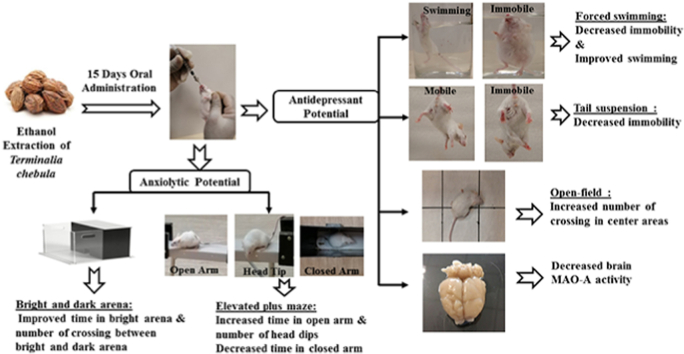
Abstract
Terminalia chebula (T.chebula) fruit is referred as “King of Medicines” in Tibet and is listed as a key plant in “Ayurvedic Materia Medica” due to its diverse pharmacological activity. The present study was aimed to investigate the comorbid antidepressant-like and anxiolytic-like effects of ethanol extract from T.chebula fruit using experimental behavioral tests in mice. In addition, the study explored the effects of extract on monoamine oxidase –A (MAO-A) levels in mouse brain. Two doses of the T.chebula extract (100 or 200 mg/kg, p.o.) were treated continuously for fifteen days to mice. Regarding antidepressant-like effects, the treatment of T.chebula extract at both dose (100 or 200 mg/kg, p.o.) levels resulted with significant (p < 0.001) reduction in duration of immobility time and increase in swimming time as compared to control group in forced swimming test. Moreover, both doses declined the duration of immobility time in the tail suspension test and increased the number of crossing in the center area using open-field test. Additionally, the dose 200 mg/kg treatment showed a significant reduction (p < 0.05) in MAO-A activity in mouse brain. For anxiolytic activity, both doses significantly (p < 0.001) improved the time spent in open arm and the number of head dips in elevated plus maze test. The higher duration of time spent in light chamber and higher number of crossing between the light and dark chambers by extract treatment in light-dark box test also supported the anxiolytic behavior. The obtained results supported the antidepressant-like and anxiolytic-like effects of ethanol extract of T.chebula in mice.
Keywords: Mood disorders, Forced swimming test, Tail suspension test, Open-field test, Monoamine oxidase, Elevated plus maze, Light-dark box test
Graphical abstract
Highlights
-
•
Repeated administration of T.chebula ethanol extract (100 and 200 mg/kg) resulted antidepressant-like effects in mice.
-
•
Repeated administration of T.chebula ethanol extract (100 and 200 mg/kg) resulted anxiolytic-like effects in mice.
-
•
Repeated administration of T.chebula ethanol extract (200 mg/kg) declined MAO-A level in the mouse brain.
List of abbreviations
- ANOVA
Analysis of Variance
- EPM
Elevated Plus Maze
- FST
Forced Swimming Test
- i.p
intraperitoneal injection
- LDB
Light-Dark Box
- MAO-A
Monoamine Oxidase –A
- OECD
Organization for Economic Co-operation
- OFT
Open-Field Test
- p.o
per oral
- SEM
Standard Error
- T.chebula
Terminalia chebula
- TST
Tail Suspension Test
1. Introduction
Medicinal plants have always been important sources of drugs for the treatment of various diseases. Their use is well documented from ancient days of history where they were popular, effective and safe for human treatment.1 Terminalia chebula (Family: Combratacea) is a medium-large sized tree found in sunny forests of Asia. The plant is widely known as black- or chebulic myrobalan in English. In Sanskrit, it is called as ‘Haritak’ that referees away from all the diseases.2 The fruit of the plant has huge medicinal values and can be found in the Ayurvedic, Chinese and Tibetan medicine literatures.3 Also, it is a main constituent of an a traditional herbal medicine triphala, which is potentially effective for several clinical uses in Ayurvedic system.2 Several pharmacological activities have been reported for T.chebula. Earlier preclinical studies suggest that administration of the crude drug extract possesses antianaphylactic, antispasmodic, prokinetic, immunosuppressive, cardiotonic, antioxidant, antihepatoxic, antimicrobial, antimutagenic/anticarcinogenic, cytoprotective, radioprotective, antidiabetic and retinoprotective activities.3, 4, 5, 6
Several active phytochemical constituents have been reported to be present in the T.chebula. The chief among them are tannins such as neochebulinic acid, chebulinic acid, chebulagic acid, chebulanin, corilagin, punicalagin, gallic acid and ellagic acid.7 Besides these compounds, fructose, betasitosterol, succinic acid, amino acids, resin and purgative principle of sennoside and anthroquinone have also been reported.8 Triterpenoids, flavonol glycosides, coumarin conjugated with gallic acids called chebulin and other phenolic compounds were also identified in T.chebula.9
Anxiety, stress and depression are characterized as highly comorbid psychological conditions that are defined as negative emotional experiences. Anxiety and depression disorders are reported to be among the most common illnesses in the community, affecting 2–6% of the global population.10 Around 25% of general practice patients are reported with comorbid depression and anxiety disorders. An epidemiological report revealed that nearly 85% of depression patients have been found with significant anxiety, on the other hand about 90% of the anxiety related disorder patients have depression. Occurrences of comorbid psychological conditions are reported to be associated with poor diagnosis and medical interventions, affecting the functioning of the patients at their workplace.11
Several mechanisms for the comorbid psychological diseases have been proposed such as increased corticotropic-releasing factor, alteration of peptides and hormones of hypothalamic-pituitary adrenal axis, neuroinflammatory, oxidative and nitrosative pathways. These episodes in the patients most likely to be triggered by external psychosocial stressor at first instance and, after three or more episodes, the pathological changes in patients spontaneously occur without any external stimulus.12
Medical interventions such as antidepressants, cognitive behavior therapy, occasionally with antipsychotics have been reported to benefit the comorbid conditions of depression and anxiety. Benzodiazepines are most popular agents that help in alleviating insomnia and anxiety but not depression. The drugs have the tendency to cause dependence and withdrawal complications and also increase the risks of falls in elderly patients.13 Selective antidepressant therapies are also known to cause several adverse reactions such as weight gain, sexual dysfunction, insomnia and blurred vision. Considering these complications, many patients tend to discontinue their medications leading to precipitation of the disease. There is a need for the safe therapy, which will be effective for the treatment of comorbid psychological conditions in patients.14 Additionally, the significant number of patients suffering from depression and anxiety is not responding to currently available medications and thus new medications are required to treat patients.
Plant based drugs for the treatment of psychological diseases has been reported in the literature. The natural components per se are safe and reported to be devoid of several adverse reactions.1,4 Previous studies conducted on experimental animals suggested the antidepressant and anxiolytic activities of aqueous extract of T.chebula.15,16 The aqueous extract of the T.chebula fruit pulp is reported to exhibit an acute anxiolytic effect at the doses 9, 18 and 36 mg/kg in mice. However, the reported activity was found to be inconsistent with respect to dose-relationship characteristic.16 This type of variation is pharmacological activity is reported to be common in psychological diseases such as anxiety and depression.17 Moreover, extraction of crude drugs by aqueous solvent is reported to contain limited number of active constituents.18 Also, in an experimental study in rodents, it was reported that chebulic acid isolated in ethanol exhibited the antidepressant and anxiolytic activities.19 Hence based on this information, we planned to test the chronic administration of ethanolic extract of T.chebula at the doses most frequently used (100 and 200 mg/kg) for the anxiolytic-like and antidepressant-like activities in mice. Also, the study was extended to determine the effect of the treatment on the brain MAO-A levels.
