Abstract
Abstract
Outbreaks of invasive meningococcal disease (IMD) are unpredictable, can be sudden and have devastating consequences. We conducted a non-systematic review of the literature in PubMed (1997–2020) to assess outbreak response strategies and the impact of vaccine interventions. Since 1997, IMD outbreaks due to serogroups A, B, C, W, Y and X have occurred globally. Reactive emergency mass vaccination campaigns have encompassed single institutions (schools, universities) through to whole sections of the population at regional/national levels (e.g. serogroup B outbreaks in Saguenay–Lac-Saint-Jean region, Canada and New Zealand). Emergency vaccination responses to IMD outbreaks consistently incurred substantial costs (expenditure on vaccine supplies, personnel costs and interruption of other programmes). Impediments included the limited pace of transmission of information to parents/communities/healthcare workers; issues around collection of informed consents; poor vaccine uptake by older adolescents/young adults, often a target age group; issues of reimbursement, particularly in the USA; and difficulties in swift supply of large quantities of vaccines. For serogroup B outbreaks, the need for two doses was a significant issue that contributed substantially to costs, delayed onset of protection and non-compliance with dose 2. Real-world descriptions of outbreak control strategies and the associated challenges systematically show that reactive outbreak management is administratively, logistically and financially costly, and that its impact can be difficult to measure. In view of the unpredictability, fast pace and potential lethality of outbreak-associated IMD, prevention through routine vaccination appears the most effective mitigation tool. Highly effective vaccines covering five of six disease-causing serogroups are available. Preparedness through routine vaccination programmes will enhance the speed and effectiveness of outbreak responses, should they be needed (ready access to vaccines and need for a single booster dose rather than a primary series).
Plain Language Summary
Keywords: Epidemic, Neisseria meningitidis, Meningitis, Outbreak, Vaccine
Digital Features
This article is published with digital features, including a graphical plain language summary, to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.14912220.
Introduction
Neisseria meningitidis: A Moving Target
Neisseria meningitidis is a Gram-negative diplococcus that is an exclusive human pathogen. It exists as a commensal in the nasopharynx but causes meningitis or fulminant, life-threatening sepsis if it becomes invasive. Six serogroups (A, B, C, X, Y and W) cause the majority of invasive meningococcal disease (IMD). All six can cause endemic disease and all have epidemic potential. The predominant serogroups vary geographically and also temporally in response to antigenic change or vaccine pressure [1, 2]. The polysaccharide (PS) capsule is the major virulence factor, forming the basis of all currently available meningococcal conjugate vaccines (Men-CV) against serogroups A, C, W and Y (Table 1). Other surface proteins contributing to virulence, immune evasion and survival generally show wide genetic diversity across strains [3]. The two broadly protective serogroup B (MenB) vaccines include surface antigens and not the MenB PS capsule because of its poor immunogenicity and potential homology with human neural glycoproteins [4]. As yet, none of the available vaccines target serogroup X (MenX), although pentavalent ACWYX conjugate vaccines are in clinical development [5].
Table 1.
Vaccine abbreviations
| Abbreviation | Vaccine |
|---|---|
| MenA-CV | Meningococcal A conjugate vaccine |
| MenC-CV | Meningococcal C conjugate vaccine |
| MenAC-PS | Bivalent meningococcal AC polysaccharide vaccine |
| MenACW-PS | Trivalent meningococcal ACW polysaccharide vaccine |
| MenACWY-CV | Quadrivalent meningococcal ACWY conjugate vaccine |
| MenACWY-PS | Quadrivalent meningococcal ACWY polysaccharide vaccine |
| 4CMenB | 4-component meningococcal serogroup B vaccine |
| MenB-FHbp | Meningococcal serogroup B vaccine containing Factor H binding protein |
| MenACWY-TT | Quadrivalent meningococcal tetanus toxoid conjugate vaccine |
| MeNZB | Meningococcal serogroup B strain-specific outer membrane vesicle vaccine |
| MenBVac | Meningococcal serogroup B outer membrane vesicle vaccine |
The key feature of N. meningitidis driving the epidemiology of IMD is its ability to undergo continuous antigenic change, including capsular switching, mainly via horizontal gene transfer [6, 7]. The result is continuous strain evolution and the possibility for outbreaks to occur should a virulent strain meet a population with low underlying immunity to that particular strain. The presence of risk factors including settings of close contact, social behaviours, lack of immunity and the carriage of hypervirulent strains all increase the likelihood that an outbreak of IMD may occur. Nevertheless, outbreaks of IMD occur unpredictably, supporting a preventative approach rather than a reactive approach to their management.
There is no globally accepted definition of a meningococcal disease cluster, outbreak or epidemic. The World Health Organization (WHO) has established epidemic thresholds for the sub-Saharan region of Africa. Since 2014, the WHO epidemic threshold for populations of between 30,000 and 100,000 inhabitants is an attack rate of 10 cases per 100,000 inhabitants in 1 week. For populations of fewer than 30,000 inhabitants, the threshold is an incidence of 5 cases in 1 week, or the doubling of the number of cases over a 3-week period [8]. However, individual countries apply their own thresholds based on local epidemiology. For example, the United States (US) Centers for Disease Control and Prevention (CDC) moved away from a threshold-based definition and now defines a community IMD outbreak as multiple outbreak-associated cases with an incidence that is above the expected incidence for a community during a 3-month period. An organisation-based outbreak is defined when 2–3 outbreak-associated cases occur within an organisation during a 3-month period [9]. In this review, we loosely refer to very large outbreaks as epidemics, and use the terminology (outbreaks/epidemics) as employed by individual authors when we discuss their publications.
Outbreaks can be sudden and have devastating consequences. In recent history, the largest epidemics with the greatest human toll have occurred in sub-Saharan Africa, in the so-called meningitis belt. The region was subject to periodic seasonal serogroup A (MenA) epidemics until the introduction of MenA conjugate vaccination (MenA-CV) in 2010. During these epidemics in the pre-vaccine era, the number of cases ran into the hundreds of thousands, with tens of thousands of deaths [10]. In complete contrast, IMD outbreaks can also begin insidiously and be prolonged. For example, New Zealand and the United Kingdom (UK) experienced prolonged epidemics spanning many years during the 1990s that were caused by MenB and serogroup C (MenC), respectively [11, 12].
Meningococcal disease is characterised by unpredictability, rapid onset, a fulminant clinical course with a substantial risk of poor outcomes due to permanent sequelae and death. Early and rapid intervention is required to arrest outbreaks and prevent further deaths. However, pre-emptive planning and management are substantially impaired by the inability to predict when or where an outbreak will occur, or which serogroup will be responsible. Ideally, outbreaks are detected early, with rapid identification of the causative strain and at-risk group to provide the information needed to guide vaccine choice should reactive emergency vaccination be needed. The response to outbreaks requires contact tracing, antibiotic prophylaxis of contacts, and emergency vaccination campaigns. The last of these can be complex and expensive to deploy, and difficult to evaluate in terms of effectiveness. We reviewed outbreaks of IMD and the associated response over the last 20 years with the aim of collating lessons on key aspects of their prevention and management.
Methods
We conducted a non-systematic, narrative literature review to assess the epidemiology of IMD outbreaks globally from 1997 to 2020, a period coinciding with the largest recorded MenA epidemic in Africa and capturing the introduction of the first MenC conjugate vaccine (MenC-CV) into a national immunisation programme (NIP) for epidemic control in the UK in 1999. We describe vaccination responses to these outbreaks in terms of vaccination strategies and, where available, the impact of the intervention.
The search terms were (outbreak[Title/Abstract] OR epidemics[Title/Abstract]) AND (meningitis[Title/Abstract] OR meningococcal meningitis[Title/Abstract] OR meningococcal disease[Title/Abstract] OR invasive meningococcal disease[Title/Abstract] OR IMD[Title/Abstract]) to identify English language papers describing meningococcal outbreaks or epidemics that were published between 1997 and 2020. All articles with potentially relevant titles were reviewed. No quality assessments or additional analyses were conducted as part of this narrative review. The literature search acted as a foundation for the review and numerous additional articles were identified from review of citations in the articles, or from other sources such as on-line outbreak websites maintained by the WHO and US CDC. Where several articles described the same outbreak, the article providing the most recent data or the most comprehensive data was cited. We did not attempt to differentiate between outbreaks and epidemics but included all reports of clusters of IMD due to the same serogroup in a specific region. The results are tabulated chronologically by region.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
The articles encompassed descriptions of a single outbreak/epidemic or group of outbreaks/epidemics, review articles on meningococcal outbreaks, and vaccination programmes implemented in response to an outbreak/epidemic. The information in many articles was incomplete as details on how vaccination interventions were conducted as well as on the outcome of the intervention were missing.
Epidemiology
Europe
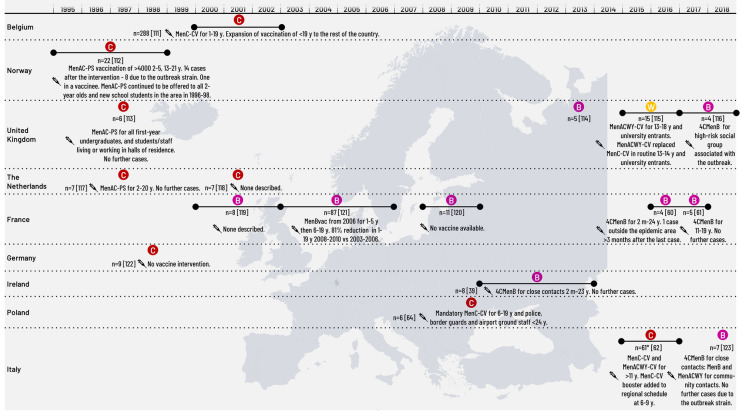
MenC was a common cause of IMD outbreaks in Europe until the availability of MenC-CVs from 1999. After the introduction of MenC-CV into routine NIPs, the majority of IMD outbreaks in Europe have been caused by MenB (Table 2; Fig. 1). Seven of these outbreaks were managed by reactive vaccination using MenB vaccines. From 2010, cases of serogroup W (MenW) IMD, associated with a hypervirulent MenWcc11 sublineage originating from South America, began to increase rapidly in the UK, with 170 cases reported in 2014–2015 (case fatality rate [CFR] 12%) compared with 46 in 2012–2013 of whom more than half developed septicaemia [13]. Since 2014–2015, MenW IMD has increased in other European countries [1].
Table 2.
Characteristics of meningococcal outbreaks/epidemics in Europe 1997–2020
| Region, setting | Outbreak years | Serogroup | Age group primarily affected | No. of cases | CFR (%) | Vaccine intervention | Outcome of intervention | |
|---|---|---|---|---|---|---|---|---|
| Belgium | ||||||||
| Community | 2000–2002 | C | 0–19 y | 288 | 5 | MenC-CV for 1–19 y | Expansion of vaccination of < 19 y to the rest of the country | [111] |
| Norway | ||||||||
| Community | 1995–1998 | C | 15–21 y | 22 | 14 | MenAC-PS vaccination of > 4000 2–5 y, 13–21 y | 14 cases after the intervention—8 due to the outbreak strain. One in a vaccinee. MenAC-PS continued to be offered to all 2-year-olds and new school students in the area in 1996–1998 | [112] |
| UK | ||||||||
| University | 1997 | C | 18–19 y | 6 | 50 | MenAC-PS for all first-year undergraduates, and students/staff living or working in halls of residence | No further cases | [113] |
| Nursery | 2013 | B | 2 y | 5 | – | No vaccine intervention | – | [114] |
| Community | 2015–2016 | W | 15–19 y | 15 | 40 | MenACWY-CV for 13–18 y and university entrants | MenACWY-CV replaced MenC-CV in routine 13–14 y and university entrants | [115] |
| College | 2016–2017 | B | 16–19 y | 4 | 50 | 4CMenB for high-risk social group associated with the outbreak | – | [116] |
| Netherlands | ||||||||
| Community | 1997 | C | 13–18 y | 7 | – | MenAC-PS for 2–20 y | No further cases | [117] |
| Community | 2001 | C | < 24 y | 7 | – | None described | – | [118] |
| France | ||||||||
| Community | 2000–2002 | B | 14–28 y | 8 | 13 | None described | [119] | |
| Community | 2008–2009 | B | ≤ 24 y | 11 | 9 | No vaccine available | – | [120] |
| Community | 2003–2006 | B | 1–19 y | 87 | 19 (confirmed cases) | MenBvac from 2006 for 1–5 y then 6–19 y | 81% reduction in 1–19 y 2008–2010 vs 2003–2006 | [121] |
| Community | 2016 | B | 17 y | 4 | 0 | 4CMenB for 2 m–24 y | 1 case outside the epidemic area > 3 months after the last case | [60] |
| Community | 2016–2017 | B | 11–19 y | 5 | 0 | 4CMenB for 11–19 y | No further cases | [61] |
| Germany | ||||||||
| Disco attendees | 1998 | C | 13–16 y | 9 | 11 | No vaccine intervention | – | [122] |
| Ireland | ||||||||
| (Indigenous minority) | 2010–2013 | B | 5–46 m | 8 | 0 | 4CMenB for close contacts 2 m-23 y | No further cases | [39] |
| Poland | ||||||||
| Community | 2009 | C | 13–17 y | 6 | 0 | Mandatory MenC-CV for 6–19 y, + police, border guards and airport ground staff < 24 y | – | [64] |
| Tuscany, Italy | ||||||||
| Community | 2015–2016 after infant MenC-CV programme introduced in 2005 | C | Median 14.5 y in vaccine failures, 28 y in previously unvaccinated | 61, 12 were vaccinate failures | 21 |
MenC-CV and MenACWY-CV for > 11 y |
MenC-CV booster added to regional schedule at 6–9 y | [62] |
| Sardinia | ||||||||
| Family cluster and nightclub attendees | 2018 | B | 18–21 y | 7 | 29 | 4CMenB for close contacts: MenB and MenACWY for community contacts | No further cases due to the outbreak strain | [123] |
4CMenB 4-component meningococcal serogroup B vaccine, CFR case fatality rate, CV conjugate vaccine, Men(A/C/W/Y) meningococcal vaccine containing one serogroup or combinations of serogroups A, C, W and Y, m months of age, MenBVac meningococcal serogroup B outer membrane protein vaccine, PS polysaccharide vaccine, Ref source reference, y years of age
Fig. 1.
Meningococcal outbreaks/epidemics in Europe 1997–2020. 4CMenB, 4-component meningococcal serogroup B vaccine; CV, conjugate vaccine, m, months of age; MenBVac, meningococcal serogroup B outer membrane protein vaccine; Men(A/C/W/Y), meningococcal vaccine containing one serogroup or combinations of serogroups A, C, W and Y; n, number of cases; PS, polysaccharide vaccine; y, years of age
The Americas
USA
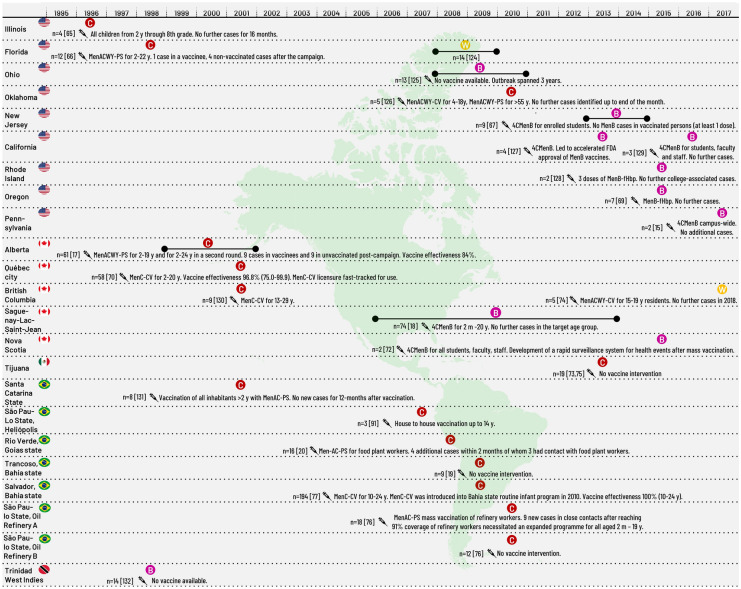
Meningococcal outbreaks occur periodically in the USA but not all are reported in the peer-reviewed literature. Prior to 2009, MenC community-based outbreaks were reported in Illinois (4 IMD cases) and Florida (12 IMD cases), triggering mass immunisation of children and adolescents (and young adults in Florida) with Men-PS vaccines (Table 3; Fig. 2).
Table 3.
Characteristics of meningococcal outbreaks/epidemics in the Americas 1997–2020
| Region | Outbreak years | Serogroup | Age group primarily affected | No. of cases | Attack rate per 100,000 | CFR (%) | Vaccine intervention | Outcome of intervention | Ref |
|---|---|---|---|---|---|---|---|---|---|
| USA | |||||||||
| Community outbreaks | |||||||||
| Illinois | 1996 | C | 3–6 y | 4 | < 10 y: 205 | – | All children from 2 y through 8th grade | No further cases for 16 months | [65] |
| Florida | 1998 | C | 2–18 y | 12 | 15 | 17 | MenACWY-PS for 2–22 y | 1 case in a vaccinee, 4 non-vaccinated cases after the campaign | [66] |
| Florida | 2008–2009 | W | Adults | 14 | – | 29 | None | – | [124] |
| University and college outbreaks | |||||||||
| Ohio | 2008–2010 | B | 18–23 y | 13 |
13 Freshmen: 50 Greek Club members: 60 |
8 | No vaccine available | Outbreak spanned 3 years | [125] |
| Oklahoma | 2010 | C | 5–7 y | 5 | 162 | 40 |
MenACWY-CV for 4–18 y MenACWY-PS for > 55 y |
No further cases identified up to end of the month | [126] |
| New Jersey | 2013–2014 | B | 17–21 y | 9 | 134 | 11 | 4CMenB for enrolled students | No MenB cases in vaccinated persons (at least 1 dose) | [67] |
| California | 2013 | B | – | 4 | – | – | 4CMenB | Led to accelerated FDA approval of MenB vaccines | [127] |
| Rhode Island | 2015 | B | – | 2 | 44 | 0 | 3 doses of MenB-fHbp | No further college-associated cases | [128] |
| Oregon | 2015 | B | Undergraduate students | 7 | 30.5 | 14 | MenB-FHbp | No further cases | [69] |
| California | 2016 | B | Undergraduate students | 3 | – | 0 | 4CMenB for students, faculty and staff | No additional cases | [129] |
| Pennsylvania | 2017 | B | Undergraduate students | 2 | – | 0 | 4CMenB campus-wide | No additional cases | [15] |
| Canada | |||||||||
| Alberta | 1999–2001 | C | < 24 y | 61 |
0–1 y: 50 15–19 y: 35.7 |
3 | MenACWY-PS for 2–19 y and for 2–24 y in a second round | 9 cases in vaccinees and 9 in unvaccinated post-campaign. Vaccine effectiveness 84% | [17] |
| Quebec City | 2001 | C | 2 m–20 y | 58 | – | – | MenC-CV for 2–20 y |
Vaccine effectiveness 96.8% (95%CI 75.0–99.9) MenC-CV licensure fast-tracked for use |
[70] |
| British Columbia | 2001 | C | 17–27 y | 9 | – | 22 | MenC-CV for 13–29 y | – | [130] |
| Saguenay–Lac-Saint-Jean | 2006–2013 | B | ≤ 20 y | 74 | – | 0 | 4CMenB for 2 m–20 y | No further cases in the target age group | [18] |
| Nova Scotia | 2015 | B | University students | 2 | – | 50 | 4CMenB for all students, faculty, staff | Development of a rapid surveillance system for health events after mass vaccination | [72] |
| British Columbia | 2017 | W | 15–19 y | 5 | – | – | MenACWY-CV for 15-19y residents | No further cases in 2018 | [74] |
| Mexico | |||||||||
| Tijuana | 2013 | C | > 13 y | 19 | 1.07 | 37 | No vaccine intervention | – | [73, 75] |
| Brazil | |||||||||
| Santa Catarina State | 2001 | C | 15–19 y | 8 | 367.5 | 13 | Vaccination of all inhabitants > 2 y with MenAC-PS | No new cases for 12 months after vaccination | [131] |
| Heliópolis community, São Paulo State | 2007 | C | ≤ 9 y | 3 | 89.5 | 0 | House to house vaccination up to 14 y | – | [91] |
| Rio Verde, Goias State | 2008 | C | 1–19 y | 16 |
12 (residents) 77 (1–4 y) 60 (food processing plant workers) |
31 | MenAC-PS for food plant workers | 4 additional cases within 2 months of whom 3 had contact with food plant workers | [20] |
| Trancoso, Bahia State | 2009 | C | 14–25 y | 9 | – | 67 | No vaccine intervention | – | [19] |
| Salvador, Bahia State | 2009 | C | – | 194 | 1.5 | 39 | MenC-CV for 10–24 y |
MenC-CV was introduced into Bahia state routine infant programme in 2010 Vaccine effectiveness 100% (10–24 y) |
[77] |
|
São Paulo State, Oil refinery A |
2010 | C |
Oil refinery workers, family members < 4 y |
18 | 34.1 | 16.7 | MenAC-PS mass vaccination of refinery workers | 9 new cases in close contacts after reaching 91% coverage of refinery workers necessitated an expanded programme for all aged 2 m–19 y | [76] |
| Oil refinery B | 2010 | C | 12 | 12.5 | 50 | No vaccine intervention | – | ||
| West Indies | |||||||||
| Trinidad | 1998 | B | < 5 y | 14 | – | 57 |
No vaccine available |
– | [132] |
4CMenB 4-component meningococcal serogroup B vaccine, CI confidence interval, CFR case fatality rate, CV conjugate vaccine, FDA Food and Drug Administration, FHbp factor H binding protein, Men meningococcal disease vaccine, m months of age, Men(A/C/W/Y) meningococcal vaccine containing one serogroup or combinations of serogroups A, C, W and Y, PS polysaccharide vaccine, Ref source reference, y years of age
Fig. 2.
Meningococcal outbreaks/epidemics in the Americas 1997–2020. 4CMenB, 4-component meningococcal serogroup B vaccine; CV, conjugate vaccine; FDA, Food and Drug Administration; m, months of age; MenB-fHbp, meningococcal serogroup B vaccine containing Factor H binding protein; Men(A/C/W/Y), meningococcal vaccine containing one serogroup or combinations of serogroups A, C, W and Y; n, number of cases; PS, polysaccharide vaccine; y, years of age
In a CDC review covering the years 2009 to 2013, there were 36 IMD outbreaks listed that included 180 cases, corresponding to 4.9% of all IMD cases reported for that period, and 43 deaths (CFR 24%) [14]. Serogroups A, B, C, W and Y were implicated in these outbreaks, with MenB (15/36 outbreaks) predominantly detected in university-based outbreaks, and MenC (16/36 outbreaks) in community- and organisation-based outbreaks (schools, sports clubs, childcare and residential facilities, and in men who have sex with men [MSM)]) [14]. Since 2013, MenB outbreaks continue to occur in universities, with two reported in the literature in 2013, two in 2015, four in 2016, two in 2017, one in 2018 and two in 2019 [15, 16].
Canada
Canada experienced endemic MenC disease and periodic MenC outbreaks until 2006 (Table 3; Fig. 2). A large MenC outbreak in Alberta was controlled after the administration of MenACWY-PS to residents aged 2–24 years from 2000–2002 [17]. A MenC outbreak in Quebec City in 2001 continued to evolve despite reactive MenC-PS-containing vaccination of secondary school students and extended to include young children. As a result, the national approval of MenC-CV was fast-tracked for use in a mass immunisation programme commencing a few months after the onset of outbreak. The next meningococcal outbreak in Quebec was prolonged (2006–2013) and caused by MenB [18]. Subsequently, a MenB outbreak occurred in a university in Nova Scotia in 2015. The first MenW (sequence type [ST]-11) outbreak occurred in British Columbia in 2017 with five cases reported in 15–19-year-olds.
Brazil
Seven outbreaks were reported between 1995 and 2010, with the CFR reaching up to 67% during an outbreak in young people who attended the same dance party (Table 3; Fig. 2). All were MenC outbreaks that predominantly affected children and adolescents. Three were community-based, triggering large-scale interventions for mass vaccination of local residents. Four outbreaks were linked to specific gatherings: one affected attendees of a large dance party [19], one occurred in employees of a large food-processing plant [20] and two affected workers in two oil refineries in São Paulo State and their family contacts.
Asia–Pacific
Australia
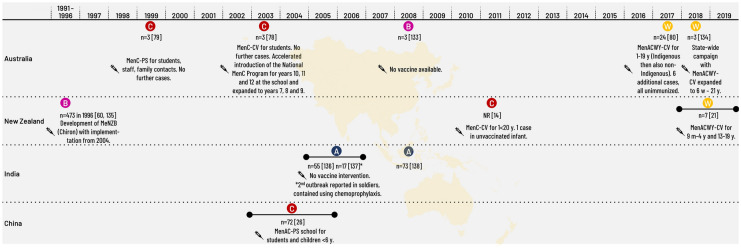
IMD outbreaks in Australia have predominantly affected school-age children, with the exception of a MenW outbreak of 24 cases that affected remote indigenous communities, in which more than 50% of cases were at most 4 years of age (Table 4; Fig. 3).
Table 4.
Characteristics of meningococcal outbreaks/epidemics in the Asia–Pacific 1997–2020
| Region, setting | Outbreak years | Serogroup | Age group primarily affected | No. of cases | Attack rate per 100,000 | CFR (%) | Vaccine intervention and outcome | Ref |
|---|---|---|---|---|---|---|---|---|
| Australia | ||||||||
|
Victoria Secondary school |
1999 | C | Year 9 and 10 students | 3 | 187 | 0 |
MenC-PS for students, staff, family contacts No further cases |
[79] |
| New South Wales Secondary school | 2003 | C | High school children | 3 | – | 0 |
MenC-CV for students No further cases. Accelerated introduction of the National MenC Programme for years 10, 11 and 12 at the school and expanded to years 7, 8 and 9 |
[78] |
| Sydney | 2008 | B | 2–13 y | 3 | 10.7 | 0 | No vaccine available | [133] |
| Central Australia Indigenous communities | 2017 | W | ≤ 4 y | 24 | 10.9 | 0 | MenACWY-CV for 1–19 y (indigenous then also non-indigenous). 6 additional cases, all unimmunised | [80] |
| Tasmania | 2018 | W | – | 3 | – | – | State-wide campaign with MenACWY-CV expanded to 6 w–21 y | [134] |
| New Zealand | ||||||||
| New Zealand | 1991–1996 | B | < 5 y | 473 in 1996 |
14.0 (142 in < 1 y, 549.6 in Maori < 1 y) |
~ 5 | Development of MeNZB (Chiron) with implementation from 2004 | [60, 135] |
| Northland | 2011 | C | – | – | – | – |
MenC-CV for 1 to < 20 y 1 case in unvaccinated infant |
[14] |
| Northland | 2018–2019 | W | Children, adolescents | 7 | 8.1 (22.7 for Maori) | 43 | MenACWY-CV for 9 m–4 y and 13–19 y | [21] |
| India | ||||||||
| New Delhi | 2005–2006 | A | 78% in 6–29 y | 55 | – | 15 | No vaccine intervention | [136] |
| Soldiers deployed in operations | 2006 | A | 21–26 y | 17 | – | 12 | No vaccine intervention Outbreak contained using chemoprophylaxis | [137] |
| Delhi community outbreak | 2008 | A | – | 73 | – | – | – | [138] |
| China | ||||||||
|
Anhui province 8 middle schools, cooking school, police school |
2003–2005 | C | Middle school attendees | 72 | – | 7 | MenAC-PS for school students and children < 6 y | [26] |
CV conjugate vaccine, CFR case fatality rate, m months of age, MeNZB meningococcal serogroup B outer membrane protein vaccine, Men(A/C/W/Y) meningococcal vaccine containing one serogroup or combinations of serogroups A, C, W and Y, PS polysaccharide vaccine, Ref source reference, y years of age
Fig. 3.
Meningococcal outbreaks/epidemics in the Asia–Pacific 1997–2020. CV, conjugate vaccine; m, months of age; MeNZB, meningococcal serogroup B strain-specific outer membrane vesicle vaccine; Men(A/C/W/Y), meningococcal vaccine containing one serogroup or combinations of serogroups A, C, W and Y; NR, not reported; n, number of cases; PS, polysaccharide vaccine; y, years of age
New Zealand
New Zealand experienced a prolonged MenB epidemic from 1991 to 2007, which resulted in 6128 cases and an incidence peak at 17.4 per 100,000 in 2001. Rates in the Pacific and Maori communities were several-fold higher than the European population [11]. The most recent outbreak in New Zealand was due to MenW in Northland in 2018, with an overall incidence of 8.1 per 100,000 and 22.7 per 100,000 amongst Maori people [21].
India
MenA epidemics have been recorded in India for more than a century [22]. In Delhi, 616 cases of MenA IMD were reported during an outbreak in 1966, 1731 cases and 569 deaths (CFR 33%) in 1985, and 6133 cases and 799 deaths (CFR 13%) in 1986 [22, 23]. In the last 2 decades, numerous outbreaks of MenA disease have been reported across the country [24]. A large MenA outbreak occurred in 2005 (Table 4; Fig. 3). Although exact numbers are not known, more than 500 suspected cases were reported in Delhi, with a CFR of around 10% [23, 25]. Outbreak management appeared to be mainly through chemoprophylaxis of contacts.
China
Until a nationwide vaccination campaign with MenA-PS took place in 1982, large MenA epidemics occurred periodically in China [26]. Low levels of MenA disease continued to occur until 2003 to 2005, when a series of 10 outbreaks, caused by a novel ST of MenC, occurred in Anhui province (mainly in middle schools). In 2004–2005, there were 221 cases and 17 deaths. Mass vaccination with MenAC-PS was undertaken and nationwide surveillance was implemented [26]. To date, IMD management in China is mainly through use of a bivalent MenAC-PS vaccine. However, the epidemiology of IMD in China may be changing; a case of IMD caused by MenX was reported in 2007, cases due to MenW were reported in 2011–2012 in south-eastern China, and MenB was isolated from healthy carriers in Beijing [27–30].
Africa
The epidemiology of IMD in sub-Saharan Africa is unique compared to the rest of the world, characterised by high rates of endemic IMD with periodic large, devastating epidemics. The African meningitis belt comprises 26 countries prone to seasonal IMD outbreaks during the hot and dry months from December to April.
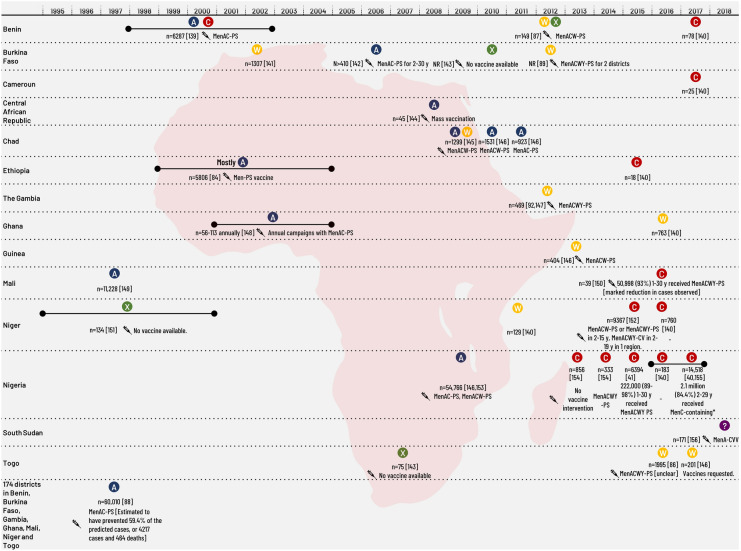
Prior to the introduction of MenA-CV in 2010, MenA caused the vast majority of IMD epidemics in the region (Table 5). The largest known outbreak in 1996–1997, affected Burkina Faso, Mali, Nigeria and Niger and caused at least 250,000 cases and 25,000 deaths, although significant underreporting was likely [31, 32].
Table 5.
Characteristics of meningococcal outbreaks/epidemics in Africa 1997–2020*
| Country (year of MenA-CV introduction [10]) | Outbreak years | Serogroup | Age group Primarily affected | No. of cases** | Cumulative attack rate per 100,000 population | CFR (%) | Vaccination intervention and outcome (when available) | Ref |
|---|---|---|---|---|---|---|---|---|
| Benin (2012) | 1998–2002 | A and C | – | 6287 | 20.4 to 619 by district | 2–15 | MenAC-PS | [139] |
| 2012 |
81% W 15% X |
0–14 y | 149 | 46.8 to 211 by district | – | MenACW-PS | [87] | |
| 2017 | C | – | 78 | 189 | 8 | – | [140] | |
| Burkina Faso (2010) | 2002 | W | Infants, young children | 1307 |
251 overall, < 1 y 1092, 1–4 y 660 |
10 | – | [141] |
| 2006 | A | Median 7 y | > 410 | Up to 2800 by village | 7 | MenAC-PS for 2–30 y | [142] | |
| 2010 | X | 5–14 y | – | Up to 130 | 11 | No vaccine available | [143] | |
| 2012 | W | – | – | 51.2 to 105.2 by district | – | MenACWY-PS for 2 districts | [89] | |
| Cameroon (2011) | 2017 | C | – | 25 | – | 32 | – | [140] |
| Central African Republic (2016) | 2008 | A | – | 45 | – | 11 | Mass vaccination | [144] |
| Chad (2011) | 2009 | A and W | – | 1299 | – | 11 | MenACW-PS | [145] |
| 2010 | A | – | 1531 | – | 10 | MenACW-PS | [146] | |
| 2011 | A | – | 923 | – | 6 | MenAC-PS | [146] | |
| Ethiopia (2013) | 60 outbreaks 1999–2004 | Mostly A | – | 5806 | 8.6 | – | Men-PS vaccine | [84] |
| 2015 | C | – | 18 | – | 0 | – | [140] | |
| Gambia (2014) | 2012 | W | 1–14 y | 469 | 111 | 8 | MenACWY-PS | [92, 147] |
| Ghana (2012) | 2001–2004 | A | Median 10 y | 56–113 annually | Up to 80 | 5 | Annual campaigns with MenAC-PS | [148] |
| 2016 | W | – | 763 | 97 | 6 | – | [140] | |
| Guinea (2015) | 2013 | W | – | 404 | – | 9 | MenACW-PS | [146] |
| Mali (2010) | 1997 | A | 0–14 y | 11,228 | – | 10 | – | [149] |
| 2016 | C | 6–14 y | 39 | 23 | 15 | 50,998 (93%) 1–30 y received MenACWY-PS [marked reduction in cases observed] | [150] | |
| Niger (2010) | 1995–2000 | X | Mean 9.2 y | 134 | – | 12 | No vaccine available | [151] |
| 2011 | W | – | 129 | 43 | 10 | – | [140] | |
| 2015 | C | < 30 y | 9367 | 50.6 | 6 | MenACW-PS or MenACWY-PS in 2–15 y, MenACWY-CV in 2–19 y in 1 region | [152] | |
| 2016 | C | – | 760 | 83 | 3 | – | [140] | |
| Nigeria (2011) | 2009 | A | – | 54,766 | 2.8 to 236.2 | 2 to 11 (5 overall) | MenAC-PS, MenACW-PS | [146, 153] |
| 2013 | C | 5–29 y | 856 | 673 | 7 | No vaccine intervention | [154] | |
| 2014 | C | 5–29 y | 333 | 165 | 11 | MenACWY-PS | [154] | |
| 2015 | C | 5–29 y | 6394 | 282 | 5 | 222,000 (89–98%) 1–30 y received MenACWY-PS | [41] | |
| 2016 | C | – | 183 | 183 | 4 | – | [140] | |
| 2016–2017 | C | 1–14 y | 14,518 | – | 8 | 2.1 million (84.4%) 2–29 y received MenC-containing [Immediate drop in case numbers. Outbreak declared over within 2 months] | [40, 155] | |
| South Sudan (2016) | 2018 | ? | – | – | Up to 987 | 18 | MenA-CV | [156] |
| Togo (2014) | 2007 | X | 75 | 33 | 10 | No vaccine available | [143] | |
| 2016 | W | < 15 y | 1995 | 78.8 | 6 | MenACWY-PS [unclear] | [86] | |
| 2017 | W | – | 201 | 12.4 | 9 | Vaccines requested | [146] | |
| 174 districts in Benin, Burkina Faso, Gambia, Ghana, Mali, Niger, Togo, other countries | 1997 | A | – | 60,010 | – | > 10% | MenAC-PS [Estimated to have prevented 59.4% of the predicted cases, or 4217 cases and 464 deaths] | [88] |
CFR case fatality rate, CV conjugate vaccine, Men(A/C/W/Y) meningococcal vaccine containing one serogroup or combinations of serogroups A, C, W and Y, PS polysaccharide vaccine, Ref source reference, y years of age
*Enhanced surveillance networks were established progressively from 2003
**Suspected or confirmed
The introduction of MenA-CV in Africa in 2010 had an immediate and profound impact on the epidemiology of MenA IMD. More than 235 million 1–29-year-olds were vaccinated in 16 countries from 2010 to 2015, and more than 304 million were vaccinated in 22 countries by the end of 2018 [33, 34]. Amongst fully vaccinated populations from nine countries of the meningitis belt, the incidence of confirmed MenA disease from 2010 to 2015 was reduced by more than 99% [35], a reduction that continues to be sustained [36]. In 2015, the WHO recommended that MenA-CV be introduced into routine childhood immunisation programmes (one dose at 9–18 months of age) within 1–5 years after the mass vaccination campaigns [37]. By 2018, eight countries had introduced MenA-CV into their NIP [34].
The first large MenW outbreak in Africa occurred in 2000 in Burkina Faso among returned Hajj pilgrims [38] (Table 5; Fig. 4). Since then, MenW (mainly ST-11) continues to cause large epidemics across the meningitis belt. Outbreaks of MenX, previously confined to Niger, have increased in geographic range and severity since 2006, affecting Burkina Faso, Togo, Benin, Ghana, Uganda and Kenya [38, 39]. Prior to 2013, MenC caused minimal disease in the meningitis belt but is increasingly responsible for large outbreaks (Fig. 5). From December 2016 until June 2017, the world’s largest MenC outbreak occurred in Nigeria and was associated with a newly identified ST-10217 lineage. There were 14,518 suspected cases reported with 1166 deaths (CFR 8%) [40]. MenC now impacts an extensive geographic region across the meningitis belt, causing large epidemics previously typical of MenA [41]. In Africa, children, adolescents and young adults are the age groups most commonly affected by IMD, regardless of causal serogroup.
Fig. 4.
Meningococcal outbreaks/epidemics in Africa 1997–2020. CV, conjugate vaccine, Men(A/C/W/Y), meningococcal vaccine containing one serogroup or combinations of serogroups A, C, W and Y; n, number of cases; NR, not reported; PS, polysaccharide vaccine; y, years of age
Fig. 5.
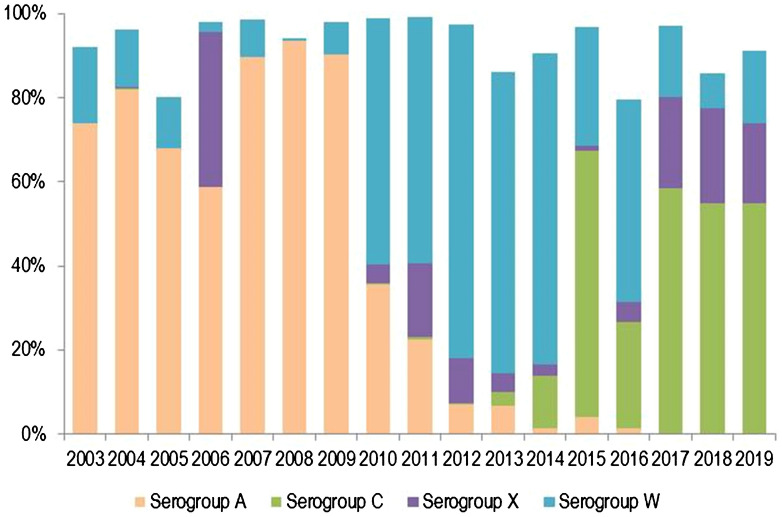
Distribution of invasive meningococcal disease caused by serogroups A, C, X and W over time in the African meningitis belt from 2003 to 2019. Data from enhanced meningococcal surveillance, World Health Organization [71, 160]. *Serogroups C and X were not reported until 2010. Cases of serogroup B and Y were minimal or zero in all years
Outbreaks in Special Groups
Men Who Have Sex with Men (MSM)
Outbreaks of IMD, usually caused by MenC, have been reported since 2001 in gay communities in Australia, Canada, France, Germany and the USA [42–47]. In the USA, seven MenC IMD outbreaks occurred in four cities (Chicago, Los Angeles County/Southern California, Miami and New York City) between 2003 and 2015 [48].
Recreational Drug Use
We found one study that described an outbreak of MenC IMD in recreational drug users in New York (USA) in 2005–2006 [49]. The median age of the 23 cases was 41 years, 19 were recreational drug users and the CFR was 30%.
Outbreaks Associated with Mass Gatherings
Outbreaks of IMD have occurred in settings where large numbers of people gather, and published reports describe outbreaks associated with a football tournament/football club, funeral and an international scout jamboree (Table 6) [50–53]. Crowded conditions facilitating meningococcal transmission can lead to high attack rates. Of 150 attendees of a funeral in Liberia, 28 (19%) developed MenC IMD and 13 died (CFR 46%) [51]. A potentially even greater risk associated with mass gatherings is the subsequent dispersal of attendees and transmission of disease across communities and countries.
Table 6.
Outbreaks and outbreak dissemination associated with mass gatherings
| Event and location | Year | Serogroup | Cases | Age group primarily affected | Attack rate per 100,000 | CFR (%) | Comment | Country of dissemination | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Hajj, Saudi Arabia |
2000 |
37% W 24% A |
264 | Median 40 y |
8.9 (W) 5.8 (A) |
28 | MenACWY required for all pilgrims from May 2001 | 90 cases, 14 deaths (15.6%) in UK, France, the Netherlands, Germany, Finland, Sweden, Belgium, Switzerland, Norway | [56, 157] | ||
|
Hajj, Saudi Arabia |
2001 | W (50%) | 109 | – | – | 32 | 8 cases of W disease in contacts in a London Muslim community (all children) suggested ongoing transmission | [55, 158] | |||
|
Hajj, Pilgrims and contacts in Singapore |
2000–2001 | W | 8 | – |
Pilgrims: 25 in 2000, 0 in 2001 Contacts: 18 in 2000, 28 in 2001 |
38 | No cases in pilgrims after administration of MenACWY (2001) | – | [159] | ||
| Football tournament, Belgium | 1997 | C | 11 | 12–18 y | – | – | Cases in participants from the Netherlands, Germany, Denmark. Accurate contact tracing and chemoprophylaxis/vaccination. No cases in their contacts | [50] | |||
|
Funeral, Liberia |
2017 | C | 28 | 10 to ≥ 50 y | 10% in funeral attendees | 46 | – | [51] | |||
|
World Scout Jamboree, Japan |
2015 | W | 6 | 14–17 y | 240.4 in the affected camp | 0 | – | – | [52] | ||
CFR case fatality rate, Men meningococcal disease vaccine, Men(A/C/W/Y) meningococcal vaccine containing one serogroup or combinations of serogroups A, C, W and Y, Ref source reference, y years of age
One of the great mixing pots for the dissemination of meningococci is the annual Hajj, which brings more than 2 million pilgrims from across the globe to a single location in Saudi Arabia who acquire and carry strains of meningococcus back to their home countries. The first well-documented Hajj-related international outbreak of IMD was in 1987. It affected 1841 pilgrims and triggered outbreaks in neighbouring Gulf states, sub-Saharan Africa, Europe and North America, and led to the establishment of a new clonal group in Africa [54, 55]. In 1987, the Saudi authorities implemented compulsory MenAC-PS vaccination for all pilgrims [55]. Hajj-related MenA IMD outbreaks reduced in magnitude but continued to occur until 2000, when there was a major shift in the predominating strain with emergence of MenW. After the 2000 Hajj, more than 400 cases of MenW IMD were reported in pilgrims or their close contacts in 16 countries in the Middle East, Asia and Europe, and MenACWY vaccination became mandatory for pilgrims [55, 56] (Table 6).
Public Health Responses to IMD Outbreaks
The first step in management of an IMD outbreak is to recognise that it is occurring on the basis of WHO-based definitions or local guidance, and to identify the causative serogroup. The public health response is frequently a multipronged effort that includes chemoprophylaxis of close contacts and administration of a vaccine, if available, to the population identified as at risk. In practice, the implementation of these principles is highly complex, resource intensive, expensive and success can be difficult to measure. The outbreak response is highly specific to the location and target group and requires individualised planning to maximise the success of the campaign.
Europe
UK
Increasing rates of MenC IMD coupled with local outbreaks of MenC disease in high schools during the 1990s prompted UK health authorities to approach manufacturers to collaborate on the development of MenC-CV. Between 1999 and 2000, three MenC vaccines were approved for use in the UK. Vaccination of 15 million children aged 2 months–17 years was progressively rolled out, beginning with 15–17-year-olds, the age group most at risk. Vaccines were administered by general practitioners (GPs) and through school-based programmes to children individually called up using national immunisation computing systems or school enrolment lists. Enhanced reporting of adverse events was expanded to capture all events regardless of severity. Compared with 1998–1999, cases of MenC IMD in the target age group had reduced by 81% in 2000–2001 [12].
The increase in MenW IMD recorded in the UK in 2014–2015 triggered an outbreak response targeting 13–18-year-olds that was launched in 2015, with time-limited catch-up for unvaccinated university entrants aged up to 25 years. The quadrivalent (serogroups A, C, W, Y) meningococcal conjugate vaccine (MenACWY-CV) replaced the 13–14 year MenC booster from 2015 in the NIP [13]. Cases of MenW IMD were 69% lower than predicted in the first year of the programme [57].
France
MenBVac (outer membrane vesicle vaccine) was employed during an outbreak in Normandy from 2006, but its implementation was delayed because of vaccine shortages [58]. The vaccine was changed to the four-component meningococcal serogroup B vaccine (4CMenB) once it became available [59].
An outbreak of four cases of MenB IMD in Beaujolais in 2016 triggered implementation of 4CMenB vaccination for the population of 4331 residents aged 2 months–24 years. Information was communicated by press releases, radio networks, local newspapers, posters and flyers, the health agency’s website and printed information put directly into letterboxes. A toll-free hotline was established and public information meetings for parents occurred at school prior to vaccination days. Vaccines were administered at clinics set up in schools and day care centres, ad hoc clinics provided by local councils, and public health centres. No further cases occurred within the epidemic area [60].
Two vaccination campaigns with 4CMenB were conducted in two schools in response to a MenB outbreak in 2017 in Brittany. The initial campaign targeted the school where two cases had first occurred. After two additional cases were identified at another school, a second campaign was initiated to also include all 11–19-year-olds at the second school located in the hyperendemic region. Public information meetings were held for parents, and a hotline was established. Information was transmitted to local mayors, GPs, private nurses and pharmacies. School students and staff were vaccinated in the school over a 2-day period for each dose, whereas community residents were vaccinated by GPs or private nurses. The coverage of the first dose was 43% in the target population. No further cases occurred [61].
4CMenB is not recommended as part of the routine immunisation schedule in France, so stock levels are generally low or absent in local pharmacies. In the outbreaks in Beaujolais and Brittany, the regional health agencies were required to procure stock either from pharmacy wholesale distributors or directly from the manufacturer.
Italy
MenC-CV was introduced for 13–15-month-olds in 2005 and a MenACWY-CV booster for 11–20-year-olds in 2007. Ten years later, 61 cases of MenC IMD, including 12 cases in vaccinated individuals, occurred in Tuscany [62]. A reactive vaccination campaign using MenC-CV or MenACWY-CV was initiated in 2015 for 11–45-year-olds. In 2017, the regional health authority introduced a MenC-CV booster dose at age 6–9 years into the regional schedule [62]. A MenACWY-CV vaccine might be considered for this booster dose to maintain control over MenC while broadening protection against other serogroups [63].
Poland
Poland experienced at least six outbreaks of MenB IMD in different parts of the country between 2006 and 2008. In 2009, six cases of MenC IMD occurred in Goleniów County. A reactive vaccination campaign in the affected communities targeted approximately 6500 individuals aged 6–19 years. Police, border guards and airport workers up to age 24 years were also vaccinated [64].
The Americas
USA
The financial burden of the 1996 Illinois MenC outbreak included employee salaries, overtime, vaccine costs, and the costs of chemoprophylaxis and supplies [65]. During the mass vaccination intervention undertaken in Florida in 1998, 13,535 persons were immunised. This campaign required the resources of county and state health departments, the fire department, law enforcement personnel, local healthcare providers, a dedicated hotline and numerous volunteers. Vaccines were administered at the local school at 6–18 parallel stations, each manned by registered nurses, physicians and backed up by 3–10 nurses supplying them with syringes previously filled in the school canteen. Vaccination was to be restricted to individuals living in the affected region, but this proved impossible as the centre was overwhelmed with persons from outside the area requesting vaccination. The cost of the vaccine itself accounted for 65% of the total cost of the 1998 Florida outbreak [66].
4CMenB was first used in two university outbreaks in 2013, under an expanded access investigational new drug programme preceding licensure in the USA [67, 68].
MenB outbreaks in universities since 2013 have triggered mass immunisation campaigns for college students, requiring major logistical ventures, emergency planning and significant funds [16]. Capitano et al. [69] described the management of an outbreak at an Oregon university in detail. Within 48 h of identification of the first MenB case, and each time a case was identified, the incident management team, comprising public information officers, university health centre representatives and the registrar’s office, activated a standard protocol. The protocol activities included coordination with the County Public Health Department, implementation of a communication plan, notification of university officials and close contacts, and administration of chemoprophylaxis to close contacts. A vaccination response using MenB vaccine containing factor H binding protein (MenB-FHbp) was recommended when an outbreak was officially declared after the fourth case. Vaccines were administered in mass immunisation clinics and in smaller clinics for incoming freshmen. The immunisation programme required 2000 person-hours of university staff time, in addition to the activities of pharmacist immunisers and medical and administrative support staff. Each individual was required to go through six stations to address issues of eligibility and to collect informed consent prior to vaccination. Significant issues that needed to be addressed were student apathy to vaccination (only 10% received the full three-dose course of MenB-FHbp), issues of reimbursement related to lacking or lapsed health insurance, and resource reallocation to the detriment of other university programmes. The cost of the campaign was estimated to be close to US $600,000, of which 90% was for vaccine supplies. The lifetime costs associated with death or sequelae of cases that may have been prevented through routine mass immunisation were not included [69].
Canada
Approval of MenC-CV was fast-tracked in Canada in response to an emerging MenC outbreak that was not controlled with a MenC-PS vaccination campaign. Approximately 1.5 million individuals aged 2 months to 20 years received at least one dose of MenC-CV during the programme. The incidence of MenC IMD in the target age group decreased from 21.47 per million person-years in 2001 to 3.26 per million person-years in 2002, and the estimated vaccine effectiveness was 96.8% (95% confidence interval [CI] 75.0–99.9) [70]. MenC-CV was introduced into the provincial immunisation schedules progressively from 2002.
A mass immunisation programme was undertaken in the Saguenay–Lac-Saint-Jean region which was the region most severely affected by a prolonged MenB epidemic (2006–2013). From May 2014, all 2-month-olds to 20-year-olds were offered 4CMenB. More than 48,000 persons, representing 82% of the target population, received at least one dose of 4CMenB. There were no cases in vaccinated persons during 2015–2016 [18]. The incidence of MenB IMD decreased in 20-year-olds and younger the year after the vaccination campaign, from 11.4 per 100,000 to 0.0 per 100,000. The decrease in MenB IMD in the target population was 100% in the first 3 years and 96% after 4 years [18, 71].
4CMenB was also used in response to a MenB outbreak in a university in Nova Scotia in 2015. Of the campus population of 3500, 70% received two doses of 4CMenB administered in vaccination units set up on site [72].
In response to the first MenW (ST-11) outbreak in British Columbia, mass immunisation of local residents was conducted, with administration of 11,417 doses of MenACWY-CV to 68% of the population. Mobilisation of vaccine supplies, coordination of vaccination clinics and data management related to vaccine uptake were identified as the main challenges while executing the programme [73]. No cases were subsequently observed throughout 2018 [74].
Mexico
A MenC (ST-11) outbreak, causing 19 cases and 7 deaths (CFR 37%), was reported in Tijuana in 2013. Widespread chemoprophylaxis of contacts was implemented without vaccination. The continued circulation of the ST-11 clone remains a concern in the region [75].
Brazil
A MenC outbreak in an oil refinery in Brazil triggered mass vaccination of workers with MenAC-PS. Despite reaching 91% coverage with MenAC-PS among workers, cases in family contacts continued to occur, necessitating mass vaccination of individuals aged 2 months to 19 years in the neighbouring city. The failure of MenAC-PS to control the outbreak and prevent transmission to close contacts triggered a change in the recommendations for outbreak control in Brazil, from Men-PS to Men-CV vaccines [76]. MenC-CV was introduced into the NIP in 2010 [77]. An outbreak of 194 MenC IMD cases in Salvador in 2009 led to mass vaccination of 10–24-year-olds with MenC-CV, with estimated vaccine effectiveness of one dose of 100% (95% CI 79–100). Fifty-two vaccination clinics were set up around the city. The vaccination period was extended and additional clinics were set up in large universities to counter poor uptake by the 20–24-year-old population [77].
Asia–Pacific
Australia
Two MenC outbreaks, each confined to a single high school, were managed with reactive vaccination campaigns using MenC-PS in 1998 and MenC-CV in 2003 [78, 79]. The 1998 campaign targeted 1600 staff, students, and families of cases, and incurred a total cost in excess of Australian $65,000 [79].
A MenW outbreak was declared in 2017 after nine cases of MenW disease were admitted to the Alice Springs hospital, a catchment hospital for 58 surrounding remote communities. Given the remote location of the population at risk, a fever protocol specifying the case definition, investigative and management procedures was implemented in primary and secondary healthcare centres across the region. Patients from remote communities were evacuated to the central hospital by the medical retrieval service. Chemoprophylaxis was administered to 465 close contacts and a vaccination campaign with MenACWY-CV targeted all indigenous Australians and Torres Strait Islanders aged 1–19 years living in remote communities. The programme was subsequently expanded to non-indigenous Australians. No patient died and the number of cases of MenW IMD decreased rapidly after the onset of the vaccination campaign. The remoteness of the Central Australian indigenous population and frequent movements between communities were challenges specific to this outbreak that necessitated a different approach compared to outbreaks in urban centres, including the use of a fever protocol and presumptive antibiotic treatment for febrile illnesses [80].
New Zealand
In responses to New Zealand’s prolonged MenB epidemic (1991–2007), a strain-specific outer membrane vesicle vaccine (MeNZB) was developed by Chiron Corporation in collaboration with the Norwegian Institute of Public Health. Clinical trials commenced in 2002 and approval was received in 2004. A vaccination programme was progressively rolled out across the country to children aged 6 weeks–19 years. By mid-2006, almost 3 million vaccine doses had been administered to 80% of the eligible population [81]. Estimates of vaccine effectiveness are thought to be between 53% and 77% [82].
The outbreak response to the 2018 MenW outbreak in Northland included the purchase and administration of 25,000 doses of MenACWY-CV to residents aged 13–20 years, primarily through pharmacies in the region [21].
Africa
WHO epidemic response guidelines recommend antibiotic prophylaxis for contacts and reactive vaccination campaigns once the pre-defined thresholds are reached [8]. Outbreak response activities require large-scale mobilisation of local and national healthcare personnel to raise awareness, provide education, encourage reduced overcrowding, and administer chemoprophylaxis and/or vaccines.
The success of reactive vaccination programmes is difficult to measure. Modelling shows that more cases are prevented if vaccination is given early in an epidemic [83–85]. In practice, however, many outbreaks are intense and of short duration, which means that mass vaccination programmes only begin after the epidemic peaks, and their impact may be modest. It has been estimated that 62% of outbreak cases occur within the first 6 weeks of an epidemic, and that the maximum cases preventable by vaccination would be 30% (of the total predicted) if vaccination started at week 8 [84].
Confirmation of the causative organism from multiple specimens in an outbreak is required before a vaccination campaign can begin. However, remote villages and regions may have low capacity to perform lumbar puncture, communication can be delayed, specimens need to be sent to reference laboratories, and delays to obtain results can be substantial. Authorities require documentation confirming that threshold levels have been reached. The disease can spread to other regions before the epidemic threshold is reached and preventative measures taken in one district [84, 86, 87]. Access to vaccines requires application to the International Coordinating Group (ICG) on Vaccine Provision [33]. If approved, vaccines need to be shipped, cleared by customs, distributed without disruption of the cold chain, and administered by trained local staff. Supply does not always equal demand and vaccination may therefore be limited to the most at-risk age groups. During a 2016 MenW outbreak in Togo, the time between ICG approval for each request and implementation amounted to 2–4 weeks [86]. Considering such potential delays, vaccines may only be administered once the outbreak is waning. In the meantime, regions on the leading edge of the outbreak might not qualify for reactive vaccination. Unsurprisingly, in many of the reports provided in Table 5, vaccination commenced after the number of cases began to decrease and therefore any effect of vaccination is unclear [86–90].
Almost all reactive vaccination conducted in African countries is with PS vaccines stockpiled by the ICG. Immunity is short-lived, revaccination is required in the event of another outbreak and repeated vaccination can be associated with hyporesponsiveness. Furthermore, PS vaccination has no discernible effect on carriage and thus does not interrupt transmission to susceptible contacts [44]. The quadrivalent (serogroups A, C, W, Y) meningococcal conjugate vaccine (MenACWY-CV) was used in some regions in Niger in 2017 during a MenC outbreak, but the longer-term impact on IMD in the region is not known.
Outbreaks in Special Groups
Men Who Have Sex with Men (MSM)
In the USA, MenC IMD outbreaks in MSM between 2003 and 2015 triggered targeted immunisation campaigns, often with MenACWY-CV, for all MSM or for individuals with specific risk factors such as more than one male partner, human immunodeficiency virus (HIV) infection or exposure to risk activities specific to the outbreak (e.g. attendance at bars, bath houses) [43, 45].
In Australia, a government-funded time-limited (approximately 12 months) vaccination programme with MenACWY-CV was instituted in Victoria, following an outbreak of MenC IMD among MSM in 2017. Vaccines were administered through general practices, sexual health clinics and registered immunisation providers. Vaccine coverage at a sexual health clinic in Melbourne was 67.4%. The number of MenC IMD cases in this population reduced from seven in 2017 to one in 2018 [47].
Recreational Drug Use
A vaccination programme was launched in New York in response to an outbreak of MenC IMD in recreational drug users. The programme targeted adults aged over 18 years with a recent history of recreational drug use and their household contacts who were aged 2 years or more. Contact tracing was minimally successful because of the reluctance of patients and contacts to identify associates. MenACWY-CV was administered to 11–55-year-olds and MenACWY-PS was given to individuals aged 2–10 or over 55 years, in accordance with recommendations in place at the time. Vaccination was provided at community health centres, soup kitchens, homeless shelters, and correctional healthcare facilities. It was also provided at sites for methadone maintenance treatment, syringe exchange, day or residential drug treatment. The programme incurred significant costs, with vaccines and staff salaries costing more than US $1 million. Sporadic cases of the outbreak strain continued to occur but there were no cases in vaccinated individuals, and no further cases were reported in recreational drug users after 2006 [49].
Outbreaks Associated with Mass Gatherings
Rapid responses to outbreaks of IMD at mass gatherings have successfully limited their impact. Rapid and thorough contact tracing for chemoprophylaxis with or without vaccination prevented the international spread of IMD from participants at a Belgium football game [50]. After a scout jamboree in Japan, public health agencies in Scotland and Sweden were successful in restricting disease cases to participants, with no cases in contacts in their country of origin [52].
The Hajj is one of the only mass gatherings where prophylactic vaccination (with MenACWY) is mandatory for attendance [55, 56]. A strict vaccination policy, the use of chemoprophylaxis for pilgrims from some countries and enhanced IMD surveillance during the Hajj have successfully prevented IMD outbreaks in recent years. However, MenB vaccination is not currently required for Hajj attendees, no MenX vaccine currently exists, and both of these serogroups have epidemic potential.
Challenges in Outbreak Management
Many of the challenges associated with implementing mass vaccination campaigns were common to high- and low-income countries and were centred around acquiring sufficient numbers of vaccine doses in a short time frame, rapid dissemination of information to healthcare professionals and the affected community, and accessing the target population. Vaccine availability was most problematic in countries where the vaccine was not included in the NIP, adding to delays between outbreak onset and implementation of a vaccination programme [58, 60, 61, 73, 86–90]. The logistics involved with accessing the target population differed in high- versus low-income countries, with apathy, particularly among students and young adults, or reluctance to identify close contacts (recreational drug users) negatively impacting uptake in high/upper-middle-income countries [49, 69, 77]. Accessing the target population posed specific challenges when they were located in remote settings such as central Australian indigenous communities [80], remote communities in African countries requiring the use of mobile laboratories [87] and in slum areas of large cities where house-by-house vaccination was undertaken [91].
Challenges specific to high-income countries were those related to health insurance and reimbursement (mainly the USA) [69], whereas the main challenge specific to low-income countries was the risk of cumulative delay between outbreak identification and vaccination due to delays in serogroup identification, the need to reach outbreak threshold before intervention can be actioned, and bureaucracy associated with requests for vaccines, shipping and customs [84, 86, 87].
Impact of Outbreaks on NIPs
The exact timing and location of infectious diseases outbreaks, including IMD, cannot be predicted with any precision. Infectious disease control is therefore most successful using preventative rather than reactive strategies. IMD outbreaks combined with epidemiological surveillance have prompted some countries to introduce meningococcal vaccines into NIPs [62, 70, 92, 93].
Since 2014–2015, MenW IMD has increased across European countries [1], leading several to introduce a MenACWY-CV booster dose into their adolescent vaccination schedule. As of 2020, MenACWY-CV is recommended for all adolescents in eight countries in Europe [94].
Vaccination recommendations for meningococcal disease in the USA have moved forward by increments. MenACWY-CV was added to the US immunisation schedule in 2005, with recommendation for 11–12-year-olds and for 11–55-year-olds at increased risk of IMD [95]. Routine vaccination was also recommended for college freshmen living in dormitories and for other populations at increased risk. The recommendation was extended to all 11–18-year-olds and to children aged 2–10 years at increased risk of IMD in 2007 [96, 97], to 2- to 55-year-olds and 9–23-month-olds at increased risk of IMD in 2011 [98, 99], and to 2–23-month-olds at increased risk of IMD in 2013 [100]. Since 2010, a booster dose of MenACWY-CV is recommended for all adolescents at age 16 years [101]. In 2015, the Advisory Committee on Immunization Practices (ACIP) recommended that persons aged 10 years and over at increased risk for MenB IMD, and that persons aged 16–23 years receive a MenB vaccine series [102, 103]. The recommendation for 16–23-year-olds was initially a category B recommendation (for individual clinical decision making), and then on the basis of shared clinical decision-making from 2020.
As of 2020, ACIP recommends routine MenACWY vaccination for 11–12-year-olds with a booster dose at age 16 years, routine MenACWY vaccination for persons aged 2 months and older at increased risk if IMD including unvaccinated or incompletely vaccinated first-year college students living in residence halls, and MenACWY booster doses for vaccinated persons who become or remain at increased risk. ACIP continued to recommend routine MenB vaccination in persons aged 10 years and older who are at increased risk for MenB IMD, and MenB booster doses for previously vaccinated persons who become or remain at increased risk. During an outbreak, a single MenB booster dose is recommended for individuals with at least 1 year since completion of the primary series [103].
At present, MenACWY-CV continues to be recommended for unvaccinated or undervaccinated college freshmen living in residence halls, while there is no similar recommendation for MenB vaccines [103], even though all college-associated outbreaks between 2011 and 2019 were caused by MenB [104]. The incidence of IMD in 18–24-year-old college students is estimated to be more than five times higher than those who do not attend college [104].
A booster dose of MenC-CV or quadrivalent meningococcal tetanus toxoid conjugate vaccine (MenACWY-TT) was added to the Canadian recommended vaccination schedule for adolescents in 2017 [105]. All provinces now provide a MenACWY-CV booster, except Quebec, which uses MenC-CV as booster [106].
In Australia, the 2017 MenW outbreak in remote indigenous communities led to the replacement of the 12-month MenC-CV dose with MenACWY-CV in 2017, and the inclusion of a booster dose of MenACWY-CV for all adolescents living in the Northern Territory [93]. 4CMenB was added to the NIP for Aboriginal and Torres Strait Islander infants in 2020, with a catch-up program up to 2 years of age until 2023. Individuals with complement deficiencies or functional or anatomical asplenia are also eligible to receive 4CMenB [107].
Discussion
Meningococcal epidemiology evolves constantly, requires continual surveillance and proactive review of vaccination policies to maintain control as the epidemiology changes and as new vaccines become available. Because of genetic flexibility, the emergence and clonal expansion of new strains of N. meningitidis means that human populations with underlying immunity to historical strains may be susceptible to novel strains, with potentially devastating consequences. The role of surveillance in monitoring changes in carriage and disease isolates of N. meningitidis cannot be overemphasised for this rapidly evolving pathogen.
This non-systematic review highlights that IMD outbreaks/epidemics are a global phenomenon that can potentially affect persons of all ages and occur unpredictably. Attempts to classify risk groups by age, social-sexual-recreational behaviours, or by proximity of social contacts in defined settings are of limited practical use because of their wide catchment and non-specificity.
Reactive management of outbreaks, with contact tracing, enhanced surveillance, chemoprophylaxis and vaccination, can be effective, but only if it starts early and proceeds very rapidly. Reactive outbreak management does not prevent disease and death in index cases, and lives can be lost while waiting for pre-defined threshold levels of disease to occur. The latter is observed most acutely in Africa, where a combination of diagnostic, administrative and logistical delays can substantially reduce the effectiveness of reactive vaccination campaigns and the number of cases averted.
Emergency campaigns during MenA or MenC outbreaks often employ mono- or bivalent vaccines. These vaccines may achieve the goal of outbreak control but leave the population susceptible to other serogroups. For example, despite the success of MenA-CV, IMD is not controlled in the African meningitis belt (Fig. 5). In 2019 there were 22,414 suspected IMD meningitis cases with a CFR of 6% across 24 countries. IMD epidemiology has changed, and most IMD in the African meningitis belt is now caused by MenC, MenW and MenX (Fig. 5). MenC is rapidly emerging as an urgent epidemic threat and appears to cause epidemics resembling those caused by MenA. Provision of affordable multivalent conjugate vaccines targeting serogroups A, C, W, Y and X, for use in mass vaccination campaigns followed by routine immunisation of infants, will be critical to long-term IMD control in Africa [37, 108].
While the burden of disease is less pronounced in the rest of the world, the predominating serogroups are changing, with evidence of increased MenW in South America, China and Australia, and serogroup Y in Scandinavian countries and Australia [1]. In light of the unpredictability of outbreaks, the extreme efforts and substantial costs involved in reactive outbreak management, and the need to preserve of the maximum number of lives, prevention of IMD through routine immunisation using broadly protective meningococcal vaccines is the most effective way to prevent IMD morbidity and mortality.
The articles we found in this review consistently reported that emergency vaccination responses to IMD outbreaks incurred substantial costs in terms of direct monetary expenditure on vaccine supplies, personnel costs and interruption to other programmes. One USA-based programme cost more than US $1 million to vaccinate a relatively modest number of 2763 individuals at risk due to an outbreak among recreational drug users [49]. These costs do not consider the lifetime costs incurred by victims of the disease, nor do they give a picture of the cumulative cost of outbreak management over decades. Frequently identified impediments to a successful emergency response were the need for transmission of information to parents, communities, healthcare workers, issues around the collection of informed consent, poor uptake of vaccination by older adolescents and young adults who were one of the most frequently targeted age groups, issues of reimbursement particularly in the USA, and difficulties in obtaining large quantities of vaccine very quickly. For the management of MenB outbreaks, the need to administer two doses was a significant issue. Both available MenB vaccines require primary vaccination with two doses. The interval between doses should be 1 month for 4CMenB and 6 months for MenB-FHbp. The need for a second dose in an emergency setting contributes substantially to the overall cost, delays the onset of protection and leads to substantial non-compliance with the second dose. For MenB vaccines, where herd protection effects have not been demonstrated because of the lack of effect against carriage [109], individual protection is critical for disease prevention and requires completion of the vaccine series.
Currently, ACIP recommends administration of a single MenB booster dose during an outbreak, when more than 1 year has elapsed since completion of the primary series [103]. Uptake of MenB vaccines remains low in the USA; 21.8% of 17-year-olds received at least one dose of MenB vaccine in 2019, which is in contrast to 88.9% of 13–17-year-olds who received MenACWY-CV [110]. This could reflect the difference in the wording around the respective recommendations for these vaccines (shared decision-making versus a direct recommendation). Current recommendations for MenB vaccines seem insufficient to markedly impact the occurrence of sporadic MenB outbreaks in the USA. Mandated MenB vaccination prior to school or university entry would improve uptake amongst this at-risk population but would not impact other age groups. Higher coverage of MenB achieved through routine vaccination platforms would vastly simplify outbreak responses should they be needed. Several outbreak reports note that vaccine acquisition was one of the most challenging aspects of reactive emergency vaccination campaigns, especially when the vaccine was not used routinely in that country [61, 86]. It follows that routine vaccination programmes engender a setting of ‘preparedness’, not only inducing a baseline level of population protection but also increasing the speed at which emergency interventions can occur. Early access to vaccines and the requirement for a single booster dose all work to maximise the efficiency and effectiveness of an emergency intervention. Furthermore, administration of a single dose when it has been at least 1 year since completion of the primary series would likely improve coverage and levels of direct protection during outbreaks, avoiding the need for a second dose and the associated costs and compliance issues.
It is unlikely that the present review captures all IMD outbreaks that have occurred globally since 1997. Despite this limitation, a comprehensive summary of outbreaks from all world regions is presented, with details on the vaccine interventions undertaken and their effects, when available. Although the quality of the information captured is variable, the key message from the data is nevertheless clear—that although rare, IMD outbreaks occur suddenly and unpredictably, and their management is challenging and resource intensive.
Conclusion
As a result of continual antigenic change, the epidemiology of IMD is fluid, and changes can be unexpected. Novel meningococcal strains regularly emerge, and virulent strains may cause outbreaks or epidemics if they are transmitted within populations with low underlying immunity. Real-world descriptions of outbreak control strategies and the associated challenges show that without exception, reactive outbreak management is administratively, logistically and financially costly, and the benefit or impact difficult to measure. Highly effective vaccines are available that together cover five of the six disease-causing serogroups. In view of the unpredictability, rapidity and potential lethality of outbreak-associated IMD, prevention of outbreak-associated IMD through routine vaccination is the most effective means of control.
Acknowledgements
The authors thank Johannes E. Schmidt for his input during the review of this manuscript.
Funding
GlaxoSmithKline Biologicals SA took in charge all costs associated with the development and the publishing of the present manuscript.
Medical Writing and/or Editorial Assistance
The authors thank Business & Decision Life Sciences platform for editorial assistance, writing support, manuscript coordination and design support for the digital illustrations, on behalf of GSK. Joanne Wolter provided writing support. Bruno Baudoux coordinated manuscript development and editorial support.
Authorship
All named authors meet the ICMJE criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors participated in the development and the review of the manuscript and approved the final submitted version.
Disclosures
Nevena Vicic is employed by the GSK group of companies. Véronique Abitbol, Rafik Bekkat-Berkani, Lamine Soumahoro are employed by the GSK group of companies and hold shares in the GSK group of companies as part of their employee remuneration. Marco AP Safadi reports research grants and personal fees for advisory boards from the GSK group of companies, Pfizer, and Sanofi. All authors declare no other financial and non-financial relationships and activities.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
- 1.Parikh SR, Campbell H, Bettinger JA, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81:483–498. doi: 10.1016/j.jinf.2020.05.079. [DOI] [PubMed] [Google Scholar]
- 2.Topaz N, Caugant DA, Taha MK, et al. Phylogenetic relationships and regional spread of meningococcal strains in the meningitis belt, 2011–2016. EBioMedicine. 2019;41:488–496. doi: 10.1016/j.ebiom.2019.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooda Y, Shin HE, Bateman TJ, Moraes TF. Neisserial surface lipoproteins: structure, function and biogenesis. Pathog Dis. 2017;75:ftx010. [DOI] [PubMed]
- 4.Finne J, Leinonen M, Mäkelä PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen WH, Neuzil KM, Boyce CR, et al. Safety and immunogenicity of a pentavalent meningococcal conjugate vaccine containing serogroups A, C, Y, W, and X in healthy adults: a phase 1, single-centre, double-blind, randomised, controlled study. Lancet Infect Dis. 2018;18:1088–1096. doi: 10.1016/S1473-3099(18)30400-6. [DOI] [PubMed] [Google Scholar]
- 6.Harrison LH, Jolley KA, Shutt KA, et al. Antigenic shift and increased incidence of meningococcal disease. J Infect Dis. 2006;193:1266–1274. doi: 10.1086/501371. [DOI] [PubMed] [Google Scholar]
- 7.Swartley JS, Marfin AA, Edupuganti S, et al. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA. 1997;94:271–276. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guidelines Review Committee. Meningitis outbreak response in sub-Saharan Africa: WHO guideline. Geneva: WHO; 2014. [PubMed]
- 9.Gully PR. Pandemics, regional outbreaks, and sudden-onset disasters. Healthc Manage Forum. 2020:33(4):164–9. [DOI] [PMC free article] [PubMed]
- 10.Mustapha MM, Harrison LH. Vaccine prevention of meningococcal disease in Africa: major advances, remaining challenges. Hum Vaccin Immunother. 2018;14:1107–1115. doi: 10.1080/21645515.2017.1412020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin D, Lopez L, McDowell R. The epidemiology of meningococcal disease in New Zealand in 2005. Report prepared for the Ministry of Health by the Institute of Environmental Science and Research Limited (ESR). Wellington: Ministry of Health. 2006. https://www.moh.govt.nz/notebook/nbbooks.nsf/0/8CF3A9486FE3DDE8CC257CED007E3AC8/$file/2005.pdf. Accessed 24 June 2021.
- 12.Miller E, Salisbury D, Ramsay M. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine. 2001;20(Suppl 1):S58–S67. doi: 10.1016/s0264-410x(01)00299-7. [DOI] [PubMed] [Google Scholar]
- 13.Campbell H, Saliba V, Borrow R, Ramsay M, Ladhani SN. Targeted vaccination of teenagers following continued rapid endemic expansion of a single meningococcal group W clone (sequence type 11 clonal complex), United Kingdom 2015. Euro Surveill. 2015;20:21188. doi: 10.2807/1560-7917.es2015.20.28.21188. [DOI] [PubMed] [Google Scholar]
- 14.Mbaeyi SA, Blain A, Whaley MJ, et al. Epidemiology of meningococcal disease outbreaks in the United States, 2009–2013. Clin Infect Dis. 2019;68:580–585. doi: 10.1093/cid/ciy548. [DOI] [PubMed] [Google Scholar]
- 15.Soeters HM, McNamara LA, Blain AE, et al. University-based outbreaks of meningococcal disease caused by serogroup B, United States, 2013–2018. Emerg Infect Dis. 2019;25:434–440. doi: 10.3201/eid2503.181574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alderfer J, Isturiz RE, Srivastava A. Lessons from mass vaccination response to meningococcal B outbreaks at US universities. Postgrad Med. 2020:132(7):614–23. [DOI] [PubMed]
- 17.Tyrrell GJ, Chui L, Johnson M, et al. Outbreak of Neisseria meningitidis, Edmonton, Alberta. Can Emerg Infect Dis. 2002;8:519–521. doi: 10.3201/eid0805.010337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Wals P, Deceuninck G, Lefebvre B, et al. Impact of an immunization campaign to control an increased incidence of serogroup B meningococcal disease in one region of Quebec. Can Clin Infect Dis. 2017;64:1263–1267. doi: 10.1093/cid/cix154. [DOI] [PubMed] [Google Scholar]
- 19.Gorla MC, de Lemos AP, Quaresma M, et al. Phenotypic and molecular characterization of serogroup C Neisseria meningitidis associated with an outbreak in Bahia. Brazil Enferm Infecc Microbiol Clin. 2012;30:56–59. doi: 10.1016/j.eimc.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iser BP, Lima HC, de Moraes C, et al. Outbreak of Neisseria meningitidis C in workers at a large food-processing plant in Brazil: challenges of controlling disease spread to the larger community. Epidemiol Infect. 2012;140:906–915. doi: 10.1017/S0950268811001610. [DOI] [PubMed] [Google Scholar]
- 21.Lahra MM, Enriquez RP, Hogan TP. Australian Meningococcal Surveillance Programme annual report, 2018. Commun Dis Intell. 2018;2020:44. doi: 10.33321/cdi.2020.44.10. [DOI] [PubMed] [Google Scholar]
- 22.Sinclair D, Preziosi MP, Jacob John T, Greenwood B. The epidemiology of meningococcal disease in India. Trop Med Int Health. 2010;15:1421–1435. doi: 10.1111/j.1365-3156.2010.02660.x. [DOI] [PubMed] [Google Scholar]
- 23.Sachdeva A, Kukreja S, Jain V, Dutta AK. Meningococcal disease—outbreak in Delhi. Indian Pediatr. 2005;42:547–556. [PubMed] [Google Scholar]
- 24.Dutta AK, Swaminathan S, Abitbol V, Kolhapure S, Sathyanarayanan S. A comprehensive review of meningococcal disease burden in India. Infect Dis Ther. 2020;9:537–559. doi: 10.1007/s40121-020-00323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duggal S, Duggal N, Charoo H, Mahajan RK. Recent outbreak of meningococcal meningitis—a microbiological study with brief review of literature. J Commun Dis. 2007;39:209–216. [PubMed] [Google Scholar]
- 26.Shao Z, Li W, Ren J, et al. Identification of a new Neisseria meningitidis serogroup C clone from Anhui province. China Lancet. 2006;367:419–423. doi: 10.1016/S0140-6736(06)68141-5. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Zhang TG, He JG, et al. A first meningococcal meningitis case caused by serogroup X Neisseria meningitidis strains in China. Chin Med J (Engl) 2008;121:664–666. [PubMed] [Google Scholar]
- 28.Zhang TG, He JG, He X, et al. The molecular characterization of serogroup C Neisseria meningitidis strains circulating in Beijing. J Microbiol. 2006;44:685–688. [PubMed] [Google Scholar]
- 29.Zhou H, Liu W, Xu L, et al. Spread of Neisseria meningitidis serogroup W clone. China Emerg Infect Dis. 2013;19:1496–1499. doi: 10.3201/eid1909.130160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Shao Z, Liu G, et al. Meningococcal disease and control in China: findings and updates from the Global Meningococcal Initiative (GMI) J Infect. 2018;76:429–437. doi: 10.1016/j.jinf.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Mohammed I, Iliyasu G, Habib AG. Emergence and control of epidemic meningococcal meningitis in sub-Saharan Africa. Pathog Glob Health. 2017;111:1–6. doi: 10.1080/20477724.2016.1274068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammed I, Nasidi A, Alkali AS, et al. A severe epidemic of meningococcal meningitis in Nigeria, 1996. Trans R Soc Trop Med Hyg. 2000;94:265–270. doi: 10.1016/s0035-9203(00)90316-x. [DOI] [PubMed] [Google Scholar]
- 33.Fall A, Bita AF, Lingani C, et al. Elimination of epidemic meningitis in the African region: progress and challenges: 2010–2016. J Immunol Sci. 2018;Suppl:41–5. [PMC free article] [PubMed]
- 34.Bwaka A, Bita A, Lingani C, et al. Status of the rollout of the meningococcal serogroup A conjugate vaccine in African meningitis belt countries in 2018. J Infect Dis. 2019;220:S140–S147. doi: 10.1093/infdis/jiz336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trotter CL, Lingani C, Fernandez K, et al. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: an analysis of surveillance data. Lancet Infect Dis. 2017;17:867–872. doi: 10.1016/S1473-3099(17)30301-8. [DOI] [PubMed] [Google Scholar]
- 36.Caro JJ, Möller J, Getsios D, et al. Invasive meningococcal disease epidemiology and control measures: a framework for evaluation. BMC Public Health. 2007;7:130. doi: 10.1186/1471-2458-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alderson MR, LaForce FM, Sobanjo-Ter Meulen A, et al. Eliminating meningococcal epidemics from the African meningitis belt: the case for advanced prevention and control using next-generation meningococcal conjugate vaccines. J Infect Dis. 2019;220:S274–S278. doi: 10.1093/infdis/jiz297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mustapha MM, Marsh JW, Harrison LH. Global epidemiology of capsular group W meningococcal disease (1970–2015): multifocal emergence and persistence of hypervirulent sequence type (ST)-11 clonal complex. Vaccine. 2016;34:1515–1523. doi: 10.1016/j.vaccine.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor L, Ward M, Bennett D, et al. A prolonged outbreak of invasive meningococcal disease in an extended Irish traveller family across three Health Service Executive (HSE) areas in Ireland, 2010 to 2013. Euro Surveill. 2015;20:21139. doi: 10.2807/1560-7917.es2015.20.21.21139. [DOI] [PubMed] [Google Scholar]
- 40.Nnadi C, Oladejo J, Yennan S, et al. Large outbreak of Neisseria meningitidis serogroup C—Nigeria, December 2016-June 2017. MMWR Morb Mortal Wkly Rep. 2017;66:1352–1356. doi: 10.15585/mmwr.mm6649a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chow J, Uadiale K, Bestman A, et al. Third consecutive outbreak of a new strain. PLoS Curr. 2015;2016:8. doi: 10.1371/currents.outbreaks.06d10b6b4e690917d8b0a04268906143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aubert L, Taha M, Boo N, et al. Serogroup C invasive meningococcal disease among men who have sex with men and in gay-oriented social venues in the Paris region: July 2013 to December 2014. Euro Surveill. 2015;20:21016. doi: 10.2807/1560-7917.es2015.20.3.21016. [DOI] [PubMed] [Google Scholar]
- 43.Nanduri S, Foo C, Ngo V, et al. Outbreak of serogroup C meningococcal disease primarily affecting men who have sex with men—Southern California, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:939–940. doi: 10.15585/mmwr.mm6535e1. [DOI] [PubMed] [Google Scholar]
- 44.Hellenbrand W, Claus H, Schink S, et al. Risk of invasive meningococcal disease in men who have sex with men: lessons learned from an outbreak in Germany, 2012–2013. PLoS ONE. 2016;11:e0160126. doi: 10.1371/journal.pone.0160126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kratz MM, Weiss D, Ridpath A, et al. Community-based outbreak of Neisseria meningitidis serogroup C infection in men who have sex with men, New York City, New York, USA, 2010–2013. Emerg Infect Dis. 2015;21:1379–1386. doi: 10.3201/eid2108.141837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsang RS, Kiefer L, Law DK, et al. Outbreak of serogroup C meningococcal disease caused by a variant of Neisseria meningitidis serotype 2a ET-15 in a community of men who have sex with men. J Clin Microbiol. 2003;41:4411–4414. doi: 10.1128/JCM.41.9.4411-4414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martín-Sánchez M, Fairley CK, Bradshaw CS, Chen MY, Chow EPF. Meningococcal vaccine uptake among men who have sex with men in response to an invasive meningococcal C disease outbreak in Melbourne. Aust Sex Transm Infect. 2020;96:246–250. doi: 10.1136/sextrans-2019-054318. [DOI] [PubMed] [Google Scholar]
- 48.Bozio CH, Blain A, MacNeil J, et al. Meningococcal disease surveillance in men who have sex with men—United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67:1060–1063. doi: 10.15585/mmwr.mm6738a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss D, Stern EJ, Zimmerman C, et al. Epidemiologic investigation and targeted vaccination initiative in response to an outbreak of meningococcal disease among illicit drug users in Brooklyn. New York Clin Infect Dis. 2009;48:894–901. doi: 10.1086/597257. [DOI] [PubMed] [Google Scholar]
- 50.Reintjes R, Kistemann T, MacLehose L, et al. Detection and response to a meningococcal disease outbreak following a youth football tournament with teams from four European countries. Int J Hyg Environ Health. 2002;205:291–296. doi: 10.1078/1438-4639-00156. [DOI] [PubMed] [Google Scholar]
- 51.Bozio CH, Vuong J, Dokubo EK, et al. Outbreak of Neisseria meningitidis serogroup C outside the meningitis belt-Liberia, 2017: an epidemiological and laboratory investigation. Lancet Infect Dis. 2018;18:1360–1367. doi: 10.1016/S1473-3099(18)30476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanai M, Kamiya H, Smith-Palmer A, et al. Meningococcal disease outbreak related to the World Scout Jamboree in Japan, 2015. Western Pac Surveill Response J. 2017;8:25–30. doi: 10.5365/WPSAR.2016.7.3.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koh YM, Barnes GH, Kaczmarski E, Stuart JM. Outbreak of meningococcal disease linked to a sports club. Lancet. 1998;352:706–707. doi: 10.1016/S0140-6736(05)60823-9. [DOI] [PubMed] [Google Scholar]
- 54.Moore PS, Reeves MW, Schwartz B, Gellin BG, Broome CV. Intercontinental spread of an epidemic group A Neisseria meningitidis strain. Lancet. 1989;2:260–263. doi: 10.1016/s0140-6736(89)90439-x. [DOI] [PubMed] [Google Scholar]
- 55.Yezli S, Assiri AM, Alhakeem RF, Turkistani AM, Alotaibi B. Meningococcal disease during the Hajj and Umrah mass gatherings. Int J Infect Dis. 2016;47:60–64. doi: 10.1016/j.ijid.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 56.Lingappa JR, Al-Rabeah AM, Hajjeh R, et al. Serogroup W-135 meningococcal disease during the Hajj, 2000. Emerg Infect Dis. 2003;9:665–671. doi: 10.3201/eid0906.020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell H, Edelstein M, Andrews N, et al. Emergency meningococcal ACWY vaccination program for teenagers to control group W meningococcal disease, England, 2015–2016. Emerg Infect Dis. 2017;23:1184–1187. doi: 10.3201/eid2307.170236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delbos V, Lemée L, Bénichou J, et al. Meningococcal carriage during a clonal meningococcal B outbreak in France. Eur J Clin Microbiol Infect Dis. 2013;32:1451–1459. doi: 10.1007/s10096-013-1897-6. [DOI] [PubMed] [Google Scholar]
- 59.Marshall HS, Lally N, Flood L, Phillips P. First statewide meningococcal B vaccine program in infants, children and adolescents: evidence for implementation in South Australia. Med J Aust. 2020;212:89–93. doi: 10.5694/mja2.50481. [DOI] [PubMed] [Google Scholar]
- 60.Thabuis A, Tararbit K, Taha MK, et al. Community outbreak of serogroup B invasive meningococcal disease in Beaujolais, France, February to June 2016: from alert to targeted vaccination. Euro Surveill. 2018;23:1700590. doi: 10.2807/1560-7917.ES.2018.23.28.1700590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pivette M, Taha MK, Barret AS, et al. Targeted vaccination campaigns of teenagers after two clusters of B invasive meningococcal disease in Brittany, France, 2017. BMC Public Health. 2020;20:1382. doi: 10.1186/s12889-020-09487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pezzotti P, Miglietta A, Neri A, et al. Meningococcal C conjugate vaccine effectiveness before and during an outbreak of invasive meningococcal disease due to Neisseria meningitidis serogroup C/cc11, Tuscany. Italy Vaccine. 2018;36:4222–4227. doi: 10.1016/j.vaccine.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Booy R, Gentile A, Nissen M, Whelan J, Abitbol V. Recent changes in the epidemiology of Neisseria meningitidis serogroup W across the world, current vaccination policy choices and possible future strategies. Hum Vaccin Immunother. 2019;15:470–480. doi: 10.1080/21645515.2018.1532248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skoczyńska A, Wasko I, Kuch A, et al. Outbreak of invasive meningococcal disease in Goleniow County, northwest Poland, March 2009. Euro Surveill. 2010;15:19646. [PubMed] [Google Scholar]
- 65.Austin CC, Fingar AR, Langkop C. Outbreak of serogroup C meningococcal disease among preschool-aged children: Illinois, 1996. Am J Public Health. 1998;88:685. doi: 10.2105/ajph.88.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krause G, Blackmore C, Wiersma S, et al. Mass vaccination campaign following community outbreak of meningococcal disease. Emerg Infect Dis. 2002;8:1398–1403. doi: 10.3201/eid0812.010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNamara LA, Shumate AM, Johnsen P, et al. First use of a serogroup B meningococcal vaccine in the US in response to a university outbreak. Pediatrics. 2015;135:798–804. doi: 10.1542/peds.2014-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Breakwell L, Vogt TM, Fleming D, et al. Understanding factors affecting university a students' decision to receive an unlicensed serogroup B meningococcal vaccine. J Adolesc Health. 2016;59:457–464. doi: 10.1016/j.jadohealth.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 69.Capitano B, Dillon K, LeDuc A, Atkinson B, Burman C. Experience implementing a university-based mass immunization program in response to a meningococcal B outbreak. Hum Vaccin Immunother. 2019;15:717–724. doi: 10.1080/21645515.2018.1547606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Wals P, Deceuninck G, Boulianne N, De Serres G. Effectiveness of a mass immunization campaign using serogroup C meningococcal conjugate vaccine. JAMA. 2004;292:2491–2494. doi: 10.1001/jama.292.20.2491. [DOI] [PubMed] [Google Scholar]
- 71.Deceuninck G, Lefebvre B, Tsang R, et al. Impact of a mass vaccination campaign against serogroup B meningococcal disease in the Saguenay-Lac-Saint-Jean region of Quebec four years after its launch. Vaccine. 2019;37:4243–4245. doi: 10.1016/j.vaccine.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 72.Langley JM, MacDougall DM, Halperin BA, et al. Rapid surveillance for health events following a mass meningococcal B vaccine program in a university setting: a Canadian Immunization Research Network study. Vaccine. 2016;34:4046–4049. doi: 10.1016/j.vaccine.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 73.Andrews M, Frosst G, Spence J, et al. Outbreak of invasive meningococcal disease serogroup W in the Okanagan, British Columbia. In proceedings of the 2018 Canadian Immunization Conference, Ottawa, Canada, December 2018. https://cic-cci.ca/wp-content/uploads/2018/11/CIC18_Oral-Presentations.pdf. Accessed 24 June 2021.
- 74.De Wals P. Epidemiology and control of meningococcal disease in Canada: a long, complex, and unfinished story. Can J Infect Dis Med Microbiol. 2019;2019:8901847. doi: 10.1155/2019/8901847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chacon-Cruz E, Espinosa-De Los Monteros LE, Navarro-Alvarez S, et al. An outbreak of serogroup C (ST-11) meningococcal disease in Tijuana, Mexico. Ther Adv Vaccines. 2014;2:71–6. [DOI] [PMC free article] [PubMed]
- 76.Safadi MA, Carvalhanas TR, Paula de Lemos A, et al. Carriage rate and effects of vaccination after outbreaks of serogroup C meningococcal disease, Brazil, 2010. Emerg Infect Dis. 2014;20:806–11. [DOI] [PMC free article] [PubMed]
- 77.Cardoso CW, Pinto LL, Reis MG, Flannery B, Reis JN. Impact of vaccination during an epidemic of serogroup C meningococcal disease in Salvador. Brazil Vaccine. 2012;30:5541–5546. doi: 10.1016/j.vaccine.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 78.Miles TA, Lewis PR, Cook L, Bruderlin KI. An outbreak of meningococcal disease in a secondary school-implications for public health practice. Commun Dis Intell Q Rep. 2004;28:345–347. doi: 10.33321/cdi.2004.28.39. [DOI] [PubMed] [Google Scholar]
- 79.Robinson P, Taylor K, Tallis G, et al. An outbreak of serogroup C meningococcal disease associated with a secondary school. Commun Dis Intell Q Rep. 2001;25:121–125. doi: 10.33321/cdi.2001.25.24. [DOI] [PubMed] [Google Scholar]
- 80.Sudbury EL, O'Sullivan S, Lister D, Varghese D, Satharasinghe K. Case manifestations and public health response for outbreak of meningococcal W disease, Central Australia, 2017. Emerg Infect Dis. 2020;26:1355–1363. doi: 10.3201/eid2607.181941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taha MK, Gaudelus J, Deghmane AE, Caron F. Recent changes of invasive meningococcal disease in France: arguments to revise the vaccination strategy in view of those of other countries. Hum Vaccin Immunother. 2020:16(10):2518–23. [DOI] [PMC free article] [PubMed]
- 82.Arnold R, Galloway Y, McNicholas A, O'Hallahan J. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine. 2011;29:7100–7106. doi: 10.1016/j.vaccine.2011.06.120. [DOI] [PubMed] [Google Scholar]
- 83.Trotter CL, Cibrelus L, Fernandez K, et al. Response thresholds for epidemic meningitis in sub-Saharan Africa following the introduction of MenAfriVac®. Vaccine. 2015;33:6212–6217. doi: 10.1016/j.vaccine.2015.09.107. [DOI] [PubMed] [Google Scholar]
- 84.Cuevas LE, Savory EC, Hart CA, Thomson MC, Yassin MA. Effect of reactive vaccination on meningitis epidemics in Southern Ethiopia. J Infect. 2007;55:425–430. doi: 10.1016/j.jinf.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 85.Ferrari MJ, Fermon F, Nackers F, et al. Time is (still) of the essence: quantifying the impact of emergency meningitis vaccination response in Katsina State. Nigeria Int Health. 2014;6:282–290. doi: 10.1093/inthealth/ihu062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mounkoro D, Nikiema CS, Maman I, et al. Neisseria meningitidis serogroup W meningitis epidemic in Togo, 2016. J Infect Dis. 2019;220:S216–S224. doi: 10.1093/infdis/jiz330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Njanpop-Lafourcade BM, Hugonnet S, Djogbe H, et al. Mobile microbiological laboratory support for evaluation of a meningitis epidemic in Northern Benin. PLoS ONE. 2013;8:e68401. doi: 10.1371/journal.pone.0068401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leake JA, Kone ML, Yada AA, et al. Early detection and response to meningococcal disease epidemics in sub-Saharan Africa: appraisal of the WHO strategy. Bull World Health Organ. 2002;80:342–349. [PMC free article] [PubMed] [Google Scholar]
- 89.Cibrelus L, Medah I, Koussoubé D, et al. Serogroup W meningitis outbreak at the subdistrict level, Burkina Faso, 2012. Emerg Infect Dis. 2015;21:2063–2066. doi: 10.3201/eid2111.150304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Outbreak news. Meningococcal disease, African meningitis belt. Wkly Epidemiol Rec. 2009;84:117–8. [PubMed]
- 91.[Inquiry of meningococcal disease outbreak in São Paulo, July 2007]. Rev Saude Publica. 2007;41:873–8. [DOI] [PubMed]
- 92.Osuorah D, Shah B, Manjang A, et al. Outbreak of serotype W135 Neisseria meningitidis in central river region of the Gambia between February and June 2012: a hospital-based review of paediatric cases. Niger J Clin Pract. 2015;18:41–47. doi: 10.4103/1119-3077.146977. [DOI] [PubMed] [Google Scholar]
- 93.Webby R. Northern Territory Government Centre for Disease Control. Northern Territory meningococcal ACWY vaccination program rollout and coverage, June 2019 Disease Control Bulletin. 2019;26:1–2.
- 94.Ladhani SN, Andrews N, Parikh SR, et al. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med. 2020;382:309–317. doi: 10.1056/NEJMoa1901229. [DOI] [PubMed] [Google Scholar]
- 95.Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54:1–21. [PubMed] [Google Scholar]
- 96.Centers for Disease Control and Prevention Notice to Readers: Recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate Vaccine (MCV4) in children aged 2–10 years at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep. 2007;56:1265–1266. [Google Scholar]
- 97.Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11–18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2007;56:794–5. [PubMed]
- 98.Centers for Disease Control and Prevention (CDC). Licensure of a meningococcal conjugate vaccine for children aged 2 through 10 years and updated booster dose guidance for adolescents and other persons at increased risk for meningococcal disease–Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1018–9. [PubMed]
- 99.Centers for Disease Control and Prevention (CDC). Recommendation of the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MenACWY-D) among children aged 9 through 23 months at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep. 2011;60:1391–2. [PubMed]
- 100.MacNeil JR, Rubin L, McNamara L, et al. Use of MenACWY-CRM vaccine in children aged 2 through 23 months at increased risk for meningococcal disease: recommendations of the Advisory Committee on Immunization Practices, 2013. MMWR Morb Mortal Wkly Rep. 2014;63:527–530. [PMC free article] [PubMed] [Google Scholar]
- 101.Centers for Disease Control and Prevention (CDC). Updated recommendations for use of meningococcal conjugate vaccines - Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72–6. [PubMed]
- 102.MacNeil JR, Rubin L, Folaranmi T, et al. Use of serogroup B meningococcal vaccines in adolescents and young adults: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1171–1176. doi: 10.15585/mmwr.mm6441a3. [DOI] [PubMed] [Google Scholar]
- 103.Mbaeyi S, Bozio C, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MWR Morb Mortal Wkly Rep. 2020;69:1–41. doi: 10.15585/mmwr.rr6909a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marshall GS, Dempsey AF, Srivastava A, Isturiz RE. US college students are at increased risk for serogroup B meningococcal disease. J Pediatric Infect Dis Soc. 2020;9:244–247. doi: 10.1093/jpids/piz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robinson JL. Update on invasive meningococcal vaccination for Canadian children and youth. Paediatr Child Health. 2018;23:e1–e4. doi: 10.1093/pch/pxx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Danila RN, Bahta L. Recent outbreaks of meningococcal disease among men who have sex with men. Minn Med. 2015;98:47–48. [PubMed] [Google Scholar]
- 107.Australian Government , Department of Health. Clinical update: National Immunisation Program (NIP) schedule changes from 1 July 2020 – advice for vaccination providers. 3 June 2020. https://www.health.gov.au/news/clinical-update-national-immunisation-program-nip-schedule-changes-from-1-july-2020-advice-for-vaccination-providers. Accessed 24 June 2021
- 108.World Health Organization. Summary of WHO position paper on meningococcal A conjugate vaccine: updated guidance, February 2015. https://www.who.int/immunization/policy/position_papers/pp_menA_2015_summary.pdf?ua=1. Accessed 24 June 2021. [DOI] [PubMed]
- 109.Marshall HS, McMillan M, Koehler AP, et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med. 2020;382:318–327. doi: 10.1056/NEJMoa1900236. [DOI] [PubMed] [Google Scholar]
- 110.Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69:1109–1116. doi: 10.15585/mmwr.mm6933a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Schrijver K, Maes I. An outbreak of serogroup C meningococcal disease in the province of Antwerp (Belgium) in 2001–2002. Eur J Epidemiol. 2003;18:1073–1077. doi: 10.1023/a:1026100321871. [DOI] [PubMed] [Google Scholar]
- 112.Smith I, Lehmann AK, Lie L, et al. Outbreak of meningococcal disease in western Norway due to a new serogroup C variant of the ET-5 clone: effect of vaccination and selective carriage eradication. Epidemiol Infect. 1999;123:373–382. doi: 10.1017/s0950268899003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gilmore A, Jones G, Barker M, Soltanpoor N, Stuart JM. Meningococcal disease at the University of Southampton: outbreak investigation. Epidemiol Infect. 1999;123:185–192. doi: 10.1017/s0950268899002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chatt C, Gajraj R, Hawker J, et al. Four-month outbreak of invasive meningococcal disease caused by a rare serogroup B strain, identified through the use of molecular PorA subtyping, England, 2013. Euro Surveill. 2014;19:20949. doi: 10.2807/1560-7917.es2014.19.44.20949. [DOI] [PubMed] [Google Scholar]
- 115.Campbell H, Ladhani S. The importance of surveillance: group W meningococcal disease outbreak response and control in England. Int Health. 2016;8:369–371. doi: 10.1093/inthealth/ihw037. [DOI] [PubMed] [Google Scholar]
- 116.Clark SA, Lucidarme J, Angel G, et al. Outbreak strain characterisation and pharyngeal carriage detection following a protracted group B meningococcal outbreak in adolescents in South-West England. Sci Rep. 2019;9:9990. doi: 10.1038/s41598-019-46483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Conyn-van Spaendonck MA, Reintjes R, Spanjaard L, et al. Meningococcal carriage in relation to an outbreak of invasive disease due to Neisseria meningitidis serogroup C in the Netherlands. J Infect. 1999;39:42–48. doi: 10.1016/s0163-4453(99)90101-9. [DOI] [PubMed] [Google Scholar]
- 118.van der Ende A, Hopman CT, Keijzers WC, et al. Outbreak of meningococcal disease caused by PorA-deficient meningococci. J Infect Dis. 2003;187:869–871. doi: 10.1086/367899. [DOI] [PubMed] [Google Scholar]
- 119.Grodet C, Dequin PF, Watt S, et al. Outbreak in France of Neisseria meningitidis B:15:P1.12 belonging to sequence type 1403. Clin Microbiol Infect. 2004;10:845–848. doi: 10.1111/j.1469-0691.2004.00935.x. [DOI] [PubMed] [Google Scholar]
- 120.Delisle E, Larrieu S, Simões J, et al. Community outbreak of group B meningococcal disease in southwest France—December 2008 to September 2009. Euro Surveill. 2010;15:19665. [PubMed] [Google Scholar]
- 121.Caron F, du Chatelet IP, Leroy JP, et al. From tailor-made to ready-to-wear meningococcal B vaccines: longitudinal study of a clonal meningococcal B outbreak. Lancet Infect Dis. 2011;11:455–463. doi: 10.1016/S1473-3099(11)70027-5. [DOI] [PubMed] [Google Scholar]
- 122.Hauri AM, Ehrhard I, Frank U, et al. Serogroup C meningococcal disease outbreak associated with discotheque attendance during carnival. Epidemiol Infect. 2000;124:69–73. doi: 10.1017/s0950268899003416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stefanelli P, Fazio C, Vacca P, et al. An outbreak of severe invasive meningococcal disease due to a capsular switched Neisseria meningitidis hypervirulent strain B:cc11. Clin Microbiol Infect. 2019;25:111.e1–.e4. [DOI] [PubMed]
- 124.Doyle T, Mejia-Echeverry A, Fiorella P, et al. Cluster of serogroup W135 meningococci, Southeastern Florida, 2008–2009. Emerg Infect Dis. 2010;16:113–115. doi: 10.3201/eid1601.091026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mandal S, Wu HM, MacNeil JR, et al. Prolonged university outbreak of meningococcal disease associated with a serogroup B strain rarely seen in the United States. Clin Infect Dis. 2013;57:344–348. doi: 10.1093/cid/cit243. [DOI] [PubMed] [Google Scholar]
- 126.Centers for Disease Control and Prevention (CDC). Outbreak of meningococcal disease associated with an elementary school – Oklahoma, March 2010. MMWR Morb Mortal Wkly Rep. 2012;61:217–21. [PubMed]
- 127.Hao L, Holden MTG, Wang X, et al. Distinct evolutionary patterns of Neisseria meningitidis serogroup B disease outbreaks at two universities in the USA. Microb Genom. 2018;4:e000155. [DOI] [PMC free article] [PubMed]
- 128.Soeters HM, McNamara LA, Whaley M, et al. Serogroup B meningococcal disease outbreak and carriage evaluation at a college—Rhode Island, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:606–607. [PMC free article] [PubMed] [Google Scholar]
- 129.Biswas HH, Han GS, Wendorf K, et al. Notes from the field: outbreak of serogroup b meningococcal disease at a university—California, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:520–521. doi: 10.15585/mmwr.mm6520a3. [DOI] [PubMed] [Google Scholar]
- 130.Patrick DM, Champagne S, Goh SH, et al. Neisseria meningitidis carriage during an outbreak of serogroup C disease. Clin Infect Dis. 2003;37:1183–1188. doi: 10.1086/378743. [DOI] [PubMed] [Google Scholar]
- 131.Puricelli RC, Kupek E, Bertoncini Rde C. [Control of a community outbreak of group C meningococcal meningitis in Corupá, Santa Catarina State, Brazil, based on rapid and effective epidemiological surveillance and immunization] Cad Saude Publica. 2004;20:959–967. doi: 10.1590/s0102-311x2004000400010. [DOI] [PubMed] [Google Scholar]
- 132.Chadee D, Lee R, Ferdinand A, et al. Meningococcal meningitis outbreak in Trinidad, 1998. Eur J Gen Med. 2006;3:49–53. [Google Scholar]
- 133.Jardine A, Truman G, Sheppeard V, et al. A community outbreak of meningococcal serogroup B disease in western Sydney: the challenges of identification and significance. Commun Dis Intell Q Rep. 2009;33:221–224. doi: 10.33321/cdi.2009.33.23. [DOI] [PubMed] [Google Scholar]
- 134.Muttalif AR, Presa JV, Haridy H, et al. Incidence and prevention of invasive meningococcal disease in global mass gathering events. Infect Dis Ther. 2019;8:569–579. doi: 10.1007/s40121-019-00262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martin DR, Walker SJ, Baker MG, Lennon DR. New Zealand epidemic of meningococcal disease identified by a strain with phenotype B:4:P1.4. J Infect Dis. 1998;177:497–500. doi: 10.1086/517385. [DOI] [PubMed] [Google Scholar]
- 136.Nair D, Dawar R, Deb M, et al. Outbreak of meningococcal disease in and around New Delhi, India, 2005–2006: a report from a tertiary care hospital. Epidemiol Infect. 2009;137:570–576. doi: 10.1017/S0950268808001398. [DOI] [PubMed] [Google Scholar]
- 137.Kushwaha AS, Aggarwal SK, Arora MM. Outbreak of meningococcal infection amongst soldiers deployed in operations. Med J Armed Forces India. 2010;66:4–8. doi: 10.1016/S0377-1237(10)80082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Negi S, Grover S, Rautela S, et al. Direct detection and serogroup characterization of Neisseria meningitidis from outbreak of meningococcal meningitis in Delhi. Iran J Microbiol. 2010;2:73–79. [PMC free article] [PubMed] [Google Scholar]
- 139.Fourn L, Makoutodé M, Ouendo M, Tounkara B. Meningococcal meningitis epidemics in Benin. Sante. 2004;14:153–159. [PubMed] [Google Scholar]
- 140.Fernandez K, Lingani C, Aderinola OM, et al. Meningococcal meningitis outbreaks in the African meningitis belt after meningococcal serogroup a conjugate vaccine introduction, 2011–2017. J Infect Dis. 2019;220(Suppl 4):S225–S232. doi: 10.1093/infdis/jiz355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nathan N, Rose AM, Legros D, et al. Meningitis serogroup W135 outbreak, Burkina Faso, 2002. Emerg Infect Dis. 2007;13:920–923. doi: 10.3201/eid1306.060940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sié A, Pflüger V, Coulibaly B, et al. ST2859 serogroup A meningococcal meningitis outbreak in Nouna Health District, Burkina Faso: a prospective study. Trop Med Int Health. 2008;13:861–868. doi: 10.1111/j.1365-3156.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 143.Delrieu I, Yaro S, Tamekloe TA, et al. Emergence of epidemic Neisseria meningitidis serogroup X meningitis in Togo and Burkina Faso. PLoS One. 2011;6:e19513. [DOI] [PMC free article] [PubMed]
- 144.Outbreak news. