Abstract
Background
The relationship between hospital surgical volume and outcome after colorectal cancer surgery has thoroughly been studied. However, few studies have assessed hospital surgical volume and failure-to-rescue (FTR) after colon and rectal cancer surgery. The aim of the current study is to evaluate FTR following colorectal cancer surgery between clinics based on procedure volume.
Methods
Patients undergoing colorectal cancer surgery in Sweden from January 2015 to January 2020 were recruited through the Swedish Colorectal Cancer Registry. The primary endpoint was FTR, defined as the proportion of patients with 30-day mortality after severe postoperative complications in colorectal cancer surgery. Severe postoperative complications were defined as Clavien–Dindo ≥ 3. FTR incidence rate ratios (IRR) were calculated comparing center volume stratified in low-volume (≤ 200 cases/year) and high-volume centers (> 200 cases/year), as well as with an alternative stratification comparing low-volume (< 50 cases/year), medium-volume (50–150 cases/year) and high-volume centers (> 150 cases/year).
Results
A total of 23,351 patients were included in this study, of whom 2964 suffered severe postoperative complication(s). Adjusted IRR showed no significant differences between high- and low-volume centers with an IRR of 0.97 (0.75–1.26, p = 0.844) in high-volume centers in the first stratification and an IRR of 2.06 (0.80–5.31, p = 0.134) for high-volume centers and 2.15 (0.83–5.56, p = 0.116) for medium-volume centers in the second stratification.
Conclusion
This nationwide retrospectively analyzed cohort study fails to demonstrate a significant association between hospital surgical volume and FTR after colorectal cancer surgery. Future studies should explore alternative characteristics and their correlation with FTR to identify possible interventions for the improvement of quality of care after colorectal cancer surgery.
Introduction
The association between postoperative outcomes after cancer surgery and center volumes has been the topic of much debate, and the assumption that centers with higher caseload perform advanced operations with improved outcomes has driven centralization [1]. Several studies have associated this centralization of complex oncologic resections, including esophagectomy, pancreatectomy, liver resection, and pulmonary lobectomy, with improved outcomes [2–4]. Equipoise remains, however, in colorectal cancer surgery, with several studies demonstrating improvements in overall survival for high-volume centers [5–7], while other studies failed to detect any morbidity or mortality difference with increased procedure volume [8–11].
Failure-to-rescue (FTR), an emerging surgical outcome metric reflecting center-level quality of postoperative care, is described as "the mortality rate among patients with complications"[12]. Literature on center volume and its effect on FTR after colorectal cancer surgery is scarce. Some studies have shown that higher-volume centers (HVCs) have lower FTR rates than low-volume centers (LVCs), indicating better quality of care [13, 14], while others have not been able to do so [15, 16].
In a recently published study by our group, we noted a strong association between university hospital status and decreased FTR in colorectal cancer surgery across Sweden [17]. In this study, we aim to evaluate the effects of procedure volume on FTR in colorectal cancer surgery in the same study cohort. We hypothesize that HVCs will show higher survival rates after severe postoperative complications compared to LVCs.
Material and methods
Patient selection
Ethical approval was granted by the Swedish Ethics Review Authority (reference 2020-01622). This study complies with the principles of the Declaration of Helsinki and the STROBE guidelines (Supplementary Table) [18]. All patients who underwent colorectal cancer surgery from January 1, 2015, to January 1, 2020, in Sweden were retrieved from the prospectively accrued national Swedish Colorectal Cancer Registry (SCRCR). SCRCR has recently been validated to have high quality, with data completeness of > 98% [19]. Retrieved data included demographics, surgical and oncological treatments, and outcomes for all patients undergoing colorectal cancer surgery within the study period. Only colorectal cancer resections were included. Severe complications were defined according to Clavien-Dindo as a score of ≥ 3[20]. The primary outcome of interest was failure-to-rescue, defined in accordance with previous FTR studies [16, 21], as the ratio of patients with a severe complication who died within 30 days of surgery to the total number of patients with a severe complication.
Statistical analysis
Centers were divided into cohorts based on their procedure volumes, where hospitals with an average of ≤ 200 cases/year were classified as low volume and hospitals with an average of > 200 cases/year were classified as high volume [15]. An alternative stratification method was also used with LVCs defined as < 50 cases/year, medium-volume centers (MVCs) between 50 and 150 cases/year, and HVCs > 150 cases/year.
Categorical variables between the cohorts were compared using the Chi-square test or Fisher's exact test, while the Student's t-test was used for normally distributed continuous variables. The Mann–Whitney U test was used for non-normally distributed continuous variables. A Poisson regression model with robust standard errors of variance was carried out for calculating the incidence risk ratios (IRR) for FTR. For each stratification method, a univariable and multivariable model was calculated. The multivariable Poisson regressions were adjusted for age, sex, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, cancer stage, surgical technique (minimally invasive or open surgery), type of surgery, and unplanned reoperation.
Results
A total of 23,351 patients underwent colorectal cancer surgery in Sweden during the five-year inclusion period. Patient demographics for the entire cohort are presented in Table 1. Depicted in Table 2 are demographics of patient who suffered a severe complication. The majority of patients (n = 16,943, 72.6%) underwent surgery for colon cancer. Eighty-nine percent of operations were elective cases. Most cases were performed using open rather than a minimally invasive surgery (57.6% vs. 42.3%). A total of 6,463 patients (27.7%) suffered some form of complication, and 2,964 patients (12.7%) had a Clavien–Dindo score of ≥ 3 (Table 1).
Table 1.
