Abstract
Background and aim
Genistein (GEN) and exercise (Ex) may be regarded as an alternative treatment for non-alcoholic steatohepatitis (NASH). However, the mechanisms behind their therapeutic effects in NASH are not well-understood.
Experimental procedure
This study investigated the roles of histone deacetylase (HDAC)3 and interleukin-(IL-)13 in the NASH model of ovariectomized (OVX) rats fed with high fat high fructose (HFHF) diet.
Results and conclusion
Nine weeks after being fed with HFHF diet, severe NASH pathology with mild fibrosis were seen along with an increase in HDAC3, IL-13 and matrix metalloelastase (MMP-12) expressions in OVX rats. Five weeks of either GEN or Ex treatments abrogated the increase in both HDAC3 and IL-13 expressions in OVX rats fed with HFHF diet and ameliorated NASH features, liver fibrosis and MMP-12 expression. The combination of Gen and Ex, however, did not provide additional benefits on NASH features in OVX rats fed with HFHF diet. These results suggested that GEN and Ex treatments improved HFHF diet induced NASH in OVX rats through the suppression of HDAC3, IL-13 and MMP-12 expression.
Keywords: Estrogen deficiency, Genistein, Exercise, Nonalcoholic steatohepatitis, Ovariectomized
Abbreviations: NAFLD, Nonalcoholic fatty liver disease; NASH, Nonalcoholic steatohepatitis; OVX, ovariectomized; HFHF, High-fat high-fructose; FFA, Free fatty acid; HDAC3, histone deacetylase 3; IL-13, Interleukin-13; MMP-12, matrix metalloelastase 12; TBA, Thiobarbituric acid-reactive substances; ELISA, Enzyme-linked immunosorbent assay; DMSO, Dimethyl sulfoxide; DAB, Diaminobenzidine
Graphical abstract
Highlights
•Estrogen deficiency leads to NASH development.
•Either genistein or exercise modulated lipid metabolism reducing steatohepatitis.
•Either genistein or exercise attenuated liver fibrosis improving NASH.
•Combining genistein and exercise did not provide additional benefits.
•Genistein and exercise have beneficial effects in post-menopausal women with NASH.
1. Introduction
NASH is characterized histologically by macrovesicular steatosis, hepatocyte ballooning, lobular inflammation and in some cases bridging fibrosis.1 Major risk factors of NASH include genetic and epigenetic factors, sedentary lifestyle, high-calorie diet and gender differences. Physical inactivity and dietary factors are important risk factors for the NASH development in humans. Recent reports suggest that high-calorie foods that use fructose as a sweetener are associated with the greater severity in patients with nonalcoholic fatty liver disease (NAFLD).2 Mice fed with 30%fructose in water developed hepatic triglyceride deposition, increased markers of insulin resistance and hepatic lipid peroxidation and TNF-alpha levels.3 In terms of gender differences, NASH appears to be more common in men, and its prevalence increases with age in women, especially after menopause.4 In this study, HFHF diet induced NASH in a post-menopausal condition is therefore chosen as a model of studying.
Genistein (4, 5, 7-trihydroxyisoflavone) is a subclass of isoflavone. GEN has structural similarities to endogenous 17β-estradiol and shows higher binding affinity for estrogen receptor β (ER-β) than for estrogen receptor α (ER-α).5 GEN exerts its actions through estrogenic and antioxidant activities. Previous studies suggested that genistein (16 mg/kg) improved histopathology of NASH through the upregulation of PPARγ and reduction of oxidative stress in male rats fed with high fat diet (HFD) for six weeks.6 Aerobic exercise induced lipolysis in adipose tissues, leading to acetyl-CoA production and up-regulation of β–oxidation.7 In type 2 diabetes rats with HFD-induced NASH, hepatic MMP-12 expression was elevated, and after 12 weeks of treadmill running exercise, its expression reduced.1 In addition, five weeks of both treadmill running exercise and isoflavone supplementation could reduce plasma triglyceride (TG) in male rats.8
To characterize the mechanism of GEN and Ex treatments on NASH, we attempted to unravel the connection between HDAC3, IL-13, and MMP-12 induced by HFHF diet in OVX rats. HDAC3 is a class I histone deacetylase, whose deletion in liver may result in hepatic steatosis due to the increase in lipogenesis and lipid accumulation through the fatty acid synthase and sterol regulatory element binding protein (SREBP) 1 upregulation9,10 and the reduction of fatty acid oxidation.10,11 Excessive liver fat accumulation precedes NASH and can cause end-stage liver diseases. Thus, HDAC3 plays an important role in lipid metabolism. IL-13 is recognized as a pro-fibrotic cytokine in the liver.12 IL-13 can induce expression of collagen type I and connective tissue growth factor (CTGF) in hepatic stellate cells.12 Elevated IL-13 levels in the serum were seen in NASH patients as compared to healthy controls.13 The aforementioned evidence suggested that IL-13 played a role in liver fibrosis.14 Genistein (5 mg/kg BW) given via intra-gastric route for 12 weeks in rats could inhibit the activation of hepatic stellate cells by increasing hepatic Smad7 expression and inhibiting the TGF-β signaling resulting in decreased alpha smooth muscle actin and collagen matrix accumulation.15 In a pre-clinical study, hepatic MMP-12 expression increased in type 2 diabetes rats with high fat diet induced NASH and treadmill running exercise for 12 weeks decreased it.1 Prior evidence supported the roles of HDAC3, IL-13 and MMP-12 in the development of NASH and liver fibrosis, and that GEN and Ex improved NASH pathology. It remains unclear whether the therapeutic effects of GEN and Ex have any correlation with HDAC3, IL-13 or MMP-12 expressions in the liver. The aims of this study were to evaluate the effects of GEN and Ex on HFHF diet induced NASH in OVX rats and their association with HDAC3, IL-13 and MMP-12.
