Abstract
Vine tea has been used as an herbal tea by several ethnic minorities for hundreds of years in China. Flavonoids, a kind of indispensable component in a variety of nutraceutical, pharmaceutical and cosmetic applications, are identified to be the major metabolites and bioactive ingredients in vine tea. Interestingly, vine tea exhibits a wide range of significant bioactivities including anti-oxidant, anti-inflammatory, anti-tumor, antidiabetic, neuroprotective and other activities, but no toxicity. These bioactivities, to some extent, enrich the understanding about the role of vine tea in disease prevention and therapy. The health benefits of vine tea, particularly dihydromyricetin and myricetin, are widely investigated. However, there is currently no comprehensive review available on vine tea. Therefore, this report summarizes the most recent studies investigating bioactive constituents, pharmacological effects and possible mechanisms of vine tea, which will provide a better understanding about the health benefits and preclinical assessment of novel application of vine tea.
Keywords: Vine tea, Flavonoids, Dihydromyricetin, Myricetin, Pharmacological effects, Molecular mechanism
Graphical abstract
Highlights
-
•
Vine tea has been consumed as a folk herbal tea for hundreds of years.
-
•
Flavonoids were the major bioactive ingredients in vine tea.
-
•
Dihydromyricetin and myricetin are major bioactives in vine tea.
-
•
Vine tea exhibits a wide range of significant bioactivities.
-
•
Vine tea may be a potential, functional herbal tea.
1. Introduction
Tea culture has been carried forward in China for thousands of years. Teas are broadly divided into green tea, oolong tea and black tea, i.e., nonfermented, semifermented and fermented as a result of their diverse manufacturing processes [1]. Since ancient times, many herbs have always been used to prepare tea-like beverages locally capable of improving health or inhibiting all kinds of diseases in various regions of China on the basis of Camellia sinensis [2,3]. These herbs, so called non-Camellia tea varieties, such as vine tea and bitter tea, form a sub-culture in the Chinese tea culture [4].
The tender stems and leaves of Ampelopsis grossedentata, named vine tea or Mao Yan Mei, have traditionally been used as a health tea and folk herbal medicine by Tujia, Yao and other ethnic minorities in China [5]. According to the records in the Chinese Materia, vine tea exhibits a wide variety of beneficial properties, such as clearing away heat, detoxification, diuresis, activating blood circulation and dissipating blood stasis. In addition, it was traditionally used as a folk medicine by most local people to treat pyretic fever and cough, stab wounds, bruises, jaundice hepatitis and pain in pharynx and larynx [6]. A study reported that high levels of flavonoids [7], especially dihydromyricetin (DMY, also known as ampelopsin), myricetin and myricitrin [8], are present in vine tea. It is well known that flavonoids possess many special functions and are one of the most potent nutraceuticals in food and phytopharmaceutical products [9].
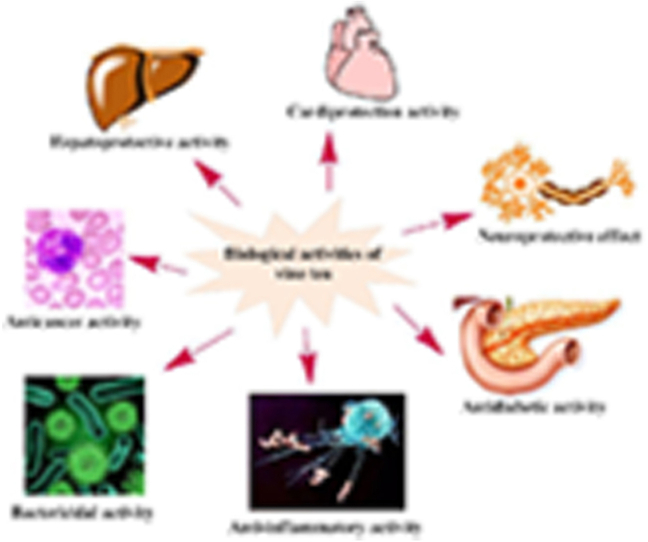
Modern investigations indicate that vine tea exhibits a number of beneficial pharmacological properties (Fig. 1), such as hypoglycemic [10,11], antioxidant [5,12,13], anti-thrombosis function [14], anti-tumor [15,16], anti-inflammatory [10,17] and antibacterial activities [18], but no toxicity [19]. In addition, the main pharmacological effects of vine tea are similar to those of Camellia sinensis, i.e., anti-inflammatory, antioxidant, anticancer, antidiabetic, anti-obesity, cardioprotective activity and other activities [[20], [21], [22]]. With the Chinese and English words of “vine tea”, “Ampelopsis grossedentata”, “dihydromyricetin”, “myricetin”, “flavonoids”, “extraction”, “separation”, “purification” and “pharmacological activity” as keywords, we combined and searched the relevant literatures published in PubMed, Web of Science, ScienceDirect, China National Knowledge Internet (CNKI), Wanfang Data and Weipu from 1990 to 2019. The advancements in the study design in investigating bioactive constituents, pharmacological effects and possible mechanisms of vine tea are summarized in this paper, which will provide a better understanding about the health benefits of vine tea.
Fig. 1.
Different pharmacological effects of vine tea.
2. Chemical constituents
2.1. Flavonoids
It is well known that flavonoids have many peculiar effects and have usually been used as nutraceuticals because they are widely found in many foods and herbs [23]. Flavonoids have the same basic C6–C3–C6 structure. According to whether there is a carbonyl group at C4 and whether there is a double bond at C2–C3, flavonoids can be segmented into different sub-groups: flavones, flavonols, flavanols, flavanones, isoflavones, anthocyanins, chalcone and aurones [24].
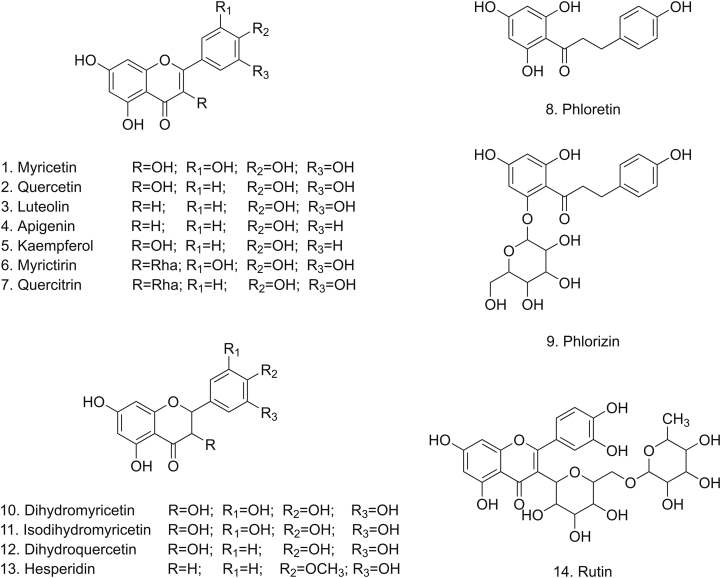
Vine tea is a flavonoid-rich wild plant. It has been reported that more than 20 different flavonoids have been isolated and identified from vine tea. Fig. 2 presents the structures of some common flavonoids in vine tea. Table 1 lists the flavonoids in vine tea [6,[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]]. Moreover, dihydromyricetin (DMY) (up to 30%, m/m), myricetin and myricitrin are found to be the most abundant and bioactive flavonoids in vine tea [41,42].
Fig. 2.
Structures of some common flavonoids in vine tea.
Table 1.
The flavonoids in vine tea.