Demographics of patients stratified by hospital caseload, according to Henneman et al.
| Low volume (≤ 200 cases/year) (N = 14,246) |
High volume (> 200 cases/year) (N = 9,105) |
P-value | |
|---|---|---|---|
| Age in years | |||
| Mean (SD) | 72.0 (± 11.0) | 70.3 (± 11.7) | < 0.001 |
| Median [IQR] | 73 [66–80] | 72 [64–79] | < 0.001 |
| BMI | |||
| Mean (SD) | 26.2 (± 4.6) | 26.1 (± 4.7) | 0.175 |
| Median [IQR] | 26 [23–29] | 26 [23–29] | 0.085 |
| Missing, n (%) | 6026 (42.3) | 4151 (45.6) | |
| Sex, n (%) | 0.350 | ||
| Female | 6809 (47.8) | 4294 (47.2) | |
| Male | 7437 (52.2) | 4811 (52.8) | |
| ASA classification, n (%) | < 0.001 | ||
| 1 | 1624 (11.4) | 1035 (11.4) | |
| 2 | 7507 (52.7) | 4368 (48.0) | |
| 3 | 4380 (30.7) | 3213 (35.3) | |
| 4 | 348 (2.4) | 305 (3.3) | |
| 5 | 9 (0.1) | 6 (0.1) | |
| Missing | 378 (2.7) | 178 (2.0) | |
| Cancer stage, n (%) | < 0.001 | ||
| 1 | 3047 (21.4) | 1858 (20.4) | |
| 2 | 4895 (34.4) | 2888 (31.7) | |
| 3 | 4778 (33.5) | 3203 (35.2) | |
| 4 | 1038 (7.3) | 734 (8.1) | |
| Missing | 488 (3.4) | 422 (4.6) | |
| Tumor location, n (%) | < 0.001 | ||
| Colon | 10561 (74.1) | 6382 (70.1) | |
| Rectum | 3685 (25.9) | 2723 (29.9) | |
| Type of surgery, n (%) | < 0.001 | ||
| Ileocecal resection | 91 (0.6) | 57 (0.6) | |
| Right hemicolectomy | 5859 (41.1) | 3370 (37.0) | |
| Transverse colon resection | 167 (1.2) | 117 (1.3) | |
| Left hemicolectomy | 1069 (7.5) | 678 (7.4) | |
| Sigmoid colon resection | 2193 (15.4) | 1312 (14.4) | |
| Total colectomy | 534 (3.7) | 405 (4.4) | |
| Anterior resection | 2157 (15.1) | 1560 (17.1) | |
| Abdomino-perineal excision | 1399 (9.8) | 1088 (11.9) | |
| Hartmann's operation | 777 (5.5) | 518 (5.7) | |
| Neoadjuvant therapy, n (%) | 1005 (7.1) | 938 (10.3) | < 0.001 |
| Missing | 73 (0.5) | 42 (0.5) | |
| Curative treatment, n (%) | 12773 (89.7) | 8175 (89.8) | 0.775 |
| Surgical setting, n (%) | 0.008 | ||
| Elective | 12762 (89.6) | 8057 (88.5) | |
| Acute | 1477 (10.4) | 1046 (11.5) | |
| Missing | 7 (0.0) | 2 (0.0) | |
| Minimally invasive surgery, n (%) | 5867 (41.2) | 4013 (44.1) | < 0.001 |
| Missing | 25 (0.2) | 7 (0.1) | |
| Reoperation, n (%) | 1218 (8.5) | 718 (7.9) | 0.140 |
| Missing | 323 (2.3) | 325 (3.6) | |
| Length of stay, median [IQR] | 7.0 [4.0–10] | 7.0 [4.0–12] | < 0.001 |
| Missing, n (%) | 395 (2.8) | 396 (4.3) | |
| Length of ICU stay, median [IQR] | 3.0 [1.0–6.0] | 2.0 [1.0–5.0] | 0.110 |
| Missing, n (%) | 13533 (95.0) | 8742 (96.0) | |
| 30-day mortality, n (%) | 230 (1.6) | 148 (1.6) | 0.991 |
| Any complication, n (%) | 3527 (24.8) | 2936 (32.2) | < 0.001 |
| Severe complication, n (%) | 1754 (12.3) | 1210 (13.3) | 0.030 |
SD, standard deviation; IQR, interquartile range; BMI, body mass index; ASA, American Society of Anesthesiologists; ICU, intensive care unit
Table 2.
Demographics of patients with severe complications stratified by hospital caseload, according to Henneman et al.
