Abstract
Background and aim
Phytoestrogens are traditionally used for cardiovascular risks but direct effects on the ischemic heart remain unclear. Plants with phytoestrogens are used for reducing menopausic symptoms and they could also be cardioprotectives. Here we investigated whether maca (Lepidium meyenii) contains isoflavones and prevents cardiac stunning, in comparison to soy isoflavones.
Experimental procedure
Both products were orally and daily administered to rats during 1 week before exposing isolated hearts to ischemia/reperfusion (I/R). Young male (YM), female (YF) and aged female (AgF) rats treated with maca (MACA, 1 g/kg/day) or soy isoflavones (ISOF, 100 mg/kg/day) were compared to acute daidzein (DAZ, 5 mg/kg i.p.) and non-treated rat groups. Isolated ventricles were perfused inside a calorimeter to simultaneously measure contractile and calorimetrical signals before and during I/R.
Results and conclusions
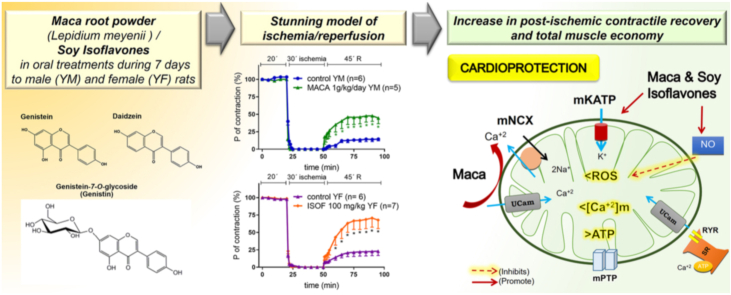
Maca has genistein and daidzein. MACA and ISOF improved the post-ischemic contractile recovery (PICR) and muscle economy (P/Ht) in YM and YF hearts, but not in AgF hearts. DAZ improved PICR and P/Ht more in YM than in YF. The mKATP channels blockade reduced both PICR and P/Ht in DAZ-treated YM hearts, without affecting them in ISOF or MACA-treated YM hearts. In MACA treated YF hearts, the simultaneous blockade of NOS and mKATP channels, or the mNCX blockade reduced cardioprotection. Results show that subacute oral treatment with maca or with soy isoflavones was strongly preventive of cardiac ischemic dysfunction, more than the acute administration of a pure isoflavone (daidzein, genistein). Maca induced synergistic and complex mechanisms which prevented mitochondrial calcium overload.
Keywords: Maca, Daidzein, Myocardial economy, Mitochondria, mKATP channels, mNCX, NOS, Isoflavones, Calcium, Cardiomyocytes
Abbreviations: AgF, aged female rats; CICUAL, Institutional Committee for Care of Laboratory Animals; CONICET, National Council of Scientific and Technical Research; DAZ, daidzein; DMSO, dimethylsulphoxide; F, Fisher coefficient for variance statistical test; 5-HD, 5-hydroxydecanoate; HPLC, high performance liquid chromatography; Ht, total heat rate; i.p, intraperitoneal; I/R, ischemia and reperfusion; ISOF, soy isoflavones; l-NAME, Nω-nitro-l-arginine methyl ester hydrochloride; ΔLVEDP, resting diastolic pressure; MACA, Lepidium meyenii root powder; mKATP, mitochondrial ATP-dependent K+ channels; mNCX, mitochondrial Na/Ca exchanger; NOS, nitric oxide synthases; P, maximal pressure developed in contraction; PICR, post-ischemic contractile recovery; P/Ht, muscle economy; PKC, protein-kinase C; ROS, reactive oxygen species; SEM, standard error of media; TFT, triphenyltetrazolium chloride; YF, young female rats; YM, young male
Graphical abstract
Highlights
-
•
Subacute oral administration of maca or soy isoflavones reduced rat cardiac stunning.
-
•
Maca powder contains the isoflavones genistein, daidzein and genistin.
-
•
Maca and soy isoflavones improved the postischemic recovery more than individual isoflavones, with synergic mechanisms.
-
•
The synergic mechanisms include activation of mNCX, NOS and mKATP channels.
-
•
Maca cardioprotection was lost in female aged rats.
1. Introduction
Cardiovascular diseases are the leading cause of death in the world. Prevalence of coronary disease is higher in elderly people, despite of being lower in women at earlier ages.1 Consequently, the influence of ageing and sex on both, myocardial ischemia–reperfusion and cardioprotective interventions, is a subject of interest and continuous investigation.2 Phytoestrogens are naturally occurring compounds that reduce the incidence of cardiovascular diseases, because they decrease LDL-cholesterol while increasing HDL-cholesterol.3 The most described phytoestrogens are soy isoflavones genistein and daidzein, which are also traditionally used to prevent menopausal symptoms all over the world.4 Observational studies and short-term randomized clinical trial suggest that isoflavones are anti-atherogenic, improve arterial stiffness and potentially prevent coronary heart disease.5 Li and Zhang demonstrated that isoflavones had benefits on ischemic patients after a 24-week intervention and increased the antioxidant capacities.6 The effectivity and mechanisms of action depend on experimental models of ischemic disease (either infarct or stunning), as well as the model of estrogenic deficiency (induced by ovariectomy or by ageing). In ovariectomized rats, isoflavones reduced the myocardial infarct size after coronary artery occlusion, and improved ventricle function through increasing the PI3K/Akt/eNOS pathway and decreasing oxidative stress.7 However, the effects of isoflavones on cardiac stunning were not still completely explored. Stunning was clinically defined as a postischemic contractile reduction partially and slowly reversed, without infarct. In previous works we showed that the isoflavone genistein was cardioprotective in cardiac stunning of rats with estrogenic deficiency, such as young males (YM) and aged females (AgF), but not in young female (YF) rats. Genistein cardioprotection in those models of ischemia/reperfusion (I/R) was mediated by pathways that involve PKC and mKATP channels activation.8
Some other plants have been suggested to have isoflavones. One of them is Lepidium meyenii Walp (Brassicaceae), known as “maca” and traditionally employed in the Peruvian Andean region as functional food with energetic properties and fertility improvement.9 The hypocotyl powder contains carbohydrates, lipids, proteins, fibers, unsaturated fatty acids and phytosterols.10 It also contains imidazolic alkaloids (lepidilin A and B),11 benzyl-alkamides (macamides), and macaens (benzyl fatty acids).12 The beneficial effects of maca were described in fertility and reproduction,13,14 depression,9 cerebral infarct,15 swimming capacity,16 estradiol level and symptoms of menopausic women.17 Their possibilities of preventing cardiac stunning under an ischemic episode were not still studied, and the effectivity in cerebral infarct and menopausic symptoms suggested their potential cardioprotective properties. The calorimetrical methodology allows to study whether an in vivo or ex vivo pharmacological intervention could protect the isolated heart from ischemic dysfunction, because it is sensitive to continuously detect changes in contractility and energy expenditure still during ischemia and reperfusion.18,19 Heart function is strongly coupled to mitochondrial metabolism that provides chemical energy, and this relationship can be monitored by changes in total muscle economy (as a ratio between work or contractility and energy expenditure expressed by heat release).18,19 So, cardiac calorimetry is sensitive enough to evaluate the consequences of I/R on the exothermic cellular processes, such as ATP hydrolysis and mitochondrial metabolism, which are affected by I/R. Previous in vivo treatments could improve muscle economy, and selective pharmacological inhibitors allow to evaluate the role of certain pathways in cardioprotection during the I/R.
Therefore, the aim of this work was to study whether oral subacute treatment with maca powder or soy isoflavones could prevent the mechano-energetical dysfunction in a severe stunning model of cardiac ischemia/reperfusion in rats. Moreover, the sex and age influences on cardioprotection and cellular mechanisms of these natural products were explored.
2. Materials and methods
2.1. Natural products
Commercial soy isoflavones (40% w/w isoflavones) and powder of maca root (Lepidium meyenii Walp, Brassicaceae) were suspended in water before oral administration. The quality control of commercial maca powder was done by identifying starch particles stained with Lugol on an optical microscopy, in comparison to the powder obtained from an original hypocotyl of maca. It was identified by Prof. Agr. Eng. Marta Colares (Herbarium LPAG, College of Agronomical and Forestal Sciences, UNLP).20
2.2. Phytochemical screening of maca root
Commercial powder of maca root was macerated with ethanol 70°, for 24 h at room temperature, continuously shaking. Total extract was recuperated by cold centrifugation, concentrated in a rotary vacuum evaporator and dried in vacuum stove. One portion of total extract was partitioned with ethyl-acetate (EtAcO). The ethanolic extract and their EtAcO fraction were analyzed by qualitative chemical reactions (Table 1).21 Also the extract and their fractions were analyzed for chromatographic profiles.22,23 Thin layer chromatography (TLC) system: on silicagel G60 F254, mobile phase of ethyl acetate-formic acid-glacial acetic acid-water (100:11:11:26), detection under UV light at 365 nm.23
Table 1.