2. Materials and methods
2.1. Animal experiment
The study was conducted according to the standard of animal care and use established under ethical principles and policies of the National Research Council of Thailand. The study protocol and experimental procedures were approved by the Animal Care and Use Committee, Faculty of Medicine, Chulalongkorn University (the permission No. is 032/2561).
Female Sprague-Dawley rats at 8 weeks of age (180–220 g) were purchased from the Nomura Siam International Co., Ltd., Bangkok, Thailand and housed in a 12-h light/dark cycle at the temperature of 25±1 °C. After a 1-week acclimatization period, all rats were divided into six groups (n = 6 in each group) as follows: (1) control group (CON), rats were fed with standard diet for 11 weeks; (2) OVX group fed with standard diet (OVX), rats underwent bilateral ovariectomy, which will be described in details below, and received standard diet for 11 weeks; (3) OVX group fed with HFHF diet (OVX + HFHF), rats underwent bilateral ovariectomy followed by a 2-week recovery period, then were fed with HFHF diet for the remaining 9 weeks; (4–6) Treatment groups, following a 2-week recovery period after bilateral ovariectomy, rats were fed with HFHF diet for the remaining 9 weeks, but in the last 5 weeks of the experiment, rats received either genistein (group 4, OVX + HFHF + GEN) or moderate running exercise (group 5, OVX + HFHF + Ex) or both treatment (group 6, OVX + HFHF + GEN + Ex). The duration of the experiment was 11 weeks. Sample size calculation was performed using G Power program. Rats in exercise treated group were sacrificed 24 h after the last exercise in order to prevent any acute exercise effects. Other groups were euthanized at the end of 11 weeks. Liver and serum samples were collected and stored at −80 °C until the time of analysis.
2.2. Bilateral ovariectomy
Rats were anesthesized with intraperitoneal injection of sodium pentobarbital (60 mg/kg body weight) before the procedure. Bilateral ovariectomy was performed using a bilateral dorsolateral approach.16 A week after the surgery, all OVX rats received daily vaginal smear for four days to determine the estrous cycles.17 The appearance of cornified cells was used as an indicator of estrogenic activity. The completeness of ovariectomy was determined microscopically (×10) by the predominance of leukocytes called anestrous phase.
2.3. Protocols
Standard diet had 6%fat, 25% protein and 47% carbohydrate (Perfect companion group co., Ltd, Thailand). HFHF diet was modified from Pickens MK formula,18, which contained 55% fat (vegetable oil), 10% protein (albumin), 35% carbohydrate (20% fructose and 15% starch). The percentage mentioned above was the energy percentage of the total Calories in that particular diet. HFHF diet was prepared in our laboratory. All rats were fed ad libitum.
Genistein at dose 16 mg/kg body weight (Cayman Chemical Company, USA) was dissolved in 0.1%dimethyl sulfoxide (DMSO) and given to each rat once daily by oral gavage for 5 weeks. Genistein dosage was based on a study by Susutlertpanya et al.6 Rats in Ex groups performed treadmill running exercise 5 times a week for 5 weeks as described previously.8 Briefly, speed was maintained at 15 m/min for the first three weeks then increased to 20 m/min for the remaining two weeks. The exercise duration was started at 10 min in the first week, then progressively increased by 5 min each week to the maximum duration of 30 min at week 5. Base on prior studies, this exercise regimen was considered a moderate intensity exercise.19,20
2.4. Histology
Liver samples were fixed in 10% formalin and cut into 4-μm-thick paraffin sections (Thermo electron corporation, USA). The sections were stained with hematoxylin and eosin (H&E), and then were graded for steatosis (0–3), lobular inflammation (0–3), and hepatocellular ballooning (0–2) according to the criteria described by Brunt et al.21 Every sample was evaluated by an experienced liver pathologist, who was blinded to the experimental group. Slide images were captured at ×10 magnification under light microscope. The summation of NASH activity score (NAS) was calculated according to Kleiner DE., 200522 which ranged from 0 to 8. The higher score represents the more severe form of NASH.
Liver fibrosis was examined using Masson's Trichrome stain. The collagen fibers were stained blue, cell nuclei were stained black, and cytoplasm, muscle and erythrocytes were stained red.23 The stained slides were further assessed for qualitative scoring of fibrosis severity (F0: non, F1: mild, F2: moderate, F3:severe, and F4:cirrhosis) by a liver pathologist following a Metavir fibrosis score.24
Oil Red O staining is a technique for the measurement of neutral lipid and cholesteryl esters.25 Frozen liver was thawed and embedded with Tissue-Tek O·C.T compound (Sakura Finetek, Torrance, CA, USA) on cryomold. Then, slide was stained with fresh Oil Red O (Fluka) working solution followed by counter staining with hematoxylin and rinsing with running tap water. The slides were air dried and mounted with mounting medium (Dako, CA, USA). Images of 10 different areas26 of the liver were captured with a Nikon Eclipse E200 light microscopy equipped with Nikon DS-Fi3 camera with ×40 magnification.
