Vis Sci. 2009; 50(12): 5901–5908. [DOI] [PubMed] [Google Scholar]
- 103. Yang P, Seiler MJ, Aramant RB, Whittemore SR.. In vitro isolation and expansion of human retinal progenitor cells. Exp Neurol. 2002; 177(1): 326–331. [DOI] [PubMed] [Google Scholar]
- 104. Hasan SM, Vugler AA, Miljan EA, Sinden JD, Moss SE, Greenwood J.. Immortalized human fetal retinal cells retain progenitor characteristics and represent a potential source for the treatment of retinal degenerative disease. Cell Transplant. 2010; 19(10): 1291–1306. [DOI] [PubMed] [Google Scholar]
- 105. Wright LS, Pinilla I, Saha J, et al.. VSX2 and ASCL1 Are Indicators of Neurogenic Competence in Human Retinal Progenitor Cultures. PLoS One. 2015; 10(8): e0135830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Gamm DM, Wright LS, Capowski EE, et al.. Regulation of prenatal human retinal neurosphere growth and cell fate potential by retinal pigment epithelium and Mash1. Stem Cells. 2008; 26(12): 3182–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Klassen H. Stem cells in clinical trials for treatment of retinal degeneration. Expert Opin Biol Ther. 2016; 16(1): 7–14. [DOI] [PubMed] [Google Scholar]
- 108. Klassen H, Kiilgaard JF, Zahir T, et al.. Progenitor Cells from the Porcine Neural Retina Express Photoreceptor Markers After Transplantation to the Subretinal Space of Allorecipients. Stem Cells. 2007; 25(5): 1222–1230. [DOI] [PubMed] [Google Scholar]
- 109. Semo M, Haamedi N, Stevanato L, et al.. Efficacy and Safety of Human Retinal Progenitor Cells. Transl Vis Sci Technol. 2016; 5(4): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Luo J, Baranov P, Patel S, et al.. Human retinal progenitor cell transplantation preserves vision. J Biol Chem. 2014; 289(10): 6362–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al.. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282(5391): 1145–1147. [DOI] [PubMed] [Google Scholar]
- 112. Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R.. Derivation and Comparative Assessment of Retinal Pigment Epithelium from Human Embryonic Stem Cells Using Transcriptomics. Cloning and Stem Cells. 2004; 6(3): 217–245. [DOI] [PubMed] [Google Scholar]
- 113. Ikeda H, Osakada F, Watanabe K, et al.. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci USA. 2005; 102(32): 11331–11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhao X, Liu J, Ahmad I.. Differentiation of embryonic stem cells into retinal neurons. Biochem Biophys Res Commun. 2002; 297(2): 177–184. [DOI] [PubMed] [Google Scholar]
- 115. Banin E, Obolensky A, Idelson M, et al.. Retinal Incorporation and Differentiation of Neural Precursors Derived from Human Embryonic Stem Cells. Stem Cells. 2006; 24(2): 246–257. [DOI] [PubMed] [Google Scholar]
- 116. Lamba DA, Karl MO, Ware CB, Reh TA.. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA. 2006; 103(34): 12769–12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Osakada F, Ikeda H, Mandai M, et al.. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008; 26(2): 215–224. [DOI] [PubMed] [Google Scholar]
- 118. Yu J, Vodyanik MA, Smuga-Otto K, et al.. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318(5858): 1917–1920. [DOI] [PubMed] [Google Scholar]
- 119. Takahashi K, Tanabe K, Ohnuki M, et al.. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007; 131(5): 861–872. [DOI] [PubMed] [Google Scholar]
- 120. Pankratz MT, Li X-J, LaVaute TM, Lyons EA, Chen X, Zhang S-C.. Directed Neural Differentiation of Human Embryonic Stem Cells via an Obligated Primitive Anterior Stage. Stem Cells. 2007; 25(6): 1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hirami Y, Osakada F, Takahashi K, et al.. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009; 458(3): 126–131. [DOI] [PubMed] [Google Scholar]
