Before the development of the inactivated (IPV) and live oral poliovirus (OPV) vaccines, sporadic outbreaks of poliomyelitis were reported to cause as many as 18,000 cases of paralysis and over 3,000 deaths in the United States alone.1 The straightforward oral administration, high efficacy and relatively low cost of OPV was fundamental to the dramatic reduction in polio achieved by mass vaccination campaigns. Wild polioviruses were certified by the World Health Organization (WHO) as eliminated throughout the Americas in 1994. However, an adverse effect of OPV is vaccine-associated paralytic polio. Among those countries exclusively using OPV in 2012, an estimated 400 cases of vaccine-associated paralytic polio occurred that year.2 This burden is more than double the incidence of wild polio in 2019.3 Vaccine-derived polioviruses (VDPV) can also spread from person-to-person, a phenomenon which led to more than 250 additional cases of paralysis during 2019.4 The risk of OPV-associated paralytic polio spurred many countries to switch to the safer IPV vaccine. While IPV elicits a much weaker mucosal immune response than OPV,5 and is thus less effective at averting transmission, it is very protective against disease. In the Americas, Canada transitioned to exclusive IPV use in 1995, the US in 2000, Costa Rica in 2010 and Uruguay in 2012. However, the remaining 31 countries in the Americas (Table 1) continue to administer at least one dose of OPV.
Table 1:
Country-specific poliovirus vaccine policies and income strata across the Americas (December 2019).33
| Country | IPV dosesab | OPV dosesa | Income levelc |
|---|---|---|---|
| Antigua and Barbuda | 2 | 3 | High |
| Argentina | 2 | 3 | High |
| Bahamas | 2 | 2 | High |
| Barbados | 1 | 5 | High |
| Belize | 1 | 4 | Upper middle |
| Bolivia | 1 | 4 | Lower middle |
| Brazil | 3 | 2 | Upper middle |
| Canada | 5 | 0 | High |
| Chile | 2 | 2 | High |
| Colombia | 2 | 3 | Upper middle |
| Costa Rica | 5 | 0 | Upper middle |
| Cuba | 2d | 4 | Upper middle |
| Dominica | 1 | 5 | Upper middle |
| Dominican Republic | 1 | 4 | Upper middle |
| Ecuador | 2d | 3 | Upper middle |
| El Salvador | 1 | 5 | Lower middle |
| Grenada | 1 | 5 | Upper middle |
| Guatemala | 1 | 4 | Upper middle |
| Guyana | 1 | 4 | Upper middle |
| Haiti | 1b | 4 | Low |
| Honduras | 2 | 2 | Lower middle |
| Jamaica | 1 | 4 | Upper middle |
| Mexico | 4 | 1 | Upper middle |
| Nicaragua | 1 | 2 | Lower middle |
| Panama | 3 | 2 | Upper middle |
| Paraguay | 1 | 4 | Upper middle |
| Peru | 2 | 3 | Upper middle |
| Saint Kitts and Nevis | 1 | 5 | High |
| Saint Lucia | 1 | 5 | Upper middle |
| Saint Vincent and the Grenadines | 2 | 3 | Upper middle |
| Suriname | 1 | 4 | Upper middle |
| Trinidad and Tobago | 1 | 4 | High |
| United States of America | 4e | 0 | High |
| Uruguay | 4 | 0 | High |
| Venezuela | 1 | 4 | Upper middle |
Doses recommended for immunocompetent children.
Countries that use OPV apply all IPV doses before any OPV, with the exception of Haiti which administers OPV at birth, followed by IPV six weeks later.
World Bank Classification.
Fractional dose.
IPV vaccination schedule in the US typically includes four doses, but five doses are also possible depending on the formulation of combined vaccine used.