| No. | Compound name | Sub-class | Refs. |
|---|---|---|---|
| 1 | Quercetin-3-O-β-D-glucoside | Flavonol | [25] |
| 2 | Apiin | Flavone | [25] |
| 3 | Dihydromyricetin | Flavanol | [6,[25], [26], [27], [28], [29], [30], [31], [32], [33]] |
| 4 | Dihydroquercetin | Flavanol | [[25], [26], [27],33] |
| 5 | Myricetin-3-O-β-D-glucoside | Flavonol | [29,33] |
| 6 | 3-dihydroxyquercetin | Flavonol | [33] |
| 7 | Quercetin-3-O-β-D-xyloside | Flavonol | [33] |
| 8 | Kaempferol-3-O-α-L-rhamnoside | Flavonol | [33] |
| 9 | Phloretin | Chalcone | [33] |
| 10 | Phloridzin | Chalcone | [33] |
| 11 | Quercetin-3-O-α-L-rhamnoside | Flavonol | [26,27,33,34] |
| 12 | Quercetin | Flavonol | [25,34] |
| 13 | Quercetin-3-galactoside | Flavonol | [34] |
| 14 | Luteolin | Flavone | [34] |
| 15 | Vitexin | Flavanone | [34] |
| 16 | Vitexin-2′-O-rhamnoside | Flavanone | [34] |
| 17 | Isodihydromyricetin | Flavanol | [26,33,35] |
| 18 | Myricetin | Flavonol | [25,26,[28], [29], [30],32,33,35,36] |
| 19 | Myricetin-3-O-α-L-rhamnoside | Flavonol | [[26], [27], [28], [29], [30],32,33,37] |
| 20 | Myricetin-3-O-β-D-galactopyranoside | Flavonol | [37] |
| 21 | Apigenin | Flavone | [34,38] |
| 22 | Hesperidin | Flavanone | [33,38] |
| 23 | Kaempferol | Flavonol | [33,34,38] |
| 24 | Rutin | Flavonol | [34,39] |
| 25 | 6,7-dihydroxy-3′-methoxy-4′,5′-methylenedioxy isoflavone | Isoflavone | [40] |
| 26 | 6,7-dihydroxy-3′-methoxy-4′,5′-methylenedioxy isoflavone 6-O-β-D-glucopyranoside | Isoflavone | [40] |
| 27 | 6,7-dihydroxy-3′-methoxy-4′,5′-methylenedioxy isoflavone 6-O-α-L-rhamnopyranoside | Isoflavone | [40] |
| 28 | 6,7-dihydroxy-3′-methoxy-4′,5′-methylenedioxy isoflavone 6-O-β-D-xylopyranosyl-(1–6)-β-D-glucopyranoside | Isoflavone | [40] |
2.2. Polysaccharides
Several studies have shown that plant polysaccharides, especially water-soluble polysaccharides extracted from Chinese medicinal materials, such as ginkgo polysaccharide, have anti-virus, anti-aging, stimulated hematopoiesis, immune regulation, antitumor and other biological effects [43,44].
It has been suggested that vine tea contains water-soluble polysaccharides. These polysaccharides such as Ampelopsis grossedentata polysaccharides obtained from leaves (ALPS) and Ampelopsis grossedentata polysaccharides obtained from stems (ASPS) are acid protein-bound heteropolysaccharides and have strong antioxidant and α-glucosidase inhibitory activity in vitro [45].
2.3. Organic acids, steroids and others
Vine tea contains organic acids and steroids. According to the references, gallic acid, palmitic acid, oleanolic acid and sitosterol have been isolated and identified in vine tea [25,32,38,39]. Studies revealed that vine tea also possesses many aroma components, which account for 74% of all essential oils. The main aroma components contain trans-2-hexenal, phenylacetaldehyde, methyl salicylate, geraniol, azelone, cis-jasmonone, cedar alcohol and so on [46].
3. Biological activities of vine tea
3.1. Antioxidant activity
It is commonly acknowledged that the antioxidant properties of vine tea, particularly DMY and myricetin, have been extensively reported. The antioxidant effect of vine tea and its possible mechanisms are summarized in Table 2 [[26], [33], [47], [48], [49], [50], [51]]. In studies by Ma et al. [26] and Gao et al. [33], the ethyl acetate and n-butanol fraction of vine tea extract showed high 1,1-diphenyl-2-picrylhydrazyl radical (DPPH・) scavenging activities in comparison to the ascorbic acid (VC) group. The polysaccharides (ALPS and ASPS) showed effective DPPH・ scavenging ability. Moreover, according to in vitro evaluation, ASPS has a stronger antioxidant activity potential thanALPS [45].
Table 2.
Antioxidant activity of vine tea and its main flavonoids (dihydromyricetin and myricetin).
| In vitro | In vivo | Compound | Observed effects | Refs. |
|---|---|---|---|---|
| scavenging assay | Vine tea extract | scavenging activity | [26,33] | |
| scavenging assay; | Piglets | Dihydromyricetin | , ABTS・+ and O2・–scavenging activities; | [47] |
| ABTS・+ scavenging assay; | H2O2 scavenging activity; | |||
| FRAP assay; | Ferric reducing ability; | |||
| H2O2 scavenging activity; | ↑SOD activity; | |||
| O2・– scavenging activity; | ↓Lipid peroxidation; | |||
| Measurement of erythrocyte SOD activity and lipidperoxidation level | ↓MDA | |||
| ABTS・+ and scavenging assay | Myricetin | and ABTS・+ scavenging activities | [48] | |
| scavenging assay | Myricetin | scavenging activities | [49] | |
| Chinese hamster lung fibroblasts (V79-4) cells | Myricetin | scavenging activity; | [50] | |
| ↓ROS; ↑Activities of SOD, CAT and GPx; | ||||
| ↑PI3K/Akt activation; | ||||
| ↑MAPK activation; | ||||
| ↓H2A.X expression; Preventing the DNA damage; | ||||
| ↓Lipid peroxidation | ||||
| HepG2 cells | Myricetin | ↑Nrf2 activation | [51] |
: 1,1-diphenyl-2-picrylhydrazyl radical; ABTS·+: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt radical cation; FRAP: Ferric-reducing antioxidant power; SOD: Superoxide dismutase; ROS: Reactive oxygen species; MDA: Malondialdehyde; GPx: Glutathione peroxidase; CAT: Catalase; Nrf2: Nuclear factor E2-related factor 2; MAPK: Mitogen-activated protein kinase; PI3K/Akt: Phosphatidylinositol 3-kinase/protein kinase B; H2A.X: Nuclear phosphorylation histone.
Hou et al. [47] reported that DMY shows effective DPPH・, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt radical cation (ABTS・+) and O2・–radical scavenging activities. In addition, DMY has the ability to increase the activity of total superoxide dismutase and to decrease the level of lipid peroxidation. Moreover, DMY can also decrease the levels of malondialdehyde in LPS-treated piglets. The studies revealed that DMY has the ability to decrease lipopolysaccharide (LPS)-induced oxidative stress, which may be attributed to its reactive oxygen species scavenging activities.
Myricetin can scavenge many radicals and ions. Examples of such radicals include ABTS・+ and DPPH・ [48]. However, it cannot scavenge reactive oxygen species (ROS) in menadione-stressed modeling [49]. Moreover, myricetin protects cells against H2O2-induced cell damage via inhibition of ROS generation and activation of antioxidant enzymes [50]. A study by Qin et al. [51] reported that the antioxidant effect of myricetin on hepatic human hepatocellular carcinomas (HepG2) cells is related to the activation of the nuclear factor E2-related factor 2 (Nrf2)-induced antioxidant reaction element. Altogether, vine tea might be developed as a novel antioxidant agent.
3.2. Anti-inflammatory activity
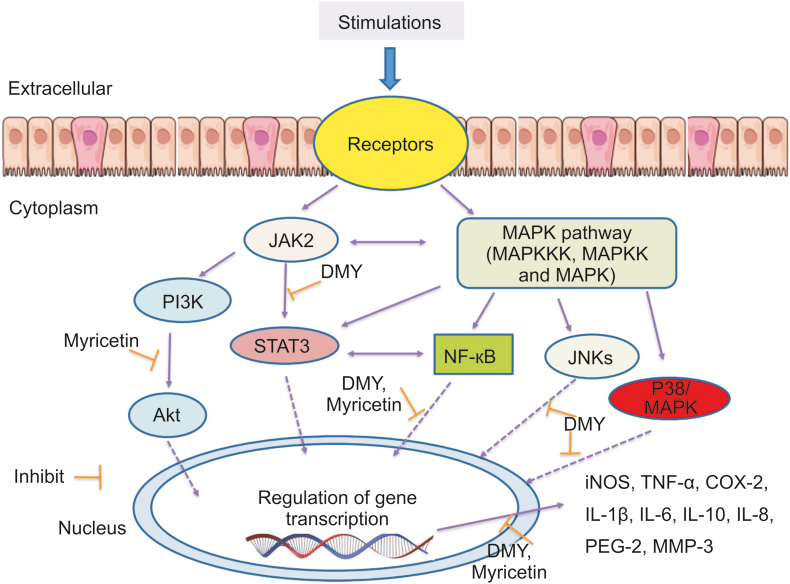
Studies have determined that vine tea and its main flavonoids are potent anti-inflammatory agents. Signaling pathways involved in anti-inflammatory mechanisms of vine tea are shown in Fig. 3. A study reported that Ampelopsis grossedentata can alleviate ulcerative colitis via the interleukin-1 receptor-associated kinase 1/TNF receptor associated factor 6/nuclear factor-κB (IRAK1/TRAF6/NF-κB) signaling pathways [52].
Fig. 3.
Signaling pathways involved in anti-inflammatory mechanisms of vine tea.
