Abstract
Purpose
To investigate the biometric differences of anterior segment parameters between fellow eyes of acute primary angle closure (F-APAC) and chronic primary angle closure glaucoma (F-CPACG) to get information about differences between APAC and CPAC.
Methods
Patients with F-APAC and F-CPACG without prior treatment were enrolled from glaucoma clinics. Parameters were measured on ultrasound biomicroscopy images, including pupil diameter, lens vault (LV), anterior chamber depth, anterior chamber width, iris area, iris thickness (IT 750 and 2000), angle-opening distance (AOD 500 and 750), trabecular-iris space area (TISA 500 and 750), trabecular iris angle (TIA 500 and 750), trabecular–ciliary angle, and ciliary process area. Multivariate logistic regression analysis was performed to determine the most important parameters associated with F-APAC compared with F-CPACG.
Results
Fifty-five patients with APAC and 55 patients with CPACG were examined. The anterior chamber depth, IT 750, AOD 750, trabecular iris angle 750, and trabecular–ciliary angle were smaller, and LV and ciliary process area were greater in F-APAC as compared with F-CPACG (P ≤ 0.01). Multivariate logistic regression showed that thinner IT 750, smaller AOD 750, and larger LV were significantly associated with F-APAC (P < 0.01). IT 750 (area under the curve, 0.703) performed relatively better than AOD 750 (area under the curve, 0.696) in distinguishing F-APAC from F-CPACG, with the best cutoff of 0.404 mm and 0.126 mm, respectively.
Conclusions
Compared with F-CPACG, F-APAC had thinner peripheral iris, narrower anterior chamber angle, shallower anterior chamber depth, greater LV, larger and anteriorly positioned ciliary body. IT 750, AOD 750, and LV played important roles in distinguishing eyes predisposed to APAC or CPAC.
Keywords: acute primary angle closure, chronic primary angle closure, glaucoma anterior segment, ultrasound biomicroscopy, fellow eyes
Glaucoma is currently the leading cause of irreversible blindness worldwide.1 It is estimated that the condition affects 80 million people in 2020 and will increase to 112 million by 2040.2,3 A large proportion of the patients suffer from primary angle closure diseases (PACDs) in East Asia.2,3
PACDs refer to a spectrum of diseases, including primary angle closure suspect (PACS), primary angle closure (PAC), and PAC glaucoma (PACG).4 Among them, acute PAC (APAC) is an ophthalmic emergency characterized by a sudden episode of ocular or periocular pain, blurred vision, headache, nausea, and/or vomiting accompanied by an IOP elevation.5,6 In contrast, patients with chronic PAC (CPAC) rarely experience the dramatic symptoms and are associated with a gradual and insidious onset.5,6 Anatomical structural differences must exist between APAC and CPAC eyes to explain their differences in presentation and clinical course.
A number of studies have compared the anatomic structural differences in subtypes of PACDs, whereas there has been little research in comparisons between eyes with APAC and CPAC.7–12 Besides, the sequelae of the diseases such as iris atrophy and pupillary changes following APAC, and extensive peripheral anterior synechia (PAS) in CPAC could impede the measurement and evaluation of the original anatomical characteristics. The fellow eyes of APAC (F-APAC) and fellow eyes of CPACG (F-CPACG) could perform better in evaluation of the initial anatomical characteristics of APAC and CPAC, because PACDs have been described as a bilateral condition typically with asymmetric severity between eyes, and the F-APAC and F-CPACG have been confirmed to aggravate to APAC and CPACG, respectively, if not treated.13–15
A recent study compared the differences between F-APAC and F-CPACG using ultrasound biomicroscopy (UBM), and showed smaller anterior segment dimensions, higher lens vault (LV), more posterior iris insertion, greater iris curvature, and more anteriorly rotated ciliary body in F-APAC compared with F-CPACG.12 However, the peripheral iris thickness, angle width, and the area of ciliary process were not well-studied. In this study, we used UBM to compare anterior segment parameters between F-APAC and F-CPACG, with a special focus on peripheral iris thickness, angle width, and the area of ciliary process, to further investigate the anatomic structural differences between APAC and CPAC.
Methods
Patients
This was an observational comparative study of Chinese subjects approved by the ethics committee of Peking University People's Hospital and adhering to the tenets of the Declaration of Helsinki. Written informed consent was obtained for all subjects.
Subjects diagnosed with APAC and CPACG were recruited from the glaucoma clinics of Peking University People's Hospital from September 2016 through May 2021. A total of 116 eyes of 116 patients were recruited from the clinic, of whom 6 were excluded for poor UBM image quality. Finally, 110 eyes of 110 patients were enrolled.
APAC was defined as eyes with two of the following symptoms: ocular or periocular pain, headache, nausea and/or vomiting, blurred vision, and halos around lights; and the following ophthalmologic findings: an IOP of more than 30 mm Hg, conjunctival hyperemia, corneal epithelial oedema, shallow anterior chamber with angle closure, iris bombe, and mid-dilated pupil. The fellow eyes of APAC (F-APAC) were defined as the fellow eyes of patients with a recent unilateral APAC that never experienced an acute attack and showed no signs of a prior acute attack (no PAS).
