Abstract
Aims
Emerging evidence suggests that remnant cholesterol (RC) promotes atherosclerotic cardiovascular disease (ASCVD). We aimed to estimate RC-related risk beyond low-density lipoprotein cholesterol (LDL-C) and apolipoprotein B (apoB) in patients without known ASCVD.
Methods and results
We pooled data from 17 532 ASCVD-free individuals from the Atherosclerosis Risk in Communities study (n = 9748), the Multi-Ethnic Study of Atherosclerosis (n = 3049), and the Coronary Artery Risk Development in Young Adults (n = 4735). RC was calculated as non-high-density lipoprotein cholesterol (non-HDL-C) minus calculated LDL-C. Adjusted Cox models were used to estimate the risk for incident ASCVD associated with log RC levels. We also performed discordance analyses examining relative ASCVD risk in RC vs. LDL-C discordant/concordant groups using difference in percentile units (>10 units) and clinically relevant LDL-C targets. The mean age of participants was 52.3 ± 17.9 years, 56.7% were women and 34% black. There were 2143 ASCVD events over the median follow-up of 18.7 years. After multivariable adjustment including LDL-C and apoB, log RC was associated with higher ASCVD risk [hazard ratio (HR) 1.65, 95% confidence interval (CI) 1.45–1.89]. Moreover, the discordant high RC/low LDL-C group, but not the low RC/high LDL-C group, was associated with increased ASCVD risk compared to the concordant group (HR 1.21, 95% CI 1.08–1.34). Similar results were shown when examining discordance across clinical cutpoints.
Conclusions
In ASCVD-free individuals, elevated RC levels were associated with ASCVD independent of traditional risk factors, LDL-C, and apoB levels. The mechanisms of RC association with ASCVD, surprisingly beyond apoB, and the potential value of targeted RC-lowering in primary prevention need to be further investigated.
Keywords: LDL-cholesterol, Remnant cholesterol, Apolipoprotein B, Primary prevention
Graphical Abstract
See page 4333 for the editorial comment for this article ‘Keeping remnants in perspective’, by J. Borén and C.J. Packard, https://doi.org/10.1093/eurheartj/ehab531.
Introduction
Pharmacological lowering of low-density lipoprotein cholesterol (LDL-C), primarily via statin therapy, has an unequivocal impact on the reduction of atherosclerotic cardiovascular diseases (ASCVD). 1 However, significant residual cardiovascular risk has been reported among statin-treated individuals, even with otherwise low LDL-C levels.2 , 3
Continuous efforts have been made to identify strategies to approach residual risk.4 , 5 Given the failure of high-density lipoprotein cholesterol (HDL-C) raising therapies in reducing ASCVD events,6 , 7 the research focus has swung to triglycerides (TG) and triglyceride-rich lipoproteins (TGRL), which have been associated with the development of ASCVD.8–13 Remnant particles are TGRL that have been partially lipolyzed by the action of lipoprotein lipase; they circulate in plasma and accumulate in the subendothelial space,14 , 15 contributing to endothelial dysfunction, inflammation, and ultimately atherogenesis.16 , 17 About one third of the cholesterol load carried by apolipoprotein B (apoB) containing lipoprotein particles is transported via remnant particles in non-fasting conditions.18 Mounting evidence from Mendelian randomization studies has established the cholesterol content in remnant particles to be causally associated with ischaemic heart disease,19 , 20 and most recently with the risk of aortic valve stenosis.21 Recent studies suggest that cholesterol rather than TG content of remnant particles is the causal culprit in ASCVD and that TG levels indirectly reflect remnant particles and their cholesterol content.22
Although some data suggest that TG or remnant cholesterol (RC) associated ASCVD risk is proportional to apoB changes,23 a recent study in patients with coronary artery disease demonstrated that RC is associated with coronary atheroma progression independent of apoB levels.24 These unexpected observations have prompted a call to further validate these findings in other populations and re-evaluate the atherogenic mechanisms of RC beyond apoB particle concentration.25 In this study, we aim to identify the risk associated with RC independent of LDL-C and apoB in patients without known ASCVD in a large pooled primary prevention cohort with long-term follow-up.
Methods
Setting
For the present study, we used individual-level data from three landmark US cohorts: the Atherosclerosis Risk in Communities (ARIC) study, the Multi-Ethnic Study of Atherosclerosis (MESA), and the Coronary Artery Risk Development in Young Adults (CARDIA). The cohorts data are available to qualifying investigators directly from the study. These cohorts were included to increase precision and generalizability of our findings. In addition, they share key features that were particularly helpful for this study such as non-clinical referral, inclusion of mostly primary prevention US individuals, and full lipid and apolipoprotein measurements, such as apoB and apolipoprotein A1 (apoA1). The respective study protocols were approved by the institutional review committee of each of the sites participating in each of the studies. Institutional review boards at all participating institutions approved the three studies. All participants provided written informed consent at each study visit.
Study design
We conducted a study pooling individual-level data from the three cohorts. For the present analysis, the baseline visit was defined as the time of lipid and apolipoprotein measurement in each cohort: Visit 1 in MESA (years 2000–2002), Visit 1 in CARDIA (year 1985), and Visit 4 in ARIC (1996–98). End of follow-up time was 31 December 2013 for MESA, 31, December 2011 for CARDIA, and 31, December 2016 for ARIC.
Study population
All participants from baseline visits were screened for inclusion. Of them, we excluded individuals with prevalent ASCVD at baseline (n = 1292), in addition to those with missing values for lipids or apolipoproteins (n = 2213), information regarding outcomes and/or follow-up times (n = 6). To increase homogeneity of our study population, we only included Whites and Blacks; therefore, we excluded Hispanics (n = 1496) and Chinese (n = 803) from MESA, and those who were neither black nor white from ARIC (n = 69).
Lipid measurements
In ARIC, total cholesterol (TC) and TG were determined by enzymatic methods, and HDL-C was measured after dextran-magnesium precipitation (https://www2.cscc.unc.edu/aric/cohort-manuals). ApoB was measured using World Health Organization/International Federation of Clinical Chemistry (WHO/IFCC) standardized reference materials [intra-coefficient of variation (CV): 2.1%, inter-CV: 4.5%]. In CARDIA, TC and HDL-C were measured using serum from a fasting blood draw that was separated into plasma frozen at −70°C before analysis in a central laboratory. ApoB was measured by enzyme-linked immunosorbent assay (intra-CV: <5%). In MESA, fasting blood samples were collected and stored at −70°C. Lipids were measured on eDTA plasma at a central laboratory within approximately 2 weeks of collection. TC was measured via cholesterol oxidase methods, and TG were measured using TG GB on a Roche COBAS FARA centrifugal analyser (Roche Diagnostics, Indianapolis, IN, USA). HDL-C was quantified by cholesterol oxidase methods after precipitation of non-HDL-C by magnesium/dextran (Roche Diagnostics). ApoB was quantified using the Tina-quant apoB ver.2 immunoassay on a Roche Modular P analyser (Roche Diagnostics) (intra-CV: <5%, inter-CV: <5%). High-sensitivity C-reactive protein (hsCRP) was only available in ARIC and MESA and was measured by the immunoturbidimetric assay using the BNII analyser (Dade Behring, Deerfield, IL).