Following the recognition that most VDPV is caused by serotype 2 poliovirus, the WHO coordinated a global switch in 2016, replacing trivalent OPV with bivalent OPV and recommending at least one dose of IPV before OPV.6 The switch to bivalent OPV eliminated serotype 2 vaccine-associated paralytic polio, whereas the use of IPV before OPV substantially reduces the risk of vaccine-associated paralytic polio linked to other serotypes. In countries where vaccine-associated polio was a significant problem, such as in Hungary, those adverse effects disappeared after introducing an IPV dose before the administration of bivalent OPV.7 Additionally, the use of bivalent instead of trivalent OPV reduces the de novo instigation of VDPV transmission. However, the regimen does not confer sufficient protection against serotype 2. Although wild serotype 2 has been eradicated worldwide, the risk of serotype 2 VDPV remains in all regions, including the Americas, due to long-term excretion8 and importation from other regions.4 Immunocompromised individuals, for example, are at elevated risk of prolonged excretion,9 15 of whom have been documented in the Americas.10
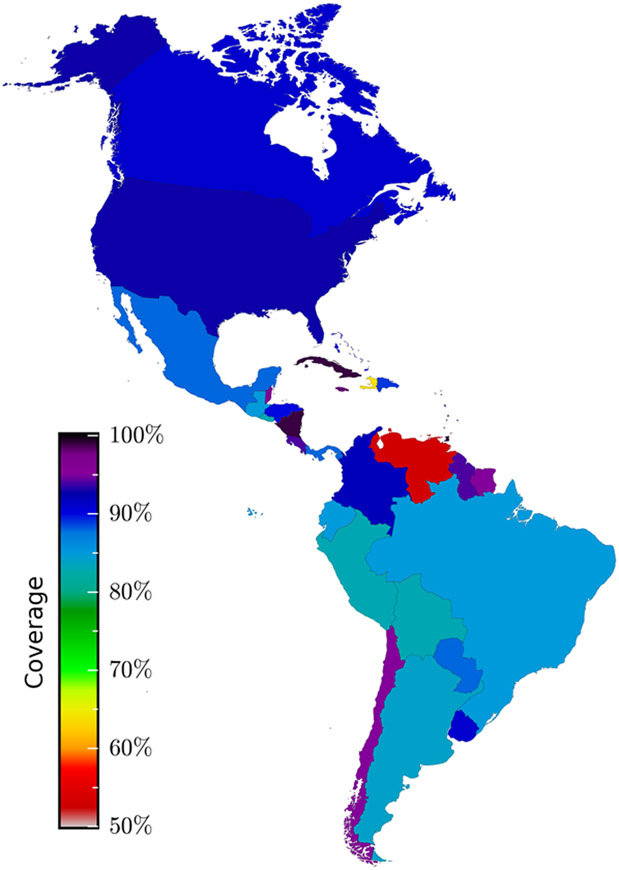
While the clinical efficacy of IPV has only been established for one and two doses against serotype 1 (as 36% and 89%, respectively),11 seroconversion can be used as a proxy for efficacy. In the absence of an OPV booster, three doses of IPV are required to elicit an efficacy approaching complete protection against all serotypes.12,13 If followed by OPV, two doses of IPV may be sufficient to provide an efficacy of more than 90%.13,14 Under the vaccination schedule of most countries in the Americas, which includes only one IPV dose followed by OPV (Table 1), at best 77% seroconversion against serotype 2 would be achieved.13 Worryingly, the herd immunity threshold necessary to prevent sustained transmission has been estimated to range between 80% to 97%.15 Therefore, outbreak risk is a concern in any country where the schedule includes only a single dose of IPV, and even a two-dose schedule may be insufficient to avert an outbreak, irrespective of the coverage attained. Unfortunately, coverage in many countries is incomplete (Figure 1), and the confluence of the global rise in vaccine hesitancy with the adverse effects of OPV could fuel refusal of poliovirus vaccines and even impact the uptake of other vaccines.
Figure 1:
Coverage for three doses of poliovirus vaccine in the Americas. Coverage reported by each country to the World Health Organization for the year 2018.32
The risk of a polio outbreak is especially perilous in Venezuela and Haiti, where the schedule includes only one dose of IPV, and where humanitarian crises are undermining surveillance and vaccination (Figure 1). A serotype 2 outbreak in these countries would endanger the many other nations whose vaccination schedules include fewer than three IPV doses (Table 1). The very real possibility of an international outbreak propagating from a local origin is evidenced by the measles epidemic that spread from Venezuela into neighbouring countries, two years after the Americas had been declared free of measles.
To address these challenges, the Polio Eradication and Endgame Strategic Plan prepared by the WHO calls for the complete replacement of OPV. However, IPV supply shortages have delayed progress towards this goal and vaccine manufacturers are not on track to supply even two doses of IPV worldwide until at least 2023.6 Exacerbating the problem, two doses may not be enough, for the reasons outlined above. We should set our sights higher and aim for three doses of IPV worldwide on this timeline, if not more quickly.