2.5. Immunostaining
Tissue microarray (TMA) was first described by Battifora H., 1986.27 and has been proven to be an effective and efficient tool for immunohistochemical (IHC) study. Briefly, tissue core (2 mm diameter, 8–10 cores/rat) was removed from the area of interest in the histopathology block (the donor block) and placed into an empty paraffin block (the recipient block) at a specifically designated location.28 TMA tissue sections of 4 μm thickness were cut on a microtome. One was stained with H&E for histological validation by the pathologist and the other one was for the immunohistochemical study of IL-13. The primary antibody, anti-IL-13 (A130D 12G5 1E4, NBP1-52466, Novus Biologicals), was used at the dilution of 1:100 and incubated overnight at 4 °C. Subsequently, slides were incubated with secondary antibody. All the antibodies, reagents and equipment except for the anti-IL-13 antibody were obtained from Dako, USA. Histologic slides of TMA at 40× resolution were scanned using a whole slide scanner, which is an Aperio ScanScope CS system (Leica Biosystems Imaging, USA). The images were viewed using the Aperio ImageScope software. The positive cells were stained with brown color in nucleus. The intensity of positive staining was graded as absent, weak, moderate, or intense by using Positive pixel count V9 algorithm from the Aperio ImageScope software.
2.6. Western blot analysis
The levels of HDAC3 and MMP-12 expressions were determined by Western blot. Liver tissue was homogenized in RIPA buffer with protease inhibitor cocktail (Sigma) on ice under the supplier's instruction. The supernatant was collected, and protein concentration was measured using a Pierce BCA Protein Assay Kit (Thermo Scientific, USA). The protein was run in Mini-PROTEAN TGX Precast Gels (BioRad, USA) for polyacrylamide gel electrophoresis (SDS-PAGE), then transferred to the nitrocellulose membrane by Semi-Dry transfer method. After keeping with 5%skim milk at room temperature for 1 h, then antibodies against HDAC3 (1:2000, ab32369, Abcam) molecular weight 49 kDa, MMP-12 (1:2000, NBP2-67344, Novus Biologicals) molecular weight 48 kDA, and actin, molecular weight 36 kDa (1:10,000, Santa Cruz Biotechnology) were added, and the membrane was incubated overnight at 4 °C. After washing with TBST, the membrane was incubated with secondary antibody, conjugated horseradish peroxidase (HRP), (goat anti-mouse and anti-rabbit, 1:10,000, Santa Cruz Biotechnology) at room temperature for 1 h. The protein bands were visualized by ECL reagent. The amount of protein levels was calculated using Bio-Rad ChemiDoc Touch Imaging System (BioRad, USA). Actin served as an internal control.
2.7. Statistical analysis
Data were presented as mean ± standard error of the mean (SEM) and the Statistics Package for the Social Sciences (SPSS) software version 22.0 for windows was used for all analyses. The comparison among groups was determined using one-way analysis of variance (one-way ANOVA) and the post hoc Tukey test. Differences were considered statistically significant at p < 0.05.
3. Results
The completeness of bilateral ovariectomy was confirmed in each rat using daily vaginal smear for four day. The estrous cycle was found in the control group (Fig. 1a). Vaginal smear from OVX rats did not show any cornified cells (Fig. 1b) and these findings were consistent with anestrous. At the beginning of the experiment, the body weight of rats in each group were not different (p = 0.988). At the end of the experiment, the body weight of ovariectomized (OVX) rats was markedly increased compared with control group (ΔBody weight 165.67 ± 5.51 vs. 78.13 ± 4.76 g, p < 0.01). A significant reduction in the body weight was observed in the all groups of OVX rats fed with high fat high fructose (HFHF) diet compared with rats fed with standard diet. The body weight changes after treatment with genistein, running exercise, or combining genistein and exercise were not significantly different among treatment groups compared with OVX rats fed with HFHF diet (ΔBody weight, −22.04 ± 9.22 vs. −27.68 ± 8.87 vs. −29.32 ± 12.03 vs. −36.13 ± 8.93 g, respectively). We suspected body weight losses in rats fed with HFHF diet to be from the resemblance between our diet formula and a methionine and choline-deficient (MCD) diet.
Fig. 1.
Representative images of vaginal smears in (a) upper panel: CON and (b) lower panel: OVX rats for four consecutive days. Upper panel composed of proestrus, estrus, metestrus, and diestrus, respectively in CON rats. Lower panel shows an anestrous stage in OVX rats. Image magnification ×100.