2. Materials & methods
2.1. Plant material and preparation of extract
Dried fruits of T.chebula were purchased from the local market of Buraydah, Al-Qassim, Saudi Arabia. The fruits were identified in the Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, Qassim University, Buraydah, Saudi Arabia and the voucher specimen was deposited in the college herbarium. The collected fruits were washed with running tap water to remove the adhering debris. After drying, the fruits were ground to a course powder using an electric grinder. For extraction, the fruit powder (500 g) was macerated in five litters of 98% ethanol for three days with occasional shaking at room temperature. The extracted solvent was filtered and removed using a rotary evaporator at 40ο C. The extract was freeze dried and stored at −20ο C for further studies. The resulting yield of the extract was 58.6g of dry weight.
2.2. Experimental animals
Groups of young (age of 8–12 weeks) male Swiss albino mice weighing between 25 and 35g were used for antidepressant and anxiolytic studies. All experimental animals were subjected to inclusion and exclusion criteria before grouping them. Age, sex, body weight as well the motor activity, and aggressive behavior were tested before selecting the animals for each study. The same strains of young female mice were used for acute oral toxicity study. Animals were obtained from animal facility from the Department of Pharmacology and Toxicology, College of Pharmacy, Qassim University, Saudi Arabia. Each group of six mice was housed in a polyacrylic cage and maintained at room temperature between 21°C-25 °C under a 12h light:12h dark cycle. All the animals were fed with standard diet and water ad libitum and acclimatized for at least 5 days before the experiments. The experimental procedures of the present study were approved by ethical research committee, College of Pharmacy, Qassim University, Buraydah, Saudi Arabia (Reference Number: 2017-CP-9) and the animals were maintained according to the procedures from the National Research Council (USA) Guide for the care and Use of Laboratory Animals.
2.3. Drugs and treatment
Fruit extract of T.chebula (100 or 200 mg/kg) and a standard drug imipramine hydrochloride (20 mg/kg, for antidepressant activity; purchased from Sigma-Aldrich, USA) were suspended with 0.5% w/v carboxymethylcellulose sodium (CMC) and administered orally to the respective groups of mice. The second standard drug diazepam (1 mg/kg, for anxiolytic activity; as injection colleted form Medical City, Qassim University, Saudi Arabia) was diluted using sterile saline solution and injected intraperitoneally.
2.4. Acute oral toxicity study
The acute oral toxicity study of T.chebula was followed according to 423 guideline of the Organization for Economic Co-operation and Development (OECD guideline 423).20 Three female mice were selected by random sampling technique for each dose. The selected animals were fasted for minimum 4 h with free access to water. Initially, the mice were treated orally with a dose of 5 mg/kg T.chebula extract and observed the general toxic signs for the first 4 h carefully as well as a number of deaths for three days. If the death was recorded two out of three animals, the treated dose (5 mg/kg, p.o.) was concluded as a toxic dose. And if the death was recorded one out of three animals and then the same dose was repeated with another three mice. Further, if there was no record of deaths and then the higher doses (50, 300 and 2000 mg/kg) were used to study the toxicity of the extract.
2.5. Evaluation of antidepressant potential
2.5.1. Experimental design
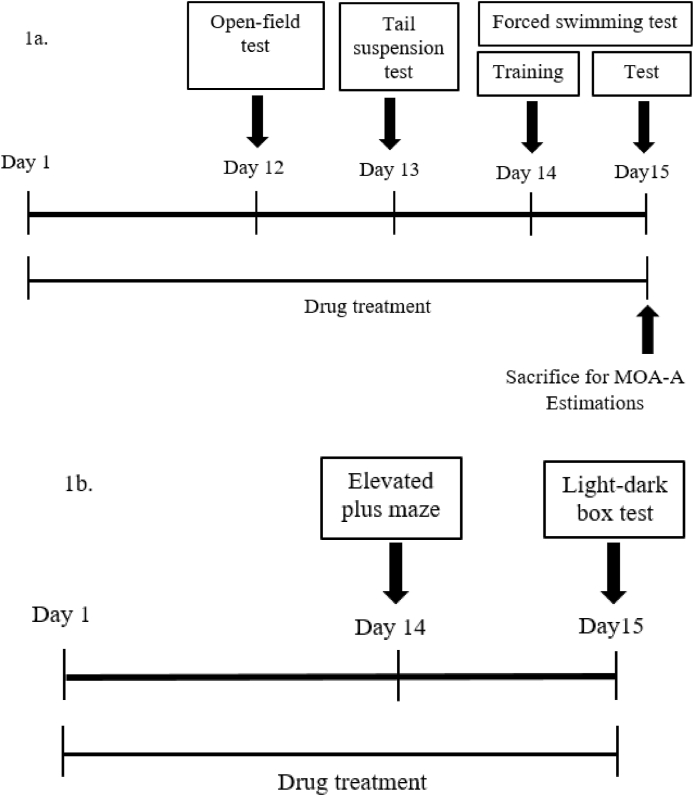
For evaluating the antidepressant potential of T.chebula extract, 24 mice were divided randomly into four groups comprising of six animals in each group. First group served as a control that was treated with 10 ml/kg vehicle (0.5% w/v of carboxymethyl cellulose) by oral route. The second group considered as standard and that was treated with imipramine (20 mg/kg, p.o.).21 The third and fourth groups considered as test groups and were treated with 100 or 200 mg/kg of T.chebula extract, respectively. The doses (100 or 200 mg/kg) of the extract have selected according to the results of acute toxicity study and previous literature.22 The animals were treated with vehicle, imipramine or T.chebula extract for fifteen days continuously according to the respective group. The groups of animals were subjected to various tests such as FST, TST and OFT from day 12 to day 15 during the treatment (Fig. 1a). After completing the behavioral studies, all the animals were sacrificed and the brain tissues were collected for estimation of MAO-A levels.
Fig. 1.
Timeline of drug administration for Evaluation of antidepressant (1a) and anxiolytic (1b) potentials T.chebula extract.
2.5.2. Forced swimming test (FST)
The FST was conducted according to the method of Porsolt et al. (1977) with minor modification.23 A glass cylinder with 20 cm in height and 14 cm in diameter was used in this experiment. The container was filled with fresh water to the height of 10 cm and the temperature was maintained around 24–26 °C. The experimental animals were forced to swim individually in a glass cylinder for 6 min (on the 14th day of treatment). At the end of the session, the mouse was removed from the cylinder, dried it using a towel and returned to the home cage. The similar session was repeated after 24 h (on the 15th day of treatment) for 6 min and recorded the duration of immobility referred the time at which animal became motionlessness of total body excepting little needful move to remain mouse head's over the water. Also the total swimming time in seconds for 6 min was recorded in the same session.