Eyes with CPACG were defined as eyes without symptoms or signs of a prior acute attack, including glaucomatous fleck, keratic precipitates, or iris atrophy. Patients had more than three cumulative clock-hours of PAS and a chronically elevated IOP (>21 mm Hg), along with glaucomatous optic neuropathy or visual field defect in CPACG eyes. F-CPACG was defined as the less severe fellow eyes of CPACG, namely, the PACS/PAC eyes with no PAS or less than three cumulative clock-hours of PAS without glaucomatous optic neuropathy or visual field defect.
Based on the International Society of Geographic and Epidemiologic Ophthalmology classification, in F-APAC and F-CPACG, an eye with appositional contact between the peripheral iris and the posterior trabecular meshwork was defined as PACS; an eye with iridotrabecular contact and an elevated IOP or PAS with no secondary cause for the PAS, but without glaucomatous optic neuropathy was defined as PAC.4
Exclusion criteria included (1) secondary angle closure such as iris neovascularization, trauma, tumor, uveitis, and lens intumescence and subluxation, (2) plateau iris and nanophthalmos, (3) prior laser or intraocular surgery, (4) the use of topical antiglaucoma medicine in F-APAC and F-CPACG eyes, and (5) an inability to tolerate gonioscopy or UBM examinations.
Ophthalmologic Examinations
Each recruited subject underwent a comprehensive ophthalmologic examination, including visual acuity, IOP measurement by Goldmann applanation tonometry (Haag-Streit, Koniz, Switzerland), slit-lamp examination, stereoscopic evaluation of the optic disc using a 90-diopter lens (Volk Optical, Inc., Mentor, OH). Gonioscopy was performed in dimly lit room by a glaucoma specialist (H.J.W.) using a Zeiss-style four-mirror gonioscopy lens (Model G-4, Volk Optical, Inc., Mentor, OH) at 16× magnification with and without indentation. An occludable angle was defined as the invisibility of the posterior trabecular meshwork under a dynamic compression technique. Axial length and flat and steep keratometry were measured by IOLMaster biometry (Carl Zeiss Meditec, Inc., Dublin, CA). Five IOLMaster measurements with a signal-to-noise ratio of more than 100 were taken, the mean of which was used for analysis.
Ultrasound Biomicroscopy
UBM (Aviso, Quantel Medical, Inc., Bozeman, MT) measurements were performed using a 50-MHz transducer by an experienced operator (Y.Y.W.) who was masked to the clinical data. All subjects underwent UBM examinations in a supine position in room light (illumination 120 lux, measured with a luminance meter [Model ST-92, Beijing Teachers University Photoelectricity Instrument Factory, Beijing, China]). After topical anesthesia, a plastic eyecup containing normal saline as a coupling agent was used to carefully separate the lids. The UBM transducer was held perpendicular to the ocular structures at the limbal region to be examined. Care was taken not to exert pressure on the globe, which could cause changes in the angle configuration. Patients were asked to fixate with the contralateral eye at a distant target on the ceiling to minimize accommodation. Both eyes of each subject were measured in the superior, inferior, temporal, and nasal quadrants as well as nasal and temporal scans centered on the pupil to obtain complete images of the anterior segment. Only images with a clear view of the scleral spur, angle, ciliary body, iris and anterior surface of the lens were included for analysis.
Images of F-APAC and F-CPACG were measured quantitatively in all the four quadrants by a single examiner (K.Y.Y.) masked to clinical data with an in-built caliper in the UBM software. The scleral spur was identified based on the differential tissue density between the collagen fibers of the scleral spur and the longitudinal muscle of the ciliary body. The anterior segment parameters were measured according to the methods of Pavlin et al.16–18 and recent studies as follow (Figs. 1 and 2):
-
(1)
Pupil diameter: the shortest distance between the pupil edges of the iris cross-sections.
-
(2)
LV: the perpendicular distance from the anterior pole of the lens to the horizontal line between the scleral spurs.
-
(3)
Anterior chamber depth (ACD): the axial distance between the corneal endothelium and the anterior lens surface.19
-
(4)
Anterior chamber width (ACW): the distance between the two scleral spurs.20
-
(5)
Iris area: the cumulative cross-sectional area of the full length (from spur to pupil) of the iris.
-
(6)
Iris thickness at 750 µm (IT 750) and at 2000 µm (IT 2000): iris thickness at 750 µm and 2000 µm from the scleral spur.
-
(7)
Angle-opening distance at 500 µm (AOD 500) and at 750 µm (AOD 750): the distance between the posterior corneal surface and the anterior iris surface on a line perpendicular to the trabecular meshwork 500 µm and 750 µm from the scleral spur.21
-
(8)
Trabecular–iris space area at 500 µm (TISA 500) and at 750 µm (TISA 750): the area bounded anteriorly by AOD 500 and AOD 750 as determined, posteriorly by a line drawn from the scleral spur perpendicular to the plane of the inner scleral wall to the iris, superiorly by the inner corneoscleral wall, and inferiorly by the iris surface.21
-
(9)
Trabecular iris angle at 500 µm (TIA 500) and at 750 µm (TIA 750): the apex of the angle at the iris recess and the arms of the angle passing through a point on the trabecular meshwork at 500 µm and 750 µm from the scleral spur and the point on the iris perpendicularly opposite.
-
(10)
Ciliary process area (CPA): a new parameter defined as the cross-sectional area of ciliary process bounded laterally by a line connecting the insertion location of iris into the ciliary body and the cross-point of a line at 500 µm from the scleral spur perpendicular to the plane of the inner scleral wall to the ciliary process, and internally by the ciliary process surface.