3.1. Effects of treatments on NASH features in OVX rats fed with HFHF diet
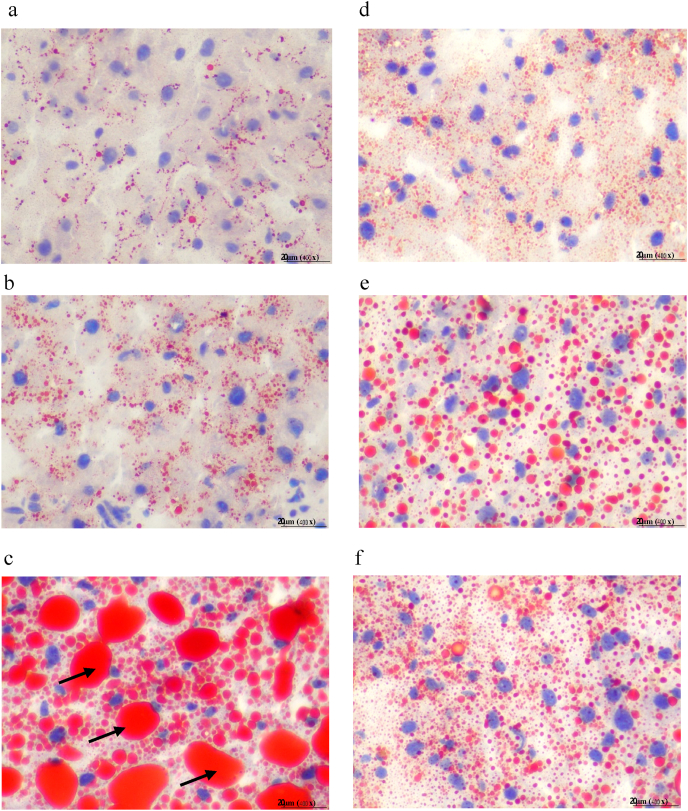
Liver histology in each group is presented in Fig. 2. After ovariectomy, mild steatosis, inflammation and hepatocyte ballooning were observed in some rats. Higher NASH severity scores were seen in OVX + HFHF group as compared with CON and OVX groups. No pathological changes were observed in livers of control rats. All treatments could improve NASH features compared with OVX + HFHF group. The summary of steatosis, inflammation and ballooning scores in each group were presented as the number of rats in each grading score in Table 1. Lipid droplets were stained red with Oil Red O and visualized under light microscopy with ×40 magnification as shown in Fig. 3. The OVX + HFHF group showed abundant, larger red staining of lipid droplets compared with OVX rats and control group. In all treatment groups, smaller and fewer droplets of Oil Red O staining were seen in the livers compared with OVX + HFHF group.
Fig. 2.
Representative images of H&E stains of liver sections from (a) CON, (b) OVX, (c) OVX + HFHF, (d) OVX + HFHF + GEN, (e) OVX + HFHF + Ex, (f) OVX + HFHF + GEN + Ex groups. All treatments could improve NASH features compared with OVX + HFHF group. Red arrows indicated steatosis. Image magnification ×100.
Table 1.
Summary of steatosis, lobular inflammation, hepatocyte ballooning and the mean NASH activity score grading according to the Brunt's criteria.
| Group | n | Steatosis |
Lobular inflammation |
Hepatocyte ballooning |
NASH activity score |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | mean ± SEM | ||
| CON | 6 | 6 | – | – | – | 6 | – | – | – | 6 | – | – | 0.00 ± 0.00 |
| OVX | 6 | 5 | 1 | – | – | 3 | 3 | – | – | 2 | 4 | – | 1.33 ± 0.33a |
| OVX + HFHF | 6 | – | – | – | 6 | – | 6 | – | – | – | 5 | 1 | 5.17 ± 0.17a,b |
| OVX + HFHF + GEN | 6 | 6 | – | – | – | 6 | – | – | – | 3 | 3 | – | 0.50 ± 0.22c |
| OVX + HFHF + Ex | 6 | 5 | 1 | – | – | 6 | – | – | – | 4 | 2 | – | 0.50 ± 0.34c |
| OVX + HFHF + GEN + Ex | 6 | 2 | 4 | – | – | 6 | – | – | – | 4 | 2 | – | 1.00 ± 0.37c |
Data are expressed as the number of rats in each of histology grading score. NASH activity score is shown in mean ± SEM, n = 6 in each group. ap < 0.01; vs. control; bp < 0.01; vs. OVX; cp < 0.01; vs. OVX + HFHF.
Fig. 3.
Oil Red O stained sections, from (a) CON, (b) OVX, (c) OVX + HFHF, (d) OVX + HFHF + GEN, (e) OVX + HFHF + Ex, (f) OVX + HFHF + GEN + Ex groups. The amount of intracellular lipid droplets (black arrows) in hepatocytes were higher in OVX + HFHF group and lower after treatments. Image magnification ×400.
3.2. Effects of treatments on HDAC3 expression and fibrosis markers in OVX rats fed with HFHF diet
The degree of liver fibrosis was determined by the presence of collagen fiber (stained blue on Masson's trichrome staining) as shown in Fig. 4. F1 fibrosis (portal fibrosis without septa) was seen in OVX + HFHF groups compared with OVX rats and CON group which had F0 (no substantial fibrosis). After treatment with GEN, Ex or in combination, F0 fibrosis were observed in all treatment groups.
Fig. 4.
Masson's trichrome stained sections, from (a) CON, (b) OVX, (c) OVX + HFHF, (d) OVX + HFHF + GEN, (e) OVX + HFHF + Ex, (f) OVX + HFHF + GEN + Ex groups. Black arrows indicate pericellular fibrosis. Image magnification ×400.
To evaluate the fibrosis markers, hepatic MMP-12 expression was detected by Western blot (Fig. 5A). Results showed that MMP-12 maintained a relatively low basal expression in livers of OVX rats compared with CON group (0.29 ± 0.02 vs. 1.00 ± 0.00, p < 0.01) but higher expression in OVX + HFHF group compared with CON group (1.28 ± 0.03 vs. 1.00 ± 0.00, p < 0.01). After treatment with GEN, Ex and a combination, MMP-12 expression decreased compared with OVX + HFHF group (0.38 ± 0.03 vs. 0.68 ± 0.06 vs. 0.56 ± 0.04 vs. 1.28 ± 0.03, respectively, p < 0.01). Genistein treatment had a higher effect in reducing the MMP-12 expression than that observed with exercise or combined treatment (0.38 ± 0.03 vs. 0.68 ± 0.06 vs. 0.56 ± 0.04, respectively, p < 0.01).
Fig. 5.
