Abstract
The rise of e-cigarette popularity has sparked interest in the role of palatable flavors on nicotine use. Despite growing evidence that sweet flavorants enhance nicotine reward, their influence on nicotine consumption has not been studied extensively. In addition, the impact that flavored nicotine use in adolescence could have on nicotine reward and dependence in adulthood remains unclear. This study examined the role of flavored nicotine access on nicotine preference and consumption longitudinally, from adolescence to adulthood. Male and female adolescent mice preferred a fruit-flavored nicotine solution over an unflavored nicotine solution. However, only adolescent female mice with access to flavored nicotine consumed higher doses. Furthermore, while adolescent male mice escalated consumption of both flavored and unflavored nicotine, female mice only escalated when given access to flavored nicotine. As mice matured into adulthood, there was no evidence that a history of flavored-nicotine access altered preference for unflavored nicotine compared to a nicotine-free control in a classic two-bottle choice design. However, when the nicotine concentration was progressively reduced, mice that had consumed strawberry-flavored nicotine in adolescence maintained baseline nicotine consumption levels longer than mice that initiated nicotine use without flavor in adolescence. Finally, addition of fruit-flavorants into the nicotine solution during adulthood led to increased levels of nicotine consumption, regardless of previous flavored-nicotine access or of familiarity with the selected flavorant. These results indicate that flavorants increase nicotine consumption independent of life stage, possibly posing a disproportionate risk to adolescent females. Our results also point to an effect of adolescent flavored-nicotine use on nicotine dose maintenance in adulthood, which could have implications for the success of future quit attempts.
1. Introduction:
E-cigarettes arrived on the market in the early 2000s and they have been increasing in popularity among adolescents for the past decade (Gentzke et al., 2019; National Academies of Sciences, et al., 2018). E-cigarettes are available in over 15,000 distinct flavors that are attractive to adolescents (e.g. ‘cotton candy’, ‘tropical blue slushie’ and ‘crazy berry’) (Hsu et al., 2018). These sweet and ‘characterizing’ flavors have played a major role in e-cigarette uptake and popularity among young people, with nationally representative data sets regularly showing that ‘flavor availability’ is among the top two reasons for e-cigarette use and experimentation in students (Ambrose et al., 2015; Bold et al., 2016; Kong et al., 2015; Patten and De Biasi, 2020).
The flavoring compounds used by e-liquid manufacturers are the same as those that children grow up consuming in candy and sugary drinks (Brown et al., 2014). As a result of repeated pairing with highly palatable and caloric foods, these flavorants have established positive associations well in advance of nicotine exposure (Beauchamp and Cowart, 1985; Fanselow and Birk, 1982; Harris et al., 2004; Mennella et al., 2016). Compared to adults, adolescents have a heightened preference for both sweetness and the foods and flavors paired with sweetness (Cooke and Wardle, 2005; Desor and Beauchamp, 1987; Hoffman et al., 2016; Mennella et al., 2016). Therefore, it is not surprising that sweet and fruit-flavored e-cigarettes are attractive to adolescents. Between 80–98% of adolescents and 60–95% of young adults initiate e-cigarette use with a flavored e-cigarette, underscoring flavor’s importance in e-cigarette use among these populations (Harrell et al., 2017; Villanti et al., 2017). In this context, it is important to note that the 2020 FDA ban on flavored e-liquid cartridges (https://www.fda.gov/) only affects cartridge-based products such as JUUL while the sales of flavored e-liquids designed for open-tank systems and disposable cartridge-based products remain unaffected.
In addition to their role in e-cigarette uptake, flavorants may impact nicotine reward and consumption during e-cigarette use. Characterizing flavors enhance subjective reward of nicotine-containing e-cigarettes and can increase vaping patterns, such as number of puffs taken, volume of e-liquid used, and duration of puffs (Audrain-McGovern et al., 2016; Goldenson et al., 2016; Jackson et al., 2020; Kim et al., 2016; Leventhal et al., 2019a; St.Helen et al., 2018). However, changes in these vaping behaviors do not easily extrapolate to total nicotine exposure. For example, in one study, although longer puff durations were observed when participants vaped a strawberry-flavored e-cigarette, increased puff duration was not associated with a statistically significant increase in systemic nicotine exposure (St.Helen et al., 2018). Another study in adult male rats showed that licorice flavor enhances nicotine self-administration (i.e. consumption). However, this study did not address how flavors (and more specifically, how flavors popular with young people) could impact nicotine intake during adolescence, a period when users are particularly vulnerable to both nicotine and flavorings (Palmatier et al., 2019). Notably, to our knowledge, all preclinical studies which could have informed about the role of sex differences in flavored nicotine consumption include only male subjects (Cooper et al., 2021; Palmatier et al., 2019; Wong et al., 2020). Women show a slightly higher preference for- and use of- e-cigarettes with characterizing flavors, and they may be more sensitive to the sensory components of vaping/smoking (Kistler et al., 2017; Patten and De Biasi, 2020; Perkins, 1999; Perkins et al., 2001; Soneji et al., 2019; Xiao et al., 2019). Overall, there is a need to better understand the influence of flavored additives on nicotine intake levels, especially in females and in adolescents.
Individuals who report a positive first-experience with smoking are more likely to go on to become regular smokers (Chen et al., 2003; DiFranza et al., 2007; Mantey et al., 2017; Rodriguez and Audrain-McGovern, 2004; Sartor et al., 2010; Urbán, 2010). Additional research suggests that reducing the initial aversion to a bitter taste (such as that of nicotine), could reduce aversion to nicotine if and when adolescents transition to combustible tobacco use (Capaldi and Privitera, 2008; Hoffman et al., 2016). It is a public health concern that this generation of young ‘vapers’ could experience more severe long-term consequences due to their early experimentation with flavored nicotine vaping, such as increases in nicotine dependence.
For the first time since the 1990s, adolescent tobacco product use has increased, and the vast majority of adolescents are initiating nicotine use with flavored e-cigarettes (CDC, 2019). Although there is evidence from clinical and preclinical studies suggesting that flavorants increase nicotine reward in adult subjects, there is very little research on how e-cigarette flavorants affect nicotine reward and consumption during adolescence. Furthermore, we do not understand how initiation of nicotine with a palatable fruit-flavored product could affect long-term preferences for and consumption of nicotine as young vapers age. First-use of a flavored nicotine product could possibly increase the severity of nicotine addiction in a new generation of nicotine users.
This study was designed both to determine whether a fruit-flavorant can alter nicotine consumption in adolescent male and females, and to model the potential long-term consequences of initiating nicotine use with flavored products in adolescence.
2. Materials and methods
2.1. Animals
C57BL/6J mice were housed with a reverse 12 hr light/dark cycle (lights off at 10 A.M.) in a temperature-controlled room (24 ± 2 °C, relative humidity 55 ± 10%). Mice were weaned into single-housed cages with enrichment on post-natal day 21 (PND 21) with ad libitum access to food pellets (Labdiet 5001, PMI, Brentwood, MO) and to a source of liquid (see procedures below for more details). All acute behavioral testing occurred in the dark-phase, but drinking behavior was measured for 24-hours. Mice were evaluated in adolescence (PND 31–49), young adulthood (PND 50–70), and adulthood (PND 70+). Due to our interest in sex differences and the high “branching” nature of our longitudinal experimental, we estimated needing ~75 mice to explore the possibility of sex differences at all phases of our experiment. This experiment was repeated in 4 separate groups of mice whose behavior was measured at different times throughout the year. Animals were bred in-house and were the offspring of 14 different breeding pairs. All procedures were approved by the institutional animal care and use committee and followed the guidelines for animal intramural research from the National Institute of Health.
2.2. Solutions
Both control and nicotine solutions contained 2% saccharin in filtered water, which was used to mask the bitter flavor of nicotine in nicotine-containing solutions (Perez et al., 2015; Salas et al., 2009; Zhang et al., 2012). For the majority of the experiment, nicotine bottles contained 0.1 mg/ml free-base nicotine (or 0.2804 mg/ml of nicotine hydrogen tartrate salt from Sigma Aldrich, St Louis, MO). During the “Nicotine Fading + Flavor Reintroduction” experiment, the nicotine concentration was sequentially reduced to 0.075, 0.05, 0.025, and 0.000 mg/ml (free-base). Nicotine solutions were kept in dark bottles to protect against photodegradation and were prepared fresh every 4 days. Kool-Aid ® solutions were made according to package instructions (0.14 oz powder in 2 quarts of 2% saccharin solution). Kool-Aid ® powder (Kool-Aid ® Strawberry Drink Mix Unsweetened, Kraft Foods, Chicago IL) contains flavorants, but does not contain any additional sweeteners. In other words, the only sweetener in the Kool-Aid ® solutions was the saccharin that was added during preparation of the solution.
2.3. Measuring Longitudinal Flavor and Nicotine Preference and Consumption
The experiments described below were performed longitudinally. For a schematic of the experimental timeline see Figure 1. We utilized an adaptation of the two bottle choice paradigm (2-BC) to evaluate the effect of flavorants on the voluntary consumption of nicotine. The volume of fluid consumed by animals was measured indirectly by weighing bottles (g) at each drinking time point. Assuming our solutions, which were made in filtered water, had a relative density of 1.0 g/mL, the loss of weight (g) from each bottle was considered to be equal to the volume consumed by the mouse in milliliters (mL). Mice were weighed daily during adolescence, and then every other day for the remainder of the experiment. The position of the bottles was alternated daily to prevent confounds due to side preferences. For each phase of the experiment, a control cage contained matching solutions, but no mouse. These control solutions were weighed to correct for solution loss that could have occurred due to evaporation and/or accidental movement of the housing rack. This control “drip volume” (on average ~0.2mL) was subtracted from each animal’s “consumed volume” before preference and dose calculations were then performed. Each phase in the experiment is described in detail below.
Figure 1. Timeline showing the sequence of events, drug treatments, and behavioral testing.
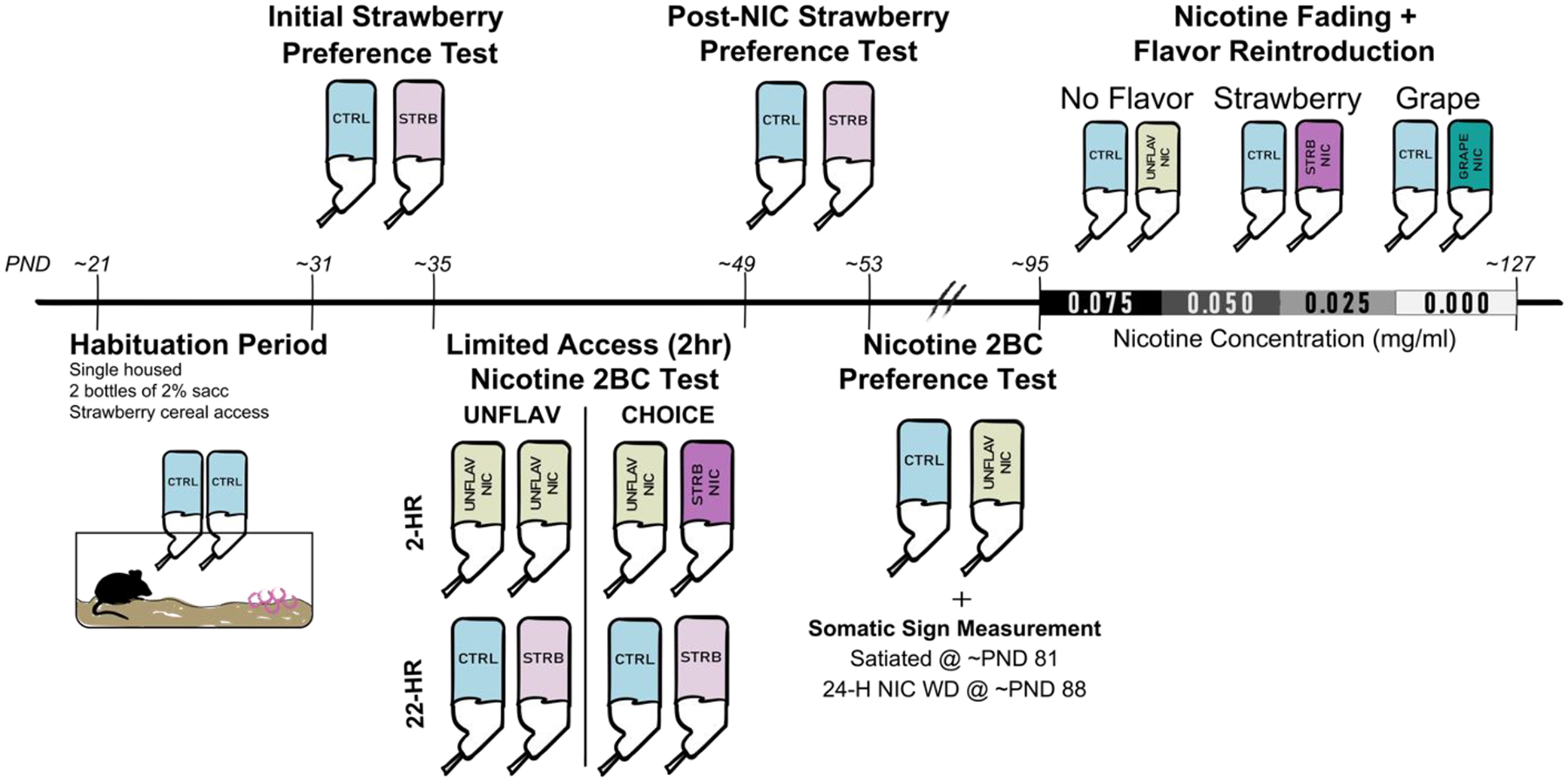
Mice were divided into groups at two points in the experimental timeline. First, at the start of the “Limited Access (2hr) Nicotine 2 Bottle Choice (2BC) Test” mice were separated into either the “UNFLAV” or “CHOICE” groups. Data from the “Initial Strawberry Preference Test” ensured that animals in each group had approximately equal baseline strawberry preference. All mice then followed the same treatment pattern, until “Nicotine Fading and Flavor Re-introduction”, when groups were further divided into one of three nicotine flavors: No Flavor, Strawberry Flavor, or Grape Flavor.