| Low volume (≤ 200 cases/year) (N = 1754) |
High volume (> 200 cases/year) (N = 1210) |
P-value | |
|---|---|---|---|
| Age in years | |||
| Mean (SD) | 72.1 (± 10.5) | 69.8 (± 11.8) | < 0.001 |
| Median [IQR] | 73 [66–80] | 72 [64–78] | < 0.001 |
| BMI | |||
| Mean (SD) | 26.7 (± 5.0) | 26.5 (± 5.3) | 0.438 |
| Median [IQR] | 26 [24–29] | 26 [23–29] | 0.453 |
| Missing, n (%) | 789 (45.0) | 584 (48.3) | |
| Sex, n (%) | 0.781 | ||
| Female | 664 (37.9) | 465 (38.4) | |
| Male | 1090 (62.1) | 745 (61.6) | |
| ASA classification, n (%) | 0.006 | ||
| 1 | 158 (9.0) | 118 (9.8) | |
| 2 | 833 (47.5) | 506 (41.8) | |
| 3 | 638 (36.4) | 482 (39.8) | |
| 4 | 69 (3.9) | 70 (5.8) | |
| 5 | 2 (0.1) | 1 (0.1) | |
| Missing | 54 (3.1) | 33 (2.7) | |
| Cancer stage, n (%) | < 0.001 | ||
| 1 | 357 (20.4) | 203 (16.8) | |
| 2 | 621 (35.4) | 376 (31.1) | |
| 3 | 596 (34.0) | 436 (36.0) | |
| 4 | 135 (7.7) | 147 (12.1) | |
| Missing | 45 (2.6) | 48 (4.0) | |
| Tumor location, n (%) | < 0.001 | ||
| Colon | 1205 (68.7) | 726 (60.0) | |
| Rectum | 549 (31.3) | 484 (40.0) | |
| Type of surgery, n (%) | < 0.001 | ||
| Ileocecal resection | 10 (0.6) | 14 (1.2) | |
| Right hemicolectomy | 611 (34.8) | 340 (28.1) | |
| Transverse colon resection | 19 (1.1) | 14 (1.2) | |
| Left hemicolectomy | 145 (8.3) | 98 (8.1) | |
| Sigmoid colon resection | 241 (13.7) | 128 (10.6) | |
| Total colectomy | 125 (7.1) | 76 (6.3) | |
| Anterior resection | 293 (16.7) | 274 (22.6) | |
| Abdomino-perineal excision | 190 (10.8) | 182 (15.0) | |
| Hartmann's operation | 120 (6.8) | 84 (6.9) | |
| Neoadjuvant therapy, n (%) | 146 (8.3) | 167 (13.8) | < 0.001 |
| Missing | 9 (0.5) | 8 (0.7) | |
| Curative treatment, n (%) | 1535 (87.5) | 1036 (85.6) | 0.150 |
| Surgical setting, n (%) | 0.038 | ||
| Elective | 1523 (86.8) | 1017 (84.0) | |
| Acute | 231 (13.2) | 193 (16.0) | |
| Minimally invasive surgery, n (%) | 588 (33.5) | 452 (37.4) | 0.035 |
| Missing | 2 (0.1) | 1 (0.1) | |
| Reoperation, n (%) | 1118 (63.7) | 679 (56.1) | < 0.001 |
| Length of stay, median [IQR] | 15 [8.0–23] | 15 [9.0–24] | 0.112 |
| Missing, n (%) | 13 (0.7) | 18 (1.5) | |
| Length of ICU stay, median [IQR] | 4.0 [2.0–8.0] | 3.0 [2.0–6.0] | 0.012 |
| 30-day mortality, n (%) | 171 (9.7) | 114 (9.4) | 0.815 |
SD, standard deviation; IQR, interquartile range; BMI, body mass index; ASA, American Society of Anesthesiologists; ICU, intensive care unit
When centers were stratified using a 200 case/year threshold, patients were older in LVCs [mean age (SD): 72 (11) vs. 70 (12) years, p < 0.001]. HVCs treated a greater proportion of patients with stage 3–4 cancer (43.3% vs. 40.8%, p < 0.001) compared to LVCs. A significantly higher percentage of patients with rectal cancer were operated in HVCs than LVCs (29.9% vs. 25.9%, p < 0.001), congruent with a national strategy favoring volume-cohorting of rectal cancers. Crude results showed that HVCs had higher rates of overall complications compared to LVCs (32.2% vs. 24.8%, p < 0.001), as well as severe complications (13.3% vs. 12.3%, p = 0.03). Despite this, 30-day mortality did not differ between high-volume and low-volume hospitals (1.6%. vs. 1.6%, p = 0.991). There were also no statistically significant differences in unplanned reoperations or ICU length of stay (LOS) between HVCs and LVCs (Table 1). Patients with severe complications were older in LVCs. Most severe postoperative complications occurred after colon cancer surgery, corresponding to 68.7% of all severe complications in LVCs compared to 60.0% in HVCs. Reoperations after severe complications were required more frequently in LVCs than HVCs, with a reoperation frequency of 63.7% and 56.1%, respectively. There was no significant difference in 30-day mortality rates for patients with severe complications (9.7% vs. 9.4%, p = 0.815) (Table 2).
When classified according to our alternative 3-level stratification method, there was a significant decrease in the proportion of patients with stage 3–4 cancer when comparing low-, medium- and high-volume centers (42.3%, vs. 41.9% vs. 41.7%, p = 0.010). Compared to LVCs, medium- and HVCs had higher proportions of patients with rectal cancer (10.5% vs. 29.4% and 25.8%, p < 0.001). Unplanned reoperations were more common in LVCs compared to medium- and HVCs (12.6% vs. 8.7% and 7.8%, p < 0.001). Crude results showed that HVCs and LVCs had higher rates of overall complications compared to medium-volume centers (29.4% and 28.4% vs. 24.7%, p < 0.001). There was no significant difference in severe complications (14.4%, 12.8%, 12.5%, p = 0.256) or 30-day crude mortality (1.5% vs. 1.7% vs. 1.8%, p = 0.498) between centers (Table 3). When comparing the stratified volume groups, the mean age for patients with severe complications was higher in low- and medium-volume centers than HVCs. Most severe postoperative complications occurred after colon cancer surgery, corresponding to 87.5% of all severe complications in LVCs compared to 68.3% and 61.7% in medium- and HVCs, respectively. Reoperations were required more frequently in LVCs than medium- and HVCs, (81.7% compared to 61.5% and 58.6%, respectively, p = 0.010). There was no significant difference in 30-day mortality rates for patients with severe complications (5.8% vs. 10.3% vs. 9.4%, p = 0.269) (Table 4). Figure 1 shows the association between mortality following a severe complication and procedure volume and demonstrates no correlation.
