Abstract
Novel treatments that target neurobiological alterations associated with childhood trauma, particularly among those with posttraumatic stress disorder (PTSD), could mitigate negative outcomes for these at-risk individuals. PTSD is characterized by abnormalities within the brain’s salience network and reward circuitry, which are modulated by intranasal oxytocin. Using a double-blind, randomized, placebo-controlled crossover design, we tested whether intranasal oxytocin (24 international units) influenced functional coupling of the amygdala with the anterior insula (AI), dorsal anterior cingulate cortex, and nucleus accumbens in response to implicitly presented fearful, angry, and happy faces among childhood trauma-exposed individuals with (n = 16, 9 women) and without PTSD (n = 18, 12 women). Psychophysiological interaction analyses revealed that oxytocin effects were limited to amygdala-AI connectivity in the fear condition, distinct for men and women, and not impacted by PTSD diagnosis. In response to fear faces, oxytocin reduced left amygdala-left AI connectivity for women but not men; reduced left amygdala-right AI connectivity among women, but increased this connectivity in men; and reduced right amygdala-right anterior insula connectivity for men, but increased it for women. Results suggest that intranasal oxytocin modulates threat salience among childhood trauma-exposed individuals and that these effects vary as a function of gender and hemisphere.
Keywords: trauma, fMRI, psychophysiological interaction, emotion, fear, anger, happiness, facial affect
1. Introduction
Exposure to childhood trauma has broad and long-lasting effects on developing neurocognitive systems, including brain regions that mediate threat processing, reward processing, and emotion regulation (Hart et al., 2018; Herringa, 2017; Pechtel and Pizzagalli, 2011). Childhood trauma is also associated with elevated risk for posttraumatic stress disorder (PTSD; Green et al., 2010; Kessler et al., 1995; Koenen et al., 2007), high rates of psychiatric and physical health comorbidities (Agorastos et al., 2014; Briggs-Gowan et al., 2012; Goodwin and Stein, 2004; Widom et al., 2012), and poor treatment response (Nanni et al., 2012; Tunnard et al., 2014). Treatment approaches that target the neurobiological alterations associated with childhood trauma and/or PTSD could help to improve outcomes in this population.
1.1. Abnormalities in Threat and Reward Processing in PTSD
Enhanced processing of perceived threat contributes to the development and maintenance of PTSD (Ehlers and Clark, 2000; McNally, 2019). Neuroimaging studies indicate that PTSD is characterized by dysregulated threat detection reflected by hyperreactivity within the salience network, which is responsible for detecting and responding to biologically and psychosocially salient stimuli (Abdallah et al., 2019; Shalev et al., 2017). During rest and task fMRI, PTSD is associated with hyperreactivity within each of the network’s nodes (i.e., the amygdala, anterior insula [AI], and dorsal anterior cingulate cortex [dACC]) and with hyperconnectivity among these nodes (Hayes et al., 2012; Koch et al., 2016c; Patel et al., 2012). Meta-analytic findings further suggest that dACC and AI hyperreactivity are related to PTSD, whereas amygdala hyperreactivity may be a function of trauma exposure more generally (Hayes et al., 2012; Patel et al., 2012; Sartory et al., 2013). Given that overactive threat processing predicts poorer treatment response (Joshi et al., 2020), novel interventions that reduce threat processing either before or in conjunction with conventional therapies could be useful.
PTSD is also characterized by alterations in the detection, interpretation, and response to positive, rewarding environmental cues (Fonzo, 2018; Nawijn et al., 2015). Abnormalities in reward processing are also related to early childhood stress (Pechtel and Pizzagalli, 2011). Reward processing is mediated by the “reward circuit,” which includes the ventral striatum (i.e., nucleus accumbens [NA] and olfactory tubercle), amygdala, insula, ACC, and medial orbitofrontal cortex (Haber and Knutson, 2010; Mobbs et al., 2003). For example, among male veterans with PTSD, reduced NA activation was related to less sensitivity to monetary reward and more severe emotional numbing symptoms (Hopper et al., 2008). Happy faces provide a more externally valid probe of sensitivity to the rewarding nature of interpersonal relationships, a key mechanisms of recovery from PTSD (Monson et al., 2010), than monetary reward paradigms. Results of the few studies with happy face stimuli in PTSD are mixed, however, with evidence of decreased responsivity of the anterior insula (Nawijn et al., 2017), amygdala, and ventral striatum to overtly presented happy faces (Felmingham et al., 2014) but inconsistent findings for implicitly presented faces. For example, Herringa and colleagues (2013) found that PTSD symptoms were not related to neural reactivity to implicit happy faces. In contrast, Killgore et al. (2014) found increased amygdala reactivity to implicit happy faces versus neutral faces among participants with PTSD compared to healthy controls (Killgore et al., 2014). Better understanding of social reward processing abnormalities in PTSD could yield opportunities for interventions that could improve responsivity to social support, a key factor in trauma recovery (Monson et al., 2010).
1.2. Intranasal Oxytocin Modulates Threat and Reward Processing
Novel treatment approaches that target neural circuits mediating both threat and reward could be particularly powerful in enhancing recovery from PTSD. Intranasal oxytocin, a 9-amino acid neuropeptide, is one such candidate (Flanagan et al., 2018; Giovanna et al., 2020; Olff et al., 2010). Intranasal oxytocin acts on nodes and connections within the salience and reward processing networks, with findings for the amygdala and insula being the most consistent across studies—although directionalty of effects vary (Grace et al., 2018). In addition to its anxiolytic effects (Koch et al., 2014; Koch et al., 2016a), oxytocin increases sensitivity to socially rewarding cues via increased activation within the NA (Scheele et al., 2013). Meta-analytic evidence indicates that oxytocin improves recognition of fear, anger, and happiness among healthy individuals (Leppanen et al., 2017; Shahrestani et al., 2013; Van IJzendoorn and Bakermans-Kranenburg, 2012), with larger effects for happy and angry faces when presented briefly (i.e., < 300 ms). Oxytocin has also been shown to shift attention toward briefly presented (< 100 ms) happy faces (Domes et al., 2013) and enhance detection accuracy of implicitly presented angry and happy faces (Schulze et al., 2011). Thus, oxytocin may most robustly affect sensitivity to, and accurate identification of, preconscious social cues, which is critical for successful, nimble navigation of both rewarding and threatening situations.
Emerging evidence suggests that intranasal oxytocin could be used to address amygdala hyperreactivity to threat and dulled reward processing in PTSD. For example, intranasal oxytocin has been shown to reduce amygdala responsivity to emotional faces among individuals with PTSD (and increase responsivity within trauma-exposed controls) (Koch et al., 2016a). A recent study showed that individuals with PTSD who reported more severe childhood trauma exhibited more greatly attenuated amygdala reactivity to implicitly-presented fear faces after oxytocin administration (Flanagan et al., 2019). Among healthy men, intranasal oxytocin has also reduced connectivity between the amygdala and mid-brain in response to fearful images (Kirsch et al., 2005). With respect to reward, oxytocin increased activation in the striatum, dACC, and insula during anticipation of monetary reward (Nawijn et al., 2016) and normalized hypoactivation in the AI and increased striatal activation in response to social reward (Nawijn et al., 2017) among police officers with PTSD. Most neuroimaging studies of the effects of intranasal oxytocin have examined activation within specific brain regions during a task or examined functional connectivity at rest (Bethlehem et al., 2013) versus connectivity during tasks (with some exceptions, e.g., Kirsch et al., 2005; Rilling et al., 2012). Studies that examine connectivity between brain regions in response to emotional stimuli are better positioned to inform on the functional consequences of intranasal oxytocin on social information processing.