Hou et al. [53] demonstrated that DMY downregulates the levels of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6) in LPS-induced mouse mononuclear macrophage leukemic cells (RAW 264.7) macrophages. Similar results showed that DMY increases the content of interleukin-10 (IL-10) in LPS-induced mice. In addition, it also attenuates the protein expression of inducible nitric oxide synthase (iNOS), TNF-α, and cyclooxygenase-2 (COX-2) in macrophages. Moreover, DMY inhibits the activation of NF-κB, NF-κB alpha (IκBα), p38 mitogen-activated protein kinase (p38) and c-Jun NH2-terminal kinase (JNK) signaling pathways. The occurrence of Alzheimer disease (AD) and Parkinson's disease (PD) may be attributed to the microglia-induced neuroinflammation. The mechanism experiment indicated that the NF-κB and janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) pathways play the main role in the suppression of neuroinflammation of DMY [54]. DMY could alleviate inflammation in collagen induced arthritis (CIA) rats and attenuate IL-1β–induced activities in fibroblast-like synoviocytes through suppression of NF-κB signaling [55].
Several researches have demonstrated that myricetin has anti-inflammatory activity [50]. Myricetin may be an effective agent for the therapy of periodontitis by inhibiting the production of IL-6, interleukin-8 (IL-8) and matrix metalloproteinase-3 (MMP-3) [56]. Moreover, myricetin alleviates periodontitis by activating extracellular signal-regulated kinases-1/2 (ERK-1/2), Akt and p38, inhibiting COX-2 activation in human gingival fibroblasts, as well as inhibiting the degradation of IκB and the synthesis and expression of prostaglandin E2 (PGE2) [57]. Similar results showed that myricetin can decrease the levels of NO and PGE2 as well as the expression of iNOS and COX-2 in RAW 264.7 macrophages [58]. In addition, myricetin suppresses the expression of phorbol ester-induced COX-2 via NF-κB signaling pathway [59]. Moreover, myricetin has anti-rheumatoid arthritis activity via JNK and p38 mitogen-activated protein kinase (MAPK) signaling pathways in human synovial sarcoma (SW982) cells [60]. The results indicated that myricitrin possesses activity against acute colitis by inhibiting the over-expression of inflammatory cytokines via phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway in mice [61]. These results revealed that vine tea has a great potential to be an anti-inflammatory drug.
3.3. Antidiabetic activity
Several researches have revealed that vine tea may have the ability to improve glucose and lipid homeostasis and increase the insulin sensitivity. The antidiabetic activity of vine tea and its possible mechanisms are summarized in Table 3 [[30], [62], [63], [64], [65], [66], [67], [68], [69], [70]]. α-glucosidase plays a critical role in the degradation of carbohydrates. Chen et al. [30] investigated the α-glucosidase inhibitory activity and hypoglycemic activity of three major components (myricetin, DMY and myricitrin) from Ampelopsis grossedentata as well as six new flavonoid derivatives. The results showed that myricetin analogs exert effective α-glucosidase inhibition in vitro and in streptozotocin (STZ)-induced diabetic mice. In an experiment by Wan et al. [62], the results showed that vine tea regulates glucose and lipid metabolism, increases the insulin sensitivity and improves hepatic lipid accumulation in high-fat diet (HFD)-induced rats. Moreover, the mechanism may be related to the improvement of energy-related metabolism and the decrease of lipid accumulation. Ran et al. [63] reported that Ampelopsis grossedentata is helpful in ameliorating the glucose levels in patients with type 2 diabetes. Wang et al. [45] investigated the α-glucosidase inhibition of two polysaccharides isolated from vine tea. The results exhibited that the two polysaccharides have a stronger α-glucosidase inhibitory potential.
Table 3.
Antidiabetic activity of vine tea and its main flavonoids (dihydromyricetin and myricetin).
| In vitro | In vivo | Compound | Observed effects | Refs. |
|---|---|---|---|---|
| α-glucosidase inhibition assay | Myricetin, Dihydromyricetin | α-glucosidase inhibitory activity | [30] | |
| High-fat diet (HFD)-induced rats | Vine tea extract | Regulation of glucose and lipid metabolism; | [62] | |
| Regulation of tricarboxylic acid (TCA)cycle, amino acid metabolism and purine metabolism | ||||
| ↑Insulin sensitivity; | ||||
| Improvement of hepatic lipid accumulation; | ||||
| Patients with type 2 diabetes mellitus | Vine tea | ↓Fasting plasma glucose(FPG); | [63] | |
| ↓Glycated albumin(GA); | ||||
| ↓Retinol binding protein-4(RBP4); | ||||
| ↓Cystatin C | ||||
| HepG2 cells | Male Sprague-Dawley(SD) rats | Dihydromyricetin | Improvement of serum glucose and lipid homeostasis; | [64] |
| ↑Insulin sensitivity; | ||||
| Regulation of TCA cycle; | ||||
| ↑Glucose uptake; | ||||
| Improvement of the translocation of glucose transporter 1; | ||||
| ↑Phosphorylation of AMP-activated protein kinase (AMPK); | ||||
| ↑Glycogen synthase-3β (GSK-3β) phosphorylation; | ||||
| ↑Phosphorylation of protein kinase B (Akt2) Ser474 and insulin receptor substrate-1 (IRS-1) Ser612 | ||||
| Mouse skeletal muscle C2C12 myoblast cells | Male 129/SvJ (wild type, WT) and SIRT3−/− 129/SvJ mice | Dihydromyricetin | Improvement of insulin sensitivity; | [65] |
| ↓Autophagy; | ||||
| Regulation of Sirt3 functions; | ||||
| Activation of the AMP-activated protein kinase (AMPK)- peroxisome proliferator-activated receptor coactivator-1α (PGC-1a) signaling pathways; | ||||
| Improvement of insulin resistance | ||||
| Streptozotocin (STZ) induced C57BL/6J mice | Dihydromyricetin | Improvement of cardiac function in STZ induced diabetic mice; | [66] | |
| ↓Oxidative stress; | ||||
| ↓Levels of inflammation factors (Interleukin-6, Tumor necrosis factor-α); | ||||
| Improvement of mitochondrial function | ||||
| Male Wistar rats | Myricetin | ↓Serum glucose levels; | [67] | |
| ↑Hepatic glycogen synthesis; | ||||
| ↑Absorption and utilization of glucose in peripheral tissues; | ||||
| Regulation of glucose metabolism | ||||
| E. coli cells | Myricetin | Inhibition of aggregation of islet amyloid polypeptide (IAPP); ↓Death of pancreatic β-islet cells |
[68] | |
| α-glucosidase inhibition assay | Male Sprague-Dawley rats | Myricetin | Inhibition of α-glucosidase activity; Improvement of postprandial hyperglycemia; Alleviation of fasting hyperglycemia |
[69] |
| Lean (Fa/Fa or Fa/fa) and obese (Fa/Fa) Strains of Zucker rats | Myricetin | ↓Plasma glucose levels; | [70] | |
| Improvement of insulin resistance; | ||||
| ↑Expression of glucose transporter subtype 4 (GLUT4); | ||||
| ↑The protein and phosphorylation of insulin receptor substrate-1(IRS-1); | ||||
| ↑Activation of phosphatidylinositol 3-kinase (PI3K) |
In a study by Le et al. [64], the results indicated that DMY improves insulin resistance in HFD-treated rats. Furthermore, DMY improves insulin resistance, which is related to the activation of the AMP-activated protein kinase (AMPK) signaling pathway to increase glucose uptake. In addition, this activity of DMY is also related to the reduction of the glucose production via the insulin receptor substrates (IRS)/PI3K/Akt pathways. Insulin resistance experiment revealed that DMY can improve the insulin sensitivity in skeletal muscle cells in diabetic patients. Moreover, the autophagy enhancement is good for increasing the insulin sensitivity by DMY. Besides, the protective effect may be related to the activation of AMPK-peroxisome proliferator-activated receptor coactivator-1α (PGC-1α)-sirtuin-3 (SIRT3) [65]. Wu et al. [66] reported that DMY has a significant effect on cardiac function and pathological changes in the myocardium in streptozotocin-induced model, and AMPK signaling pathway plays a main role in the protective effect on diabetic cardiomyopathy.
Studies verified the regulatory effect of myricetin on type 2 diabetes by increasing the absorption and utilization of glucose in peripheral tissues. Furthermore, results demonstrated that myricetin has the potential in the alleviation in hyperglycemia and promotion of synthesis of liver glycogen without any serious hepatotoxicity in streptozotocin-induced diabetic rats [67]. In addition, the activity of myricetin is related to its improvement of glycogen metabolism and inhibition of aggregation of islet amyloid polypeptide (IAPP) [68]. Kang et al. [69] found that myricetin can also have a pharmacological effect on glucose metabolism and insulin sensitivity, which is probably related to enhancing the production of β-endorphin and improving the phosphorylation of insulin receptor. A further study by Liu et al. [70] exhibited that myricetin plays a significant effect in regulating insulin resistance by enhancing the post-receptor insulin signaling in experimental rats. These results indicated that vine tea has a great potential to be an effective drug for diabetes.