Table 3.
Demographics of patients stratified by hospital caseload, according to the alternative stratification
| Low volume (< 50 cases/year) (N = 831) |
Medium volume (50–150 cases/year) (N = 8494) |
High volume (> 150 cases/year) (N = 14,026) |
P-value | |
|---|---|---|---|---|
| Age in years | ||||
| Mean (SD) | 73.0 (± 10.7) | 72.0 (± 10.8) | 70.8 (± 11.6) | < 0.001 |
| Median [IQR] | 74 [67–81] | 73 [66–80] | 72 [64–79] | < 0.001 |
| BMI | ||||
| Mean (SD) | 26.2 (± 4.7) | 26.3 (± 4.6) | 26.1 (± 4.7) | 0.073 |
| Median [IQR] | 26 [23–29] | 26 [23–29] | 26 [23–29] | 0.056 |
| Missing, n (%) | 373 (44.9) | 3594 (42.3) | 6210 (44.3) | |
| Sex, n (%) | 0.413 | |||
| Female | 413 (49.7) | 4017 (47.3) | 6673 (47.6) | |
| Male | 418 (50.3) | 4477 (52.7) | 7353 (52.4) | |
| ASA classification, n (%) | < 0.001 | |||
| 1 | 74 (8.9) | 959 (11.3) | 1626 (11.6) | |
| 2 | 373 (44.9) | 4576 (53.9) | 6926 (49.4) | |
| 3 | 338 (40.7) | 2530 (29.8) | 4725 (33.7) | |
| 4 | 15 (1.8) | 208 (2.4) | 430 (3.1) | |
| 5 | 0 (0.0) | 6 (0.1) | 9 (0.1) | |
| Missing | 31 (3.7) | 215 (2.5) | 310 (2.2) | |
| Cancer stage, n (%) | 0.010 | |||
| 1 | 152 (18.3) | 1731 (20.4) | 3022 (21.5) | |
| 2 | 306 (36.8) | 2933 (34.5) | 4544 (32.4) | |
| 3 | 291 (35.0) | 2894 (34.1) | 4796 (34.2) | |
| 4 | 61 (7.3) | 662 (7.8) | 1049 (7.5) | |
| Missing | 21 (2.5) | 274 (3.2) | 615 (4.4) | |
| Tumor location, n (%) | < 0.001 | |||
| Colon | 744 (89.5) | 6301 (74.2) | 9898 (70.6) | |
| Rectum | 87 (10.5) | 2193 (25.8) | 4128 (29.4) | |
| Type of surgery, n (%) | < 0.001 | |||
| Ileocecal resection | 9 (1.1) | 55 (0.6) | 84 (0.6) | |
| Right hemicolectomy | 419 (50.4) | 3513 (41.4) | 5297 (37.8) | |
| Transverse colon resection | 6 (0.7) | 93 (1.1) | 185 (1.3) | |
| Left hemicolectomy | 84 (10.1) | 668 (7.9) | 995 (7.1) | |
| Sigmoid colon resection | 167 (20.1) | 1322 (15.6) | 2016 (14.4) | |
| Total colectomy | 29 (3.5) | 323 (3.8) | 587 (4.2) | |
| Anterior resection | 53 (6.4) | 1144 (13.5) | 2520 (18.0) | |
| Abdomino-perineal excision | 37 (4.5) | 870 (10.2) | 1580 (11.3) | |
| Hartmann's operation | 27 (3.2) | 506 (6.0) | 762 (5.4) | |
| Neoadjuvant therapy, n (%) | 41 (4.9) | 516 (6.1) | 1386 (9.9) | < 0.001 |
| Missing | 5 (0.6) | 26 (0.3) | 84 (0.6) | |
| Curative treatment, n (%) | 744 (89.5) | 7520 (88.5) | 12684 (90.4) | < 0.001 |
| Surgical setting, n (%) | 0.668 | |||
| Elective | 733 (88.2) | 7582 (89.3) | 12504 (89.1) | |
| Acute | 97 (11.7) | 907 (10.7) | 1519 (10.8) | |
| Missing | 1 (0.1) | 5 (0.1) | 3 (0.0) | |
| Minimally invasive surgery, n (%) | 381 (45.8) | 3314 (39.0) | 6185 (44.1) | < 0.001 |
| Missing | 3 (0.4) | 18 (0.2) | 11 (0.1) | |
| Reoperation, n (%) | 105 (12.6) | 742 (8.7) | 1089 (7.8) | < 0.001 |
| Missing | 33 (4.0) | 214 (2.5) | 401 (2.9) | |
| Length of stay, median [IQR] | 6.0 [4.0–10] | 6.0 [4.0–10] | 7.0 [4.0–11] | < 0.001 |
| Missing, n (%) | 36 (4.3) | 262 (3.1) | 493 (3.5) | |
| Length of ICU stay, median [IQR] | 3.0 [2.0–5.0] | 3.0 [1.0–6.0] | 2.0 [1.0–5.0] | 0.135 |
| Missing, n (%) | 774 (93.1) | 8052 (94.8) | 13449 (95.9) | |
| 30-day mortality, n (%) | 15 (1.8) | 147 (1.7) | 216 (1.5) | 0.498 |
| Any complication, n (%) | 236 (28.4) | 2097 (24.7) | 4130 (29.4) | < 0.001 |
| Severe complication, n (%) | 120 (14.4) | 1087 (12.8) | 1757 (12.5) | 0.256 |
SD, standard deviation; IQR, interquartile range; BMI, body mass index; ASA, American Society of Anesthesiologists; ICU, intensive care unit
Table 4.