Preclinical research and meta-analytic findings indicate that effects of intranasal oxytocin may be sex- and gender-dependent (Dumais et al., 2013; Grace et al., 2018; Neumann and Slattery, 2016). However, most previous studies of the effects of intranasal oxytocin on facial affect processing among humans have included all-male samples (Domes et al., 2013; Schulze et al., 2011), making it difficult to examine these differences (Van IJzendoorn and Bakermans-Kranenburg, 2012). Among healthy men and women, Luo and colleagues (2017) observed gender-dependent effects of oxytocin on neural reactivity and functional connectivity during implicit face processing; specifically, oxytocin reduced inferior frontal gyrus, dorsal ACC, and AI reactivity to negatively valenced faces in men while increasing neural reactivity in women (Luo et al., 2017). Oxytocin also reduced connectivity between the amygdala, ACC, and inferior frontal gyrus in women, but not in men. Findings in PTSD samples are mixed. For example, gender did not emerge as a predictor of oxytocin effects in a study of amygdala reactivity to emotional faces in PTSD patients versus controls (Koch et al., 2016a), but in the same sample, men and women showed distinct oxytocin-related changes in resting state functional connectivity that also varied by PTSD status (Koch et al., 2016b). Given the mixed findings, potential gender-specific effects of intranasal oxytocin on brain connectivity during processing of threatening and rewarding faces in PTSD warrants further exploration.
1.3. The Current Study
To date, no study has examined the effects of intranasal oxytocin on brain connectivity in response to implicitly presented facial affect cues among individuals with PTSD. Filling this gap is critical for informing the field about the potential clinical utility of intranasal oxytocin in modulating threat and reward processing abnormalities in PTSD, particularly those that occur at early stages of cognitive processing and can have important downstream effects on behavior. To address this gap, we analyzed data from a randomized, placebo-controlled cross-over study of intranasal oxytocin in a sample of men and women who had experienced childhood trauma, approximately half of whom had probable PTSD (Flanagan et al., 2019). We tested the effects of intranasal oxytocin versus placebo on functional connectivity between the amygdala and the left and right AI, dACC, and NA in response to implicitly-presented faces depicting threat (i.e., fear and anger) and reward (i.e., happiness) using psychophysiological interaction (PPI) analyses. We included fearful faces because they are most frequently used to probe the salience network (Bryant et al., 2008; Fonzo et al., 2010; Rauch et al., 2000; Shin et al., 2005) and angry faces because of their salience due to the high levels of anger exhibited by perpetrators of interpersonal trauma (Rodriguez and Green, 1997). We also explored whether effects of oxytocin varied as a function of gender.
Based on prior findings, we expected that oxytocin would decrease threat salience in the PTSD group and increase reward salience in both groups. Specifically, we hypothesized the following: (1) in response to angry faces, intranasal oxytocin would (1a) weaken amygdala-AI and (1b) amygdala-dACC functional connectivity in the PTSD group but not the control group; (2) similarly, in response to fearful faces, intranasal oxytocin would (2a) weaken amygdala-AI and (2b) amygdala-dACC functional connectivity in the PTSD group but not the control group; (3) in response to happy faces, intranasal oxytocin would (3a) strengthen amygdala-AI and (3b) amygdala-NA functional connectivity in both the trauma-exposed control and PTSD groups.
2. Methods
2.1. Participants
A total of 38 individuals enrolled in the parent study, which was designed to assess effects of intranasal oxytocin on BOLD signal change in the amygdala (see Flanagan et al., 2019). Participants were recruited over 33 months via local media advertisements. IRB-approved advertisements for a research study involving “healthy adults with a history of childhood adversity and trauma” were disseminated through multiple media and community settings, including flyers posted in the area, local television, newspaper, and word of mouth among participants. We also posted IRB-approved materials in various local clinics.
Four participants were omitted from analyses due to excessive head motion (n = 2) or missing data (n = 2). The functional connectivity analyses in the current study were based on the final sample of 34 participants, which included 16 individuals in the PTSD group (n = 9 women) and 18 individuals in the childhood-trauma exposed control group (n = 12 women). The PTSD and control groups were matched on gender, age, education, and smoking status (n = 6 smokers; 3 in each group).
Inclusion criteria were: (1) scores of moderate to severe (> 3) on at least one item on a minimum of one of the five subscales of the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 2003) and (2) exposure to a trauma that met Diagnostic and Statistical Manual of Mental Disorders-IV [DSM-IV] Criterion A for PTSD (i.e., experiencing, witnessing, or confronting an event(s) that occurred prior to age 18 that involved actual or threatened death or serious injury or a threat to the physical integrity of oneself or others; peritraumatic response involved intense fear, helplessness, and/or horror). Exclusion criteria were: (1) current pregnancy, nursing, or ineffective means of birth control; (2) history or evidence of head trauma, neurological disorders, seizures, or unconsciousness; (3) current psychotic or bipolar disorder; (4) psychoactive substance use in the past 30 days per participant report or urine drug screen; (5) unwillingness or inability to be abstinent of caffeine and alcohol for 24 hours and drugs of abuse for 72 hours prior to study visits; (6) ferrous metal implants/pacemaker; (7) claustrophobia; (8) history of or current significant hematological, endocrine, cardiovascular, pulmonary, renal, or gastrointestinal diseases; and (9) use of psychotropic medications in the previous 30 days or less than five half-lives of the specific medication.
Participants in the PTSD group were also required to meet full DSM-IV diagnostic criteria for current PTSD (i.e., past six months; see Assessments) and excluded if they met DSM-IV criteria for substance dependence in the past year with the exception of alcohol dependence. PTSD participants reported the following comorbid psychiatric diagnoses: major depression (n = 2), attention deficit hyperactivity disorder (n = 1), alcohol use disorder (n = 1), panic disorder (n = 2), agoraphobia (n = 1), and dysthymia (n = 1). Participants were excluded from the childhood trauma-exposed control group if they met criteria for (1) mood or anxiety disorders in the past 90 days or (2) current/past substance dependence.
2.2. Assessments
Psychiatric diagnoses with the exception of current and lifetime substance use disorders were established with the Mini-International Neuropsychiatric Interview for DSM-IV (Sheehan et al., 1998); substance use was assessed with the Structured Clinical Interview for DSM-IV module (SCID-I; First et al., 2000). PTSD assessments were anchored to a childhood trauma index event. The Clinician-Administered PTSD Scale (CAPS; Blake et al., 1995) was initially used to assess PTSD diagnosis and symptom severity (n = 7), with the exception of one participant who was assessed for PTSD with the MINI and assigned to the control group for not meeting PTSD criteria. For the remainder of the sample, the research team assessed PTSD with the self-report Posttraumatic Diagnostic Scale (PDS; Foa, 1995) to reduce participant burden (n = 26; 13 from each group). When compared to the CAPS, the PDS has good sensitivity (.97) in survivors of interpersonal trauma (Griffin et al., 2004). In addition to determining eligibility, the CTQ (Bernstein et al., 2003) was used to measure severity of exposure to potentially traumatic events including abuse and neglect during childhood. Medical exclusion criteria were assessed with a medical history form and physical examination.
2.3. Procedures
Procedures were approved by the IRB of record of the principal investigator and conducted consistent with the Declaration of Helsinki. Written informed consent was obtained prior to study procedures. Participants who met eligibility criteria were scheduled to complete two laboratory sessions. Women were scheduled for their laboratory sessions during the luteal phase of their menstrual cycles to control for menstrual cycle variations in oxytocin response. All participants completed breathalyzer and urine drug screens; women also completed a urine pregnancy test. MUSC’s research pharmacy compounded and dispensed the oxytocin (24 IU) and placebo (saline) nasal sprays. The pharmacy was also responsible for medication randomization. The dosage of 24 IU was chosen for its safety profile and extant research showing its effectiveness at modifying neural responses and behavior; later dose-response research confirmed that dose exerts maximal effects on the amygdala in response to emotional faces (Spengler et al., 2017). Under supervision of study staff, participants self-administered the nasal sprays approximately 45 minutes before the functional magnetic resonance imaging (fMRI) scan. All but three participants completed the fMRI scans within one week of each other, with 16 participants (n = 10 in the PTSD group, 6 in the control group) completing the second scan within 24 hours, a wash-out period commonly employed in the clinical oxytocin literature (Koch et al., 2016b; Palgi et al., 2016). The shortest duration between scans among participants in this sample was 23 hours.
