Abstract
Background and aim
Hind limb ischemia is one of the peripheral arterial diseases affecting majority of the people with atherosclerosis, diabetes and chronic cigarette smokers. Hind limb ischemic-reperfusion injury is also one of the exacerbating events in these peoples, resulting in hind limb dysfunction. The aim of this study was to identify the effects of ethanolic extracts of mangifera indica (EEMI) on reversing hind limb dysfunction in diabetic rats with acute hind limb ischemia-reperfusion injury.
Experimental procedure
Unilateral femoral artery ligated diabetic rats were orally fed with EEMI (0.2 and 0.4 g/kg) for 14 days. At the end of the study, plasma levels of pro-inflammatory cytokines and relevant biochemical parameters were measured. The isolated gastrocnemius muscles were used for gene expression and histopathological studies.
Results
There was a significant reduction (p < 0.05) in the plasma levels of pro-inflammatory cytokines, nitric oxide, malondialdehyde; and the expression levels of mRNA of induced nitric oxide synthase and intercellular adhesion molecule −1; and increase in anti-inflammatory cytokine, in isolated gastrocnemius muscles of animals treated with 0.2 and 0.4 g/kg of EEMI in comparison to disease control. In addition, histopathological study of gastrocnemius muscle and hind limb function test indicated the recovery of tissue damage from ischemic reperfusion at 0.2 and 0.4 g/kg of EEMI in comparison to disease control.
Conclusion
We conclude that 14-day EEMI treatment of rats with acute hind limb ischemia/reperfusion in diabetic rats recovered from ischemic/reperfusion injury by modulating (decreasing) oxidative stress and inflammation.
Keywords: Streptozotocin, Mangifera indica, Inflammation, Oxidative stress, Vascular diseases
Graphical abstract
Highlights
-
•
Ethanolic extracts of M.indica (EEMI) was effective in diabetic rats with acute hind limb ischemia-reperfusion (AHLI-R) injury.
-
•
EEMI attenuated plasma levels of pro-inflammatory cytokines and augmented IL-10 in diabetic rats with AHLI-R injury.
-
•
EEMI augmented cell-cell adhesion molecule, ICAM-1 in gastrocnemius muscle of diabetic rats with AHLI-R injury.
-
•
EEMI reversed I/R injury and hind limb dysfunction by modulating mechanisms of oxidative stress and inflammation.
List of abbreviations
- EEMI
Ethanolic Extract of Mangifera indica.L
- STZ
Streptozotocin
- SD
Sprague-Dawley
- IR
Ischemia-Reperfusion
- DM
Diabetes Mellitus
- PAD
Peripheral Arterial Disease
- PAOD
Peripheral Arterial Occlusive Disorder
- ALI
Acute Limb Ischemia
- CLI
Critical Limb Ischemia
- AHLI-RI
Acute hind limb ischemia-Reperfusion injury
- FLPI
Full field Laser Perfusion Imaging
- ELISA
Enzyme linked immunosorbent Assay
- ICAM-1
Intercellular Adhesion Molecule 1
- GAPDH
Glyceraldehyde 3-Phosphate dehydrogenase
- IL
Interleukin
- TNF-α
Tumour Necrosis Factor-α
- PCR
Polymerase Chain Reaction
- RNA
Ribonucleic acid
- DNA
Deoxyribonucleic acid
- cDNA
Complementary Deoxyribonucleic acid
- DEPC
Diethyl pyro carbonate
- dNTP
deoxyribonucleotide triphosphate
- HPLC
High Performance Liquid Chromatography
1. Introduction
Peripheral arterial disease (PAD) or peripheral arterial occlusive disease (PAOD) is one of the vascular diseases where progressive narrowing of blood vessels reduces blood flow to the lower limbs.1 PAD typically causes pain and fatigue, often in legs, and especially during exercise and usually improves with rest.2 The spectrum of manifestation of PAD are asymptomatic, atypical, intermittent claudication, chronic critical limb ischemia (CLI) and acute limb ischemia (ALI).1 People with PAD experiences pain in their lower extremity due to either CLI or ALI which usually occurs at rest.2 The primary cause of PADs is due to atherosclerosis of the arteries supplying the upper and lower extremities.1 The underlying risk factors of PAD are cigarette smoking, diabetes mellitus, hypertension, dyslipidemia, obesity, alcohol consumption, race and ethnicity, elevated levels of homocysteine, C - reactive protein, fibrinogen, chronic kidney disease, genetic factors and other risk factors.1 Epidemiological data indicates that more than 200 million people suffer from PAD worldwide; where men and women from low- and middle-income countries are affected modestly with lower PAD rates than those in high income countries.3 It has also been reported that the rate of mortality due to PAD exceeds that of breast cancer and coronary artery disease.4 PAD is uncommon in younger people; however, prevalence of PAD rises with ageing (>10%) and affects a substantial proportion of the elderly or geriatric population.3
The complications of PAD arose from life-threatening situation such as amputations, if left unnoticed or untreated.1 This is most probably due to ischemia-reperfusion injury of the supplying organs.1 In hind limb ischemia, the mechanisms underlying the pathogenesis of ischemia-reperfusion injury are primarily due to enhanced oxidative stress and inflammation.5
Firstly, in skeletal muscles ischemia-reperfusion increases accumulation of oxygen free radicals known as reactive oxygen species (ROS) from the cellular components such as mitochondria, sarcoplasmic reticulum, transverse tubules, sarcolemma and enzymes (xanthine and NADPH-oxidase).6 Accumulation of ROS in skeletal muscle impairs membrane lipids, proteins and DNA of the cell.6 By the way, increased duration of exposure to high levels of ROS affects the structure and function of myofilaments, where it gets oxidized and the function is also impaired.7 In particular, myosin heavy chain molecules and Troponin C are also affected by ROS, where oxidation of these proteins results in impaired function.6
Following ischemia-reperfusion and ROS accumulation, a secondary source of ROS arose from non-muscle sources, especially phagocytic white blood cells which play an important role in affecting muscle redox state, which leads to tissue injury.8 In general, invasion of macrophages and other phagocytic cells is to carry out fiber regeneration; however this process involves enhanced release of ROS from phagocytic cells which results in damage of intact muscle cells and thereby exacerbates muscle fiber injury.9
Secondly, during reperfusion, DAMPs released from the endothelium of the blood vessels supplying the necrotic tissue (due to ischemia) acts on its corresponding receptors and enhances inflammatory cascades.10 Similarly ROS can also induce inflammatory cascade through induction of nuclear factor (NF)-κB which leads to accumulation of pro-inflammatory cytokines, which attracts white blood cells, followed by ROS production and further tissue damage.11 Thus ischemia -reperfusion undergoes vicious cycle of events and leads into tissue damage.
Hence resolving ischemia promptly either pharmacotherapeutically or surgically is essential in clinical settings, otherwise it leads to severe leg necrosis and amputation.1 Treatment of PAD depends upon the location of the ischemia and its severity.12 The management of PAD is mainly focused on controlling the risk factors, in particular smoking cessation (if a smoker) and pharmacotherapeutically by increasing the blood supply to the ischemic area.13 The blood supply to the ischemic area can be increased by medications, exercise and surgery. Anti-hypertensive (vasodilators) and anti-hyperlipidemic agents are commonly used to control blood pressure and lipid levels respectively.14 In addition, analgesic and anti-infective agents are also prescribed to reduce pain and to treat any infections.15 However, majority of the people with diabetes presented with any of the PAD are also more prone to infection and amputation of the hind limbs.16 This was the rationale in our study to employ and investigate the effect of herbal extracts in diabetic rats with concomitant acute hind limb ischemia/reperfusion model. As aforementioned, people with PAD are more prone to amputation; hence an adjuvant therapy is essential in an order to overcome these complications. Alternative and complementary medicines were proven effective for the management of some chronic and metabolic diseases.17 A number of herbal extracts were also reported for their anti-oxidant, anti-diabetic and anti-inflammatory activity.17 Out of all Mangifera indica L. (Family: Anacardiaceae), commonly known as mango tree, indigenous and native to the Indian subcontinent was also reported for anti-diabetic and anti-inflammatory activity.18 The seed kernels, fruit, leaves and bark of M. indica were found to contain diverse classes of phytoconstituents such as xanthonoids (e.g., mangiferin), flavonoids, saponins and triterpenoids.18 Mangiferin, a bio-active compound found in the pulp, pericarp, leaves and seed kernel of the M. indica18
In this study, our aim was to determine does seed kernels of ethanolic extracts of M. indica (EEMI) reverses hind limb dysfunction in the acute hind limb ischemia/reperfusion injury in STZ induced diabetic rats.