Chemical screening of the ethanolic extract of maca root powder.
| Compounds evaluated | Reactive | Result |
|---|---|---|
| phenols | Ferric chloride | ++ (2-OH) |
| lipids | Iodine | + |
| peptides, aminoacids | Ninhidrine | + |
| carbohydrates | Molisch | ++ |
| anthraquinones | Bornträger | – |
| steroids | Liebermann-Burchard | + |
| triterpenes | Liebermann-Burchard | – |
| flavonoids | Shinoda | + |
| tannins | Gelatin | – |
| alkaloids | Dragendorff | ++ |
2.3. Analysis of isoflavones composition
The isoflavones composition of the commercial samples of soy isoflavones and maca powder were analyzed by using a similar high-performance liquid chromatography in reverse phase (RP-HPLC-DAD) system. Soy isoflavones sample was suspended in water for analysis. For maca, the fraction obtained with ethyl acetate from the ethanolic extract was analyzed. The analysis was performed in a HPLC system with photodiode array detector and a symmetric reverse-phase C-18 column (see details in Supplementary Table A.1). As mobile phase, acetonitrile (solvent A) and water-acetic acid (40:1, solvent B) were used. The elution gradient consisted in 15% solvent A and 85% solvent B, for 15 min, followed by a linear gradient to 65% solvent B for 30 min and then a sharp transition to 0% solvent B in 2 min. The chromatographic separation was performed at room temperature at a flow rate of 1.0 ml/min. The elution was monitored at 254 and 360 nm. The identification of each compound was achieved by comparing retention times and UV spectra with those of standards.
2.4. Animals and experimental groups
The experiments were approved by the Laboratory Animal Care and Research Committee (CICUAL, protocol number 015-5-2015, renewed on 2019) of the School of Exact Sciences, National University of La Plata (UNLP), Argentina. Animal experiments were conducted according to the recommendations of the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, Eight edition; 2011), the latest directives of the European Union for laboratory animal care (2010), and the Resolution 1047, Annex II of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina).
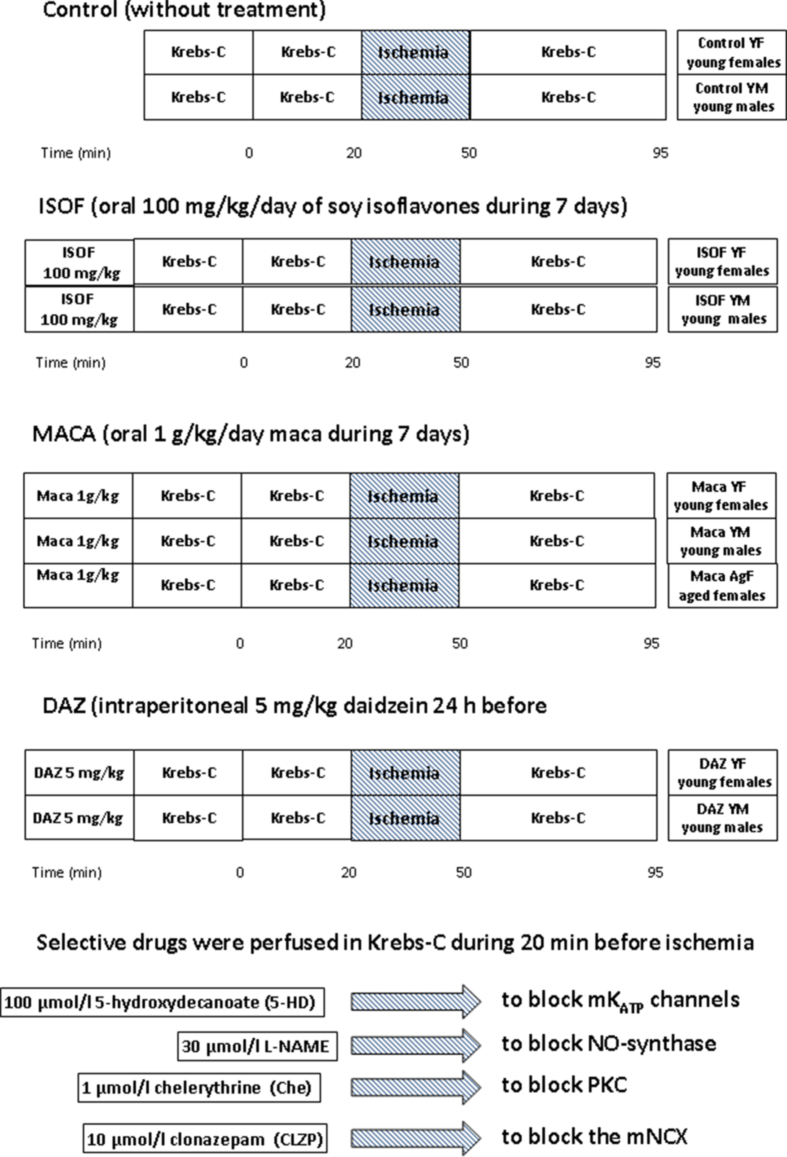
Young male (6–9 months), young female (6–9 months) Sprague Dawley rats (230–280 g) and aged female Wistar rats (over 20 months old, 320–380 g) were maintained in the Bioterium of Pharmacology with standard pellet food and water ad libitum. Young male (YM), young female (YF) and aged female (AgF) rats were randomly divided in several groups with the following treatments (See Supplementary Fig. A.2): Control: without treatment; ISOF: with 100 mg/kg/day of soy isoflavones by intragastric administration during 7 days; MACA: with 1 g/kg/day maca powder by intragastric administration during 7 days; DAZ: 5 mg/kg of daidzein was given by intraperitoneal administration 24 h before the ex vivo experiment (positive control).
2.5. Pharmacological studies
2.5.1. Isolated rat heart and I/R model
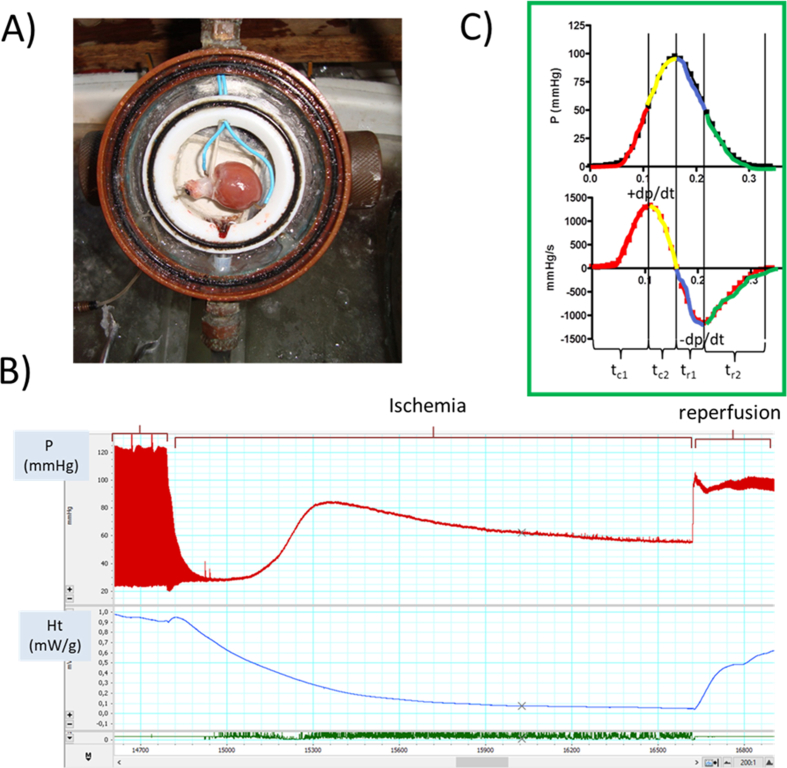
Rats were anesthetized with an intraperitoneal injection of pentobarbital (40 mg/kg i.p.) and received heparine (2000 IU) and subcutaneous tramadol for deep analgesia (10–20 mg/kg s.c.). Heart was excised and the coronaries were perfused by the Langendorff technique with Krebs solution, at a constant flow of 7 ml/min/g with a peristaltic pump, as previously described.8 Atria were excised and spontaneous beating was stopped. A latex balloon was inserted in the left ventricle to measure intraventricular pressure through a cannula with water connected to a pressure transducer. Perfused ventricles were introduced in the chamber of a flow calorimeter,19 and it was submerged in a water bath at constant temperature of 37 °C. After equilibration, the left ventricular pressure (LVP) and total heat rate (Ht) were simultaneously measured (See Supplementary Fig. A.1). The calorimeter is a cylindrical mass of copper that contains a chamber with 2 Peltier units (127 thermocouples each one and ceramic walls) which have contact with the heart. The units additively record the temperature differences between the internal (heart) and external (bath) calorimetrical walls, and is calibrated in potency (heat rate, in mW) units. Perfused ventricles were electrically stimulated at 3 Hz (5 V, 5 ms). Signals of LVP and Ht were simultaneously recorded in a computer (details in Supplementary Table A.1). Total muscle heat rate (Ht, in mW/g) was calculated from the calorimetrical signal continuously recorded with ventricle after subtraction of the base line and multiplying for the calibration factor, either with or without perfusion, as previously described.8 Maximal pressure of contraction (P), changes in the diastolic pressure over initial (ΔLVEDP), maximal rates of contraction (+dP/dt = +P) and relaxation (-dP/dt = -P), and the periods of contraction (tC1 and tC2) and relaxation (tR1 and tR2) were calculated (See Supplementary Fig. A.1), as well as total muscle economy (P/Ht in mmHg.g/mW). For comparison among protocols, P and P/Ht were expressed as percentage of their initial values (shown in Supplementary Table A.2).