2.4. Facial Affect Recognition Task
As shown in Figure 1, the facial affect recognition task included brief (50ms) presentations of emotional adult faces followed by neutral face masks, for which participants identified the model’s gender. This task is based on previous work using a similar task to target automatic processing of threat cues (Rauch et al., 2006) and evidence that presenting stimuli for 50ms or less with visual masking and redirection of executive processing effectively induces automatic processing (Kouider and Dehaene, 2007). Faces in three emotion categories (anger, happiness, and fear) were selected from multiple standardized stimuli sets (Ekman and Friesen, 1976; Langner et al., 2010; Tottenham et al., 2009), standardized in size, converted to grayscale, and enclosed in an oval surround. The stimulus set included White, Black, and Asian male and female faces.
Figure 1. Facial Affect Recognition Task.
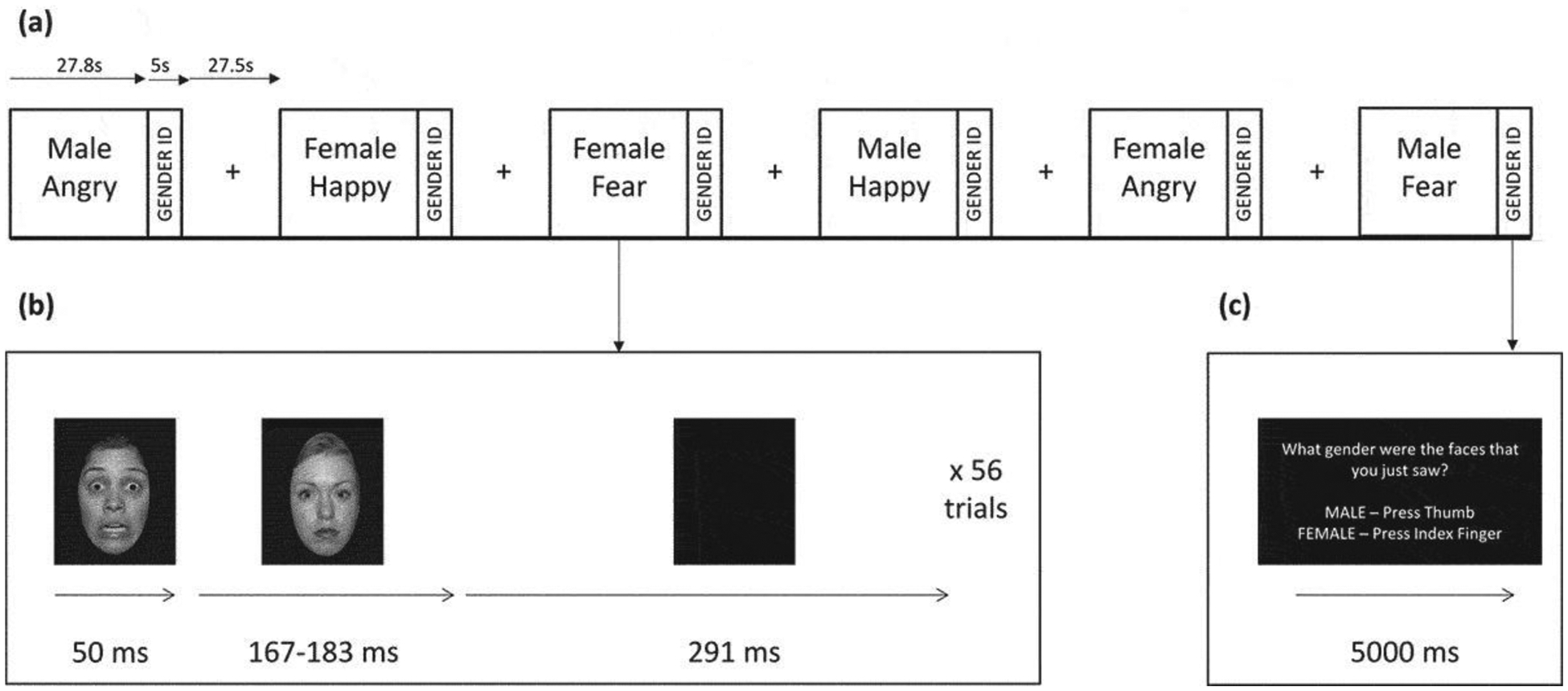
(a) Six task blocks depicting male or female faces expressing angry, happy, or fear emotions were interleaved with rest blocks consisting of a central crosshair for visual fixation. Each task block lasted 27.8 s followed by a 5-s gender identification block. Each rest block lasted 27.5 s. (b) Illustration of a single trial within each task block. An emotional face was presented for 50 ms followed by a neutral face mask for 167 or 183 ms followed by a completely blank screen for 291 ms. Fifty-six trials were presented in a single task block. (c) Illustration of the gender identification block, which lasted 5 s. Subjects were asked ‘What gender were the faces that you just saw? MALE – Press Thumb, FEMALE – Press Index Finger’.
Faces were presented in a block design. Within each block, participants viewed a series of 56 same-gender faces depicting the same emotion; at the end of the block they were asked to identify the gender of the faces using two buttons on a response pad. Each emotional face was presented for 50ms, followed by a neutral face mask of a different individual for 167ms and then a blank screen for 291ms. The task included six pseudo-randomized task blocks (3 emotions × 2 genders) and seven rest blocks (27.5s each) consisting of presentation of a crosshair.
2.5. fMRI Data Acquisition
Images were acquired on a Siemens Trio 3.0 Tesla scanner with a 12-channel head coil (Siemens Medical, Erlangen, Germany). Participants were shown 20 relaxation images for 30s each during initial scanner tuning, localizing, and structural scanning. A high-resolution T1-weighted MPRAGE anatomical scan (TR = 8.1ms, TE = 3.7ms, flip angle = 8°, field of view = 256mm, 1.0mm) of the entire brain and positioned using a sagittal scout image was acquired for co-registration and normalization of functional images. T2*-weighted gradient echo EPI images were acquired with the following parameters: TR = 2500ms, TE = 27ms, flip angle = 77°, 40 axial slices (FOV = 224 × 224mm, thickness = 3.5mm voxels with 0.5mm gap, in interleaved order, 160 volumes). The scanning planes were oriented parallel to the anterior commissure– posterior commissure line.
2.6. fMRI Data Preprocessing
Data were preprocessed using fMRI Expert Analysis Tool (FEAT) Version 5.63, part of FMRIB’s Software Library (FSL; www.fmrib.ox.ac.uk/fsl). The 4D images for each participant were corrected for head motion using MCFLIRT, slice timing corrected, spatially smoothed (FWHM = 7.0 mm) and temporally filtered (cutoff = 60s). MCFLIRT was used to register each participant’s 4D image to MNI standard space using the high-resolution T1 MPRAGE image.
2.7. Psychophysiological Interaction Analysis with Regions of Interest
A PPI analysis was conducted to examine functional connectivity change as a function of experimental condition (Friston et al., 1997). Following initial preprocessing, left and right amygdala seeds for the PPI analysis were defined as 10mm-diameter spheres: left amygdala (−22, −6, −14) and right amygdala (22, −6, −14) in MNI space. Time series were extracted in each amygdala seed region after resampling the seed regions into each subject’s native space.