2.5.1. Habituation Period
On postnatal day (PND) 21 (± 1 day), mice were single housed and given access to 2 bottles, each containing 2% saccharin in filtered water. Mice were also given 3 pieces of Strawberry Power O’s Cereal (Love Grown Goods, Denver, CO) daily, for 10 days (PND21-PND30). This was done to acclimate animals to strawberry flavoring in a naturalistic environment before experimentation with the flavor began.
2.5.2. Initial Strawberry Preference Test
On PND31, after cereal feeding, mice began a two-bottle choice (2-BC) test in which each mouse had access to a bottle containing 2% saccharin in filtered water and a strawberry-flavored bottle (Strawberry Kool-Aid ® in the same 2% saccharin solution). Bottles were weighed at 24-hour intervals for 4 days.
2.5.3. Limited Access (2-hr) Nicotine Two-bottle Choice (2-BC) Test
For the remainder of adolescence (~PND 35–50), mice were provided with 2 hours/day of nicotine access. Nicotine access began 2 hours after the start of the dark-phase (12 PM – 2 PM). The experimental group, referred to as the “CHOICE” group, had access to one bottle of unflavored nicotine (0.1 mg/ml nicotine (free-base) dissolved in 2% saccharin solution) and one bottle of strawberry-flavored nicotine (0.1 mg/ml nicotine (free-base) dissolved in Strawberry Kool-Aid ® + 2% saccharin solution). A control group, referred to as the “UNFLAV” group, had access to two bottles of unflavored nicotine. Mice were sorted into treatment groups so that they had approximately equal initial strawberry preference (relative to an unflavored but equally sweetened control) (Supplementary Figure 1). Experimenters weighed both nicotine bottles before and after the 2-hour session. Mice were excluded if they consumed less than 0.2 mL of nicotine during the 2-hr session on more than 2 days since this was similar to the average “drip” value measured in control-cage bottles. After the 2-hr drinking session, all animals were provided with 2 nicotine-free bottles for the remainder of the day (22-h). One of these bottles contained a 2% saccharin solution and the other contained a strawberry-flavored 2% saccharin solution.
2.5.4. Post-Nicotine Strawberry Preference Test
24 hours after the last limited-access nicotine session (~PND 51), mice were given one bottle of control solution (saccharin only) and one bottle of 0.1 mg/ml unflavored nicotine + saccharin in the home cage. Access to these solutions continued for 96-h. This approach provided continued nicotine access, but allowed for a strawberry-flavor “wash-out” period before testing their post-nicotine strawberry preference. On ~PND 55 mice began the ‘Post-Nicotine Strawberry Preference Test’, which was performed using an identical procedure to that detailed in the ‘initial strawberry preference test’ section.
2.5.5. Maturation Nicotine 2-BC
For the remainder of maturation (~PND 59–95), mice had 24-h access to two bottles in the home cage. One bottle contained 0.1 mg/ml of free-base nicotine dissolved in a 2% saccharin solution and a second (control) bottle contained a 2% saccharin solution. Bottles were weighed at 24-h intervals. Drinking continued for ~5 weeks.
2.5.6. Spontaneous Withdrawal Testing
Approximately 3 weeks into the ‘Maturation Nicotine 2-BC’ phase, mice were observed while satiated with nicotine (i.e. at baseline) for the following physical signs: head shaking, scratching, grooming, chewing, jumping, paw licking, as described previously (Salas et al., 2009, 2004). Mice were then moved into a behavioral testing room with moderate lighting (Lux = 9–12) and allowed to acclimate for 1 hour. After acclimation, mice were placed in a new cage and their behavior was recorded on video for 20 min. One week later, the same procedure was repeated; however, to monitor signs of nicotine withdrawal, this time mice were recorded 24 hours after the start of nicotine deprivation. Previous work from our lab and others have identified 24 hours post-nicotine removal as a peak time for expression of spontaneous signs of nicotine withdrawal (Bagdas et al., 2019; Perez et al., 2015). An individual blinded to animal ID, treatment, and state (e.g. satiated or withdrawn) measured the occurrence and the duration of the somatic signs. The total number of signs was used for comparison.
2.5.7. Nicotine Fading and Flavor Reintroduction
At ~PND 95, mice were sorted into 6 treatment groups based on “Adolescent Treatment” (e.g. CHOICE vs. UNFLAV) and an assigned “Nicotine Flavor” (e.g. ‘No Flavor’, ‘Strawberry’, or ‘Grape’). Mice were divided so that groups had approximately equal preference for strawberry at the end of adolescence, and approximately equal dose consumed- and preference for- nicotine during maturation. During the “Nicotine Fading and Flavor Reintroduction” phase, mice had continued 24-hr access to one nicotine-containing bottle and one nicotine-free bottle, with two additional experimental manipulations. First, the concentration of nicotine in the nicotine-containing bottle was reduced, or “faded” from 1.000, to 0.075, to 0.050, to 0.025, and finally to 0.000 mg/ml nicotine (free-base). Second, animals assigned to either a strawberry- or a grape-nicotine flavor had, in addition to a sequential reduction in nicotine concentration, their assigned nicotine flavor introduced (or reintroduced, in the case of the CHOICE + ‘Strawberry’ fading flavor mice) into the nicotine bottle. All mice had a control bottle that contained a 2% saccharin solution. Exposure to each nicotine concentration lasted for 8 days. The volume consumed by each animal from the nicotine-containing and the saccharin bottles were weighed so that we could determine the dose of nicotine (mg/kg) consumed of and the preference for nicotine. Data is reported as the dose consumed of nicotine (mg/kg), the preference for the nicotine bottle (%), and as a % change in nicotine dose from baseline drinking. To determine the % change in nicotine dose, nicotine consumption levels (mg/kg) at all nicotine concentrations were measured and compared to each animal’s baseline consumption level (mg/kg) that was established during the “Nicotine 2BC Preference test”, at which point mice had access to one bottle of 0.100 mg/ml nicotine + 2% saccharin (unflavored) and one bottle of 2% saccharin (unflavored).
2.6. Data Analysis and Statistics
For drinking experiments, data were averaged over the course of two days (i.e. one session) so that each data point represents counterbalanced data in order to protect against misinterpretation due to innate side preferences of mice. Two mice were excluded from the study (one female from the “UNFLAV” group and one female from the “CHOICE” group) after being identified as having drank too little nicotine during the “Limited Access (2-hr) Nicotine Two-bottle Choice (2-BC) Test”. We defined criteria for drinking too little nicotine as: an animal drank < 0.2 mL of total nicotine on two or more days during the adolescent 2 bottle choice test. This was based on the fact that 0.2 mL was around the volume which would “drip” from our bottles during the 2-hour nicotine drinking session. In one case, an animal was improperly labeled as a “low drinking mouse” and therefore data was not collected for this mouse after adolescence. Finally, there was a spilled bottle in one animal’s cage in the final days of the fading experiment (0 mg/ml nicotine). This spill prevented data collection at that timepoint and there is one data point missing for that animal at the 0 mg/ml nicotine concentration. This is why a Repeated Measures Mixed-Effects Analysis, rather than a RMANOVA, which cannot handle missing data points, was used to analyze the nicotine preference and fluid consumption data during the “Nicotine Fading and Flavor Re-introduction” phase for mice that originated in the adolescent CHOICE group. All data sets were tested for normality using the D’Agostino Pearson Test prior to selecting appropriate methods of analysis (non-parametric vs. parametric). The only exception being the data in supplementary Figures 5 and 6, where the sample sizes were too small to run the D’Agostino Pearson Test and normality had to be assumed. Non-parametric data was analyzed using Wilcoxon tests. Parametric tests employed in this study include t-tests, fixed effects ANOVA and RMANOVA, and RM Mixed Effects Analyses. Data are represented as +/− SEM for all data. GraphPad Prism (San Diego, CA) was used for the data analyses. In all figures, asterisks (*) indicate a significant difference from a comparison group, a plus sign (+) indicates a significant difference from a baseline measurement in repeated measures testing, and a hashtag (#) indicates a significant difference from a theoretical value (e.g. chance, or 50%).
3. Results
3.1. Adolescent mice prefer a strawberry-flavored nicotine solution over an unflavored nicotine solution
Strawberry-flavored (vs. unflavored) nicotine preference was measured in adolescent mice during the “Limited Access (2-hr) Nicotine Two-bottle Choice (2-BC) Test” (see Figure 1 for experimental schematic). Mice were divided in two experimental groups: one group had access to two bottles of unflavored nicotine (i.e. “UNFLAV” group) and a second group had access to one bottle of unflavored nicotine and one bottle of strawberry-flavored nicotine (i.e. “CHOICE” group) during a 2-hour nicotine access period. An initial two-way ANOVA of strawberry preference and strawberry-nicotine preference data with factors of sex and drinking solution detected a significant effect of solution and an interaction between solution and sex (Fsolution(1,33) = 53.81, ****P<0.0001, (F 1,33)interaction = 5.243, *p < 0.05). As a result, male and female data are displayed and analyzed separately. Mice in both the UNFLAV and CHOICE groups had a highly variable initial preference for nicotine-free strawberry flavored solution compared to a nicotine-free unflavored solution (control). Mice were sorted into treatment groups such that their initial preference for the nicotine-free strawberry solution was not significantly different (Supplementary Figure 1). Male and female mice display a significant preference (statistically higher than chance, i.e. 50%) when presented with a strawberry flavored nicotine solution (female: one sample t test, t(16) = 8.96, ****p<0.0001; male: Wilcoxon Signed Rank test, n=18, *p<0.05). Preference for the strawberry-flavored nicotine solution is also significantly greater than the preference measured for the nicotine-free strawberry bottle during the ‘Initial Strawberry Preference test’ (female: paired t-test,: t(16) = 7.214, ****p <0.0001, male: Wilcoxon matched-pairs signed rank test, n=18, **p<0.01) (Figure 2A, B). In contrast, mice with two bottles of identical and unflavored solution (UNFLAV group) showed no preference for either bottle (Figure 2C).
Figure 2. Male and female adolescent mice prefer strawberry-flavored nicotine over a nicotine-free strawberry and an unflavored nicotine solution.
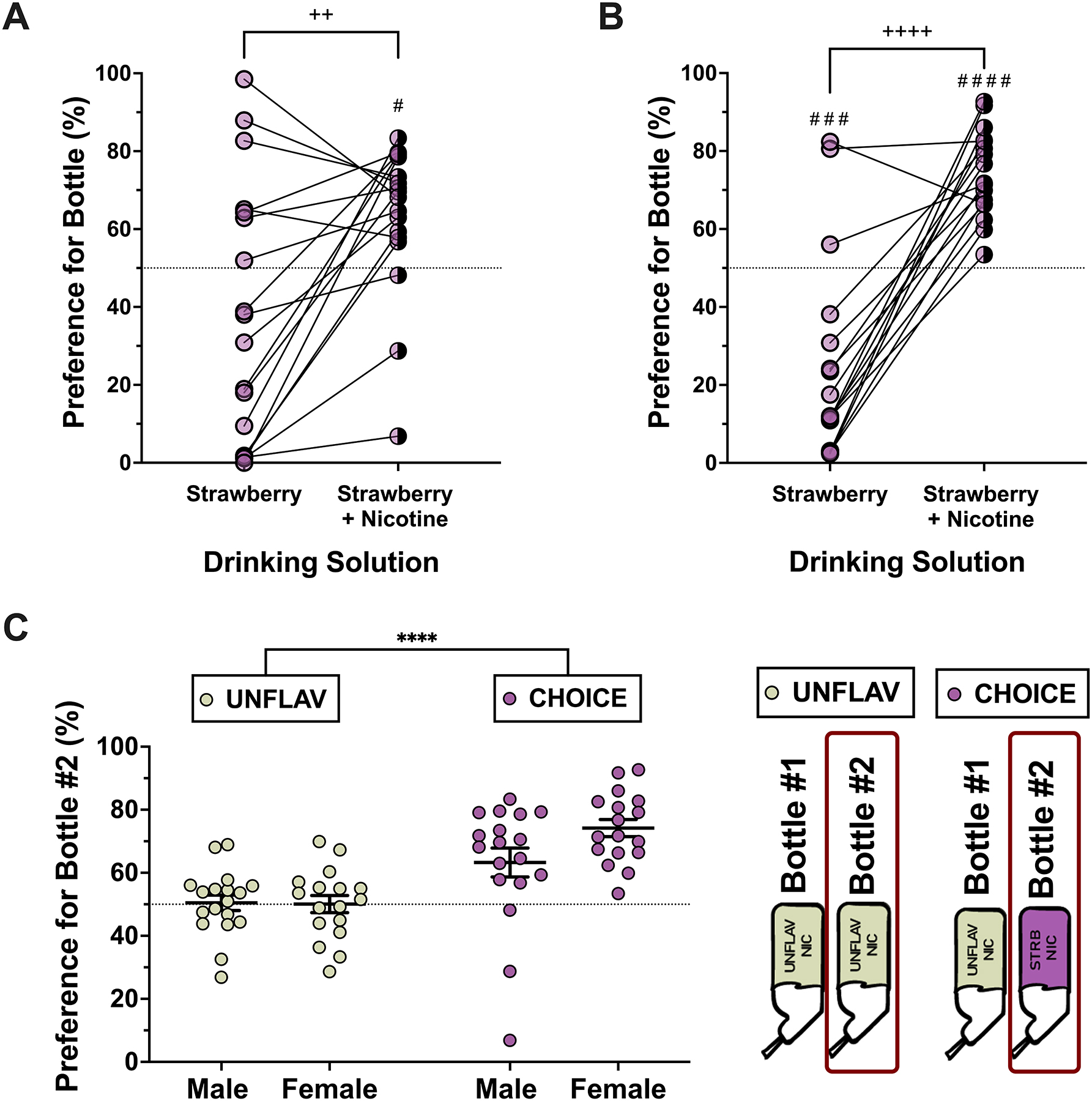
A-B) Before and after plots display the individual values of preference for a bottle containing strawberry flavor only and a bottle containing nicotine + strawberry flavor in males and females, respectively (paired t-test or Wilcoxon signed rank test). C) The preferences for bottle #2 (unflavored in the UNFLAV group but strawberry-flavored in the CHOICE group) are displayed by sex and according to treatment groups. Lines and error bars represent summary data (mean +/− SEM). (Two-way ANOVA, Fsex(1,66) = 2.616, p=0.111, Ftreatment(1,66) = 32.07,**** p<0.0001, Finteraction(1,66) = 3.015, p=0.0872).(Male, UNFLAV: n=18, CHOICE: n= 18; Female, UNFLAV: n=17, CHOICE: n=17).