2. Materials and methods
2.1. Materials
M.indica dry seed kernel powder was purchased from a local vendor in the region of Coimbatore, Tamil Nadu, India. All primers and reagents were purchased from Merck (Sigma), Germany. Tumor necrosis factor-α, Interleukin(IL)-6, IL-10, and IL-1β assay kits were purchased from R&D Systems, USA. All solvents used for the chromatographic analysis are of high performance liquid chromatography grade.
2.2. M.Indica dry seed kernel powder and quality control tests
The M. indica seed kernel powder obtained from the vendor was evaluated for its quality as per the guidelines of world health organisation (WHO) on Quality Control Methods on Herbal Materials.19 The procedures20,21 for all of the tests used to determine the quality of M. indica seed kernel powder are also shown (Supplementary file.S.1, S.2, and S.3).
2.3. Preparation and phytochemical characterization of ethanolic extract of M.indica (EEMI)
EEMI was prepared by cold maceration technique (Supplementary file S.4). The phytochemicals present in EEMI was qualitatively determined through chemical tests (Supplementary file S.5); and diluted samples of EEMI were analysed using high performance liquid chromatography coupled with Ultraviolet–Visible detection (Supplementary file S.6 and S.7).
2.4. Animals
Institutional Animal Ethical Committee (158/PO/ReBi-S/Re-L/99/CPCSEA) approved the study; Sprague-Dawley rats weighing about 150–200 g were acquired from central animal facility, PSGIMSR, Peelamedu, Coimbatore. The animals were maintained under a room temperature of 23 ± 1 °C, humidity of 55 ± 5%, with 12 h of light & 12 h of dark. A total of 25 animals were divided into 5 groups (5 animals per group, Total, n = 5 × 5 = 25); Group I – Healthy; Group II - sham control; Group III – disease control; Group, IV and V – 0.2 and 0.4 g/kg of EEMI for 14 days respectively. Note: Group III, IV, and V were unilateral femoral artery ligation/reperfusion-STZ induced diabetic rats.
2.5. Hind limb function assessment
Animals were tested for hind limb function22 during the course of the study for a period of 14 days from the day of reperfusion (day 14–28) and their performance was evaluated through Tarlov scoring (Supplementary file S.8).
2.6. Diabetes induction, unilateral femoral artery ligation, reperfusion and oral administration of EEMI
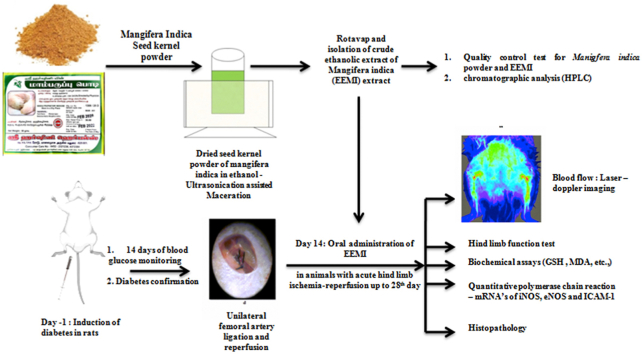
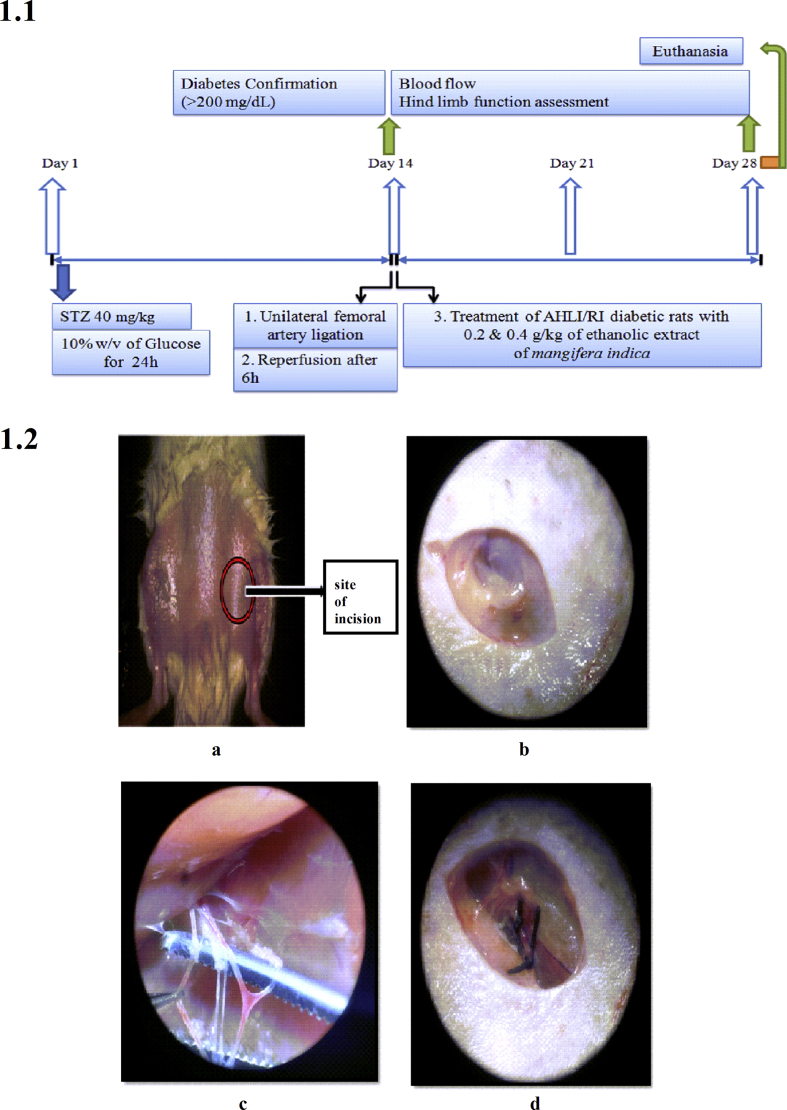
Acclimatized rats were injected with freshly prepared streptozotocin in citrate buffer (pH4.5) (40 mg/kg, intraperitoneal (i.p.)). During the course of study (Fig. 1.1), blood samples were collected through rat tail vein and fasting blood glucose levels were monitored using glucose detection strips (Avantor Gluco Sphera diagnostics) every day for 2 weeks till 14th day and thereafter up to end of the study. On 14th day, animals from group II, III, IV and V were anesthetized as per Committee for the Purpose of Control and Supervision of Experiments on Animals (CPSCEA) guidelines by intraperitoneal (i.p.) injection of ketamine (40 mg/kg) and xylazine (10 mg/kg). Anesthetized animal was placed in supine position; fore and hind limbs were secured using surgical tape. The site of surgery was cleaned (Fig. 1.2a) and an incision was made at the left inguinal region (Fig. 1.2b) for about 1 cm, the skin was separated and superficial femoral artery adhered along with vein and nerve was identified. Superficial femoral artery was separated from the adhering femoral vein and femoral nerve (Fig. 1.2c). A unilateral femoral artery ligation (UFAL) was made using a suture (4.0 sized) at proximal and distal region of superficial femoral artery to achieve acute hind limb ischemia (AHLI) (Fig. 1.2d). Post-ligation, the skin was sutured (3.0 sized) and all the animals with AHLI were placed individually in a separate cage. After 6 h, the UFAL was removed for reperfusion. Post-reperfusion, the skin was sutured (3.0 sized) and the animals were placed individually in a separate cage; and observed until its recovery from anesthesia.23 The flow of blood through femoral artery was visualized by Moor-Full-field laser Doppler imaging (Supplementary file S.9). The corresponding animals recovered from anesthesia (Group IV and V) were treated daily, using gastric gavage of EEMI (0.2 and 0.4 g/kg) for next 14 days till 28th day. (Note: EEMI was prepared by mixing 1 g of dry crude extract in 10 mL of 0.5% carboxymethylcellulose (CMC)).