For the PPI analysis, left and right amygdala time series served as the physiological regressors (with no convolution, temporal derivatives or temporal filtering applied). Each experimental condition (angry, fear, happy, and gender identification) was an explanatory variable with double-gamma HRF convolution applied and temporal derivatives added. The angry, fear, and happy conditions were the psychological variables. Six interaction terms (PPI EV’s) were examined: 2 seed regions (left, right amygdala) × 3 experimental conditions (angry, fear, happy). Although the gender identification phase of each block was modeled, it was not a variable of interest. The six rotations and displacements of head movement and head motion outliers (isolated using fsl_motion_outliers) were added as confound variables. As stated above, two of the originally 38 participants were excluded from analyses due to excessive head motion.
Coordinates for the central seed of each ROI were derived from an MNI-based probabilistic structural atlas defining the regions. Regions of interest (ROIs: right and left AI, NA, and dACC) were represented by 10mm-diameter spheres centered on the following MNI coordinates: left AI (−32, +24, 0), right AI (+32, +24, 0), left NA (−8, +8, −8), right NA (+8, +8, −8), left dACC (−5, 1, 32), and right dACC (5, 5, 31). Featquery was used to extract parameter estimates for each PPI EV and each subject, with parameter estimate values converted to percent signal change. As in our prior work (Flanagan et al., 2019), the effect of drug was expressed as a difference score. These difference score values were then submitted to the analyses described below. In the current analyses, we examined placebo minus oxytocin (P-O), such that a positive score indicates a greater reduction in fMRI connectivity due to oxytocin.
We ran a total of 24 models using IBM SPSS Statistics v. 25 (Chicago, IL). Sixteen models tested hypotheses for anger and fear faces: 2 valences (fear, anger) × 2 seeds (R, L amygdala) × 2 ROIs (insula, ACC) × 2 ROI hemispheres (L, R). Eight models tested hypotheses for happy faces: 2 seeds (R, L amygdala) × 2 ROIs (insula, NA) × 2 ROI hemispheres (R, L). For each model, we tested the effect of subject group (PTSD, trauma control), gender, and the interaction, with P-O as the outcome variable. We also controlled for the effects of order of treatment condition (oxytocin or placebo first). Because of the preliminary nature of our investigation, we interpreted results that were statistically significant at an uncorrected level, but also report which findings survived correction for 24 simultaneous tests for any effect in a model using false discovery rate correction (FDR; Benjamini and Hochberg, 1995) with a false discovery rate of .25. Results are reported using Wald chi-square (χ2). Effect sizes are reported using Hedge’s g due to the small sample sizes (Hedges and Olkin, 1985).
3. Results
3.1. Demographics and Clinical Characteristics
Sample characteristics are presented in Table 1. Most participants (82%) had either completed some college (control group n = 7, PTSD group n = 4) or had a college degree (control group n = 10, PTSD group n = 7). The PTSD group reported more severe histories of childhood trauma and greater PTSD symptom severity than the trauma-exposed control group.
Table 1.
Demographic and Clinical Characteristics by Group
| PTSD (n = 16) | Trauma Control (n = 18) | ||||
|---|---|---|---|---|---|
| Variable/Measure | Range | N (%) or M (SD) | N (%) or M (SD) | χ2 / t / U | P |
| Gender (women) | -- | 9 (56%) | 12 (67%) | 0.39 | .533 |
| Education (college degree) | -- | 7 (44%) | 10 (56%) | 0.47 | .492 |
| Age (years) | 21–60 | 35.44 (9.61) | 38.44 (11.78) | −.81 | .424 |
| CTQ Total | 27–112 | 77.50 (19.27) | 52.67 (21.46) | 3.53 | .001 |
| CTQ Emotional Abuse | 5–25 | 15.44 (5.42) | 11.33 (5.99) | 2.09 | .045 |
| CTQ Physical Abuse | 5–25 | 14.69 (5.84) | 9.94 (3.95) | 2.80 | .009 |
| CTQ Sexual Abuse | 5–25 | 18.50 (7.14) | 12.06 (7.90) | 2.48 | .018 |
| CTQ Emotional Neglect | 5–25 | 17.13 (4.44) | 11.33 (6.44) | 3.01 | .005 |
| CTQ Physical Neglect | 5–19 | 12.53 (4.82) | 8.00 (3.11) | 3.14 | .005 |
| PDS PTSD severity* | 0–45 | 19.77 (10.79) | 5.38 (4.98) | 8.00 | .000 |
Note. PTSD = Posttraumatic stress disorder. CTQ = Childhood Trauma Questionnaire. PDS = Posttraumatic Diagnostic Scale.
PDS administered to n = 26 (76.5% of the sample). Chi-square tests were used to examine group difference on gender and education; t-tests were used for all other variables except the PDS, for which a Mann-Whitney U test was used due to non-normality.
3.2. Head Motion
A generalized linear mixed effects model revealed that absolute head motion did not vary as a function of drug condition (χ21 = 1.8, p = .186), diagnostic group (χ21 = .54, p = .464), or their interaction (χ21 = .10, p = .755).
3.3. PPI with Regions of Interest Analyses
3.3.1. Amygdala and anterior insula.
Hypothesis 1a (anger):
The interaction of subject group and gender for the drug effect for left amygdala-right AI connectivity (χ21 = 3.8, uncorrected p = .053; corrected p = .042) revealed that oxytocin weakened connectivity for males with PTSD more than for any other group (Hedge’s g = 1.01 for the comparison of male control vs. male PTSD; see Figure 2a). However, this interaction did not survive FDR-correction.
Figure 2. Psychophysiological Interaction Analysis with Regions of Interest.
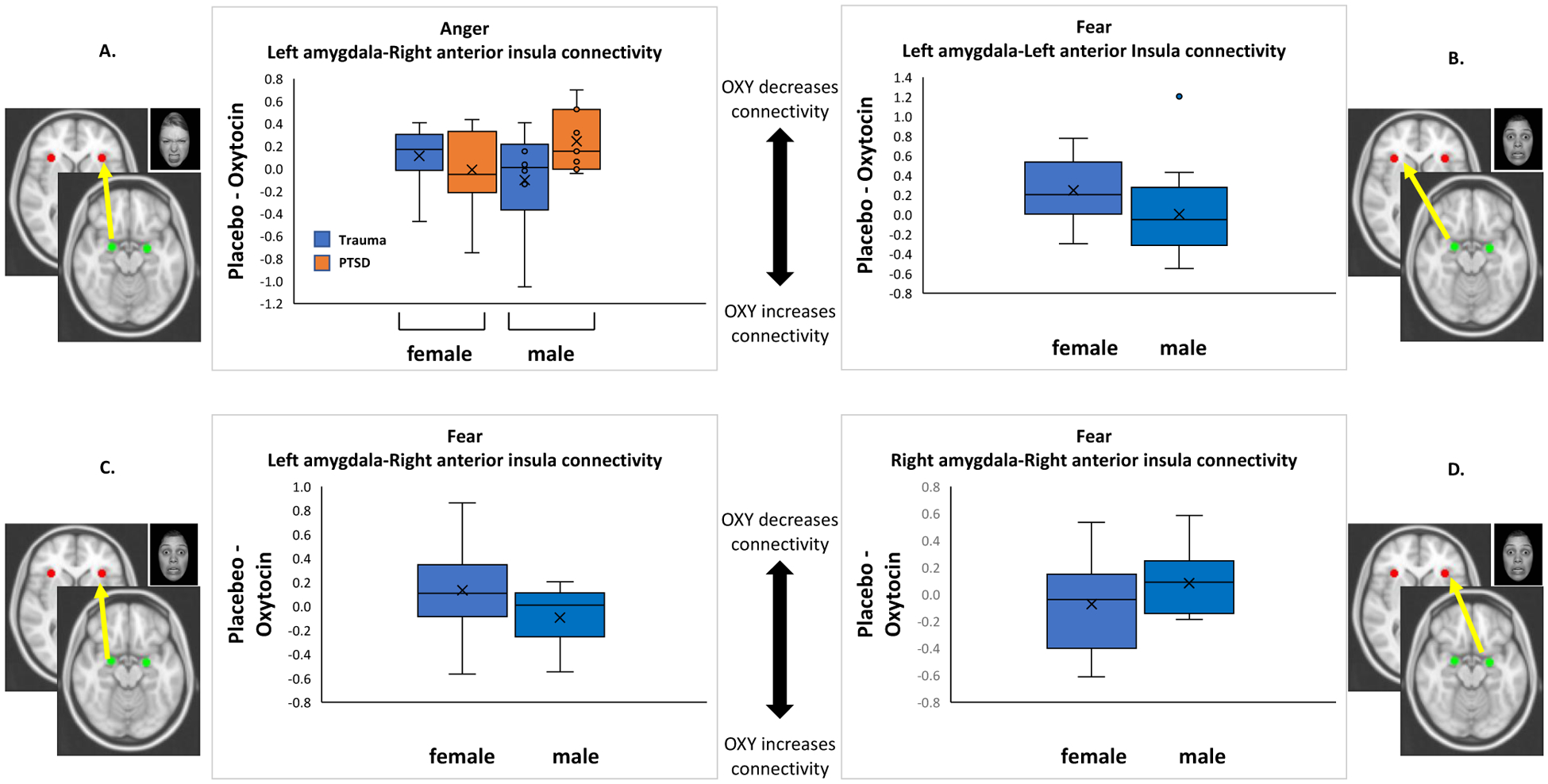
Note: Effect of drug expressed as a difference score (Placebo – Oxytocin); positive score indicates a greater decrease in fMRI signal due to oxytocin. †p < .10, *p < .05, **p < .01. Only the main effect of gender on left amygdala-right AI connectivity (panel C) survived FDR correction.