After stabilization, the respective perfusion protocol was applied followed by 30 min of no-flow ischemia (I) and 45 min reperfusion (R) with Krebs solution. The protocols applied to YM, YF or AgF hearts consisted in perfusing only Krebs solution (control) or the following selective inhibitors during 20 min before ischemia (see Supplementary Fig. A.2): 100 μmol/l 5-hydroxydecanoate (5HD, to inhibit mKATP channels); 100 μmol/l l-NAME (to inhibit NO-synthases); 1 μmol/l chelerythrine (Che, to block PKC); 10 μmol/l clonazepam (Clzp, to block mNCX).8,19
At the end of experiment, the calculated water content of hearts resulted 82.3 ± 0.4% of the wet weight (0.875 ± 0.040 g) dried weight: 0.153 ± 0.007 g (n = 28).
2.5.2. Determination of myocardial infarcted area
To measure the infarcted area in some hearts at the end of reperfusion, it was used 1% triphenyltetrazolium chloride (TFT) tinction to incubate 4 transverse slices cut from apex to base. After 5 min, slices were scanned and their areas were measured, in red (viable sections) and unstained (infarcted). The infarcted area was expressed as a percentage of the total ventricular area (AI/VI%), as previously described.8
2.5.3. Determination of calcium in cardiomyocytes with confocal microscopy
Ventricular myocytes were isolated from YF rat hearts by the previously described method.8 Heart was perfused with a Krebs–Hepes solution in order to isolate cells, by using collagenase P and protease XIV.8 Cardiomyocytes were loaded with Fluo-4 AM during 15 min for measuring cytosolic Ca2+ signals, and with Rhod-2 AM for 1h at 4 °C and washing 1 h at 37 °C for mitochondrial Ca2+ signals. Resting cells were superfused in a laminin-precoated chamber with Krebs–Hepes solution containing 2 mmol/l Ca2+ (C) up to stabilization. Changes in fluorescence were recorded in a confocal microscope, and changes in F/Fo were calculated over the control baseline (C) as previously decribed.8 The protocol consisted in perfusing C (10 min), C+10 mmol/l caffeine-36 mmol/l Na+ (10 min), C (5 min). This protocol was carried out in cardiomyocytes from non-treated YF rats and those treated with maca 1 g/kg/day by 7 days.
2.6. Drugs and solutions
The brand and origin of drugs is shown on Table A.1. For the phytochemical study, chemicals and solvents of analytical AR grade and standards of isoflavones (genistin, genistein, daidzein and glycitein) were used. In the pharmacological evaluation, daidzein, 5-hydroxydecanoate, l-NAME, chelerythrine and clonazepam were dissolved in dimethyl sulfoxide (DMSO), conserved at −20 °C and diluted in Krebs solution. The compositions of Krebs solution for ventricles perfusion and Krebs-Hepes solution for cardiomyocytes were similar to the previously described.8
2.7. Statistical analysis
Results are expressed as means ± SEM. Statistical multiple comparisons were done by two-way ANOVA with the variables treatment and time during I/R (Table A.4). A posteriori Tukey’s tests for paired comparisons were applied and shown on figures. One-way ANOVA was also used for comparing each initial cardiac parameter of hearts among the in vivo treatments, with Tukey’s post-hoc tests. Student t-test was used for comparing 2 treatments in aged hearts (Table 2). Statistical analyses were made by using Graph Pad Prism 6.0 software. In all tests, p values < 0.05 were considered significant.
Table 2.
Absolute initial values of mechano-calorimetrical parameters in each experimental hearts group before applying I/R, and statistical results of one-way ANOVA. ∗p < 0.05 vs. the respective non-treated rats (control).
| Experimental group (n) | P (mm Hg) | +P/P (s−1) | -P/P (s−1) | tc1 (s) | tc2 (s) | tR1 (s) | tR2 (s) | Ht (mW/g) | P/Ht (mmHg.g/mW) |
|---|---|---|---|---|---|---|---|---|---|
| Young male rats (YM) | |||||||||
| Control (n = 6) | 84.4 ± 6.0 | 13.2 ± 0.9 | −15.0 ± 2.1 | 0.12 ± 0.01 | 0.05 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.02 | 19.0 ± 1.8 | 4.6 ± 0.6 |
| DAZ (n = 9) | 86.9 ± 7.9 | 19.8 ± 1.1∗ | −13.1 ± 0.4 | 0.09 ± 0.01 | 0.03 ± 0.01∗ | 0.06 ± 0.01 | 0.07 ± 0.01 | 18.5 ± 1.1 | 4.7 ± 0.3 |
| ISOF (n = 18) | 71.5 ± 6.1 | 19.7 ± 1.1∗ | −15.1 ± 0.8 | 0.07 ± 0.01∗ | 0.03 ± 0.01∗ | 0.05 ± 0.01 | 0.07 ± 0.01 | 16.2 ± 0.9 | 4.4 ± 0.3 |
| MACA (n = 15) | 68.7 ± 5.8 | 21.1 ± 0.9∗ | −16.4 ± 0.7 | 0.05 ± 0.01∗ | 0.04 ± 0.01∗ | 0.05 ± 0.01 | 0.06 ± 0.01 | 14.2 ± 1.3 | 5.0 ± 0.4 |
| 1-way ANOVA | F = 1.578 P > 0.1, ns |
F = 4.512 P < 0.01 |
F = 2.279 P > 0.05, ns |
F = 7.768 P < 0.001 |
F = 4.653 P < 0.01 |
F = 0.4769 P > 0.5, ns |
F = 0.3236 P > 0.5, ns |
F = 2.947 P < 0.05 |
F = 0.5894 P > 0.5, ns |
| Young female rats (YF) | |||||||||
| Control (n = 6) | 71.0 ± 14.0 | 18.0 ± 1.5 | −15.7 ± 1.3 | 0.13 ± 0.02 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.01 | 14.4 ± 2.5 | 4.9 ± 0.2 |
| DAZ (n = 4) | 89.4 ± 28.2 | 20.9 ± 2.1 | −11.9 ± 1.0∗ | 0.06 ± 0.01∗ | 0.03 ± 0.01 | 0.07 ± 0.01∗ | 0.10 ± 0.01 | 19.5 ± 6.3 | 4.6 ± 0.3 |
| ISOF (n = 7) | 55.6 ± 10.6 | 17.7 ± 1.1 | −14.5 ± 0.8 | 0.12 ± 0.02 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.02 | 13.1 ± 2.1 | 4.2 ± 0.2 |
| MACA (n = 18) | 93.3 ± 6.1 | 18.5 ± 0.8 | −13.3 ± 0.3∗ | 0.06 ± 0.01∗ | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.08 ± 0.01 | 20.3 ± 1.4 | 4.8 ± 0.4 |
| 1-way ANOVA | F = 2.664 P > 0.05, ns |
F = 0.8901 P > 0.1, ns |
F = 3.543 P < 0.05 |
F = 10.35 P < 0.0001 |
F = 4.360 P < 0.05 |
F = 6.001 P < 0.01 |
F = 1.440 P > 0.1, ns |
F = 2.493 P > 0.05, ns |
F = 0.3885 P > 0.5, ns |
| Aged female rats (AgF) | |||||||||
| Control (n = 5) | 65.5 ± 7.0 | 20.7 ± 0.9 | −19.4 ± 1.0 | Not measured | Not measured | Not measured | Not measured | 17.0 ± 1.2 | 4.8 ± 0.4 |
| MACA (n = 4) | 35.0 ± 10.0 | 22.5 ± 3.4 | −18.4 ± 3.7 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.08 ± 0.02 | 17.0 ± 5.0 | 3.0 ± 1.0 |
| t-test | t = 2.584 df = 7, p < 0.05 |
t = 0.5681, df = 7, ns |
t = 0.2862 df = 7, ns |
t = 0.004341 df = 7, ns | t = 0.9202 df = 7, ns | ||||
3. Results
3.1. Phytochemical analysis
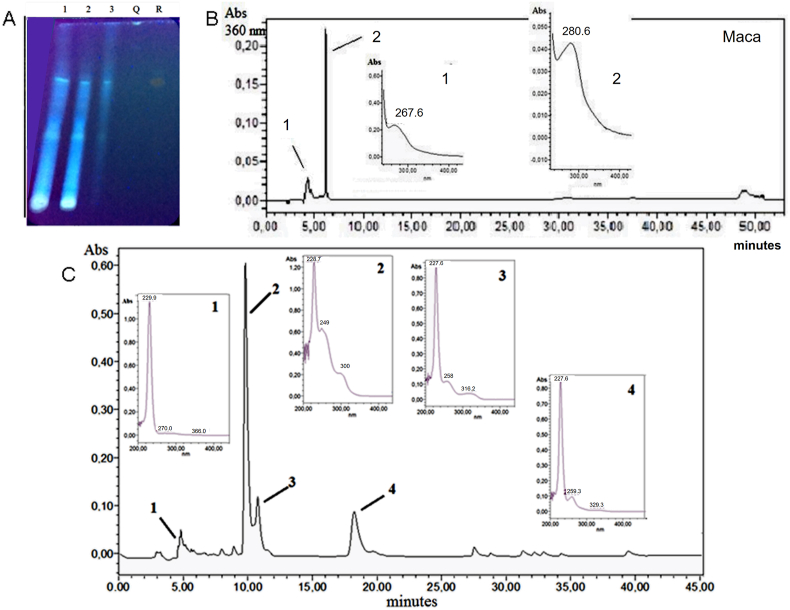
Chemical qualitative screening of maca powder evidenced carbohydrates, lipids, peptides, phenolic compounds (with 2 adjacent OH), flavonoids, steroids and alkaloids (Table 1). Ethanolic extract and their aqueous and ethyl acetate fractions were analyzed by planar chromatographic profile. Blue fluorescent zones at Rf 0.45–0.50 and 0.60–0.75 correspond to isoflavones (Fig. 1A). As a constituent of the flavonoids evidenced in maca, the presence of isoflavones was determined by RP-HPLC-DAD (Fig. 1B). These compounds could be purified with ethyl acetate, genistein-7-O-glycoside called genistin (19.30%), daidzein (1.36%) and genistein (78.11%) being identified in this fraction. Another possible isoflavone (3′, 4′, 7-tri-OH) not yet identified was also observed (1.23%).