2.5.3. Tail suspension test (TST)
The TST was carried out (on the 13th day of drug treatment) according to the procedure reported by Steru et al. (1985).24 The mouse was individually tied by its tail with an adhesive tape in such a way that the animal was suspended at a distance of 1 cm from top and 60 cm from the bottom surface. The test was performed in quite, calm and dark atmosphere, and the recording was taken for 6 min. The duration of immobility in seconds was noted from second minute onwards (first minute for stabilization) until the end. The immobility was considered only when the mouse hung passively and was completely motionless.
2.5.4. Open-field test (OFT)
The OFT (on the 12th day of drug treatment) was followed as per the procedure described by Yu et al. (2002) with minor modification.25 The apparatus was consisted of a circular base with 80 cm in diameter and 20 cm in height. The floor of the apparatus was divided into three concentric circles with radius of 14, 18, 24 cm and also divided further into equal 36 units without walls. The center of the apparatus was illuminated by a 60 W LED lamp, hanged at a height of 40 cm. The experimental room was maintained for minimum light with quiet and calm atmosphere. Each mouse was placed individually in the center of apparatus and allowed the animals to explore freely. The total number of crossing and number of crossing in the center area with all four paws was noted for 3 min of time.
2.5.5. Monoamine oxidase- A (MAO-A) assay
At the end of the FST tests, total number of 24 mice from all four groups were sacrificed by cervical decapitation under light ether anesthesia on the 15th day of drug treatment. The whole brain was isolated immediately and homogenized with cold phosphate buffered saline (pH 7.4) for the evaluation of MAO-A level. The assay of MAO-A levels in mouse brain was measured using enzyme-linked immunosorbent assay kit (Cloud-Clone Corp, KATY, USA).26 The assay procedure was followed according to the manufacturers’ manual. The Pre-coated 96-well strip plate was used for this assay. The diluted standard, blank and sample was prepared and mixed with detection reagent A according to the standard procedure. The 100 μL of standard or sample to each well and kept for 1 h incubation at 37 °C. Then added 100 μL prepared detection reagent A again kept 1 h at 37 °C in incubation. After the three washes, added 100 μL prepared detection reagent B and was incubate 30 min at 37 °C. After five washes, the 90 μL substrate solution was added and incubated for 10–20 min at 37 °C. Finally, added 50 μL stop solution and the strip plate run under the microplate reader at 450 nm.
2.6. Evaluation of anxiolytic potential
2.6.1. Experimental design
A total of 24 mice were divided into four groups randomly similar to antidepressant activity. However, the second group considered as a standard, which was treated with diazepam (1 mg/kg, i.p.).16 Analogous to antidepressant study, the third and fourth groups served as test groups and were treated with 100 or 200 mg/kg of T.chebula extract, respectively. The animals received their respective treatments for fifteen days daily and on 14th and 15th day of their treatment; animals were subjected to behavioral tests like EPM and LDB tests (Fig. 1b).
2.6.2. Elevated plus maze (EPM)
The EPM model is a commonly used and widely accepted behavioral model for determining the anxiolytic potential of various targeted agents in experimental animals.19 The apparatus consists of four arms. Two of them are open arms (30 cm long and 5 cm wide) and other two arms are closed (30 cm long, 5 cm wide and 25 cm sidewall with open roof). The arms are extended from a central platform (5 cm × 5 cm). During the experiment (on the 14th day of drug treatment) the apparatus was elevated 50 cm from the floor. Each of the mice was placed on the center of the platform, facing the open arm and initially allowed to explore 5 min and recorded the time spent in open arm, time spent in closed arm and numbers of head dips to evaluate the anxiolytic potential of the test agents.
2.6.3. Light-dark box test (LDB)
The design and procedure for LDB was followed as per the research of Mohan et al. (2011).27 The instrument is used to measure the potential of test compounds on unconditioned anxiety-like behavior of rodents. The apparatus is made of a Plexiglas box with two equal compartments (20 cm × 20 cm each), one is dark and other one is bright compartment illuminated with a white light. Each of the mouse was placed (on the 15th day of drug treatment) at the junction of the bright and dark, facing the illuminated compartment. In the total test duration of 5 min, the time spent in bright arena and number of crossing between bright and dark arena was recorded using stopwatch. At the end of the each test the apparatus was carefully cleaned with 10% ethanol to remove any residue or odor.
2.7. Statistical analysis
The results from various behavioral studies and MAO-A levels were represented as mean ± standard error (SEM). The collected data were analyzed using one-way analysis of variance (ANOVA) and compared the difference between groups by Tukey–Kramer multiple comparisons test. Graph Pad version 6 (GraphPad Software Inc., United States) was employed for statistical analysis. P-values <0.05 were considered as statistically significant.
3. Results
3.1. Acute toxicity study
The mice were treated with different doses of T.chebula fruit extract by oral route until the highest dose of 2000 mg/kg and the death rate was observed for 3 days. There were no deaths resulted till the higher dose 2000 mg/kg. However, some of the minor symptoms of toxicity such as urination, abdominal cramping, elongation of right leg, abdominal distension and muscle twitches were observed at 2000 mg/kg. Therefore, the doses of the extract were fixed at 100 mg/kg and 200 mg/kg for further pharmacological evolution. A previous report also supports the selection of these doses.28
3.2. Evaluation of antidepressant potential
3.2.1. Effect of T.chebula extract on the duration of immobility and swimming time in the forced swimming test (FST)
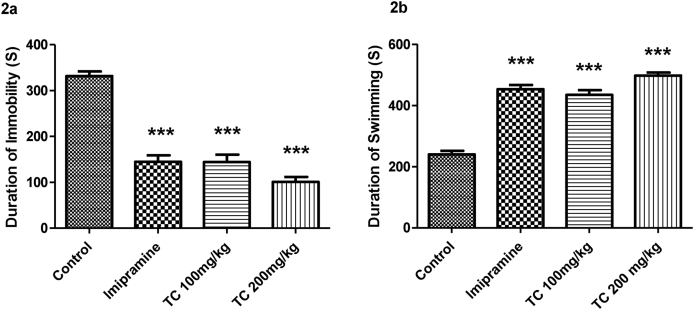
Fig. 2a and 2b and have highlighted the effect of ethanol extract of T.chebula on the duration of immobility and swimming time in seconds respectively. The statistical analysis using one-way ANOVA highlights the significant differences among the groups in duration of immobility (F (3,20) = 61.89, P < 0.001) and swimming time (F (3,20) = 77.01, P < 0.001) in FST. The post hoc analysis is referred that the groups of mice treated with T.chebula extract at 100 or 200 mg/kg by oral route showed a significant (P < 0.001) decline in duration of immobility time as compared to control animals. Additionally, the treatment of a standard drug imipramine also showed a significant (P < 0.001) decrease in duration of immobility time as compared to control animals. Moreover, there was no significant difference in duration of immobility time between imipramine (20 mg/kg, p.o.) and both doses of extract (100 and 200 mg/kg, p.o.) groups. It is indicated that both of test groups established the effects on immobility time are like to the standard drug effects. Regarding another parameter, the duration of the swimming time was significantly increased (P < 0.001) by both doses (100 or 200 mg/kg, p.o.) of T.chebula extract treatment when compared to control group. Treatment of imipramine at the dose 20 mg/kg also significantly (P < 0.001) increased the swimming time. The both results supported the antidepressant activity of T.chebula in FST.