- 122. Meyer JS, Shearer RL, Capowski EE, et al.. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci USA. 2009; 106(39): 16698–16703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lamba DA, Gust J, Reh TA.. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009; 4(1): 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, Reh TA.. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010; 5(1): e8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Meyer JS, Howden SE, Wallace KA, et al.. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011; 29(8): 1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Nakano T, Ando S, Takata N, et al.. Self-Formation of Optic Cups and Storable Stratified Neural Retina from Human ESCs. Cell Stem Cell. 2012; 10(6): 771–785. [DOI] [PubMed] [Google Scholar]
- 127. Zhong X, Gutierrez C, Xue T, et al.. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014; 5: 4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kuwahara A, Ozone C, Nakano T, Saito K, Eiraku M, Sasai Y.. Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat Commun. 2015; 6: 6286. [DOI] [PubMed] [Google Scholar]
- 129. Capowski EE, Samimi K, Mayerl SJ, et al.. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development. 2019; 146(1): dev171686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Phillips MJ, Wallace KA, Dickerson SJ, et al.. Blood-Derived Human iPS Cells Generate Optic Vesicle–Like Structures with the Capacity to Form Retinal Laminae and Develop Synapses Production of Retina from Human Blood iPS Cells. Invest Ophthalmol Vis Sci. 2012; 53(4): 2007–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hallam D, Hilgen G, Dorgau B, et al.. Human-Induced Pluripotent Stem Cells Generate Light Responsive Retinal Organoids with Variable and Nutrient-Dependent Efficiency. Stem Cells (Dayton, Ohio). 2018; 36(10): 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Kim S, Lowe A, Dharmat R, et al.. Generation, transcriptome profiling, and functional validation of cone-rich human retinal organoids. Proc Natl Acad Sci USA. 2019; 116(22): 10824–10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bell CM, Zack DJ, Berlinicke CA.. Human Organoids for the Study of Retinal Development and Disease. Annu Rev Vis Sci. 2020; 6: 91–114. [DOI] [PubMed] [Google Scholar]
- 134. Cowan CS, Renner M, De Gennaro M, et al.. Cell Types of the Human Retina and Its Organoids at Single-Cell Resolution. Cell. 2020; 182(6): 1623–1640.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Phillips MJ, Jiang P, Howden S, et al.. A Novel Approach to Single Cell RNA-Sequence Analysis Facilitates In Silico Gene Reporting of Human Pluripotent Stem Cell-Derived Retinal Cell Types. Stem Cells. 2018; 36(3): 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Assawachananont J, Mandai M, Okamoto S, et al.. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports. 2014; 2(5): 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Decembrini S, Koch U, Radtke F, Moulin A, Arsenijevic Y.. Derivation of Traceable and Transplantable Photoreceptors from Mouse Embryonic Stem Cells. Stem Cell Reports. 2014; 2(6): 853–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Gonzalez-Cordero A, West EL, Pearson RA, et al.. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013; 31(8): 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Kruczek K, Gonzalez-Cordero A, Goh D, et al.. Differentiation and Transplantation of Embryonic Stem Cell-Derived Cone Photoreceptors into a Mouse Model of End-Stage Retinal Degeneration. Stem Cell Reports. 2017; 8(6): 1659–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Santos-Ferreira T, Völkner M, Borsch O, et al.. Stem Cell–Derived Photoreceptor Transplants Differentially Integrate Into Mouse Models of Cone-Rod Dystrophy. Invest Ophthalmol Vis Sci. 2016; 57(7): 3509–3520. [DOI] [PubMed] [Google Scholar]