To quantify the gap between projected supply and required supply, we calculated the number of vaccines that would achieve a 95% coverage with two and with three IPV doses. For each country, we computed the doses required for an IPV vaccination schedule at 2 and 4 months, or at 2, 4 and 12 months of age based on country-specific population size, population growth, birth rate, as well as neonatal and infant mortalities (Supplement). To achieve IPV schedules with two or with three doses, our projections suggest that 29.6 or 44.4 million doses would be required in the Americas, and 277.7 or 416.0 million doses globally, respectively. We then adjusted for the use of multivalent IPV-containing vaccines, as these combination vaccines are manufactured separately from IPV-exclusive formulations. Consequently, we estimated that 14.1 or 21.1 million IPV-exclusive doses would be required annually in the Americas for an IPV schedule of two or three doses, respectively. On a global scale, the number of IPV-exclusive doses required would be an order of magnitude greater than those needed in the Americas: 232.6 or 348.7 million IPV-exclusive doses for a schedule with two or three IPV doses, respectively. These estimates are conservative given that in addition catch-up campaigns should be implemented among those who have not received the full schedule. Compared to the global requirement for 348.7 million doses, only between 80 and 100 million doses are expected to be available in 2020.16 This shortfall underscores the urgent need for action to expand capacity and accelerate production.
Another obstacle delaying the switch to IPV is the comparative price of the two vaccines. The price of OPV ranges from $0.12 to $0.18,17 substantially less than the $1.00 to $3.28 range for IPV.18 Added to the price of IPV are the costs of syringes, needles and training of healthcare workers for delivery via injection, as well as risks associated with safe disposal of used syringes. Counterbalancing this investment, the VDPV outbreaks that can originate from OPV impose substantial economic and public health costs.19 Cost-effectiveness analyses that take into account the morbidity and mortality as well as the economic costs of controlling these outbreaks are needed to comprehensively evaluate the true toll of continuing OPV vaccination.
One interim solution successfully adopted by India and Sri Lanka to address the difficulty of insufficient supply and higher cost of IPV is dilution to produce fractional dose IPV. This approach has also been implemented in Ecuador and Cuba, while seven other Latin American countries have plans to do so.20 The major difficulty facing implementation of a fractional dose strategy is that this formulation requires intradermal administration, which challenges countries without a robust health system infrastructure. Promisingly, a recent study found that intramuscular fractional doses of IPV could provide seroconversion rates even higher than those of intradermal fractional IPV.21 Nevertheless, the efficacy of fractional doses has not been evaluated and antibody titers are lower when compared to a full dose.22 Therefore, although potentially useful as an interim strategy to overcome IPV shortages, fractional dosing should not be considered sustainable long-term approach until high efficacy can be assured.
A stockpile of OPV doses should be maintained until eradication efforts are complete. For emergency response to an outbreak, the WHO recommends that the monovalent oral vaccine against the outbreak strain should be used as part of a multistage vaccination strategy targeting specific at-risk populations and locations informed by risk assessments from an expert advisory group.23 While IPV efficaciously protects against symptomatic poliomyelitis and paralysis, OPV elicits a stronger intestinal immunity which in turn is more effective in preventing viral shedding and thus transmission to others.5 This consideration is particularly pertinent for settings characterized by low income and poor sanitation, in which polioviruses introduced might disseminate widely before detection. Indeed, Israel experienced persistent silent circulation of wild poliovirus, which was only brought under control through an OPV campaign. It is noteworthy, however, that recent silent outbreaks in the Americas have been of OPV-derived viruses, not wild poliovirus.24,25 This history suggests that OPV poses a greater risk to the Americas than do wild introductions, and that a switch to an IPV schedule in the Americas would therefore improve overall safety. To protect against any risk of silent carriage in an exclusive IPV setting, surveillance systems must have the capacity to monitor sewage. If poliovirus circulation is detected, we would further advocate for emergency OPV until the outbreak is controlled, simultaneous with or followed immediately by an intensive IPV vaccination program.26 IPV will boost the protection elicited by OPV as well as prevent VDPV disease.23,27 The IPV campaign should be maintained until the vaccination coverage of the population is sufficient to avert further outbreaks.