Fig. 2.
Effect of ethanol extract of T.chebula on duration of immobility (2a) and duration of swimming (2b) using forced swimming test. The results are expressed by mean ± SEM (n = 6). One-way ANOVA [F (3,20) = 61.89, P < 0.001 for duration of immobility and F (3,20) = 77.01, P < 0.001 for duration of swimming] followed by Tukey-Kramer multiple comparisons test. ∗∗∗P < 0.001 as compared to control group.
3.2.2. Effect of T.chebula extract on the duration of immobility in the tail suspension test (TST)
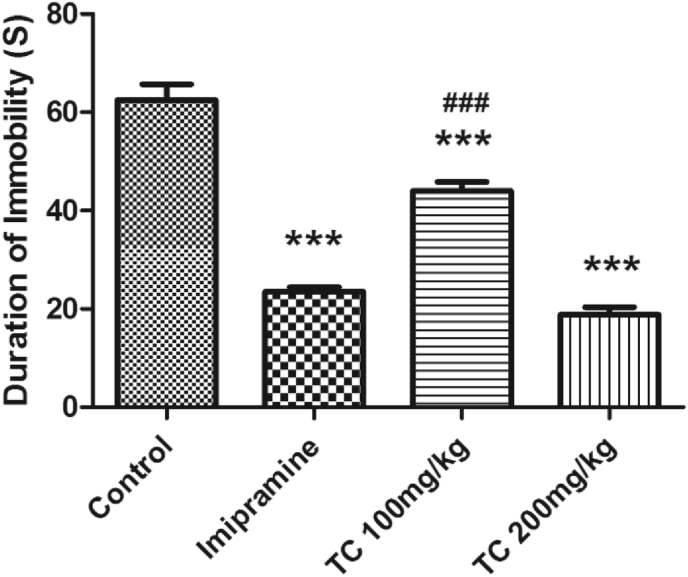
The results of T.chebula extract effect on the duration of immobility using TST is shown in Fig. 3. There was a significant difference between all groups (F (3,20) = 96.17, P < 0.001) by using one-way ANOVA analysis. The groups of mice orally treated with 100 or 200 mg/kg of T.chebula extract and standard drug imipramine (20 mg/kg) showed a significant (P < 0.001) reduction in the immobility time as compared to the control animals which were treated with vehicle only. Among the two doses, the effect of higher dose (200 mg/kg, p.o.) of T.chebula extract like to imipramine (20 mg/kg, p.o.) in TST.
Fig. 3.
Effect of ethanol extract of T.chebula on duration of immobility using tail suspension test. The results are expressed by mean ± SEM (n = 6). One-way ANOVA [F (3,20) = 96.17, P < 0.001] followed by Tukey-Kramer multiple comparisons test. ∗∗∗P < 0.001 as compared to control group, and ###P < 0.001 as compared to imipramine group.
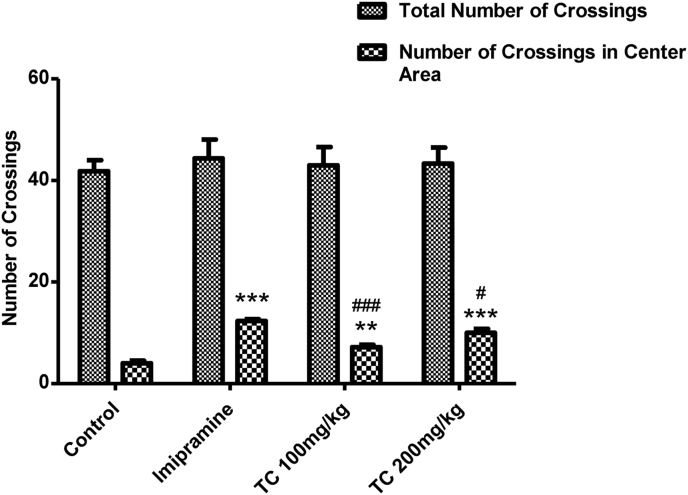
3.2.3. Effect of T.chebula extract on total number of crossing and number of crossing in center areas in the open-field test (OFT)
Fig. 4 highlights the results of T.chebula extract on total number of crossing and number of crossing in center areas using OFT. Regarding the parameter of total number of crossing, there were no significant differences (F (3,20) = 0.2281, P > 0.05) among all the treated groups. Interestingly, analysis of one-way ANOVA showed a significal difference between the groups (F (3,20) = 43.03, P < 0.001) on number of crossing in center areas using OFT. Furthermore, the oral administration of T.chebula extract at the dose level 200 mg/kg or imipramine (20 mg/kg) resulted a significant (P < 0.001) increase in number of crossing in the center area as compared to control animals. On the other hand, the lower dose (100 mg/kg, p.o.) of T.chebula extract showed the significant increase in number of crossing in center area at the level of P < 0.01.
Fig. 4.
Effect of ethanol extract of T.chebula on total number of crossings and number of crossings in center area using open-field test. The results are expressed by mean ± SEM (n = 6). One-way ANOVA [F (3,20) = 43.03, P < 0.001) for number of crossings in center area] followed by Tukey-Kramer multiple comparisons test. ∗∗P < 0.01 and ∗∗∗P < 0.001 as compared to control group. #P < 0.05 and ###P < 0.001 as compared to standard group.
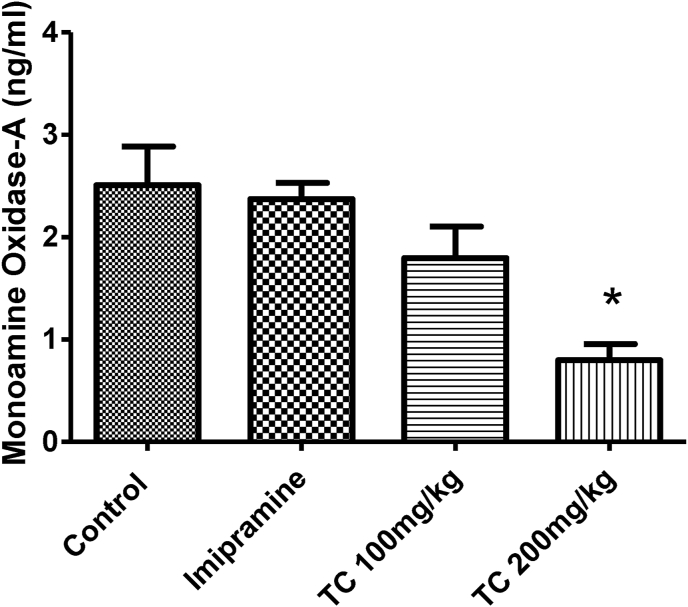
3.2.4. Effect of T.chebula extract on monoamine oxidase- A (MAO-A) levels in mouse whole brain
The treatment of T.chebula extract and imipramine for 15 days on the brain MAO-A levels show in Fig. 5. The one-way ANOVA analysis of between the groups revealed a significant difference (F (3,20) = 8.415, P < 0.05) in brain MAO-A levels. In extension of comparison between the groups, the animals treated with low dose (100 mg/kg, p.o.) of T.chebula extract and imipramine (20 mg/kg, p.o.) did not show any significant changes in brain MAO-A levels when compared to control group. The MAO-A levels of the control group was recorded as 2.51 ± 0.38 ng/ml, while 2.37 ± 0.16 ng/ml and 1.80 ± 0.31 ng/ml for T.chebula extract at 100 mg/kg and imipramine (20 mg/kg, p.o.) treated groups, respectively. Interestingly, administration of high dose (200 mg/kg, p.o.) of T.chebula extract reduced the brain MAO-A levels (0.80 ± 0.16 ng/ml) significantly (P < 0.05) as compared to control animals. It is indicated a weak MAO-A inhibitory capability of the T.chebula extract at the high dose 200 mg/kg.