- 141. Mandai M, Fujii M, Hashiguchi T, et al.. iPSC-Derived Retina Transplants Improve Vision in rd1 End-Stage Retinal-Degeneration Mice. Stem Cell Reports. 2017; 8(1): 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tucker BA, Park I-H, Qi SD, et al.. Transplantation of Adult Mouse iPS Cell-Derived Photoreceptor Precursors Restores Retinal Structure and Function in Degenerative Mice. PLoS One. 2011; 6(4): e18992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. West EL, Gonzalez-Cordero A, Hippert C, et al.. Defining the integration capacity of embryonic stem cell-derived photoreceptor precursors. Stem Cells. 2012; 30(7): 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Gonzalez-Cordero A, Kruczek K, Naeem A, et al.. Recapitulation of Human Retinal Development from Human Pluripotent Stem Cells Generates Transplantable Populations of Cone Photoreceptors. Stem Cell Reports. 2017; 9(3): 820–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zhu J, Cifuentes H, Reynolds J, Lamba DA.. Immunosuppression via Loss of IL2rγ Enhances Long-Term Functional Integration of hESC-Derived Photoreceptors in the Mouse Retina. Cell Stem Cell. 2017; 20(3): 374–384.e5. [DOI] [PubMed] [Google Scholar]
- 146. Iraha S, Tu H-Y, Yamasaki S, et al.. Establishment of Immunodeficient Retinal Degeneration Model Mice and Functional Maturation of Human ESC-Derived Retinal Sheets after Transplantation. Stem Cell Reports. 2018; 10(3): 1059–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tu H-Y, Watanabe T, Shirai H, et al.. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine. 2019; 39: 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Shirai H, Mandai M, Matsushita K, et al.. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci U S A. 2016; 113(1): E81–E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. McLelland BT, Lin B, Mathur A, et al.. Transplanted hESC-Derived Retina Organoid Sheets Differentiate, Integrate, and Improve Visual Function in Retinal Degenerate Rats. Invest Ophthalmol Vis Sci. 2018; 59(6): 2586–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Seiler MJ, Aramant RB, Jones MK, Ferguson DL, Bryda EC, Keirstead HS.. A new immunodeficient pigmented retinal degenerate rat strain to study transplantation of human cells without immunosuppression. Graefes Arch Clin Exp Ophthalmol. 2014; 252(7): 1079–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hambright D, Park KY, Brooks M, McKay R, Swaroop A, Nasonkin IO.. Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina. Molecular Vision. 2012; 18: 920–936. [PMC free article] [PubMed] [Google Scholar]
- 152. Singh MS, Balmer J, Barnard AR, et al.. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat Commun. 2016; 7: 13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Pearson RA, Gonzalez-Cordero A, West EL, et al.. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat Commun. 2016; 7: 13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Santos-Ferreira T, Llonch S, Borsch O, Postel K, Haas J, Ader M.. Retinal transplantation of photoreceptors results in donor-host cytoplasmic exchange. Nat Commun. 2016; 7: 13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ortin-Martinez A, Tsai ELS, Nickerson PE, et al.. A Reinterpretation of Cell Transplantation: GFP Transfer From Donor to Host Photoreceptors. Stem Cells. 2017; 35(4): 932–939. [DOI] [PubMed] [Google Scholar]
- 156. Decembrini S, Martin C, Sennlaub F, et al.. Cone Genesis Tracing by the Chrnb4-EGFP Mouse Line: Evidences of Cellular Material Fusion after Cone Precursor Transplantation. Mol Ther. 2017; 25(3): 634–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Waldron PV, Di Marco F, Kruczek K, et al.. Transplanted Donor- or Stem Cell-Derived Cone Photoreceptors Can Both Integrate and Undergo Material Transfer in an Environment-Dependent Manner. Stem Cell Reports. 2018; 10(2): 406–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. MacLaren RE. Cone fusion confusion in photoreceptor transplantation. Stem Cell Investig. 2017; 4: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Barnea-Cramer AO, Wang W, Lu S-J, et al.. Function of human pluripotent stem cell-derived photoreceptor progenitors in blind mice. Sci Rep. 2016; 6: 29784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Aboualizadeh E, Phillips MJ, McGregor JE, et al.. Imaging Transplanted Photoreceptors in Living Nonhuman Primates with Single-Cell Resolution. Stem Cell Reports. 