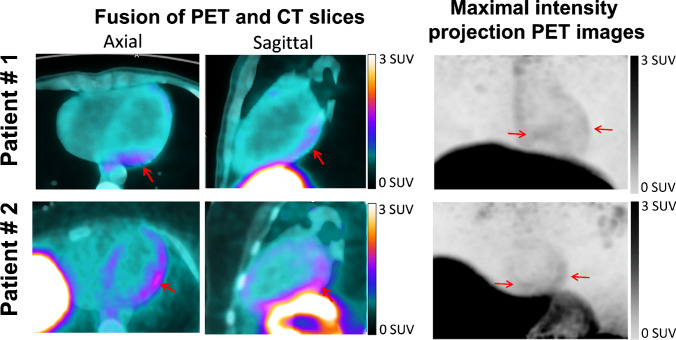
Acute myocarditis was recently reported after mRNA COVID-19 vaccination. Here we present images from two male (18 and 21 years old) patients that were recorded with a digital-PET/CT system (Vereos, Philips) 1 h after the injection of 2 MBq/kg of 68 Ga-DOTATOC, as part of an ongoing clinical study (NCT03347760 on ClinicalTrials.gov). Both patients experienced myocarditis 2 to 3 days after the second dose of an mRNA COVID-19 vaccine (Moderna and Pfizer, respectively) and fulfilled the cardiovascular magnetic resonance 2018 Lake Louise criteria for myocarditis, associated with increased plasma troponin (peak troponin: 771 ng/L and 10 830 ng/L) but normal plasma fibrinogen. Plasma C-reactive protein was increased in the 21-year-old patient (41 mg/L).
The DOTATOC-PET images, recorded at 1 to 3 days from peak troponin, showed an increase in myocardial uptake relative to blood activity, predominantly in the lateral and inferior walls (red arrows) and which are even better depicted on the gated-PET cine-loops in the online supplement. Myocardial/blood SUVmax ratio was > 2.2 in both cases and, thus, higher than what we commonly observe in non-myocarditis patients. This likely reflects a myocardial infiltrate of inflammatory cells overexpressing somatostatin receptors (lymphocytes, macrophages, activated monocytes) [1–4], presumably within specific antigenic sites.
Supplementary Information
Below is the link to the electronic supplementary material.
Data availability
All data are available on request.
Declarations
Informed consent
A written informed consent for the procedure and for the publication of images was obtained from all participants.
Conflict of interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Image of the month
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lichtenauer-Kaligis EG, Dalm VA, Oomen SP, Mooij DM, van Hagen PM, Lamberts SW, et al. Differential expression of somatostatin receptor subtypes in human peripheral blood mononuclear cell subsets. Eur J Endocrinol. 2004;150(4):565–577. doi: 10.1530/eje.0.1500565. [DOI] [PubMed] [Google Scholar]
- 2.Lapa C, Reiter T, Li X, Werner RA, Samnick S, Jahns R, et al. Imaging of myocardial inflammation with somatostatin receptor based PET/CT - a comparison to cardiac MRI. Int J Cardiol. 2015;194:44–49. doi: 10.1016/j.ijcard.2015.05.073. [DOI] [PubMed] [Google Scholar]
- 3.Pizarro C, Kluenker F, Dabir D, Thomas D, Gaertner FC, Essler M, et al. Cardiovascular magnetic resonance imaging and clinical performance of somatostatin receptor positron emission tomography in cardiac sarcoidosis. ESC Heart Fail. 2018;5:249–261. doi: 10.1002/ehf2.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amini A, Dehdar F, Jafari E, Gholamrezanezhad A, Assadi M. Somatostatin receptor scintigraphy in a patient with myocarditis. Mol Imaging Radionucl Ther. 2021;30:50–53. doi: 10.4274/mirt.galenos.2019.03164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available on request.