3.2. Female mice only escalate nicotine consumption during adolescence if a strawberry-flavored nicotine solution is provided
Males in both the CHOICE group and the UNFLAV group escalated nicotine consumption over the course of adolescence (Figure 3A). Escalation is indicated by a significant effect of session in a two-way RMANOVA (Fsession(6,204) = 10.28, ****p<0.0001, Ftreatment(1,34) = 2.91, p=0.100, Finteraction(6,204) = 0.4418, p=0.850). Females were less sensitive to nicotine escalation overall, possibly because they started out drinking higher levels at session 1; however, there was a strong trend towards a session * treatment interaction, suggesting that females in the CHOICE group were more likely to increase their nicotine consumption over the two-week adolescent testing period (P35–49). (Fsession(6,192) = 0.7995, p = 0.571, Ftreatment(1,32) = 3.69, p=0.064, Finteraction(6,192) = 2.095, p=0.056) (Figure 3C).
Figure 3. Adolescent female mice escalate nicotine consumption and consume higher doses of nicotine when given access to a strawberry flavored nicotine solution.
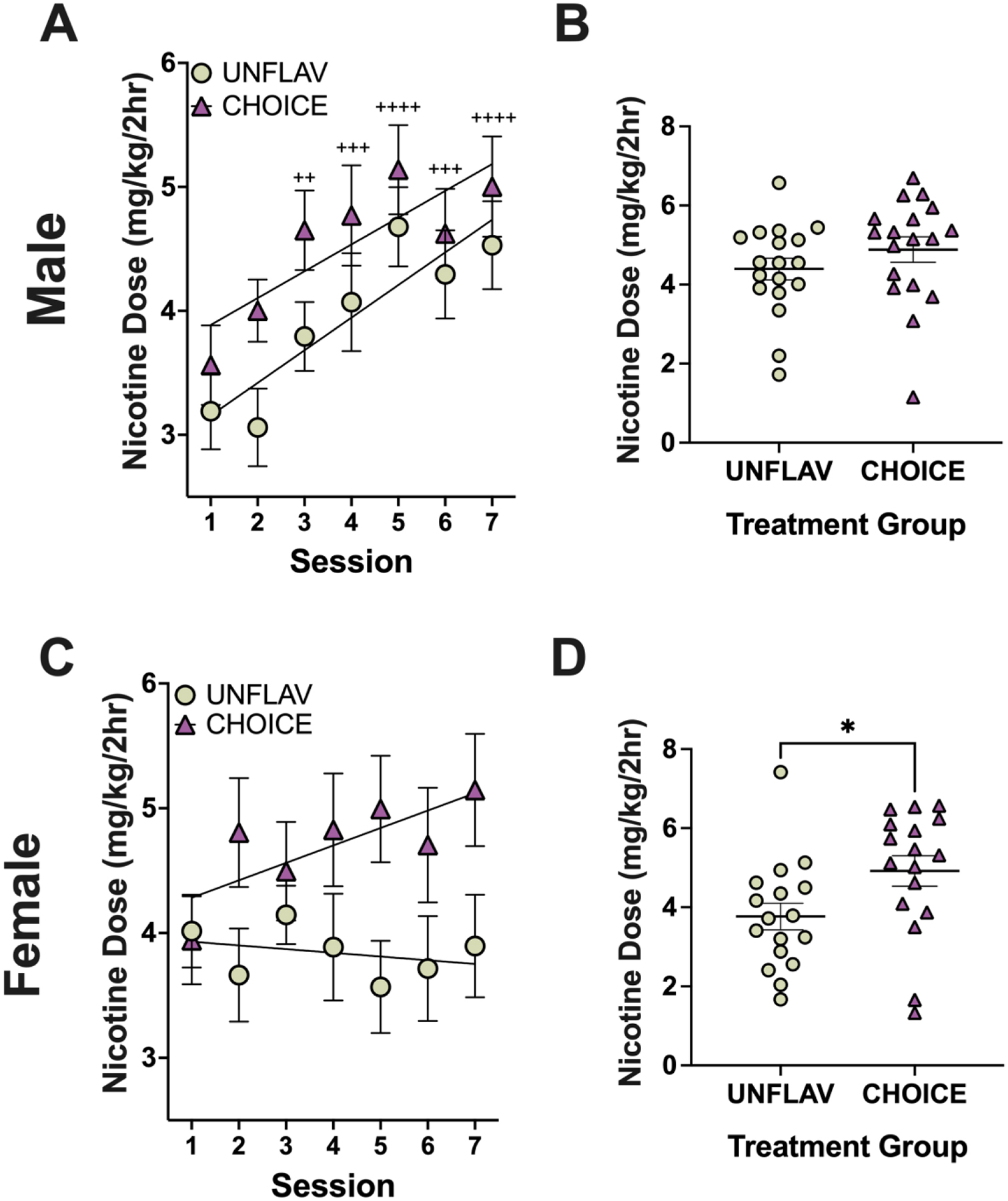
A,C) Line graphs display the summary data (mean +/− SEM) of the total dose consumed by adolescent mice during a 2-hour nicotine access period over the course of 2-weeks in adolescence. A line of best-fit is overlayed for each data set. The dose consumed by mice that had access to two bottles of unflavored nicotine (UNFLAV) is compared to mice with access to one bottle of unflavored nicotine and one bottle of strawberry-flavored nicotine (CHOICE). (2-way RMANOVA, followed by Dunnett’s multiple comparison relative to session 1 consumption). B,D) Points display the average nicotine consumption of each mouse during the last week of adolescence (Sessions 4–7) (Unpaired t-tests). *P < 0.05. **P < 0.01, and ****P < 0.0001 for all comparisons. (Male, UNFLAV: n=18, CHOICE: n= 18; Female, UNFLAV: n=17, CHOICE: n=17).
3.3. Adolescent female mice drink a higher dose of nicotine when given access to a strawberry-flavored nicotine solution
The total amount of nicotine consumed (in mg/kg body weight) during adolescence was measured following each 2-hour drinking period of the “Limited Access (2-hr) Nicotine Two-bottle Choice (2-BC) Test”. On average, during the last week of adolescence (sessions 4–7) male mice in the CHOICE group (i.e. mice with access to strawberry-flavored nicotine) drank 11.2% more nicotine (4.885 ± 0.318 mg/kg) than their counterparts in the UNFLAV group (4.393 ± 0.275 mg/kg) (Figure 3B). Whereas, female mice in the CHOICE group drank 30.5% more nicotine (4.918 ± 0.386 mg/kg) on average during the last week of adolescence than their counterparts in the UNFLAV group (3.768 ± 0.335 mg/kg), which rose to the level of significance (unpaired t-test, t(32) = 2.251, *p=0.031) (Figure 3D). Although mice in the CHOICE group tend to drink a significantly greater volume of fluid during the 2-hour nicotine session resulting in the observed higher nicotine doses, the volume of fluid consumed by animals on average during 24-hours does not differ (Supplementary Figure 3).
3.4. Mice that initiate nicotine consumption in adolescence with a fruit-flavorant resist reductions in nicotine consumption in adulthood
We used two-way ANOVAs to investigate the possibility of sex effects at each nicotine concentration during the “Nicotine Fading and Flavor Re-introduction” experiment. On average, females drank more nicotine (mg/kg/day) than males at every concentration of nicotine except 0.100 mg/ml (Baseline). Despite this difference in total nicotine consumption, there were no sex*flavor interactions and males and females were equally represented in the data. In general, sex was not a factor for the nicotine preference data in adulthood. As a result, to preserve statistical power, data for both sexes were pooled for all ‘fading’ experimental data in the main body of the text (see supplementary Figures 5 and 6 for data separated by sex).
Prior to initiating the “Nicotine Fading and Flavor Re-introduction” experiment and while mice were maturing into adulthood, mice had access to one bottle containing 2% saccharin and one bottle containing 0.1 mg/ml nicotine in 2% saccharin, i.e. “Maturation Nicotine 2-BC” phase (see Figure 1 for experimental schematic). Neither sex nor adolescent treatment (i.e. UNFLAV or CHOICE) had an effect on preference for- or dose consumed of- nicotine during this maturation phase, when mice had access to 0.10 mg/ml unflavored nicotine (free base) 24h/day (2-way ANOVAs, Preference: Fsex(1,65) = 1.154, p=0.219, Ftreatment(1,65) = 0.433, p=0.513, Finteraction(1,65) = 1.540, p=0.219; Dose: Fsex(1,65) = 1.243, p=0.269, Ftreatment(1,65) = 0.066, p=0.798, Finteraction(1,65) = 0.499, p=0.482) (Figure 4). However, differences based on adolescent treatment emerged when the nicotine concentration was decreased over time during the “Nicotine Fading and Flavor Re-introduction” phase.
Figure 4. Access to strawberry-flavored nicotine in adolescence does not increase preference for or consumption of unflavored nicotine during maturation.
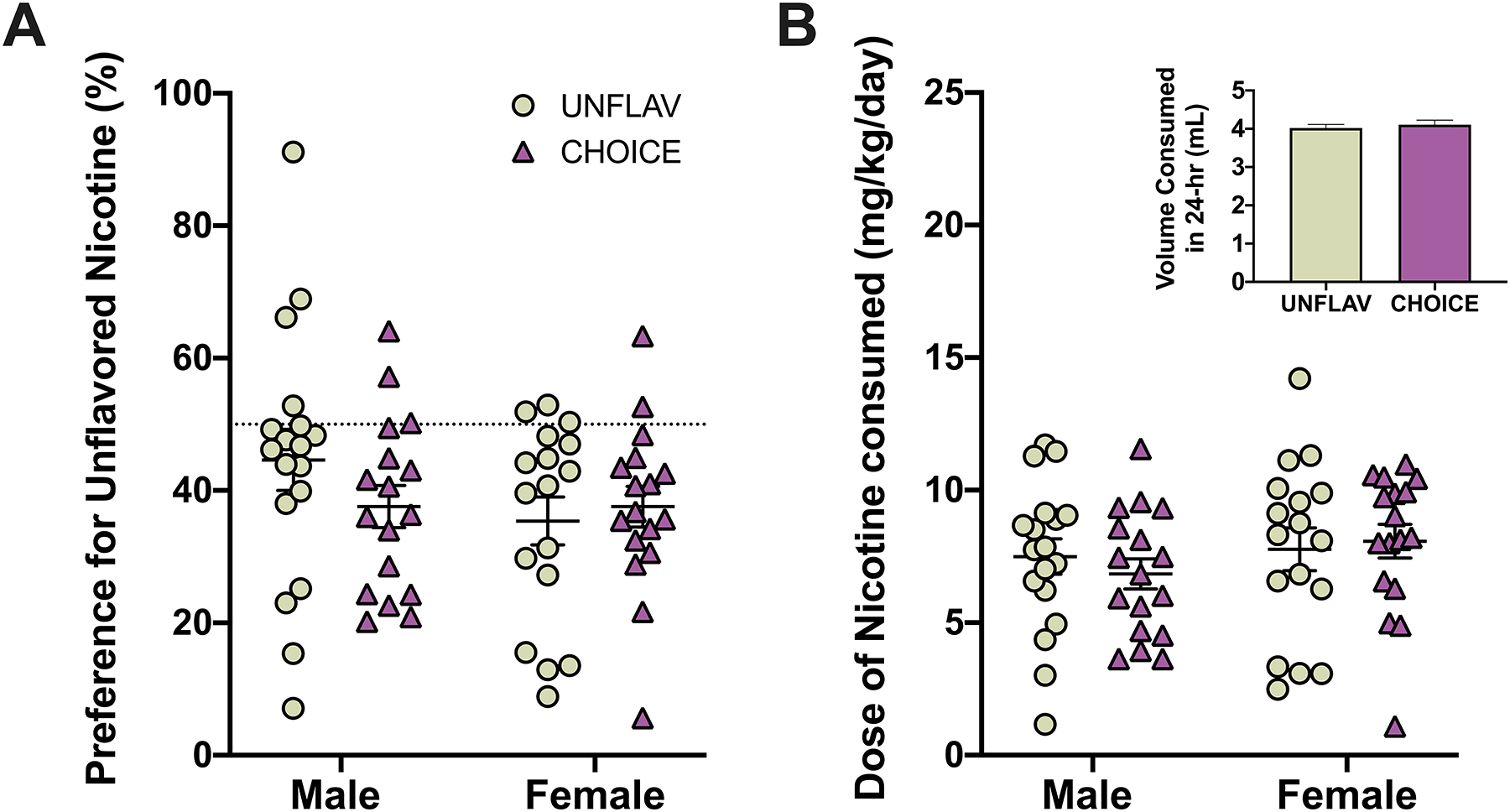
Data points represent the response of individual animals and lines and error bars represent summary data (mean +/− SEM). In panel B, the inlayed graph represents an average of the volume consumed by mice of both sexes during 24-hours across all maturation drinking sessions. (Male, UNFLAV: n=18, CHOICE: n= 17; Female, UNFLAV: n=17, CHOICE: n=17).