-
(11)
Trabecular–ciliary angle (TCA): the angle between the posterior corneal surface and the anterior surface of the ciliary body.
Figure 1.
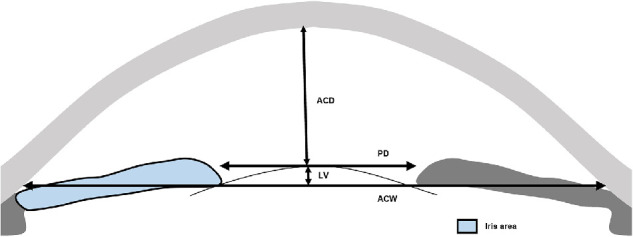
Anterior segment parameters measured by UBM. (Illustrator: Shuqi You). Pupil diameter (PD), the shortest distance between the pupil edges of the iris cross-sections; LV, the perpendicular distance from the anterior pole of the lens to the horizontal line between the scleral spurs; ACD, the axial distance between the corneal endothelium and the anterior lens surface; ACW, the distance between the two scleral spurs; Iris area, the cumulative cross-sectional area of the full length (from spur to pupil) of the iris.
Figure 2.
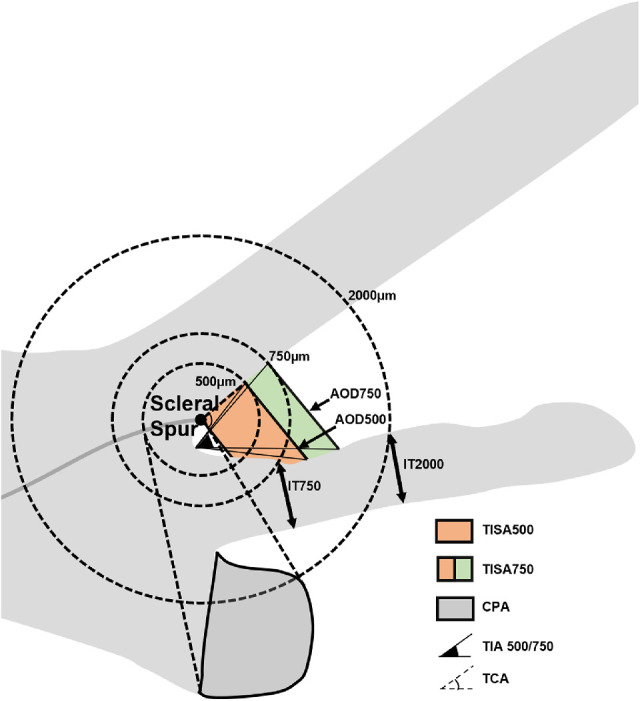
Anterior segment parameters measured by UBM. (Illustrator: Shuqi You). IT 750/2000: iris thickness at 750 µm and 2000 µm from the scleral spur; AOD 500/750: the distance between the posterior corneal surface and the anterior iris surface on a line perpendicular to the trabecular meshwork 500 µm and 750 µm from the scleral spur; TISA 500/750: the area bounded anteriorly by AOD 500 and AOD 750 as determined, posteriorly by a line drawn from the scleral spur perpendicular to the plane of the inner scleral wall to the iris, superiorly by the inner corneoscleral wall, and inferiorly by the iris surface; TIA 500/750: the apex of the angle at the iris recess and the arms of the angle passing through a point on the trabecular meshwork at 500 µm and 750 µm from the scleral spur and the point on the iris perpendicularly opposite; CPA: the cross-sectional area of ciliary process bounded laterally by a line connecting the insertion location of iris into the ciliary body and the cross-point of a line at 500 µm from the scleral spur perpendicular to the plane of the inner scleral wall to the ciliary process, and internally by the ciliary process surface; TCA: the angle between the posterior corneal surface and the anterior surface of the ciliary body.
All measurements of linear parameters were expressed in millimeters and angular parameters in degrees. To assess the repeatability and reproducibility of UBM measurements, 12 images were randomly selected and regraded by the same observer (K.Y.Y.) twice separated by an interval of 1 week to assess intraobserver variability, and by a second observer (S.Q.Y.) to assess interobserver variability.
Statistical Analysis
Statistical analysis was performed using SPSS version 23.0 (SPSS, Inc., Chicago, IL). The averages and standard deviations were calculated for continuous data. The averages of the superior, nasal, inferior, and temporal iris area were calculated and used for final analysis. Mann–Whitney U tests and χ2 tests were used to compare the continuous variables and categorical variables, respectively, between F-APAC and F-CPACG. The coefficient of the intraclass correlation was used to assess the intraobserver and interobserver variability. Univariate and multivariate logistic regression analysis were performed to determine the most important parameters differentiating F-APAC and F-CPACG. Receiver operator characteristic curve was generated and the area under the receiver operator characteristic curve (AUC) was used to discriminate F-APAC from F-CPACG. The best cut-off value was determined based on Youden's index for parameters. Spearman correlation coefficients were calculated between anterior segment parameters and IT 750, AOD750, LV, CPA, respectively. A P value of less than 0.05 was considered statistically significant.
Results
A total of 110 eyes of 110 patients, 55 F-APAC and 55 F-CPACG, were enrolled. The mean age was 66.46 ± 9.34 years in F-APAC and 67.31 ± 10.52 years in F-CPACG. There were no significant differences between the two groups in terms of age, gender, diagnosis, axial length, flat and steep keratometry, cup-to-disc ratio, or lens nucleus opacity. The IOP in F-CPACG was significantly higher than that of F-APAC (P = 0.03). The demographic characteristics and clinical examination data of the two groups are summarized in Table 1.
