Abstract
Background and Aim
Despite advances in modern medicine, the development and growth of calculi continues to be a source of concern for mankind, as there is no effective treatment for kidney stones. In the present study we investigated antiurolithiatic activity of Bryophyllum pinnatum Lam against sodium oxalate (NaOx) induced urolithiasis in rats.
Experimental procedure
In rats with renal calculi caused by sodium oxalate (NaOx, 70 mg/kg, i.p.); the antiurolithiatic action of Bryophyllum pinnatum hydroalcoholic extract (BPHE) was studied. BPHE was given every day orally at doses of 50, 200 mg/kg for 14 days to rats to examine activity against sodium oxalate (NaOx) mediated urolithiasis, with Cystone (500 mg/kg, p.o.) as a reference standard. The effect of the extract on urine oxalate, creatinine and phosphate retention and excretion in the kidney, as well as serum and biochemical analysis of kidney homogenate and histopathological examinations were studied.
Results and conclusion
Oral administration of BPHE at doses of 50,100, and 200 mg/kg to rats with sodium oxalate-mediated renal calculi showed dose-dependent substantial (P<0.05) antiurolithiatic potential, with notable reversal of NaOx-induced ion excretion and urinary CaOx concentration. These findings justify the traditional use of Bryophyllum pinnatum hydroalcoholic extract (BPHE) in the treatment of renal calculi.
Keywords: Bryophyllum pinnatum Lam., Cystone, Sodium oxalate, Urolithiasis
Graphical abstract
Highlights
-
•
Supporting its traditional use in renal calculi.
-
•
Demonstrated dose-dependent antiurolithiatic capacity.
-
•
A notable reversal of NaOx-induced ion excretion.
1. Introduction
Kidney stone disease is a multifactorial condition caused by the interaction of epidemiological, biochemical, and genetic risk factors. It affects both men and women, but the risk is higher in men, and it is becoming more prevalent among young women.1 According to studies, calcium oxalate and calcium phosphate make up 80% of renal stones, while struvite, which contains magnesium ammonium phosphate, makes up 10%. Uric acid 9% and cystine or ammonium acid urate are the causes of drug-related renal stones 1%.2,3
The formation of stone is thought to be caused by an abnormal increase in urinary levels of calcium, oxalate, and uric acid, which reduces urinary citrate levels.4 Citrate and magnesium are the main inhibitors of stone formation in the urinary tract, and decreased levels or a lack of these inhibitors in the urine causes stone formation.5
Urolithiasis affects both men and women between the ages of 20 and 49 years.6 Surgical procedures, lithotripsy, and local calculus destruction with a high-powered laser are all common methods for removing the calculi. However, these treatments are costly, and recurrence is common.7 Without preventive care, the recurrence rate is about 10% after one year, 33% after five years, and 50% after ten years.8 Various drugs, such as thiazide diuretics and alkali-citrate, are used to avoid recurrence, but clinical evidence for their effectiveness is lacking.9
Except for some composite herbal drugs and plants, most remedies in traditional systems of medicine, including Ayurveda, were derived from plants and were found to be effective, even though the rational for their use was not well established through systematic pharmacological and clinical studies. These plant products are said to reduce the recurrence rate of renal calculi while having no side effects.
Bryophyllum pinnatum (Lam.) (Crassulaceae) Kalanchoe pinnata (Lam.) Pers., Kalancho In tropical America, China, India, Australia, and tropical Africa, it is commonly known as life leaf and air plant and is widely cultivated as a garden ornamental. Bryophyllum pinnatum leaves are commonly used in traditional and ethnomedical practice to treat urinary insufficiency and stone disorders.
Various tribes of Muzaffarnagar (Uttar Pradesh), Midnapur and Murshidabad districts of West Bengal, Aurangabad (Maharashtra), Nath people and Sonowal kacharis of Bhekulajan village in Dibrugarh district of Assam use fresh leaf juice or powder of 2–3 black peppers (Piper nigrum Linn.) as folklore medicine for kidney and gall bladder stones.10, 11, 12, 13, 14, 15 Tribes in Trinidad and Tobago, Nigeria, and Pakistan's Mianwali district use 10–15 leaves in ethnomedical activities to treat urinary calculi.16,17 Furthermore, the leaves of this plant were an essential component of poly-herbal formulations used for antilithiatic purposes.18,19
Leaves have antimicrobial, antiulcer, uterine contractility, antinociceptive, anti-inflammatory, and antidiabetic properties, as well as antihypertensive and nephroprotective properties.20, 21, 22, 23, 24, 25, 26 The existence of alkanes, alkanols, triterpenes and sterols, triterpenoids and phenanthrenes, flavonoids, bufadienolides, alkaloids, glycosides, and lipids is attributed to the medicinal and pharmacological properties of Bryophyllum pinnatum.27, 28, 29
In-vitro inhibitory activity of Bryophyllum pinnatum leaves on calcium oxalate crystallisation is well documented.30,31 It was thought worthwhile to investigate the effect of Bryophyllum pinnatum leaves on an experimentally induced in-vivo lithiasis model because of the traditional and ethno medicinal uses of the leaves for the treatment of kidney and bladder stones, as well as urinary insufficiency. As a result, we tested the anti-urolithiatic activity of Bryophyllum pinnatum Lam. against sodium oxalate (NaOx)-induced urolithiasis in rats in the current study.