The responses of mice that received unflavored nicotine during the “Nicotine Fading and Flavor Re-introduction” varied based on adolescent treatment. In mice that initiated nicotine use without flavorant in adolescence (i.e., UNFLAV mice), the drop in nicotine concentration from 0.100 to 0.075 mg/ml was associated with a significant decrease in nicotine consumption below baseline levels (Two-way RMANOVA: Fnic.conc.(3,96) = 48.10, ****p<0.0001, Fflavor(2,32) = 2.89, p=0.070, Finteraction(6,96) = 2.434, *p=0.031, Sidak’s test for multiple comparisons)((Figure 5B). However, mice that initiated nicotine use with strawberry flavor (i.e. CHOICE mice) maintained baseline nicotine consumption despite the reduction in nicotine concentration from 0.100 to 0.075 mg/ml (Two-way RMANOVA: Fnic.conc.(3,93) = 76.77, ****p<0.0001, Fflavor(2,31) = 0.790, p=463, Finteraction(6,93) = 3.601, **p<0.01, Sidak’s test for multiple comparisons) (Figure 5C). In order to determine if this difference in dose-maintenance was related to differences in nicotine dependence, we measured the number of somatic behaviors (e.g. grooming, chewing, licking, etc.) displayed by animals in videos taken during both during nicotine satiety and 24-hours into nicotine withdrawal. Although on average mice did not display significant signs of spontaneous nicotine withdrawal, there was a trend towards an increase in the proportion ‘CHOICE’ mice that displayed symptoms of nicotine withdrawal (defined by ≥ 15% increase in the number of somatic signs performed) (Supplementary Figure 4).
Figure 5. Mice that initiate nicotine use in adolescence with flavored nicotine maintain baseline levels of nicotine consumption for longer than counterparts who initiate with unflavored nicotine and are more sensitive to increased consumption of flavored nicotine in adulthood.
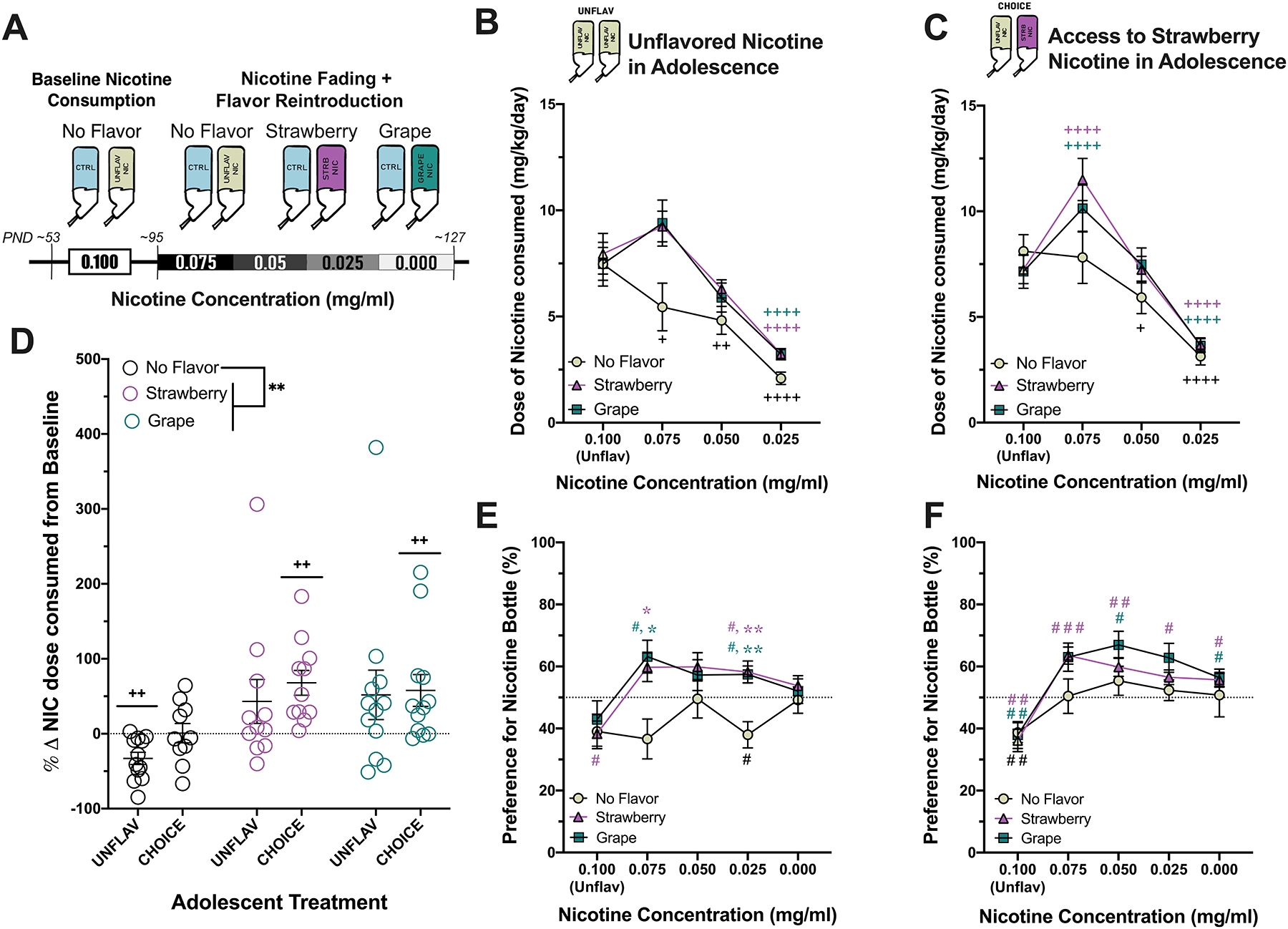
Data from mice that only had access to unflavored nicotine in adolescence (UNFLAV) (B,E) and mice that had access to strawberry flavored nicotine in adolescence (CHOICE) (C, F) are displayed vertically. A) In adulthood (starting at ~PND95), the concentration of nicotine was reduced sequentially, and mice were either left with an unflavored nicotine, had strawberry Kool-Aid® added to the nicotine bottle, or had Grape Kool-Aid® added to the nicotine bottle. All mice had access to a second bottle that did not contain flavor or nicotine. B-C) Line graphs display the dose of nicotine consumed (mg/kg) during the baseline nicotine consumption phase and during the nicotine fading experiment of UNFLAV and of CHOICE mice, respectively (mean +/− SEM). +P < 0.05. ++P < 0.01, and ++++P < 0.0001 represent a significant change from each group’s baseline consumption (Two-way RMANOVA, Sidak’s multiple comparisons test). D) Data displayed is the % change in nicotine consumption from baseline for each mouse when the nicotine solution contained 0.075 mg/ml nicotine. A significant increase in % change of dose consumed by animals that received flavored solutions compared to unflavored solution is indicated by **P < 0.01 near the figure legend (Two way ANOVA, Sidak’s multiple comparisons test). On the graph, ++P < 0.01 indicates a significant change in % consumed of each group from their own baseline consumption (one sample t-test or Wilcoxon test, depending on distribution of data). E-F) Line graphs display the preference for the nicotine bottle (%) during the baseline nicotine consumption phase and during the nicotine fading experiment of UNFLAV and of CHOICE mice, respectively (mean +/− SEM). Preference for the nicotine bottle in UNFLAV group depended on nicotine concentration, flavor and an interaction (Two-way ANOVA, Tukey’s test for multiple comparisons). *P<0.05 and **P<0.01. Whereas, preference for the nicotine bottle in the CHOICE group only depended on nicotine concentration (Repeated Measures Mixed Effects Model). One sample t-tests and Wilcoxon tests were used to measure either aversion to or preference for the nicotine bottle at each concentration (one sample t-tests) # P < 0.05. ## P < 0.01, and ### P < 0.001 represent a significant difference from theoretical chance (i.e., 50% preference) (UNFLAV in adolescence, ‘No Flavor’: n=12, ‘Strawberry’: n= 11, ‘Grape’: n=12; CHOICE in adolescence, ‘No Flavor’: n=10, ‘Strawberry’: n= 11, ‘Grape’: n=12).
3.5. Mice increase nicotine consumption if a palatable fruit-flavorant is added to nicotine solutions in adulthood
We next examined the effect of adding palatable flavors, both familiar (strawberry) and unfamiliar (grape) on nicotine consumption during adulthood. We were interested in the hypothesis that strawberry flavor would serve as a conditioned reinforcer only in the mice that initiated nicotine use with strawberry flavor in adolescence (i.e., the adolescent CHOICE mice). Conditioned reinforcers are nicotine-associated cues which can both make nicotine more rewarding, and become rewarding on their own, in the absence of nicotine (Patten and De Biasi, 2020).Therefore, if strawberry became a conditioned reinforcer only for the adolescent ‘CHOICE’ mice, we would expect only that group (i.e. not the adolescent “UNFLAV’ group) to increase nicotine consumption when strawberry was re-introduced to their bottle. However, an alternative hypothesis is that regardless of previous experience with a flavor, that flavor will be able to mask the ‘harshness’ of nicotine and will increase nicotine consumption via increasing its palatability. As a result, grape was added to the experiment in the “Nicotine Fading and Flavor Re-introduction” phase as a novel fruit flavorant, to help us differentiate between these two possible outcomes.
Mice that initiated nicotine use with flavored nicotine in adolescence (i.e., CHOICE mice) appeared to be more sensitive to flavored nicotine during adulthood, as indicated by a significant increase in the dose of nicotine consumed above baseline levels when flavor was added to the nicotine bottle (0.075 mg/ml nicotine) (Figure 5C). A trend towards an increase in dose was observed for mice in the UNFLAV group at the same concentration, but it did not reach significance (Figure 5B). The flavor-induced increase in nicotine consumption was not flavor-specific, since mice that received either strawberry-flavored or grape-flavored nicotine in adulthood similarly increased the dose of nicotine consumed (Figure 5B–D). These data were analyzed further by calculating the % change from the baseline nicotine consumption to when the nicotine bottles contained 0.075 mg/ml nicotine. A two-way ANOVA of this data indicated a significant effect of flavor on the % change from baseline at this concentration, but no effect of adolescent treatment or an interaction (Fflavor(2,62) = 6.649, **p<0.002, Fadolescent_treatment(1,62) = 1.383, p=0.244, Finteraction(2,62) = 0.206, p=0.815, Sidak’s test for multiple comparisons)(Figure 5D). Finally, using one sample t-tests or Wilcoxon signed-rank tests, we determined which groups significantly changed their nicotine consumption relative to baseline (i.e. 0% change) when the nicotine bottle contained 0.075 mg/ml nicotine concentration Mice from the adolescent “UNFLAV” group given unflavored nicotine in adulthood significantly decreased their dose from baseline consumption levels (one sample t-test: t(11) = 4.03, ++p<0.01). In addition, mice from the adolescent “CHOICE” group significantly increased consumption when given either strawberry- or grape- flavored nicotine in adulthood (one sample t-test: strawberry: t(10) = 4.11, ++p<0.01, Wilcoxon signed-rank test: grape:, ++p<0.01) (Figure 5D). These statistical findings are in agreement with the results of the more rigorous RMANOVA and associated multiple comparisons tests reported above and in Figure 5.
3.6. Adult mice show a preference for fruit-flavored nicotine solutions over a nicotine-free control solution, regardless of a history of using flavored nicotine or of familiarity with the fruit-flavorant.
Regardless of exposure to flavored nicotine in adolescence, mice showed a fairly consistent preference for a flavored nicotine bottle (compared to chance, i.e., 50%) as the nicotine concentration was gradually faded out (Figure 5E, F, one sample t-tests, theoretical mean = 50%). Interestingly, only adolescent ‘CHOICE’ mice continued to show a preference for the flavored bottles, even after nicotine was no longer present (Figure 5E, F). Repeated measures testing was also used to determine an effect of nicotine concentration and flavor on preference for the nicotine bottle. Nicotine concentration, flavor and an interaction significantly affected the preference for nicotine compared to a control bottle of mice who initiated nicotine in adolescence without flavor (i.e. adolescent UNFLAV mice). (Two-way RMANOVA, Tukey’s multiple comparison tests, Fnic.conc(3.116, 99.72) = 6.432, ***p< 0.001, Fflavor(2,32)=4.808, *p<0.05, Finteraction(8,128) = 2.250, *p<0.05)(Figure 5E). However, only nicotine concentration affected nicotine preference in the adolescent CHOICE group, who had access to strawberry-flavored nicotine in adolescence (Mixed Effects Model (REML), Tukey’s multiple comparisons test, Fnic.conc(2.807, 86.31) = 21.96, ****p<0.0001, Fflavor(2,31)=2.070, p=0.1433, Finteraction(8,123) = 1.102, p=0.427)(Figure 5F). This was most likely due to the increase in preference for the unflavored nicotine bottle observed in these mice, seemingly to maintain baseline nicotine levels. The higher preference for unflavored nicotine in these animals minimized the ability to detect a significant difference in the preference for unflavored vs. flavored nicotine. Still, these mice showed a preference for flavored nicotine (>50%); whereas, preference for unflavored nicotine was near 50% at all nicotine concentrations below 0.100 mg/ml.