Fig. 1.
Chromatographic profiles of maca in TLC (A) and HPLC (B), and soy isoflavones in HPLC (C). In A: ethanolic extract (1); aqueous (2) and ethyl-acetate (3) fractions of the ethanolic extract, respectively, quercetin (Q), and rutin (R). In B: main isoflavones in ethyl-acetate fraction of maca (1- genistin, 2- genistein). In C: main soy isoflavones found in commercial sample (1- genistin, 2-daidzein, 3-glycitein, 4-genistein).
A sample of soy isoflavones was analyzed by the same HPLC system described for maca. The presence of 4 isoflavones was determined, the most important being genistein (51.5%). Fig. 1C shows the corresponding chromatogram and the spectra obtained. The retention times (in minutes) and composition (% of separated isoflavones) were the following: genistin (4.59, 21.1%), daidzein (9.81, 22.6%), glycitein (10.76, 4.8%) and genistein (18.23, 51.5%).
3.2. Pharmacological effects on the I/R model in hearts
Before ischemia, the treatments with MACA, ISOF and DAZ induced changes in some of the contractile parameters. Table 2 shows that treatments with MACA, ISOF and DAZ in YM rat hearts increased the relative maximal rate of contraction (+P/P ratio), and MACA and ISOF reduced the times of contraction, tc1 and/or tc2. However, these treatments did not affect the relative rates of relaxation (-P/P) nor their times (tR1 and tR2). At a difference, in YF rat hearts the +P/P ratio was not significantly changed by the treatments, although tc1 was reduced by MACA and DAZ. Moreover, both DAZ and MACA reduced the -P/P ratio, and DAZ increased tR1. In AgF rat hearts, MACA reduced P, without significantly modifying other parameters (Table 2). The model of stunning in rat hearts stimulated at 3 Hz and exposed to 30 min ischemia/45 min reperfusion (I/R) was previously characterized.8,24 The infarction size (AI/VI%) after R in the different groups studied here was very low, considering that hearts resulted severely stunned without significant infarction (Supplementary Table A.3).
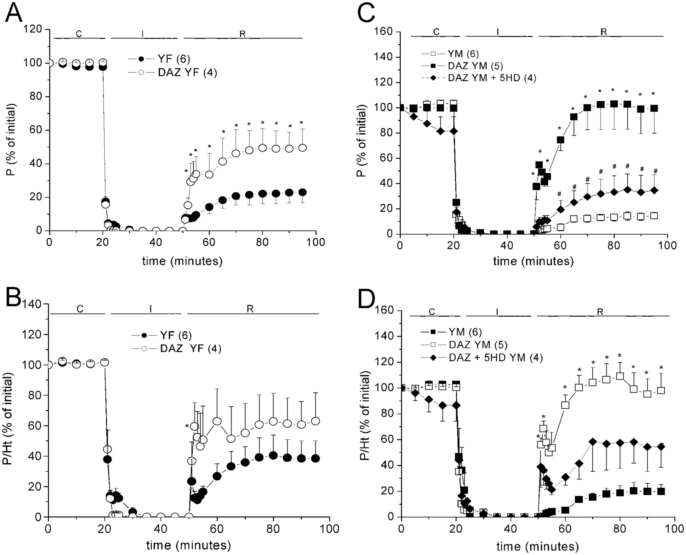
3.2.1. Effects of daidzein
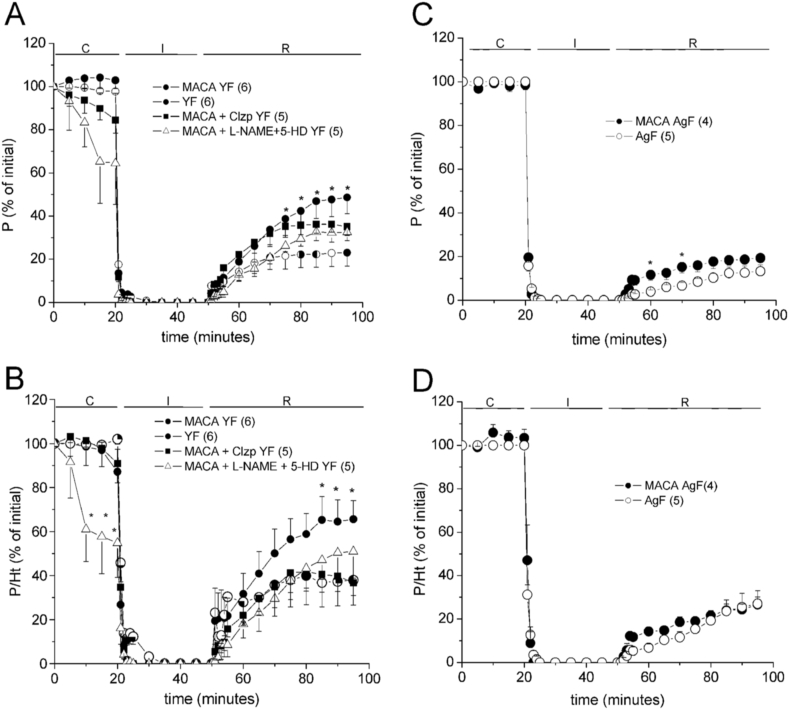
The i.p. administration of 5 mg/kg DAZ one day before the I/R experiment increased the post ischemic contractile recovery (PICR) in both sexes (Fig. 2), without modifying the Ht recovery nor the diastolic contracture (ΔLVEDP) typical of I/R (Table 3). The P/Ht ratio was also significantly improved, and DAZ cardioprotection was higher in YM than in YF rat hearts (Fig. 2). For investigating whether daidzein cardioprotection depends on mKATP channels opening, other group of DAZ-treated YM rat hearts was perfused with 5-HD before I/R. Fig. 2 (C-D) shows that this intervention significantly reduced the P and P/Ht recoveries, as well as it strongly increased ΔLVEDP during R (Table 3), indicating that DAZ activates mKATP channels.
Fig. 2.
Effects of 5 mg/kg i.p. daidzein (DAZ) on the contractile recovery (P, % of initial) of young female (YF, in A) and young male (YM, in C) rat hearts in the absence and the presence of 5-HD. The respective recoveries in total muscle economy (P/Ht, % of initial) are shown in B and D. The 2-way ANOVA results are shown in Supplementary Table A.4, post-hoc tests: ∗p < 0.05 vs non-treated hearts, #p < 0.05 vs DAZ-treated group.