Fig. 1.
Study design and surgery. 1.1. Time line of the study; 1.2. Unilateral femoral artery ligation. a. The site of incision b. Incision at the left inguinal region of about 1 cm c. superficial femoral artery and vein adhered with nerve d. Unilateral femoral artery ligation (ligation at proximal and distal site of femoral artery).
2.7. Blood collection
At the end of study, all animals were anesthetized (section 2.6) and blood was collected through cardiac puncture in sodium citrate (4%) tubes, centrifuged immediately at 2000 g for 15 min. The supernatant (plasma) was collected and stored at −80 °C.
2.8. Griess assay
The plasma level of nitrite was determined through Griess assay.24 The plasma sample was deproteinized with trichloroacetic acid; 50 μL of deproteinized plasma supernatant or different concentration of NaNO2 (200–0.1 μM) is added to 50 μL of Griess reagent (1% Sulphanilamide and 0.1% N-(Naphthyl) ethylene diamine in 5% HCl) in 96 well microtiter plate and incubated for 10 min in dark. Post-incubation, the absorbance was measured at 540 nm in a 96-well plate reader. Finally, the concentrations of nitrite in the samples were quantified by using linearity equation derived from reference standard (NaNO2).
2.9. Enzyme linked immunosorbent assay (ELISA)
The plasma levels of pro-inflammatory markers such as TNF-α, IL-6, Il-1β and anti-inflammatory cytokine IL-10 was measured using sandwich ELISA as per manufacturer's instruction (R&D Systems).
2.10. Determination of malondialdehyde and reduced glutathione
The plasma levels of malondialdehyde (MDA) and reduced glutathione (GSH) was determined by thiobarbituric acid (TBA) and spectrophotometric kinetic assay respectively. MDA reacts with TBA at 90 °C to produce a thiobarbituric acid-malondialdehyde (TBA-MDA) adduct25 which was measured at 532 nm. The level of reduced glutathione was determined through 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB) assay.26 The detailed protocol of determination of MDA and GSH is shown (Supplementary file S.10 and S.11).
2.11. Isolation of gastrocnemius muscle
Animals were euthanized, the left hind limb portion was dissected out and the calf muscles were isolated. Calf muscle comprising gastrocnemius and soleus muscles were separated; and gastrocnemius muscle was dissected into two parts at the mid-point. One part of the muscle was fixed in 10% formalin solution for histopathology and the other was stored at −80 °C for RNA isolation.
2.12. RNA isolation and reverse transcriptase-polymerase chain reaction
The expression level of ICAM-1, eNOS and iNOS in gastrocnemius muscle were determined by real time reverse transcriptase polymerase chain reaction (RT-PCR). The protocol was followed as per the manufacturer's instruction (Applied Biosystems) (Supplementary file S.12). The relative expression of the mRNA was expressed as 2^ΔΔCt and normalized to fold change.
2.13. Histopathology
The gastrocnemius muscle tissue suspended in formalin was used for histopathological study after 24 h of fixation. The gastrocnemius muscle sections were stained with hematoxylin and eosin (H & E) to visualize muscle fibre degenerations/regeneration, nucleus location and inflammatory cells. The detailed protocol of tissue preparation, sectioning and staining is shown (Supplementary file S.13).
2.14. Statistical analysis
All the data were tabulated and statistical analysis was done through GraphPad Prism V 5.03. The data are represented as mean ± standard deviation (SD); the significance between groups was analysed through one-way ANOVA and post-hoc Dunnett's multiple comparison test at 95% confidence intervals (p < 0.05).
3. Results
3.1. Quality control tests
The M. indica dry seed kernel powder was coarse, brown colour in appearance with bitter taste.
3.1.1. The physico-chemical parameters of M.indica dry seed kernel powder
The physico-chemical parameters of M. indica dry seed kernel powder was evaluated for foreign matter, particle size, foaming index, swelling index, acid insoluble ash value, water soluble ash value, total ash and hexane extractive value; and the results of each of the parameters are also shown (Supplementary file S.14).
3.1.2. Phytochemical characterization of EEMI
EEMI was subjected to qualitative analysis to determine the presence of alkaloids, tannins, triterpenoids, saponins, flavonoids and glycosides (Supplementary file S.15). In addition, HPLC of 10-fold diluted samples of EEMI also revealed the presence of one of the biologically active phytoconstituents, mangiferin (0.5%) (Supplementary file S.16). The inorganic elements such as sodium, potassium, iron, sulphate, phosphate, chloride, carbonate and nitrates were also found to be present (Supplementary file S.17).
3.2. Blood glucose and body weight
There was a significant increase in the blood glucose of all STZ treated animals (Group II, III, IV and V) on day 7 (Range: 148–172 mg/dL) and 14 (Range: 195–225 mg/dL)in comparison to healthy controls (86 ± 7 mg/dL). Post – reperfusion, treatment with EEMI (day 14–21) and examination of blood glucose on day 21, a gradual reduction in blood glucose was observed; and thereafter on day 28, a significant reduction was observed in animals from group IV and V. As sham animals are also STZ treated, there was an elevated level of blood glucose in comparison to healthy controls (Supplementary file S.18). The body weight of all STZ treated animals were found to be reduced on day 7 and 14; whereas in disease control there was continuous reduction in body weight during the course of study (on day 21 and 28) in comparison to healthy and EEMI (0.2 and 0.4 g/kg) treated animals (Supplementary file S.19).
3.3. Effect of EEMI on hind limb blood flow
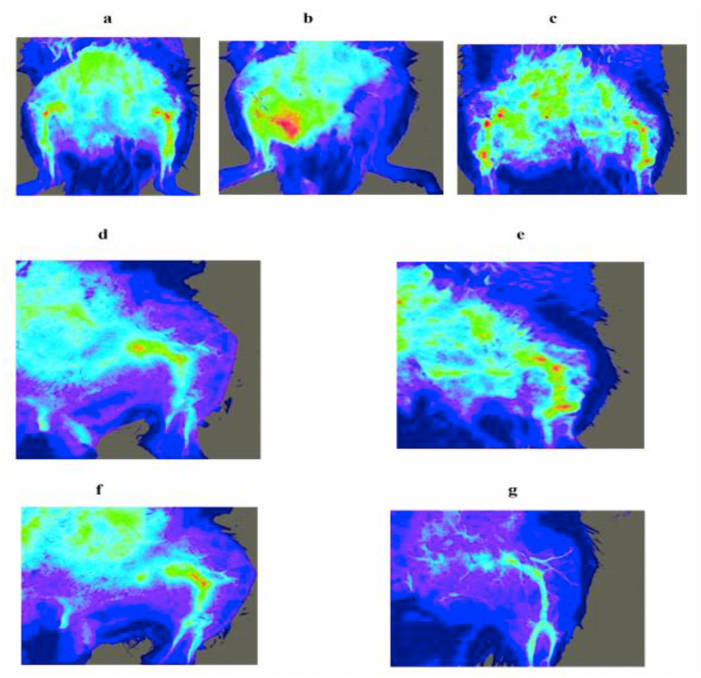
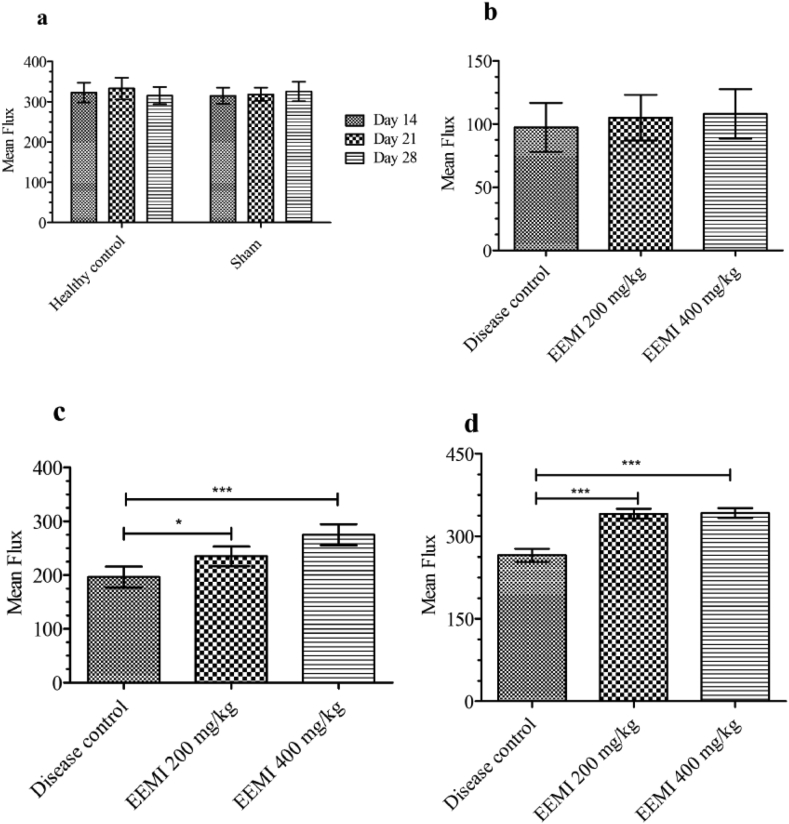
The obstruction of blood flow to distal area of hind limb was visualized by laser Doppler imaging (Moor FLPI) and the mean flux was recorded. The blood flow to distal area of the hind limb through left femoral artery in healthy and sham controls was also shown (Fig.2a and c) and their corresponding mean flux (Fig. 3a) was found to be 323 ± 24.4 and 315 ± 19.9 units.