Since the Friedewald equation is known to underestimate LDL-C in the presence of hypertriglyceridaemia,26 we used the Martin/Hopkins equation. This method estimates LDL-C using 1 of 180 different factors for the TG to very low-density lipoprotein cholesterol (VLDL-C) ratio according to non-HDL-C and TG levels27 and has been externally validated by groups inside and outside the US.28–30 Levels of RC were estimated as TC minus HDL-C minus calculated LDL-C. Although currently there is no standard method to estimate RC, this equation has been frequently used in previous studies because it is available from the standard lipid profile.8 , 24 , 31 Furthermore, we have previously demonstrated that using Martin/Hopkins LDL-C in the equation provides a more accurate estimate of RC than using Friedewald LDL-C.32 Non-HDL-C was calculated as TC minus HDL-C.
Discordance definition
As there is no physiological cutpoint for discordance between different lipid or lipoprotein measures, we used different approaches to define discordance. First, we defined discordance using >10 difference in percentile units (RC percentile minus LDL-C percentile). The population was divided into: (i) RC percentile < LDL-C percentile (discordantly low RC) by >10 percentile units; (ii) concordant RC and LDL-C within ±10 percentile units; and (iii) RC percentile > LDL-C percentile (discordantly high RC) by >10 percentile units. We also used several clinical cutpoints to define discordance and to assess the robustness of our findings. Although existing literature has used medians as cutpoints,33–36 we focused on clinically relevant LDL-C cutpoints (70, 100, and 130 mg/dL) that were obtained from worldwide guideline recommendations.37–39 Respective RC cutpoints were identified using equivalent population percentiles from the pooled cohort corresponding to these LDL-C values.
Other covariates
Demographics (age, sex, race/ethnicity) and cardiovascular risk factors were obtained from history, physical examination, and laboratory data at each visit selected as baseline for our study. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of height in metres. Smoking status was similarly categorized in each cohort as never, former, and current smoker. Diabetes mellitus was defined as a fasting (≥8 h) serum glucose ≥126 mg/dL, non-fasting glucose ≥200 mg/dL, self-reported physician diagnosis of diabetes mellitus, or use of hypoglycaemic agents. Blood pressure (BP) was measured three times, and the mean of the second and third measurements was used in MESA and CARDIA, whereas it was measured two times and the mean of both measurements was used in ARIC. Hypertension was defined as self-reported physician diagnosis of hypertension or reported use of antihypertensive medications. Use of lipid-lowering and antihypertensive medications was self-reported.
Study outcomes
Details on the event ascertainment methods used in each cohort have been reported previously and were similar across studies. The primary outcome was incident ASCVD determined from hospital discharge codes or death certificates. Incident ASCVD was similarly defined in the three cohorts as definite or probable myocardial infarction, definite coronary death, and definite or probable stroke. In addition, ARIC investigators conducted continuous surveillance for all cardiovascular disease-related hospitalizations and deaths. All ASCVD events were adjudicated by the ARIC study investigators. Study participants contributed follow-up time from the date of the participant’s baseline visit until the date of incident ASCVD event, death, loss to follow-up, or the administrative censoring at December 31, 2016, whichever came first. For CARDIA, incident ASCVD events were recorded through September 2011. Medical records were requested for participants who had been hospitalized or received an outpatient revascularization procedure. Two physician members of the CARDIA endpoints surveillance and adjudication committee independently classified events and assigned incident dates. If they disagreed, the full committee made the final decision. For MESA, event data were collected through follow-up telephone calls, patient information at MESA visits, and medical records. Two independent physicians, blinded to participant data, served as adjudicators.40
Statistical analyses
Baseline characteristics of the study population by concordance/discordance categories between LDL-C vs. RC were described using medians (25th–75th percentiles) for continuous and proportions for categorical variables. Comparisons were performed using Kruskal–Wallis test and chi-squared test, respectively, between the four categories.
For our prospective analysis, we constructed nested Cox proportional hazard models to assess the independent association between continuous log-transformed RC levels (given non-normal distribution) and incident ASCVD. Model 1 was adjusted by age, sex, race/ethnicity, smoking status, systolic BP (SBP), use of antihypertensive and lipid-lowering medications. Model 2 was additionally adjusted by apoB, and Model 3 was further adjusted by apoA1. Additional models (Supplementary material online) included non-HDL-C, HDL-C and hsCRP (available only in ARIC and MESA). We incorporated apoA1 in the model in order to explore if the observed association was independent of apoA1 particles, therefore avoiding potential over-adjustment that may result from using HDL-C given the known close association between estimated RC and both TG and HDL-C.41 Furthermore, baseline variables that are known to be in the causal pathway for increase in RC levels, such as obesity (i.e. BMI) and diabetes were not included in the models.
Using the same models, we assessed the association between RC and LDL-C concordant/discordant groups and incident ASCVD using difference in percentile units, LDL-C clinical cutpoints and medians. We also explored discordance in a continuous manner, defined as standardized difference in percentile units (RC percentile minus LDL-C percentile), adjusted for traditional risk factors in addition to apolipoproteins. In an exploratory analysis, we also performed discordance analyses between RC vs. apoB medians.
To explore whether our findings varied by individual cohorts, we performed continuous analyses stratified by race (Blacks and Whites), as well as by study/cohort (ARIC, CARDIA, and MESA). We additionally explored both components of the primary outcome (myocardial infarction/coronary death and stroke) separately.
Finally, we performed sensitivity analyses excluding individuals on statin therapy at baseline (n = 1148), and an additional model adjusting for hsCRP only in ARIC and MESA individuals as this was available only in this cohort.
Results
The pooled cohort for this study included 17532 participants (MESA = 3049; ARIC = 9748; CARDIA = 4735); the mean age at baseline was 52.3 ± 17.9 years; 56.7% were women, and 34% were Black. At baseline visit, median levels were RC: 20 mg/dL, LDL-C: 118 mg/dL, non-HDL-C: 140 mg/dL, apoB: 97 mg/dL. The proportion of discordance between RC and LDL-C was 69.8% (35.6% had discordantly low RC, and 34.2% had discordantly high RC).
Table 1 shows the baseline characteristics of the study population by concordant/discordant categories between LDL-C and RC. Individuals with discordantly high RC had older age, higher BMI and SBP, as well as greater proportion of diabetes and use of cholesterol-lowering and antihypertensive medications (P < 0.001) compared to those with concordant and discordantly low RC, in addition to greater levels of TG but lower levels of HDL-C. Importantly, those with discordantly high RC had lower non-HDL-C and apoB levels compared to those with discordantly low RC.
Table 1.