Fig. 5.
Effect of ethanol extract of T.chebula on level of monoamine oxidase- A. The results are expressed by mean ± SEM (n = 6). One-way ANOVA [F (3,20) = 8.415, P < 0.05] followed by Tukey-Kramer multiple comparisons test. ∗P < 0.05 as compared to control group.
3.3. Evaluation of anxiolytic potential
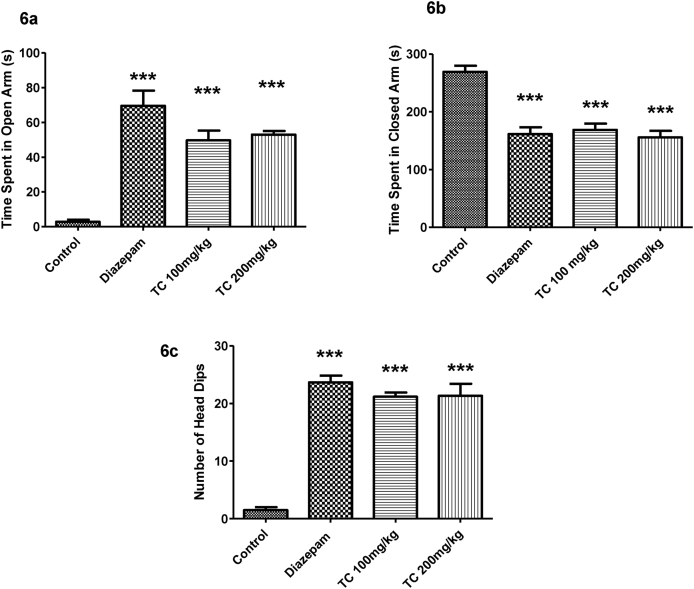
3.3.1. Effect of T.chebula extract on elevated plus maze (EPM) test
Fig. 6a–6c demonstrate the effect of T.chebula extract on time spent in open arm, time spent in closed arm and the number of head dips in EPM test. Analysis by one-way ANOVA showed that a significant difference in time spend in open arm (F (3,20) = 28.73, P < 0.001), time spend in closed arm (F (3,20) = 23.07, P < 0.001) and number of head dips (F (3,20) = 65.14, P < 0.001) using EPM test. Further post hoc analysis explained that, the control mice showed anxiety-like behavior by spending more time in closed arm and less time in open arm as well as less number in head dips. Mice treated with standard drug diazepam (1 mg/kg, i.p.) increased the time spent in of open arm and decreased time spent in the closed arm significantly (P < 0.001) as compared with control group as expected. Also the diazepam (1 mg/kg, i.p.) treatment significantly increased (P < 0.001) the number of head dips. Continuous fifteen day treatment with T.chebula extract with both doses (100 or 200 mg/kg, p.o.) showed anxiolytic effects with elevation in the duration of time spent in open arm and decline in time spent in the closed arm significantly (P < 0.001) as compared to control animals. The number of head dips also increased significantly (P < 0.001) when compared to control.
Fig. 6.
Effect of ethanol extract of T.chebula on time spend in open arm (6a), time spend in closed arm (6b) and number of head dips (6c) using elevated plus maze. The results are expressed by mean ± SEM (n = 6). One-way ANOVA [F (3,20) = 28.73, P < 0.001 for time spend in open arm, F (3,20) = 23.07, P < 0.001 for time spend in closed arm and F (3,20) = 65.14, P < 0.001 for number of head dips] followed by Tukey-Kramer multiple comparisons test. ∗∗∗P < 0.001 as compared to control group.
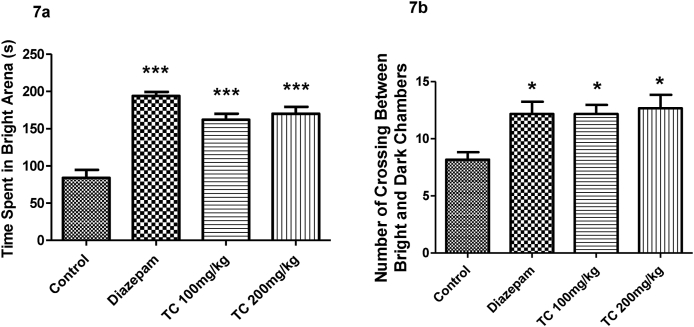
3.3.2. Effect of T.chebula extract on time spent in bright arena and number of crossing between bright and dark chambers using light-dark box test (LDB)
Fig. 7a and 7b represented the results of T.chebula extract effects on time spent in bright arena, and number of crossing between light and dark chambers in LDB. The comparision among the groups showed the significant differences on time spent in the bright arena (F (3,20) = 30.36, P < 0.001) and number of crossing between bright and dark chambers (F (3,20) = 4.892, P < 0.05) in the LDB test. The decreased in time spent in bright arena and crossing behavior between two chambers by control animals highlighted the anxiety-like behavior. The treatment of T.chebula extract with 100 or 200 mg/kg by oral route significantly improved both parameters such as time spent in the bright arena (P < 0.001) and, number of crossing between bright and dark arena (P < 0.05) compared to control animals. The observations supported the anxiolytic effects of the extract treatment. Similarly, the standard drug diazepam (1 mg/kg, i.p.) significantly increased both the parameters such as time spent in the bright arena (P < 0.001) and a number of crossings (P < 0.05) as compared to the normal saline group.
Fig. 7.
Effect of ethanol extract of T.chebula on time spent in bright arena (7a) and number of crossing between bright and dark chambers (7b) using light-dark box test. The results are expressed by mean ± SEM (n = 6). One-way ANOVA [F (3,20) = 30.36, P < 0.001 for time spent in bright arena and F (3,20) = 4.892, P < 0.05 for number of crossing between bright and dark chambers] followed by Tukey-Kramer multiple comparisons test. ∗P < 0.05 and ∗∗∗P < 0.001 as compared to control group.