Fig. 2.
Laser-Doppler imaging. Hind limb blood flow to distal areas visualized using laser Doppler imaging (Moor FLPI) system. a. Healthy control; b. Disease control model (Acute hind limb ischemia (AHLI) by unilateral femoral artery ligation) - Loss of blood flow to hind limbs; c) Sham control on day 28 d) Blood flow in animals treated with 0.2 g/kg EEMI on day 21 e) Blood flow in animals treated with 0.4 g/kg EEMI on day 21 f) Blood flow in animals treated with 0.2 g/kg EEMI on day 28 g) Blood flow in animals treated with 0.4 g/kg EEMI on day 21.
Fig. 3.
Hind limb blood flow - Mean flux. Blood flow through femoral artery was expressed as mean flux units (Laser-Doppler imaging) a) Mean flux measurements of healthy and sham control on day 14, 21 and 28 b) Mean flux of disease control, animals treated with 0.2 and 0.4 g/kg EEMI on day 14 after 6 h of reperfusion. c) Mean flux of disease control, animals treated at 0.2 and 0.4 g/kg EEMI on day 21. d) Mean flux of disease control, animals treated at 0.2 and 0.4 g/kg EEMI on day 28. The significance between the groups were analysed using one-way ANOVA and post-hoc Dunnett's multiple comparison test. The mean flux in treatment groups (0.2 and 0.4 g/kg) were significant on days 21 (∗ and ∗∗∗p < 0.05) and 28 (∗∗∗p < 0.05) in comparison to disease control.
On day 14, after UFAL surgery the blood flow to distal area of the hind limb through left femoral artery in disease control (Fig. 2b) and animals treated with 0.2 and 0.4 g/kg EEMI was completely blocked. On the same day after 6 h of blockade and post-reperfusion, the mean flux was found to be reduced (Fig. 3b) in disease control (97.4 ± 19.3 units) and animals treated with 0.2 and 0.4 g/kg EEMI (105.03 ± 18.1 and 108.1 ± 19.5 units) in comparison to healthy and sham (Fig. 3a). In comparison with disease control, the blood flow (Fig. 2d, e, f and g) and mean flux (Fig. 3c and d) was found to be significantly greater (p < 0.05) on days 21 and 28 from the day of reperfusion in animals treated with 0.2 and 0.4 g/kg EEMI.
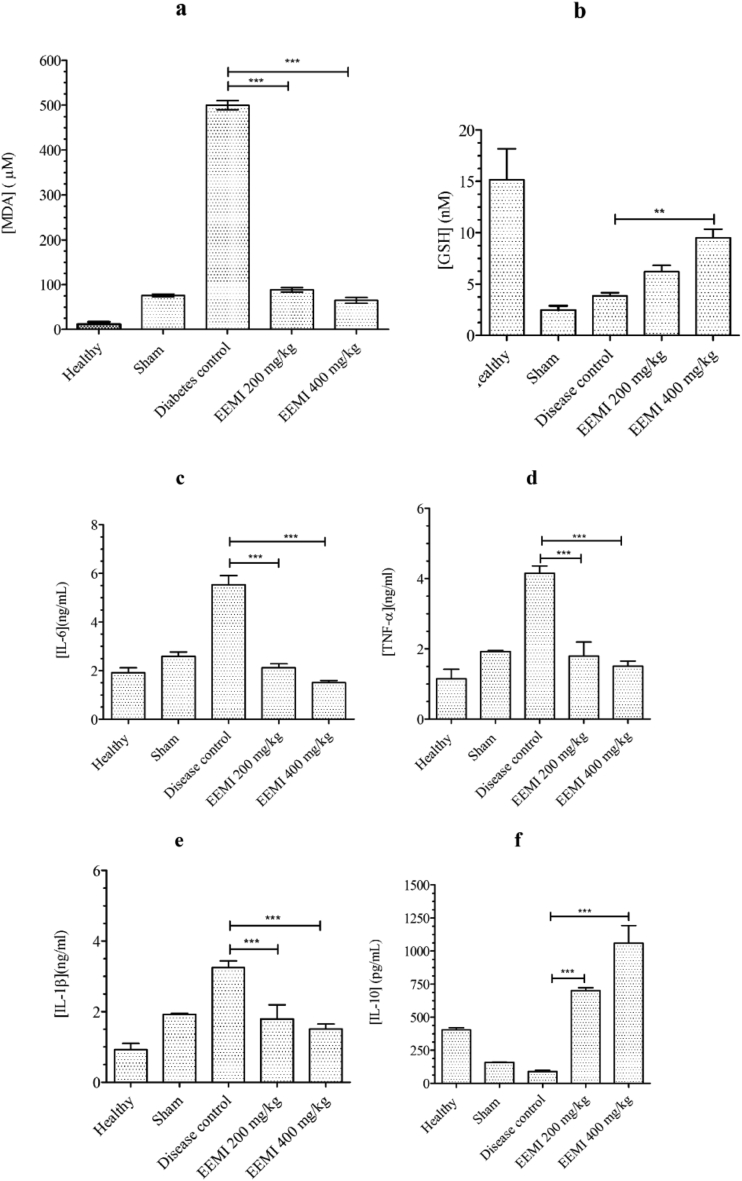
3.4. Effect of EEMI on malondialdehyde and reduced glutathione
There was a strong increase in the levels of plasma MDA of disease control (499.83 ± 10.01 μM) in comparison to healthy animals (11.73 ± 4.9 μM). However, such increases were not seen in animals treated with EEMI (88.39 ± 1.05 and 64.77 ± 2.45 μM) at 0.2 and 0.4 g/kg (Fig. 4a). The plasma levels of GSH (9.49 ± 0.82 Vs 3.85 ± 0.29 nM) was highly significant (P < 0.05) at 400 mg/kg of EEMI in comparison to disease control, respectively. Even though the levels of GSH in animals treated with 0.2 g/kg of EEMI is not significant, there was a 0.6-fold increase in GSH levels in comparison to disease control (Fig. 4b).
Fig. 4.
Plasma levels of (a) Malondialdehyde (MDA) and (b) Glutathione (GSH), (c) IL-6 (d), TNF-α (e) IL-1β and (f) IL-10. The significance between the groups were analysed using one way ANOVA and post-hoc Dunnett's multiple comparison test at 95% confidence intervals (p < 0.05).