To achieve and maintain high IPV coverage in low and lower-middle income countries, vaccination programs must be efficient. In nations with fragile health infrastructure, particular emphasis should be placed on the development of implementation plans that are evidence-based, data-driven and accountable. These plans should include rigorously maintained surveillance and monitoring systems, as well as engage civil society networks and community health workers.28 Community engagement is one critical component of ameliorating vaccine hesitancy, and likewise the comparative safety of IPV may boost public confidence in the vaccine recommendation. On the supply side, signaling a change in vaccination policy is a fundamental step, as an expected change in demand incentivizes the entry of new suppliers into markets.29 Beyond expansion of supply, methods to lower IPV costs include combining delivery with other vaccines and subsidies for manufacturers to produce low-cost IPV.30 Additionally, governments and other health actors should prioritize research aimed at improving the safety and efficacy of vaccines to prevent both disease and transmission.
While OPV was instrumental to humanity’s progress towards worldwide polio virus elimination, it has now become the principal cause of poliomyelitis in the Americas. Unless OPV vaccines that are less likely to revert to virulent strains become available,31 the end-game strategy in the region will entail a switch to the exclusive use of IPV with a high vaccination coverage of at least three doses. Despite the logistical obstacles of achieving herd immunity against all polio serotypes, the successful implementation of high coverage of IPV in both high and middle-income countries demonstrates the feasibility of the goal.
Supplementary Material
Acknowledgments
JAA-M was funded by the Notsew Orm Sands Foundation. MCF was supported by funding from the National Institute of Allergy and Infectious Diseases (K01AI141576). APG was supported by the Burnett and Stender Families’ Endowment. The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The corresponding authors confirm that they had full access to all the data in the study and had final responsibility for the decision to submit for publication.
References
- 1.Centers for Disease Control and Prevention. The Pinkbook ∣ Appendix E: Data and Statistics. 2018; published online May 10. https://www.cdc.gov/vaccines/pubs/pinkbook/appendix/appdx-e.html (accessed Jan 16, 2020).
- 2.Platt LR, Estívariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden. J Infect Dis 2014; 210 Suppl 1: S380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Polio Eradication Initiative. Polio Now. http://polioeradication.org/polio-today/polio-now/ (accessed Jan 16, 2020).
- 4.Global Polio Eradication Initiative. Circulating vaccine-derived poliovirus. http://polioeradication.org/polio-today/polio-now/this-week/circulating-vaccine-derived-poliovirus/ (accessed Jan 16, 2020). [DOI] [PMC free article] [PubMed]
- 5.Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog 2012; 8: e1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UNICEF. Inactivated Polio Vaccine: Supply Update. 2018; published online May 28. https://www.unicef.org/supply/files/Inactivated_Polio_Vaccine_Supply_Update.pdf (accessed Jan 16, 2020).
- 7.Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: past, present and future. Future Microbiol 2015; 10: 791–808. [DOI] [PubMed] [Google Scholar]
- 8.Hull HF, Minor PD. When can we stop using oral poliovirus vaccine? J. Infect. Dis 2005; 192: 2033–5. [DOI] [PubMed] [Google Scholar]
- 9.Duintjer Tebbens RJ, Pallansch MA, Thompson KM. Modeling the prevalence of immunodeficiency-associated long-term vaccine-derived poliovirus excretors and the potential benefits of antiviral drugs. BMC Infect Dis 2015; 15: 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macklin G, Liao Y, Takane M, et al. Prolonged Excretion of Poliovirus among Individuals with Primary Immunodeficiency Disorder: An Analysis of the World Health Organization Registry. Front Immunol 2017; 8: 1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson SE, Traverso HP, Drucker JA, et al. Clinical efficacy of a new, enhanced-potency, inactivated poliovirus vaccine. Lancet 1988; 1: 897–9. [DOI] [PubMed] [Google Scholar]
- 12.Asturias EJ, Dueger EL, Omer SB, et al. Randomized trial of inactivated and live polio vaccine schedules in Guatemalan infants. J Infect Dis 2007; 196: 692–8. [DOI] [PubMed] [Google Scholar]
- 13.O’Ryan M, Bandyopadhyay AS, Villena R, et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study. Lancet Infect Dis 2015; 15: 1273–82. [DOI] [PubMed] [Google Scholar]
- 14.Grassly NC. Immunogenicity and effectiveness of routine immunization with 1 or 2 doses of inactivated poliovirus vaccine: systematic review and meta-analysis. J Infect Dis 2014; 210 Suppl 1: S439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patriarca PA, Sutter RW, Oostvogel PM. Outbreaks of Paralytic Poliomyelitis, 1976–1995. J Infect Dis 1997; 175: S165–72. [DOI] [PubMed] [Google Scholar]
- 16.Gavi-Global Polio Eradication Initiative. Supply and procurement roadmap. Inactivated Polio Vaccine (IPV). https://www.gavi.org/sites/default/files/document/ipv-roadmap-public-summarypdf.pdf (accessed Jan 16, 2020).