3.4. Neuroprotective effect
Growing evidence has shown that myricetin has a regulatory effect on the development of neurodegenerative diseases, such as PD and AD. Many researches reported that myricetin can mitigate neurodegenerative diseases by blocking the hyperphosphorylation of tau proteins and interfering with the formation of β-amyloid fibril [71].
The high levels of glutamate can lead to brain disorders. Myricetin has the ability to decrease the content of glutamate from cerebrocortical synaptosomes by blocking voltage-dependent Ca2+ channel [72]. Further evidence showed that myricetin inhibits the production of α-synuclein (αS) fibrils and suppresses the αS to change into oligomers, the vital mechanisms for the development of PD [73]. Myricetin can significantly block the activation of catechol O-methyltransferase (COMT), a key enzyme for the metabolism of levodopa in vitro [74]. Moreover, Moonrungsee et al. [75] demonstrated that myricetin has the ability to reduce tyramine oxidase, which is related to the inactivation of neurotransmitters. In addition, it was found to block the expression of ROS and the peroxidation of lipid, prevent glutathione (GSH) depletion, and promote γ-aminobutyric acid (GABA) activity in the neurons [76].
As is known to all, the increase of the level of microRNAs (miRNAs) is related to the aging-related diseases. Devastating neurodegeneration and cognitive dysfunction are the main characteristics of degenerative diseases. DMY adjusts cognitive dysfunction and mitigates neuron necrosis in the brain aging model of rats. Moreover, it can up-regulate sirtuin 1 (SIRT1) and down-regulate p53/p21 [77]. A growing body of evidence suggested that DMY has the ability to attenuate the activity of COMT [78]. Further experiments found that DMY is related to restoring the behavioral damage, protecting DA neurons, and reducing ROS production after dopaminergic neurotoxic compound stimulation [74]. Major depressive disorder (MDD) is a very common disease. A study revealed that DMY-mitigated MDD is attributed to the elevated brain derived neurotrophic factor (BDNF) signaling pathway [79]. Further experiments found that DMY can prevent neurodegeneration and enhance hypoxia induced cognitive function [80]. The above results indicate that DMY may be an effectively agent for the improvement of neurodegenerative diseases.
3.5. Anticancer activity
A number of researches have reported the prevention of vine tea flavonoids on cancer. Studies confirmed that DMY can promote mitochondria-induced apoptosis in HepG2 cells by down-regulating Akt/Bax and B-cell lymphoma-2 (Bcl-2) associated death promoter (Bad) pathway and inactivating the mammalian target of rapamycin (mTOR) via ERK1/2, AMPK and PI3K/Akt pathways [81]. In a study, the results showed that DMY might be an effective agent for non-small cell lung cancer (NSCLC) through increasing intracellular peroxide and sustaining the expression of ERK1/2 and JNK1/2 [82]. Another experiment investigated that DMY prevents osteosarcoma, which may be related to suppressing the activation of glycogen synthase-3β (GSK3β) through the activation of AMPKα and p38 signaling pathway [83]. Further experiment revealed that DMY prevents osteosarcoma by inhibiting the expressions of caspase and Bcl-2 [84]. Xu et al. [85] confirmed that DMY might be an effective therapeutic medication for ovarian cancer through inducing cell apoptosis by p53-induced surviving down-regulation. In addition, the ability to treat gastric cancer of DMY may be related to inhibiting proliferation, inducing cell cytotoxicity and promoting apoptosis in human gastric cancer cells [86]. Zhou et al. [87] reported that DMY might be a new therapeutic drug for breast cancer. The results showed that DMY can inhibit cell viability and induce apoptosis in breast cancer cells (MCF-7 and MDA-MB-231 cells) without cytotoxicity in human normal breast epithelial cells (MCF-10A cells) through ROS generation and endoplasmic reticulum (ER) stress pathway. Extensive research indicated that myricetin possesses an anti-proliferative activity against human cancer cell lines, such as hepatic, skin, pancreatic and colon cancer cells, as well as suppresses key enzymes related to the development of cancer. Previous studies showed that myricetin induces the apoptosis of human chronic and acute leukemia cells [88]. Further experiment exhibited that myricetin also inhibits the ability of topoisomerase (topo) I and topo II in K562 cells. Moreover, this activity may be attributed to the hydroxy and carbonyl groups in this compound [89]. Regulating the activity of mitogen-activated protein kinase (MEK), janus kinase 1 (JAK1), Akt and mitogen-activated protein kinase 4 (MKK4) is a prerequisite for anticancer activities of myricetin [90]. Besides, a study reported that myricetin can attenuate STAT3 activity and reduce the activation of STAT3 at tyrosine 705 (Tyr705) and serine 727 (Ser727) [91]. In vitro, myricetin has a significant effect on human laryngeal carcinoma HEp2 cells and prostate cancer PC-3 cells [92]. A mechanism-based study revealed that myricetin attenuates the phosphorylation of Akt and promotes the phosphorylation of p38 MAPK in HepG2 cells [93]. In addition, myricetin was also found to possess anti-proliferative activity against medulloblastoma and lung carcinoma [94].
3.6. Antihypertensive activity
The in vivo and in vitro experiments demonstrated that myricetin possesses an antihypertensive activity, which may be closely related to the decrease of hypertension and oxidative stress. Similarly, studies also revealed that this compound has the capability to modify systolic blood pressure, vascular reactivity and heart rate [95].
3.7. Hepatoprotective activity
A growing body of evidence suggests that vine tea might possess a hepatoprotective activity. The inhibition of apoptosis and adjustment of autophagy-related genes are possibly the main mechanisms for this activity of DMY [96]. Qiu et al. [97] verified that DMY has the ability to improve lipid metabolism and decrease inflammatory cytokines in alcohol-induced liver injury mice. Furthermore, DMY could also increase the level of GSH and decrease the level of malondialdehyde (MDA) in the liver via NF-κB signaling pathway. Studies revealed that DMY has a regulatory effect on nonalcoholic fatty liver disease (NAFLD) by improving lipid metabolism and decreasing oxidative stress. In addition, mechanism research indicated that it can effectively increase the level of SIRT3 via AMPK/PGC-1α/estrogen-related receptor-α (ERRα) signaling pathway [98]. Furthermore, previous studies have suggested that the hepatoprotective function of DMY may be related to the antioxidant activity [99]. Moreover, growing clinical trials proved the effect of DMY on NAFLD [100].
It was found that myricetin exerts hepatoprotective effects through decreasing the level of serum enzymes and total bilirubin as well as attenuating DNA damage in the liver [101]. Moreover, myricetin can lessen liver uric acid levels and attenuate liver xanthine oxidase activity in potassium oxonate-induced hyperuricemic mice [102].
3.8. Activity against cardiovascular diseases
Inflammation is a critical mechanism in atherosclerosis. DMY possesses the ability to decrease the release of interleukin-1b (IL-1b), inhibit caspase-1 cleavage, and suppress ROS generation during atherosclerosis [103]. Zeng et al. [104] revealed that DMY improves lipid metabolism probably as an effective agent for atherosclerosis. Furthermore, ATP-binding cassette transporter A1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1) are related to these activities. Several studies revealed that DMY has an anti-atherosclerotic function through many mechanisms. It can ameliorate hyperlipidemia and decrease the levels of inflammatory cytokines as well as inhibit the expression of NF-κB [105].
The cardioprotective effect of DMY is mainly attributed to its antioxidant activity and apoptosis inhibition in vivo and in vitro [106]. One experiment exhibited that DMY has the ability to attenuate the toxicity of adriamycin on the heart [107]. DMY can treat arrhythmia by inhibiting sodium currents and enhancing calcium current [108]. Furthermore, several studies revealed that DMY might be an effective agent to improve myocardial remodeling and pulmonary hypertension [109]. Altogether, the above data indicate that DMY may be an effective agent for the improvement of cardiovascular diseases.
Studies revealed that myricetin exerts protective activity for cardiomyocytes by inhibiting the voltage-dependent Ca2+ channel [110]. Myricetin can reduce the apoptosis of neonatal cardiomyocytes under hypoxic conditions [111]. Tiwari et al. [112] reported that myricetin possesses the ability to reduce heart rate and change vascular reactivity induced by isoproterenol. In addition, myricetin exerts lipid lowering activity and effective function in hyperlipidemia and related cardiovascular diseases [113].
3.9. Other activities
In addition to the above-mentioned activities, vine tea also has bactericidal activity, anti-rheumatoid arthritis and anti-osteoporosis activity, immunomodulatory activity, anti-allergic activity, anti-asthma and other activities. DMY exerts bactericidal activity by disrupting integrity and fluidity of membrane and interacting with intracellular DNA in Staphylococcus aureus [114]. Several studies revealed that DMY may be beneficial to the prevention of osteoclast-related diseases through multiple pathways [115].