4. Discussion:
The potential of flavored e-cigarettes to increase adolescent nicotine reward and consumption has been a major public health concern. In addition, individuals who report a positive first-experience with smoking are more likely to go on to become regular smokers (Chen et al., 2003; DiFranza et al., 2007; Mantey et al., 2017; Rodriguez and Audrain-McGovern, 2004; Sartor et al., 2010; Urbán, 2010). Therefore, it is important to consider how initiating nicotine use with palatable flavored e-cigarettes could impact acute adolescent nicotine consumption, as well as long-term nicotine consumption and reward. In this study, we investigated the effect that fruit flavorants could have on nicotine consumption in adolescent C57BL/6J mice and we examined how this “palatable/positive” first nicotine exposure might impact later nicotine consumption and preference with and without flavorants. To examine the long-term abuse liability of flavored nicotine, from adolescence to adulthood, we chose the 2BC model of voluntary oral self-administration and preference. Compared to the intravenous self-administration paradigm, this approach allowed us to overcome the challenges associated with the small size of young mice and the need for prolonged catheter patency. Despite resulting in slower pharmacokinetics compared to intravenous nicotine self-administration, the 2BC model produces an expected inverted U-shape dose-response curve for nicotine and, when nicotine concentrations in the drinking solution are high, leads to symptoms of withdrawal when nicotine is withheld (Bagdas et al., 2019; Collins et al., 2012).
Both male and female adolescent mice preferred a nicotine solution when it was flavored with a palatable fruit flavorant (i.e. strawberry) compared to when the solution was left unflavored; however, this preference was slightly more prominent in adolescent female mice. The majority of mice preferred a combination of strawberry and nicotine over a nicotine-free strawberry solution. This is important, since it is evidence that mice did not simply like strawberry and therefore also drank strawberry flavored nicotine. In other words, our data cannot be simply interpreted as a survey of strawberry preference in the mouse. In fact, there was no correlation between the initial preference for strawberry and the preference for strawberry-flavored nicotine. Nicotine has previously been shown to make other drugs, rewards, and non-pharmacological cues more rewarding (Buffalari et al., 2014; Patten and De Biasi, 2020; Perkins et al., 2017), and it has been shown that fruity flavorants are able to increase ratings of sweetness, a natural reward (Frank and Byram, 1988; Labbe et al., 2007; Rao et al., 2018; Smith et al., 2020). Mass spectrometry of Kool-Aid ® solutions revealed that the major component of both the strawberry and grape Kool-Aid ® solutions was ethyl butyrate (data not shown). Ethyl butyrate (also known as ethyl butanoate) was recently determined to be the second most common flavorant in a survey of 277 e-cigarette refill liquids (Omaiye et al., 2019) and has been shown to increase ratings of the ‘sweetness’ of solutions (Rao et al., 2018). Therefore, it is possible that nicotine was acting through this mechanism to enhance a preference for the strawberry-flavored nicotine bottle in adolescence.
Access to flavored nicotine had a unique effect on adolescent female nicotine consumption. Whereas male mice escalated nicotine consumption over the course of adolescence regardless of their access to flavored nicotine, female mice only increased nicotine consumption if they had access to the palatable strawberry-flavored nicotine solution. In addition, during the last week of adolescence, female mice with access to strawberry-flavored nicotine drank ~30.5% more nicotine (vs. males who drank ~11.2% more) than their counterparts, which only had access to unflavored nicotine. Evidence from human literature supports the idea of sex differences in relation to perceived palatably of flavored tobacco products. Women are more likely than men to use characterizing flavored products and to value flavor availability (Kistler et al., 2017; Patten and De Biasi, 2020; Perkins, 1999; Perkins et al., 2001; Soneji et al., 2019; Xiao et al., 2019). Furthermore, there is evidence that women are more driven by the sensory aspect of smoking, as opposed to the pharmacological effects of nicotine (Perkins, 1999; Perkins et al., 2001a; US Surgeon General, 2001). There is a history within the tobacco industry for flavored products to be marketed towards women and now e-cigarette distributors might be taking further advantage of young girls’ and women’s risk by creating products with ‘feminine’ attributes (e.g. pink and jeweled packaging) (Patten and De Biasi, 2020; Yao et al., 2016). The combination of a possible susceptibility of girls toward flavored products and adolescents towards nicotine is particularly troubling, since it is well understood that adolescence is a period of sensitive neurodevelopment and nicotine exposure during adolescence is linked to a multitude of health complications, including cognitive deficits and increased probability of becoming a dependent smoker in adulthood (England et al., 2017, 2015; Goriounova and Mansvelder, 2012; US HHS, 2016; Omelchenko et al., 2016; Walker and Loprinzi, 2014; Yuan et al., 2015).
A recent meta-analysis showed a strong and consistent association between initial e-cigarette use and subsequent cigarette smoking initiation (Soneji et al., 2017). In addition, flavored e-cigarette use (rather than e-cigarette use generally) has been associated with higher rates of vaping and increased risk of subsequent cigarette initiation (Barrington-Trimis et al., 2018; Chen et al., 2016; Dai and Hao, 2016; Leventhal et al., 2019b). Therefore, we were interested in the possibility that mice with access to flavored nicotine in adolescence (i.e. CHOICE mice) would have higher preferences for nicotine and consume higher doses of nicotine during maturation. Our data did not indicate an increase in nicotine preference or consumption during young adulthood, once flavor was removed from the bottle. Importantly, not all research suggests that flavors are associated with a progression to combustible use (Audrain-McGovern et al., 2019). Furthermore, CDC data published in 2019 only shows an increase in e-cigarette, not combustible smoking, among adolescent populations (CDC, 2019). Therefore, the role of flavored e-cigarettes on the ability to promote progression to combustible cigarettes is still unclear.
Although adolescent access to fruit-flavored nicotine did not alter nicotine consumption at a stable nicotine concentration (0.100 mg/ml) during young adulthood, when the concentration of the nicotine solution was reduced by 25% (to 0.075 mg/ml) later on in adulthood, only mice that initiated nicotine use with flavored nicotine (i.e., the CHOICE group) maintained baseline (unflavored) nicotine consumption (Figure 5 C, D), which was achieved by increasing their preference toward the nicotine bottle and away from the nicotine-free control bottle (Figure 5F). In contrast, adult mice that initiated nicotine use without a fruit-flavorant in adolescence significantly reduced (unflavored) nicotine consumption relative to their baseline levels, as preference for the nicotine bottle at 0.075 mg/ml remained unchanged from baseline preference (Figure 5B,E). This suggests that mice that initated nicotine use with flavor (i.e., CHOICE adolescent group) were uniquely motivated to maintain baseline nicotine consumption levels. We hypothesized that these mice would be more dependent on nicotine. However, somatic sign testing did not show increases in signs of nicotine withdrawal prior to the start of the fading experiment. We believe this is due to the relatively low doses of nicotine consumed by animals in this study, compared to those in which spontaneous withdrawal from oral nicotine administration has been reported. For example, in a recent publication spontaneous signs of nicotine withdrawal mice were observed in mice consuming a 0.960 mg/ml nicotine solution and reaching doses of nicotine ranging from 25–40 mg/kg prior to nicotine abstinence (Bagdas et al., 2019). In comparison, in this study mice were drinking a 0.100 mg/ml nicotine solution and drank approximately 7 mg/kg/day.
We were next interested in how animals would respond if a flavor was introduced into the nicotine bottle in adulthood. One hypothesis for a flavorant-induced increase in dose/preference for nicotine in adulthood is that strawberry could serve as a conditioned reinforcer and cause a sort of “cue-induced” escalation. As mentioned earlier, conditioned reinforcers are developed over time as the cue is associated with nicotine reward. Once conditioned, the cue can both enhance nicotine reward and be rewarding in its own right (Patten and De Biasi, 2020). In this case, if strawberry became a conditioned reinforcer for mice during adolescence, one would hypothesize that nicotine consumption would only increase in mice for which nicotine and strawberry were previously paired (i.e., the adolescent CHOICE group). An alternative hypothesis is that an increase in dose/preference for strawberry-flavored nicotine would be based on a simpler explanation that flavor could increase palatability or reduce harshness of the nicotine solution. Tobacco company documents openly state that inclusion of sweet and fruity flavor additives (e.g. “vanilla beans, peach, apricot, licorice, cocoa, and many others”) are meant to cover up “objectionable off flavors” (Fries and Brother and Triest; Kostygina et al., 2014; Industry document, 1966) and the addition of characterizing flavors can suppress ‘unappealing’ sensations of nicotine in e-cigarettes and of other bittering agents (Isogai and Wise, 2016; Leventhal et al., 2019a). Therefore, it is possible that in our study, flavorants overpowered the bitterness in their nicotine solutions and led to increased consumption of the more palatable solution by all mice.
We thought it was important to include a novel fruit flavor (i.e. grape) as a control at this stage of the experiment to help us determine if any changes were impacted by familiarity or by conditioned reinforcement after pairing with nicotine. Furthermore, if adolescent CHOICE mice increased nicotine consumption when strawberry flavor was re-introduced to the nicotine bottle in adulthood, but not when grape flavor was added, it would suggest that strawberry was behaving as a flavor-specific conditioned reinforcer. Interestingly, only mice that initiated nicotine use in adolescence with access to strawberry-flavored nicotine (i.e., CHOICE mice) significantly increased the dose of nicotine consumed over their baseline consumption levels and only at the first dilution of 0.075 mg/ml nicotine (Figure 5 C, D). As mentioned above, conditioned reinforcers can work to increase the rewarding value of nicotine and suggests that the flavor could be acting as a conditioned reinforcer only in the CHOICE mice. However, a tendency in the response of UNFLAV mice to increase nicotine consumption at this concentration of nicotine and the immediate and dramatic shift to a preference for the flavored nicotine bottles compared to the control bottle seen in all mice suggests that palatability is also playing a role. There is a general trend towards an indifference for the nicotine bottle (an approach towards 50%) as nicotine is removed from the bottle. In mice that initiated nicotine use without flavor, once nicotine is completely removed from the bottle all aversion and preference is neutralized and mice drink about 50% from the control bottle and from the bottle that used to contain nicotine. This suggests that the fruit flavorant on its own did not confer a preference for the bottle, but that it made nicotine more palatable. In contrast, mice that were exposed to strawberry-flavored nicotine in adolescence (i.e. adolescent CHOICE mice) maintain a preference for the flavored bottles, even in the absence of nicotine. Again, this supports the idea that flavorants became secondary or conditioned reinforcers in this group of mice. At two other time points in this longitudinal design we measured animals’ preference for a nicotine-free strawberry solution. Initially, mice did not prefer for the strawberry solution over an equally sweet control solution. However, after adolescent nicotine exposure, mice from both the UNFLAV and CHOICE adolescent treatment groups preferred a nicotine-free strawberry solution (Supplementary Figure 2). With these data in mind, it is not surprising that when strawberry flavor was added to the nicotine bottles in adulthood, mice showed a preference for the strawberry flavored nicotine bottle, or even for the strawberry-only bottle, since a preference for strawberry had already been established. Still, this fails to explain why preference for the grape flavored bottle persisted in the adolescent CHOICE mice, or why the preference for strawberry did not persist in the adolescent UNFLAV mice, who had also established a preference for strawberry during adolescence. A recent paper investigating a different flavor’s (licorice’s) ability to serve as a conditioned reinforcer paired IV nicotine self-administration with oral licorice delivery. In this study, similar to ours, they found that the flavor increased self-administration of nicotine and that rats would continue to self-administer licorice, even in the absence of nicotine (Palmatier et al., 2019).
Ultimately, we think it is most likely that mice that initiated nicotine use with flavor (i.e., adolescent CHOICE mice) are more sensitive to flavorants as conditioned reinforcers in adulthood, and that palatability played a role in the increased preference for and consumption of nicotine in both groups, regardless of their adolescent treatment. More research would be needed to fully elucidate these mechanisms. Although nicotine oral self-administration experiments, such as this one, are useful to measure preference and voluntary consumption of nicotine, they cannot fully differentiate between the ability of the flavorants to increase nicotine consumption due to an increase in palatability/reduction in aversiveness and the ability of flavorants to increase the reinforcing properties of nicotine. This could be more appropriately addressed using an operant response based self-administration procedure on a progressive ratio schedule of reinforcement. A recent publication using a vapor self-administration paradigm and the green apple flavorant, farsenol, showed an increase in the amount of nicotine puffs earned on low effort schedules of reinforcement (Cooper et al., 2021). However, they only measured the effects of flavorant on self-administration in male mice and did not explore the ability of the flavorant to increase reinforcement on a progressive ratio schedule. Still, the fact that multiple flavors comprising of different flavor chemicals (e.g. farsenol vs. ethyl butyrate) increases nicotine consumption in studies across multiple modes of nicotine self-administration all builds towards an important and consistent finding: that flavorants can increase consumed doses of nicotine (Cooper et al., 2021; Palmatier et al., 2019; Wong et al., 2020).
This research highlights the importance of studying flavored nicotine using techniques that allow for the sensory components of flavorants to be perceived by research subjects, as well as the importance of studying both male and female subjects. One weakness in our experimental design is that we are unable to distinguish between changes in nicotine consumption due to increased reward or reduced aversion of nicotine. Future studies which combine both pharmacological and sensory components of popular e-cigarette flavorants, such as via vapor inhalation, might help address these questions.
5. Conclusion
In summary, our results indicate that fruit-flavorants have a significant impact on nicotine preference and consumption, having the ability to increase nicotine consumption, regardless of life-stage and could potentially interfere with one’s ability to quit later in life after exposure to flavored nicotine during adolescence.