2020; 15(2): 482–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ribeiro J, Procyk CA, West EL, et al.. Restoration of visual function in advanced disease after transplantation of purified human pluripotent stem cell-derived cone photoreceptors. Cell Reports. 2021; 35(3): 109022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Gagliardi G, Ben M’Barek K, Chaffiol A, et al.. Characterization and Transplantation of CD73-Positive Photoreceptors Isolated from Human iPSC-Derived Retinal Organoids. Stem Cell Reports. 2018; 11(3): 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Garita-Hernandez M, Lampič M, Chaffiol A, et al.. Restoration of visual function by transplantation of optogenetically engineered photoreceptors. Nature Communications. 2019; 10(1): 4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wiley LA, Burnight ER, DeLuca AP, et al.. cGMP production of patient-specific iPSCs and photoreceptor precursor cells to treat retinal degenerative blindness. Sci Rep. 2016; 6: 30742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Reichman S, Slembrouck A, Gagliardi G, et al.. Generation of Storable Retinal Organoids and Retinal Pigmented Epithelium from Adherent Human iPS Cells in Xeno-Free and Feeder-Free Conditions. Stem Cells (Dayton, Ohio). 2017; 35(5): 1176–1188. [DOI] [PubMed] [Google Scholar]
- 166. Stone NE, Voigt AP, Mullins RF, Sulchek T, Tucker BA.. Microfluidic processing of stem cells for autologous cell replacement. Stem Cells Transl Med. 2021; 10(10): 1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Huang C-Y, Liu C-L, Ting C-Y, et al.. Human iPSC banking: barriers and opportunities. J Biomed Sci. 2019; 26: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kramer J, Chirco KR, Lamba DA.. Immunological Considerations for Retinal Stem Cell Therapy. In: Bharti K, ed. Pluripotent Stem Cells in Eye Disease Therapy. Advances in Experimental Medicine and Biology. New York, NY: Springer International Publishing; 2019: 99–119. [DOI] [PubMed] [Google Scholar]
- 169. Petrus-Reurer S, Winblad N, Kumar P, et al.. Generation of Retinal Pigment Epithelial Cells Derived from Human Embryonic Stem Cells Lacking Human Leukocyte Antigen Class I and II. Stem Cell Reports. 2020; 14(4): 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Creasey AA, Stacey G, Bharti K, Sato Y, Lubiniecki A.. A strategic road map to filing a Biologics License Application for a pluripotent stem cell derived therapeutic product. Biologicals. 2019; 59: 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wright LS, Phillips MJ, Pinilla I, Hei D, Gamm DM.. Induced pluripotent stem cells as custom therapeutics for retinal repair: Progress and rationale. Exp Eye Res. 2014; 123: 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ovando-Roche P, West EL, Branch MJ, et al.. Use of bioreactors for culturing human retinal organoids improves photoreceptor yields. Stem Cell Res Ther. 2018; 9(1): 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Xue Y, Seiler MJ, Tang WC, et al.. Retinal organoids on-a-chip: a micro-millifluidic bioreactor for long-term organoid maintenance. Lab Chip. 2021; 21(17): 3361–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Koso H, Minami C, Tabata Y, et al.. CD73, a novel cell surface antigen that characterizes retinal photoreceptor precursor cells. Invest Ophthalmol Vis Sci. 2009; 50(11): 5411–5418. [DOI] [PubMed] [Google Scholar]
- 175. Stone NE, Voigt AP, Cooke JA, et al.. Label-free microfluidic enrichment of photoreceptor cells. Exp Eye Res. 2020; 199: 108166. [DOI] [PubMed] [Google Scholar]
- 176. Lakowski J, Welby E, Budinger D, et al.. Isolation of Human Photoreceptor Precursors via a Cell Surface Marker Panel from Stem Cell-Derived Retinal Organoids and Fetal Retinae. Stem Cells. 2018; 36(5): 709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Collin J, Zerti D, Queen R, et al.. CRX Expression in Pluripotent Stem Cell-Derived Photoreceptors Marks a Transplantable Subpopulation of Early Cones. Stem Cells. 2019; 37(5): 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ludwig A, Phillips J, Jager L, et al.. Transplantation of human pluripotent stem cell-derived photoreceptors on a biocompatible scaffold in the S334ter rat. Invest Ophthalmol Vis Sci. 2019; 60(9): 2886–2886. [Google Scholar]