Table 1.
Demographic Characteristics and Clinical Examination Data of Subjects
| Parameters | F-APAC | F-CPACG | P Value |
|---|---|---|---|
| No. of eyes | 55 | 55 | – |
| Gender (M/F) | 12/43 | 17/38 | 0.279* |
| Age (years), mean ± SD | 66.46 ± 9.34 | 67.31 ± 10.52 | 0.388 |
| Diagnosis (PACS/PAC) | 48/7 | 47/8 | 0.781* |
| AXL (mm), mean ± SD | 22.23 ± 0.80 | 22.87 ± 1.44 | 0.432 |
| IOP (mm Hg), mean ± SD | 14.26 ± 3.13 | 18.05 ± 6.04 | 0.03† |
| Flat K (D), mean ± SD | 45.05 ± 0.87 | 43.7 ± 1.97 | 0.149 |
| Steep K (D), mean ± SD | 45.92 ± 0.93 | 44.82 ± 2.33 | 0.530 |
| C/D ratio, mean ± SD | 0.38 ± 0.02 | 0.47 ± 0.03 | 0.057 |
| Lens nucleus opacity (LOCS III), mean ± SD | 2.00 ± 1.05 | 2.42 ± 1.08 | 0.287 |
test.
P < 0.05.
AXL, axial length; C/D, cup-to-disc ratio; flat K, flat keratometry; LOCS III, Lens Opacities Classification System III; steep K, steep keratometry.
UBM parameters used in this study were manually measured by the researcher. The intraobserver and interobserver intraclass correlation were 0.904 to 0.997 and 0.824 to 0.995, respectively (Supplementary Table S1), which demonstrated good repeatability and reproducibility of UBM measurements in this study.
The results of anterior segment UBM parameters are shown in Table 2. Compared with F-CPACG, F-APAC had larger LV and CPA, smaller ACD, thinner IT 750, smaller AOD 750, and narrower TIA 750 and TCA (P ≤ 0.01). No significant differences were found regarding pupil diameter, ACW, Iris area, IT 2000, AOD 500, TIA 500, TISA 500 and TISA 750 (P > 0.05).
Table 2.
Comparison of Anterior Segment Parameters Between F-APAC and F-CPACG
| Parameters | F-APAC | F-CPACG | P Value |
|---|---|---|---|
| PD (mm) | 2.30 ± 0.77 | 2.55 ± 0.57 | 0.081 |
| LV (mm) | 1.01 ± 0.39 | 0.79 ± 0.31 | 0.001* |
| ACW (mm) | 11.01 ± 0.55 | 10.94 ± 0.66 | 0.727 |
| ACD (mm) | 1.75 ± 0.25 | 1.96 ± 0.32 | <0.001* |
| Iris area (mm2) | 1.88 ± 0.23 | 2.10 ± 1.56 | 0.907 |
| IT 750 (mm) | 0.38 ± 0.07 | 0.42 ± 0.04 | <0.001* |
| IT 2000 (mm) | 0.45 ± 0.07 | 0.47 ± 0.05 | 0.141 |
| AOD 500 (mm) | 0.12 ± 0.06 | 0.15 ± 0.08 | 0.056 |
| AOD 750 (mm) | 0.15 ± 0.08 | 0.22 ± 0.11 | <0.001* |
| TISA 500 (mm2) | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.695 |
| TISA 750 (mm2) | 0.08 ± 0.03 | 0.09 ± 0.04 | 0.381 |
| TIA 500 (°) | 12.36 ± 5.89 | 14.42 ± 6.98 | 0.111 |
| TIA 750 (°) | 10.17 ± 5.46 | 14.34 ± 7.05 | 0.001* |
| CPA (mm2) | 0.62 ± 0.16 | 0.54 ± 0.14 | 0.010* |
| TCA (°) | 52.94 ± 10.76 | 59.49 ± 12.40 | 0.005* |
PD, pupil diameter.
*P < 0.05.
A multivariate logistic regression analysis was performed (Table 3) to determine which among the anterior segment parameters were associated with the occurrence of F-APAC. In univariate logistic regression analysis, ACD, IT 750, AOD 750, TIA 750, and TCA were significantly smaller, whereas the LV and CPA were significantly larger in the F-APAC compared with the F-CPACG when adjusted for age and gender (P < 0.05). In forward multivariate logistic regression analysis, only LV, IT 750, and AOD 750 were left with significance (P < 0.01). For every 1-mm increase in IT 750 and AOD 750, the odds of developing F-APAC were 0.000005 and 0.001, respectively, and a 1-mm increase in LV, the OR was 8.63 (P < 0.01).
Table 3.