Supplementary Material
Supplementary Figure 1. Mice are divided into treatment groups, such that groups have approximately equal initial preference for a nicotine-free strawberry solution. Lines and error bars represent summary data (mean +/− SEM). The UNFLAV group showed an initial preference for nicotine-free strawberry solution equal to 39.64 ± 5.47 %, which is similar to those sorted into the CHOICE group, with an initial preference for nicotine-free strawberry solution equal to 31.29 ± 5.08 %. Two-way ANOVA; Fsex(1,66) = 1.980, p=0.164, Ftreatment(1,66) = 1.271, p=0.263, Finteraction(1,66) = 0.075, p=0.784. Male, UNFLAV: n=18, CHOICE: n= 17; Female, UNFLAV: n=17, CHOICE: n=17).
Supplementary Figure 2. Regardless of a direct-pairing with nicotine, preference for a strawberry solution increases following a 2-week nicotine two-bottle choice test in adolescence. A) Diagram shows a simplified experimental timeline, including a baseline (pre-nicotine) strawberry preference test, a limited access (2-hr/day) nicotine 2-bottle choice test with or without flavored nicotine, and a post-nicotine exposure strawberry preference test. B) In general, mice did not prefer a strawberry solution prior to the “Limited Access (2hr) Nicotine 2BC test”. However, following the 2BC experiment, regardless of treatment group or sex, mice significantly increased their preference for a nicotine-free strawberry solution (Three-way ANOVA, Ftime(1,66) = 55.010, ****p<0.0001, Fsex(1,66) = 3.176, p=0.079, Fadolescent_treatment(1,66) = 2.299, p = 0.134, Ftime*sex(1, 66) = 0.328, p=0.569, Ftime*adolescent_treatment(1,66) = 0.131, p=0.719, Fsex*adolescent_treatment(1,66) = 0.131, p=0.718, Ftime*sex*adolescent_treatment(1,66) = 0.800, p=0.374, Sidak’s multiple comparisons test). ++P < 0.01 ++++P < 0.0001 represent a significant difference from the Pre-NIC value (Sidak’s multiple comparisons test), while #P<0.05, ##P<0.01, ###P<0.001, ####P<0.0001 represent a significant difference from theoretical chance (50%). Male, UNFLAV: n=18, CHOICE: n= 18; Female, UNFLAV: n=17, CHOICE: n=17.
Supplementary Figure 3. Mice with access to strawberry flavored nicotine tend to drink a larger volume of nicotine during the 2-hour nicotine access session, but do not drink more fluid overall. A-B) The summary data of total volume (mL) consumed during the 2-hour nicotine access period in adolescence is shown for males and females separately (mean +/− SEM). 2-way RMANOVAs (treatment × session) detected a trend but no significant effect of treatment group on the volume consumed during the 2-hour nicotine session among males (Fgroup(1,34) =2.868, p=0.100) and B) among females (Fgroup(1,32) = 3.676, p=0.064). There was a significant effect of session for males (Fsession(6,204)=13.22, ****p<0.0001), but not females (Fsession(6,192)=1.664, p=0.132), and no significant interactions (Male: Finteraction(6,204)=0.3227, p= 0.925; Female: Finteraction(6,192)=1.460, p=0.194). C-D) Data shows the average volume consumed by mice over 24-hr. Lines and error bars represent summary data (mean +/− SEM). The volume consumed over a 24-hr period is not significantly different between treatment groups in either C) male (unpaired t-test, t(34) = 1.428, p=0.162) or D) female mice (unpaired t-test, t(32) = 0.924, p=0.3623). (Male, UNFLAV: n=18, CHOICE: n= 18; Female, UNFLAV: n=17, CHOICE: n=17).
Supplementary Figure 4. Mice do not exhibit spontaneous signs of nicotine withdrawal after 4 weeks of a free choice between 0.100 mg/ml nicotine and a control bottle. A) The number of somatic signs displayed by animals during two trials (satiated vs. 24-hr nicotine abstinent) were compared. A two-way RMANOVA did not detect a significant effect of state (i.e. satiety vs. abstinent) (Fstate(1, 60) = 0.7993, p = 0.3749). The results indicate that, on average, the 4 weeklong 24-h nicotine choice, at the low nicotine doses administered, was not sufficient to produce spontaneous signs of nicotine dependence in these animals. B) There is however, a trend towards an increase in the proportion ‘CHOICE’ mice that displayed symptoms of nicotine withdrawal (defined by ≥ 15% increase in the number of somatic signs displayed) compared to ‘UNFLAV’ mice (Fisher’s exact test, p=0.109) (adolescent UNFLAV mice, Unflav: n=12, Strb: n=10, Grape: n=12; adolescent CHOICE mice, Unflav: n=9, Strb: n=11, Grape: n=12)
Supplementary Figure 5. Mice in all groups drink equivalent volumes of fluid across all concentrations of nicotine. Line graph represents summary data (mean +/− SEM). Mice that were in the UNFLAV and in the CHOICE adolescent group drink statistically equivalent volumes of fluid, regardless of nicotine flavor assignment, throughout the “Fading and Flavor Reintroduction” experiment. There was a significant effect of nicotine concentration, but no effect of flavor or an interaction (RM Mixed Effects Analysis, Fnicotine conc.(2.665,167.2) = 48.75, ****p<0.0001, Ftreatment(5, 63) = 1.696, p=0.149, Finteraction(20, 251) =0.916, p=0.567, Dunnet’s multiple comparisons test). (UNFLAV in adolescence, ‘No Flavor’: n=12, ‘Strawberry’: n= 11, ‘Grape’: n=12; CHOICE in adolescence, ‘No Flavor’: n=10, ‘Strawberry’: n= 11, ‘Grape’: n=13)
Supplementary Figure 6. Female C57/BL6 mice consume more nicotine compared to males as the concentration of nicotine is reduced from the nicotine bottle. All mice established baseline consumption levels (mg/kg/day) with an unflavored nicotine solution containing 0.100 mg/ml nicotine during a “Baseline Nicotine Consumption” phase. The concentration of nicotine was sequentially reduced (0.075, 0.050, 0.025, 0.000) following 8 days of drinking at each concentration. Bar graphs represent summary data (mean +/− SEM) and points represent the doses consumed by individual mice. Data is arranged so that mice that only had access to unflavored nicotine in adolescence (i.e. UNLFAV group) are on the left (panels A,C, E, G) and mice who had access to strawberry flavored nicotine in adolescence are on the right (panels B,D,F, H). A-B) Mice were assigned to a flavor for the “Nicotine Fading and Flavor Re-introduction” phase such that there were no differences in the dose established between groups with unflavored 0.100 mg/ml nicotine solution. C-D) Once the concentration of nicotine was reduced to 0.075 mg/ml, female mice began to drink significantly more than males, regardless of adolescent treatment (Two-way ANOVA, Sidak’s multiple comparisons test; UNFLAV in adolescence:, Fsex(1,29) = 7.269, *p=0.012, Fflavor.(2, 29) = 6.733, **p=0.004, Finteraction(2,29) =1.156, p=0.329; CHOICE in adolescence: Fsex(1,28) = 8.901, **p=0.006, Fflavor.(2, 28) = 3.273, p=0.053, Finteraction(2,28) =0.028, p=0.973). E-F) Sex differences in total dose consumed were still present when the concentration of nicotine was reduced to 0.050 mg/ml nicotine (Two-way ANOVA, Sidak’s multiple comparisons test; UNFLAV in adolescence: Fsex(1,29) = 8.084, **p=0.008, Fflavor.(2, 29) = 2.289, p=0.119, Finteraction(2,29) =1.858, p=0.174); CHOICE in adolescence: Fsex(1,28) = 5.757, *p=0.023, Fflavor.(2, 28) = 1.285, p=0.293, Finteraction(2,28) =0.107, p=0.899). G-H) Females continued to drink a higher dose of nicotine when the concentration was reduced to its lowest concentration of 0.025 mg/ml nicotine (Two-way ANOVA, Sidak’s multiple comparisons test; UNFLAV in adolescence: Fsex(1,29) = 9.612, **p=0.004, Fflavor.(2, 29) = 8.934, ***p=0.0009, Finteraction(2,29) =0.3146, p=0.733; CHOICE in adolescence: Fsex(1,28) = 12.35, **p=0.002, Fflavor.(2, 28) = 0.9298, p=0.407, Finteraction(2,28) =0.1891, p=0.829). *P < 0.05. **P < 0.01 for all comparisons. (Unflavored nicotine in adolescence: males (UNFLAV, STRB, GRAPE), n= 6,5,7, females(UNFLAV, STRB, GRAPE), n= 6,6,5; Access to strawberry nicotine in adolescence: males (UNFLAV, STRB, GRAPE), n= 5,6,6, females(UNFLAV, STRB, GRAPE), n= 5,5,7).
Supplementary Figure 7. Preference for a nicotine bottle generally increases as the concentration of nicotine in the bottle is reduced. All mice established baseline nicotine preference (%) with an unflavored nicotine solution containing 0.100 mg/ml nicotine during a “Baseline Nicotine Consumption” phase. The concentration of nicotine was sequentially reduced (0.075, 0.050, 0.025, 0.000) following 8 days of drinking at each concentration. Bar graphs represent summary data (mean +/− SEM) and points represent the nicotine preferences of individual mice. Data is arranged so that mice who had access to only unflavored nicotine in adolescence (i.e. UNLFAV group) are on the left (panels A, C, E, G, I) and mice who had access to strawberry flavored nicotine in adolescence are on the right (panels B,D,F, H, J). A-B) Mice were assigned to a flavor for the “Nicotine Fading and Flavor Re-introduction” phase such that there were no differences in the nicotine preference established between groups with an unflavored 0.100 mg/ml nicotine solution. Preferences were generally <50% at this concentration. C-D) Once the concentration of nicotine was reduced to 0.075 mg/ml, the preference for nicotine generally increased. Additionally, mice in Unflavored adolescent treatment group showed a preference for fruit-flavored nicotine solutions compared to an unflavored nicotine solution (Two-way ANOVA, Fsex(1,29) = 0.360, p=0.553, Fflavor.(2, 29) = 6.761, **p=0.004, Finteraction(2,29) =0.502, p=0.611, Sidak’s multiple comparisons tests). E-F) Preferences for flavored nicotine solutions remained similar when the nicotine concentration was reduced to 0.05 mg/ml nicotine. Mice also had the highest preference for unflavored nicotine at this concentration of nicotine. G-H) At a concentration of 0.025 mg/ml nicotine mice that had access to strawberry flavored nicotine in adolescence (i.e. adolescent CHOICE mice) maintained similar preferences for nicotine solutions in adulthood, but a preference for flavored nicotine was re-established in mice that only had access to unflavored nicotine in adolescence (i.e., adolescent UNFLAV mice) (Two-way ANOVA, Fsex(1,29) = 0.517, p=0.478, Fflavor.(2, 29) = 10.950, ***p=0.0003, Finteraction(2,29) =1.248, p=0.302, Sidak’s multiple comparisons tests). I-J) When nicotine was completely removed from the bottle flavor did not affect preference for the nicotine bottle in any group of mice; however, in mice who only had access to unflavored nicotine in adolescence, females had a higher preference for the nicotine bottle across all flavors (Two-way ANOVA, Fsex(1,29) = 7.222, *p=0.012, Fflavor.(2, 29) = 0.535, p=0.591, Finteraction(2,29) =1.182, p=0.321). *P < 0.05. **P < 0.01, ***P <0.001 for all comparisons. (Unflavored nicotine in adolescence: males (UNFLAV, STRB, GRAPE), n= 6,5,7, females(UNFLAV, STRB, GRAPE), n= 6,6,5; Access to strawberry nicotine in adolescence: males (UNFLAV, STRB, GRAPE), n= 5,6,6, females(UNFLAV, STRB, GRAPE), n= 5,5,7).
Highlights:
Adolescent mice prefer a fruit-flavored nicotine solution over an unflavored nicotine solution
Adolescent female mice consume more nicotine if it is flavored
Adolescent female mice escalate nicotine consumption only when flavored
Adult mice with a history of flavored nicotine use resist nicotine dose reductions
Adult mice increase nicotine consumption when flavor is added
Acknowledgements:
The authors would like to acknowledge Kimberly Halberstadter for her help with the drinking experiments and Annie Luo for scoring the odor discrimination videos (data not shown).
Funding:
This work was supported by the National Institute on Drug Abuse (NIDA) grantsDA044205 and DA049545 to MDB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: The authors declare no competing interests.
References:
- Ambrose BK, Day HR, Rostron B, Conway KP, Borek N, Hyland A, Villanti AC, 2015. Flavored Tobacco Product Use Among US Youth Aged 12–17 Years, 2013–2014. JAMA 314, 1871–1873. 10.1001/jama.2015.13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Pianin S, Alexander E, 2019. Initial e-cigarette flavoring and nicotine exposure and e-cigarette uptake among adolescents. Drug Alcohol Depend 202, 149–155. 10.1016/j.drugalcdep.2019.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Strasser AA, Wileyto EP, 2016. The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug Alcohol Depend 166, 263–267. 10.1016/j.drugalcdep.2016.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, Diester CM, Riley J, Carper M, Alkhlaif Y, AlOmari D, Alayoubi H, Poklis JL, Damaj MI, 2019. Assessing nicotine dependence using an oral nicotine free-choice paradigm in mice. Neuropharmacology 157, 107669. 10.1016/j.neuropharm.2019.107669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Kong G, Leventhal AM, Liu F, Mayer M, Cruz TB, Krishnan-Sarin S, McConnell R, 2018. E-cigarette use and subsequent smoking frequency among adolescents. Pediatrics 142. 10.1542/peds.2018-0486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp GK, Cowart BJ, 1985. Congenital and experiential factors in the development of human flavor preferences. Appetite 6, 357–372. 10.1016/S0195-6663(85)80004-0 [DOI] [PubMed] [Google Scholar]
- Bold KW, Kong G, Cavallo DA, Camenga DR, Krishnan-Sarin S, 2016. Reasons for Trying E-cigarettes and Risk of Continued Use. Pediatrics 138, e20160895. 10.1542/PEDS.2016-0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Luo W, Isabelle LM, Pankow JF, 2014. Candy Flavorings in Tobacco. N. Engl. J. Med 370, 2250–2252. 10.1056/NEJMc1403015 [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Marfo NYA, Smith TT, Levin ME, Weaver MT, Thiels E, Sved AF, Donny EC, 2014. Nicotine enhances the expression of a sucrose or cocaine conditioned place preference in adult male rats. Pharmacol. Biochem. Behav 124, 320–325. 10.1016/j.pbb.2014.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi ED, Privitera GJ, 2008. Decreasing dislike for sour and bitter in children and adults. Appetite 50, 139–145. 10.1016/j.appet.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2019. Progress Erased: Youth Tobacco Use Increased During 2017–2018 [WWW Document]. URL https://www.cdc.gov/media/releases/2019/p0211-youth-tobacco-use-increased.html (accessed 1.23.20).