(A). The ratio of MMP-12 to actin are presented as mean ± SEM from 3 liver specimens in each group. (a: p < 0.01; vs. CON); (b: p < 0.01; vs. OVX); (c: p < 0.01; vs. OVX + HFHF); (d: p < 0.01; vs. OVX + HFHF + GEN). (B). The ratio of HDAC3 to actin are presented as mean ± SEM from 3 liver specimens in each group. (a: p < 0.01; vs. CON); (b: p < 0.01; vs. OVX); (c: p < 0.01; vs. OVX + HFHF); (d: p < 0.01; vs. OVX + HFHF + GEN).
The protein levels of HDAC3 ratio to actin decreased in OVX rats compared with CON group (0.60 ± 0.03 vs. 1.00 ± 0.00 protein relative levels, respectively, p < 0.01) but increased significantly in OVX + HFHF group compared with CON group. (HDAC3; 1.44 ± 0.11 vs. 1.00 ± 0.00 protein relative levels, respectively, p < 0.01). A significant reduction in HDAC3 expressions were clearly detected in genistein, exercise and a combination treatment groups compared with OVX + HFHF group (0.80 ± 0.05 vs. 0.47 ± 0.04 vs. 0.51 ± 0.01 vs. 1.44 ± 0.11, protein relative levels, respectively, p < 0.01). Interestingly, exercise treatment could decrease the level of HDAC3 expression more than genistein treatment (0.47 ± 0.04 vs. 0.80 ± 0.05 protein relative levels, respectively, p < 0.01) (Fig. 5B).
We also performed an IHC assay to monitor IL-13 expression in liver tissue (Fig. 6A). IL-13 positive cells were detected in the OVX + HFHF group, while a significantly negative staining (absence staining) of IL-13 expression was observed in genistein, exercise and a combination treatment. Quantification of IL-13 positive staining was analyzed using the Spectrum Analysis algorithm package and ImageScope analysis software (version 9, Aperio Technologies, Inc.) (Fig. 6B). The algorithms calculated the area of positive staining. Each TMA core was viewed at a size corresponding to a square viewing field of 215 μm × 215 μm = 0.046 mm2 at ×40 zoom magnification. Brown colors denote an immunopositive staining. Comparison of the percentage of IL-13 positive staining obtained on 10 TMA cores per rat were assessed in each experiment. A significant increase in the percentage of positive IL-13 staining/area (mm2) were observed in OVX + HFHF groups compared with OVX and CON group (3.93 ± 0.61 vs. 0.12 ± 0.06 vs. 0.10 ± 0.02, respectively; p < 0.01) (Fig. 6B). Treatment with genistein, exercise or in combination could decrease positive IL-13 staining compared with OVX + HFHF group (0.42 ± 0.08 vs. 0.43 ± 0.14 vs. 0.26 ± 0.06 vs. 3.93 ± 0.61, respectively; p < 0.01).
Fig. 6.
(A) The representative images of IHC study for IL-13 from (a) CON, (b) OVX, (c) OVX + HFHF, (d) OVX + HFHF + GEN, (e) OVX + HFHF + Ex, (f) OVX + HFHF + GEN + Ex groups. Black arrow indicates positive staining and (B) quantitative assessment of IL-13 immunostaining. Data are presented as mean ± SEM, n = 6 in each group. The letters indicated the comparison with OVX + HFHF group (c: p < 0.01). Image magnification ×400.
4. Discussion
4.1. Estrogen deficiency on NASH features
The results of our study suggested that estrogen deficiency increased the risk of NASH by increasing lipogenesis as evidenced by histological changes, Oil Red O staining and decreased HDAC3 protein expression. Prior studies proposed that HDAC3 deletion in the liver resulted in increasing lipogenesis through the fatty acid synthase (FAS) and sterol regulatory element binding protein (SREBP1) upregulation9,10 and decreasing fatty acid oxidation.10,11 The prevalence of NAFLD was lower in postmenopausal women who used hormone replacement therapy by oral or transdermal routes for more than 6 months as compared with postmenopausal women who did not.29
In visceral fat depots, elastin fibers form a mesh-like net that becomes denser in diet-induced obesity mice and its formation might be partly regulated by MMP-12.30 Endogenous estrogen was shown to induce MMP-12 expression by the binding of estrogen receptor-α with MMP-12 promoter estrogen response element in a model of prostate cancer.31 The lack of estrogen likely explained the decreased in MMP-12 expression in OVX rats and thus the increase in body weight. These finding are in line with the results of previous study in mice which showed that MMP-12 deficiency promoted adipose tissue expansion and increased body weight changes during high fat diet-induced obesity as compared with wild-type mice.32 The results of our study indicate that female sex hormones play an essential role in the hepatic HDAC3 and MMP-12 mediated NASH pathophysiology in OVX rats.
4.2. High fat high fructose exacerbated the NASH features in OVX rats
High fructose consumption in animal was associated with increased expression of lipogenic genes, such as Acc1, FAS and stearoyl CoA desaturase (SCD1) when compared with high fat diet alone.33 Moreover, fructose metabolites could directly activate transcriptional factors SREBP-1c and carbohydrate-response element binding protein (ChREBP), thus enhancing hepatic lipogenesis.33 Fructokinase is a key enzyme responsible for converting fructose into fructose-1-phosphate and generating acetyl-CoA which is a precursor molecule for triglyceride production in the liver.34 In addition, fructose metabolism also leads to hepatic ATP depletion, formation of uric acid, ROS production and liver inflammation.35 Previous study reported that HFHF diet was associated with higher NASH activity scores, hepatic fat accumulation, inflammation, oxidative stress and hepatocytes apoptosis in OVX rats than in non-OVX rats.36 Similarly, our results showed that HFHF diet perpetuated the effect of estrogen deficiency in a more severe form of NASH in OVX + HFHF group compared with OVX alone.