Logistic Regression Analysis of Determinants of F-APAC
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||
|---|---|---|---|---|---|---|
| Parameters | OR | 95% CI | P Value | OR | 95% CI | P Value |
| PD | 0.60 | 0.34 to 1.06 | 0.083 | |||
| LV | 9.19 | 2.14 to 39.45 | 0.003* | 8.63 | 1.83 to 40.65 | 0.006* |
| ACW | 1.21 | 0.65 to 2.26 | 0.539 | |||
| ACD | 0.06 | 0.01 to 0.33 | 0.001* | |||
| Iris area | 0.72 | 0.29 to 1.74 | 0.471 | |||
| IT 750 | <0.01 | <0.01 to 0.01 | 0.001* | 0.000005 | <0.01 to 0.02 | 0.003* |
| IT 2000 | 0.01 | <0.01 to 2.43 | 0.095 | |||
| AOD 500 | <0.01 | <0.01 to 1.00 | 0.050 | |||
| AOD 750 | <0.01 | <0.01 to 0.07 | 0.002* | 0.001 | <0.01 to 0.17 | 0.008* |
| TISA 500 | 1.15 | <0.01 to 2649764.03 | 0.985 | |||
| TISA 750 | <0.01 | <0.01 to 21.67 | 0.201 | |||
| TIA 500 | 0.95 | 0.89 to 1.01 | 0.097 | |||
| TIA 750 | 0.89 | 0.83 to 0.96 | 0.002* | |||
| CPA | 31.14 | 2.04 to 475.03 | 0.013* | |||
| TCA | 0.95 | 0.92 to 0.99 | 0.007* | |||
*P < 0.05.
PD, pupil diameter.
The performance of LV, IT 750, and AOD 750 as determinants for discrimination of F-APAC from F-CPACG was summarized in Table 4, which showed AUCs of 0.703 and 0.696 for IT 750 and AOD 750, respectively, with best cut-off values of 0.404 mm and 0.126 mm for IT 750 and TIA 750, respectively; LV performed relatively poorer in diagnostic accuracy (AUC, 0.315).
Table 4.
Areas Under Curve, Best Cut-off Values, Sensitivities and Specificity of Biometric Parameters for Differentiation of F-APAC and F-CPACG
| Parameters(mm) | AUC | P Value | Best Cut-Off | Sensitivity | Specificity |
|---|---|---|---|---|---|
| LV | 0.315 | 0.001* | −0.83 | 1 | 0 |
| IT 750 | 0.703 | <0.0001* | 0.404583 | 0.759 | 0.608 |
| AOD 750 | 0.696 | <0.0001* | 0.12625 | 0.796 | 0.527 |
*P < 0.05.
Among all the patients collectively, IT 750 had positive correlations with IT 2000, pupil diameter, ACD, and TCA, and negative correlation with CPA (P < 0.05); AOD 750 was positively correlated with ACD, ACW, TIA 500/750, AOD 500, TISA 500/750, and TCA, and negatively correlated with CPA (P < 0.05); LV had a positive correlation with ACW and CPA and a negative correlation with ACD and IT2000 (P < 0.05); CPA was positively correlated with and LV and negatively correlated with TCA, IT 750/2000,TIA 500/750, AOD 500/750, and TISA 750 (P < 0.05) (Table 5).
Table 5.
Correlation Between Anterior Segment Parameters with IT 750, AOD 750, LV and CPA in All eyes
| IT 750 | AOD 750 | LV | CPA | |||||
|---|---|---|---|---|---|---|---|---|
| Parameters | r | P Value | r | P Value | r | P value | r | P Value |
| PD | 0.446 | <0.001* | −0.14 | 0.889 | −0.052 | 0.592 | −0.099 | 0.313 |
| LV | −0.058 | 0.546 | −0.155 | 0.107 | – | – | 0.209 | 0.032* |
| ACW | 0.115 | 0.232 | 0.258 | 0.007* | 0.420 | <0.001* | 0.160 | 0.102 |
| ACD | 0.270 | 0.004* | 0.509 | <0.001* | −0.436 | <0.001* | −0.106 | 0.280 |
| Iris area | 0.076 | 0.432 | 0.103 | 0.288 | 0.017 | 0.857 | 0.01 | 0.918 |
| IT 750 | – | – | 0.126 | 0.191 | −0.058 | 0.546 | −0.273 | 0.005* |
| IT 2000 | 0.636 | <0.001* | 0.025 | 0.794 | −0.225 | 0.018* | −0.258 | 0.008* |
| AOD 500 | 0.095 | 0.322 | 0.908 | <0.001* | −0.061 | 0.524 | −0.269 | 0.005* |
| AOD 750 | 0.126 | 0.191 | – | – | −0.155 | 0.107 | −0.276 | 0.005* |
| TISA 500 | 0.065 | 0.498 | 0.670 | <0.001* | 0.059 | 0.543 | −0.140 | 0.155 |
| TISA 750 | 0.078 | 0.417 | 0.924 | <0.001* | −0.056 | 0.559 | −0.203 | 0.038* |
| TIA 500 | 0.061 | 0.530 | 0.867 | <0.001* | −0.061 | 0.529 | −0.273 | 0.005* |
| TIA 750 | 0.132 | 0.168 | 0.971 | <0.001* | −0.152 | 0.113 | −0.307 | 0.001* |
| CPA | −0.273 | 0.005* | −0.276 | 0.005* | 0.209 | 0.032* | – | – |
| TCA | 0.245 | 0.012* | 0.400 | <0.001* | −0.181 | 0.065 | −0.640 | <0.001* |
*P < 0.05.
PD, pupil diameter.
Discussion
Anterior segment structural differences between PACG eyes and normal eyes, or between APAC eyes and the fellow eyes have been studied extensively; however, few studies have been focused on the differences between fellow eyes of APAC and CPACG.16,22,23 The current study confirms and extends previously published research on findings of differences between F-APAC and F-CPACG, and a new parameter, CPA, is demonstrated. To our knowledge, no research has quantitatively studied this parameter previously. In addition, it is the first time that the significance of peripheral iris thickness and AOD in the development of APAC has been described.