- Centers for Disease Control and Prevention, 2002. Women and smoking : a report of the Surgeon General [WWW Document]. MMWR. URL https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5112a4.htm (accessed 2.26.20). [PubMed]
- Chen JC, Das B, Mead EL, Borzekowski DLG, 2016. Flavored E-cigarette Use and Cigarette Smoking Susceptibility among Youth. Tob. Regul. Sci 3, 68–80. 10.18001/trs.3.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Stacy A, Zheng H, Shan J, Spruijt-Metz D, Unger JB, Gong J, Gallaher P, Liu C, Azen S, Shakib S, Johnson CA, 2003. Sensations from initial exposure to nicotine predicting adolescent smoking in China: A potential measure of vulnerability to nicotine. Nicotine Tob. Res 5, 455–463. 10.1080/14622200307239 [DOI] [PubMed] [Google Scholar]
- Collins AC, Pogun S, Nesil T, Kanit L, 2012. Oral Nicotine Self-Administration in Rodents. J. Addict. Res. Ther S2, 004. 10.4172/2155-6105.s2-004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke LJ, Wardle J, 2005. Age and gender differences in children’s food preferences. Br. J. Nutr 93, 741–746. 10.1079/bjn20051389 [DOI] [PubMed] [Google Scholar]
- Cooper SY, Akers AT, Henderson BJ, 2021. Flavors Enhance Nicotine Vapor Self-administration in Male Mice. Nicotine Tob. Res 23, 566–572. 10.1093/ntr/ntaa165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Hao J, 2016. Flavored Electronic Cigarette Use and Smoking Among Youth. Pediatrics 138, e20162513. 10.1542/peds.2016-2513 [DOI] [PubMed] [Google Scholar]
- Desor JA, Beauchamp GK, 1987. Longitudinal changes in sweet preferences in humans. Physiol. Behav 39, 639–641. 10.1016/0031-9384(87)90166-1 [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Pbert L, O’Loughlin J, McNeill AD, Ockene JK, Friedman K, Hazelton J, Wood C, Dussault G, Wellman RJ, 2007. Susceptibility to nicotine dependence: The development and assessment of nicotine dependence in youth 2 study. Pediatrics 120, e974–e983. 10.1542/peds.2007-0027 [DOI] [PubMed] [Google Scholar]
- England LJ, Aagaard K, Bloch M, Conway K, Cosgrove K, Grana R, Gould TJ, Hatsukami D, Jensen F, Kandel D, Lanphear B, Leslie F, Pauly JR, Neiderhiser J, Rubinstein M, Slotkin TA, Spindel E, Stroud L, Wakschlag L, 2017. Developmental toxicity of nicotine: A transdisciplinary synthesis and implications for emerging tobacco products. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA, 2015. Nicotine and the Developing Human: A Neglected Element in the Electronic Cigarette Debate. Am. J. Prev. Med 10.1016/j.amepre.2015.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Birk J, 1982. Flavor-flavor associations induce hedonic shifts in taste preference. Anim. Learn. Behav 10, 223–228. 10.3758/BF03212274 [DOI] [Google Scholar]
- Frank RA, Byram J, 1988. Taste–smell interactions are tastant and odorant dependent. Chem. Senses 13, 445–455. 10.1093/chemse/13.3.445 [DOI] [Google Scholar]
- Fries and Brother, Triest, F., n.d. Function of Tobacco Flavor; Bates: 4590172 [WWW Document]. URL https://www.industrydocuments.ucsf.edu/tobacco/docs/#id=jmvn0046 (accessed 1.17.20).
- Gentzke AS, Creamer M, Cullen KA, Ambrose BK, Willis G, Jamal A, King BA, 2019. Vital Signs: Tobacco Product Use Among Middle and High School Students — United States, 2011–2018. MMWR. Morb. Mortal. Wkly. Rep 68, 157–164. 10.15585/mmwr.mm6806e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenson NI, Kirkpatrick MG, Barrington-Trimis JL, Pang RD, McBeth JF, Pentz MA, Samet JM, Leventhal AM, 2016. Effects of sweet flavorings and nicotine on the appeal and sensory properties of e-cigarettes among young adult vapers: Application of a novel methodology. Drug Alcohol Depend 168, 176–180. 10.1016/j.drugalcdep.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriounova NA, Mansvelder HD, 2012. Short- and Long-Term Consequences of Nicotine Exposure during Adolescence for Prefrontal Cortex Neuronal Network Function. Cold Spring Harb. Perspect. Med 2, a012120. 10.1101/cshperspect.a012120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell MB, Weaver SR, Loukas A, Creamer M, Marti CN, Jackson CD, Heath JW, Nayak P, Perry CL, Pechacek TF, Eriksen MP, 2017. Flavored e-cigarette use: Characterizing youth, young adult, and adult users. Prev. Med. reports 5, 33–40. 10.1016/j.pmedr.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Shand FL, Carroll LQ, Westbrook RF, 2004. Persistence of Preference for a Flavor Presented in Simultaneous Compound With Sucrose. J. Exp. Psychol. Anim. Behav. Process 30, 177–189. 10.1037/0097-7403.30.3.177 [DOI] [PubMed] [Google Scholar]
- Hoffman AC, Salgado RV, Dresler C, Faller RW, Bartlett C, 2016. Flavour preferences in youth versus adults: a review. Tob. Control 25, ii32–ii39. 10.1136/tobaccocontrol-2016-053192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu G, Sun JY, Zhu S-H, 2018. Evolution of Electronic Cigarette Brands From 2013–2014 to 2016–2017: Analysis of Brand Websites. J. Med. Internet Res 20, e80. 10.2196/jmir.8550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai T, Wise PM, 2016. The effects of odor quality and temporal asynchrony on modulation of taste intensity by retronasal odor. Chem. Senses 41, 557–566. 10.1093/chemse/bjw059 [DOI] [PubMed] [Google Scholar]
- Jackson A, Green B, Erythropel HC, Kong G, Cavallo DA, Eid T, Gueorguieva R, Buta E, O’Malley SS, Krishnan-Sarin S, 2020. Influence of menthol and green apple e-liquids containing different nicotine concentrations among youth e-cigarette users. Exp. Clin. Psychopharmacol 10.1037/pha0000368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lim J, Buehler SS, Brinkman MC, Johnson NM, Wilson L, Cross KS, Clark PI, 2016. Role of sweet and other flavours in liking and disliking of electronic cigarettes. Tob. Control 25, ii55–ii61. 10.1136/tobaccocontrol-2016-053221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler C, Crutchfield T, Sutfin E, Ranney L, Berman M, Zarkin G, Goldstein A, 2017. Consumers’ Preferences for Electronic Nicotine Delivery System Product Features: A Structured Content Analysis. Int. J. Environ. Res. Public Health 14, 613. 10.3390/ijerph14060613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Morean ME, Cavallo DA, Camenga DR, Krishnan-Sarin S, 2015. Reasons for Electronic Cigarette Experimentation and Discontinuation Among Adolescents and Young Adults. Nicotine Tob. Res 17, 847–854. 10.1093/ntr/ntu257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostygina G, Glantz SA, Ling PM, 2014. Tobacco industry use of flavours to recruit new users of little cigars and cigarillos. Tob. Control 25, 66–74. 10.1136/tobaccocontrol-2014-051830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe D, Rytz A, Morgenegg C, Ali S, Martin N, 2007. Subthreshold olfactory stimulation can enhance sweetness. Chem. Senses 32, 205–214. 10.1093/chemse/bjl040 [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Goldenson NI, Barrington-Trimis JL, Pang RD, Kirkpatrick MG, 2019a. Effects of non-tobacco flavors and nicotine on e-cigarette product appeal among young adult never, former, and current smokers. Drug Alcohol Depend 203, 99–106. 10.1016/j.drugalcdep.2019.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Goldenson NI, Cho J, Kirkpatrick MG, McConnell RS, Stone MD, Pang RD, Audrain-McGovern J, Barrington-Trimis JL, 2019b. Flavored E-cigarette Use and Progression of Vaping in Adolescents. Pediatrics 144, e20190789. 10.1542/peds.2019-0789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantey DS, Harrell MB, Case K, Crook B, Kelder SH, Perry CL, 2017. Subjective experiences at first use of cigarette, e-cigarettes, hookah, and cigar products among Texas adolescents. Drug Alcohol Depend 173, 10–16. 10.1016/j.drugalcdep.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Bobowski NK, Reed DR, 2016. The development of sweet taste: From biology to hedonics. Rev. Endocr. Metab. Disord 17, 171–178. 10.1007/s11154-016-9360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences Engineering and Medicine, 2018. Public Health Consequences of E-Cigarettes National Academies Press (US), Washington, DC. 10.17226/24952 [DOI] [PubMed] [Google Scholar]
- Omaiye EE, McWhirter KJ, Luo W, Tierney PA, Pankow JF, Talbot P, 2019. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep 9, 2468. 10.1038/s41598-019-39550-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Roy P, Balcita-Pedicino JJ, Poloyac S, Sesack SR, 2016. Impact of prenatal nicotine on the structure of midbrain dopamine regions in the rat. Brain Struct. Funct 221, 1939–53. 10.1007/s00429-015-1014-y [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Smith AL, Odineal EM, Williams EA, Sheppard AB, Bradley CA, 2019. Nicotine Self-Administration With Tobacco Flavor Additives in Male Rats. Nicotine Tob. Res 22, 224–231. 10.1093/ntr/ntz053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten T, De Biasi M, 2020. History repeats itself: Role of characterizing flavors on nicotine use and abuse. Neuropharmacology 177, 108162. 10.1016/j.neuropharm.2020.108162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez E, Quijano-Cardé N, De Biasi M, 2015. Nicotinic Mechanisms Modulate Ethanol Withdrawal and Modify Time Course and Symptoms Severity of Simultaneous Withdrawal from Alcohol and Nicotine. Neuropsychopharmacology 40, 2327–2336. 10.1038/npp.2015.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, 1999. Nicotine discrimination in men and women. Pharmacol. Biochem. Behav 64, 295–299. 10.1016/S0091-3057(99)00085-4 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S, 2001. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob. Res 3, 141–50. 10.1080/14622200110043059 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Boldry MC, 2017. Nicotine acutely enhances reinforcement from non-drug rewards in humans. Front. Psychiatry 10.3389/fpsyt.2017.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PD, Husile N, Strasser AA, Wise PM, 2018. Pilot Experiment: the Effect of Added Flavorants on the Taste and Pleasantness of Mixtures of Glycerol and Propylene Glycol. Chemosens. Percept 11, 1–9. 10.1007/s12078-017-9231-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez D, Audrain-McGovern J, 2004. Construct validity analysis of the early smoking experience questionnaire for adolescents. Addict. Behav 29, 1053–1057. 10.1016/j.addbeh.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M, 2004. Decreased signs of nicotine withdrawal in mice null for the β4 nicotinic acetylcholine receptor subunit. J. Neurosci 24, 10035–10039. 10.1523/JNEUROSCI.1939-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M, 2009. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J. Neurosci 29, 3014–3018. 10.1523/JNEUROSCI.4934-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Lessov-Schlaggar CN, Scherrer JF, Bucholz KK, Madden PAF, Pergadia ML, Grant JD, Jacob T, Xian H, 2010. Initial response to cigarettes predicts rate of progression to regular smoking: Findings from an offspring-of-twins design. Addict. Behav 35, 771–778. 10.1016/j.addbeh.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TT, Heckman BW, Wahlquist AE, Cummings KM, Carpenter MJ, 2020. The Impact of E-liquid Propylene Glycol and Vegetable Glycerin Ratio on Ratings of Subjective Effects, Reinforcement Value, and Use in Current Smokers. Nicotine Tob. Res 22, 791–797. 10.1093/ntr/ntz130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneji S, Barrington-Trimis JL, Wills TA, Leventhal AM, Unger JB, Gibson LA, Yang J, Primack BA, Andrews JA, Miech RA, Spindle TR, Dick DM, Eissenberg T, Hornik RC, Dang R, Sargent JD, 2017. Association Between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults. JAMA Pediatr 171, 788–797. 10.1001/jamapediatrics.2017.1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneji SS, Knutzen KE, Villanti AC, 2019. Use of Flavored E-Cigarettes Among Adolescents, Young Adults, and Older Adults: Findings From the Population Assessment for Tobacco and Health Study. Public Health Rep 134, 282–292. 10.1177/0033354919830967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St.Helen G, Shahid M, Chu S, Benowitz NL, 2018. Impact of e-liquid flavors on e-cigarette vaping behavior. Drug Alcohol Depend 189, 42–48. 10.1016/j.drugalcdep.2018.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, 2016. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General
- Unknown, 1966. Our Affiliates Buy, Sort, Pack or Process Each Each Kind of Tobacco to Give You the Best of Quality, Bates: 4590173–4590175 [WWW Document]. US Tob. Rec. Smokeless Tobacco. URL https://www.industrydocuments.ucsf.edu/tobacco/docs/#id=ynhd0044 (accessed 1.17.20).
