Abstract
Rapid detection of human immunodeficiency virus (HIV) infection can result in improved patient care and/or faster implementation of public health preventive measures. A new rapid test, Determine (Abbott, Abbott Park, Ill.), detects HIV type 1 (HIV-1) and HIV-2 antibodies within 15 min by using 50 μl of serum or plasma. No specialized equipment or ancillary supplies are required, and results are read visually. A positive result is noted by the appearance of a red line. An operational control (red line) indicates proper test performance. We evaluated the Determine rapid HIV detection test with a group of well-characterized serum samples (CD4 counts and viral loads were known) and serum samples from HIV-positive individuals at field sites in Honduras and the Dominican Republic. In the field evaluations, the results obtained by the Determine assay were compared to those obtained by local in-country HIV screening procedures. We evaluated serum from 100 HIV-positive patients and 66 HIV-negative patients. All samples gave the expected results. In a companion study, 42 HIV-positive samples from a Miami, Fla., serum bank were tested by the Determine assay. The samples had been characterized in terms of CD4 counts and viral loads. Fifteen patients had CD4 counts <200 cells/mm3, while 27 patients had CD4 counts >200 cells/mm3. Viral loads ranged from 630 to 873,746 log10 copies/ml. All samples from the Miami serum bank were positive by the Determine test. Combined results from the multicenter studies indicated that the correct results were obtained by the Determine assay for 100% (142 of 142) of the HIV-positive serum samples and 100% (66 of 66) of the HIV-negative serum samples. The Determine test was simple to perform and the results were easy to interpret. The Determine test provides a valuable new method for the rapid identification of HIV-positive individuals, especially in developing countries with limited laboratory infrastructures.
Diagnosis and counseling are the cornerstones of prevention and care strategies for human immunodeficiency virus (HIV)-infected individuals. Moreover, effective public health surveillance is essential to track the spread of the HIV pandemic, guide research needs, and provide a focus for prevention activities (2). A major challenge to the diagnosis of HIV infection and surveillance activities in developing countries may be attributed to the fact that many clinics or point-of-care facilities in these areas are poorly equipped and often lack diagnostic capabilities or the equipment needed to perform a standard enzyme-linked immunosorbent assay (ELISA) and/or confirmatory Western blotting to identify those infected with HIV. Compounding this problem in rural areas is the possibility that electricity and refrigeration for storage of diagnostic test kits and reagents may be unavailable. Also, individuals must often travel long distances to reach a health care facility, and the chances that the person will travel back to the clinic to receive results may be slim due to transportation hardships. Additionally, samples are often sent hundreds of miles away from the point-of-care facility for testing or even to reference laboratories outside of the country. This impedes effective and timely management of patients infected with HIV.
Rapid screening for HIV infection performed on-site by tests that do not require laboratory infrastructure or highly skilled personnel can help identify those who may be infected with the virus and can facilitate immediate counseling to help prevent the individual from spreading the virus to others by introducing them to risk-reducing behaviors. The study presented in this paper details the results from a field evaluation of a new diagnostic test, the Determine test (Abbott Laboratories, Abbott Park, Ill.), that permits rapid detection of HIV infection. We evaluated the new tests using known HIV-seropositive samples at two independent laboratories in Honduras and the Dominican Republic to assess the performance of the Determine HIV rapid test and determine the level of agreement with in-country tests. Tests were also performed with a bank of serum samples from HIV-positive individuals in the United States. The latter serum samples had been characterized in terms of CD4 counts and viral loads.
MATERIALS AND METHODS
Study sites.
This multicenter study was performed at the Social Security Hospital in San Pedro Sula, Honduras, and the National Public Health Laboratory in Santo Domingo, Dominican Republic. In addition, serum samples that had been characterized in terms of CD4 counts and viral loads were obtained from HIV-infected individuals in Florida and were also tested.
Field study patient samples and diagnostic parameters.
Rapid test evaluations were performed with serum samples from patients who had previously been found to be HIV positive or negative. The serum samples had been collected by venipuncture over a 1-month period prior to our visit and were stored at 0°C in the freezer area of a regular refrigerator. This is representative of standard storage conditions in many developing countries. In Honduras, we tested serum samples from 50 HIV-positive and 46 HIV-negative patients, including 15 samples from patients who were HIV negative but who were positive for Chagas' disease and 5 who were HIV negative but who were positive for leishmaniasis. We included serum from individuals with Chagas' disease and leishmania infection because these two diseases are prevalent in Honduras and testing for evidence of Chagas' disease is always included in blood bank screening. In the Dominican Republic, we tested 50 samples from HIV-positive patients and 20 samples from HIV-negative patients.