Table 3.
Changes in the diastolic intraventricular pressure (ΔLVEDP, in mm Hg) over the preischemic values, at 5 and 30 min ischemia (I) and at 5 and 45 min of reperfusion (R) in the different protocols assessed. Two way- ANOVA results, ∗p < 0.05 vs control (n).
| Experimental group (n) | I 5′ | I 30′ | R 5′ | R 45′ | |||
|---|---|---|---|---|---|---|---|
| Young male rats (YM) | |||||||
| Control (6) | −6.3 ± 3.0 | 7.5 ± 3.8 | 17.9 ± 2.6 | 16.2 ± 3.8 | |||
| DAZ (5) | 5.8 ± 1.5 | 44.8 ± 6.3 | 43.5 ± 4.7 | 25.9 ± 2.2 | |||
| ISOF (5) | 20.0 ± 6.4 | 22.8 ± 7.4 | 42.8 ± 6.8 | 23.3 ± 5.7 | |||
| MACA (5) | 14.2 ± 7.9 | 27.0 ± 5.2 | 51.4 ± 14.9 | 34.2 ± 12.9 | |||
| DAZ + 5HD (4) | 0.5 ± 2.5 | 44.8 ± 8.4 | 109.6 ± 14.5∗ | 69.9 ± 6.7∗ | |||
| ISOF + 5HD (4) | 26.4 ± 16.3 | 59.7 ± 5.4∗ | 78.7 ± 12.2∗ | 47.7 ± 8.6 | |||
| ISOF + l-NAME (4) | 8.5 ± 4.3 | 38.5 ± 3.7 | 73.1 ± 11.1∗ | 49.6 ± 5.4 | |||
| ISOF + l-NAME + 5HD (5) | 7.6 ± 6.3 | 48.6 ± 6.5∗ | 83.8 ± 7.9∗ | 75.0 ± 7.4∗ | |||
| MACA + Che (5) | 18.4 ± 8.9 | 17.0 ± 6.2 | 42.4 ± 7.1 | 9.8 ± 6.6 | |||
| MACA + 5HD (5) | 0.8 ± 2.0 | 32.6 ± 6.1 | 43.5 ± 10.1 | 28.0 ± 7.9 | |||
| Two-way ANOVA: | |||||||
| By treatment: F = 18.07, DFn = 9, DFd = 152, P < 0.0001 | |||||||
| By time: F = 71.70, DFn = 3, DFd = 152, P < 0.0001 | |||||||
| Young female rats (YF) | |||||||
| Control (6) | 17.1 ± 12.6 | 12.5 ± 4.9 | 52.6 ± 5.0 | 31.4 ± 3.2 | |||
| DAZ (4) | −23.1 ± 7.8 | 13.5 ± 12.9 | 56.6 ± 20.7 | 30.1 ± 19.9 | |||
| ISOF (7) | 2.0 ± 7.5 | 15.6 ± 8.2 | 37.3 ± 4.1 | 10.9 ± 6.0 | |||
| MACA (6) | −7.7 ± 6.1 | 27.9 ± 8.4 | 88.7 ± 18.4∗ | 74.4 ± 20.2 | |||
| MACA + l-NAME + 5HD (5) | 14.8 ± 20.0 | 29.4 ± 12.9 | 78.8 ± 13.2 | 62.1 ± 14.2 | |||
| MACA + Clzp (5) | −9.6 ± 5.2 | 10.3 ± 12.5 | 49.8 ± 14.8 | 40.3 ± 12.6 | |||
| Two-way ANOVA: | |||||||
| By treatment: F = 5.094, DFn = 5, DFd = 108, P < 0.001 | |||||||
| By time: F = 30.48, DFn = 3, DFd = 108, P < 0.0001 | |||||||
| Aged female rats (AgF) | |||||||
| Control (5) | 16.2 ± 6.8 | 23.8 ± 4.7 | 80.3 ± 6.7 | 74.9 ± 9.1 | |||
| MACA (4) | −3.8 ± 2.8 | 5.0 ± 3.7 | 32.6 ± 13.7∗ | 19.4 ± 11.1∗ | |||
| Two-way ANOVA: | |||||||
| By treatment: F = 39.64, DFn = 1, DFd = 28, P < 0.0001 | |||||||
| By time: F = 18.88, DFn = 3, DFd = 28, P < 0.0001 | |||||||
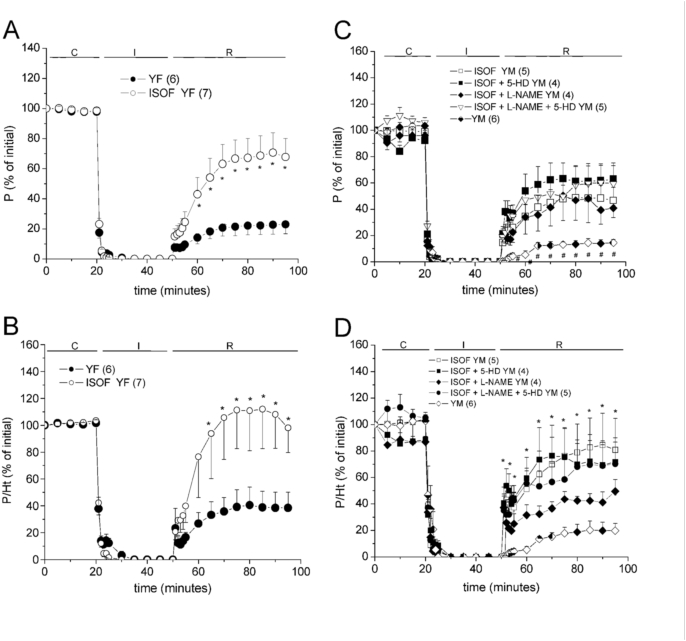
3.2.2. Effects of soy isoflavones
One week of oral administration of ISOF improved PICR as well as muscle economy (P/Ht) in both sexes (Fig. 3), with a non-significant increase in ΔLVEDP during I/R respect to the non-treated rat hearts (Table 3). So, ISOF cardioprotection was independent on the sex.
Fig. 3.
Effects of oral administration of 100 mg/kg/day soy isoflavones (ISOF) during 1 week on the contractile recovery (P, % of initial) and muscle economy (P/Ht, % of initial) of young female (YF, A-B) and young male (YM, C-D) rat hearts. C-D also show the effects of perfusing 100 μmol/L 5-HD, 100 μmol/L l-NAME and both. The 2-way ANOVA results are shown in Supplementary Table A.4, post-hoc tests: ∗p < 0.05 vs non-treated hearts, #p < 0.05 vs all the others.
3.2.3. Effects of Lepidium meyenii
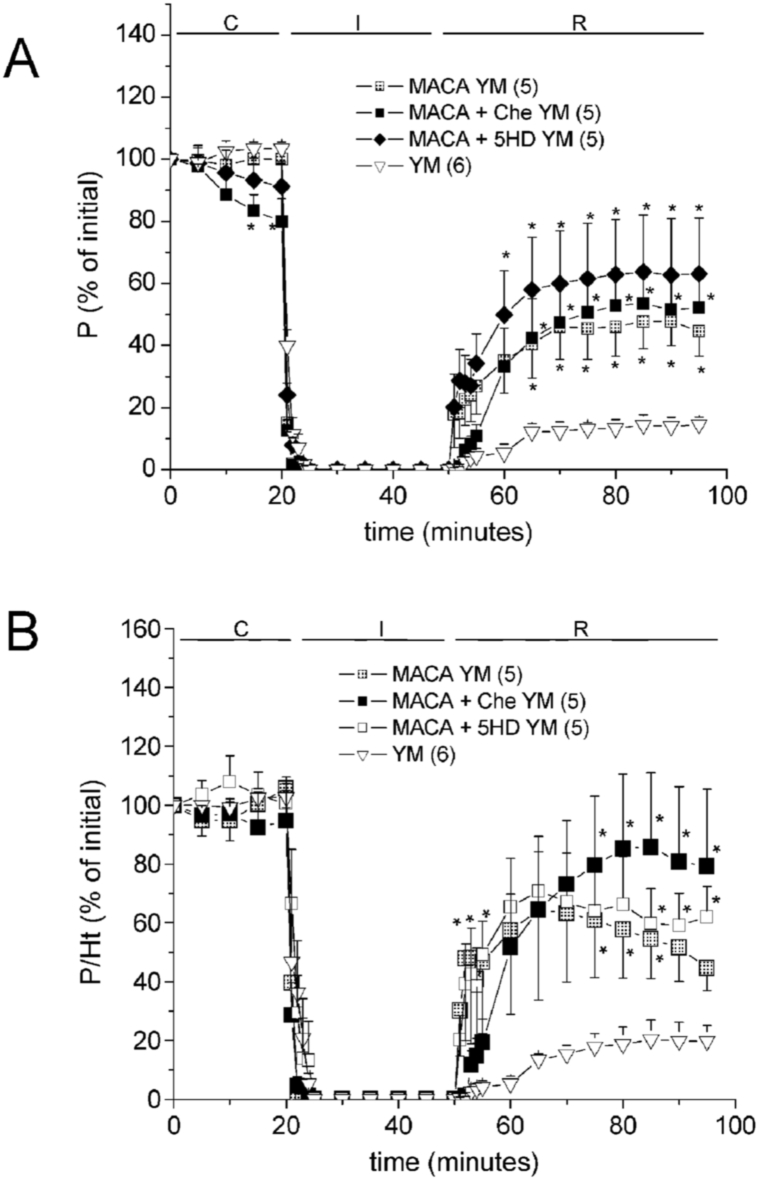
One week of oral administration of MACA was cardioprotective in both sexes. In YM rat hearts MACA increased the recovery of both, P and P/Ht ratio (Fig. 4). In YF rat hearts, MACA also significantly improved the PICR with a tendency to rise P/Ht (Fig. 5A–B), but it increased ΔLVEDP during I/R (Table 3). Contrarily, the administration of MACA in AgF rats did not induce an important cardioprotection, since recoveries of P and P/Ht were almost not modified (Fig. 5C–D). However, the ΔLVEDP during R was slightly reduced by MACA in AgF hearts (Table 3).