2. Materials and methods
2.1. Plant material and preparation of extract
Fresh leaves of Bryophyllum pinnatum Lam. (family: Crassulaceae), were collected from Mahatma Phule Krishi Vidyapeeth's medicinal and aromatic herb garden in Rahuri, Maharashtra. It was then authenticated by Dr. J. Jayanti, Botanist, Botanical Survey of India (BSI), Pune (BSI/WRC/Tech./2013/462). The dried leaves were coarsely powdered (passed through sieve no. 40) and immersed in a stopper cup for maceration with 70% v/v ethanol in water for 3 days. This extracts of plant was then evaporated at 45 °C and then dried in oven. The dried extract was stored in airtight container.
2.2. Chemicals and apparatus
Sodium oxalate (Fine Chemicals, Mumbai), Cystone (Himalaya Herbal Healthcare, Bangalore, India) were used for the study. All other chemicals and reagents used were of analytical grade and procured from approved suppliers. Apparatus such as the metabolic cage (Dolphin, Mumbai), the biochemistry analyzer (Benspera Clinical Chemistry Analyzer C-61), UV-spectrophotometer (Jasco model V- 630) and centrifuge (Remi) were used in the study.
2.3. Animals
Adult male Wistar rats (Rattus norvegicus) weighing 150–200 g were acquired from MES's College of Pharmacy, Sonai's animal house. Rats were housed in polypropylene cages lined with husk in standard environmental conditions (temperature 25±2 °C; relative humidity 55 ± 10%; and 12:12 light: dark cycle). The rats were fed on a standard pellet diet (Amrut rat and mice feed, Sangli, India) ad libitum and had free access to water. The animals were acclimatised to the laboratory conditions for at least one week prior to the experiment. The experimental protocol was approved by Institutional Animal Ethical Committee (MES/COP/IAEC/02/2013-14) of MES's College of Pharmacy, Sonai and studies were carried out accordance with current CPCSEA regulations.
2.4. NaOx -induced urolithiasis in rats
Sodium oxalate-induced urolithiasis model was used to evaluate antiurolithiatic effect in rats.32 The Wistar albino rats were divided into six groups, each consisting of six animals (n = 6): Group I served as Vehicle Control, received 0.5% (w/v) gum acacia solution (5 ml/kg p.o.), Group II served as lithiatic control, received Sodium oxalate 70 mg/kg, i.p., Group III served as positive control, received standard drug Cystone (500 mg/kg, p.o.) + Sodium oxalate 70 mg/kg, i.p., and Groups IV, V and VI received Sodium oxalate 70 mg/kg, i.p.,+ BPHE 50, 100 and 200 mg/kg, p.o. respectively. The above treatment repeated up to 14 days.32
2.5. Collection and analysis of urine sample
Animals were kept in separate metabolic cages on the 14th day, and urine samples were taken after 24 h. The amount and pH of the urine is determined. After collection, urine samples were preserved by adding 2 drops thymol as a preservative before being tested for oxalate, calcium, uric acid, magnesium and phosphate content in a clinical semi autoanalyzer using a diagnostic kit (Span Diagnostics Pvt. Ltd., India). Various urine parameters such as calcium, oxalate, magnesium, phosphate and uric acid, were tested according to the instructions included with the various kits.33, 34, 35, 36, 37, 38, 39
2.6. Serum analysis
Blood samples were withdrawn from the retro-orbital plexus using a capillary procedure under mild ether anaesthesia. Blood samples were centrifuged at 10,000 g for 10 min and serum was separated for sodium, potassium, uric acid and creatinine analysis using diagnostic kits (Span Diagnostics Pvt. Ltd., India) according to the instructions included with the kits.
2.7. Kidney homogenate and histopathological study
The rats were sacrificed and kidneys were removed. Left kidney was taken and washed thoroughly with ice-cold 0.1 M phosphate buffered saline (pH 7.4). It was blotted dry and homogenized in 1.15% KCl to prepare a 10% w/v suspension. This suspension was centrifuged at 16,000×g in a cooling centrifuge at 0 °C. The supernatant obtained was further employed for estimation of malondialdehyde (MDA),40 glutathione (GSH),41catalase (CAT)42 and superoxide dismutase (SOD).43 Protein content of the supernatant was estimated by Biuret method.44 MDA and GSH were expressed as nmol/mg protein and SOD and CAT were expressed as U/mg protein. To confirm the incidence of lithiasis, right kidney of each animal was preserved in 10% neutralized formalin for further histopathological study. The isolated kidney was embedded in paraffin and cut into 5 μm thin sections and stained with hematoxylin-eosin dye. The slides were examined under binocular microscope for histopathological changes in kidney architecture, renal cellular and tubular necrosis and presence of calcium oxalate crystals.
2.8. Statistical analysis
The results are expressed as mean ± SEM. The statistical significance was assessed using One-way Analysis of Variance (ANOVA) followed by Dunnette's multiple comparison test and value of p < 0.05 was considered statistically significant.
3. Results
3.1. Urine analysis
There was a significant (p < 0.01) decrease in urine volume with no any significant changes in pH in NaOx treated control rats in comparison to normal rats. Cystone treated rats showed a significant (p < 0.05) increase in urine volume when compared to lithiatic control group. BPHE treated rats at doses of 50, 100 and 200 mg/kg body weight caused a significant (p < 0.05 to 0.01) increase in urine volume without any significant change in pH when compared to NaOx treated control rats [Table 1].
Table 1.