In this study, F-APAC had more crowded anterior segment structures compared with F-CPACG. The ACD, IT 750, AOD 750, TIA 750, and TCA were smaller, and LV and CPA were larger in F-APAC compared with F-CPACG. A smaller IT 750 and AOD 750, as well as a greater LV were risk factors of F-APAC. For the reason that 40% to 80% F-APAC, if untreated, will develop an acute attack in 5 to 10 years,14,15 we speculate that PACS/PAC eyes with smaller IT 750 and AOD 750, and greater LV are probable to have an acute attack.
Previous studies have revealed that PAC eyes had thicker peripheral iris than normal eyes.24,25 A thicker peripheral iris could crowd the angle and subsequently contribute to angle closure.26 However, the iris thickness differences between APAC and CPAC eyes remain to be clarified. Chen et al.11 found a thinner IT 500 in F-APAC than in F-CPACG under dark condition, whereas Li et al.12 demonstrated no significant difference in IT 500 between F-APAC(G) and F-CPAC(G) in light condition. The discrepancy in IT 500 between these two studies may be due to the different illumination and the former included patients who had undergone laser peripheral iridotomy (LPI), which led to alteration of iris configuration. To observe the original status of anterior segment structure, eyes with prior-treatment were excluded and measurements were performed in room light in the present study. We found that F-APAC had markedly thinner IT 750 compared with F-CPACG, but no difference of IT 2000 was noted between F-APAC and F-CPACG. This finding implied that iris was just thinner at 750 µm from the sclera spur. Moreover, a thinner IT 750 was confirmed to be a risk factor for F-APAC, and the IT 750 was sensitive for discriminating F-APAC and F-CPACG (AUC, 0.703). It has been proved that up to 50% of F-APAC patients would have an acute attack within 5 years if left untreated.15 Thus, we proposed that, in PAC eyes, the thinner the IT 750, the greater the risk of an acute attack.
The difference of the iris area between F-APAC and F-CPACG was not significant. This finding is not surprising, because the iris area is a cross-sectional area of the whole iris and is not indicative of iris configuration.
Extensive measurements of the anterior chamber angle parameters were performed in our study, including the AOD 500/750, TIA 500/750, and TISA 500/750. Previous studies reported acute PACG eyes with a smaller AOD 500 and TISA 500/750 compared with CPACG eyes.8,21,27 However, there was no significant difference in AOD 500 and TISA 500/750 between the F-APAC and F-CPACG in this study. These discrepant findings may be because the morphology of the angle is changing continuously in the process of PACD. The AOD 750 was smaller in F-APAC than that in F-CPACG and was found to be sensitive for discriminating F-APAC and F-CPACG (AUC, 0.696), which is inconsistent with the prior study demonstrating the AOD 750 to be the most sensitive measurement for identifying narrow angles among AOD 250/500/750, TISA 500/750, and angle recess area.28 We also demonstrated a narrower TIA 750 in F-APAC compared with F-CPACG, which is in line with prior studies.7,8 These findings revealed that the angle width was smaller in F-APAC compared with F-CPACG at 750 µm from the sclera spur; meanwhile, the iris was thinner at this location in F-APAC. We speculate that the thinner peripheral iris at 750 µm from the sclera is more likely to be pushed anteriorly and cover the near trabecular meshwork to develop an acute attack.
In agreement with previous reports, the LV was significantly greater in F-APAC than in F-CPACG in the present study.12,21,27,29 A greater LV could push the iris more anteriorly and narrow the anterior chamber angle. This finding was confirmed in our study by significantly less AOD 750 and TIA 750 in F-APAC than in F-CPACG. A greater LV could also increase iridolenticular contact and aggravate pupillary block and lead to an acute attack, as was proved in the current study that higher LV was significantly associated with F-APAC.
ACD and ACW are parameters of anterior segment dimensions. Similar to previous results, the ACD was shallower in F-APAC than in F-CPACG in this study.11,12 The shallower ACD may be partly due to a greater LV, which was proved by the positive correlation between LV and ACD in this study. We found no significant difference in ACW between groups. However, Li et al.12 reported a smaller ACW in F-APAC(G) compared with F-CPAC(G). The PAS in F-CPACG could potentially obscure the determination of scleral spur and explain this discrepancy.
The trabecular–ciliary process angle (TCA) has been regarded as an important parameter representing the anterior position of the ciliary body.30 Consistent with a previous study, a smaller TCA was found in F-APAC than in F-CPACG in current study, suggesting that the ciliary body was more anteriorly rotated in F-APAC.12 Further, we provided a new parameter, CPA, to characterize ciliary body. CPA was measured larger in F-APAC than in F-CPACG. Also, CPA was positively associated with LV and negatively correlated to TIA and AOD in the present study. Therefore, we speculated that the larger CPA may cause a loosening of zonules and a more anterior position or protrusion of the lens, consequently, push the iris forward to narrow the anterior chamber angle. In addition, a large ciliary process also might decrease the distance between the ciliary body and lens, which might induce a ciliary block and might be a potential reason for angle closure.31
A multivariable logistic regression analysis showed a greater LV and smaller IT 750 and AOD 750 were the three parameters that could differentiate F-APAC and F-CPACG. An increase in the LV and a decrease in the IT 750 and AOD 750 significantly increased the risk of F-APAC. This finding may correspond with the two different forms of angle closure. In APAC eyes, a greater LV expands the contact area between the lens and the posterior face of the iris, which increases aqueous flow resistance and generates a pressure gradient between the anterior and posterior chambers.32,33 It is easier for the pressure gradient to push the thinner peripheral iris forward to occlude the nearer trabecular meshwork and cause an acute attack. In CPACG eyes, the mechanism of angle closure may be “creeping closure” or “zipper up” for crowded angle caused by thicker peripheral iris.