Myricetin can ameliorate physiological disorders through the improvement of the release of melatonin [116]. Moreover, several studies exhibited that myricetin has an effectively antiphotoaging function by inhibiting free radicals in the skin [117]. Myricetin exerts immunosuppressive effects by promoting antibody production or suppressing the activity of white blood cells (WBCs) [118]. Moreover, results indicated that myricetin possesses an anti-allergic activity [119]. Furthermore, Hagenacker et al. [120] reported that myricetin exerts an effective analgesic action in a neuropathic pain model. Besides, several researches showed that myricetin possesses antibacterial and antiviral activities through different organisms [121,122]. In addition, previous studies revealed that myricetin ameliorates the development of cataract [123].
4. Conclusions and future perspectives
Ampelopsis grossedentata has been used as healthy tea, beverage and herbal medicine for hundreds of years, and flavonoids are identified to be the main bioactive ingredient in it. Apart from DMY and myricetin, the most abundant flavonoids in vine tea, it also contains quercetin, kaempferol, hesperidin, apigenin, rutin and other common flavonoids. It is worth noting that quercetin, kaempferol and rutin also exhibit a wide range of significant bioactivities including anti-oxidant, anti-inflammatory, anti-tumor, antidiabetic, and neuroprotective effects [124]. Therefore, vine tea, a functional nutraceutical, possesses a variety of biological activities and highly health promoting properties.
Although researchers have investigated the various bioactivities of vine tea, so far, the specific mechanism of vine tea remains unclear, which may be attributed to the anti-oxidation, anti-inflammation, anti-apoptosis and other pathways. As we all know, most common flavonoids usually have low oral bioavailability [125]. However, vine tea possesses various pharmacological activities. Therefore, extensive knowledge of the absorption and metabolic mechanisms as well as the bioavailability of vine tea is essential if its pharmacological activities are to be understood in the near future. On the one hand, the pharmacological effects of natural products depend on integrating ingredients rather than single or two main ingredients. Furthermore, some low content compounds are proved to exert significant bioactivities. Moreover, some low content ingredients have been developed into clinical drugs or lead compounds, such as paclitaxel and vincristine [126]. On the other hand, only compounds absorbed by the body can exert an effect; however, metabolites formed during liver metabolism and microbial catabolism of the parent compounds may be responsible for the effects. Consequently, it is important to comprehensively investigate bioactive compounds in vine tea and serum pharmacology and serum pharmacochemistry characteristics of vine tea in the near future.
Anyway, regarding its pharmacological activities, vine tea is an excellent herbal medicine, part of the Chinese tea culture. It is a promising herbal medicine for preventing initiation and progression of several diseases. Most reports to date have focused on bioactivity testing, so comprehensively serum pharmacology and serum pharmacochemistry as well as exhaustive mechanistic and toxicological studies or preclinical studies on vine tea are necessary for the development of commercial drugs in the future.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Xi’an Jiaotong University.
References
- 1.Yang C.S., Chen G., Wu Q. Recent scientific studies of a traditional Chinese medicine, tea, on prevention of chronic diseases. J. Tradit. Complem. Med. 2014;4:17–23. doi: 10.4103/2225-4110.124326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao W., Peng Y., Xu L. Preliminary exploration of the diversity of the Chinese tea culture. Mod. Chin. Med. 2011;13:52. 53, 59. [Google Scholar]
- 3.He Z.D., Peng Y., Xiao P.G. Science Press; Beijing: 2010. Kudingcha Research and Development; pp. 1–25. [Google Scholar]
- 4.Xiao W., Peng Y., Xu L. The origin of the tea culture and the “chew-drink” concept. Mod. Chin. Med. 2011;13:45–46. [Google Scholar]
- 5.Ye L.Y., Wang H.J., Duncan S.E. Antioxidant activities of vine tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chem. 2015;172:416–422. doi: 10.1016/j.foodchem.2014.09.090. [DOI] [PubMed] [Google Scholar]
- 6.Gao J.H., Liu B.G., Ning Z.X. Characterization and antioxidant activity of flavonoid-rich extracts from leaves of Ampelopsis grossedentata. J. Food Biochem. 2009;336:808–820. [Google Scholar]
- 7.Fu M., Li X.Y., Wang D.Y. Flavonoid constituents of leaves of Ampelopsis grossedentata (Hand-Mazz) W.T. Wang, Chin. Pharmaceut. J. 2015;50:574–578. [Google Scholar]
- 8.Wang C., Xiong W., Reddy Perumalla S. Solid-state characterization of optically pure (+) dihydromyricetin extracted from Ampelopsis grossedentata leaves. Int. J. Pharm. 2016;511:245–252. doi: 10.1016/j.ijpharm.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Georgiev V., Ananga A., Tsolova V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients. 2014;6:391–415. doi: 10.3390/nu6010391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S., Zhao X., Wan J. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: a randomized controlled trial. Pharm. Res. (N. Y.) 2015;99:74–81. doi: 10.1016/j.phrs.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y., Wu B., Wan J. Rattan tea extracts improve insulin resistance in type 2 diabetes rats. J. Third Mil. Med. Univ. 2015;37:454–458. [Google Scholar]
- 12.Jiang B.P., Le L., Pan H.M. Dihydromyricetin ameliorates the oxidative stress response induced by methylglyoxal via the AMPK/GLUT4 signaling pathway in PC12 cells. Brain Res. Bull. 2014;109:117–126. doi: 10.1016/j.brainresbull.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Ying L., Xu P., Huang S.R. Antioxidant activity of bioactive compounds extracted from Ampelopsis grossedentata leaves by optimized supercritical carbon dioxide. J. Med. Plants Res. 2011;5:4373–4381. [Google Scholar]
- 14.Ye Y., Ou X.H., Huang Q.J. Antithrombotic effect of total flavonoids and monomeric compounds from Ampelopsis grossedentata. Tradit. Chin. Drug Res. Clin. Pharmacol. 2013;24:33–36. [Google Scholar]
- 15.Liu J., Shu Y., Zhang Q. Dihydromyricetin induces apoptosis and inhibits proliferation in hepatocellular carcinoma cells. Oncol. Lett. 2014;8:1645–1651. doi: 10.3892/ol.2014.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Y., Liang X.Y., Chang H. Ampelopsin-induced autophagy protects breast cancer cells from apoptosis through Akt-mTOR pathway via endoplasmic reticulum stress. Canc. Sci. 2014;105:1279–1287. doi: 10.1111/cas.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou X.L., Tong Q., Wang W.Q. Suppression of inflammatory responses by dihydromyricetin, a flavonoid from Ampelopsis grossedentata, via inhibiting the activation of NF-kappa B and MAPK signaling pathways. J. Nat. Prod. 2015;78:1689–1696. doi: 10.1021/acs.jnatprod.5b00275. [DOI] [PubMed] [Google Scholar]
- 18.Zeng C.H., Yang K., Xu M.G. Antibacterial mechanisms of total flavonoids from Ampelopsis grossedentata on Staphylococcus aureus. Chin. J. Exp. Tradit. Med. Formulae. 2013;19:249–252. [Google Scholar]
- 19.Zhou Y.C., Hu Y.X., Zang X.B. Toxicological assessment on Ampelopsis grossedentata and its immune regulation study. Pract. Prev. Med. 2001;8:412–414. [Google Scholar]
- 20.Naveed M., BiBi J., Kamboh A.A. Pharmacological values and therapeutic properties of black tea (Camellia sinensis): a comprehensive overview. Biomed. Pharmacother. 2018;100:521–531. doi: 10.1016/j.biopha.2018.02.048. [DOI] [PubMed] [Google Scholar]
- 21.Aboulwafa M.M., Youssef F.S., Gad H.A. A comprehensive insight on the health benefits and phytoconstituents of camellia sinensis and recent approaches for its quality control. Antioxidants. 2019;8:455. doi: 10.3390/antiox8100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J.Y., Wang M., Zhao J.P. Yellow tea (Camellia sinensis L.), a promising Chinese tea: processing, chemical constituents and health benefits. Food Res. Int. 2018;107:567–577. doi: 10.1016/j.foodres.2018.01.063. [DOI] [PubMed] [Google Scholar]