The in-country HIV test methods used in Honduras included the Abbot Retrocell and Bio-Rad Western blot assays. At the Dominican Republic site, HIV infection had been diagnosed by the Abbott recombinant HIV 1/2 EIA, the Serodia (Tokyo, Japan) agglutination test, and the Genelabs (Redwood City, Calif.) Western Blot assay for confirmation of the results for positive samples.
Characterized serum sample bank.
In order to evaluate the effectiveness of the Determine test in detecting HIV antibodies in those with different stages of HIV disease, we tested serum from HIV-positive patients in Miami, Fla., with known CD4 counts and viral loads. A total of 42 samples from 16 women and 26 men ranging in age from 22 to 56 years were tested. These samples had been collected by venipuncture in EDTA-coated Vacutainer tubes up to 6 months prior to our testing and had been stored at −70°C. Countries of origin in this population included the United States (n = 25), Haiti (n = 5), Jamaica (n = 4), Honduras (n = 2), Nicaragua (n = 2), Cuba (n = 2) and unknown (the individuals were Hispanic; n = 2). Fifteen samples were from patients who had CD4 counts <200 cells/mm3, 15 samples were from patients with CD4 counts between 200 and 500 cells/mm3, and 12 samples were from patients with CD4 counts >500 cells/mm3 (Table 1). Viral loads ranged from 630 to 873,746 log10 copies/ml. Opportunistic infections present at the time of serum collection in this population included Pneumocystis carinii pneumonia, toxoplasmosis, community-acquired pneumonia, Kaposi's sarcoma, herpes zoster, and candidiasis.
TABLE 1.
CD4 counts and viral loads for patients in Miami studya
| Patient no. | Age (yr) | Sex | Viral load (log10 copies/ml) | CD4 count (cells/mm3) | Coinfection | Ethnicity | Country of origin |
|---|---|---|---|---|---|---|---|
| 1 | 48 | Female | 3,000 | 952 | None | Black | USA |
| 2 | 39 | Male | 750,000 | 5 | Toxo | Hispanic | Cuba |
| 3 | 46 | Male | 243,465 | 242 | PCP | Black | USA |
| 4 | 24 | Female | 15,896 | 663 | None | Black | USA |
| 5 | 37 | Female | 53,082 | 151 | None | Black | USA |
| 6 | 51 | Female | 18,966 | 330 | None | Black | USA |
| 7 | 37 | Male | 158,381 | 46 | PCP | Black | USA |
| 8 | 38 | Male | 172,274 | 357 | None | Black | USA |
| 9 | 38 | Female | 507,984 | 29 | Toxo | Hispanic | Honduras |
| 10 | 38 | Male | 208,320 | 23 | PCP | Black | Jamaica |
| 11 | 25 | Female | 257,314 | 322 | None | White | USA |
| 12 | 25 | Male | 750,000 | 36 | KS | White | USA |
| 13 | 37 | Female | 1,903 | 36 | None | Black | USA |
| 14 | 47 | Male | 86,806 | 500 | None | Black | Jamaica |
| 15 | 44 | Male | 1,223 | 670 | None | Black | Jamaica |
| 16 | 35 | Male | 8,218 | 885 | None | Black | USA |
| 17 | 30 | Male | 166,002 | 66 | None | Black | Haiti |
| 18 | 28 | Male | 10,583 | 574 | None | Hispanic | Nicaragua |
| 19 | 22 | Male | 7,204 | 602 | None | Black | USA |
| 20 | 56 | Male | 139,839 | 35 | PCP | Hispanic | Cuba |
| 21 | 34 | Male | 21,000 | 620 | None | Black | USA |
| 22 | 23 | Female | 750,000 | 11 | None | Black | USA |
| 23 | 35 | Female | 27,453 | 171 | Zoster | Hispanic | Honduras |
| 24 | 48 | Male | 116,000 | 721 | None | Black | USA |
| 25 | 39 | Female | 2,442 | 271 | None | Black | Haiti |
| 26 | 44 | Male | 16,275 | 604 | None | Black | Haiti |
| 27 | 33 | Female | 42,085 | 587 | None | Black | USA |
| 28 | 36 | Male | 5,933 | 444 | None | Hispanic | Unknown |
| 29 | 44 | Male | NT | 9 | PCP | Black | Jamaica |
| 30 | 32 | Female | NT | 857 | None | Black | USA |
| 31 | 45 | Male | 73,629 | 333 | None | Black | USA |
| 32 | 55 | Female | 243,292 | 144 | None | Black | USA |
| 33 | 38 | Female | 166,621 | 287 | None | White | USA |
| 34 | 36 | Male | 56,938 | 227 | None | Hispanic | Unknown |
| 35 | 30 | Male | 873,746 | 334 | PCP, Can | Black | USA |
| 36 | 44 | Female | 23,282 | 591 | None | Black | USA |
| 37 | 42 | Male | 28,286 | 272 | None | Black | Haiti |
| 38 | 42 | Male | 630 | 110 | CAP | Black | Haiti |
| 39 | 36 | Male | 3,818 | 203 | None | Black | USA |
| 40 | 30 | Male | 19,856 | 5 | Toxo, PCP, Can | Black | USA |
| 41 | 49 | Male | 2,562 | 227 | None | Black | USA |
| 42 | 43 | Female | NT | 388 | None | Hispanic | Nicaragua |
Abbreviations: NT, not tested; Toxo, toxoplasmosis; CAP, community-acquired pneumonia; PCP, pneumocystis carini pneumonia; KS, Kaposi's sarcoma; Zoster, herpes zoster; Can, candidiasis; USA, United States.