Effect of BPHE against sodium oxalate induced urolithiasis in rats - urine parameters.
| Sr. no | Groups | Treatment | Urine output ml/24hrs | Urine pH |
|---|---|---|---|---|
| 1 | Normal | 0.5% (w/v) gum acacia solution (5 ml/kg p.o.) | 12.50 ± 0.42 | 6.50 ± 0.03 |
| 2 | Control | Sodium oxalate 70 mg/kg, i.p. | 10.33 ± 0.55 | 7.75 ± 0.042 |
| 3 | Standard | 500 mg/kg, p.o. Cystone suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 11.66 ± 0.42∗ | 6.74 ± 0.043ns |
| 4 | BPHE | 50 mg/kg,b.wt.p.o suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 13.50 ± 0.76∗ | 7.15 ± 0.042ns |
| 5 | BPHE | 100 mg/kg,b.wt.p.o suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 14.66 ± 0.71∗∗ | 7.04 ± 0.046ns |
| 6 | BPHE | 200 mg/kg,b.wt.p.o suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 16.00 ± 0.73∗∗ | 6.85 ± 0.048ns |
ns- Nonsignificant, ∗P < 0.05, ∗∗P < 0.01 values are mean ± SEM, n = 6, when compared with control by using one-way ANOVA followed by Dunnette's multiple comparison test. ANOVA: Analysis of variance, SEM: Standard error of mean, BPHE: Bryophyllum pinnatum hydroalcoholic extracts.
In the present experiment, administration of Sodium oxalate 70 mg/kg, i.p as a renal stone inducer caused hyperoxaluria, which was identified by an increase in urinary excretion of oxalate, uric acid, creatinine, phosphorous and decrease in magnesium. However, repeated administration of Cystone 500 mg/kg and BPHE at doses of 50, 100 and 200 mg/kg body weight for 14 days effectively avoided these increase in urinary oxalate, uric acid, creatinine, phosphorous and decrease in magnesium in a dose-dependent manner when compared with NaOx-treated control group and recovered it to normal levels [Table 2].
Table 2.
Effect of BPHE against sodium oxalate induced urolithiasis in rats - Urine parameters.
| Sr. no | Groups | Treatment | Oxalate (mg/dL) | Uric acid (mg/dL) | Magnesium (mg/dL) | Creatinine (mg/dL) | Phosphorus mg/dl |
|---|---|---|---|---|---|---|---|
| 1 | Normal | 0.5% (w/v) gum acacia solution (5 ml/kg p.o.) | 0.73 ± 0.004 | 0.16 ± 0.006 | 0.83 ± 0.005 | 1.59 ± 0.03 | 0.87 ± 0.01 |
| 2 | Control | Sodium oxalate 70 mg/kg, i.p. | 1.93 ± 0.004 | 2.32 ± 0.02 | 0.34 ± 0.006 | 3.61 ± 0.14 | 1.43 ± 0.01 |
| 3 | Standard | 500 mg/kg, p.o. Cystone suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 0.87 ± 0.005∗∗ | 1.60 ± 0.02∗∗ | 0.69 ± 0.005∗∗ | 1.65 ± 0.04∗∗ | 0.82 ± 0.02∗∗ |
| 4 | BPHE | 50 mg/kg,b.wt.p.o suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 1.31 ± 0.04∗ | 1.93 ± 0.01∗ | 0.45 ± 0.007∗ | 2.60 ± 0.05∗ | 1.02 ± 0.01∗ |
| 5 | BPHE | 100 mg/kg,b.wt.p.o suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 0.95 ± 0.004∗∗ | 1.80 ± 0.01∗ | 0.55 ± 0.008∗ | 1.90 ± 0.02∗ | 0.94 ± 0.01∗ |
| 6 | BPHE | 200 mg/kg,b.wt.p.o suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 0.91 ± 0.006∗∗ | 1.63 ± 0.01∗∗ | 0.64 ± 0.008∗∗ | 1.79 ± 0.02∗∗ | 0.94 ± 0.01∗ |
∗P < 0.05, ∗∗P < 0.01 values are mean ± SEM, n = 6, when compared with control by using one way ANOVA followed by Dunnette's multiple comparison test. ANOVA: Analysis of variance, SEM: Standard error of mean, BPHE: Bryophyllum pinnatum hydroalcoholic extracts.
3.2. Serum analysis
The induction of renal stone by NaOx treatment resulted in impaired renal function as evidenced by elevated serum sodium, potassium, creatinine and uric acid, which are markers of glomerular and tubular injury. However, repeated administration of Cystone 500 mg/kg and BPHE at doses of 50, 100 and 200 mg/kg body weight for 14 days effectively avoided these changes when compared to the NaOx-treated control group [Table 3].
Table 3.
Effect of BPHE against sodium oxalate induced urolithiasis in rats - serum parameters.
| Sr. no | Groups | Treatment | Sodium mEq/l | Potassium mEq/l | Creatinine mg/dl | Uric acid mg/dl |
|---|---|---|---|---|---|---|
| 1 | Normal | 0.5% (w/v) gum acacia solution (5 ml/kg p.o.) | 3606.7 ± 25.77 | 25.08 ± 0.32 | 0.57 ± 0.01 | 2.52 ± 0.01 |
| 2 | Control | Sodium oxalate 70 mg/kg, i.p. | 5496.7 ± 26.16 | 54.00 ± 0.57 | 1.46 ± 0.01 | 4.73 ± 0.01 |
| 3 | Standard | 500 mg/kg, p.o. Cystone suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 4145 ± 16.88∗∗ | 30.50 ± 0.42∗ | 0.59 ± 0.01∗∗ | 2.57 ± 0.01∗∗ |
| 4 | BPHE | 50 mg/kg,b.wt.p.o suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 4371.7 ± 23.58∗∗ | 34.50 ± 0.42∗ | 0.63 ± 0.01∗∗ | 2.67 ± 0.01∗∗ |
| 5 | BPHE | 100 mg/kg,b.wt.p.o suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 4283.3 ± 17.06∗∗ | 32.00 ± 0.36∗ | 0.65 ± 0.01∗∗ | 2.64 ± 0.01∗∗ |
| 6 | BPHE | 200 mg/kg,b.wt.p.o suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 4201.7 ± 13.01∗∗ | 30.83 ± 0.30∗ | 0.65 ± 0.01∗∗ | 2.64 ± 0.01∗∗ |
∗P < 0.05, ∗∗P < 0.01 values are mean ± SEM, n = 6, when compared with control by using one-way ANOVA followed by Dunnette's multiple comparison test. ANOVA: Analysis of variance, SEM: Standard error of mean, BPHE: Bryophyllum pinnatum hydroalcoholic extracts.
3.3. Kidney homogenate analysis
Sodium oxalate administration significantly (p < 0.01) increased the MDA level and decreased GSH content and activities of CAT and SOD in NaOx treated lithiatic control rats when compared to normal control rats. The treatment with Cystone 500 mg/kg significantly (p < 0.01) reduced the levels of MDA and increased GSH content, CAT and SOD activities when compared to lithiatic control rats. However, repetitive administration of BPHE at doses of 50, 100 and 200 mg/kg body weight for 14 days significantly (p < 0.01) reduced MDA and increased GSH content, CAT and SOD activities in a dose dependent manner when compared to NaOx lithiatic control rats.
In the NaOx-treated urolithiatic control rats, calcium deposition in the renal tissue was also increased. In the prophylactic types, however, BPHE at doses of 50, 100, and 200 mg/kg body weight for 14 days slightly (p < 0.01) decreased the increase in calcium in renal tissue [Table 4].
Table 4.
Effect of BPHE against sodium oxalate induced urolithiasis in rats – kidney homogenate parameters.
| Sr. no | Groups | Treatment | Kidney (mg/g) Calcium |
MDA (nmol/mg of protein) | GSH (nmol/mg of protein) | CAT (U/mg protein) | SOD (U/mg protein) |
|---|---|---|---|---|---|---|---|
| 1 | Normal | 0.5% (w/v) gum acacia solution (5 ml/kg p.o.) | 0.12 ± 0.003 | 3.85 ± 0.07 | 3.50 ± 0.05 | 3.30 ± 0.05 | 9.18 ± 0.18 |
| 2 | Control | Sodium oxalate 70 mg/kg, i.p. | 0.65 ± 0.009 | 9.00 ± 0.05 | 0.84 ± 0.02 | 0.86 ± 0.04 | 4.15 ± 0.11 |
| 3 | Standard | 500 mg/kg, p.o. Cystone suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 0.16 ± 0.008∗∗ | 4.15 ± 0.07∗∗ | 3.25 ± 0.04∗∗ | 3.12 ± 0.02∗∗ | 7.51 ± 0.06∗∗ |
| 4 | BPHE | 50 mg/kg,b.wt.p.o suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 0.33 ± 0.006∗ | 7.06 ± 0.08∗ | 1.05 ± 0.06∗ | 1.24 ± 0.02∗ | 5.05 ± 0.07∗ |
| 5 | BPHE | 100 mg/kg,b.wt.p.o suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 0.23 ± 0.009∗∗ | 5.98 ± 0.06∗ | 1.99 ± 0.05∗ | 2.11 ± 0.06∗ | 6.01 ± 0.09∗ |
| 6 | BPHE | 200 mg/kg,b.wt.p.o suspended in gum acacia 0.5% + Sodium oxalate 70 mg/kg | 0.19 ± 0.006∗∗ | 4.88 ± 0.10∗∗ | 3.00 ± 0.05∗∗ | 2.99 ± 0.04∗∗ | 7.01 ± 0.06∗∗ |
∗P < 0.05, ∗∗P < 0.01 values are mean ± SEM, n = 6, when compared with control by using one way ANOVA followed by Dunnette's multiple comparison test. ANOVA: Analysis of variance, SEM: Standard error of mean, BPHE: Bryophyllum pinnatum hydroalcoholic extracts.
3.4. Kidney histopathology
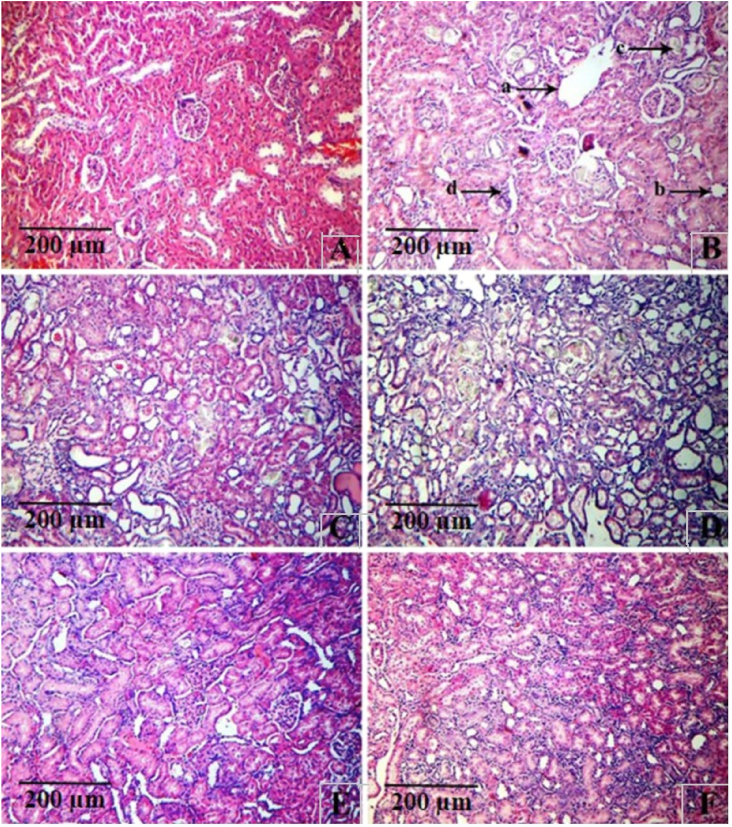
The induction of renal stone by NaOx treatment caused marked histological changes such as tubular dilatation and initial cystic changes, tubular atrophy, calcium oxalate crystal deposits and interstitial mononuclear cell infiltration in the kidneys. However, repeated administration of Cystone 500 mg/kg and BPHE at doses of 50, 100 and 200 mg/kg body weight for 14 days substantially reduced the amount of calcium oxalate deposits and other anomalies in the renal tubules in a dose-dependent manner [Fig. 1].
Fig. 1.
Histopathology of kidney tissue of (A) vehicle control, (B) Na oxalate induced calculi group, showing severe histological changes including tubular dilatation and initial cystic changes marked as (a), tubular atrophy (b), calcium oxalate crystals deposits (c) and interstitial mononuclear cell infiltration (d); crystal of calcium oxalate shown in the box (C) Cystone, standard drug treated and (D, E,and F) prophylactic treatment with BPHE at the dose 50,100 and 200 mg/kg showing moderate histological changes respectively; Microscopic magnification: 40 × .
4. Discussion
Epidemiological studies have shown that the majority of stones are commonly composed of calcium oxalate (CaOx). A number of animal models using rats have been used to induce calcium oxalate urolithiasis. Out of these models, sodium oxalate induced hyperoxaluria rat model causes rapid formation calcium oxalate crystals in renal tubules of experimental animals and hence commonly employed for rapid screening of antiurolithiatic drugs. The intraperitoneal injection of NaOx into rats may cause fast crystal formation in kidneys. The injection dose and interval will affect the size, number and location of crystals. A high dose of NaOx (10 mg/kg) may cause tubular necrosis and dilatation of the rat's kidney.45,46
Bryophyllum pinnatum is generally known as Pattharcaṭṭa in Indian traditional systems of medicine which suggests its stone dissolving power. The leaves of the plant are often used by tribal and other communities for treatment of stones. Despite its widespread traditional usage, scientific research on its antilithiatic efficacy is limited. The purpose of this study was to verify the ethno medicinal usage of Bryophyllum pinnatum leaves in the treatment of kidney stones and urine insufficiency in traditional approaches. The current study found that hydro-alcoholic extracts of Bryophyllum pinnatum had a beneficial impact in reducing NaOx-induced changes in urine and serum biochemical parameters, oxidative stress and histology of kidney.
Since conventional drugs are usually taken orally, the anti-urolithiatic action of Bryophyllum pinnatum Lam. against sodium oxalate (NaOx) mediated urolithiasis in rats was tested using the same method. Male rats were chosen to induce urolithiasis because their urinary systems are similar to humans and previous experiments have shown that female rats develop kidney stones at a lower rate than male rats.37
Result of preliminary phytochemical analysis of Bryophyllum pinnatum Lam. hydroalcoholic extract showed presence of Alkaloids, glycosides, flavonoids, tannins, anthraquinones, fixed oils and fats, saponins, sugars, terpenoids, and steroids.
In the present study, Sodium oxalate -induced hyperoxaluria model was used to assess the antilithiatic activity in wistar rats.47 Chronic administration of Sodium oxalate (70 mg/kg, i.p.) solution to male wistar rats resulted in hyperoxaluria. Oxalate, calcium and phosphate excretion were increased in the calculi-induced (Group II) animals. The biochemical mechanism involved in this process is associated with a raise in the urinary concentration of oxalate. Stone formation in Sodium oxalate -injected animals is caused by hyperoxaluria, which causes increased renal retention and excretion of oxalate.48
The formation of CaOx crystals and their retention resulted in a reduction in urine volume in lithiatic control rats. CaOx crystal formation reduces glomerular filtration rate, which further reduces Na +, Cl-, and Ca +2 excretion and increases stone formation.49, 50 Treatment with BPHE increased urination and prevented stone formation salts from becoming supersaturated in the urinary tract. In lithiatic control rats, urine uric acid, phosphorus, calcium, and oxalate levels were all elevated.
Because it is well believed that hypercalciuria is a greater risk factor for the development of renal calculi than hyperoxaluria, urine oxalate levels are more essential than calcium levels.51 Furthermore, elevated uric acid levels in the urine interfere with calcium oxalate solubility and binds to natural stone inhibitors, glycosaminoglycans, reducing their stone-inhibitory action. The amounts of these stone-forming compounds in urine were reduced by BPHE. Aqueous extract of the plant has previously been shown to have an inhibitory effect on urinary oxalate, which validates the findings of the current investigation.52
Creatinine clearance and urine magnesium levels are decreasing, indicating that they are accumulating in the blood, increasing the risk of urolithiasis. Treatment with BPHE increased creatinine clearance and urine magnesium levels while reducing crystallisation. Glycolic acid oxidase activity is increased by NaOx, resulting in the generation of glycolate and oxalate, which increases free radicals, lipid peroxidation, and nitrogenous compounds (creatinine, urea, and uric acid), finally leading to acute tubular necrosis in rats' kidneys.53 In lithiatic control rats, serum creatinine, urea, and uric acid levels were all increased, indicating severe kidney injury. Reduced levels of these nitrogenous compounds after treatment with Bryophyllum pinnatum extract or Cystone indicates that kidney injury can be avoided. The effectiveness of the extract is consistent with a previous research in which an aqueous extract of Bryophyllum pinnatum decreased serum creatinine and urea levels. In a dose-dependent way, BPHE treatment restored calcium, phosphorus, oxalate, and magnesium levels, minimizing and lowering the risk of lithiasis.
NaOx administration has been linked to an increase in the formation of reactive oxygen species (ROS) and oxidative stress in the kidney. In the kidneys of the lithiatic control group, injection of NaOx raised MDA concentration and lowered antioxidant enzyme activity. By lowering MDA levels and correcting alterations in GSH content, CAT, and SOD activity, Cystone or BPHE dramatically reduced oxidative stress. Cystone or extracts reduced lipid peroxidation and enhanced antioxidant enzyme activity, indicating a protective action against oxidative damage. Thus, therapy with Bryophyllum pinnatum extract or Cystone protected renal tissue damage.
In diverse areas of the kidney of rats, NaOx therapy caused significant CaOx crystal deposition and cellular damage, as well as oxidative damage. CaOx crystals were seen in tubular and interstitial areas in lithiatic control rats' kidney parts, along with glomerular congestion and tubular necrosis. Treatment with Cystone or BPHE inhibited the production of CaOx crystals in several sections of the renal tubules, probably by speeding up the breakdown of preformed stone and/or inhibiting the production of new crystals. The findings are supported by the fact that extract of the plant prevented CaOx crystal depositions.
CaOx crystals can obstruct renal tubular flow, resulting in glomerular congestion and tubular degeneration. By preventing the accumulation and retention of CaOx crystals in renal tubules, BPHE was able to protect the kidneys from damage. The fact that extracts have these effects suggests that they can dissolve stones. Bryophyllum pinnatum extract had previously been shown to have an anti-crystallisation effect in vitro, which supports the current findings.54 Plant saponin is said to help with diuretic and stone dissolving properties.55 Previous research has found that the plants' antioxidant properties are important in their antilithiatic properties.56
The presence of saponins and antioxidant phytochemicals such as flavonoids and polyphenols were found in quantitative investigations of Bryophyllum pinnatum extracts, which may have contributed to antilithiatic activity through stone dissolving and antioxidant action. However, more research is needed to determine the specific phytochemical ingredients and mode of action of Bryophyllum pinnatum in urolithiasis prevention.
5. Conclusion
The anti-urolithiatic effect of hydroalcoholic extracts of Bryophyllum pinnatum leaves on the development of urinary calculi (urolithiasis) in Na oxalate-induced lithiasis in rats was demonstrated in this research. As a result, BPHE can help to avoid calcium oxalate crystal deposition in the kidney by preventing hyperoxaluria-induced peroxidative damage to the renal tubular membrane surface, which can lead to calcium oxalate crystal attachment and kidney stone formation. These findings also showed that administering Bryophyllum pinnatum leaf hydroalcoholic extracts decreased and avoided the formation of urinary stones. As a result of its action on early stages of stone growth, Bryophyllum pinnatum leaves hydroalcoholic extracts are useful in preventing disease recurrence.
The mechanism behind this effect may be due to antioxidant nephroprotective properties as well as a reduction in urinary stone-forming constituent concentrations. However, further research is needed to determine the precise mechanism of this behaviour.
Financial support and sponsorship
Nil.
Declaration of competing interest
There are no conflicts of interest.
Acknowledgment
The first author is sincerely thank to Hon. Prashant Patil Gadakh President, Mula Education Society, Sonai, Dr. V. K. Deshmukh Principal, MES's College of Pharmacy, Sonai, for encouragement and availing of the laboratory facilities during course of investigation.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Selvam P., Kalaiselvi P., Govindaraj A., Murugan V.B., Sathishkumar A.S. Effect of A. lanata leaf extract and vediuppu chunnam on the urinary risk factors of calcium oxalate urolithiasis during experimental hyperoxaluria. Pharmacol Res. 2001;43:89–93. doi: 10.1006/phrs.2000.0745. [DOI] [PubMed] [Google Scholar]
- 2.Coe F.L., Evan A., Worcester E. Kidney stone disease. J Clin Invest. 2005;115:2598–2608. doi: 10.1172/JCI26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Divakar K., Pawar A., Chandrasekhar S., Dighe S., Divakar G. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol. 2010;48:1013–1018. doi: 10.1016/j.fct.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Hongshi X., Anna L.Z., Fredric L.C., Elaine M.W. Kidney stones: an update on current pharmacological management and future directions. Expet Opin Pharmacother. 2013;14:435–447. doi: 10.1517/14656566.2013.775250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleisch H. Inhibitors and promoters of stone formation. Kidney Int. 1978;13:361–371. doi: 10.1038/ki.1978.54. [DOI] [PubMed] [Google Scholar]
- 6.Alelign T., Petros B. Kidney stone disease: an update on current concepts. Adv. Urol. 2018:3068365. doi: 10.1155/2018/3068365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prasad K., Sujatha D., Bharathi K. Herbal drugs in urolithiasis - a review. Pharm Rev. 2007;1:175–179. [Google Scholar]
- 8.Doddametikurke R.B., Biyani C.S., Browning A.J., Cartledge J.J. The role of urinary kidney stone inhibitors and promoters in the pathogenesis of calcium containing renal stones EAU-EBU Update. Series. 2007;5:126–136. [Google Scholar]
- 9.Bashir S., Gilani A.H. Antiurolithiatic effect of Bergenia ligulata rhizome: an explanation of the underlying mechanisms. J Ethnopharmacol. 2009;122:106–116. doi: 10.1016/j.jep.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Prachi, Chauhan N., Kumar D., Kasana M.S. Medicinal plants of Muzaffarnagar district used in treatment of urinary tract and kidney stones. Indian J Tradit Knowl. 2009;8:191–195. [Google Scholar]
- 11.Mondal T., Samanta S. An ethnobotanical survey on medicinal plants of Ghatal block, West Midnapur district, West Bengal, India. Int J Curr Res Biosci Plant Biol. 2014;1:35–37. [Google Scholar]
- 12.Mistry J. Traditional medicinal plants used by local people of Murshidabad district, West Bengal, India. World J Pharm Pharmaceut Sci. 2015;4:1225–1235. [Google Scholar]
- 13.Zahid I.H., Bawazir A.S., Naser R. Plant based native therapy for the treatment of kidney stones in Aurangabad (M.S) J Pharmacogn Phytochem. 2013;1:189–193. [Google Scholar]
- 14.Sikdar M., Dutta U. Traditional phytotherapy among the Nath people of Assam. Ethno-Med. 2008;2:39–45. [Google Scholar]
- 15.Moromi T. Ethnomedicinal plants used by the sonowal kacharis of Bhekulajan village in Dibrugarh district, Assam, NE India. Int Res J Environ Sci. 2014;3:54–57. [Google Scholar]
- 16.Lans C.A. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J Ethnobiol Ethnomed. 2006;2:45. doi: 10.1186/1746-4269-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad M., Khan M.R., Manzoor M., Zafar M., Sultana S. Check list of medicinal flora of tehsil Isakhel, district Mianwali-Pakistan. Ethnobot Leaflets. 2006;10:41–48. [Google Scholar]
- 18.Barik L.D., Ratha K.K., Das M., Hazra J. HPTLC method for quantitative determination of quercetin in a polyherbal compound for urolithiasis. Int J Pharmacogn Phytochem Res. 2016;8:1187–1190. [Google Scholar]
- 19.Jain S., Argal A. Effect of a polyherbal formulation on glycolic acid-induced urolithiasis in rats. Bull Pharmaceut Res. 2013;3:40–43. [Google Scholar]
- 20.Ahmed S., Hasan M.M., Alam Mahmood Z. Antiurolithiatic plants: formulations used in different countries and cultures. Pak J Pharm Sci. 2016;29(6):2129–2139. [PubMed] [Google Scholar]
- 21.Yemitan O.K., Salahdeen H.M. Neurosedative and muscle relaxant activities of aqueous extract of Bryophyllum pinnatum. Fitoterapia. 2005;76:187–193. doi: 10.1016/j.fitote.2004.11.009. [PubMed] [DOI] [PubMed] [Google Scholar]
- 22.Okwu D.E., Nnamdi F.U. Two novel flavonoids from Bryophyllum pinnatum and their antimicrobial activity. J Chem Pharmaceut Res. 2011;3:1–10. [Google Scholar]
- 23.Pal S., Nag Chaudhuri A.K. Studies on the anti-ulcer activity of a Bryophyllum pinnatum leaf extract in experimental animals. J Ethnopharmacol. 1991;33:97–102. doi: 10.1016/0378-8741(91)90168-d. [DOI] [PubMed] [Google Scholar]
- 24.Gwehenberger B., Rist L., Huch R., von Mandach U. Effect of Bryophyllum pinnatum versus fenoterol on uterine contractility. Eur J Obstet Gynecol Reprod Biol. 2004;113:164–171. doi: 10.1016/S0301-2115(03)00370-1. [DOI] [PubMed] [Google Scholar]
- 25.Ojewole J.A. Antinociceptive, anti-inflammatory and antidiabetic effects of Bryophyllum pinnatum (Crassulaceae) leaf aqueous extract. J Ethnopharmacol. 2005;99:13–19. doi: 10.1016/j.jep.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Ojewole J.A. Antihypertension properties of Bryophyllum pinnatum (Lam) (oken) leaf extracts. Am J Hypertens. 2002;15:34–39. [Google Scholar]
- 27.Harlalka G.V., Patil C.R., Patil M.R. Protective effect of Kalanchoe pinnata pers. on gentamycin induced nephrotoxicity in rats. Indian J Pharmacol. 2007;39:201–205. [Google Scholar]
- 28.Ahmed S., Hasan M.M., Mahmood Z.A. Antiurolithiatic plants: multidimensional pharmacology. J Pharmacogn Phytochem. 2016;5(2):4–24. [Google Scholar]
- 29.Gaind K.N., Gupta R.L. Alkanes, alkanols, triterpenes, and sterols of Kalanchoe pinnata. Phytochemistry. 1972;11:1500–1502. [Google Scholar]
- 30.Siddiqui S., Faizi S., Siddiqui B.S., Sultana N. Triterpenoids and phenanthrenes from leaves of Bryophyllum pinnatum. Phytochemistry. 1989;28:2433–2438. [Google Scholar]
- 31.Yamagishi T., Haruna M., Yan X.Z., Chang J.J., Lee K.H. Antitumor agents, 110. Bryophyllin B, a novel potent cytotoxic bufadienolide from Bryophyllum pinnatum. J Nat Prod. 1989;52:1071–1079. doi: 10.1021/np50065a025. [DOI] [PubMed] [Google Scholar]
- 32.Rahul V., Takawale, Mali Vishal R. Effect of Lagenaria siceraria fruit powder on sodium oxalate induced urolithiasis in Wistar rats. J Ayurveda Integr Med. 2012;3(2):75–79. doi: 10.4103/0975-9476.96522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yasir F., Waqar M.A. Effect of indigenous plant extracts on calcium oxalate crystallization having a role in urolithiasis. Urol Res. 2011;39:345–350. doi: 10.1007/s00240-011-0374-x. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed S., Hasan M.M., Mahmood Z.A. In vitro urolithiasis models: an evaluation of prophylactic management against kidney stones. J Pharmacogn Phytochem. 2016;5(3):28–35. [Google Scholar]
- 35.Fiske C.H., Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–381. [Google Scholar]
- 36.Heaton F.W. Determination of magnesium by the Titan yellow and ammonium phosphate methods. J Clin Pathol. 1960;13:358–360. doi: 10.1136/jcp.13.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodgkinson A. Determination of oxalic acid in biological material. Clin Chem. 1970;16:547–557. [Google Scholar]
- 38.Neill D.W., Neely R.A. The estimation of magnesium in serum using titan yellow. J Clin Pathol. 1956;9:162–163. doi: 10.1136/jcp.9.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verley H. CBS Publishers; New Delhi: 2003. Practical Clinical Biochemistry; pp. 356–361. [Google Scholar]
- 40.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 41.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 42.Takahara S., Hamilton H.B., Neel J.V., Kobara T.Y., Ogura Y., Nishimura E.T. Hypocatalasemia: a new genetic carrier state. J Clin Invest. 1960;39:610–619. doi: 10.1172/JCI104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 44.Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 45.Liu J., Cao Z., Zhang Z. A comparative study on several models of experimental renal calcium oxalate stones formation in rats. J Huazhong Univ Sci Technol - Med Sci. 2007;27:83–87. doi: 10.1007/s11596-007-0124-z. [DOI] [PubMed] [Google Scholar]
- 46.Gupta P., Patel N., Bhatt L. Anti-urolithiatic effect of petroleum ether extract stem bark of Crataeva adansonii in rats. Pharm Biol. 2006;44:160–165. [Google Scholar]
- 47.Mitra S.K., Gopumadhavan S., Venkatarangannna M.V., Sundaram R. Effect of cystone: a herbal formulation, on glycolic acid-induced urolithiasis in rats. Phytother Res. 1998;12:372–374. [Google Scholar]
- 48.Mondal T., Samanta S. An ethnobotanical survey on medicinal plants of Ghatal block, West Midnapur district, West Bengal, India. Int J Curr Res Biosci Plant Biol. 2014;1:35–37. [Google Scholar]
- 49.Fleisch H. Inhibitors and promoters of stone formation. Kidney Int. 1978;13:361–371. doi: 10.1038/ki.1978.54. [DOI] [PubMed] [Google Scholar]
- 50.Karadi R.V., Gadge N.B., Alagawadi K.R., Savadi R.V. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 2006;105:306–311. doi: 10.1016/j.jep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 51.Agarwal M.M., Singh S.K., Mavuduru R., Mandal A.K. Preventive fluid and dietary therapy for urolithiasis: an appraisal of strength, controversies and lacunae of current literature. Indian J Urol. 2011;27:310–319. doi: 10.4103/0970-1591.85423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shukla A.B., Mandavia D.R., Barvaliya M.J., Baxi S.N., Tripathi C.R. Evaluation of anti-urolithiatic effect of aqueous extract of Bryophyllum pinnatum (Lam.) leaves using ethylene glycol-induced renal calculi. Avicenna J Phytomed. 2014;4:151–159. [PMC free article] [PubMed] [Google Scholar]
- 53.Sumathi R., Jayanthi S., Kalpanadevi V., Varalakshmi P. Effect of DL alpha-lipoic acid on tissue lipid peroxidation and antioxidant systems in normal and glycollate treated rats. Pharmacol Res. 1993;27:309–318. doi: 10.1006/phrs.1993.1031. [DOI] [PubMed] [Google Scholar]
- 54.Yasir F., Waqar M.A. Effect of indigenous plant extracts on calcium oxalate crystallization having a role in urolithiasis. Urol Res. 2011;39:345–350. doi: 10.1007/s00240-011-0374-x. [DOI] [PubMed] [Google Scholar]
- 55.Patel P.K., Patel M.A., Vyas B.A., Shah D.R., Gandhi T.R. Antiurolithiatic activity of saponin rich fraction from the fruits of Solanum xanthocarpum Schrad. & Wendl. (Solanaceae) against ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 2012;144:160–170. doi: 10.1016/j.jep.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 56.Mishra L.C. CRC Press; Boca Raton, New York: 2004. Scientific Basis for Ayurvedic Therapies; pp. 535–550. [Google Scholar]