Demographics of patients with severe complications stratified by hospital caseload, according to the alternative stratification
| Low volume (< 50 cases/year) (N = 120) |
Medium volume (50–150 cases/year) (N = 1,087) |
High volume (> 150 cases/year) (N = 1,757) |
P-value | |
|---|---|---|---|---|
| Age in years | ||||
| Mean (SD) | 72.4 (± 11.4) | 72.4 (± 10.1) | 70.3 (± 11.6) | < 0.001 |
| Median [IQR] | 74 [67–81] | 73 [67–80] | 72 [64–78] | < 0.001 |
| BMI | ||||
| Mean (SD) | 27.7 (± 5.9) | 26.7 (± 4.9) | 26.5 (± 5.1) | 0.187 |
| Median [IQR] | 28 [23–31] | 26 [24–29] | 26 [23–29] | 0.321 |
| Missing, n (%) | 54 (45.0) | 484 (44.5) | 835 (47.5) | |
| Sex, n (%) | 0.549 | |||
| Female | 40 (33.3) | 416 (38.3) | 673 (38.3) | |
| Male | 80 (66.7) | 671 (61.7) | 1084 (61.7) | |
| ASA classification, n (%) | < 0.001 | |||
| 1 | 3 (2.5) | 103 (9.5) | 170 (9.7) | |
| 2 | 43 (35.8) | 523 (48.1) | 773 (44.0) | |
| 3 | 70 (58.3) | 378 (34.8) | 672 (38.2) | |
| 4 | 1 (0.8) | 47 (4.3) | 91 (5.2) | |
| 5 | 0 (0.0) | 2 (0.2) | 1 (0.1) | |
| Missing | 3 (2.5) | 34 (3.1) | 50 (2.8) | |
| Cancer stage, n (%) | 0.114 | |||
| 1 | 23 (19.2) | 211 (19.4) | 326 (18.6) | |
| 2 | 47 (39.2) | 384 (35.3) | 566 (32.2) | |
| 3 | 41 (34.2) | 383 (35.2) | 608 (34.6) | |
| 4 | 8 (6.7) | 85 (7.8) | 189 (10.8) | |
| Missing | 1 (0.8) | 24 (2.2) | 68 (3.9) | |
| Tumor location, n (%) | < 0.001 | |||
| Colon | 105 (87.5) | 742 (68.3) | 1084 (61.7) | |
| Rectum | 15 (12.5) | 345 (31.7) | 673 (38.3) | |
| Type of surgery, n (%) | < 0.001 | |||
| Ileocecal resection | 1 (0.8) | 5 (0.5) | 18 (1.0) | |
| Right hemicolectomy | 46 (38.3) | 396 (36.4) | 509 (29.0) | |
| Transverse colon resection | 1 (0.8) | 10 (0.9) | 22 (1.3) | |
| Left hemicolectomy | 19 (15.8) | 89 (8.2) | 135 (7.7) | |
| Sigmoid colon resection | 23 (19.2) | 137 (12.6) | 209 (11.9) | |
| Total colectomy | 11 (9.2) | 75 (6.9) | 115 (6.5) | |
| Anterior resection | 10 (8.3) | 169 (15.5) | 388 (22.1) | |
| Abdomino-perineal excision | 4 (3.3) | 127 (11.7) | 241 (13.7) | |
| Hartmann's operation | 5 (4.2) | 79 (7.3) | 120 (6.8) | |
| Neoadjuvant therapy, n (%) | 5 (4.2) | 77 (7.1) | 231 (13.1) | < 0.001 |
| Missing | 0 (0.0) | 5 (0.5) | 12 (0.7) | |
| Curative treatment, n (%) | 107 (89.2) | 938 (86.3) | 1526 (86.9) | 0.663 |
| Surgical setting, n (%) | 0.847 | |||
| Elective | 101 (84.2) | 935 (86.0) | 1504 (85.6) | |
| Acute | 19 (15.8) | 152 (14.0) | 253 (14.4) | |
| Minimally invasive surgery, n (%) | 41 (34.2) | 372 (34.2) | 627 (35.7) | 0.709 |
| Missing | 0 (0.0) | 1 (0.1) | 2 (0.1) | |
| Reoperation, n (%) | 98 (81.7) | 669 (61.5) | 1030 (58.6) | < 0.001 |
| Length of stay, median [IQR] | 14 [9.0–23] | 14 [8.0–24] | 16 [9.0–24] | 0.054 |
| Missing, n (%) | 0 (0.0) | 9 (0.8) | 22 (1.3) | |
| Length of ICU stay, median [IQR] | 4.0 [2.0–8.0] | 4.0 [2.0–8.0] | 3.0 [2.0–7.0] | 0.213 |
| 30-day mortality, n (%) | 7 (5.8) | 112 (10.3) | 166 (9.4) | 0.269 |
SD, standard deviation; IQR, interquartile range; BMI, body mass index; ASA, American Society of Anesthesiologists; ICU, intensive care unit
Fig. 1.