A recent community-based randomized controlled trial recommended against widespread prophylactic LPI for PACS from the perspective of health economics, because of the low incidence of angle closure disease among PACS and the limited benefit of prophylactic LPI.34 The results of current study proposed that PACS/PAC eyes with a high LV, thin IT 750, and small AOD 750 deserve special attention, because they are significant risk factors for F-APAC, which confers a high risk of acute attack, and we suggested an LPI might be taken to into consideration for these eyes to prevent an acute attack. A future randomized controlled trial to directly study the incidence of acute attack with and without prophylactic LPI is needed.
This study has some limitations. Owing to the nature of the UBM examination in which the patient lies a supine and presses the eyes, the UBM examination results may be affected by the examiner or the situation, despite the care taken not to press on the eyes during the UBM examination. In addition, although the intraobserver and interobserver ICCs were good, the manual subjective evaluation of UBM images may affect the measurement of parameters. Finally, this study is limited by its cross-sectional nature, and a longitudinal study would be needed to further clarify the relationships between UBM-guided anterior chamber parameters and angle closure.
In conclusion, significant differences in anterior segment UBM parameters between F-APAC and F-CPACG were detected. F-APAC had thinner peripheral iris and narrower angle width at 750 µm from the sclera spur, shallower ACD, greater LV, larger and anteriorly positioned ciliary body compared with those with F-CPACG. The IT 750, AOD 750, and LV were the three most important parameters distinguishing these two forms.
Supplementary Material
Acknowledgments
The authors thank Yongzhen Bao, Yong Liang, Zeqin Ren, and Xianru Hou for recruiting patients, and thank Yanyan Wang for performing UBM for all the subjects.
Supported by the program of development and cultivation of medical innovative varieties and industrial support, Beijing Municipal Science and Technology Commission [Z191100007619045]; and National Natural Science Foundation of China [61634006].
Disclosure: S. You, None; Z. Liang, None; K. Yang, None; Y. Zhang, None; J. Oatts, None; Y. Han,None; H. Wu, None
References
- 1. Bourne RRA, Flaxman SR, Braithwaite T, et al.. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Heal. 2017; 5(9): e888–e897. [DOI] [PubMed] [Google Scholar]
- 2. Tham Y, Li X, Wong TY, Quigley HA, Aung T, Cheng C.. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121(11): 2081–2090. [DOI] [PubMed] [Google Scholar]
- 3. Quigley HA, Broman AT.. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90(3): 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foster PJ, Buhrmann R, Quigley HA, Johnson GJ.. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002; 86(2): 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun X, Dai Y, Chen Y, et al.. Primary angle closure glaucoma: what we know and what we don't know. Prog Retin Eye Res. 2017; 57: 26–45. [DOI] [PubMed] [Google Scholar]
- 6. Razeghinejad MR, Myers JS.. Contemporary approach to the diagnosis and management of primary angle-closure disease. Surv Ophthalmol. 2018; 63(6): 754–768. [DOI] [PubMed] [Google Scholar]
- 7. Marchini G, Pagliarusco A, Toscano A, Tosi R, Brunelli C, Bonomi L.. Ultrasound biomicroscopic and conventional ultrasonographic study of ocular dimensions in primary angle-closure glaucoma. Ophthalmology. 1998; 105(11): 2091–2098. [DOI] [PubMed] [Google Scholar]
- 8. Sihota R, Dada T, Gupta R, Lakshminarayan P, Pandey RM.. Ultrasound biomicroscopy in the subtypes of primary angle closure glaucoma. J Glaucoma. 2005; 14(5): 387–391. [DOI] [PubMed] [Google Scholar]
- 9. Sihota R, Lakshmaiah NC, Agarwal HC, Pandey RM, Titiyal JS.. Ocular parameters in the subgroups of angle closure glaucoma. Clin Exp Ophthalmol. 2000; 28(4): 253–258. [DOI] [PubMed] [Google Scholar]
- 10. Mochizuki H, Takenaka J, Sugimoto Y, Takamatsu M, Kiuchi Y.. Comparison of the prevalence of plateau iris configurations between angle-closure glaucoma and open-angle glaucoma using ultrasound biomicroscopy. J Glaucoma. 2011; 20(5): 315–318. [DOI] [PubMed] [Google Scholar]
- 11. Chen HJ, Wang X, Yan YJ, Wu LL.. Postiridotomy ultrasound biomicroscopy features in the fellow eye of Chinese patients with acute primary angle-closure and chronic primary angle-closure glaucoma. J Glaucoma. 2015; 24(3): 233–237. [DOI] [PubMed] [Google Scholar]