4. Discussion
According to the recent reports, depression and anxiety are considered as the major psychiatric disorders around the world particularly in the Western region.29,30 It is well known that both of the disorders affect the daily life of the human beings. At present number of drugs are available in the markets for depression and anxiety disorders. Particularly for depression disorder, most of the drugs target monoamine theories with elevation of serotonin and norepinephrine. These drugs have been reported to be effective for around 30% of the depressive populations and also most of the drugs are listed with consider a number of adverse effects related majorly hypotension, arrhythmia, insomnia and sexual dysfunction.31 Therefore, presently more attention has been developed in exploring the possible beneficial effects of alternative medication in addition to the therapeutic repertoire of various psychiatric disorders. In the present report, we established the preliminary effect of T.chebula ethanol extract from fruits at two dose levels (100 or 200 mg/kg, p.o.) on various antidepressants and anxiolytic behavioral models. Also, the study was extended to find the effect of the extract on mouse brain monoamine oxidase –A (MAO-A) level at the same doses. The results of these behavioral studies supported the antidepressant and anxiolytic effects of T.chebula fruits extract.
The antidepressant activity of T.chebula fruits extract was evaluated using three common behavioral models like forced swimming test (FST), tail suspension test (TST) and open-field test (OFT). Among them, the FST is an established behavioral model for assessment of sadness in rodents. The state of immobility reflects hopelessness or lowered mood when the rodents allowed to swim forcefully in the restricted area where they don't have hope to escape.32 The previous reports pointed that the immobility stage of rodents in FST mimicking the common trait of depression in human.33,34 The decrease in the duration of immobility time and higher the duration in swimming time represents the antidepressant effect of the targeted drugs.33 From our results, T.chebula extract showed a significant decline in duration of immobility time and also improved the swimming duration of the mice at doses 100 and 200 mg/kg by oral with continuous administration for 15 days of the experimental period as like the standard drug imipramine using FST.
Similar patterns of the results were observed with treatment of T.chebula fruits extract in the duration of immobility time using TST. Like the FST, the mouse is placed in an inescapable position but in a moderate stressful situation. The state of immobility expresses the lack of escape related behavior in TST. Moreover, it is also best validated model for the evaluation of the preliminary antidepressant potential of the drugs.35 Additionally, the results of OFT in our study supported the antidepressant activity of T.chebula extract at both dose levels (200 mg/kg and 100 mg/kg). The decline of depression behavior in mice treated with T.chebula extract and imipramine (20 mg/kg, p.o.) indicated through a significant higher number of crossing in central area rather than time spent more in the outer edge area in OFT. A previous studies using OFT, with treatment of an antidepressant drug pargyline and extract of Fraxinus rhynchophylla significantly improved the center field performance of rats in stress-induced depression model.36,37 The other study with the treatment of crude extract and solvent fractions of Rosa abyssinica using open field activity was also supported the present results of antidepressant behavior of T.chebula extract treatment.38
The enzyme MAOs play a vital role in the regulation of various monoamine amine neurotransmitters such as noradrenaline, 5-hydroxytryptamine and dopamine. MAO-A and B are two isomers from MAO family and are having a significant physiological role in several neurological disorders. Major depressive disorder is linked with an elevation of MAO-A levels and the higher level of MAO-B is reported with neurodegenerative disorders such as Parkinson's disease as well as Alzheimer's disease.39 Besides, the selective inhibitors of MAO-A enzymes in the CNS are established for the treatment of mood disorders, including major depression, while selective MAO-B inhibitors are used in the treatment of Parkinson's disease.40 In the present investigation, the group of mice treated with higher dose (200 mg/kg, p.o.) of T.chebula extract showed a weak inhibition potential of the enzyme MAO-A in mouse brain.
Now-a-days, the prescription of monoamine oxidase inhibitors (MAOIs) therapy for the management of depression is limited due to their adverse effects. Some of them are considered to be life-threatening, especially the hypertensive crisis that results after consumption of tyramine containing foods.41 Further, the drug-drug and drug-food interactions are also frequent with the MAOIs therapy. Particularly, the serotonin syndrome is reported to occur due to interaction between MAOIs and Selective serotonin reuptake inhibitors (SSRIs). Still, the MAOIs represents an important therapy for the management of treatment-resistant depression (TRD) by combining with other classes of antidepressants. The MAOIs can still be used effectively due to its selectivity on enzyme inhibitory action and by following suitable dietary restriction and by avoiding with the potential interacting agents.42,43 Furthermore, the extensive researches related to beneficial mechanisms and toxicity profiles of the plant will support its therapeutic uses on various types of depressive disorders.
Present investigation also extended with the anxiolytic activity of T.chebula extract using two different behavioral models such as elevated plus maze (EPM) and, light-dark box (LDB) tests. It is well known that the EPM is a behavioral model which is used commonly for evaluating the anxiolytic potential of various experimental lead drugs in rodents. In the EPM test, due to the elevation of apparatus aggravates of fear and anxiety behavior in the open arm, when the animal placed on the apparatus. Besides, the animals’ favor to remain in the safer area (i.e. closed arm) as compared to the open area (i.e. open arm) in anxiety stage. On the other hand, the treatment with anxiolytic agents motivates the exploratory activities like number of head dips and enhances the duration of time spent in the open arm.44 In the present study, the control animals showed a reduction in the duration of time spent in the open arm as well as number of head dips and also higher duration of activity in the closed arm explained the anxiety behavior of the mice. However, the standard drug diazepam (1 mg/kg, i.p.) and both doses (100 and 200 mg/kg, p.o.) of the extract established a significant anxiolytic effect in mice by increasing the open arm activities and reducing the duration of time spent in the closed arm.
The present investigation also demonstrated the anxiolytic effects of T.chebula fruit extract and diazepam in mice by using LDB test. Generally, the rodents desire to stay in darker area as compared to bright area. However, when they are presented in a novel environment, the animals tend to explore the area. Both of these two conflicting emotions have reported to cause the anxiety like behavior in animals. If the animal treated with any anxiolytic drugs increases the duration of time spent in bright arena and locomotion.45 There was significant improvement in duration of time spent in bright arena and number of crossing between compartments at 100 or 200 mg/kg of extract treatment by oral and standard drug diazepam 1 mg/kg by intraperitoneal injection.
T.chebula fruits consist rich sources of various chemicals, including total phenolic and tannin. Moreover, tannins from T.chebula have reported as antioxidants as well as neuroprotective agents and showed their beneficial effects in various neurological disorders including neurodegenerative diseases.46 Recently, a report from Chandrasekhar et al. (2018) demonstrated the effect of tannin-rich extract from T.chebula on various neurotransmitters and mRNA and protein expression in mouse model. The supplementation of the extract significantly reduced the serum cortisol levels and elevated the monoamine neurotransmitters such as 5-hydroxytryptamine (5-HT), dopamine and norepinephrine levels in brain tissues. Also, a gene expressions study revealed up-regulation of the brain-derived neurotrophic factor (BDNF), cAMP response element-binding protein (CREB), GABAA and 5-HT1A were up-regulated by tannin-rich extract from T.chebula treatment, which are lined with a facilitating number of mood disorders including antidepressant and anxiolytic. Additionally, the treatment of the same extract showed the significant anxiolytic activity against picrotoxin-induced anxiety model.47 The present study indicated elevation of monoamine neurotransmitters by inhibition of brain MAO-A levels at the dose level of 200 mg/kg of the T.chebula extract treatment. This beneficial activity might be correlated with the presence of tannin rich compounds in T.chebula fruits.