3.5. Effect of EEMI on levels of pro-inflammatory cytokines
The levels of plasma IL-6 (2.12 ± 0.16 and 1.51 ± 0.08 Vs 5.53 ± 0.38 ng/mL), IL-1β (1.79 ± 0.39 and 1.51 ± 0.14 Vs 3.25 ± 0.19 ng/mL) and TNF-α (1.79 ± 0.39 and 1.51 ± 0.14 Vs 4.16 ± 0.20 ng/mL) were decreased significantly (P < 0.05) in animals treated with 0.2 and 0.4 g/kg EEMI in comparison to disease control, respectively (Fig. 4c, d and e). Whereas, the levels of plasma anti - inflammatory cytokine, IL-10 (69.9 ± 0.22 and 105.90 ± 1.33 Vs 8.95 ± 0.08 pg/mL ×10−2) increased significantly (P < 0.05) in animals treated with 0.2 and 0.4 g/kg EEMI in comparison to disease control, respectively (Fig. 4f). Since sham controls were diabetic animals, there was also significant (P < 0.05) increases in pro-inflammatory (Fig. 4c, d and e) and decreases in anti-inflammatory cytokine, IL-10 in comparison to healthy animals (Fig. 4f).
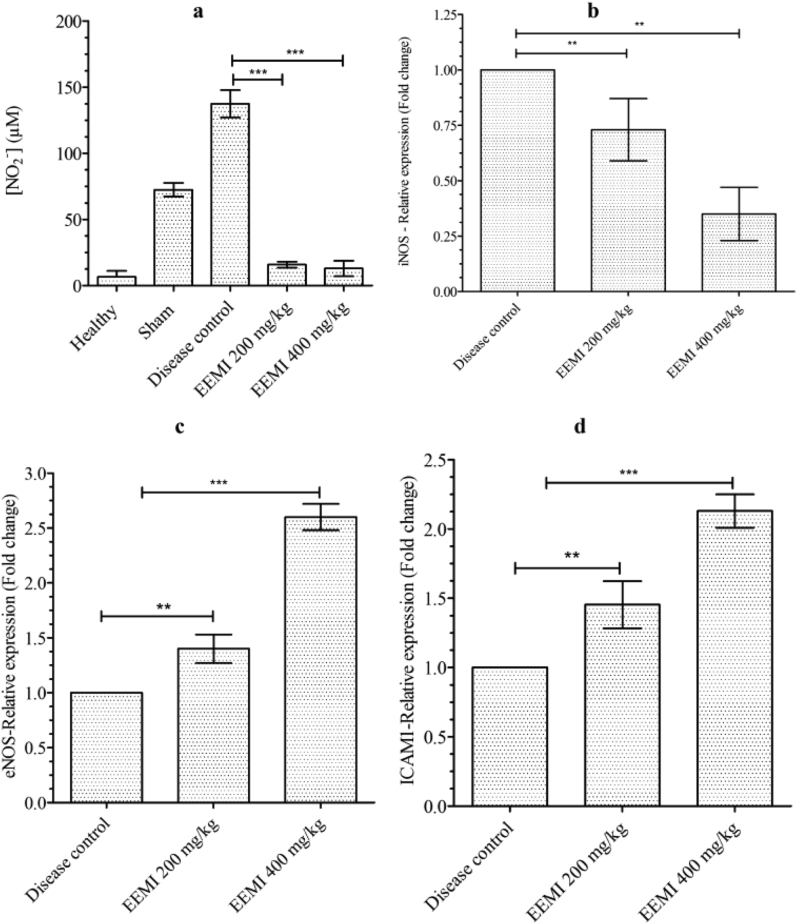
3.6. Effect of EEMI on nitric oxide (nitrite)
The unstable nitric oxide (NO) released into the plasma gets converted to nitrite (NO2−) 27, 28, 29 and determination of nitrite is an indirect measurement of NO. Even though the levels of nitrite in animals treated with 0.2 and 0.4 g/kg of EEMI (15.84 ± 2.26 and 12.91 ± 5.92 μM), respectively was not in a dose-dependent manner, however there was a significant (P < 0.05) decrease in the plasma levels of nitrite against disease control, respectively (137.42 ± 10.31 μM) (Fig. 5a).
Fig. 5.
Levels of nitrite and Relative expression of mRNAs. a) NO−2 b) iNOS c) eNOS d) ICAM-1. The data represented as mean ± SD against corresponding groups. The significance between the groups were analysed using one way ANOVA and post-hoc Dunnett's multiple comparison test at 95% confidence intervals (p < 0.05).
3.7. Effect of EEMI on expression of mRNA of intercellular adhesion molecule (ICAM)-1, endothelial nitric oxide synthase (eNOS) & induced nitric oxide synthase (iNOS) in the gastrocnemius muscle
The expression of mRNA of iNOS, eNOS and ICAM-1in gastrocnemius muscle was quantified by RT-PCR. The expression of iNOS was elevated in disease control and the treatment of animals at 0.2 and 0.4 g/kg of EEMI significantly (P < 0.05) reduced the expression of iNOS with a fold change (mean ± S. D) of 0.73 ± 0.14 and 0.35 ± 0.12 respectively (Fig. 5b). The expression levels of eNOS and ICAM-1 was contrary to iNOS, where treatment of animals at 0.2 and 0.4 g/kg of EEMI showed significant increases in the expression of eNOS with a fold change of 1.40 ± 0.13 and 2.60 ± 0.12, respectively (Fig. 5c) in comparison to disease control. A dose dependent and significant (P < 0.05) increases in the fold change of about 1.45 ± 0.17 and 2.13 ± 0.12 was observed in the levels of expression of mRNA of ICAM-1 in animals treated with 0.2 and 0.4 g/kg of EEMI respectively in comparison to disease control (Fig. 5d).
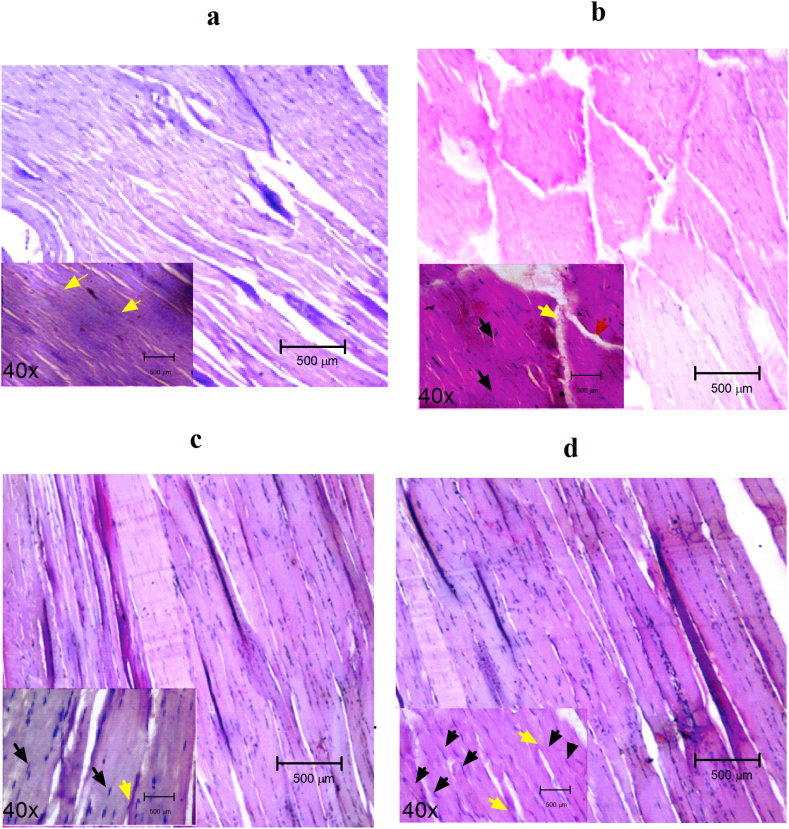
3.8. Effect of EEMI on histology of gastrocnemius muscle (GM)
The tissue section of healthy controls stained darker and was presented with homogenous, polygonal myofibers, intact sarcolemma and nuclei located peripherally (Fig. 6a). Due to reperfusion injury and inflammation, the tissue section of GM of disease controls stained lighter, irregularly shaped myofibers, very smaller number of nuclei (at centre) and some of the fibers with immune cells (stained by haemotoxylin) (Fig. 6b). However, this was not the case in the tissue sections of GM of animals treated with EEMI at 0.2 and 0.4 g/kg of EEMI, where the tissue stained darker, most of the cells were presented with centred nucleus with absence of immune cells (Fig. 6c and d).