Fig. 4.
Effects of oral administration of 1 g/kg/day maca powder (MACA) during 1 week on the contractile recovery (P, % of initial, in A) and muscle economy (P/Ht, % of initial, in B) of young male rat hearts (YM), in the absence and the presence of 100 μmol/L 5-HD or 1 μmol/L chelerythrine (Che). The 2-way ANOVA results are shown in Supplementary Table A.4, post-hoc tests: ∗p < 0.05 vs non-treated hearts.
Fig. 5.
Effects of oral administration of 1 g/kg/day maca powder (MACA) during 1 week on the contractile recovery (P, % of initial) and muscle economy (P/Ht, % of initial) of young female rat hearts (YF, in A-B), in the absence and the presence of 100 μmol/L 5-HD + 100 μmol/L l-NAME, or 10 μmol/L clonazepam (Clzp). C-D show the effects of MACA on aged female rat hearts (AgF). The 2-way ANOVA results are shown in Supplementary Table A.4, post-hoc tests: ∗p < 0.05 vs non-treated hearts.
3.3. Cardioprotective mechanisms of maca and soy isoflavones
Considering that ISOF and MACA samples contain genistein and daidzein, it was of interest to know whether all them share the mechanism of action. Then, the role of mKATP channels in ISOF effects was investigated with 5-HD perfusion. However, this blocker did not avoid the strong cardioprotection in ISOF-treated YM hearts, since P and P/Ht recovered similarly to those without 5-HD (Fig. 3C–D). On the other hand, the role of NOS was evaluated in ISOF-treated YM rat hearts with l-NAME perfusion before I/R. Fig. 3 (C-D) also shows that P and P/Ht recoveries in ISOF treated YM rat hearts were not significantly altered by l-NAME, either alone or with the simultaneous blockade of mKATP channels with 5-HD. However, these drugs increased the diastolic contracture during I/R (Table 3) giving a tendency to reduce the economy. Results suggest that ISOF cardioprotection is barely related to NO production or mKATP channels activation, but it was mainly maintained by another mechanism.
Under the same hypothesis, it was evaluated the mechanism of MACA cardioprotection. Fig. 4 shows that in YM rat hearts, MACA effects on P and P/Ht were not significantly reduced by 5-HD or by chelerythrine (Che, a PKC inhibitor). Neither the diastolic contracture was significantly affected (Table 3). However, when both, NO-synthases and mKATP channels were simultaneously inhibited by l-NAME and 5-HD perfusion, the P and P/Ht recoveries in MACA treated YF rat hearts were reduced up to values not significantly different from those of control YF rat hearts at the end of R (Fig. 5 AB).
On the other hand, the role of mNCX was evaluated by perfusing MACA-treated YF rat hearts with the selective inhibitor clonazepam (Clzp). It was also observed that PICR and P/Ht fell at the end of R to values not significantly different from those of control hearts (Fig. 5A–B), as well as ΔLVEDP (Table 3). These results suggest that MACA cardioprotection depends on the synergism of more than a pathway, involving NO production, mKATP channels and mNCX activation.
3.4. Effects of Lepidium meyenii on isolated cardiomyocytes
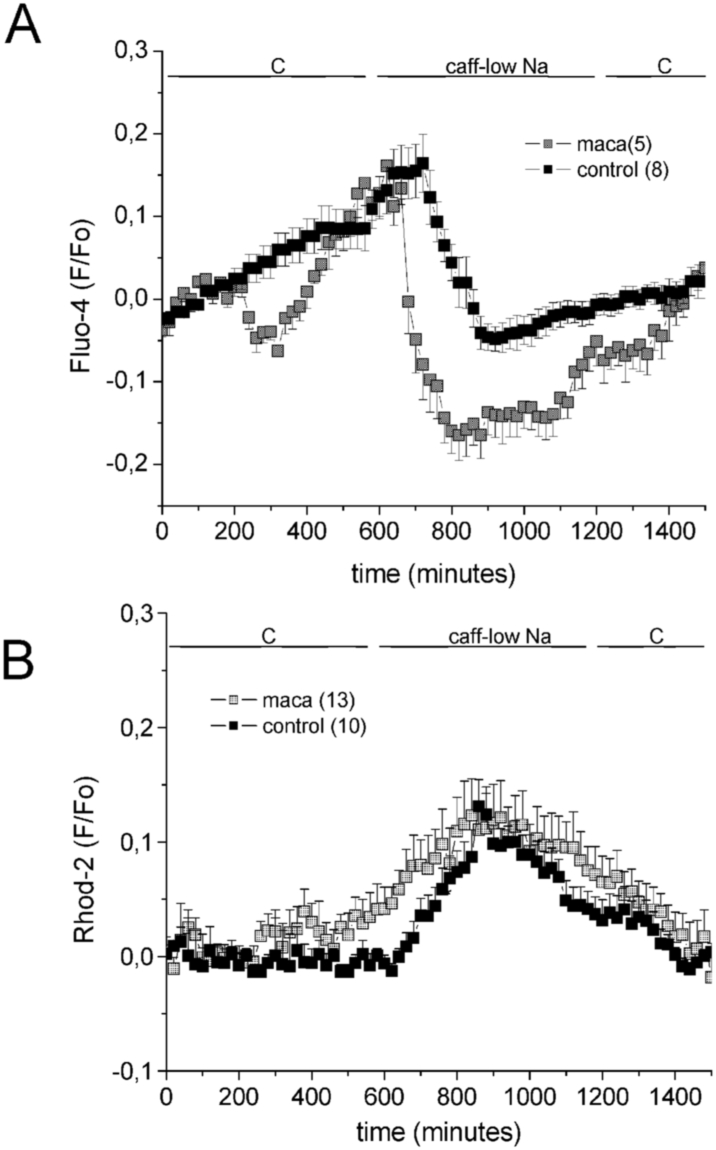
Fig. 6 shows that Fluo-4 ΔF/Fo signal (cytosolic Ca2+) increased when the sarcorreticular Ca2+ release was induced by 10 mM caffeine-low Na+ media, and then fell in temporal coincidence with the slow increase in the Rhod-2 ΔF/Fo signal (mitochondrial Ca2+). In MACA treated rat cardiomyocytes both, the fall in Fluo-4 signal and the increase in Rhod-2 signal were speeded up, suggesting that mitochondrial uptake was stimulated.
Fig. 6.
Effects of maca treatment on the changes in the fluorometrically Ca2+ signals (ΔF/Fo) of Fluo-4 (A) and Rhod-2 (B) in rat cardiomyocytes, when sarcoreticular Ca2+ was released by 10 mM caffeine in a 36 mM Na+ Krebs solution. See that maca speeded up the fall in Fluo-4 signal and the increase in Rhod-2 signal.
4. Discussion
This work demonstrates the effectivity of two subacute oral treatments, with maca powder and with the commercial soy isoflavones, to prevent the cardiac stunning induced by ischemia and reperfusion.