- 23.Mattila P.H., Hellström J., Karhu S. High variability in flavonoid contents and composition between different North European currant (Ribes spp.) varieties. Food Chem. 2016;204:14–20. doi: 10.1016/j.foodchem.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 24.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang D.Y., Liu J.M., Zhang J.D. Study on chemical constituents of Ampelopsis grossedentata (vine tea) Subtropical. Plant. Sci. 1998;27:39–44. [Google Scholar]
- 26.Ma R.Y., Zhou R.G., Tong R.N. At-line hyphenation of high-speed countercurrent chromatography with Sephadex LH-20 column chromatography for bioassay-guided separation of antioxidants from vine tea (Ampelopsis grossedentata) J. Chromatogr. B. 2017;1040:112–117. doi: 10.1016/j.jchromb.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 27.Du Q.Z., Cai W.J., Xia M. Purification of (+)-dihydromyricetin from leaves extract of Ampelopsis grossedentata using high-speed countercurrent chromatography with scale-up triple columns. J. Chromatogr. A. 2002;973:217–220. doi: 10.1016/s0021-9673(02)01092-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H.B., Xie G.Y., Tian M. Optimization of the ultrasonic-assisted extraction of bioactive flavonoids from Ampelopsis grossedentata and subsequent separation and purification of two flavonoid aglycones by high-speed counter-current chromatography. Molecules. 2016;21:1096. doi: 10.3390/molecules21081096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Zhang Q., Wang B. Chemical constituents from Ampelopsis grossedentata. J. Chin. Pharmaceut. Sci. 2006;15:211–214. [Google Scholar]
- 30.Chen J., Wu Y.C., Zou J.W. α-Glucosidase inhibition and antihyperglycemic activity of flavonoids from Ampelopsis grossedentata and the flavonoid derivatives. Bioorg. Med. Chem. 2016;24:1488–1494. doi: 10.1016/j.bmc.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Zhou T.D., Zhou X.X. Isolation, structure determination and pharmacological activity of dihydroflavanol from Ampelopsis grossdentata. Chin. J. Pharma. 1996;31:458–461. [Google Scholar]
- 32.Yuan A.X., Huang X.M., Chen J. Study on chemical composition of Ampelopsis grossedentata. Chin. J. Tradit. Chin. Med. 1998;23:359–360. [PubMed] [Google Scholar]
- 33.Gao Q.P., Ma R.Y., Chen L. Antioxidant profiling of vine tea (Ampelopsis grossedentata): off-line coupling heart-cutting HSCCC with HPLC–DAD–QTOF-MS/MS. Food Chem. 2017;225:55–61. doi: 10.1016/j.foodchem.2016.11.122. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y.F., Ying L., Sun D. Supercritical carbon dioxide extraction of bioactive compounds from Ampelopsis grossedentata stems: process optimization and antioxidant activity. Int. J. Mol. Sci. 2011;12:6856–6870. doi: 10.3390/ijms12106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y.S., Que S., Yang X.W. Isolation and identification of metabolites from dihydromyricetin. Magn. Reson. Chem. 2007;45:909–916. doi: 10.1002/mrc.2051. [DOI] [PubMed] [Google Scholar]
- 36.He G.X., Pei G., Zhou T.D. Isolation and structural identification of myricetin from yaozu vine tea. J. Med. Pharm. Chin. Minorities. 2000;6:40–41. [Google Scholar]
- 37.Zhang Y.S., Zhang Q.Y., Wang B. Study on chemical constituents of Ampelopsis grossedentata. J. Chin. Pharmaceut. Sci. 2006;15:211–214. [Google Scholar]
- 38.He G.X., Pei G., Du F.L. Study on chemical constituents of rattan tea, Modern Chin. Med. 2007;9:11–13. [Google Scholar]
- 39.Zhang Y.S., Yang W.L., Cui C. Study on chemical constituents of Ampelopsis grossedentata. Chin. Herb. Med. 2003;5:402–403. [Google Scholar]
- 40.Wang D.Y., Zheng Z.Z., Xu S.Y. Four new isoflavones from Ampelopsis grossedentata. J. Asian Nat. Prod. Res. 2002;4:303–308. doi: 10.1080/1028602021000049104. [DOI] [PubMed] [Google Scholar]
- 41.Wang J., He L., Zheng N. Dihydromyricetin in Ampelosis grossedentata collected from different habitats. Chin. Tradit. Patent. Med. 2014;36:145–147. [Google Scholar]
- 42.Zhang J.Y., Chen Y., Luo H.Q. Recent update on the pharmacological effects and mechanisms of dihydromyricetin. Front. Pharmacol. 2018;9:1204. doi: 10.3389/fphar.2018.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mizuno M. Anti-tumor polysaccharides from mushrooms during storage. BioFactors. 2000;12:275–281. doi: 10.1002/biof.5520120141. [DOI] [PubMed] [Google Scholar]
- 44.Chen L., Huang G.L. Antitumor activity of polysaccharides: an overview. Curr. Drug Targets. 2018;19:89–96. doi: 10.2174/1389450118666170704143018. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y.F., Bian X.Y., Park J. Physicochemical properties, in vitro antioxidant activities and inhibitory potential against α-Glucosidase of polysaccharides from Ampelopsis grossedentata leaves and stems. Molecules. 2011;16:7762–7772. doi: 10.3390/molecules16097762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H.F., You X.Q. Determination of aroma components of Ampelopsis grossedentata by gas chromatography. Nat. Prod. Res. Dev. 1996;8:47–50. [Google Scholar]
- 47.Hou X., Zhang J.F., Ahmad H. Evaluation of antioxidant activities of ampelopsin and its protective effect in lipopolysaccharide-induced oxidative stress piglets. PloS One. 2014;9 doi: 10.1371/journal.pone.0108314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jimenez M., Garcia-Carmona F. Myricetin, an antioxidant flavonol, is a substrate of polyphenol oxidase. J. Sci. Food Agric. 1999;79:1993–2000. [Google Scholar]
- 49.Rusak G., Gutzeit H.O., Müller J.L. Structurally related flavonoids with antioxidative properties differentially affect cell cycle progression and apoptosis of human acute leukemia cells. Nutr. Res. 2005;25:143–155. [Google Scholar]
- 50.Wang Z.H., Ah Kang K., Zhang R. Myricetin suppresses oxidative stress-induced cell damage via both direct and indirect antioxidant action. Environ. Toxicol. Pharmacol. 2010;29:12–18. doi: 10.1016/j.etap.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Qin S., Chen J., Tanigawa S. Microarray and pathway analysis highlight Nrf2/ARE-mediated expression profiling by polyphenolic myricetin. Mol. Nutr. Food Res. 2013;57:435–446. doi: 10.1002/mnfr.201200563. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y.L., Zhang Y.L., Dai Y.C. Systems pharmacology approach reveals the anti-inflammatory effects of Ampelopsis grossedentata on dextran sodium sulfate- induced colitis. World J. Gastroenterol. 2018;24:1398–1409. doi: 10.3748/wjg.v24.i13.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hou X.L., Tong Q., Wang W.Q. Suppression of inflammatory responses by dihydromyricetin, a flavonoid from Ampelopsis grossedentata, via inhibiting the activation of NF-κB and MAPK signaling pathways. J. Nat. Prod. 2015;78:1689–1696. doi: 10.1021/acs.jnatprod.5b00275. [DOI] [PubMed] [Google Scholar]
- 54.Weng L., Zhang H., Li X. Ampelopsin attenuates lipopolysaccharide-induced inflammatory response through the inhibition of the NF-κB and JAK2/STAT3 signaling pathways in microglia. Int. Immunopharm. 2017;44:1–8. doi: 10.1016/j.intimp.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Wu J., Zhao F.T., Fan K.J. Dihydromyricetin inhibits inflammation of fibroblast-like synoviocytes through regulation of nuclear factor-κB signaling in rats with collagen-induced arthritis. J. Pharmacol. Exp. Therapeut. 2019;368:218–228. doi: 10.1124/jpet.118.253369. [DOI] [PubMed] [Google Scholar]
- 56.Grenier D., Chen H., Lagha A.B. Dual action of myricetin on Porphyromonas gingivalis and the inflammatory response of host cells: a promising therapeutic molecule for periodontal diseases. PloS One. 2015;10 doi: 10.1371/journal.pone.0131758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gutiérrez-Venegas G., Luna O.A., Arreguín-Cano J.A. Myricetin blocks lipoteichoic acid-induced COX-2 expression in human gingival fibroblasts. Cell. Mol. Biol. Lett. 2014;19:126–139. doi: 10.2478/s11658-014-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H.H., Kim D.H., Kim M.H. Flavonoid constituents in the leaves of Myrica rubra sieb. et zucc. with anti-inflammatory activity. Arch Pharm. Res. (Seoul) 2013;36:1533–1540. doi: 10.1007/s12272-013-0147-x. [DOI] [PubMed] [Google Scholar]