Determine assay.
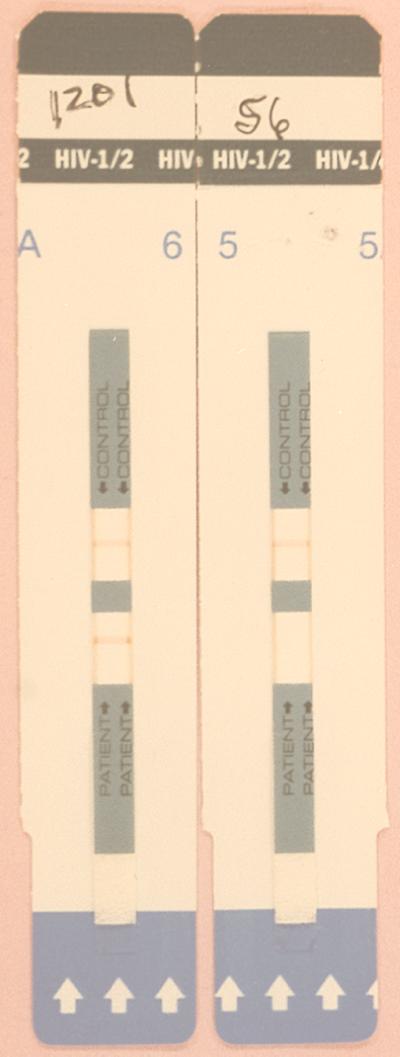
The Determine test (Abbott Laboratories) is an immunochromatographic test for the qualitative detection of HIV type 1 (HIV-1) and HIV-2. The test is performed by applying 50 μl of serum to the test pad at the bottom of the strip. As the sample migrates, it reconstitutes and mixes with a selenium colloid-antigen conjugate. This mixture continues to migrate through a solid phase until it reaches immobilized recombinant antigens and synthetic peptides in the window where the patient's results are displayed. If antibodies to HIV-1 or HIV-2 are present, a red line forms in the window where the patient's results are displayed. A procedural control window is included on each strip where a red line forms to ensure quality control of individual strips (Fig. 1). Results from the strips are interpreted visually.
FIG. 1.
Determine test strips. positive and negative results are displayed.
RESULTS
Honduras and Dominican Republic.
The results for the samples from HIV-positive and HIV-negative patients in Honduras and the Dominican Republic indicated 100% agreement between the Determine test and previous test results. Thus, both the sensitivity and the specificity were 100%. The results for 100 of the HIV-positive serum samples and all 66 of the HIV-negative serum samples that were tested by the Determine test agreed with previous test results obtained by the in-country test methods.
U.S. serum bank.
Similar to our experience with tests performed in Honduras and the Dominican Republic, we found that the Determine test was 100% sensitive in evaluating sera from patients with known CD4 counts and viral loads. Even patients with CD4 counts less than 10 cells/mm3 were positive by the Determine test, demonstrating that this test is extremely sensitive and specific even with samples from patients in the advanced stages of the disease, when the immune system is severely compromised.
DISCUSSION
This three-country multicenter study has shown that the results of the new Determine rapid HIV detection test were equivalent to in-country test results for detection of antibodies to HIV in serum samples from Honduras and the Dominican Republic and a small set of well-characterized serum samples from the United States. Importantly, the rapid test detected HIV infection in serum from patients with CD4 counts as low as 5 cells/mm3 and/or with multiple opportunistic infections. This was an important finding since in many instances, patients are not tested for HIV until they present with opportunistic infections and/or are in advanced stages of the disease, by which time their immune systems may be severely depressed.