4.1. Effects of soy isoflavones
Cardiovascular effects of isoflavones were reported in clinical studies.3, 4, 5, 6 Although isoflavones reduce the vascular risk factors,3,25 myocardial mechanisms are not fully known. Experimentally, soy isoflavones were assessed in infarction models,7 but they were not studied on a stunning condition, as a model of incipient coronary risk. This work explored a preventive strategy with isoflavones effects on a severe stunning model without significant infarction. Recently we had shown that an acute i.p. administration of genistein was cardioprotective in this model through the PKC and mKATP channels activation.8 Here, we studied as positive control other pure isoflavone, daidzein, which was also cardioprotective against stunning with mKATP channels activation, as well as genistein was. Our results agree with the daidzein-induced reduction of infarction reported in rats with coronary artery ligation and reperfusion, in that case related to a decrease in inflammatory cytokine expression.26 It is known that many cellular pathways participate in ischemic dysfunction, especially under different ischemic periods.2, 31, 32, 33 Although both isoflavones, genistein and daidzein, acutely administered were more cardioprotectives in males than in female rat hearts exposed to our stunning model, the beneficial effect of oral subacute mixed soy isoflavones was similar in both sexes. Moreover, l-NAME and/or 5-HD perfusion did not significantly reduce the postischemic recoveries of P and P/Ht in ISOF-treated hearts, but strongly increased diastolic contracture. These results suggest that isoflavones contribute to maintain a low diastolic cytosolic and mitochondrial calcium level by inducing the NOS and mKATP channels activation, and consequently attenuate the risk of hypercontracture by mPTP opening, present in this model.24 However, other cellular pathways should contribute to the ISOF-dependent increase in post-ischemic contractile recovery. It is interesting that repeated oral administration of soy isoflavones developed different pattern of mechanisms than the acute i.p. injection of genistein or daidzein. The commercial soy fraction has 40% of isoflavones and so, other active components could participate in the effects. The analysis showed the presence of 4 isoflavones, genistein being the most important in quantity, followed by daidzein, genistin and much lower amount of glycitein, according to that described by other authors.27 So, we think that cardioprotection depends on synergic effects among isoflavones and the appearance of more active metabolites, such as S-equol, after intestinal/hepatic metabolism during repeated administration.5 On this way, Huang et al. showed that soy isoflavones control the oxidative stress caused in animals, through improving intestinal morphology, as well as antioxidant and immunologic capacities.28 Moreover, Li and Zhang demonstrated that soy isoflavones improve the ischemic cardiomyopathy in patients, by increasing their antioxidant capacities such as superoxide dismutase.6 The doses of soy isoflavones used here (100 mg/kg/day) is high in comparison to that used in humans for prevention (66 mg/day, about 10 mg/kg/day during 6 months)4 or for treatment (80 mg/day for 24 weeks)5 in ischemic cardiomyopathy. However, generally rats used to need higher doses than humans, and similar isoflavones doses to ones employed here were used with beneficial endocrinological effects in rats.29
4.2. Effects of maca
Lepidium meyenii presents different phenotypes, primarily based on the hypocotyl colour. Yábar et al. described variations in the phenols composition in the hypocotyl and isoflavones in Lepidium sativum seeds.30 This work was the first report of these substances in maca hypocotyl, being genistein the most abundant followed by daidzein in lower amount. This peruvian functional food was worldwide spread as beneficial for several conditions including menopausia, but this is the first report about cardioprotection induced by daily oral administration. As well as soy isoflavones, maca increased PICR in hearts from both sexes and the P/Ht recovery in hearts from males more than in females. These effects could be due to the presence of isoflavones, since maca resulted to contain daidzein and genistein, which showed similar cardioprotective effects.9 Maca also increased the relative rates of contraction and relaxation before I/R, suggesting that heart was better prepared to the ischemic challenge, with higher energetical store and muscle economy. However, the cardioprotective mechanism of oral maca was not exactly the same than that shown by genistein and daidzein in acute i.p. administration. Perfusing YM rat hearts with 5-HD or chelerythrine did not affect maca cardioprotection, respectively concluding that mKATP channels and PKC activation were not involved in the increase of contractile recovery induced by maca. On the other hand, since estrogens release NO in cardioprotective pathways, we explored whether maca would activate this mechanism. The NOS blockade with l-NAME partially reduced maca cardioprotection (the increase in P and P/Ht) only when mKATP -channels were simultaneously blocked by 5-HD (Fig. 5). So, it seems that both mechanisms are complementary between them, in a way that only a simultaneous blockade could affect the postischemic recovery. Moreover, isoflavones could be synergistic with other phenolic compounds of maca that ordinarily have antioxidant effect.30 In addition, cardioprotection may involve the reduction of mitochondrial Ca2+ overload triggered by I/R. The fact that the selective blocker of mitochondrial Ca2+ extrusion through mNCX, clonazepam, reduced P and P/Ht recoveries of maca-treated YF rat hearts indicates that maca should stimulate this pathway. As a control, we have previously shown that non-treated rat hearts exposed to this I/R model were not significantly affected in post-ischemic recovery by this drug.24 Here, clonazepam also increased Ht and reduced ΔLVEDP, in agreement with the fact that mNCX inhibition must increase mitochondrial [Ca2+] and metabolism while reducing cytosolic [Ca2+] during I/R. On the other hand, maca increased the mitochondrial Ca2+ uptake in non-ischemic cardiomyocytes, which would rise the metabolic and energetic state to better afford the ischemic challenge, as evidenced by the higher +P/P. Afterwards, during I/R maca would stimulate Ca2+ extrusion through mNCX, so avoiding the mitochondrial Ca2+ overload and preserving metabolism and ATP synthesis to recover contractility and muscle economy.
However, oral treatment with maca did not improve PICR in aged female rat hearts, at a difference of genistein.8 We have previously shown that AgF rat hearts recovered P and P/Ht less than YF, with higher diastolic contracture.8 These results agree with reports which found a big Ca2+ leak in aged hearts exposed to I/R,31 and a high Ca2+ content in interfibrillar mitochondria with reduced capacity to avoid ROS accumulation.32 Moreover, ageing and estrogenic deficiency in rats exposed to I/R were additive by affecting mitochondrial respiration.33 So, it would be expected that an antioxidant compound was less cardioprotective in aged than in young hearts. Maca contains antioxidant phenolic compounds, which could contribute to cardioprotection in YF and YM rat hearts but would not be effective in AgF hearts. Moreover, the demonstrated mechanisms of maca on mNCX, NOS and mKATP channels activation would be also minimized in a condition of Ca2+ and ROS overload typical of ageing.32 In recent works, we have demonstrated that this model of I/R triggers the mPTP opening still in young hearts,24 and that opening of mKATP-channels by diazoxide is cardioprotective in AgF hearts.8 Taking together, the AgF hearts would develop a high mitochondrial Ca2+ overload which increases ROS production and mPTP activation, being not enough the capacity of maca to control them. Contrarily, in younger rats the oral subacute administration of maca powder was cardioprotective with the presence of several compounds which would act synergically among them. The doses used here (1 g/kg/day) is much lower than the toxicity dose estimated in rats (>7.5 g/kg without lethality) and nearer to that used in premenopausic women for reducing symptoms (2 g/day during 2 months).17 Thus, a preventive effect of maca could be a potential good strategy for the coronary dysfunction.
4.3. Conclusions
This work demonstrates the cardioprotective effects of maca powder (Lepidium meyenii) oral and daily administered during one week to rats in a severe stunning model. It contains isoflavones as genistein, genistin and daidzein in composition, as well as the soy bean. Cardioprotection was evidenced by an increase in the postischemic contractile and energetic recoveries of the isolated rat hearts. The effects of oral soy isoflavones or maca powder were both sex-independent, at a difference of the effects of acute i.p. treatment with daidzein or genistein, which were higher in male than in female rat hearts, and strongly dependent on mKATP channels activation. However, the cardioprotection of oral maca disappeared in aged female rat hearts. Results suggest that these natural products have several compounds which act synergically to prevent the mitochondrial postischemic dysfunction, involving the activation of NOS, mKATP channels and mNCX pathways. This is the first work that demonstrates cardioprotection of maca powder and soy isoflavones to prevent severe stunning after oral subacute administration. These preclinical evidences give new support to the traditional use of these natural products as complementary medicines to prevent cardiovascular illness, especially the myocardial consequences of coronary disease.
4.4. Future perspectives
Cardioprotection in ischemia/reperfusion is frequently the consequence of a very complex play of mechanisms at mitochondrial and cytosolic level, and isoflavones offers the possibility of activating some cellular antioxidant pathways. So, it is of interest to explore the occurrence of such mechanisms with soy isoflavones and maca, as well as their effects and pharmacokinetics in a chronic treatment.
Declaration of competing interest
The authors want to declare that they have no conflict of interest associated with this publication.
Acknowledgements
To Prof. Agr. Eng. Marta Colares (College of Agronomical and Forestal Sciences, UNLP) for assistance in the identification of maca powder.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.03.004.
Funding
This work was supported by Universidad Nacional de La Plata [grant number 11-X795, 2017–2020]; by Universidad Nacional de la Patagonia San Juan Bosco [grant number PI 1405] and ANPCYT-Argentina [grant number PME 2015-0362].