Chebulinic acid is an ellagitannin that was isolated from hydroalcoholic extraction of T.chebula reported to possess antidepressant and anxiolytic properties at the dose levels of 20 and 40 mg/kg.20 Also, treatment of aqueous extract of T.chebula showed antidepressant activity at two doses (780 and 1560 mg/kg, p.o.) by significant reduction in duration of immobility time using FST while in TST the higher dose 1560 mg/kg reported a significant decrease the immobility time in mice.48 Additionally, from same report the reversal of antidepressant effect of the extract by a monoaminergic antagonist prazosin highlighted its involvement of monoaminergic system. On the other hand, the aqueous extract of T.chebula fruit pulp supported the anxiolytic effect using the EPM model in rats and the dose level 18 mg/kg of the extract activity was similar to the standard drug diazepam (1 mg/kg).16 The results from our study showed antidepressant activity of ethanol extract of T.chebula by a significant reduction in duration of immobility time in both FST as well as TST in mice. Moreover, anxiolytic potential of T.chebula extract was represented with a significant improvement in duration of time spent in open arm as well as number of head dips in EPM test and increased the duration of time spent in the bright arena using LDB test. The established results from our study have been supported with above previous reports.
5. Conclusion
The present study demonstrated the possibilities of antidepressant and anxiolytic potential of T.chebula ethanol extract on different behavioral models in mice. Continuous fifteen days treatment of extract at both dose levels (100 or 200 mg/kg, p.o) showed significant improvement in the duration of immobility time in depressant models like FST and TST. In OFT, both of the doses significantly increased the number of crossing in the center area. Additionally, the high dose (200 mg/kg, p.o.) might elevate the monoamine neurotransmitters by inhibiting the metabolic enzyme MAO-A. Moreover, the anxiolytic activity of the same extract treatment was evidenced by a significant increase in duration of time spent in open arm as well as number of head dips in EPM model and improved the duration of time spent in the bright arena using LDB test. These results represent that T.chebula fruits would be an alternative lead for the treatment of numerous central nervous system disorders such as antidepressant and anxiety. However, these results are too preliminary to reach the therapeutic application and need more extensive exploration about safety profile, the precise mechanism of action and development of clinical trials.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Present and permanent addresses are same for each author.
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Contributor Information
Vasudevan Mani, Email: V.SAMY@qu.edu.sa.
Sultan Sajid, Email: su.mohammed@qu.edu.sa.
Syed Imam Rabbani, Email: s.rabbani@qu.edu.sa.
Abdulrahman Saud Alqasir, Email: d7em7x@gmail.com.
Hani Abdullah Alharbi, Email: hanialhrbi17@gmail.com.
Abdullah Alshumaym, Email: abushmaym.mc@gmail.com.
References
- 1.Vonshak A., Barazani O., Sathiyomoorthy P., Shalev R., Vardy D., Golan- Goldhirsh A. Screening of South-Indian medicinal plants for anti-fungal activity. Phyther Res. 2003;17:1123–1125. doi: 10.1002/ptr.1399. [DOI] [PubMed] [Google Scholar]
- 2.Peterson C.T., Denniston K., Chopra D. Therapeutic uses of triphala in Ayurvedic medicine. J Alternative Compl Med. 2017;23:607–614. doi: 10.1089/acm.2017.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta P. Biological and pharmacological properties of Terminalia chebula Retz. (haritaki)- an overview. Int J Pharm Pharmaceut Sci. 2012;2:2–10. [Google Scholar]
- 4.Arbind K., Manivannan E., Chandrasekar R. Ethnopharmacological review of Terminalia chebula. Bioequiv Bioavailab Int J. 2019;3:1–8. [Google Scholar]
- 5.Chattopadhyay R.R., Bhattacharyya S.K. Plant review - Terminalia chebula: an update. Pharm Rev. 2007;1:151–156. [Google Scholar]
- 6.Balunas M.J., Kinghorn A.D. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Juang L.J., Sheu S.J., Lin T.C. Determination of hydrolyzable tannins in the fruit of Terminalia chebula by high-performance liquid chromatography and capillary electrophoresis. J Separ Sci. 2004;27:718–724. doi: 10.1002/jssc.200401741. [DOI] [PubMed] [Google Scholar]
- 8.Jayaramkumar K. Effect of geographical variation on content of tannic acid, gallic acid, chebulinic acid, and ethyl gallate in Terminalia chebula fruits. Nat Prod. 2006;2:170–175. [Google Scholar]
- 9.Asish P., Sashi B. Triterpenoids and their glycosides from Terminalia chebula. Phytochemistry (Oxf) 1993;32:999–1002. [Google Scholar]
- 10.Jameson J.P., Blank M.B. Diagnosis and treatment of depression and anxiety in rural and nonrural primary care: national survey results. Psychiatr Serv. 2010;61:624–627. doi: 10.1176/ps.2010.61.6.624. [DOI] [PubMed] [Google Scholar]
- 11.Coryell W., Fiedorowicz J.G., Solomon D. Effects of anxiety on the long-term course of depressive disorders. Br J Psychiatry. 2012;200:210–215. doi: 10.1192/bjp.bp.110.081992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuijpers P., Clignet F., van Meijel B. Psychological treatment of depression in inpatients: a systematic review and meta-analysis. Clin Psychol Rev. 2011;31:353–360. doi: 10.1016/j.cpr.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Quidé Y., Witteveen A.B., El-Hage W. Differences between effects of psychological versus pharmacological treatments on functional and morphological brain alterations in anxiety disorders and major depressive disorder: a systematic review. Neurosci Biobehav Rev. 2012;36:626–644. doi: 10.1016/j.neubiorev.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Rizzo M., Creed F., Goldberg D. A systematic review of non-pharmacological treatments for depression in people with chronic physical health problems. J Psychosom Res. 2011;71:18–27. doi: 10.1016/j.jpsychores.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Chandrashekar R., Manohar V.R., Rao S.N. Antidepressant activity of aqueous extract of fruits of Terminalia chebula in rats. Int J Pharm Pharmaceut Sci. 2012;4:449–451. [Google Scholar]
- 16.Chandrashekar R., Manohar V.R., Rao S.N. Acute anxiolytic activity of aqueous extract of Terminalia chebula fruit pulp in rats. Int J Res Ayurveda Pharm. 2013;4:112–115. [Google Scholar]
- 17.Peng G.J., Tian J.S., Gao X.X., Zhou Y.Z., Qin X.M. Research on the pathological mechanism and drug treatment mechanism of depression. Curr Neuropharmacol. 2015;13:514–523. doi: 10.2174/1570159X1304150831120428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molehin O.R., Adefegha S.A. Comparative study of the aqueous and ethanolic extract of Momordica foetida on the phenolic content and antioxidant properties. Int Food Res J. 2014;21:401–405. [Google Scholar]