Fig. 6.
Histopathology. The hematoxylin and eosin-stained gastrocnemius muscle sections of (a) Healthy control, (b) Disease control, (c) 0.2 g/kg EEMI and (d) 0.4 g/kg EEMI treated animals were observed under phase-contrast microscope under magnification of 10 and 40× (inset). Black arrow: Position of nucleus; Yellow arrow: Myofibers attachment; The nuclei were visible, peripherally located in healthy (a) and EEMI (0.2 and 0.4 g/kg) treated (c and d); whereas the nuclei were moved to centre and some of the nuclei disappeared due to necrosis/injury of the tissue and damage of myofibers were also clearly visible (b).
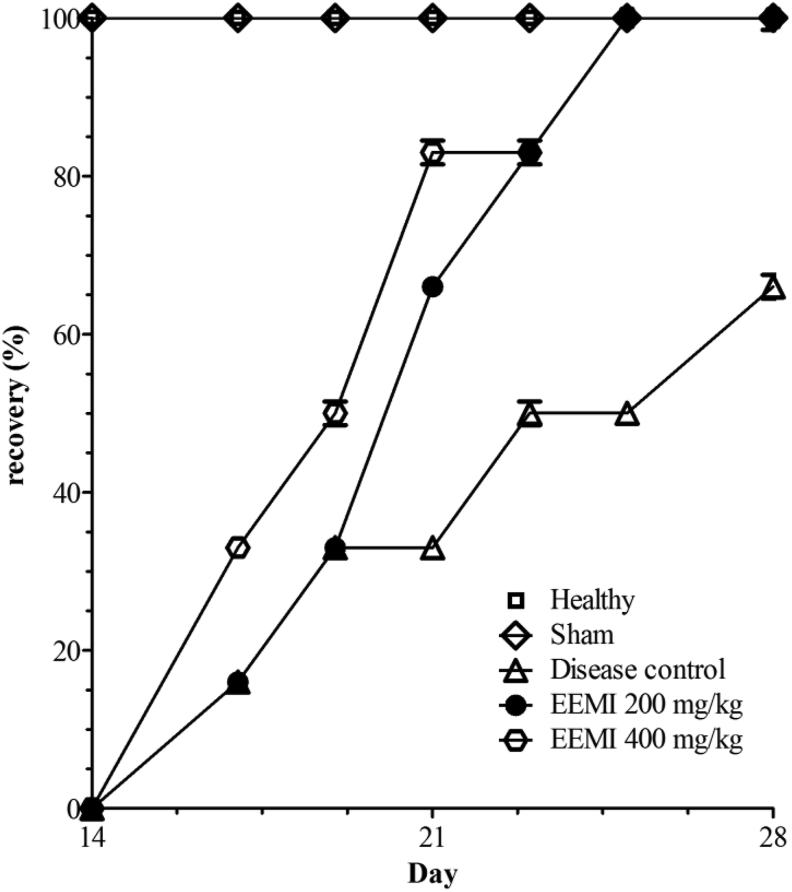
3.9. Effect of EEMI on hind limb function
The hind limb function in animals with or without ischemic-reperfusion during the 28 days of course of study was calculated using Tarlov scoring and represented as percentage recovery (Fig. 7). A significant increase in function of the hind limbs was observed in animals treated with EEMI at 0.2 and 0.4 g/kg on days 21 and 28 in comparison to disease control (Fig. 7). The healthy and sham controls were found to show full function of hind limbs as there were absence of surgical intervention of UFAL and reperfusion.
Fig. 7.
Hind limb function assessment. The function of hind limbs was represented as percentage recovery using Tarlov scoring on y axis against days on x-axis. The significance between the groups were analysed using one way ANOVA and post-hoc Dunnett's multiple comparison test at 95% confidence intervals. There was a gradual and dose-dependent recovery in EEMI treated animals at 0.2 g/kg (∗∗∗p < 0.05) and 0.4 g/kg (∗∗∗p < 0.05) on day 21 and 28 in comparison to disease control.
4. Discussion
The effect of ethanolic extract of M. indica on acute hind limb ischemia/reperfusion injury in diabetic rats was evaluated. Results indicated that animals treated with EEMI were found to attenuate critical biomarkers of LPO and inflammation with improved hind limb function in comparison to disease control.
Reperfusion increases the incidence of LPO and OS in animal models of ischemia.30 MDA is one of the by product and biomarker of LPO of cellular membranes.30 M. indica lowered serum total MDA in animal models of cognitive impairment31 and cyclophosphamide induced renal disease32 and in vitro red blood cell LPO.33 Flavonoids and C-glycosides present in the M. Indica was also found to reduce LPO.5
In general, ischemia/reperfusion leads to accumulation of free radicals (OS) in tissues and decreases normal levels of endogenous anti-oxidant enzymes, enzymes require for biosynthesis of GSH and as well as GSH.34 Loss of free radicals scavenging due to low levels of glutathione results in excessive tissue damage.34 M. Indica were reported to increase the levels of super oxide dismutase (SOD), glutathione peroxidases (GPXs) and catalase enzymes in rodent models of cadmium induced cortical damage,35 cerebral ischemia–reperfusion injury,36 transient global ischemia/reperfusion-induced brain injury.37 In our study, animals treated with EEMI were found to have significant increases in the levels of GSH. This elevation might be due to activation of nuclear factor erythroid 2-related factor (Nrf2) and its expression (e.g. glutathione transferase (GST)-γ)38; and in line with our report, in-vitro cell based and in-vivo animal studies also shown the potential of M. Indica on increasing GSH levels.37 Next, reperfusion also increases the incidence of inflammation39 and treatment of EEMI indicated decreases plasma levels of pro-inflammatory cytokines (IL-6, IL-1β and TNF-α) and nitrite. The oxidative stress and inflammation modulating effect of EEMI might be due to the presence of one of the major phytoconstituents, mangiferin. Mangiferin was reported to attenuate plasma levels of pro-inflammatory cytokines in rodent models of myocardial ischemia-reperfusion injury,40 cisplatin-induced acute kidney injury,41 intestinal ischemia/reperfusion-induced liver injury.42 In addition, we have also found EEMI treated animals with increased levels of plasma IL-10, an endogenous anti-inflammatory protein which blocks TNF-α and IL-1 induced downstream signaling pathways.43 It is highly evident through a number of in vivo studies44,45 that the leaf and seed kernel extracts of M. Indica augmented plasma IL-10 and it might be due to the presence of gallotannins in seed kernel extracts of EEMI.46 Whereas, expression levels of mRNAs of iNOS and eNOS in gastrocnemius muscles of animals treated with EEMI was contrary, where both doses of EEMI reduced the expression of mRNAs of iNOS and increased the expression of eNOS in a dose-dependent manner; and these results were also highly associated with reduced NO levels.
Herein, the cell-cell adhesion during the reversal of ischemic-reperfusion injury was evaluated through levels of mRNAs of ICAM-1 and surprisingly we found increased levels of expression of mRNA of ICAM-1 in gastrocnemius muscles of animals treated with EEMI. Given that ischemic reperfusion injury increases inflammation,5 in turn inflammation also induces adhesion molecules such as ICAM-1 which reside on the myofibers of the muscle tissue.47 Inflammation or accumulation of pro-inflammatory cytokines induced elevated levels of ICAM-1 are an adaptive mechanism in response to ischemic reperfusion injury in an order to revert the tissue for cell-to-cell adhesion.47 Accordingly, in our study, we also found that there was significant increase in levels of mRNA of ICAM-1 of disease control in comparison to healthy animals.