Hypothesis 2a (fear):
There was no main effect of subject group nor a group × gender interaction. The drug effect for left amygdala-left AI connectivity was different for men and women (main effect of gender: χ21 = 4.4, uncorrected p = .035; corrected p = .021; Hedge’s g = 1.0; Figure 2b). Oxytocin decreased connectivity for women but not for men. However this effect did not survive FDR-correction. Similarly, a main effect of gender emerged for left amygdala-right AI connectivity (χ21 = 7.6, uncorrected p = .006; corrected p = .01; Hedge’s g = 98; Figure 2c). In this case, oxytocin reduced connectivity for women, but increased connectivity for men. This effect survived FDR-correction. The effect of treatment order was also significant in this model (χ21 = 7.0, p = .008). Oxytocin delivered on Day 2 led to reduced amygdala-AI connectivity. The drug effect for right amygdala-right AI connectivity was also different for men and women (main effect of gender: χ21 = 4.3, uncorrected p = .037; corrected p = .031; Hedge’s g = .74; Figure 2d). In contrast to the gender effect for left amygdala connectivity, oxytocin reduced right amygdala-right AI connectivity for men, but increased connectivity for women. This effect did not survive FDR-correction. The effect of treatment order was also significant in this model (χ21 = 7.5, p = .006). Oxytocin delivered on Day 1 led to reduced connectivity.
Hypothesis 3a (happiness):
Amygdala-AI connectivity did not differ by PTSD group, gender or their two-way interaction.
3.3.2. Amygdala and dorsal ACC (Hypotheses 1b/anger, 2b/fear).
Connectivity among the amygdala-dACC ROIs did not differ by PTSD group, gender, or their two-way interaction.
3.3.3. Amygdala and nucleus accumbens (Hypothesis 3b/happiness).
Amygdala-NA connectivity did not differ by PTSD group, gender, or their two-way interaction.
See Supplemental Table 1 for each of the 24 test outcomes.
4. Discussion
Intranasal oxytocin is being investigated as a potential treatment for PTSD based on evidence that it modulates emotional processes that are central to PTSD pathophysiology, such as overreactive threat processing and decreased reward sensitivity. The results of the current study provide further evidence that intranasal oxytocin impacts neural markers of early-stage, implicit threat processing among childhood trauma-exposed individuals; specifically, oxytocin influenced functional connectivity between the amygdala and anterior insula (AI), key nodes of the salience network responsible for detecting threat cues. These effects varied as a function of brain region laterality and gender, and were not driven by PTSD status. Oxytocin did not, however, impact amygdala coupling with dACC or NA.
Intranasal oxytocin is a potential strategy for normalizing the hyperconnectivity within the salience network (i.e., amygdala, AI, and dACC) that has been observed in patients with PTSD (Koch et al., 2016b, 2014). In our study, as predicted, oxytocin decreased connectivity between the amygdala and AI in response to fear stimuli and was observed in the following conditions: left amygdala-left AI and left amygdala-right AI in women, and right amygdala-right AI in men. However, this effect was only in response to fearful (but not angry) faces. Interestingly, we observed increases in left amygdala-right AI connectivity among men and right amygdala-right AI among women. It is important to note that in spite of the large magnitude of these effects, only the finding that oxytocin reduced connectivity for women and increased connectivity for men survived statistical correction. Thus, these preliminary findings suggest opposite effects of oxytocin on amygdala connectivity by gender and hemisphere and require replication with larger samples.
These results are challenging to interpret. Overall, findings were fairly consistent for the left amygdala and for women. On one hand, we could conclude that by reducing connectivity between the amygdala and AI, a key circuit in the salience network, intranasal oxytocin decreased the salience of fear cues for women who experienced childhood trauma, whereas effects for men are less promising. On the other hand, the results are not that clear cut, in that women showed some increased connectivity and men showed some reduction in connectivity between the amygdala and AI. In an effort to reconcile our findings, below we address three considerations—social salience, lateralization, and participant gender.
Previous research showed that intranasal oxytocin increased functional connectivity between the amygdala and insula in response to fear faces in participants with generalized social anxiety disorder, but not among healthy controls (Gorka et al., 2015). Like them, we interpret the increases in functional connectivity in response to fear faces (left amygdala-right AI among men, right amygdala-right AI among women) that we observed in our study through the lens of the social salience hypothesis (Shamay-Tsoory and Abu-Akel, 2016). This model posits that oxytocin modulates attentional orienting to social cues in ways that are contingent on the current context and individual characteristics via interactions with the dopaminergic system. It is possible that, for trauma-exposed individuals who are administered oxytocin in a non-therapeutic context, the threat communicated by fearful faces could become more salient, and therefore increase amygdala-AI connectivity, rather than less salient with a corresponding decrease in amygdala-AI connectivity as a fear/stress theory of oxytocin would posit. This could also be the case for our study, in which oxytocin administration was paired with an fMRI scan, not with a treatment protocol or other behavioral platform that was intended to be therapeutic. And, even though we hypothesized that oxytocin would reduce amygdala-AI connectivity, there is evidence that oxytocin increases connectivity during tasks that involve social decision-making, purportedly so that the amygdala is better able to detect cues relevant to this decision-making (Rilling et al., 2012).
There is previous evidence of lateralized effects of intranasal oxytocin in response to emotional stimuli in healthy samples (Tully et al., 2018), with support for greater responsiveness within the left amygdala in clinical samples in response to task (Koch et al., 2016a) and at rest (Martins et al., 2020). For example, in their sample of police officers with PTSD, Koch and colleagues found that oxytocin effects were stronger in the left amygdala than in the right amygdala during processing of emotional faces regardless of valence (Koch et al., 2016a). The left amygdala has been shown to be sensitive to degree of arousal signaled by a specific stimulus, whereas the right amygdala is responsible for automatic detection and general arousal of emotional stimuli (Gläscher and Adolphs, 2003). It is possible that oxytocin reduced the specific threat salience of the fear faces among women (reflected in the decreased left amygdala-left AI and left amygdala-right AI connectivity) and reduced general arousal among men (reflected by reduced right amygdala-right AI connectivity). In fact, memory for emotionally arousing material is more strongly associated with left amygdala activation and glucose uptake in female healthy controls than in males, whereas the right amygdala is more strongly implicated in males than in females (Cahill et al., 2004, 2001).