- 59.Lee K.M., Kang N.J., Han J.H. Myricetin down-regulates phorbol ester-induced cyclooxygenase-2 expression in mouse epidermal cells by blocking activation of nuclear factor kappa B. J. Agric. Food Chem. 2007;55:9678–9684. doi: 10.1021/jf0717945. [DOI] [PubMed] [Google Scholar]
- 60.Lee Y.S., Choi E.M. Myricetin inhibits IL-1β-induced inflammatory mediators in SW982 human synovial sarcoma cells. Int. Immunopharm. 2010;10:812–814. doi: 10.1016/j.intimp.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Schwanke R.C., Marcon R., Meotti F.C. Oral administration of the flavonoid myricitrin prevents dextran sulfate sodium-induced experimental colitis in mice through modulation of PI3K/Akt signaling pathway. Mol. Nutr. Food Res. 2013;57:1938–1949. doi: 10.1002/mnfr.201300134. [DOI] [PubMed] [Google Scholar]
- 62.Wan W.T., Jiang B.P., Sun L. Metabolomics reveals that vine tea (Ampelopsis grossedentata) prevents high-fat diet-induced metabolism disorder by improving glucose homeostasis in rats. PloS One. 2017;12 doi: 10.1371/journal.pone.0182830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ran L., Wang X.L., Lang H.D. Ampelopsis grossedentata supplementation effectively ameliorates the glycemic control in patients with type 2 diabetes mellitus. Eur. J. Clin. Nutr. 2019;73:776–782. doi: 10.1038/s41430-018-0282-z. [DOI] [PubMed] [Google Scholar]
- 64.Le L., Jiang B.P., Wan W.T. Metabolomics reveals the protective of dihydromyricetin on glucose homeostasis by enhancing insulin sensitivity. Sci. Rep. 2016;6:36184. doi: 10.1038/srep36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi L.Y., Zhang T., Zhou Y. Dihydromyricetin improves skeletal muscle insulin sensitivity by inducing autophagy via the AMPK-PGC-1α-Sirt3 signaling pathway. Endocrine. 2015;50:378–389. doi: 10.1007/s12020-015-0599-5. [DOI] [PubMed] [Google Scholar]
- 66.Wu B., Lin J., Luo J. Dihydromyricetin protects against diabetic cardiomyopathy in streptozotocin-induced diabetic mice. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/3764370. 3764370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ong K.C., Khoo H.E. Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci. 2000;67:1695–1705. doi: 10.1016/s0024-3205(00)00758-x. [DOI] [PubMed] [Google Scholar]
- 68.Zelus C., Fox A., Calciano A. Myricetin inhibits islet amyloid polypeptide (IAPP) aggregation and rescues living mammalian cells from IAPP toxicity. Open Biochem. J. 2012;6:66–70. doi: 10.2174/1874091X01206010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kang S.J., Park J.H.Y., Choi H.N. α-glucosidase inhibitory activities of myricetin in animal models of diabetes mellitus. Food Sci. Biotechno. 2015;24:1897–1900. [Google Scholar]
- 70.Liu I.M., Tzeng T.F., Liou S.S. Improvement of insulin sensitivity in obese Zucker rats by myricetin extracted from Abelmoschus moschatus. Planta Med. 2007;73:1054–1060. doi: 10.1055/s-2007-981577. [DOI] [PubMed] [Google Scholar]
- 71.Choi Y., Kim T.D., Paik S.R. Molecular simulations for anti-amyloidogenic effect of flavonoid myricetin exerted against Alzheimer's β-amyloid fibrils formation. Bull. Kor. Chem. Soc. 2008;29:1505–1509. [Google Scholar]
- 72.Chang Y., Chang C.Y., Wang S.J. Myricetin inhibits the release of glutamate in rat cerebrocortical nerve terminals. J. Med. Food. 2015;18:516–523. doi: 10.1089/jmf.2014.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caruana M., Högen T., Levin J. Inhibition and disaggregation ofα-synuclein oligomers by natural polyphenolic compounds. FEBS Lett. 2011;585:1113–1120. doi: 10.1016/j.febslet.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 74.Zhu X., Jia Y.H. Inhibition of catechol-o-methyltransferase (COMT) by myricetin, dihydromyricetin and myricitrin. Pharmazie. 2014;69:183–186. [PubMed] [Google Scholar]
- 75.Moonrungsee N., Shimamura T., Kashiwagi T. An automated sequential injection spectrophotometric method for evaluation of tyramine oxidase inhibitory activity of some flavonoids. Talanta. 2014;122:257–263. doi: 10.1016/j.talanta.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 76.Zhang X.H., Ma Z.G., Rowlands D.K. Flavonoid myricetin modulates GABAA receptor activity through activation of Ca2+ channels and CaMK-II pathway. Evid. Based Compl. Alt. Med. 2012;2012 doi: 10.1155/2012/758097. 758097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jin Y.Y., Singh P., Chung H.J. Blood ammonia as a possible etiological agent for Alzheimer's disease. Nutrients. 2018;10:E564. doi: 10.3390/nu10050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ren Z.X., Zhao Y.F., Cao T. Dihydromyricetin protects neurons in an MPTP-induced model of Parkinson's disease by suppressing glycogen synthase kinase-3 beta activity. Acta Pharmacol. Sin. 2016;37:1315–1324. doi: 10.1038/aps.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ren Z., Yan P., Zhu L. Dihydromyricetin exerts a rapid antidepressant-like effect in association with enhancement of BDNF expression and inhibition of neuroinflammation. Psychopharmacol. (Berl) 2018;235:233–244. doi: 10.1007/s00213-017-4761-z. [DOI] [PubMed] [Google Scholar]
- 80.Liu P., Zou D., Chen K. Dihydromyricetin improves hypobaric hypoxia-induced memory impairment via modulation of SIRT3 signaling. Mol. Neurobiol. 2016;53:7200–7212. doi: 10.1007/s12035-015-9627-y. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Z., Zhang H., Chen S. Dihydromyricetin induces mitochondria-mediated apoptosis in HepG2 cells through down-regulation of the Akt/Bad pathway. Nutr. Res. 2017;38:27–33. doi: 10.1016/j.nutres.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Kao S.J., Lee W.J., Chang J.H. Suppression of reactive oxygen species-mediated ERK and JNK activation sensitizes dihydromyricetin-induced mitochondrial apoptosis in human non-small cell lung cancer. Environ. Toxicol. 2017;32:1426–1438. doi: 10.1002/tox.22336. [DOI] [PubMed] [Google Scholar]
- 83.Zhao Z.Q., Yin J.Q., Wu M.S. Dihydromyricetin activates AMP-activated protein kinase and P38(MAPK) exerting antitumor potential in osteosarcoma. Canc. Prev. Res. 2014;7:927–938. doi: 10.1158/1940-6207.CAPR-14-0067. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y.M., Wang W., Qiu E.D. Protection of oxidative stress induced apoptosis in osteosarcoma cells by dihydromyricetin through down-regulation of caspase activation and up-regulation of BcL-2. Saudi J. Biol. Sci. 2017;24:837–842. doi: 10.1016/j.sjbs.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Y.Q., Wang S.P., Chan H.F. Dihydromyricetin induces apoptosis and reverses drug resistance in ovarian cancer cells by p53-mediated downregulation of surviving. Sci. Rep. 2017;7:46060. doi: 10.1038/srep46060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ji F.J., Tian X.F., Liu X.W. Dihydromyricetin induces cell apoptosis via a p53-related pathway in AGS human gastric cancer cells. Genet. Mol. Res. 2015;14:15564–15571. doi: 10.4238/2015.December.1.7. [DOI] [PubMed] [Google Scholar]
- 87.Zhou Y., Shu F.R., Liang X.Y. Ampelopsin induces cell growth inhibition and apoptosis in breast cancer cells through ROS generation and endoplasmic reticulum stress pathway. PloS One. 2014;9 doi: 10.1371/journal.pone.0089021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Romanouskaya T.V., Grinev V.V. Cytotoxic effect of flavonoids on leukemia cells and normal cells of human blood. Bull. Exp. Biol. Med. 2009;148:57–59. doi: 10.1007/s10517-009-0633-9. [DOI] [PubMed] [Google Scholar]
- 89.López-Lázaro M., Willmore E., Austin C.A. The dietary flavonoids myricetin and fisetin act as dual inhibitors of DNA topoisomerases I and II in cells. Mutat. Res-Fund. Mol. M. 2010;696:41–47. doi: 10.1016/j.mrgentox.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 90.Kang N.J., Jung S.K., Lee K.W. Myricetin is a potent chemopreventive phytochemical in skin carcinogenesis. Ann. NY. Acad. Sci. 2011;1229:124–132. doi: 10.1111/j.1749-6632.2011.06122.x. [DOI] [PubMed] [Google Scholar]