Histopathological sections revealed altered morphology of gastrocnemius muscle tissue in disease controls in comparison to healthy controls. It is well known that reperfusion after ischemia will lead to membrane rupture around the tissue and cause cell injury, which will alter the morphology of the cells.48 In our study, histopathological findings indicated a dose-dependent repair of GM tissue of animals treated with EEMI in comparison to disease control. In addition, the animals treated with EEMI were found to show improved function of the hind limbs with increased recovery from reperfusion-injury induced hind limb dysfunction.
From our findings and available reports, we propose a probable mechanism of EEMI involved in attenuating reperfusion injury in AHL ischemic diabetic rats.
In general, ischemia/reperfusion at distal areas from the site of occlusion or ligation results in cell injury and increases the risk of OS inducing LPO and glutathione imbalance.8
On the other hand, either reperfusion induced OS or reperfusion injury itself in turn induces damage associated proteins such as damage associated molecular patterns (DAMPs) from the cellular components of endothelial cells (Syndecans, Heat Shock Proteins, HMGB1, mROS, and Calreticulin).9 DAMPs in turn can interact with receptors such as toll like receptors (e.g. TLR4, NLRP3 and CD91).10 Interaction of DAMPs with their corresponding receptors (for e.g. TLR4) induces nuclear translocation of cytosolic NF-ĸB (nuclear transcription factor of activated B cells), which interact with response elements to transcribe (gene transcription) and release corresponding mRNAs and generate nitric oxide synthases (iNOS or eNOS)11 and pro-inflammatory cytokines (IL-6, IL-1β and TNF-α).11 Both eNOS and iNOS involves in generation of nitric oxide (NO), via catalyses of their substrate Arginine (present in lumen endothelial plasma membrane).11 Released NO interacts with soluble guanylyl cyclase (sGC) and converts its substrate guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP), a second messenger induces protein kinase G resulting in vasodilation.49
Whereas secreted pro-inflammatory cytokines bind to their corresponding receptors (e.g. IL-6 with IL-6R) and expresses genes responsible for increased pro-inflammatory cytokine accumulation, cellular survival, proliferation, migration and apoptosis.50 During reperfusion injury, accumulation of pro-inflammatory cytokines predominates and results in inflammation. Overall, increased LPO, OS, glutathione imbalance and inflammation altogether exacerbate tissue injury.
In our study, EEMI reduced the levels of MDA (marker of LPO), IL-6, IL-1β, TNF-α and iNOS mRNA levels (markers of inflammation); as well increased the levels of (GSH), anti-inflammatory cytokines (IL-10) and expression of mRNAs (eNOS) in a dose-dependent manner, which are highly protective for tissues against reperfusion injury. It is also well-known that ICAM-1 is up regulated in ischemic/reperfusion injury and does adherence- and migration process of cells to assist in reversal of tissue injury.47 There was a significant increase in the levels of mRNA of ICAM-1 in GM of EEMI treated animals in comparison to disease control, which suggest in addition to ischemic/reperfusion, by this way EEMI may also promoted cell-to-cell adherence to reverse tissue injury. Overall, the probable mechanisms that drive the attenuation of tissue injury by EEMI might be through modulating OS and inflammation.
5. Conclusion
Treatment of EEMI in diabetic rats with AHLI-RI showed dose-dependent recovery and it was also strongly associated with enhanced functions of hind limb, decreased pro-inflammatory cytokines and increased anti-inflammatory cytokine in comparison to disease control. Finally, we conclude EEMI increased hind limb function with attenuated hind limb ischemia/reperfusion injury in diabetic rats by modulating (decreasing) OS and inflammation. In future, isolation and evaluation of biologically active phytoconstituents from M. indica on AHLI-RI may provide greater insights into its specific mechanism on attenuation/reversal of tissue injury.
5.1. Limitations
The effect of EEMI on animals with AHLI-RI without diabetes was not evaluated, as our primary aim of this study is to identify only the effect of EEMI on AHLI-RI in diabetic rats.
Declaration of competing interest
None to declare.
Acknowledgements
RamPravinKumar is supported through postgraduate research fund, PSG College of Pharmacy, Coimbatore. Authors acknowledge management of PSG Institutions and Prof. M. Ramanathan, Principal, PSG College of Pharmacy, Coimbatore, Tamil Nadu, India for their support during course of this study.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.05.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Olin J.W., Sealove B.A. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85(7):678–692. doi: 10.4065/mcp.2010.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartholomew J.R., Olin J.W. Pathophysiology of peripheral arterial disease and risk factors for its development. Cleve Clin J Med. 2006;73(Suppl 4):S8–S14. doi: 10.3949/ccjm.73.suppl_4.s8. [DOI] [PubMed] [Google Scholar]
- 3.Shu J., Santulli G. Update on peripheral artery disease: epidemiology and evidence-based facts. Atherosclerosis. 2018;275:379–381. doi: 10.1016/j.atherosclerosis.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman J.A., White C.J. Paclitaxel-coated balloons and eluting stents: is there a mortality risk in patients with peripheral artery disease? Circulation. 2019;140(16):1342–1351. doi: 10.1161/CIRCULATIONAHA.119.041099. [DOI] [PubMed] [Google Scholar]
- 5.Kalogeris T., Baines C.P., Krenz M., Korthuis R.J. In: Jeon K.W., editor. vol. 298. Academic Press; 2012. Chapter six - cell biology of ischemia/reperfusion injury; pp. 229–317. (International Review of Cell and Molecular Biology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers S.K., Ji L.L., Kavazis A.N., Jackson M.J. Reactive oxygen species: impact on skeletal muscle. Comp Physiol. 2011;1(2):941–969. doi: 10.1002/cphy.c100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coirault C., Guellich A., Barbry T., Samuel J.L., Riou B., Lecarpentier Y. Oxidative stress of myosin contributes to skeletal muscle dysfunction in rats with chronic heart failure. Am J Physiol Heart Circ Physiol. 2007;292(2):H1009–H1017. doi: 10.1152/ajpheart.00438.2006. [DOI] [PubMed] [Google Scholar]
- 8.Westman B., Johansson G., Luo J.-L., Söderlund K., Wernerman J., Hammarqvist F. Effects on skeletal muscle glutathione status of ischemia and reperfusion following abdominal aortic aneurysm surgery. Ann Vasc Surg. 2006;20(1):99. doi: 10.1007/s10016-005-9111-7. [DOI] [PubMed] [Google Scholar]
- 9.Slegtenhorst B.R., Dor F.J.M.F., Rodriguez H., Voskuil F.J., Tullius S.G. Ischemia/reperfusion injury and its consequences on immunity and inflammation. Curr Transplant Reports. 2014;1(3):147–154. doi: 10.1007/s40472-014-0017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubartelli A., Lotze M.T. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28(10):429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 11.El-Zayat S.R., Sibaii H., Mannaa F.A. Toll-like receptors activation, signaling, and targeting: an overview. Bull Natl Res Cent. 2019;43(1):187. doi: 10.1186/s42269-019-0227-2. [DOI] [Google Scholar]
- 12.Losordo D.W., Chung A., Chen Z., Cooke J.P. Stem Cell and Gene Therapy for Cardiovascular Disease. 2015. Peripheral arterial disease. [DOI] [Google Scholar]