Gender differences like this are not rare in the oxytocin literature. Gender-specific neural and behavioral responses to oxytocin have been observed in relation to basic processes like emotion recognition and more complex social decision-making (Feng et al., 2015; Luo et al., 2017; Rilling et al., 2014), as well as during rest. For example, during rest, oxytocin normalized hypoconnectivity between the right centromedial amygdala and left ventromedial prefrontal cortex among men with PTSD and normalized right basolateral amygdala-bilateral dACC hyperconnectivity among women with PTSD (Koch et al., 2016b). A recent meta-analysis revealed that oxytocin reduces brain activity in response to fear faces among men, whereas it increases brain activity in response to fear among women (Tully et al., 2018)—suggesting that in the context of PTSD, oxytocin could be more helpful for men. Similar results have been found for other types of stimuli in non-PTSD samples; for example, oxytocin increased amygdala reactivity to threatening scenes among women (Lischke et al., 2012) and increased right amygdala reactivity to cocaine cues among women with cocaine use disorder (CUD) and history of childhood trauma, whereas oxytocin reduced right amygdala reactivity to cocaine cues among men with CUD and childhood trauma histories (Joseph et al., 2019). Future studies designed to probe the subnuclei of the bilateral amygdala among adequate samples of men and women, and that directly compare neural activation to network connectivity at rest and in response to emotional cues, can help elucidate the lateralization of intranasal oxytocin effects and how they vary by gender under which circumstances. This work will be critical for informing the clinical utility of intranasal oxytocin.
Despite the intriguing findings of oxytocin’s opposite effects on amygdala connectivity in men and women in this study, several of our hypotheses were not supported. Most notably, functional connectivity did not vary as a function of PTSD status. Experiencing childhood trauma, regardless of whether it manifests as PTSD, may be the critical element in determining observable effects intranasal oxytocin. However, our study did not include a non-trauma-exposed control group, which would be needed to disentangle the unique effects of childhood trauma exposure and PTSD. Additionally, our measure of DSM-IV PTSD symptoms was changed early in the course of the study to reduce participant burden. The use of a self-report measure, rather than a gold-standard clinician-administered assessment, may have diminished our ability to detect PTSD diagnosis and therefore also differences in functional connectivity driven by PTSD.
The oxytocin-related decrease in connectivity between the left amygdala and right AI in response to anger faces among men in the PTSD group was in the predicted direction but did not survive statistical correction. We did not detect an effect of intranasal oxytocin on connectivity between the amygdala and dACC, another key circuit within the salience network, in response to any of the emotional faces. This may have been due to the fact that the task was meant to isolate automatic/pre-attentive processing rather than more explicit decision-making, which more strongly engages the dACC. We also did not observe the hypothesized effects of oxytocin on functional connectivity between the amygdala and both NA and AI in response to happy faces. It is possible that the facial affect stimuli were not sufficiently salient to our participants to evoke neural reactivity that could be modulated by oxytocin. This may have been particularly true for the happy faces, as previous studies have shown than patients with PTSD rate them as less intense and more negative than controls (Felmingham et al., 2014; Frewen et al., 2010). More personalized stimuli, especially those that are rewarding or that match anger faces to the age and gender of perpetrators, could add external validity and clinical relevance.
Several other limitations of this study are worth noting. With our modest sample size, even with our within-subjects cross-over design, it is likely that we did not have adequate power to detect some of our hypothesized effects, potentially leading to Type II errors. Our limited power also precluded us from exploring other potential moderators of oxytocin effects such as severity of psychopathology (Bartz et al., 2011). We did not collect behavioral data, which limits our ability to interpret our findings in light of the social salience theory; e.g., it is not clear whether participants interpreted the facial affect stimuli differently in the two medication conditions. We also did not have a non-trauma exposed control group nor data on the participants’ ages when they experienced childhood trauma, such that we could not examine the specificity of the role of trauma in relation to our outcomes nor the role of chronicity of trauma. Future studies with larger samples are needed to examine potential moderators of neural and behavioral responses to intranasal oxytocin, such as adaptive social support and variations in attachment.
This is the first study to examine the effects of intranasal oxytocin on functional connectivity between the amygdala and brain regions that mediate threat and reward processing during facial affect processing among individuals with childhood trauma exposure with and without PTSD. Our study complements and builds on previous studies that examined intranasal oxytocin in relation to amygdala reactivity (Flanagan et al., 2019; Koch et al., 2016a) and resting state connectivity (Koch et al., 2016b) in PTSD samples. While these findings suggest that it remains possible that intranasal oxytocin could be used therapeutically to reduce threat salience, it is critical to further explore how these effects vary as a function of gender and severity of psychopathology to ensure that oxytocin helps, rather than hinders, trauma recovery. The most promising pipeline for treatment development may be to deliver oxytocin in combination with complementary psychosocial interventions for individuals with elevated PTSD symptoms in which contextual factors can be carefully monitored and controlled.
Supplementary Material
Highlights.
Posttraumatic stress disorder is related to aberrant threat and reward processing
Intranasal oxytocin impacts brain systems that mediate threat and reward
We examined oxytocin effects on brain connectivity in a childhood-trauma exposed sample
Oxytocin impacted amygdala-anterior insula connectivity to fearful faces
Results varied by gender and hemisphere, but not posttraumatic stress disorder
Acknowledgements
This manuscript is the result of work supported, in part, by the National Institute of Mental Health (R21MH099619), the National Institute on Alcohol Abuse and Alcoholism (K23AA023845), the National Institute on Drug Abuse (P50DA106511), and the National Center for Advancing Translational Sciences (UL1TR001450). The views expressed in this article are those of the authors and not the NIH, the Department of Veterans Affairs, nor any U.S. government agency. Clinicaltrials.gov Identifier: NCT01963078. Data reported here were presented at the Annual Meeting of the Association for Behavioral and Cognitive Therapies, November 2019.