- 17.Global Polio Eradication Initiative. OPV. http://polioeradication.org/polio-today/polio-prevention/the-vaccines/opv/ (accessed Jan 16, 2020).
- 18.Global Polio Eradication Initiative. Availability and price of inactivated polio vaccine. http://polioeradication.org/news-post/availability-and-price-of-inactivated-polio-vaccine/ (accessed Jan 16, 2020).
- 19.Global Polio Eradication Initiative. Economic Case for Eradicating Polio. http://polioeradication.org/wp-content/uploads/2016/07/EconomicCase.pdf (accessed Jan 16, 2020).
- 20.Figureroa JP, Andrus JK, Glass R, et al. Fourth Ad-hoc Meeting of the Technical Advisory Group (TAG) on Vaccine-preventable Diseases. 2018; published online. https://www.paho.org/hq/index.php?option=com_docman&task=doc_download&gid=45756&Itemid=270&lang=pt (accessed Jan 16, 2020). [Google Scholar]
- 21.Resik S, Mach O, Tejeda A, et al. Immunogenicity of intramuscular fractional dose of inactivated poliovirus vaccine. J Infect Dis 2019; published online June 26. DOI: 10.1093/infdis/jiz323. [DOI] [PubMed] [Google Scholar]
- 22.Resik S, Tejeda A, Diaz M, et al. Boosting Immune Responses Following Fractional-Dose Inactivated Poliovirus Vaccine: A Randomized, Controlled Trial. J Infect Dis 2017; 215: 175–82. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Responding to a poliovirus event or outbreak: standard operating procedures (Version 3). 2019; published online January. http://polioeradication.org/wp-content/uploads/2016/07/sop-polio-outbreak-response-version-20193101.pdf (accessed Jan 16, 2020).
- 24.de Oliveira Pereira JS, da Silva LR, de Meireles Nunes A, de Souza Oliveira S, da Costa EV, da Silva EE. Environmental Surveillance of Polioviruses in Rio de Janeiro, Brazil, in Support to the Activities of Global Polio Eradication Initiative. Food Environ Virol 2016; 8: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troy SB, Ferreyra-Reyes L, Canizales-Quintero S, et al. Real-time Polymerase Chain Reaction Analysis of Sewage Samples to Determine Oral Polio Vaccine Circulation Duration and Mutation After Mexican National Immunization Weeks. J Pediatric Infect Dis Soc 2012; 1: 223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Global Polio Eradication Initiative. Use of fractional-dose inactivated polio vaccine (fIPV) in supplementary immunization activities (SIAs). 2017; published online November. http://polioeradication.org/wp-content/uploads/2017/11/polio-fipv-in-sias-aide-memoire-01092017-en.pdf (accessed Jan 16, 2020).
- 27.Herremans TM, Reimerink JH, Buisman AM, Kimman TG, Koopmans MP. Induction of mucosal immunity by inactivated poliovirus vaccine is dependent on previous mucosal contact with live virus. J Immunol 1999; 162: 5011–8. [PubMed] [Google Scholar]
- 28.Global Polio Eradication Initiative. Polio Endgame Strategy 2019–2023. http://polioeradication.org/wp-content/uploads/2019/06/english-polio-endgame-strategy.pdf (accessed Jan 16, 2020).
- 29.John TJ. A developing country perspective on vaccine-associated paralytic poliomyelitis. Bull World Health Organ 2004; 82: 53–7; discussion 57–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson KM, Tebbens RJD. Current polio global eradication and control policy options: perspectives from modeling and prerequisites for oral poliovirus vaccine cessation. Expert Rev Vaccines 2012; 11: 449–59. [DOI] [PubMed] [Google Scholar]
- 31.Van Damme P, De Coster I, Bandyopadhyay AS, et al. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study. Lancet 2019; 394: 148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. WHO-UNICEF estimates of Pol3 coverage. https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragepol3.html (accessed Jan 16, 2020).
- 33.World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2019. global summary. http://apps.who.int/immunization_monitoring/globalsummary/schedules (accessed Jan 16, 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.