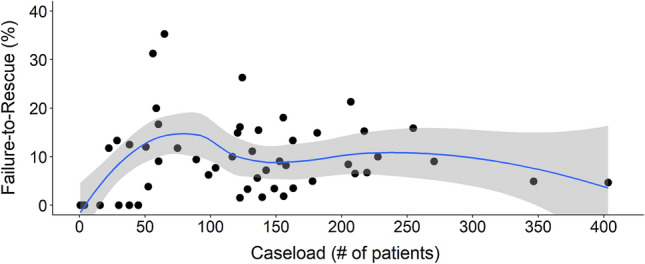
Mortality rate (%) among patients with severe complications (Clavien-Dindo ≥ 3) per colorectal unit according to their average yearly procedure volume
A multivariable analysis of FTR and hospital procedure volume is outlined in Table 5. Irrespective of the chosen stratification method, the risk of FTR was not statistically different in any of the caseload groups. This finding remained unchanged in both univariable and multivariable analyses.
Table 5.
Incidence rate ratio for FTR and hospital caseload
| Unadjusted FTR* (%) |
Unadjusted FTR IRR (95% CI) |
P-value | Adjusted FTR IRR (95% CI) |
P-value | |
|---|---|---|---|---|---|
| Hospital caseload (2 groups) | |||||
| Low (≤ 200 cases/year) | 10% | reference | reference | ||
| High (> 200cases/year) | 9% | 0.96 (0.75–1.24) | 0.79 | 0.97 (0.75–1.26) | 0.844 |
| Hospital caseload (3 groups) | |||||
| Low (< 50 cases/year) | 6% | reference | reference | ||
| Medium (50–150 cases/year) | 10% | 1.81 (0.79–4.12) | 0.159 | 2.15 (0.83–5.56) | 0.116 |
| High (> 150 cases/year) | 9% | 1.65 (0.73–3.73) | 0.231 | 2.06 (0.80–5.31) | 0.134 |
Poisson regression with robust standard errors of variance. Missing values were managed using multiple imputation by chained equations. All models were adjusted for age, sex, body mass index, American Society of Anesthesiologists classification, cancer stage, surgical technique, type of surgery, and unplanned reoperation
* FTR = 30-day mortality among patients with severe complications/total amount of severe complications
FTR, failure-to-rescue; IRR, incident rate ratio; CI, confidence interval
Discussion
In this large national observational cohort study, centers with high procedure volumes (HVCs) did not have significantly lower FTR rates than centers with lower case volumes. Centers were stratified in two different ways by their procedure volume; however, no correlation was found between volume and FTR.
Center volume, as a surrogate marker for quality, has long been considered one of several factors related to FTR after major surgery [22]. Other variables associated with lower postoperative mortality and FTR include academic teaching status [17], specialist nurse staffing, larger teams of senior doctors in surgical and medical specialties, structured transitions of care[23], advanced technology and treatment strategies in clinical practice [22, 24–26]. In the past, HVCs have been pioneers in developing efficient and safe pathways to improve logistics and surgical results. Hospitals with lower caseloads have subsequently adopted many of these protocols, which are now almost ubiquitous in modern colorectal cancer units, regardless of surgical volume. Prompt detection and amelioration of potentially severe complications by the surgical and perioperative nursing teams is necessary to improve FTR rates [24].
The results of the current study fail to demonstrate association between procedure volume and FTR of severe postoperative complications. Previous analysis of the same cohort (stratified by University Hospital status) showed a 38% (adjusted IRR 0.62, 95% CI 0.46–0.84, p = 0.002) decrease in FTR in University hospitals [17]. This apparent incongruity may in fact suggest that resource availability, such as round-the-clock radiology and dedicated ICUs, exerts a greater impact on survival after severe postoperative complications than a center’s raw caseload.
Interestingly, the LOS in HVCs was longer than lower- and medium-volume centers. This may partly be due to HVC case mix, having a higher proportion of rectal cancer surgery with high complication risks. LOS is an important surrogate marker for quality of care and treatment success following major surgery [27]. Vicendese et al. were unable to demonstrate that LOS was affected by procedure volume and postulated that other factors are of greater importance for clinical outcomes [28].
Although high procedure volume does not appear to improve FTR, many agree there should be a minimum requirement for procedure volumes in the context of cancer surgery to achieve better oncological outcomes. This idea is supported by a newly published study from Diers et al., who found up to 33% lower mortality and FTR rates after colorectal cancer surgery in centers achieving the minimum set at 30 cases per year for colon cancer and 20 cases per year for rectal cancer [14]. In future studies, FTR may be used to establish a benchmark, beyond procedure volumes and postoperative complications or oncological outcomes, for colorectal units.
Our study has several strengths and weaknesses. Data come from the SCRC registry, covering > 98% of all colorectal cancer operations in Sweden. Nonetheless, caution should be exercised when assessing postoperative complications using the SCRC registry. A recent validation study from Moberger et al. showed that complications data showed exact database agreement of only 89% for surgical complications and 84% for medical complications. In contrast, postoperative course and follow-up had an exact agreement in 98% [19]. However, including severe complications only when calculating FTR makes the accuracy of the complications data more reliable. Additionally, this is the first study to investigate FTR and procedure volume after colorectal cancer surgery in Sweden. The study aimed to assess the influence of center-level procedure volume on the ability to detect and recover severe complications and not the absolute risk of such adverse events per se. Thus, separate analyses for colon and rectal cancers were not performed, which is in line with previous studies [15, 16, 29].
There are several limitations to the current study. Firstly, this is a retrospective analysis of prospectively collected data, and individual assessments of FTR cannot be made. It would, however, be ethically challenging to address FTR and its association with procedure volume in randomized controlled trials. Secondly, in the current study we do not include surgeon-specific procedure volume nor surgical experience. Such factors may have an important impact on the peri- and postoperative outcomes but do not necessarily have a direct effect on FTR, since FTR is a metric used to evaluate the whole postoperative pathway and not the individual surgeon’s performance. Currently, there is no data specifically examining the association between characteristics of the operating surgeon and FTR [24].