- 19.Onasanwo S.A., Faborode S.O., Agrawal M., Ijiwola O.L., Jaiyesimi B.O., Narender T. Antidepressant and anxiolytic potentials of chebulinic acid in laboratory rodent. Ann Depress Anxiety. 2014;1:1032. [Google Scholar]
- 20.Ecobichon D.J. CRC Press; New York: 1997. The Basis of Toxicology Testing. [Google Scholar]
- 21.Ulrich-Merzenich G., Kelber O., Koptina A. Novel neurological and immunological targets for salicylate-based phytopharmaceuticals and for the anti-depressant imipramine. Phytomedicine. 2012;19:930–939. doi: 10.1016/j.phymed.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Choi M.K., Kim H.G., Han J.M. Hepatoprotective effect of Terminalia chebula against t-BHP-induced acute liver injury in C57/BL6 mice. Evid Based Complement Alternat Med. 2015 doi: 10.1155/2015/517350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porsolt R.D., Le Pichon M., Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 24.Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 25.Yu Z.F., Kong L.D., Chen Y. Antidepressant activity of aqueous extracts of Curcuma longa in mice. J Ethnopharmacol. 2002;83:161–165. doi: 10.1016/s0378-8741(02)00211-8. [DOI] [PubMed] [Google Scholar]
- 26.Baluchnejadmojarad T., Rabiee N., Zabihnejad S., Roghani M. Ellagic acid exerts protective effect in intrastriatal 6-hydroxydopamine rat model of Parkinson's disease: possible involvement of ERβ/Nrf 2/HO-1 signaling. Brain Res. 2017;1662:23–30. doi: 10.1016/j.brainres.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 27.Mohan L., Rao U.S.C., Gopalakrishna H.N., Nair V. Evaluation of the anxiolytic activity of NR-ANX-C (a Polyherbal Formulation) in ethanol withdrawal-induced anxiety behavior in rats. Evid Based Complementary Altern Med. 2011 doi: 10.1155/2011/327160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nageswara rao S., Palaksha M.N., Satish S., Ravishankar The effects of ethanolic extract in dried fruits of Terminalia chebula on learning and memory in mice. Asian J Biomed Pharm. 2013;3:59–62. [Google Scholar]
- 29.Alwhaibi M., Alhawassi T.M. Humanistic and economic burden of depression and anxiety among adults with migraine: a systematic review. Depress Anxiety. 2020;37:1146–1159. doi: 10.1002/da.23063. [DOI] [PubMed] [Google Scholar]
- 30.Curran E., Rosato M., Cooper J., Mc Garrigle C.A., Leavey G. Symptom profiles of late-life anxiety and depression: the influence of migration, religion and loneliness. Depress Anxiety. 2019;36:824–833. doi: 10.1002/da.22893. [DOI] [PubMed] [Google Scholar]
- 31.Wyska E. Pharmacokinetic considerations for current state-of-the-art antidepressants. Expet Opin Drug Metabol Toxicol. 2019;15:831–847. doi: 10.1080/17425255.2019.1669560. [DOI] [PubMed] [Google Scholar]
- 32.Rajput M.A., Khan R.A. Phytochemical screening, acute toxicity, anxiolytic and antidepressant activities of the Nelumbo nucifera fruit. Metab Brain Dis. 2017;32:743–749. doi: 10.1007/s11011-017-9963-x. [DOI] [PubMed] [Google Scholar]
- 33.Spini V.B.M.G., Ferreira F.R., Gomes A.O. Maternal immune activation with H1N1 or Toxoplasma gondii antigens induces behavioral impairments associated with mood disorders in rodents. Neuropsychobiology. 2020:1–8. doi: 10.1159/000510791. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimura H., Yamakawa K. Animal models for behavioral disorder in females. Brain Sci. 2000;22:49–54. [Google Scholar]
- 35.Can A., Dao D.T., Terrillion C.E., Piantadosi S.C., Bhat S., Gould T.D. The tail suspension test. JoVE. 2012;59:3769. doi: 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz R.J., Roth K.A., Carroll B.J. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- 37.Kim Y.R., Park B.K., Kim Y.H., Shim I., Kang I.C., Lee M.Y. Antidepressant effect of Fraxinus rhynchophylla Hance extract in a mouse model of chronic stress-induced depression. BioMed Res Int. 2018 doi: 10.1155/2018/8249563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fekadu N., Shibeshi W., Engidawork E. Evaluation of the antidepressant-like activity of the crude extract and solvent fractions of Rosa abyssinica Lindley (Rosaceae) using rodent models of depression. Clin Exp Pharmacol. 2016;6:1–7. [Google Scholar]
- 39.Yeung A.W.K., Georgieva M.G., Atanasov A.G., Tzvetkov N.T. Monoamine oxidases (MAOs) as privileged molecular targets in neuroscience: research literature analysis. Front Mol Neurosci. 2019;12:143. doi: 10.3389/fnmol.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzvetkov N.T., Stammler H.G., Neumann B., Hristova S., Antonov L., Gastreich M. Crystal structures, binding interactions, and ADME evaluation of brain penetrant N-substituted indazole-5-carboxamides as subnanomolar, selective monoamine oxidase B and dual MAO-A/B inhibitors. Eur J Med Chem. 2017;127:470–492. doi: 10.1016/j.ejmech.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Ramesh M., Dokurugu Y.M., Thompson M.D., Soliman M.E. Therapeutic, molecular and computational aspects of novel monoamine oxidase (MAO) inhibitors. Comb Chem High Throughput Screen. 2017;20:492–509. doi: 10.2174/1386207320666170310121337. [DOI] [PubMed] [Google Scholar]
- 42.Thomas S.J., Shin M., McInnis M.G., Bostwick J.R. Combination therapy with monoamine oxidase inhibitors and other antidepressants or stimulants: strategies for the management of treatment-resistant depression. Pharmacotherapy. 2015;35:433–449. doi: 10.1002/phar.1576. [DOI] [PubMed] [Google Scholar]
- 43.Shulman K.I., Herrmann N., Walker S.E. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27:789–797. doi: 10.1007/s40263-013-0097-3. [DOI] [PubMed] [Google Scholar]
- 44.Kraeuter A.K., Guest P.C., Sarnyai Z. The elevated plus maze test for measuring anxiety-like behavior in rodents. Methods Mol Biol. 2019;1916:69–74. doi: 10.1007/978-1-4939-8994-2_4. [DOI] [PubMed] [Google Scholar]
- 45.Ennaceur A. Tests of unconditioned anxiety — pitfalls and disappointments. Physiol Behav. 2014;135:55–71. doi: 10.1016/j.physbeh.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 46.Chang C.L., Lin C.S. Phytochemical composition, antioxidant activity, and neuroprotective effect of Terminalia chebula Retzius extracts. Evid Based Complementary Altern Med. 2012 doi: 10.1155/2012/125247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chandrasekhar Y., Kumar G.P., Navya K., Ramya E.M., Anilakumar K.R. Tannins from Terminalia chebula fruits attenuates GABA antagonist-induced anxiety-like behaviour via modulation of neurotransmitters. J Pharm Pharmacol. 2018;70:1662–1674. doi: 10.1111/jphp.13007. [DOI] [PubMed] [Google Scholar]
- 48.Dattatray B.P., Padmaja A.M., Nirmala N.R. Antidepressant activity of aqueous extracts of fruits of Terminalia chebula and Phyllanthus emblica in behavioural models of depression: involvement of monoaminergic system. Int J Pharm Pharmaceut Sci. 2014;6:615–620. [Google Scholar]