Disclosures
Dr. Paul Holtzheimer receives consulting fees from Abbott and royalties from UpToDate and Oxford University Press for work that is unrelated to this research. All other authors have no disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah CG, Averill CL, Ramage AE, Averill LA, Goktas S, Nemati S, Krystal JH, Roache JD, Resick PA, Young-McCaughan S, Peterson AL, Fox P, 2019. Salience network disruption in U.S. Army soldiers with posttraumatic stress disorder. Chronic Stress 3, 2470547019850467. 10.1177/2470547019850467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos A, Pittman J, Angkaw AC, Nievergelt CM, Hansen CJ, Aversa LH, Parisi SA, Barkauskas DA, Baker DG, 2014. The cumulative effect of different childhood trauma types on self-reported symptoms of adult male depression and PTSD, substance abuse and health-related quality of life in a large active-duty military cohort. J. Psychiatr. Res 58, 46–54. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN, 2011. Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci 15, 301–309. 10.1016/j.tics.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol 57, 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, 2003. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 27, 169–190. [DOI] [PubMed] [Google Scholar]
- Bethlehem RA, van Honk J, Auyeung B, Baron-Cohen S, 2013. Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology 38, 962–974. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM, 1995. The development of a Clinician-Administered PTSD Scale. J. Trauma. Stress 8, 75–90. [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, Ford JD, 2012. Parsing the effects of violence exposure in early childhood: Modeling developmental pathways. J. Pediatr. Psychol 37, 11–22. 10.1093/jpepsy/jsr063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM, 2008. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Hum. Brain Mapp 29, 517–523. 10.1002/hbm.20415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MT, 2001. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol. Learn. Mem 75, 1–9. 10.1006/nlme.2000.3999 [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J, 2004. Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: An fMRI investigation. Learn. Mem 11, 261–266. 10.1101/lm.70504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Steiner A, Porges SW, Heinrichs M, 2013. Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology 38, 1198–1202. 10.1016/j.psyneuen.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH, 2013. Sex differences in oxytocin receptor binding in forebrain regions: Correlations with social interest in brain region-and sex- specific ways. Horm. Behav 64, 693–701. 10.1016/j.yhbeh.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Ehlers A, Clark DM, 2000. A cognitive model of posttraumatic stress disorder. Behav. Res. Ther 38, 319–345. 10.1016/S0005-7967(99)00123-0 [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV, 1976. Measuring facial movement. Environ. Psychol. Nonverbal Behav 1, 56–75. 10.1007/BF01115465 [DOI] [Google Scholar]
- Felmingham KL, Falconer EM, Williams L, Kemp AH, Allen A, Peduto A, Bryant RA, 2014. Reduced Amygdala and Ventral Striatal Activity to Happy Faces in PTSD Is Associated with Emotional Numbing. PLoS ONE 9, e103653. 10.1371/journal.pone.0103653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK, 2015. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. 9, 754–764. 10.1007/s11682-014-9333-9 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer GM, Spitzer RL, Williams JBW, 2000. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version. Biometrics Research, New York State Psychiatric Institute, New York, NY. [Google Scholar]
- Flanagan JC, Sippel LM, Maria MMMS, Hartwell KJ, Brady KT, Joseph JE, 2019. Impact of oxytocin on the neural correlates of fearful face processing in PTSD related to childhood trauma. Eur. J. Psychotraumatology 10, 1606626. 10.1080/20008198.2019.1606626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JC, Sippel LM, Wahlquist A, Moran-Santa Maria MM, Back SE, 2018. Augmenting Prolonged Exposure therapy for PTSD with intranasal oxytocin: A randomized, placebo-controlled pilot trial. J. Psychiatr. Res 98, 64–69. 10.1016/j.jpsychires.2017.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, 1995. Posttraumatic stress diagnostic scale manual. National Computer Systems Pearson, Inc., Minneapolis, MN. [Google Scholar]
- Fonzo GA, 2018. Diminished positive affect and traumatic stress: A biobehavioral review and commentary on trauma affective neuroscience. Neurobiol. Stress 9, 214–230. 10.1016/j.ynstr.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB, 2010. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate partner violence posttraumatic stress disorder. Biol. Psychiatry 68, 433–441. 10.1016/j.biopsych.2010.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Neufeld RWJ, Densmore M, Stevens TK, Lanius RA, 2010. Social emotions and emotional valence during imagery in women with PTSD: Affective and neural correlates. Psychol. Trauma Theory Res. Pract. Policy 2, 145–157. 10.1037/a0019154 [DOI] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ, 1997. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6, 218–29. 10.1006/nimg.1997.0291 [DOI] [PubMed] [Google Scholar]
- Giovanna G, Damiani S, Fusar-Poli L, Rocchetti M, Brondino N, de Cagna F, Mori A, Politi P, 2020. Intranasal oxytocin as a potential therapeutic strategy in post-traumatic stress disorder: A systematic review. Psychoneuroendocrinology 115, 104605. 10.1016/j.psyneuen.2020.104605 [DOI] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R, 2003. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J. Neurosci 23, 10274–10282. 10.1523/JNEUROSCI.23-32-10274.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Stein MB, 2004. Association between childhood trauma and physical disorders among adults in the United States. Psychol Med 34, 509–520. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Fitzgerald DA, Labuschagne I, Hosanagar A, Wood AG, Nathan PJ, Phan KL, 2015. Oxytocin Modulation of amygdala functional connectivity to fearful faces in generalized social anxiety disorder. Neuropsychopharmacology 40, 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace SA, Rossell SL, Heinrichs M, Kordsachia C, Labuschagne I, 2018. Oxytocin and brain activity in humans: A systematic review and coordinate-based meta-analysis of functional MRI studies. Psychoneuroendocrinology 96, 6–24. 10.1016/j.psyneuen.2018.05.031 [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, et al. , 2010. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Arch Gen Psychiatry 67, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MG, Uhlmansiek MH, Resick PA, Mechanic MB, 2004. Comparison of the posttraumatic stress disorder scale versus the clinician-administered posttraumatic stress disorder scale in domestic violence survivors. J. Trauma. Stress 17, 497–503. 10.1007/s10960-004-5798-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B, 2010. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35, 4–26. 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Lim L, Mehta MA, Simmons A, Mirza KAH, Rubia K, 2018. Altered fear processing in adolescents with a history of severe childhood maltreatment: an fMRI study. Psychol. Med 48, 1092–1101. 10.1017/S0033291716003585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM, 2012. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol. Mood Anxiety Disord 2, 9. 10.1186/2045-5380-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV, Olkin I, 1985. Statistical Methods for Meta-Analysis. Academic Press. [Google Scholar]
- Herringa RJ, 2017. Trauma, PTSD, and the developing brain. Curr. Psychiatry Rep 19. 10.1007/s11920-017-0825-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper JW, Pitman RK, Su Z, Heyman GM, Lasko NB, Macklin ML, Orr SP, Lukas SE, Elman I, 2008. Probing reward function in posttraumatic stress disorder: Expectancy and satisfaction with monetary gains and losses. J. Psychiatr. Res 42, 802–807. 10.1016/j.jpsychires.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JE, McRae-Clark A, Sherman BJ, Baker NL, Moran-Santa Maria M, Brady KT, 2019. Neural correlates of oxytocin and cue reactivity in cocaine-dependent men and women with and without childhood trauma. Psychopharmacology (Berl.). 10.1007/s00213-019-05360-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi SA, Duval ER, Sheynin J, King AP, Phan KL, Martis B, Porter KE, Liberzon I, Rauch SAM, 2020. Neural correlates of emotional reactivity and regulation associated with treatment response in a randomized clinical trial for posttraumatic stress disorder. Psychiatry Res. Neuroimaging 299, 111062. 10.1016/j.pscychresns.2020.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, 1995. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 52, 1048. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Britton JC, Schwab ZJ, Price LM, Weiner MR, Gold AL, Rosso IM, Simon NM, Pollack MH, Rauch SL, 2014. Cortico-limbic responses to masked affective faces across PTSD, panic disorder, and specific phobia. Depress. Anxiety 31, 150–159. 10.1002/da.22156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A, 2005. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci 25, 11489–11493. 10.1523/JNEUROSCI.3984-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M, 2016a. Intranasal oxytocin administration dampens amygdala reactivity towards emotional faces in male and female PTSD patients. Neuropsychopharmacology 41, 1495–1504. 10.1038/npp.2015.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M, 2016b. Intranasal oxytocin normalizes amygdala functional connectivity in posttraumatic stress disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 41, 2041–2051. 10.1038/npp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M, 2014. Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: Salience processing and fear inhibition processes. Psychoneuroendocrinology 40, 242–256. 10.1016/j.psyneuen.2013.11.018 [DOI] [PubMed] [Google Scholar]