One possible confounder that we were unable to control was that of possible inter-hospital transfers in case of complications. There are no transfer data available for analysis in the SCRCR. Due to a shortage of ICU beds in Sweden, inter-hospital transfers of critically ill patients between intensive care units have increased over the last few years. However, only 6% of hospital transfers affect elective surgery cases, thereby indicating that this possible confounder should have little effect [30].
It must be noted that results from the current study may not be generalizable to all healthcare systems, when considering the unique situation of Swedish colorectal cancer surgery, that has highly specialized colorectal surgeons performing the vast majority of colorectal procedures and centralized rectal cancer surgery. All 61 hospitals are considered “teaching hospitals” as they facilitate training for residents in general surgery. Nearly all rectal cancer and > 95% of colon cancer cases are discussed in multidisciplinary teams regardless of hospital volume [31]. Moreover, rectal cancer surgery in Sweden has also been centralized over the last 20 years to ensure adequate case volumes to safeguard surgical quality [32]. These aspects must be considered when assessing any causal relationship between procedure volume and FTR.
When assessing the volume-outcome relationship in colorectal cancer surgery, both Archampong et al. and Chioreso et al. found there to be a provider variability at hospital level between different countries, especially when comparing US and non-US data. They saw a significant volume–outcome relationship after colorectal cancer surgery in US-based studies but not in European and other non-US-based studies [1, 33]. Chioreso et al. stressed that this could in part be due to the degree of centralization of rectal cancer surgery outside the USA, and especially in Europe [33]. It is plausible to assume that the same may apply to procedure volume and FTR in colorectal cancer surgery. It is, therefore, important for each healthcare system to make their own assessment.
Another important limitation is the definition of low-, medium- and high-volume centers in different studies, which severely limits generalizability of results. The authors decided therefore to use two stratification methods to facilitate comparability with available literature. The cutoffs used in the current study (stratification using a 200 cases per year threshold) were the same used by Henneman et al., the first study to evaluate hospital characteristics in colorectal cancer surgery and FTR [15]. An alternative three-level stratification was also used by the authors, considering 50 resections/year to be the cutoff for the lowest volume and 150 resections/year for the highest volume.
Finally, centers are not differentiated by factors other than procedure volume. Access to other medical and surgical specialties play a vital role in dealing with some of the most common and deadly postoperative non-surgical complications such as myocardial infarction, pulmonary embolism, sepsis and stroke.
Conclusion
This large nationwide retrospective cohort study fails to demonstrate a significant association between procedure volume and FTR after colorectal cancer surgery. It would be reasonable to deduce that characteristics other than center volume may have greater bearing on FTR rates. Therefore, we encourage future studies to explore alternative characteristics and their correlation with FTR, to identify possible interventions for the improvement of quality of care after colorectal cancer surgery.
Funding
Open access funding provided by Örebro University. No funding of any kind was received for the presented work.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Archampong D, Borowski D, Wille-Jørgensen P, Iversen LH. Workload and surgeon´s specialty for outcome after colorectal cancer surgery. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD005391.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 3.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721. doi: 10.1001/archsurg.138.7.721. [DOI] [PubMed] [Google Scholar]
- 4.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280(20):1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 5.Aquina CT, Probst CP, Becerra AZ, et al. High volume improves outcomes: the argument for centralization of rectal cancer surgery. Surgery. 2016;159:736–748. doi: 10.1016/j.surg.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Baek J-H, Alrubaie A, Guzman EA, et al. The association of hospital volume with rectal cancer surgery outcomes. Int J Colorectal Dis. 2013;28:191–196. doi: 10.1007/s00384-012-1536-1. [DOI] [PubMed] [Google Scholar]
- 7.Yeo HL, Abelson JS, Mao J, et al. Surgeon annual and cumulative volumes predict early postoperative outcomes after rectal cancer resection. Ann Surg. 2017;265:151–157. doi: 10.1097/SLA.0000000000001672. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz H, Biondo S, Codina A, et al. Hospital variability in postoperative mortality after rectal cancer surgery in the Spanish association of surgeons project: the impact of hospital volume. Cir Esp Engl Ed. 2016;94:22–30. doi: 10.1016/j.cireng.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 9.van Erning FN, van Steenbergen LN, van den Broek WT, et al. No difference between lowest and highest volume hospitals in outcome after colorectal cancer surgery in the southern Netherlands. Eur J Surg Oncol EJSO. 2013;39:1199–1206. doi: 10.1016/j.ejso.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Harling H, Bulow S, Moller LN, et al. Hospital volume and outcome of rectal cancer surgery in Denmark 1994–99. Colorectal Dis. 2005;7:90–95. doi: 10.1111/j.1463-1318.2004.00751.x. [DOI] [PubMed] [Google Scholar]