We found a higher level of HDAC3 expression in OVX + HFHF diet group. Similarly, a study in patients with type 2 diabetes found the augmentation of HDAC3 as epigenetic signature at the interface of proinflammation and insulin resistance.37 We hypothesized that the higher level of HDAC3 in OVX + HFHF diet group resulted from hypercaloric diet and insulin resistance. HDAC3 was involved in the activation of the inflammatory gene expression in macrophages.38 In addition, HDAC3 may promote ROS production and activate NF-KappaB signaling via ROS/NF-KappaB axis.39 MMP-12 expression correlated positively and significantly with insulin resistance and TNF- α expression in adipose tissue.32 Our study showed that MMP-12 expression increased in OVX + HFHF group, however, we did not measure insulin resistance. In a previous study, the authors found that the expression of MMP-12 was highly dependent on IL-4 and IL-13 mediated signaling in inducing hepatic fibrosis.40 MMP-12 was linked to fibrosis by reducing the expression of other MMPs, including MMP2 and MMP13, which promoted ECM degradation.40 In OVX rats fed with HFHF diet, hepatic fibrosis was observed on Masson's trichrome stain. In correlation with histology, IL-13 and MMP-12 overexpression was also found in rats with OVX + HFHF diet in this study.
4.3. Genistein improved NASH in OVX rats fed with HFHF
A previous study showed that, genistein treatment decreased hepatic MDA and TNF-α levels and enhanced PPAR-γ expression along with the improvement in liver histology in rats with NASH.6 Independent of the estrogen effect, genistein could also induce the expression of peroxisome proliferators-activated receptor α (PPARα) which in turn regulates fatty acid β-oxidation pathways in the liver. The activation of PPARα prevents triglyceride accumulation and is associated with histological improvement of NASH in a human study.41 Our results confirmed other observations that genistein could reduce hepatic steatosis (as evidenced by decreased NASH activity score, and fat accumulation on Oil Red O stain). Until now, the effects of genistein on HDAC3 related NASH pathophysiology remain uncertain. We found that genistein treatment improved NASH features in OVX fed with HFHF diet with suppression of HDAC3 expression. A recent study supported that genistein treatment reduced renal-aging fibrosis by inhibiting histone 3 deacetylation of Klotho promoter in mice.42 Moreover, genistein treatment reduced IL-13 and MMP-12 expression in OVX + HFHF group which was in line with the reduction in fibrosis stage in genistein-treated group. A previous study showed that genistein (5 mg/kg BW) acted through the inhibition of HSC activation by increasing hepatic Smad7 expression and inhibiting the TGF-β signaling resulting in decreased alpha smooth muscle actin and collagen matrix accumulation.15
4.4. Exercise improved NASH in OVX rats fed with HFHF
Our results showed that running exercise could elicit the improvement in NASH features in OVX + HFHF group without changes in diet. Treadmill running could significantly increase PPARα and carnitine palmitoyl transferase I (CPT-1) expression and decrease SREBP-1c, lipin1, and FAS expression leading to the improvement in NAFLD in C57BL/6 mice fed with high fat diet.43 Furthermore, continuous running exercise has been shown to decrease lipid peroxidation in mouse liver.44 The treatment effects of exercise training occurred through the activation of mitogen-activated protein kinase (MAPK), which in turn induced the NF-kB and enhanced the expression of antioxidant enzymes.45 Decreased malondialdehyde level was caused either by the decrease in the rate of free radical production or by the increase in antioxidant activity. Data are conflicting regarding the effect of exercise on oxidative stress and it appears that the intensity and type of exercise may have different effects on glutathione. Elokda and colleagues showed that combined aerobic and circuit weight training exercise increased glutathione levels.46 While Ilhan et al. reported a more prominent increase in lipid peroxidation and a reduction in glutathione in combined aerobic-anaerobic exercise group as compared to other types of exercise.46 In our study, exercise could reduce HDAC3 expression in OVX + HFHF diet group. Previous study showed that moderate intensity treadmill exercise could inhibit the inflammatory response by inhibiting HDAC3/NF-KappaB pathway in rats with osteoarthritis (OA).39 Treadmill running exercise for 12 weeks could improve NASH-related fibrosis marker (MMP-12) induced by western diet in obesity rats.1 Similarly, our study showed that running exercise decreased liver fibrosis in OVX + HFHF diet which was corresponded to the reduction in IL-13 and MMP-12 protein expression. Furthermore, the reduction in liver fibrosis in MMP-12 deficient mice was not associated with changes in TGF-β1 or tissue inhibitors of matrix metalloproteinase (TIMPs) expression.40
4.5. Combination of genistein and exercise improved NASH in OVX rats fed with HFHF
Our results indicated that combination of exercise and genistein did not provide additional benefits on NASH in OVX rat fed with HFHF diet compared with either intervention alone. It has been suggested previously that exercise training stimulates estrogen-like effects in decreasing fatty liver in postmenopausal animal models,47, which likely explains the reason that isoflavones may not produce a meaningful effect when exercise is performed. On the other hand, the other study has shown that the combination of exercise and isoflavones may be more favorable in reducing the hepatic steatosis than either treatment alone.48
5. Conclusions
Estrogen deficiency induced by ovariectomy could lead to NAFLD development in this rat model with a more severe pathology when HFHF diet was added. Genistein and exercise could modulate lipid metabolism, and reduce fibrosis markers, thus improving histological changes of NASH. Combining genistein and exercise did not provide additional benefits from either treatment alone. These results suggest a link between HDAC3, IL-13 and MMP-12 related pathways and GEN and Ex treatments in this NASH model induced by HFHF diet in OVX rats.