- Koch SBJ, Zuiden M. van, Nawijn L, Frijling JL, Veltman DJ, Olff M, 2016c. Aberrant Resting-State Brain Activity in Posttraumatic Stress Disorder: A Meta-Analysis and Systematic Review. Depress. Anxiety 33, 592–605. 10.1002/da.22478 [DOI] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A, 2007. Early childhood factors associated with the development of post-traumatic stress disorder: Results from a longitudinal birth cohort. Psychol Med 37, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouider S, Dehaene S, 2007. Levels of processing during non-conscious perception: a critical review of visual masking. Philos. Trans. R. Soc. B Biol. Sci 362, 857–875. 10.1098/rstb.2007.2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner O, Dotsch R, Bijlstra G, Wigboldus DHJ, Hawk ST, Knippenberg A. van, 2010. Presentation and validation of the Radboud Faces Database. Cogn. Emot 24, 1377–1388. 10.1080/02699930903485076 [DOI] [Google Scholar]
- Leppanen J, Ng KW, Tchanturia K, Treasure J, 2017. Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neurosci. Biobehav. Rev 78, 125–144. 10.1016/j.neubiorev.2017.04.010 [DOI] [PubMed] [Google Scholar]
- Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC, Domes G, 2012. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology 37, 1431–1438. [DOI] [PubMed] [Google Scholar]
- Luo L, Becker B, Geng Y, Zhao Z, Gao S, Zhao W, Yao S, Zheng X, Ma X, Gao Z, Hu J, Kendrick KM, 2017. Sex-dependent neural effect of oxytocin during subliminal processing of negative emotion faces. NeuroImage 162, 127–137. 10.1016/j.neuroimage.2017.08.079 [DOI] [PubMed] [Google Scholar]
- Martins D, Leslie M, Rodan S, Zelaya F, Treasure J, Paloyelis Y, 2020. Investigating resting brain perfusion abnormalities and disease target-engagement by intranasal oxytocin in women with bulimia nervosa and binge-eating disorder and healthy controls. Transl. Psychiatry 10, 1–13. 10.1038/s41398-020-00871-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ, 2019. Attentional bias for threat: Crisis or opportunity? Clin. Psychol. Rev 69, 4–13. 10.1016/j.cpr.2018.05.005 [DOI] [PubMed] [Google Scholar]
- Mobbs D, Greicius MD, Abdel-Azim E, Menon V, Reiss AL, 2003. Humor modulates the mesolimbic reward centers. Neuron 40, 1041–1048. 10.1016/S0896-6273(03)00751-7 [DOI] [PubMed] [Google Scholar]
- Monson CM, Fredman S, Dekel R, 2010. Posttraumatic stress disorder in an interpersonal context, Interpersonal processes in the anxiety disorders: Implications for understanding psychopathology and treatment. American Psychological Association, Washington, D.C. [Google Scholar]
- Nanni V, Uher R, Danese A, 2012. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am. J. Psychiatry 169, 141–151. 10.1176/appi.ajp.2011.11020335 [DOI] [PubMed] [Google Scholar]
- Nawijn L, van Zuiden M, Frijling JL, Koch SBJ, Veltman DJ, Olff M, 2015. Reward functioning in PTSD: A systematic review exploring the mechanisms underlying anhedonia. Neurosci. Biobehav. Rev 51, 189–204. 10.1016/j.neubiorev.2015.01.019 [DOI] [PubMed] [Google Scholar]
- Nawijn L, van Zuiden M, Koch SBJ, Frijling JL, Veltman DJ, Olff M, 2017. Intranasal oxytocin increases neural responses to social reward in post-traumatic stress disorder. Soc. Cogn. Affect. Neurosci 12, 212–223. 10.1093/scan/nsw123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawijn L, van Zuiden M, Koch SBJ, Frijling JL, Veltman DJ, Olff M, 2016. Intranasal oxytocin enhances neural processing of monetary reward and loss in post-traumatic stress disorder and traumatized controls. Psychoneuroendocrinology 66, 228–237. 10.1016/j.psyneuen.2016.01.020 [DOI] [PubMed] [Google Scholar]
- Neumann ID, Slattery DA, 2016. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol. Psychiatry, Oxytocin and Psychiatry: From DNA to Social Behavior 79, 213–221. 10.1016/j.biopsych.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Witteveen A, Denys D, 2010. A psychobiological rationale for oxytocin in the treatment of posttraumatic stress disorder. CNS Spectr. 15, 522–530. 10.1017/S109285290000047X [DOI] [PubMed] [Google Scholar]
- Palgi S, Klein E, Shamay-Tsoory SG, 2016. Oxytocin improves compassion toward women among patients with PTSD. Psychoneuroendocrinology 64, 143–149. 10.1016/j.psyneuen.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Patel RC, Spreng RN, Shin LM, Girard TA, 2012. Neurocircuitry models of posttraumatic stress disorder and beyond: A meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev 36, 2130–2142. 10.1016/j.neubiorev.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA, 2011. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology (Berl.) 214, 55–70. 10.1007/s00213-010-2009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA, 2006. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research—Past, present, and future. Biol. Psychiatry 60, 376–382. 10.1016/j.biopsych.2006.06.004 [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK, 2000. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol. Psychiatry 47, 769–776. 10.1016/S0006-3223(00)00828-3 [DOI] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, 2014. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology 39, 237–248. 10.1016/j.psyneuen.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G, 2012. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology 37, 447–461. 10.1016/j.psyneuen.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CM, Green AJ, 1997. Parenting stress and anger expression as predictors of child abuse potential. Child Abuse Negl. 21, 367–377. 10.1016/S0145-2134(96)00177-9 [DOI] [PubMed] [Google Scholar]
- Sartory G, Cwik J, Knuppertz H, Schürholt B, Lebens M, Seitz RJ, Schulze R, 2013. In search of the trauma memory: A meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD). PLoS ONE 8, e58150. 10.1371/journal.pone.0058150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Gunturkun O, Maier W, Hurlemann R, 2013. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc. Natl. Acad. Sci 110, 20308–20313. 10.1073/pnas.1314190110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze L, Lischke A, Greif J, Herpertz SC, Heinrichs M, Domes G, 2011. Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology 36, 1378–1382. 10.1016/j.psyneuen.2011.03.011 [DOI] [PubMed] [Google Scholar]
- Shahrestani S, Kemp AH, Guastella AJ, 2013. The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: A meta-analysis. Neuropsychopharmacology 38, 1929–1936. 10.1038/npp.2013.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev A, Liberzon I, Marmar C, 2017. Post-Traumatic Stress Disorder. N. Engl. J. Med 376, 2459–2469. 10.1056/NEJMra1612499 [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A, 2016. The social salience hypothesis of oxytocin. Biol. Psychiatry 79, 194–202. 10.1016/j.biopsych.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 22–33. [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK, 2005. Structural and functional anatomy of PTSD: Findings from neuroimaging research, in: Neuropsychology of PTSD: Biological, Cognitive, and Clinical Perspectives. The Guilford Press, New York, NY, US, pp. 59–82. [Google Scholar]
- Spengler FB, Schultz J, Scheele D, Essel M, Maier W, Heinrichs M, Hurlemann R, 2017. Kinetics and Dose Dependency of Intranasal Oxytocin Effects on Amygdala Reactivity. Biol. Psychiatry 82, 885–894. 10.1016/j.biopsych.2017.04.015 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C, 2009. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 168, 242–249. 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J, Gabay AS, Brown D, Murphy DGM, Blackwood N, 2018. The effect of intranasal oxytocin on neural response to facial emotions in healthy adults as measured by functional MRI: A systematic review. Psychiatry Res. Neuroimaging 272, 17–29. 10.1016/j.pscychresns.2017.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunnard C, Rane LJ, Wooderson SC, Markopoulou K, Poon L, Fekadu A, Juruena M, Cleare AJ, 2014. The impact of childhood adversity on suicidality and clinical course in treatment-resistant depression. J. Affect. Disord 152, 122–130. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ, 2012. A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology 37, 438–443. [DOI] [PubMed] [Google Scholar]
- Widom CS, Czaja SJ, Bentley T, Johnson MS, 2012. A prospective investigation of physical health outcomes in abused and neglected children: New findings from a 30-year follow-up. Am. J. Public Health 102, 1135–1144. 10.2105/AJPH.2011.300636 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