- 91.Kumamoto T., Fujii M., Hou D.X. Myricetin directly targets JAK1 to inhibit cell transformation. Canc. Lett. 2009;275:17–26. doi: 10.1016/j.canlet.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 92.Xu R., Zhang Y., Ye X. Inhibition effects and induction of apoptosis of flavonoids on the prostate cancer cell line PC-3 in vitro. Food Chem. 2013;138:48–53. doi: 10.1016/j.foodchem.2012.09.102. [DOI] [PubMed] [Google Scholar]
- 93.Zhang X., Ling Y., Yu H. Studies on mechanism of myricetin-induced apoptosis in human hepatocellular carcinoma HepG-2 cells, Chin. J. Chin. Mater. Med. 2010;35:1046–1050. doi: 10.4268/cjcmm20100824. [DOI] [PubMed] [Google Scholar]
- 94.Phillips P.A., Sangwan V., Borja-Cacho D. Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Canc. Lett. 2011;308:181–188. doi: 10.1016/j.canlet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Borde P., Mohan M., Kasture S. Effect of myricetin on deoxycorticosterone acetate (DOCA)-salt-hypertensive rats. Nat. Prod. Res. 2011;25:1549–1559. doi: 10.1080/14786410903335190. [DOI] [PubMed] [Google Scholar]
- 96.Chen Y., Lv L., Pi H. Dihydromyricetin protects against liver ischemia/reperfusion induced apoptosis via activation of FOXO3a-mediated autophagy. Oncotarget. 2016;7:76508–76522. doi: 10.18632/oncotarget.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qiu P., Dong Y., Li B. Dihydromyricetin modulates p62 and autophagy crosstalk with the Keap-1/Nrf2 pathway to alleviate ethanol- induced hepatic injury. Toxicol. Lett. 2017;274:31–41. doi: 10.1016/j.toxlet.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 98.Zeng X., Yang J., Hu O. Dihydromyricetin ameliorates nonalcoholic fatty liver disease by improving mitochondrial respiratory capacity and redox homeostasis through modulation of SIRT3 signaling. Antioxid. Redox Signal. 2019;30:163–183. doi: 10.1089/ars.2017.7172. [DOI] [PubMed] [Google Scholar]
- 99.Liu T.T., Zeng Y., Tang K. Dihydromyricetin ameliorates atherosclerosis in LDL receptor deficient mice. Atherosclerosis. 2017;262:39–50. doi: 10.1016/j.atherosclerosis.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 100.Chen S.H., Zhao X.L., Wan J. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: a randomized controlled trial. Pharmacol. Res. 2015;99:74–81. doi: 10.1016/j.phrs.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 101.Matić S., Stanić S., Bogojević D. Methanol extract from the stem of Cotinus coggygria Scop., and its major bioactive phytochemical constituent myricetin modulate pyrogallol-induced DNA damage and liver injury. Mutat. Res. 2013;755:81–89. doi: 10.1016/j.mrgentox.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 102.Mo S.F., Zhou F., Lv Y.Z. Hypouricemic action of selected flavonoids in mice: structure-activity relationships. Biol. Pharm. Bull. 2007;30:1551–1556. doi: 10.1248/bpb.30.1551. [DOI] [PubMed] [Google Scholar]
- 103.Hu Q., Zhang T., Yi L. Dihydromyricetin inhibits NLRP3 inflammasome-dependent pyroptosis by activating the Nrf2 signaling pathway in vascular endothelial cells. Biofactors. 2018;44:123–136. doi: 10.1002/biof.1395. [DOI] [PubMed] [Google Scholar]
- 104.Zeng Y., Peng Y., Tang K. Dihydromyricetin ameliorates foam cell formation via LXRa-ABCA1/ABCG1-dependent cholesterol efflux in macrophages. Biomed. Pharmacother. 2018;101:543–552. doi: 10.1016/j.biopha.2018.02.124. [DOI] [PubMed] [Google Scholar]
- 105.Liu T.T., Zeng Y., Tang K. Dihydromyricetin ameliorates atherosclerosis in LDL receptor deficient mice. Atherosclerosis. 2017;262:39–50. doi: 10.1016/j.atherosclerosis.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 106.Liu S., Ai Q., Feng K. The cardioprotective effect of dihydromyricetin prevents ischemia-reperfusion-induced apoptosis in vivo and in vitro via the PI3K/Akt and HIF-1α signaling pathways. Apoptosis. 2016;21:1366–1385. doi: 10.1007/s10495-016-1306-6. [DOI] [PubMed] [Google Scholar]
- 107.Zhu H., Luo P.H., Fu Y.Y. Dihydromyricetin prevents cardiotoxicity and enhances anticancer activity induced by adriamycin. Oncotarget. 2015;6:3254–3267. doi: 10.18632/oncotarget.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang Y., Fu L., Wang L. Electrophysiological study on the antiarrhythmic mechanism of ampelopsin in rats. Chin. J. Cardiovasc. Dis. 2014;42:675–679. [PubMed] [Google Scholar]
- 109.Li Q., Wang J., Zhu X. Dihydromyricetin prevents monocrotaline-induced pulmonary arterial hypertension in rats. Biomed. Pharmacother. 2017;96:825–833. doi: 10.1016/j.biopha.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 110.Gan C.L., Liu F.Z., Jiang S.X. Effects of five flavonols on [Ca2+] i in cardiomyocytes of rats. Chin. J. Epidemiol. 2007;26:624–626. [Google Scholar]
- 111.Scarabelli T.M., Mariotto S., Abdel-Azeim S. Targeting STAT1 by myricetin and delphinidin provides efficient protection of the heart from ischemia/reperfusion-induced injury. FEBS Lett. 2009;583:531–541. doi: 10.1016/j.febslet.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 112.Tiwari R., Mohan M., Kasture S. Cardioprotective potential of myricetin in isoproterenol-induced myocardial infarction in Wistar rats. Phytother Res. 2009;23:1361–1366. doi: 10.1002/ptr.2688. [DOI] [PubMed] [Google Scholar]
- 113.Bhatia G., Khanna A.K., Sonkar R. Lipid lowering and antioxidant activity of flavones in triton treated hyperlipidemic rats. Med. Chem. Res. 2011;20:1622–1626. [Google Scholar]
- 114.Wu Y., Bai J., Zhong K. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R,3Rdihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 2017;218:463–470. doi: 10.1016/j.foodchem.2016.07.090. [DOI] [PubMed] [Google Scholar]
- 115.Zhao L., Cai C., Wang J. Dihydromyricetin protects against bone loss in ovariectomized mice by suppressing osteoclast activity. Front. Pharmacol. 2017;8:928. doi: 10.3389/fphar.2017.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shin J.C., Jung H.Y., Harikishore A. The flavonoid myricetin reduces nocturnal melatonin levels in the blood through the inhibition of serotonin N-acetyltransferase. Biochem. Bioph. Res. Co. 2013;440:312–316. doi: 10.1016/j.bbrc.2013.09.076. [DOI] [PubMed] [Google Scholar]
- 117.Kumamoto T., Fujii M., Hou D.X. Akt is a direct target for myricetin to inhibit cell transformation. Mol. Cell. Biochem. 2009;332:33–41. doi: 10.1007/s11010-009-0171-9. [DOI] [PubMed] [Google Scholar]
- 118.Kang B.Y., Kim S.H., Cho D. Inhibition of interleukin-12 production in mouse macrophages via decreased nuclear factor-kappa B DNA binding activity by myricetin, a naturally occurring flavonoid. Arch Pharm. Res. (Seoul) 2005;28:274–279. doi: 10.1007/BF02977791. [DOI] [PubMed] [Google Scholar]
- 119.Park H.H., Lee S., Son H.Y. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch Pharm. Res. (Seoul) 2008;31:1303–1311. doi: 10.1007/s12272-001-2110-5. [DOI] [PubMed] [Google Scholar]
- 120.Hagenacker T., Hillebrand I., Wissmann A. Anti-allodynic effect of the flavonoid myricetin in a rat model of neuropathic pain: involvement of p38 and protein kinase C mediated modulation of Ca2+ channels. Eur. J. Pain. 2010;14:992–998. doi: 10.1016/j.ejpain.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 121.D'Souza L., Wahidulla S., Devi P. Antibacterial phenolics from the mangrove Lumnitzera racemosa. Indian J. Mar. Sci. 2010;39:294–298. [Google Scholar]
- 122.Xu H.X., Lee S.F. Activity of plant flavonoids against antibiotic-resistant bacteria. Phytother Res. 2001;15:39–43. doi: 10.1002/1099-1573(200102)15:1<39::aid-ptr684>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 123.Chen R., Hollborn M., Grosche A. Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyaniding in primary cultures of human retinal pigment epithelial cells. Mol. Vis. 2014;20:242–258. [PMC free article] [PubMed] [Google Scholar]
- 124.Russo B., Picconi F., Malandrucco I. Flavonoids and insulin-resistance: from molecular evidences to clinical trials. Int. J. Mol. Sci. 2019;20:2061. doi: 10.3390/ijms20092061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Manach C., Scalbert A., Morand C. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 126.Oberlies N.H., Kroll D.J. Camptothecin and taxol: historic achievements in natural products research. J. Nat. Prod. 2004;67:129–135. doi: 10.1021/np030498t. [DOI] [PubMed] [Google Scholar]