Baseline characteristics in concordant and discordant groups: pooled cohort (Multi-Ethnic Study of Atherosclerosis: 3049; Atherosclerosis Risk in Communities: 9748; Coronary Artery Risk Development in Young Adults: 4735)–remnant cholesterol vs. low-density lipoprotein cholesterol
| RC < LDL-C (discordantly low RC; n = 6242) | RC ∼ LDL-C (concordant; n = 5302) | RC > LDL-C (discordantly high RC; n = 5988) | P-value | Overall population (n = 17 532) | |
|---|---|---|---|---|---|
| Age, years | 56 (27–64) | 58 (29–66) | 61 (55–67) | <0.001 | 58 (30–66) |
| Female sex, n (%) | 3591 (57.5) | 3026 (57.1) | 3320 (55.4) | <0.001 | 9937 (56.7) |
| Race, n (%) | |||||
| Whites | 3449 (55.3) | 3578 (67.6) | 4528 (75.7) | <0.001 | 11 555 (66.0) |
| Blacks | 2786 (44.7) | 1711 (32.4) | 1451 (24.3) | <0.001 | 5948 (34.0) |
| BMI, kg/m2 | 26.0 (23.1–29.8) | 26.4 (23.1–30.3) | 27.8 (24.4–31.7) | <0.001 | 26.7 (23.5–30.7) |
| Lipid-lowering medication use, n (%) | 196 (3.1) | 302 (5.7) | 650 (10.9) | <0.001 | 1148 (6.6) |
| Smoking status, n (%) | |||||
| Never smoker | 3163 (51.1) | 2514 (47.7) | 2586 (43.4) | <0.001 | 8263 (47.4) |
| Former smoker | 1853 (29.9) | 1760 (33.4) | 2295 (38.5) | <0.001 | 5908 (33.9) |
| Current smoker | 1179 (19) | 997 (18.9) | 1080 (18.1) | <0.001 | 3256 (18.7) |
| Systolic BP, mmHg | 118 (107–131) | 119 (108–132) | 123 (112–136) | <0.001 | 120 (109–133) |
| Antihypertensive medications, n (%) | 1190 (19.1) | 1216 (23.0) | 1974 (33.1) | <0.001 | 4380 (25.0) |
| Diabetes, n (%) | 361 (5.8) | 433 (8.2) | 826 (13.8) | <0.001 | 1620 (9.3) |
| LDL-C, mg/dL | 134.4 (116.1–154.0) | 116.4 (89.1–143.4) | 103.8 (87.2–119.5) | <0.001 | 117.9 (97.1–140) |
| RC, mg/dL | 16.8 (14.4–20.6) | 19.6 (14.4–27.5) | 25.2 (20–32.1) | <0.001 | 20 (15.5–26.6) |
| ApoB, mg/dL | 104.7 (91–119.5) | 94.6 (75.6–116.3) | 89.3 (76–103.8) | <0.001 | 96.9 (81–113.5) |
| Non-HDL-C, mg/dL | 152 (131–175) | 136 (103.5–172) | 130 (109–151) | <0.001 | 140 (115–166) |
| Triglycerides, mg/dL | 73 (54–97) | 95 (62–149) | 145 (104–200) | <0.001 | 99 (67–147) |
| ApoA1, g/L | 139 (124.3–155) | 138.1 (122.3–156.2) | 136.2 (118.7–160.7) | <0.001 | 138 (122–156.9) |
| HDL-C, mg/dL | 52 (44–62) | 50 (41–61) | 45 (36–57) | <0.001 | 50 (41–61) |
| hsCRP, mg/La | 1.9 (0.9–4.4) | 2.3 (1.1–5.2) | 2.8 (1.2–6.0) | <0.001 | 2.3 (1.0–5.3) |
Discordant groups were defined as difference of >10 percentile units. Continuous variables are reported as median (25th–75th percentile). Medians and proportions were compared using Kruskal–Wallis and chi-squared test, respectively. To convert to SI units: HDL-C, LDL-C, RC, and non-HDL-C, multiply by 0.02586; to convert TG to SI units, multiply by 0.01129.
hsCRP data only available in ARIC and MESA.
Over a median follow-up of 18.7 years (25th–75th percentiles 13.9–24.7), there were 2143 incident ASCVD events. In our continuous analysis, we observed a significant association between log RC levels with incident ASCVD after adjusting for LDL-C in addition to several traditional cardiovascular risk factors. This association remained significant after including apoB (HR 1.65; 95% CI 1.45–1.89) and apoA1 (HR 1.59; 95% CI 1.39–1.82) in the model (Table 2). Notably, log RC continued to be associated with incident ASCVD despite adjusting for non-HDL-C, although significance was attenuated after additional adjustment for HDL-C (Supplementary material online, Table S1). In contrast, the association between log LDL-C and ASCVD was lost after adjusting for apoB as expected (Table 2).
Table 2.
Cox models (95% confidence interval) for incident atherosclerotic cardiovascular disease events for log remnant cholesterol and log LDL-C (continuous variables) in the pooled cohort
| Model 1, HR (95% CI) | Model 2, HR (95% CI) | Model 3, HR (95% CI) | |
|---|---|---|---|
| Log RC | 1.71 (1.50–1.93) | 1.65 (1.45–1.89) | 1.59 (1.39–1.82) |
| Log LDL-C | 1.32 (1.11–1.58) | 1.06 (0.80–1.41) | 1.03 (0.77–1.37) |
Model 1: adjusted for age + gender + race + smoking status + systolic blood pressure + treatment for hypertension + lipid-lowering medication use. Model 2: Model 1 + apoB. Model 3: Model 2 + apoA1.
Approximately one third of individuals (34.2%) were in the high RC/low LDL-C discordance group. Compared to the concordant group, these individuals had a significant increase in ASCVD risk after adjusting for traditional cardiovascular risk factors. This increase remained significant after adjusting for apoB (HR 1.21; 95% CI 1.08–1.34) and apoA1 (HR 1.19; 95% CI 1.07–1.34) (Table 3). On the other hand, those in the low RC/high LDL-C discordant group had similar ASCVD risk compared to the concordant group.
Table 3.
Cox models (95% confidence interval) for incident atherosclerotic cardiovascular disease events for standardized (remnant cholesterol percentile minus LDL-C percentile) in the pooled cohort
| n ASCVD events/n individuals | Model 1, HR (95% CI) | Model 2, HR (95% CI) | Model 3, HR (95% CI) | |
|---|---|---|---|---|
| Discordantly low RC | 686/6242 | 0.99 (0.88–1.10) | 0.93 (0.83–1.04) | 0.94 (0.84–1.05) |
| Concordant | 608/5296 | REF | REF | REF |
| Discordantly high RC | 849/5994 | 1.07 (0.97–1.20) | 1.21 (1.08–1.34) | 1.19 (1.07–1.34) |
Model 1: adjusted for age, gender, race + smoking status + systolic blood pressure + treatment for hypertension + lipid-lowering medication use. Model 2: Model 1 + apoB. Model 3: Model 2 + apoA1.