- 11.Comber H, Sharp L, Timmons A, Kean FBC. Quality of rectal cancer surgery and its relationship to surgeon and hospital caseload: a population-based study: caseload and quality of rectal cancer surgery. Colorectal Dis. 2012;7:90–95. doi: 10.1111/j.1463-1318.2012.03145.x. [DOI] [PubMed] [Google Scholar]
- 12.Silber JH, Williams SV, Krakauer H, Schwartz S. Hospital and patient characteristics associated with death after surgery: a study of adverse occurrence and failure to rescue. Med Care. 1992;30:615–629. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Spolverato G, Gennaro N, Zorzi M, et al. Failure to rescue as a source of variation in hospital mortality after rectal surgery: The Italian experience. Eur J Surg Oncol. 2019;45:1219–1224. doi: 10.1016/j.ejso.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Diers J, Baum P, Matthes H, et al. Mortality and complication management after surgery for colorectal cancer depending on the DKG minimum amounts for hospital volume. Eur J Surg Oncol. 2020;47:850–857. doi: 10.1016/j.ejso.2020.09.024. [DOI] [PubMed] [Google Scholar]
- 15.Henneman D, van Leersum NJ, ten Berge M, et al. Failure-to-rescue after colorectal cancer surgery and the association with three structural hospital factors. Ann Surg Oncol. 2013;20:3370–3376. doi: 10.1245/s10434-013-3037-z. [DOI] [PubMed] [Google Scholar]
- 16.van Groningen JT, Eddes EH, Fabry HFJ, et al. Hospital teaching status and patients’ outcomes after colon cancer surgery. World J Surg. 2018;42:3372–3380. doi: 10.1007/s00268-018-4580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lillo-Felipe M, Ahl Hulme R, Sjolin G, et al. Hospital academic status is associated with failure-to-rescue after colorectal cancer surgery. Surgery. 2021 doi: 10.1016/j.surg.2021.01.050. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Moberger P, Sköldberg F, Birgisson H. Evaluation of the Swedish colorectal cancer registry: an overview of completeness, timeliness, comparability and validity. Acta Oncol. 2018;57:1611–1621. doi: 10.1080/0284186X.2018.1529425. [DOI] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henneman D, Snijders HS, Fiocco M, et al. Hospital variation in failure to rescue after colorectal cancer surgery: results of the dutch surgical colorectal audit. Ann Surg Oncol. 2013;20:2117–2123. doi: 10.1245/s10434-013-2896-7. [DOI] [PubMed] [Google Scholar]
- 22.Johnston MJ, Arora S, King D, et al. A systematic review to identify the factors that affect failure to rescue and escalation of care in surgery. Surgery. 2015;157:752–763. doi: 10.1016/j.surg.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Lane-Fall MB, Pascual JL, Peifer HG, et al. A partially structured postoperative handoff protocol improves communication in 2 mixed surgical intensive care units: findings from the handoffs and transitions in critical care (hatricc) prospective cohort study. Ann Surg. 2020;271:484–493. doi: 10.1097/SLA.0000000000003137. [DOI] [PubMed] [Google Scholar]
- 24.Portuondo JI, Shah SR, Singh H, Massarweh NN. Failure to rescue as a surgical quality indicator: current concepts and future directions for improving surgical outcomes. Anesthesiology. 2019;131:426–437. doi: 10.1097/ALN.0000000000002602. [DOI] [PubMed] [Google Scholar]
- 25.Ghaferi AA, Osborne NH, Birkmeyer JD, Dimick JB. Hospital characteristics associated with failure to rescue from complications after pancreatectomy. J Am Coll Surg. 2010;211:325–330. doi: 10.1016/j.jamcollsurg.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Kauppila JH, Wahlin K, Lagergren P, Lagergren J. University hospital status and surgeon volume and risk of reoperation following surgery for esophageal cancer. Eur J Surg Oncol. 2018;44:632–637. doi: 10.1016/j.ejso.2018.02.212. [DOI] [PubMed] [Google Scholar]
- 27.Regionala cancercentrum i samverkan. Annual reports from the Swedish Colorectal Cancer Registry [cited 2021 Mar 1]. Available from: https://cancercentrum.se/globalassets/cancerdiagnoser/tjock--och-andtarm-anal/kvalitetsregister/tjock--och-andtarm-2020/kolonrapport-2019.pdf.
- 28.Regionala cancercentrum i samverkan. Annual reports from the Swedish Colorectal Cancer Registry [cited 2021 Mar 1]. Available from: https://cancercentrum.se/globalassets/cancerdiagnoser/tjock--och-andtarm-anal/kvalitetsregister/tjock--och-andtarm-2020/rektalrapport-2019.pdf.
- 29.Almoudaris AM, Burns EM, Mamidanna R, et al. Value of failure to rescue as a marker of the standard of care following reoperation for complications after colorectal resection. Br J Surg. 2011;98:1775–1783. doi: 10.1002/bjs.7648. [DOI] [PubMed] [Google Scholar]
- 30.Oras J, Strube M, Rylander C. The mortality of critically ill patients was not associated with inter-hospital transfer due to a shortage of ICU beds - a single-centre retrospective analysis. J Intensive Care. 2020;8:82. doi: 10.1186/s40560-020-00501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed J, Lim M, Khan S, et al. Predictors of length of stay in patients having elective colorectal surgery within an enhanced recovery protocol. Int J Surg. 2010;8:628–632. doi: 10.1016/j.ijsu.2010.07.294. [DOI] [PubMed] [Google Scholar]
- 32.Vicendese D, Marvelde LT, McNair PD, et al. Hospital characteristics, rather than surgical volume, predict length of stay following colorectal cancer surgery. Aust N Z J Public Health. 2020;44:73–82. doi: 10.1111/1753-6405.12932. [DOI] [PubMed] [Google Scholar]
- 33.Chioreso C, Del Vecchio N, Schweizer ML, et al. Association between hospital and surgeon volume and rectal cancer surgery outcomes in patients with rectal cancer treated since 2000: systematic literature review and meta-analysis. Dis Colon Rectum. 2018;61:1320–1332. doi: 10.1097/DCR.0000000000001198. [DOI] [PMC free article] [PubMed] [Google Scholar]