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Mosca L., Barrett-Connor E., Wenger N.K. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruiz-Meana M., Boengler K., Garcia-Dorado D. Ageing, sex, and cardioprotection. Br J Pharmacol. 2019 doi: 10.1111/bph.14951. Dec 20. 10.1111/bph.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharifi-Rad J., Rodrigues C.F., Sharopov F. Diet, Lifestyle and cardiovascular diseases: linking pathophysiology to cardioprotective effects of natural bioactive compounds. Int J Environ Res Publ Health. 2020;17(7):2326–2357. doi: 10.3390/ijerph17072326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sathyapalan T., Aye M., Rigby A.S. Soy isoflavones improve cardiovascular disease risk markers in women during the early menopause. Nutr Metabol Cardiovasc Dis. 2018;28:691–697. doi: 10.1016/j.numecd.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Sekikawa A., Ihara M., Lopez O. Effect of S-equol and soy isoflavones on heart and brain. Curr Cardiol Rev. 2019;15:114–135. doi: 10.2174/1573403X15666181205104717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y., Zhang H. Soybean isoflavones ameliorate ischemic cardiomyopathy by activating Nrf2-mediated antioxidant responses. Food Funct. 2017;8:2935–2944. doi: 10.1039/c7fo00342k. [DOI] [PubMed] [Google Scholar]
- 7.Tang Y., Li S., Zhang P. Soy isoflavone protects myocardial ischemia/reperfusion injury through increasing endothelial nitric oxide synthase and decreasing oxidative stress in ovariectomized rats. Oxid Med Cell Longev. 2016:1–14. doi: 10.1155/2016/5057405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colareda G.A., Ragone M.I., Bonazzola P., Consolini A.E. The mKATP channels and PKC are involved in the cardioprotective effects of genistein on estrogen-deficient rat hearts exposed to ischemia/reperfusion: energetic study. J Cardiovasc Pharmacol. 2020;75:460–474. doi: 10.1097/FJC.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 9.Gonzales G.F., Villaorduña L., Gasco M., Rubio J., Gonzales C. Maca (Lepidium meyenii WALP), una revisión sobre sus propiedades biológicas. Rev Peru Med Exp Salud Pública. 2014;31:100–110. [PubMed] [Google Scholar]
- 10.Dini A., Migliuolo G., Rastrelli L., Saturnino P., Schettino O. Chemical composition of maca. Food Chem. 1994;49:347–349. [Google Scholar]
- 11.Baoliang C., Bo L., Kan H., Qun Y. Imidazole alkaloids from Lepidium meyenii. J Nat Prod. 2003;66:1101–1103. doi: 10.1021/np030031i. [DOI] [PubMed] [Google Scholar]
- 12.Muhammad I., Zhao J., Chuck Dunbar D., Khan I.A. Constituents of Lepidium meyenii ‘maca’. Phytochemistry (Oxf) 2002;59:105–110. doi: 10.1016/s0031-9422(01)00395-8. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales G.F., Ruiz A., Gonzales C., Villegas L., Cordova A. Effect of Lepidium meyenii (maca) roots on spermatogenesis of male rats. Asian J Androl. 2001;3(3):231–233. [PubMed] [Google Scholar]
- 14.Gonzales G.F., Córdova A., Vega K., Chung A., Villena A. Effect of Lepidium meyenii (maca), a root with aphrodisiac and fertility-enhancing properties, on serum reproductive hormone levels in adult healthy men. J Endocrinol. 2003;176:163–168. doi: 10.1677/joe.0.1760163. [DOI] [PubMed] [Google Scholar]
- 15.Pino-Figueroa A., Nguyen D., Maher T.J. Neuroprotective effects of Lepidium meyenii (maca) Ann N Y Acad Sci. 2010;1199:77–85. doi: 10.1111/j.1749-6632.2009.05174.x. [DOI] [PubMed] [Google Scholar]
- 16.Choi E.H., Kang J.I., Cho J.Y. Supplementation of standardized lipid-soluble extract from maca (Lepidium meyenii) increases swimming endurance capacity in rats. J Funct Foods. 2012;4:568–573. [Google Scholar]
- 17.Meissner H.O., Reich-Bilinska H., Mscisz A., Kedzia B. Therapeutic effects of pre-gelatinized maca (Lepidium peruvianum, Chacon) used as a non-hormonal alternative to HRT in perimenopausal women - clinical Pilot Study. Int J Biomed Sci. 2006;2:143–159. [PMC free article] [PubMed] [Google Scholar]
- 18.Ponce-Hornos J.E., Ricchiuti N.V., Langer G.A. Online calorimetry in arterially perfused rabbit interventricular septum. Am J Physiol. 1982;243:H289–H295. doi: 10.1152/ajpheart.1982.243.2.H289. [DOI] [PubMed] [Google Scholar]
- 19.Consolini A.E., Ragone M.I., Conforti P., Volonté M.G. Mitochondrial role in ischemia-reperfusion of rat hearts exposed to high-K+ cardioplegia and clonazepam: energetic and contractile consequences. Can J Physiol Pharmacol. 2007;85:483–496. doi: 10.1139/y07-022. [DOI] [PubMed] [Google Scholar]
- 20.Holmgren P.K., Holmgren N.H., Barnett L.C. Index Herbariorum. The Herbaria of the World. eighth ed. P1: New York Botanical Gardens; Bronx, New York: 1990. [Google Scholar]
- 21.Rondina R.V.V., Coussio J.D. Estudio fitoquímico de plantas medicinales argentinas (1) Rev Invest Agropec. 1969;6:351–366. [Google Scholar]
- 22.Haborne J.B. Chapman and Hall; London: 1991. Phytochemical Methods. [Google Scholar]
- 23.Wagner H., Bladt S. Plant Drug Analysis. A Thin Layer Chromatography Atlas. second ed. Springer; 2009. [Google Scholar]
- 24.Ragone M.I., Bayley M., Bonazzola P., Colareda G.A., Consolini A.E. Cardioprotective mechanisms of hypothyroidism on ischemia/reperfusion in rats and effects of carvedilol: energetic study. J Cardiovasc Pharmacol Therapeut. 2020;25:72–85. doi: 10.1177/1074248419872957. [DOI] [PubMed] [Google Scholar]
- 25.Yamagata K. Soy isoflavones inhibit endothelial cell dysfunction and prevent cardiovascular disease. J Cardiovasc Pharmacol. 2019;74:201–209. doi: 10.1097/FJC.0000000000000708. [DOI] [PubMed] [Google Scholar]
- 26.Kim J.W., Jin Y.C., Kim Y.M., Rhie S., Chang K.C. Daidzein administration in vivo reduces myocardial injury in a rat ischemia/reperfusion model by inhibiting NF-kB activation. Life Sci. 2009;84:227–234. doi: 10.1016/j.lfs.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Peiretti P.G., Karama M., Janiak M. Phenolic composition and antioxidant activities of soybean (Glycine max (L.) Merr.) plant during growth Cccle. Agronomy. 2019;9:153. doi: 10.3390/agronomy9030153. [DOI] [Google Scholar]
- 28.Huang L., Ma X.Y., Jiang Z.Y. Effects of soybean isoflavone on intestinal antioxidant capacity and cytokines in young piglets fed oxidized fish oil. J Zhejiang Univ - Sci B. 2016;17:965–974. doi: 10.1631/jzus.B1600078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajan R.K., Kumar S.S., Balaji B. Soy isoflavones exert beneficial effects on letrozole-induced rat polycystic ovary syndrome (PCOS) model through anti-androgenic mechanism. Pharm Biol. 2017;55:242–251. doi: 10.1080/13880209.2016.1258425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yábar E., Chirinos R., Campos D. Phenolic compounds and antioxidant capacity in three maca (Lepidium meyenii Walp.) ecotypes during pre-harvest, harvest and natural post-harvest drying. Sci Agropec. 2019;10:85–97. [Google Scholar]
- 31.Hamilton S., Terentyev D. Altered intracellular calcium homeostasis and arrhythmogenesis in the aged heart. Int J Mol Sci. 2019;20(10):2386. doi: 10.3390/ijms20102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruiz-Meana M., Minguet M., Bou-Teen D. Ryanodine receptor glycation favors mitochondrial damage in the senescent heart. Circulation. 2019;139:949–964. doi: 10.1161/CIRCULATIONAHA.118.035869. [DOI] [PubMed] [Google Scholar]
- 33.Garvin A.M., Aurigemma N.C., Hackenberger J.L., Korzick D.H. Age and ischemia differentially impact mitochondrial ultrastructure and function in a novel model of age-associated estrogen deficiency in the female rat heart. Pflugers Arch- Eur J Physiol. 2017;469:1591–1602. doi: 10.1007/s00424-017-2034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.