The Determine test yielded rapid, easy to interpret results, and no specialized equipment or ancillary reagents were required to perform the test. Because of these features, the Determine test series could provide a valuable tool with which to rapidly screen patient serum samples at point-of-care facilities as well as reference laboratories. This is of particular importance for HIV testing, as it can be problematic to have patients return to the point of care for their HIV test results. It has been noted that with rapid tests, negative results can be given to the patient at the time of testing, eliminating the need for return visits. A positive, rapid HIV test result requires confirmation by another test or Western blotting; however, the patient can receive immediate feedback on the likelihood of infection with HIV and can receive counseling which encourages adoption of risk-reducing behaviors (3–5).
The World Health Organization has addressed the issues of increasing in-country capabilities for infectious disease diagnoses, particularly in the case of HIV infection, and incorporating the use of rapid diagnostic tests into standard laboratory formats and point-of-care facilities (8). For example, the strategy for HIV testing recommends sequential testing of samples by either ELISAs or rapid assays with different formats. Samples are screened by one method, and reactive samples can then be tested by a second assay. Thus, screening of samples by the rapid Determine assay could serve as first-line diagnostic support with follow-up by the secondary method only for samples that appear to be positive upon initial testing. As these tests are completed within 15 min, the patient benefits from a rapid result. This can allay fears and anxiety when negative results are obtained or can provide rapid treatment options and/or counseling in the case of a positive result. Either way, patients are immediately aware of their potential disease status.
One other recent report has detailed the use of the Determine test in Southeast Asia, in the Ivory Coast, and with commercially available serum conversion panels (1). Similar to the results of our study in the Americas (Honduras, Dominican Republic, and Florida), they reported 100% sensitivity and specificity for the Determine test. Thus, the Determine test appears to be robust as excellent results have been obtained in widely separated geographic areas.
In conclusion, it has been suggested that the ideal test for the rapid diagnosis of HIV infection should be rapid, inexpensive, highly sensitive and specific, and easy to perform; and results should be easy to interpret; the test should be able to be stored at room temperature with a long shelf life; and no additional equipment or ancillary supplies should be required to perform the test (6). In addition, rapid testing is efficient for laboratories that have a small volumes for testing, that require rapid results, and that do not have technically advanced equipment such as ELISA plate readers (7). The new Determine test for rapid detection of HIV fulfills these criteria and, as such, would provide a powerful tool for controlling the HIV pandemic in developing countries worldwide.
REFERENCES
- 1.Arai H, Petchclai B, Khupulsup K, Kurimura T, Takeda K. Evaluation of a rapid immunochromatographic test for detection of antibodies to human immunodeficiency virus. J Clin Microbiol. 1999;37:367–370. doi: 10.1128/jcm.37.2.367-370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu D J, Dondero T J, Rayfield M A, George J R, Schochetman G, Jaffe H W, Luo C, Kalish M L, Weniger B G, Pau C, Schable C A, Curran J W. The emerging genetic diversity of HIV. JAMA. 1996;275:210–216. [PubMed] [Google Scholar]
- 3.Irwin K, Olivo N, Schable C, Weber J T, Janssen R, Ernst J the CDC Bronx-Lebanon HIV Serosurvey Team. Performance characteristics of a rapid HIV antibody assay in a hospital with a high prevalence of HIV infection. Ann Intern Med. 1995;125:471–475. doi: 10.7326/0003-4819-125-6-199609150-00007. [DOI] [PubMed] [Google Scholar]
- 4.Kassler W J, Haley C, Jones W K, Gerber A, Kennedy E J, George J R. Performance of a rapid, on-site human immunodeficiency virus antibody assay in a public health setting. J Clin Microbiol. 1995;33:2899–2902. doi: 10.1128/jcm.33.11.2899-2902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassler W J. Advances in HIV testing technology and their potential impact on prevention. AIDS Educ Prevent. 1997;9(Suppl. 3):27–40. [PubMed] [Google Scholar]
- 6.Malone J D, Smith E S, Sheffield J, Bigelow D, Hyams K C, Beardsley S G, Lewis R S, Roberts C R. Comparative evaluation of six rapid serological tests for HIV-1 antibody. J Acquir Immune Defic Syndr. 1993;6:115–119. [PubMed] [Google Scholar]
- 7.Mitchell S W, Mboup S, Mingle J, Sambe D, Tukei P, Milenge K, Nyamongo J, Mubarak O, Sankale J, Hanson D, Quinn T C. Field evaluation of alternative HIV testing strategy with a rapid immunobinding assay and an agglutination assay. Lancet. 1991;337:1328–1331. doi: 10.1016/0140-6736(91)92991-a. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Recommendation for the selection and use of HIV antibody tests. Weekly Epidemiol Rev. 1992;67:145–149. [PubMed] [Google Scholar]