- 12. Li M, Chen Y, Chen X, et al.. Differences between fellow eyes of acute and chronic primary angle closure (glaucoma): an ultrasound biomicroscopy quantitative study. PLoS One. 2018; 13(2): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sihota R. Classification of primary angle closure disease. Curr Opin Ophthalmol. 2011; 22(2): 87–95. [DOI] [PubMed] [Google Scholar]
- 14. Lowe RF. The natural history and principles of treatment of primary angle-closure glaucoma. Am J Ophthalmol. 1966; 61(4): 642–651. [DOI] [PubMed] [Google Scholar]
- 15. Edwards RS. Behaviour of the fellow eye in acute angle-closure glaucoma. Br J Ophthalmol. 1982; 66(9): 576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pavlin CJ, Ritch R, Foster FS. Ultrasound biomicroscopy in plateau iris syndrome. Am J Ophthalmol. 1992; 113(4): 390–395. [DOI] [PubMed] [Google Scholar]
- 17. Pavlin CJ, Harasiewicz K, Sherar MD, Foster FS.. Clinical use of ultrasound biomicroscopy. Ophthalmology. 1991; 98(3): 287–295. [DOI] [PubMed] [Google Scholar]
- 18. Pavlin CJ, Harasiewicz K, Foster FS.. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol. 1992; 113(4): 381–389. [DOI] [PubMed] [Google Scholar]
- 19. Shabana N, Aquino MC, See J, et al.. Quantitative evaluation of anterior chamber parameters using anterior segment optical coherence tomography in primary angle closure mechanisms. Clin Exp Ophthalmol. 2012; 40(8): 792–801. [DOI] [PubMed] [Google Scholar]
- 20. Nongpiur ME, Sakata LM, Friedman DS, et al.. Novel association of smaller anterior chamber width with angle closure in Singaporeans. Ophthalmology. 2010; 117(10): 1967–1973. [DOI] [PubMed] [Google Scholar]
- 21. Moghimi S, Vahedian Z, Fakhraie G, et al.. Ocular biometry in the subtypes of angle closure: an anterior segment optical coherence tomography study. Am J Ophthalmol. 2013; 155(4): 664–673.e1. [DOI] [PubMed] [Google Scholar]
- 22. Dada T, Sihota R, Gadia R, Aggarwal A, Mandal S, Gupta V.. Comparison of anterior segment optical coherence tomography and ultrasound biomicroscopy for assessment of the anterior segment. J Cataract Refract Surg. 2007; 33(5): 837–840. [DOI] [PubMed] [Google Scholar]
- 23. Wang Z, Chen D, Zeng Y, Wang Y, Liang X, Liu X.. Comparison of anterior segment optical coherence tomography and ultrasound biomicroscopy for iris parameter measurements in patients with primary angle closure glaucoma. Eye Sci. 2013; 28(1): 1–6. [PubMed] [Google Scholar]
- 24. Jiang Y, He M, Huang W, Huang Q, Zhang J, Foster PJ.. Qualitative assessment of ultrasound biomicroscopic images using standard photographs: the Liwan Eye Study. Invest Ophthalmol Vis Sci. 2010; 51(4): 2035–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ku JY, Nongpiur ME, Park J, et al.. Qualitative evaluation of the iris and ciliary body by ultrasound biomicroscopy in subjects with angle closure. J Glaucoma. 2014; 23(9): 583–588. [DOI] [PubMed] [Google Scholar]
- 26. Wang B, Narayanaswamy A, Amerasinghe N, et al.. Increased iris thickness and association with primary angle closure glaucoma. Br J Ophthalmol. 2011; 95(1): 46–50. [DOI] [PubMed] [Google Scholar]
- 27. Guzman CP, Gong T, Nongpiur ME, et al.. Anterior segment optical coherence tomography parameters in subtypes of primary angle closure. Invest Ophthalmol Vis Sci. 2013; 54(8): 5281–5286. [DOI] [PubMed] [Google Scholar]
- 28. Narayanaswamy A, Sakata LM, He MG, et al.. Diagnostic performance of anterior chamber angle measurements for detecting eyes with narrow angles: an anterior segment OCT study. Arch Ophthalmol. 2010; 128(10): 1321–1327. [DOI] [PubMed] [Google Scholar]
- 29. Nongpiur ME, He M, Amerasinghe N, et al.. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology. 2011; 118(3): 474–479. [DOI] [PubMed] [Google Scholar]
- 30. Henzan IM, Tomidokoro A, Uejo C, et al.. Ultrasound biomicroscopic configurations of the anterior ocular segment in a population-based study: the Kumejima study. Ophthalmology. 2010; 117(9): 1720–1728.e1. [DOI] [PubMed] [Google Scholar]
- 31. Ng WT, Morgan W.. Mechanisms and treatment of primary angle closure: a review. Clin Experiment Ophthalmol. 2012; 40(4): e218–e228. [DOI] [PubMed] [Google Scholar]
- 32. Tarongoy P, Ho CL, Walton DS.. Angle-closure glaucoma: the role of the lens in the pathogenesis, prevention, and treatment. Surv Ophthalmol. 2009; 54(2): 211–225. [DOI] [PubMed] [Google Scholar]
- 33. Quigley HA, Friedman DS, Congdon NG.. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003; 12(2): 167–180. [DOI] [PubMed] [Google Scholar]
- 34. He M, Jiang Y, Huang S, et al.. Laser peripheral iridotomy for the prevention of angle closure: a single-centre, randomised controlled trial. Lancet. 2019; 393(10181): 1609–1618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