- 179. Lin B, McLelland BT, Aramant RB, et al.. Retina Organoid Transplants Develop Photoreceptors and Improve Visual Function in RCS Rats With RPE Dysfunction. Invest Ophthalmol Vis Sci. 2020; 61(11): 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Phillips MJ, Capowski EE, Petersen A, et al.. Generation of a rod-specific NRL reporter line in human pluripotent stem cells. Scientific Reports. 2018; 8(1): 2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Kaewkhaw R, Swaroop M, Homma K, et al.. Treatment Paradigms for Retinal and Macular Diseases Using 3-D Retina Cultures Derived From Human Reporter Pluripotent Stem Cell Lines. Invest Ophthalmol Vis Sci. 2016; 57(5): ORSFl1–ORSFl11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Kaewkhaw R, Kaya KD, Brooks M, et al.. Transcriptome Dynamics of Developing Photoreceptors in Three-Dimensional Retina Cultures Recapitulates Temporal Sequence of Human Cone and Rod Differentiation Revealing Cell Surface Markers and Gene Networks. Stem Cells (Dayton, Ohio). 2015; 33(12): 3504–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Vergara MN, Flores-Bellver M, Aparicio-Domingo S, et al.. Three-dimensional automated reporter quantification (3D-ARQ) technology enables quantitative screening in retinal organoids. Development. 2017; 144(20): 3698–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. McClements ME, Staurenghi F, MacLaren RE, Cehajic-Kapetanovic J.. Optogenetic Gene Therapy for the Degenerate Retina: Recent Advances. Front Neurosci. 2020; 14: 570909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Singh R, Phillips MJ, Kuai D, et al.. Functional analysis of serially expanded human iPS cell-derived RPE cultures. Invest Ophthalmol Vis Sci. 2013; 54(10): 6767–6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Sinha D, Steyer B, Shahi PK, et al.. Human iPSC Modeling Reveals Mutation-Specific Responses to Gene Therapy in a Genotypically Diverse Dominant Maculopathy. Am J Hum Genet. 2020; 107(2): 278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Engle SJ, Blaha L, Kleiman RJ.. Best Practices for Translational Disease Modeling Using Human iPSC-Derived Neurons. Neuron. 2018; 100(4): 783–797. [DOI] [PubMed] [Google Scholar]
- 188. Kruczek K, Swaroop A.. Pluripotent stem cell-derived retinal organoids for disease modeling and development of therapies. Stem Cells. 2020; 38: 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Garita-Hernandez M, Chaffiol A, Guibbal L, et al.. Control of Microbial Opsin Expression in Stem Cell Derived Cones for Improved Outcomes in Cell Therapy. Front Cell Neurosci. 2021; 15: 648210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Akiba R, Matsuyama T, Tu HY, et al.. Quantitative and Qualitative Evaluation of Photoreceptor Synapses in Developing, Degenerating and Regenerating Retinas. Frontiers in Cellular Neuroscience. 2019; 13: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Worthington KS, Wiley LA, Kaalberg EE, et al.. Two-photon polymerization for production of human iPSC-derived retinal cell grafts. Acta Biomater. 2017; 55: 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Jung YH, Phillips MJ, Lee J, et al.. 3D Microstructured Scaffolds to Support Photoreceptor Polarization and Maturation. Advanced Materials (Deerfield Beach, Fla). 2018; 30(39): e1803550. [DOI] [PubMed] [Google Scholar]
- 193. Lee I-K, Ludwig AL, Phillips MJ, et al.. Ultrathin micromolded 3D scaffolds for high-density photoreceptor layer reconstruction. Science Advances. 2021; 7(17): eabf0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sharma R, Khristov V, Rising A, et al.. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med. 2019; 11(475): eaat5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Kashani AH, Uang J, Mert M, et al.. Surgical Method for Implantation of a Biosynthetic Retinal Pigment Epithelium Monolayer for Geographic Atrophy: Experience from a Phase 1/2a Study. Ophthalmol Retina. 2020; 4: 264–273. [DOI] [PubMed] [Google Scholar]