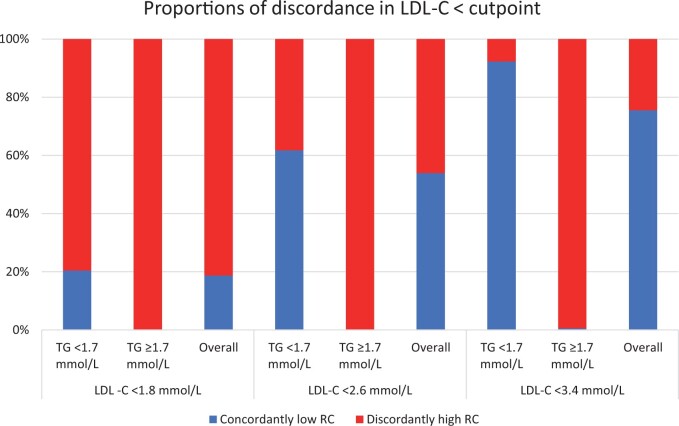
At lower LDL-C clinical cutpoints, the proportion of individuals with high RC/low LDL-C discordance increased dramatically, up to 81% in those with LDL-C < 70 mg/dL (Figure 1) and was associated with increased relative ASCVD risk (Table 4), although with lower precision given smaller sample size and fewer number of events in each category. We found similar results when using medians to define discordance (Supplementary material online, Table S2).
Figure 1.
Proportions of discordance among individuals with LDL-C below clinical cutpoints.
Table 4.
Hazard ratios (95% confidence interval) for atherosclerotic cardiovascular disease events across LDL-C vs. remnant cholesterol concordant/discordant groups by LDL-C clinical cutpoints (70, 100, and 130 mg/dL) and percentile equivalents for remnant cholesterol
| Lipid groups | RC | n ASCVD events/n individuals | Model 1, HR (95% CI) | Model 2, HR (95% CI) | Model 3, HR (95% CI) |
|---|---|---|---|---|---|
| Cutpoints: LDL-C 70 mg/dL; RC 12 mg/dL | |||||
| LDL-C< cutpoint | < cutpoint | 3/170 | REF | REF | REF |
| (n = 906) | ≥ cutpoint | 77/736 | 3.73 (1.17–11.84) | 3.68 (1.16–11.69) | 3.73 (1.17–11.85) |
| LDL-C ≥ cutpoint | < cutpoint | 21/681 | 1.82 (0.54–6.11) | 1.54 (0.46–5.18) | 1.51 (0.45–5.07) |
| (n = 16 626) | ≥ cutpoint | 2042/15 945 | 3.78 (1.21–11.78) | 2.76 (0.88–8.63) | 2.74 (0.88–8.56) |
| Cutpoints: LDL-C 100 mg/dL; RC 16 mg/dL | |||||
| LDL-C < cutpoint | < cutpoint | 163/2658 | REF | REF | REF |
| (n = 4930) | ≥ cutpoint | 269/2272 | 1.35 (1.10–1.65) | 1.29 (1.05–1.58) | 1.28 (1.05–1.57) |
| LDL-C ≥ cutpoint | < cutpoint | 193/2389 | 1.17 (0.95–1.45) | 1.01 (0.81–1.26) | 1.00 (0.81–1.25) |
| (n = 12 602) | ≥ cutpoint | 1518/10 213 | 1.62 (1.37–1.93) | 1.28 (1.06–1.56) | 1.25 (1.03–1.52) |
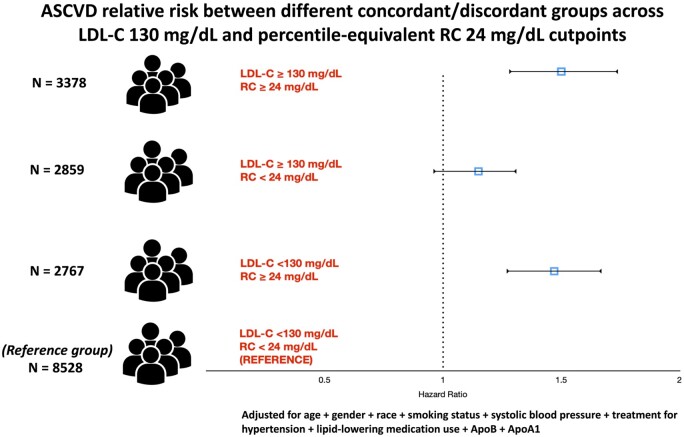
| Cutpoints: LDL-C 130 mg/dL; RC 24 mg/dL | |||||
| LDL-C < cutpoint | < cutpoint | 735/8528 | REF | REF | REF |
| (n = 11 295) | ≥ cutpoint | 451/2767 | 1.53 (1.35–1.73) | 1.47 (1.29–1.67) | 1.43 (1.26–1.63) |
| LDL-C ≥ cutpoint | < cutpoint | 349/2859 | 1.28 (1.13–1.46) | 1.15 (0.99–1.34) | 1.15 (0.99–1.34) |
| (n = 6237) | ≥ cutpoint | 608/3378 | 1.73 (1.54–1.94) | 1.50 (1.28–1.74) | 1.46 (1.25–1.70) |
Model 1: adjusted for age + gender + race + smoking status + systolic blood pressure + treatment for hypertension + lipid-lowering medication use. Model 2: Model 1 + apoB. Model 3: Model 2 + apoA1.
In a discordance continuous analysis, we observed a 15% increase (HR 1.15; 95% CI 1.09–1.20) in ASCVD for every 1 SD (∼29 percentile units) increase in the percentile unit difference between RC and LDL-C, after adjusting for several cardiovascular risk factors in addition to apoB (Supplementary material online, Table S3). We observed similar findings in all analyses when excluding individuals on lipid-lowering therapy at baseline (Supplementary material online, Table S4).
Finally, to further evaluate the independent contribution of RC from total apoB atherogenic risk, we performed a supplementary analysis showing that among individuals with apoB < median, those with discordant RC ≥ median had significantly increased risk of incident ASCVD (HR 1.30; 95% CI 1.13–1.49) (Supplementary material online, Table S5).
Of note, results were consistent when stratifying our study population by race (Supplementary material online, Table S6) and by study/cohort with the exception of MESA. The lack of association in MESA could be explained by its relatively smaller sample size and shorter duration of follow-up compared to CARDIA and ARIC (Supplementary material online, Table S7). Finally, we observed that our results were consistent when examining individual endpoints of our primary outcome (stroke and myocardial infarction/coronary death) (Supplementary material online, Table S8).
Discussion
In a diverse, representative and large population composed of three community-based US cohorts followed for more than 18 years, we found that (i) elevated RC levels were associated with risk of incident ASCVD independent of traditional cardiovascular risk factors, LDL-C, and apoB and (ii) those with high RC/low LDL-C, but not low RC/high LDL-C, discordance were associated with increased ASCVD risk in fully adjusted models including apoB compared to those with concordance (Graphical abstract) . Our findings suggest that identifying RC-related residual risk, in addition to LDL-C and apoB-related risk, is clinically relevant as we usher in a new era of targeted lipid-lowering therapies. Future studies are needed to identify novel mechanisms that explain the association of RC with ASCVD independent of total atherogenic particle burden, and whether lowering its levels improves clinical outcomes.