- Urbán R, 2010. Early smoking experience in adolescents. Addict. Behav 35, 612–615. 10.1016/j.addbeh.2009.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, Johnson AL, Ambrose BK, Cummings KM, Stanton CA, Rose SW, Feirman SP, Tworek C, Glasser AM, Pearson JL, Cohn AM, Conway KP, Niaura RS, Bansal-Travers M, Hyland A, 2017. Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013–2014). Am. J. Prev. Med 53, 139–151. 10.1016/j.amepre.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JF, Loprinzi PD, 2014. Longitudinal examination of predictors of smoking cessation in a national sample of U.S. adolescent and young adult smokers. Nicotine Tob. Res 16, 820–827. 10.1093/ntr/ntu005 [DOI] [PubMed] [Google Scholar]
- Wong AL, McElroy SM, Robinson JM, Mulloy SM, El Banna FK, Harris AC, LeSage MG, Lee AM, 2020. Flavor-specific enhancement of electronic cigarette liquid consumption and preference in mice. Drug Alcohol Depend 10.1016/j.drugalcdep.2020.107995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Heley K, Kennedy RD, Lagasse L, Moran MB, 2019. Sociodemographic differences in reasons for ENDS use among US youth within Wave 2 of the PATH study. Tob. Induc. Dis 17, 4. 10.18332/tid/99879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T, Jiang N, Grana R, Ling PM, Glantz SA, 2016. A content analysis of electronic cigarette manufacturer websites in China. Tob. Control 25, 188–194. 10.1136/tobaccocontrol-2014-051840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Cross SJ, Loughlin SE, Leslie FM, 2015. Nicotine and the adolescent brain. J. Physiol 593, 3397–3412. 10.1113/JP270492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dong Y, Doyon WM, Dani JA, 2012. Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol. Psychiatry 71, 184–191. 10.1016/j.biopsych.2011.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Mice are divided into treatment groups, such that groups have approximately equal initial preference for a nicotine-free strawberry solution. Lines and error bars represent summary data (mean +/− SEM). The UNFLAV group showed an initial preference for nicotine-free strawberry solution equal to 39.64 ± 5.47 %, which is similar to those sorted into the CHOICE group, with an initial preference for nicotine-free strawberry solution equal to 31.29 ± 5.08 %. Two-way ANOVA; Fsex(1,66) = 1.980, p=0.164, Ftreatment(1,66) = 1.271, p=0.263, Finteraction(1,66) = 0.075, p=0.784. Male, UNFLAV: n=18, CHOICE: n= 17; Female, UNFLAV: n=17, CHOICE: n=17).
Supplementary Figure 2. Regardless of a direct-pairing with nicotine, preference for a strawberry solution increases following a 2-week nicotine two-bottle choice test in adolescence. A) Diagram shows a simplified experimental timeline, including a baseline (pre-nicotine) strawberry preference test, a limited access (2-hr/day) nicotine 2-bottle choice test with or without flavored nicotine, and a post-nicotine exposure strawberry preference test. B) In general, mice did not prefer a strawberry solution prior to the “Limited Access (2hr) Nicotine 2BC test”. However, following the 2BC experiment, regardless of treatment group or sex, mice significantly increased their preference for a nicotine-free strawberry solution (Three-way ANOVA, Ftime(1,66) = 55.010, ****p<0.0001, Fsex(1,66) = 3.176, p=0.079, Fadolescent_treatment(1,66) = 2.299, p = 0.134, Ftime*sex(1, 66) = 0.328, p=0.569, Ftime*adolescent_treatment(1,66) = 0.131, p=0.719, Fsex*adolescent_treatment(1,66) = 0.131, p=0.718, Ftime*sex*adolescent_treatment(1,66) = 0.800, p=0.374, Sidak’s multiple comparisons test). ++P < 0.01 ++++P < 0.0001 represent a significant difference from the Pre-NIC value (Sidak’s multiple comparisons test), while #P<0.05, ##P<0.01, ###P<0.001, ####P<0.0001 represent a significant difference from theoretical chance (50%). Male, UNFLAV: n=18, CHOICE: n= 18; Female, UNFLAV: n=17, CHOICE: n=17.
Supplementary Figure 3. Mice with access to strawberry flavored nicotine tend to drink a larger volume of nicotine during the 2-hour nicotine access session, but do not drink more fluid overall. A-B) The summary data of total volume (mL) consumed during the 2-hour nicotine access period in adolescence is shown for males and females separately (mean +/− SEM). 2-way RMANOVAs (treatment × session) detected a trend but no significant effect of treatment group on the volume consumed during the 2-hour nicotine session among males (Fgroup(1,34) =2.868, p=0.100) and B) among females (Fgroup(1,32) = 3.676, p=0.064). There was a significant effect of session for males (Fsession(6,204)=13.22, ****p<0.0001), but not females (Fsession(6,192)=1.664, p=0.132), and no significant interactions (Male: Finteraction(6,204)=0.3227, p= 0.925; Female: Finteraction(6,192)=1.460, p=0.194). C-D) Data shows the average volume consumed by mice over 24-hr. Lines and error bars represent summary data (mean +/− SEM). The volume consumed over a 24-hr period is not significantly different between treatment groups in either C) male (unpaired t-test, t(34) = 1.428, p=0.162) or D) female mice (unpaired t-test, t(32) = 0.924, p=0.3623). (Male, UNFLAV: n=18, CHOICE: n= 18; Female, UNFLAV: n=17, CHOICE: n=17).
Supplementary Figure 4. Mice do not exhibit spontaneous signs of nicotine withdrawal after 4 weeks of a free choice between 0.100 mg/ml nicotine and a control bottle. A) The number of somatic signs displayed by animals during two trials (satiated vs. 24-hr nicotine abstinent) were compared. A two-way RMANOVA did not detect a significant effect of state (i.e. satiety vs. abstinent) (Fstate(1, 60) = 0.7993, p = 0.3749). The results indicate that, on average, the 4 weeklong 24-h nicotine choice, at the low nicotine doses administered, was not sufficient to produce spontaneous signs of nicotine dependence in these animals. B) There is however, a trend towards an increase in the proportion ‘CHOICE’ mice that displayed symptoms of nicotine withdrawal (defined by ≥ 15% increase in the number of somatic signs displayed) compared to ‘UNFLAV’ mice (Fisher’s exact test, p=0.109) (adolescent UNFLAV mice, Unflav: n=12, Strb: n=10, Grape: n=12; adolescent CHOICE mice, Unflav: n=9, Strb: n=11, Grape: n=12)
Supplementary Figure 5. Mice in all groups drink equivalent volumes of fluid across all concentrations of nicotine. Line graph represents summary data (mean +/− SEM). Mice that were in the UNFLAV and in the CHOICE adolescent group drink statistically equivalent volumes of fluid, regardless of nicotine flavor assignment, throughout the “Fading and Flavor Reintroduction” experiment. There was a significant effect of nicotine concentration, but no effect of flavor or an interaction (RM Mixed Effects Analysis, Fnicotine conc.(2.665,167.2) = 48.75, ****p<0.0001, Ftreatment(5, 63) = 1.696, p=0.149, Finteraction(20, 251) =0.916, p=0.567, Dunnet’s multiple comparisons test). (UNFLAV in adolescence, ‘No Flavor’: n=12, ‘Strawberry’: n= 11, ‘Grape’: n=12; CHOICE in adolescence, ‘No Flavor’: n=10, ‘Strawberry’: n= 11, ‘Grape’: n=13)
Supplementary Figure 6. Female C57/BL6 mice consume more nicotine compared to males as the concentration of nicotine is reduced from the nicotine bottle. All mice established baseline consumption levels (mg/kg/day) with an unflavored nicotine solution containing 0.100 mg/ml nicotine during a “Baseline Nicotine Consumption” phase. The concentration of nicotine was sequentially reduced (0.075, 0.050, 0.025, 0.000) following 8 days of drinking at each concentration. Bar graphs represent summary data (mean +/− SEM) and points represent the doses consumed by individual mice. Data is arranged so that mice that only had access to unflavored nicotine in adolescence (i.e. UNLFAV group) are on the left (panels A,C, E, G) and mice who had access to strawberry flavored nicotine in adolescence are on the right (panels B,D,F, H). A-B) Mice were assigned to a flavor for the “Nicotine Fading and Flavor Re-introduction” phase such that there were no differences in the dose established between groups with unflavored 0.100 mg/ml nicotine solution. C-D) Once the concentration of nicotine was reduced to 0.075 mg/ml, female mice began to drink significantly more than males, regardless of adolescent treatment (Two-way ANOVA, Sidak’s multiple comparisons test; UNFLAV in adolescence:, Fsex(1,29) = 7.269, *p=0.012, Fflavor.(2, 29) = 6.733, **p=0.004, Finteraction(2,29) =1.156, p=0.329; CHOICE in adolescence: Fsex(1,28) = 8.901, **p=0.006, Fflavor.(2, 28) = 3.273, p=0.053, Finteraction(2,28) =0.028, p=0.973). E-F) Sex differences in total dose consumed were still present when the concentration of nicotine was reduced to 0.050 mg/ml nicotine (Two-way ANOVA, Sidak’s multiple comparisons test; UNFLAV in adolescence: Fsex(1,29) = 8.084, **p=0.008, Fflavor.(2, 29) = 2.289, p=0.119, Finteraction(2,29) =1.858, p=0.174); CHOICE in adolescence: Fsex(1,28) = 5.757, *p=0.023, Fflavor.(2, 28) = 1.285, p=0.293, Finteraction(2,28) =0.107, p=0.899). G-H) Females continued to drink a higher dose of nicotine when the concentration was reduced to its lowest concentration of 0.025 mg/ml nicotine (Two-way ANOVA, Sidak’s multiple comparisons test; UNFLAV in adolescence: Fsex(1,29) = 9.612, **p=0.004, Fflavor.(2, 29) = 8.934, ***p=0.0009, Finteraction(2,29) =0.3146, p=0.733; CHOICE in adolescence: Fsex(1,28) = 12.35, **p=0.002, Fflavor.(2, 28) = 0.9298, p=0.407, Finteraction(2,28) =0.1891, p=0.829). *P < 0.05. **P < 0.01 for all comparisons. (Unflavored nicotine in adolescence: males (UNFLAV, STRB, GRAPE), n= 6,5,7, females(UNFLAV, STRB, GRAPE), n= 6,6,5; Access to strawberry nicotine in adolescence: males (UNFLAV, STRB, GRAPE), n= 5,6,6, females(UNFLAV, STRB, GRAPE), n= 5,5,7).
Supplementary Figure 7. Preference for a nicotine bottle generally increases as the concentration of nicotine in the bottle is reduced. All mice established baseline nicotine preference (%) with an unflavored nicotine solution containing 0.100 mg/ml nicotine during a “Baseline Nicotine Consumption” phase. The concentration of nicotine was sequentially reduced (0.075, 0.050, 0.025, 0.000) following 8 days of drinking at each concentration. Bar graphs represent summary data (mean +/− SEM) and points represent the nicotine preferences of individual mice. Data is arranged so that mice who had access to only unflavored nicotine in adolescence (i.e. UNLFAV group) are on the left (panels A, C, E, G, I) and mice who had access to strawberry flavored nicotine in adolescence are on the right (panels B,D,F, H, J). A-B) Mice were assigned to a flavor for the “Nicotine Fading and Flavor Re-introduction” phase such that there were no differences in the nicotine preference established between groups with an unflavored 0.100 mg/ml nicotine solution. Preferences were generally <50% at this concentration. C-D) Once the concentration of nicotine was reduced to 0.075 mg/ml, the preference for nicotine generally increased. Additionally, mice in Unflavored adolescent treatment group showed a preference for fruit-flavored nicotine solutions compared to an unflavored nicotine solution (Two-way ANOVA, Fsex(1,29) = 0.360, p=0.553, Fflavor.(2, 29) = 6.761, **p=0.004, Finteraction(2,29) =0.502, p=0.611, Sidak’s multiple comparisons tests). E-F) Preferences for flavored nicotine solutions remained similar when the nicotine concentration was reduced to 0.05 mg/ml nicotine. Mice also had the highest preference for unflavored nicotine at this concentration of nicotine. G-H) At a concentration of 0.025 mg/ml nicotine mice that had access to strawberry flavored nicotine in adolescence (i.e. adolescent CHOICE mice) maintained similar preferences for nicotine solutions in adulthood, but a preference for flavored nicotine was re-established in mice that only had access to unflavored nicotine in adolescence (i.e., adolescent UNFLAV mice) (Two-way ANOVA, Fsex(1,29) = 0.517, p=0.478, Fflavor.(2, 29) = 10.950, ***p=0.0003, Finteraction(2,29) =1.248, p=0.302, Sidak’s multiple comparisons tests). I-J) When nicotine was completely removed from the bottle flavor did not affect preference for the nicotine bottle in any group of mice; however, in mice who only had access to unflavored nicotine in adolescence, females had a higher preference for the nicotine bottle across all flavors (Two-way ANOVA, Fsex(1,29) = 7.222, *p=0.012, Fflavor.(2, 29) = 0.535, p=0.591, Finteraction(2,29) =1.182, p=0.321). *P < 0.05. **P < 0.01, ***P <0.001 for all comparisons. (Unflavored nicotine in adolescence: males (UNFLAV, STRB, GRAPE), n= 6,5,7, females(UNFLAV, STRB, GRAPE), n= 6,6,5; Access to strawberry nicotine in adolescence: males (UNFLAV, STRB, GRAPE), n= 5,6,6, females(UNFLAV, STRB, GRAPE), n= 5,5,7).