- 196. Thompson JR, Worthington KS, Green BJ, et al.. Two-photon polymerized poly(caprolactone) retinal cell delivery scaffolds and their systemic and retinal biocompatibility. Acta Biomaterialia. 2019; 941: 204–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Wendland RJ, Jiao C, Russell SR, et al.. The effect of retinal scaffold modulus on performance during surgical handling. Exp Eye Res. 2021; 207: 108566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Singh MS, MacLaren RE.. Stem Cell Treatment for Age-Related Macular Degeneration: the Challenges. Invest Ophthalmol Vis Sci. 2018; 59(4): AMD78–AMD82. [DOI] [PubMed] [Google Scholar]
- 199. Klassen H, Kiilgaard JF, Warfvinge K, et al.. Photoreceptor Differentiation following Transplantation of Allogeneic Retinal Progenitor Cells to the Dystrophic Rhodopsin Pro347Leu Transgenic Pig. Stem Cells International. 2012; 2012: e939801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zhao X, van Praag H.. Steps towards standardized quantification of adult neurogenesis. Nature Communications. 2020; 11(1): 4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Schmitz C, Hof PR.. Design-based stereology in neuroscience. Neuroscience. 2005; 130(4): 813–831. [DOI] [PubMed] [Google Scholar]
- 202. Schmitz C, Eastwood BS, Tappan SJ, Glaser JR, Peterson DA, Hof PR.. Current automated 3D cell detection methods are not a suitable replacement for manual stereologic cell counting. Front Neuroanat. 2014; 8: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Zerti D, Hilgen G, Dorgau B, et al.. Transplanted pluripotent stem cell-derived photoreceptor precursors elicit conventional and unusual light responses in mice with advanced retinal degeneration. Stem Cells. 2021; 39: 882–896. [DOI] [PubMed] [Google Scholar]
- 204. Zhang KY, Aguzzi EA, Johnson TV.. Retinal Ganglion Cell Transplantation: Approaches for Overcoming Challenges to Functional Integration. Cells. 2021; 10(6): 1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Zerti D, Hilgen G, Dorgau B, et al.. Transplanted pluripotent stem cell-derived photoreceptor precursors elicit conventional and unusual light responses in mice with advanced retinal degeneration. Stem Cells. 2021; 39: 882–896. [DOI] [PubMed] [Google Scholar]
- 206. Laver CRJ, Matsubara JA.. Structural divergence of essential triad ribbon synapse proteins among placental mammals – Implications for preclinical trials in photoreceptor transplantation therapy. Experimental Eye Research. 2017; 159: 156–167. [DOI] [PubMed] [Google Scholar]
- 207. Pardue MT, Phillips MJ, Yin H, et al.. Neuroprotective Effect of Subretinal Implants in the RCS Rat. Invest Ophthalmol Vis Sci. 2005; 46(2): 674–682. [DOI] [PubMed] [Google Scholar]
- 208. Bryda EC, LaVail MM.. Letter to the editor announcing the availability of RCS and transgenic rats with P23H and S334ter rhodopsin mutations with inherited retinal degenerations. Exp Eye Res. 2019; 178: 176. [DOI] [PubMed] [Google Scholar]
- 209. Fujii M, Sunagawa GA, Kondo M, Takahashi M, Mandai M.. Evaluation of micro Electroretinograms Recorded with Multiple Electrode Array to Assess Focal Retinal Function. Sci Rep. 2016; 6(1): 30719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Lyubarsky A, Bennett J.. Light Avoidance in Mice May Be not Related to Photoreceptor-Driven Input. Invest Ophthalmol Vis Sci. 2016; 57(12): 2768–2768. [Google Scholar]
- 211. Winkler PA, Occelli LM, Petersen-Jones SM.. Large Animal Models of Inherited Retinal Degenerations: A Review. Cells. 2020; 9(4): 882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Lamb TD. Evolution of vertebrate retinal photoreception. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009; 364(1531): 2911–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Zarbin M. Cell-Based Therapy for Degenerative Retinal Disease. Trends in Molecular Medicine. 2016; 22(2): 115–134. [DOI] [PubMed] [Google Scholar]
- 214. Zarbin M. Cell-Based Therapy for Retinal Disease: The New Frontier. Methods Mol Biol. 2019; 1834: 367–381. [DOI] [PubMed] [Google Scholar]