When there is overproduction of TGRLs42 (VLDL from the liver and chylomicrons from the gut), less efficient lipolysis by lipoprotein lipase (LPL) tends to occur leading to accumulation of partially metabolized remnant particles. On a particle-for-particle basis, these remnant particles have similar atherogenic potential to that of LDL particles23 but carry ∼40 times more cholesterol.43 Remnant particles can be transported across the endothelial cell as opposed to chylomicrons and VLDL particles, which are significantly larger.44 Furthermore, remnant particles have unique physicochemical characteristics that confer highly atherogenic properties. Due to the electrostatic interaction between their apolipoproteins (apoB and apolipoprotein E) and matrix proteoglycans, remnant particles are selectively retained in the subintimal space and efflux slowly in relation to their rates of entry.45 In addition to atherogenesis due to cholesterol deposition similar to LDL particles, apolipoprotein E also mediates the receptor-mediated endocytosis of remnants by surface receptors of subendothelial monocyte-derived macrophages, which leads to foam cell formation and subsequent inflammatory response contributory for atherosclerosis.46–49
RC atherogenic risk independent of apoB
It has been postulated that the atherogenic risk attributed to remnant, LDL, and lipoprotein(a) particles is reflected within total apoB particle concentration. A Mendelian randomization study showed that LPL and LDL receptor variants associated with lower TG and LDL levels, respectively, were linked with a similarly lower risk of coronary heart disease per unit difference in apoB.23 This suggested that the clinical benefit of lowering either TG, a surrogate for RC, or LDL levels is proportional to the absolute change in apoB.23 On the other hand, a recent study in a pooled cohort of intravascular ultrasound trials of patients with known coronary artery disease showed that on-treatment and changes in RC levels were linked to coronary atheroma progression after adjusting for apoB.24 Reaffirming the latter observations, we demonstrate here in individuals without known ASCVD that RC levels are associated with incident ASCVD in fully adjusted models including LDL-C and apoB. Discordance analyses also show that participants with discordantly high RC/low LDL-C or apoB had higher risk of incident ASCVD when compared to those with concordance. This increased risk was not observed in those with low RC/high LDL-C discordance.
These observations suggest that the cholesterol content of remnants may modify ASCVD risk information beyond the total atherogenic particle burden. Several hypotheses may explain the mechanisms behind this association. First, RC may be handled differently than LDL-C within macrophages and vascular walls.25 Second, increased levels of RC may be associated with increased TG enrichment of LDL particles, particularly TG-enriched small dense LDL particles that tend to be atherogenic and stay longer in circulation.47 Third, RC levels could indirectly echo the activity of key plasma lipid regulatory proteins such as apolipoprotein C3 (apoC3) or angiopoietin-like protein 3 (ANGPTL3), which may be independent of the single apoB equatorial moiety encircling TGRLs.41
Another observation worth discussing is that, in an exploratory analysis of a subset of our pooled cohort, RC remained an independent predictor of ASCVD events after additionally adjusting for hsCRP levels, despite previous evidence suggesting that RC contributes to atherosclerosis by inducing low-grade inflammation and pro-inflammatory endothelial activation.48
RC in cardiovascular risk assessment and primary prevention
HDL-C and TG concentrations are inversely related through reverse cholesterol transport,50 and for many years, the former has been presumed to be the causal partner in ASCVD.41 However, recent studies have suggested that there is a U-shaped association between HDL-C and ASCVD that could be partially explained by lipolysis of TGRLs by LPL and the transfer of free cholesterol to HDL-C.51 Moreover, recent genetic studies and HDL-C raising drug trials have cast doubt over the causal role of HDL-C levels in ASCVD turning the tide towards TGRLs; their cholesterol content is thought to be the causal culprit rather than TG, which are degraded by most cells and do not accumulate within vascular walls.42 But if RC is included within non-HDL-C, does it provide additional value to risk assessment? In our continuous analysis, we show that elevated RC levels remained associated with ASCVD even after adjusting for non-HDL-C. These observations were replicated in percentile discordance analyses where we showed that the risk of ASCVD increased as the magnitude of discordance between RC and LDL-C percentiles increased even after adjusting for non-HDL-C; incident ASCVD increased by 10% for every +29 percentile units difference between RC and LDL-C. This observation suggests that elevated levels of RC, regardless of total non-HDL-C level, may indirectly reflect risk information related to other atherogenic mechanisms such as increased apoC3 or ANGPTL3 activity rather than the risk captured in the cholesterol content of remnant particles. ApoC3 has been proposed to have proatherogenic properties mediated by heterogeneous mechanisms such as inhibiting LPL, impeding the clearance of remnant lipoproteins by the liver, and promoting inflammation and endothelial cell apoptosis.41 Several genetic studies of individuals with apoC3 loss-of-function mutations have shown lifelong reduction in TG and RC levels and a lower incidence of ASCVD.52 , 53 Our findings suggest that RC-related risk, which may reflect more complex underlying atherogenic processes, is not fully captured within non-HDL-C levels. In other words, the relative contributions of RC and LDL-C to non-HDL-C add risk information that is not inherently captured within the simple sum of the two measures. This evidence may support the use of RC levels in residual risk assessment in primary prevention beyond non-HDL-C, especially when discordant with LDL-C.
As newer generation TG or RC lowering therapies, such as apoC3 and ANGPTL3 inhibitors,54 are being evaluated in contemporary clinical trials, transitioning into a new approach that considers the individual components of non-HDL-C may be the way to move forward. Rather than a one-size-fits-all approach to managing elevated non-HDL-C, or apoB, levels, estimating LDL-C and RC levels from the standard lipid profile can help guide the use of different combinations of lipid-lowering therapies. For example, a very high-risk patient on high-dose statin with LDL-C of 85, non-HDL-C of 115 and TG of 200 mg/dL (RC 30 mg/dL) may receive a PCSK9 inhibitor leading to LDL-C lowering to 50 mg/dL and non-HDL-C to 75 mg/dL, leaving RC at 25 and TG at 170 mg/dL. While LDL-C and non-HDL-C were significantly reduced to levels below guideline-recommended targets, RC is only mildly reduced, and its residual risk could possibly be targeted with TG or RC lowering medications. Whether this approach to residual risk reduction using new generation therapies will translate into clinical benefit in primary and secondary prevention requires further evaluation in dedicated randomized trials, especially as recent data from trials examining TG lowering using n-3 polyunsaturated fatty acids have shown conflicting results.55 , 56
In our study, we estimated RC using the definition of non-HDL-C minus Martin/Hopkins LDL-C given its availability from the standard lipid panel and its superiority when compared with RC extrapolated from Friedewald LDL-C.32 Furthermore, different direct measurements and definitions of remnants have been proposed using various methods such as ultracentrifugation or immunoseparation.32 However, the accuracy of these methods has been questionable making it difficult to agree on a gold standard and a general preference to use the better standardized TG measurements in clinical trials. Although the definition utilized in our study (regardless of the LDL-C estimation method used) includes both smaller remnant particles and larger particles such as large VLDL, it has been the most commonly used in primary and secondary prevention studies showing RC association with risk and is readily available from the standard lipid profile at no extra cost.24 Reaching consensus on RC measurement or estimation from the standard lipid profile in fasting or non-fasting states is essential to facilitate its adoption in routine practice and clinical trials.
Finally, we hypothesize, based on all our observations, that estimating and considering RC may potentially yield important residual risk information beyond LDL-C and non-HDL-C or apoB, particularly in individuals with mild to moderate hypertriglyceridaemia. Although such risk may be reflected in HDL-C levels, measuring RC may be more pragmatic in an era when effective RC lowering therapies may become available in our cholesterol treatment armamentarium. However, these therapies still require validation in primary and secondary prevention outcome trials.
Strengths and limitations
Our study has several strengths. First, we were able to show the independent predictive power of RC as a continuous measure, but also its additional conferred risk by using a discordance analysis. Second, by pooling data from three landmark representative US cohorts, our results are more generalizable than epidemiological studies counterparts. Third, we used a variety of clinical cutpoints to define discordance, which showed robustness of our findings. Fourth, we used the Martin/Hopkins equation for estimation of LDL-C, which yields more accurate estimates of RC than using the Friedewald equation.32 Fifth, the design and conduction of each of the three cohorts included in this study provide carefully documented outcomes ascertained by independent adjudication committees, which makes our results more reliable.
As with all observational studies, we cannot exclude the possibility of residual confounding. Although the length of follow-up is a strength, there may be significant volatility in lipid levels that fluctuate over time. In addition, the difference in decades of enrollment between each cohort (10–15 years) adds a potential source of bias given that individuals from later enrolment visits (ARIC and MESA) were probably more likely to have been started on lipid-lowering therapies than those enrolled earlier (CARDIA). Given the primary prevention nature of our pooled cohort with median LDL-C of 118 mg/dL, our estimates—although significant—were not precise among those with LDL-C levels <70 mg/dL. Furthermore, although the Martin/Hopkins equation performs significantly better than Friedewald’s in this LDL-C range, it still has some inherent inaccuracy.57 Some studies have shown that postprandial TG and RC levels were more strongly associated with ASCVD compared with fasting levels. Therefore, the atherogenic risk driven by fasting RC levels in our study may underestimate the total RC risk. However, the use of fasting RC levels ensured standardization across all three study cohorts. Dedicated studies comparing the atherogenic risk of fasting vs. non-fasting RC levels are needed. Type III hyperlipidaemia is a rare disorder characterized by extremely elevated RC levels. It is worthwhile noting that, similar to findings from the general population,5 , 35 the prevalence of this disorder was 1.9% in our pooled cohort for which we deduce that most results were not driven by the presence of this disorder. Our data may have also been confounded by the failure to measure lipoprotein(a) levels and to correct LDL-C and apoB values for this parameter. Finally, methodologies for apoB measurements were not the same in each cohort. Although the distribution of apoB was different across the three cohorts, so was the distribution for LDL-C and non-HDL-C, which was likely a result of different inherent characteristics of each population (Supplementary material online, Table S9). Finally, we observed no evidence for assay drift (i.e. shift in slope or y-intercept) when plotting apoB vs. non-HDL-C levels in the overall population stratified by cohort (Supplementary material online, Figures S1 and S2) and their correlation was strong (Spearman correlation coefficient >0.85) in all cohorts.
Conclusions
In a representative pooled cohort of US individuals free of ASCVD, levels of RC were associated with ASCVD independent of traditional cardiovascular risk factors, LDL-C, and non-HDL-C or apoB levels. These associations were expectedly attenuated by adjusting for HDL-C; however, identifying RC residual risk may be more pragmatic as we usher in a new era of targeted RC or TG lowering therapies and given the recent failure of HDL-C boosting therapies. Advancing the use of RC in routine clinical practice requires reaching consensus on the best and most cost-effective method to measure RC. Future studies are needed to demonstrate the mechanisms by which RC is independently associated with ASCVD beyond total atherogenic particle concentration and to determine whether RC lowering to specific targets is associated with cardiovascular benefit.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
R.Q. is supported by an NIH T32 training grant (5T32HL007227). The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). The authors thank the staff and participants of the ARIC study for their important contributions. The MESA study was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). We also thank the other investigators, the staff, and the participants of MESA (Multi-Ethnic Study of Atherosclerosis) for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The CARDIA study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C and HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). The CARDIA study is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). This paper has been reviewed by CARDIA for scientific content.
Conflict of interest: S.S.M. and S.R.J. are listed as coinventors on a pending patent filed by Johns Hopkins University for LDLn‐C estimation. S.R.J. has served as an advisor to Sano/Regeneron. S.S.M. has served as a consultant to Quest Diagnostics, Sano/Regeneron, Amgen, and the Pew Research Center. R.P. has received speakers’ fees from Sanofi‐Aventis and Amgen and research honorarium from Cerenis (unrelated to the present work). Unrelated to this work, E.D.M. received an honorarium from Siemens Healthcare for being a blinded adjudicator of events in a clinical trial. J.T.W. is a consultant for NGM Biopharmaceuticals. C.M.B. has received grant/research support through his institution from Akcea, Amgen, Esperion, Novartis, and Regeneron and is a consultant for Akcea, Althera, Amarin, Amgen, Arrowhead, Astra Zeneca, Corvidia, Denka Seiken, Esperion, Gilead, Janssen, Matinas BioPharma Inc, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, and Sanofi-Synthelabo. The remaining authors have no disclosures to report.
Contributor Information
Renato Quispe, Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, MD, USA; Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Seth Shay Martin, Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, MD, USA; Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Erin Donelly Michos, Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, MD, USA; Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Isha Lamba, Department of Medicine, New York Presbyterian Hospital-Cornell, 525 East 68th Street, New York, NY, USA.
Roger Scott Blumenthal, Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, MD, USA; Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Anum Saeed, Department of Cardiovascular Medicine, Heart and Vascular Institute, University of Pittsburgh Medical Center, Pittsburgh, PA, USA.
Joao Lima, Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Division of Radiology, Johns Hopkins University, Baltimore, MD, USA.
Rishi Puri, Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic, Cleveland, OH, USA.
Sarah Nomura, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, USA.
Michael Tsai, Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, USA.
John Wilkins, Division of Cardiology and the Department of Preventive Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
Christie Mitchell Ballantyne, Department of Cardiovascular Medicine, Center for Cardiovascular Disease Prevention, Methodist DeBakey Heart and Vascular Center, Houston, TX, USA.
Stephen Nicholls, Monash Cardiovascular Research Centre, Victorian Heart Institute, Monash University, Melbourne, Australia.
Steven Richard Jones, Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, MD, USA; Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Mohamed Badreldin Elshazly, Johns Hopkins Ciccarone Center for the Prevention of Cardiovascular Disease, Baltimore, MD, USA; Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic, Cleveland, OH, USA.
References
- 1. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wong ND, Zhao Y, Quek RGW, Blumenthal RS, Budoff MJ, Cushman M, Garg P, Sandfort V, Tsai M, Lopez JAG. Residual atherosclerotic cardiovascular disease risk in statin-treated adults: the Multi-Ethnic Study of Atherosclerosis. J Clin Lipidol 2017;11:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sirimarco G, Labreuche J, Bruckert E, Goldstein LB, Fox KM, Rothwell PM, Amarenco P; PERFORM and SPARCL Investigators and Committees. Atherogenic dyslipidemia and residual cardiovascular risk in statin-treated patients. Stroke 2014;45:1429–1436. [DOI] [PubMed] [Google Scholar]
- 4. Fruchart JC, Sacks FM, Hermans MP, Assmann G, Brown WV, Ceska R, Chapman MJ, Dodson PM, Fioretto P, Ginsberg HN, Kadowaki T, Lablanche JM, Marx N, Plutzky J, Reiner Z, Rosenson RS, Staels B, Stock JK, Sy R, Wanner C, Zambon A, Zimmet P; Residual Risk Reduction Initiative (R3I). The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in dyslipidaemic patient. Diabetes Vasc Dis Res 2008;5:319–335. [DOI] [PubMed] [Google Scholar]
- 5. Joshi PH, Martin SS, Blumenthal RS. The remnants of residual risk. J Am Coll Cardiol 2015;65:2276–2278. [DOI] [PubMed] [Google Scholar]
- 6. Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Armitage CR. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–212. [DOI] [PubMed] [Google Scholar]
- 7. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W; AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 8. Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299–308. [DOI] [PubMed] [Google Scholar]
- 9. Langsted A, Freiberg JJ, Tybjaerg-Hansen A, Schnohr P, Jensen GB, Nordestgaard BG. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City Heart Study with 31 years of follow-up. J Intern Med 2011;270:65–75. [DOI] [PubMed] [Google Scholar]
- 10. Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009;119:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aday AW, Lawler PR, Cook NR, Ridker PM, Mora S, Pradhan AD. Lipoprotein particle profiles, standard lipids, and peripheral artery disease incidence. Circulation 2018;138:2330–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawler PR, Akinkuolie AO, Chu AY, Shah SH, Kraus WE, Craig D, Padmanabhan L, Glynn RJ, Ridker PM, Chasman DI, Mora S. Atherogenic lipoprotein determinants of cardiovascular disease and residual risk among individuals with low low-density lipoprotein cholesterol. J Am Heart Assoc 2017;6:e005549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC, O’Leary DH, Saad MF, Tsai MY, Sharrett AR. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2007;192:211–217. [DOI] [PubMed] [Google Scholar]
- 14. Nordestgaard BG, Wootton R, Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol 1995;15:534–542. [DOI] [PubMed] [Google Scholar]
- 15. Proctor SD, Vine DF, Mamo JC. Arterial retention of apolipoprotein B(48)- and B(100)-containing lipoproteins in atherogenesis. Curr Opin Lipidol 2002;13:461–470. [DOI] [PubMed] [Google Scholar]
- 16. Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 2007;116:1832–1844. [DOI] [PubMed] [Google Scholar]
- 17. Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T, Pennathur S; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011;123:2292–2333. [DOI] [PubMed] [Google Scholar]
- 18. Balling M, Langsted A, Afzal S, Varbo A, Davey Smith G, Nordestgaard BG. A third of nonfasting plasma cholesterol is in remnant lipoproteins: lipoprotein subclass profiling in 9293 individuals. Atherosclerosis 2019;286:97–104. [DOI] [PubMed] [Google Scholar]
- 19. Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol 2013;61:427–436. [DOI] [PubMed] [Google Scholar]
- 20. Jørgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjærg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J 2013;34:1826–1833. [DOI] [PubMed] [Google Scholar]
- 21. Kaltoft M, Langsted A, Nordestgaard BG. Triglycerides and remnant cholesterol associated with risk of aortic valve stenosis: Mendelian randomization in the Copenhagen General Population Study. Eur Heart J 2020;41:2288–2299. [DOI] [PubMed] [Google Scholar]
- 22. Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev 2019;40:537–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ference BA, Kastelein JJP, Ray KK, Ginsberg HN, Chapman MJ, Packard CJ, Laufs U, Oliver-Williams C, Wood AM, Butterworth AS, Di Angelantonio E, Danesh J, Nicholls SJ, Bhatt DL, Sabatine MS, Catapano AL. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA 2019;321:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elshazly MB, Mani P, Nissen S, Brennan DM, Clark D, Martin S, Jones SR, Quispe R, Donnellan E, Nicholls SJ, Puri R. Remnant cholesterol, coronary atheroma progression and clinical events in statin-treated patients with coronary artery disease. Eur J Prev Cardiol 2020;27:1091–1100. [DOI] [PubMed] [Google Scholar]
- 25. Pirillo A, Norata GD, Catapano AL. Beyond LDL-C levels, does remnant cholesterol estimation matter? Eur J Prev Cardiol 2020;27:1088–1090. [DOI] [PubMed] [Google Scholar]
- 26. Martin SS, Blaha MJ, Elshazly MB, Brinton EA, Toth PP, McEvoy JW, Joshi PH, Kulkarni KR, Mize PD, Kwiterovich PO, Defilippis AP, Blumenthal RS, Jones SR. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J Am Coll Cardiol 2013;62:732–739. [DOI] [PubMed] [Google Scholar]
- 27. Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA 2013;310:2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee J, Jang S, Son H. Validation of the Martin method for estimating low-density lipoprotein cholesterol levels in Korean adults: findings from the Korea National Health and Nutrition Examination Survey, 2009–2011. PLoS One 2016;11:e0148147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaen H, Kinchiku S, Miyata M, Kajiya S, Uenomachi H, Yuasa T, Takasaki K, Ohishi M. Validity of a novel method for estimation of low-density lipoprotein cholesterol levels in diabetic patients. J Atheroscler Thromb 2016;23:1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kang M, Kim J, Lee SY, Kim K, Yoon J, Ki H. Martin's equation as the most suitable method for estimation of low-density lipoprotein cholesterol levels in Korean adults. Korean J Fam Med 2017;38:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Joshi PH, Khokhar AA, Massaro JM, Lirette ST, Griswold ME, Martin SS, Blaha MJ, Kulkarni KR, Correa A, D'Agostino RB Sr., Jones SR, Toth PP. Remnant lipoprotein cholesterol and incident coronary heart disease: the Jackson Heart and Framingham Offspring Cohort studies. J Am Heart Assoc 2016;5:e002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Faridi KF, Quispe R, Martin SS, Hendrani AD, Joshi PH, Brinton EA, Cruz DE, Banach M, Toth PP, Kulkarni K, Jones SR. Comparing different assessments of remnant lipoprotein cholesterol: the very large database of lipids. J Clin Lipidol 2019;13:634–644. [DOI] [PubMed] [Google Scholar]
- 33. Quispe R, Elshazly MB, Zhao D, Toth PP, Puri R, Virani SS, Blumenthal RS, Martin SS, Jones SR, Michos ED. Total cholesterol/HDL-cholesterol ratio discordance with LDL-cholesterol and non-HDL-cholesterol and incidence of atherosclerotic cardiovascular disease in primary prevention: the ARIC study. Eur J Prev Cardiol 2020;27:1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Quispe R, Michos ED, Martin SS, Puri R, Toth PP, Al Suwaidi J, Banach M, Virani SS, Blumenthal RS, Jones SR, Elshazly MB. High-sensitivity C-reactive protein discordance with atherogenic lipid measures and incidence of atherosclerotic cardiovascular disease in primary prevention: the ARIC study. J Am Heart Assoc 2020;9:e013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mora S, Buring JE, Ridker PM. Discordance of low-density lipoprotein (LDL) cholesterol with alternative LDL-related measures and future coronary events. Circulation 2014;129:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lawler PR, Akinkuolie AO, Ridker PM, Sniderman AD, Buring JE, Glynn RJ, Chasman DI, Mora S. Discordance between circulating atherogenic cholesterol mass and lipoprotein particle concentration in relation to future coronary events in women. Clin Chem 2017;63:870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr., Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:e285–e350. [DOI] [PubMed] [Google Scholar]
- 38. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL, Cooney MT; ESC Scientific Document Group. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 39. Anderson TJ, Grégoire J, Pearson GJ, Barry AR, Couture P, Dawes M, Francis GA, Genest J Jr., Grover S, Gupta M, Hegele RA, Lau DC, Leiter LA, Lonn E, Mancini GB, McPherson R, Ngui D, Poirier P, Sievenpiper JL, Stone JA, Thanassoulis G, Ward R. 2016 Canadian Cardiovascular Society Guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2016;32:1263–1282. [DOI] [PubMed] [Google Scholar]
- 40. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 41. Libby P. Triglycerides on the rise: should we swap seats on the seesaw? Eur Heart J 2015;36:774–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014;384:626–635. [DOI] [PubMed] [Google Scholar]
- 43. Boren J, Williams KJ. The central role of arterial retention of cholesterol-rich apolipoprotein-B-containing lipoproteins in the pathogenesis of atherosclerosis: a triumph of simplicity. Curr Opin Lipidol 2016;27:473–483. [DOI] [PubMed] [Google Scholar]
- 44. Boren J, Matikainen N, Adiels M, Taskinen MR. Postprandial hypertriglyceridemia as a coronary risk factor. Clin Chim Acta 2014;431:131–142. [DOI] [PubMed] [Google Scholar]
- 45. Schwartz EA, Reaven PD. Lipolysis of triglyceride-rich lipoproteins, vascular inflammation, and atherosclerosis. Biochim Biophys Acta 2012;1821:858–866. [DOI] [PubMed] [Google Scholar]
- 46. Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res 2016;118:547–563. [DOI] [PubMed] [Google Scholar]
- 47. MäRz W, Scharnagl H, Winkler K, Tiran A, Nauck M, Boehm BO, Winkelmann BR. Low-density lipoprotein triglycerides associated with low-grade systemic inflammation, adhesion molecules, and angiographic coronary artery disease: the Ludwigshafen Risk and Cardiovascular Health study. Circulation 2004;110:3068–3074. [DOI] [PubMed] [Google Scholar]
- 48. Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013;128:1298–1309. [DOI] [PubMed] [Google Scholar]
- 49. Hansen SEJ, Madsen CM, Varbo A, Nordestgaard BG. Low-grade inflammation in the association between mild-to-moderate hypertriglyceridemia and risk of acute pancreatitis: a study of more than 115000 individuals from the general population. Clin Chem 2019;65:321–332. [DOI] [PubMed] [Google Scholar]
- 50. Langsted A, Jensen AMR, Varbo A, Nordestgaard BG. Low high-density lipoprotein cholesterol to monitor long-term average increased triglycerides. J Clin Endocrinol Metab 2020;105:dgz265. [DOI] [PubMed] [Google Scholar]
- 51. Feng M, Darabi M, Tubeuf E, Canicio A, Lhomme M, Frisdal E, Lanfranchi-Lebreton S, Matheron L, Rached F, Ponnaiah M, Serrano CV Jr., Santos RD, Brites F, Bolbach G, Gautier E, Huby T, Carrie A, Bruckert E, Guerin M, Couvert P, Giral P, Lesnik P, Le Goff W, Guillas I, Kontush A. Free cholesterol transfer to high-density lipoprotein (HDL) upon triglyceride lipolysis underlies the U-shape relationship between HDL-cholesterol and cardiovascular disease. Eur J Prev Cardiol 2020;27:1606–1616. [DOI] [PubMed] [Google Scholar]
- 52. Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014;371:32–41. [DOI] [PubMed] [Google Scholar]
- 53. Working Group Of The Exome Sequencing Project, National Heart, Lung TH, Institute; Crosby Peloso BJ, Auer GM, Crosslin PL, Stitziel DR, Lange NO, Lu LA, Tang Y, Zhang Z, Hindy ZH, Masca Stirrups GN, Kanoni K, Do S, Jun Hu RG, Kang Y, Xue HM, Goel C, Farrall A, Duga M, Merlini S, Asselta PA, Girelli R, Olivieri D, Martinelli O, Yin N, Reilly W, Speliotes D, Fox E, Hveem CS, Holmen K, Nikpay OL, Farlow M, Assimes DN, Franceschini TL, Robinson N, North J, Martin KE, DePristo LW, Gupta M, Escher N, Jansson SA, Van Zuydam JH, Palmer N, Wareham CN, Koch N, Meitinger W, Peters T, Lieb A, Erbel W, Konig R, Kruppa IR, Degenhardt J, Gottesman F, Bottinger O, O'Donnell EP, Psaty CJ, Ballantyne BM, Abecasis CM, Ordovas G, Melander JM, Watkins O, Orho-Melander H, Ardissino M, Loos D, McPherson RJ, Willer R, Erdmann CJ, Hall J, Samani AS, Deloukas NJ, Schunkert P, Wilson H, Kooperberg JG, Rich C, Tracy SS, Lin RP, Altshuler DY, Gabriel D, Nickerson S, Jarvik DA, Cupples GP, Reiner LA, Boerwinkle AP, Kathiresan E. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 2014;371:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, Yu R, Hurh E, Paz E, McEvoy BW, Baker BF, Pham NC, Digenio A, Hughes SG, Geary RS, Witztum JL, Crooke RM, Tsimikas S. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med 2017;377:222–232. [DOI] [PubMed] [Google Scholar]
- 55. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, Tardif J-C, Ballantyne CM; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 56. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Ridker PM, Ray KK, Katona BG, Himmelmann A, Loss LE, Rensfeldt M, Lundstrom T, Agrawal R, Menon V, Wolski K, Nissen SE. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA 2020;324:2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Quispe R, Hendrani A, Elshazly MB, Michos ED, McEvoy JW, Blaha MJ, Banach M, Kulkarni KR, Toth PP, Coresh J, Blumenthal RS, Jones SR, Martin SS. Accuracy of low-density lipoprotein cholesterol estimation at very low levels. BMC Med 2017;15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.