- 13.Hirsch A.T., Haskal Z.J., Hertzer N.R. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Su. Circulation. 2006;113(11):e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 14.Tsioufis C., Andrikou I., Siasos G., Filis K., Tousoulis D. Anti-hypertensive treatment in peripheral artery disease. Curr Opin Pharmacol. 2018;39:35–42. doi: 10.1016/j.coph.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Atturu G., Homer-Vanniasinkam S., Russell D.A. Pharmacology in peripheral arterial disease: what the interventional radiologist needs to know. Semin Intervent Radiol. 2014;31(4):330–337. doi: 10.1055/s-0034-1393969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thiruvoipati T., Kielhorn C.E., Armstrong E.J. Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J Diabetes. 2015;6(7):961–969. doi: 10.4239/wjd.v6.i7.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasan S.S., Ahmed S.I., Bukhari N.I., Loon W.C.W. Use of complementary and alternative medicine among patients with chronic diseases at outpatient clinics. Compl Ther Clin Pract. 2009;15(3):152–157. doi: 10.1016/j.ctcp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Barreto J.C., Trevisan M.T.S., Hull W.E. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (mangifera indica L.) J Agric Food Chem. 2008;56(14):5599–5610. doi: 10.1021/jf800738r. [DOI] [PubMed] [Google Scholar]
- 19.WHO . World Health Organization; 2011. Quality Control Methods for Herbal Materials.https://apps.who.int/iris/handle/10665/44479 Published. [Google Scholar]
- 20.Khandelwal K. Nirali Prakashan; Pune, India: 2017. Practical Pharmacognosy Techniques & Experiments. [Google Scholar]
- 21.Siddiqui and Hakim . Workshop on Standardization of Unani Drugs. Central Council for Research in Unani Medicine (CCRUM); New Delhi: 1995. Format for the pharmacopoeial analytical standards of compound formulation. [Google Scholar]
- 22.Tarlov I.M. Spinal cord compression studies. III. Time limits for recovery after gradual compression in dogs. AMA Arch Neurol Psychiatry. 1954;71(5):588–597. [PubMed] [Google Scholar]
- 23.Niiyama H., Huang N.F., Rollins M.D., Cooke J.P. Murine model of hindlimb ischemia. JoVE. 2009;23 doi: 10.3791/1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone W.L., Yang H., Qui M. Assays for nitric oxide expression. Methods Mol Biol. 2006;315:245–256. doi: 10.1385/1-59259-967-2:245. [DOI] [PubMed] [Google Scholar]
- 25.Zeb A., Ullah F. A simple spectrophotometric method for the determination of thiobarbituric acid reactive substances in fried fast foods. Calokerinos A.C., editor. J Anal Methods Chem. 2016;2016:9412767. doi: 10.1155/2016/9412767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahman I., Kode A., Biswas S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1(6):3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 27.Gulcin İ. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol. 2020 doi: 10.1007/s00204-020-02689-3. [DOI] [PubMed] [Google Scholar]
- 28.Taslimi P., Gulçin İ. Antioxidant and anticholinergic properties of olivetol. J Food Biochem. 2018 doi: 10.1111/jfbc.12516. [DOI] [Google Scholar]
- 29.Gülçin I. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012 doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- 30.Lucas D.T., Szweda L.I. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc Natl Acad Sci Unit States Am. 1998;95(2):510–514. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wattanathorn J., Muchimapura S., Thukham-Mee W., Ingkaninan K., Wittaya-Areekul S. Mangifera indica fruit extract improves memory impairment, cholinergic dysfunction, and oxidative stress damage in animal model of mild cognitive impairment. Oxid Med Cell Longev. 2014;2014:132097. doi: 10.1155/2014/132097. Minghetti L, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amien A.I., Fahmy S.R., Abd-Elgleel F.M., Elaskalany S.M. Renoprotective effect of Mangifera indica polysaccharides and silymarin against cyclophosphamide toxicity in rats. J Basic Appl Zool. 2015;72:154–162. doi: 10.1016/j.jobaz.2015.09.006. [DOI] [Google Scholar]
- 33.Rodríguez J., Di Pierro D., Gioia M. Effects of a natural extract from Mangifera indica L, and its active compound, mangiferin, on energy state and lipid peroxidation of red blood cells. Biochim Biophys Acta Gen Subj. 2006;1760(9):1333–1342. doi: 10.1016/j.bbagen.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Iskusnykh I.Y., Popova T.N., Agarkov A.A., Pinheiro de Carvalho M.Â.A., Rjevskiy S.G. Expression of glutathione peroxidase and glutathione reductase and level of free radical processes under toxic hepatitis in rats. J Toxicol. 2013;2013:870628. doi: 10.1155/2013/870628. Seagrave J, ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al omairi N.E., Radwan O.K., Alzahrani Y.A., Kassab R.B. Neuroprotective efficiency of Mangifera indica leaves extract on cadmium-induced cortical damage in rats. Metab Brain Dis. 2018;33(4):1121–1130. doi: 10.1007/s11011-018-0222-6. [DOI] [PubMed] [Google Scholar]
- 36.Yang Z., Weian C., Susu H., Hanmin W. Protective effects of mangiferin on cerebral ischemia-reperfusion injury and its mechanisms. Eur J Pharmacol. 2016 doi: 10.1016/j.ejphar.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Viswanatha G.L., Shylaja H., Mohan C.G. Alleviation of transient global ischemia/reperfusion-induced brain injury in rats with 1,2,3,4,6-penta-O-galloyl-β-d-glucopyranose isolated from Mangifera indica. Eur J Pharmacol. 2013;720(1):286–293. doi: 10.1016/j.ejphar.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Wu S., Lu H., Bai Y. Nrf2 in cancers: a double-edged sword. Canc. Med. 2019;8(5):2252–2267. doi: 10.1002/cam4.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghaly A., Marsh D.R. Ischaemia–reperfusion modulates inflammation and fibrosis of skeletal muscle after contusion injury. Int J Exp Pathol. 2010;91(3):244–255. doi: 10.1111/j.1365-2613.2010.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatia J., Suchal K., Bhargava P., Malik S., Arya D. Evaluation OF the cardioprotective effect OF mangiferin IN an experimental model OF ischemia reperfusion injury. J Hypertens. 2021 doi: 10.1097/01.hjh.0000747668.48915.20. [DOI] [Google Scholar]
- 41.Sahu A.K., Verma V.K., Mutneja E. Mangiferin attenuates cisplatin-induced acute kidney injury in rats mediating modulation of MAPK pathway. Mol Cell Biochem. 2019 doi: 10.1007/s11010-018-3420-y. [DOI] [PubMed] [Google Scholar]
- 42.El-Sayyad S.M., Soubh A.A., Awad A.S., El-Abhar H.S. Mangiferin protects against intestinal ischemia/reperfusion-induced liver injury: Involvement of PPAR-γ, GSK-3β and Wnt/β-catenin pathway. Eur J Pharmacol. 2017 doi: 10.1016/j.ejphar.2017.05.021‬‬‬‬‬‬‬‬‬. [DOI] [PubMed] [Google Scholar]
- 43.Lobo-Silva D., Carriche G.M., Castro A.G., Roque S., Saraiva M. Balancing the immune response in the brain: IL-10 and its regulation. J Neuroinflammation. 2016;13(1):297. doi: 10.1186/s12974-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramírez N.M., Toledo R.C.L., Moreira M.E.C. Anti-obesity effects of tea from Mangifera indica L. leaves of the Ubá variety in high-fat diet-induced obese rats. Biomed Pharmacother. 2017 doi: 10.1016/j.biopha.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Toledo R.C.L., Brito L.F., Caetano M.M.M. Acute treatment with Mangifera indica L. leaf extract attenuates liver inflammation in rats fed a cafeteria diet. Food Funct. 2019;10(8):4861–4867. doi: 10.1039/C9FO00651F. [DOI] [PubMed] [Google Scholar]
- 46.Kim H., Banerjee N., Ivanov I. Comparison of anti-inflammatory mechanisms of mango (Mangifera Indica L.) and pomegranate (Punica Granatum L.) in a preclinical model of colitis. Mol Nutr Food Res. 2016 doi: 10.1002/mnfr.201501008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon C.-H., Hur J., Oh I.-Y. Intercellular adhesion molecule-1 is upregulated in ischemic muscle, which mediates trafficking of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2006;26(5):1066–1072. doi: 10.1161/01.ATV.0000215001.92941.6c. [DOI] [PubMed] [Google Scholar]
- 48.Gillani S., Cao J., Suzuki T., Hak D.J. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2012;43(6):670–675. doi: 10.1016/j.injury.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 49.Denninger J.W., Marletta M.A. Guanylate cyclase and the ⋅NO/cGMP signaling pathway. Biochim Biophys Acta Bioenerg. 1999;1411(2):334–350. doi: 10.1016/S0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 50.Grunnet L.G., Aikin R., Tonnesen M.F. Proinflammatory cytokines activate the intrinsic apoptotic pathway in β-cells. Diabetes. 2009;58(8):1807–1815. doi: 10.2337/db08-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.