Funding statement
This study was funded by the 100th Anniversary Fund of Chulalongkorn University, the 90th Anniversary of Chulalongkorn University Scholarship and Ratchadaphiseksomphot Endowment Fund, Bangkok, Thailand.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of competing interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgments
The authors would like to thank financial support from the 100th Anniversary Fund of Chulalongkorn University, the 90th Anniversary of Chulalongkorn University Scholarship and Ratchadaphiseksomphot Endowment Fund, Bangkok, Thailand.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Linden M.A., Sheldon R.D., Meers G.M. Aerobic exercise training in the treatment of non-alcoholic fatty liver disease related fibrosis. J. Physiol. 2016;594(18):5271–5284. doi: 10.1113/JP272235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelmalek M.F., Suzuki A., Guy C. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51(6):1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spruss A., Kanuri G., Wagnerberger S. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50(4):1094–1104. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- 4.Clark J.M. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl 1):S5–S10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 5.Fang H., Tong W., Shi L.M. Structure-activity relationships for a large diverse set of natural, synthetic, and environmental estrogens. Chem Res Toxicol. 2001;14(3):280–294. doi: 10.1021/tx000208y. [DOI] [PubMed] [Google Scholar]
- 6.Susutlertpanya W., Werawatganon D., Siriviriyakul P., Klaikeaw N. Genistein attenuates nonalcoholic steatohepatitis and increases hepatic PPARgamma in a rat model. Evid. Based Complement. Alternat. Med. 2015;2015:509057. doi: 10.1155/2015/509057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stich V., de Glisezinski I., Berlan M. Adipose tissue lipolysis is increased during a repeated bout of aerobic exercise. J Appl Physiol. 1985;88(4):1277–1283. doi: 10.1152/jappl.2000.88.4.1277. 2000. [DOI] [PubMed] [Google Scholar]
- 8.Yoon G.A., Park S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014;8(6):618–624. doi: 10.4162/nrp.2014.8.6.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller K.M., Kornfeld J.W., Friedbichler K. Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology. 2011;54(4):1398–1409. doi: 10.1002/hep.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papazyan R., Sun Z., Kim Y.H. Physiological suppression of lipotoxic liver damage by complementary actions of HDAC3 and SCAP/SREBP. Cell Metabol. 2016;24(6):863–874. doi: 10.1016/j.cmet.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Z., Miller R.A., Patel R.T. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat Med. 2012;18(6):934–942. doi: 10.1038/nm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y., Meyer C., Muller A. IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-beta-independent Smad signaling. J Immunol. 2011;187(5):2814–2823. doi: 10.4049/jimmunol.1003260. [DOI] [PubMed] [Google Scholar]
- 13.Shimamura T., Fujisawa T., Husain S.R. Novel role of IL-13 in fibrosis induced by nonalcoholic steatohepatitis and its amelioration by IL-13R-directed cytotoxin in a rat model. J Immunol. 2008;181(7):4656–4665. doi: 10.4049/jimmunol.181.7.4656. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Munker S., Mullenbach R., Weng H.L. IL-13 signaling in liver fibrogenesis. Front Immunol. 2012;3:116. doi: 10.3389/fimmu.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganai A.A., Husain M. Genistein attenuates D-GalN induced liver fibrosis/chronic liver damage in rats by blocking the TGF-beta/Smad signaling pathways. Chem Biol Interact. 2017;261:80–85. doi: 10.1016/j.cbi.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Khajuria D.K., Razdan R., Mahapatra D.R. Description of a new method of ovariectomy in female rats. Rev Bras Reumatol. 2012;52(3):462–470. [PubMed] [Google Scholar]
- 17.Cora M.C., Kooistra L., Travlos G. Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicol Pathol. 2015;43(6):776–793. doi: 10.1177/0192623315570339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickens M.K., Ogata H., Soon R.K., Grenert J.P., Maher J.J. Dietary fructose exacerbates hepatocellular injury when incorporated into a methionine-choline-deficient diet. Liver Int. 2010;30(8):1229–1239. doi: 10.1111/j.1478-3231.2010.02285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoydal M.A., Wisloff U., Kemi O.J., Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil. 2007;14(6):753–760. doi: 10.1097/HJR.0b013e3281eacef1. [DOI] [PubMed] [Google Scholar]
- 20.Wisloff U., Helgerud J., Kemi O.J., Ellingsen O. Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2001;280(3):H1301–H1310. doi: 10.1152/ajpheart.2001.280.3.H1301. [DOI] [PubMed] [Google Scholar]
- 21.Brunt E.M., Janney C.G., Di Bisceglie A.M., Neuschwander-Tetri B.A., Bacon B.R. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 22.Kleiner D.E., Brunt E.M., Van Natta M. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 23.Garvey W. Modified elastic tissue-Masson trichrome stain. Stain Technol. 1984;59(4):213–216. doi: 10.3109/10520298409113858. [DOI] [PubMed] [Google Scholar]