- 215. Wynne N, Carroll J, Duncan JL. Promises and pitfalls of evaluating photoreceptor-based retinal disease with adaptive optics scanning light ophthalmoscopy (AOSLO). Progress in Retinal and Eye Research. 2021; 83: 100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. McGregor JE, Williams DR, Merigan WH.. Functional Assessment of Vision Restoration. Adv Exp Med Biol. 2019; 1185: 145–149. [DOI] [PubMed] [Google Scholar]
- 217. Thompson DA, Iannaccone A, Ali RR, et al.. Advancing Clinical Trials for Inherited Retinal Diseases: Recommendations from the Second Monaciano Symposium. Transl Vis Sci Technol. 2020; 9(7): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Liu YV, Sodhi SK, Xue G, et al.. Quantifiable In Vivo Imaging Biomarkers of Retinal Regeneration by Photoreceptor Cell Transplantation. Trans Vis Sci Tech. 2020; 9(7): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Reh TA. Photoreceptor Transplantation in Late Stage Retinal Degeneration. Invest Ophthalmol Vis Sci. 2016; 57(5): ORSFg1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Hippert C, Graca AB, Basche M, et al.. RNAi-mediated suppression of vimentin or glial fibrillary acidic protein prevents the establishment of Müller glial cell hypertrophy in progressive retinal degeneration. Glia. 2021; 69: 2272–2290. [DOI] [PubMed] [Google Scholar]
- 221. Chan K, Hoon M, Pattnaik BR, et al.. Vigabatrin-Induced Retinal Functional Alterations and Second-Order Neuron Plasticity in C57BL/6J Mice. Invest Ophthalmol Vis Sci. 2020; 61(2): 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Tsai ELS, Ortin-Martinez A, Gurdita A, et al.. Modeling of Photoreceptor Donor-Host Interaction Following Transplantation Reveals a Role for Crx, Müller Glia, and Rho/ROCK Signaling in Neurite Outgrowth. Stem Cells. 2019; 37(4): 529–541. [DOI] [PubMed] [Google Scholar]
- 223. Hoon M, Okawa H, Della Santina L, Wong ROL. Functional architecture of the retina: development and disease. Prog Retin Eye Res. 2014; 42: 44–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Itoh M, Mukae Y, Kitsuka T, et al.. Development of an immunodeficient pig model allowing long-term accommodation of artificial human vascular tubes. Nature Communications. 2019; 10(1): 2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Singh RK, Occelli LM, Binette F, Petersen-Jones SM, Nasonkin IO.. Transplantation of Human Embryonic Stem Cell-Derived Retinal Tissue in the Subretinal Space of the Cat Eye. Stem Cells and Development. 2019; 28(17): 1151–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Yamasaki S, Sugita S, Horiuchi M, et al.. Low Immunogenicity and Immunosuppressive Properties of Human ESC- and iPSC-Derived Retinas. Stem Cell Reports. 2021; 16(4): 851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Saraf Steven S, Cunningham Matthew A, Kuriyan Ajay E, et al.. Bilateral Retinal Detachments After Intravitreal Injection of Adipose-Derived ‘Stem Cells’ in a Patient With Exudative Macular Degeneration. Ophthalmic Surgery, Lasers and Imaging Retina. 2017; 48(9): 772–775. [DOI] [PubMed] [Google Scholar]
- 228. Leung Ella H, Flynn Harry W, Albini Thomas A, Medina Carlos A.. Retinal Detachment After Subretinal Stem Cell Transplantation. Ophthalmic Surgery, Lasers and Imaging Retina. 2016; 47(6): 600–601. [DOI] [PubMed] [Google Scholar]
- 229. Kuriyan AE, Albini TA, Townsend JH, et al.. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N Engl J Med. 2017; 376: 1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Knoepfler PS. The Stem Cell Hard Sell: Report from a Clinic's Patient Recruitment Seminar. Stem Cells Translational Medicine. 2017; 6(1): 14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Cossu G, Birchall M, Brown T, et al.. Lancet Commission: Stem cells and regenerative medicine. The Lancet. 2018; 391(10123): 883–910. [DOI] [PubMed] [Google Scholar]
- 232. Patient Resources. A Closer Look at Stem Cells. Accessed May 27, 2021, https://www.closerlookatstemcells.org/patient-resources/.
- 233. Age-Related Macular Degeneration. A Closer Look at Stem Cells. Accessed May 27, 2021, https://www.closerlookatstemcells.org/stem-cells-medicine/age-related-macular-degeneration/.
- 234. Zarbin M. What Constitutes Translational Research? Implications for the Scope of Translational Vision Science and Technology. Transl Vis Sci Technol. 2020; 9(8): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Haeckel EHPA. Anthropogenie. Germany: W. Engelmann; 1877. [Google Scholar]
- 236. Boveri: Ueber die Befruchtung der Eier von Ascaris... - Google Scholar. Accessed September 9, 2021, https://scholar.google.com/scholar_lookup?title=%C3%9Cber%20die%20Befruchtung%20der%20Eier%20von%20Ascaris%20megalocephala&author=T.%20Boveri&journal=Sitzungsberichte%20der%20Gesellschaft%20f%C3%BCr%20Morphologie%20und%20Physiologie%20in%20M%C3%BCnchen&volume=3&pages=71-80&publication_year=1887.
- 237. Häcker V. Die Kerntheilungsvorgänge bei der Mesodermund Entodermbildung von Cyclops. Archiv f mikrosk Anatomie. 1892; 39(1): 556–581. [Google Scholar]
- 238. Thomas ED, Lochte HL, Lu WC, Ferrebee JW.. Intravenous Infusion of Bone Marrow in Patients Receiving Radiation and Chemotherapy. N Engl J Med. 1957; 257(11): 491–496. [DOI] [PubMed] [Google Scholar]
- 239. Till JE, McCulloch EA.. A Direct Measurement of the Radiation Sensitivity of Normal Mouse Bone Marrow Cells. Radiation Research. 1961; 14(2): 213–222. [PubMed] [Google Scholar]
- 240. Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol. 1962; 10: 622–640. [PubMed] [Google Scholar]
- 241. Evans MJ, Kaufman MH.. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981; 292(5819): 154–156. [DOI] [PubMed] [Google Scholar]
- 242. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981; 78(12): 7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Thomson JA, Kalishman J, Golos TG, et al.. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA. 1995; 92(17): 7844–7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH.. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997; 385(6619): 810–813. [DOI] [PubMed] [Google Scholar]
- 245. Takahashi K, Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126(4): 663–676. [DOI] [PubMed] [Google Scholar]
- 246. Kondo M, Sakai T, Komeima K, et al.. Generation of a transgenic rabbit model of retinal degeneration. Invest Ophthalmol Vis Sci. 2009; 50(3): 1371–1377. [DOI] [PubMed] [Google Scholar]
- 247. Wang Z, Feng C, Yang R, et al.. Large-Area Photoreceptor Degeneration Model in Rabbits by Photocoagulation and Oxidative Stress in the Retina. Frontiers in Neuroscience. 2021; 15: 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Waide EH, Dekkers JCM, Ross JW, et al.. Not All SCID Pigs Are Created Equally: Two Independent Mutations in the Artemis Gene Cause SCID in Pigs. J Immunol. 2015; 195(7): 3171–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Stanzel B, Ader M, Liu Z, et al.. Surgical Approaches for Cell Therapeutics Delivery to the Retinal Pigment Epithelium and Retina. In: Bharti K, ed. Pluripotent Stem Cells in Eye Disease Therapy. Advances in Experimental Medicine and Biology. New York, NY: Springer International Publishing; 2019: 141–170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.