- 24.Goodman Z.D. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007;47(4):598–607. doi: 10.1016/j.jhep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Ramirez-Zacarias J.L., Castro-Munozledo F., Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97(6):493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 26.Deutsch M.J., Schriever S.C., Roscher A.A., Ensenauer R. Digital image analysis approach for lipid droplet size quantitation of Oil Red O-stained cultured cells. Anal Biochem. 2014;445:87–89. doi: 10.1016/j.ab.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Battifora H. The multitumor (sausage) tissue block: novel method for immunohistochemical antibody testing. Lab Invest. 1986;55(2):244–248. [PubMed] [Google Scholar]
- 28.Jawhar N.M. Tissue Microarray: a rapidly evolving diagnostic and research tool. Ann Saudi Med. 2009;29(2):123–127. doi: 10.4103/0256-4947.51806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Florentino G.S., Cotrim H.P., Vilar C.P. Nonalcoholic fatty liver disease in menopausal women. Arq Gastroenterol. 2013;50(3):180–185. doi: 10.1590/S0004-28032013000200032. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Santibanez G., Singer K., Cho K.W. Obesity-induced remodeling of the adipose tissue elastin network is independent of the metalloelastase MMP-12. Adipocyte. 2015;4(4):264–272. doi: 10.1080/21623945.2015.1027848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang Z., Cao J., Tian L. Aromatase-induced endogenous estrogen promotes tumour metastasis through estrogen receptor-alpha/matrix metalloproteinase 12 axis activation in castration-resistant prostate cancer. Canc Lett. 2019:72–84. doi: 10.1016/j.canlet.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.T., Pamir N., Liu N.C. Macrophage metalloelastase (MMP12) regulates adipose tissue expansion, insulin sensitivity, and expression of inducible nitric oxide synthase. Endocrinology. 2014;155(9):3409–3420. doi: 10.1210/en.2014-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Softic S., Cohen D.E., Kahn C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci. 2016;61(5):1282–1293. doi: 10.1007/s10620-016-4054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Legeza B., Marcolongo P., Gamberucci A. Fructose, glucocorticoids and adipose tissue: implications for the metabolic syndrome. Nutrients. 2017;9(5) doi: 10.3390/nu9050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim J.S., Mietus-Snyder M., Valente A., Schwarz J.M., Lustig R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7(5):251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 36.Pummoung S., Werawatganon D., Klaikeaw N., Siriviriyakul P. Genistein-attenuated hepatic steatosis and inflammation in nonalcoholic steatohepatitis with bilateral ovariectomized rats. Phcog Mag. 2018;14(55):S20–S24. [Google Scholar]
- 37.Sathishkumar C., Prabu P., Balakumar M. Augmentation of histone deacetylase 3 (HDAC3) epigenetic signature at the interface of proinflammation and insulin resistance in patients with type 2 diabetes. Clin Epigenet. 2016;8:125:1–12. doi: 10.1186/s13148-016-0293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X., Barozzi I., Termanini A. Requirement for the histone deacetylase HDAC3 for the inflammatory gene expression program in macrophages. Proc Natl Acad Sci USA. 2012;109(42):E2865–E2874. doi: 10.1073/pnas.1121131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H., Ji L., Yang Y. The therapeutic effects of treadmill exercise on osteoarthritis in rats by inhibiting the HDAC3/NF-KappaB pathway in vivo and in vitro. Front Physiol. 2019;10:1060. doi: 10.3389/fphys.2019.01060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madala S.K., Pesce J.T., Ramalingam T.R. Matrix metalloproteinase 12-deficiency augments extracellular matrix degrading metalloproteinases and attenuates IL-13-dependent fibrosis. J Immunol. 2010;184(7):3955–3963. doi: 10.4049/jimmunol.0903008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francque S., Verrijken A., Caron S. PPARalpha gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J Hepatol. 2015;63(1):164–173. doi: 10.1016/j.jhep.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Li Y., Chen F., Wei A. Klotho recovery by genistein via promoter histone acetylation and DNA demethylation mitigates renal fibrosis in mice. J Mol Med (Berl) 2019;97(4):541–552. doi: 10.1007/s00109-019-01759-z. [DOI] [PubMed] [Google Scholar]
- 43.Cho J., Lee I., Kim D. Effect of aerobic exercise training on non-alcoholic fatty liver disease induced by a high fat diet in C57BL/6 mice. J Exer Nutr Biochem. 2014;18(4):339–346. doi: 10.5717/jenb.2014.18.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.da Silva L.A., Pinho C.A., Rocha L.G. Effect of different models of physical exercise on oxidative stress markers in mouse liver. Appl Physiol Nutr Metabol. 2009;34(1):60–65. doi: 10.1139/H08-132. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Cabrera M.C., Domenech E., Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44(2):126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Elokda A.S., Nielsen D.H. Effects of exercise training on the glutathione antioxidant system. Eur J Cardiovasc Prev Rehabil. 2007;14(5):630–637. doi: 10.1097/HJR.0b013e32828622d7. [DOI] [PubMed] [Google Scholar]
- 47.Pighon A., Gutkowska J., Jankowski M., Rabasa-Lhoret R., Lavoie J.M. Exercise training in ovariectomized rats stimulates estrogenic-like effects on expression of genes involved in lipid accumulation and subclinical inflammation in liver. Metabolism. 2011;60(5):629–639. doi: 10.1016/j.metabol.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 48.Barsalani R., Riesco E., Lavoie J.M., Dionne I.J. Effect of exercise training and isoflavones on hepatic steatosis in overweight postmenopausal women. Climacteric. 2013;16(1):88–95. doi: 10.3109/13697137.2012.662251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.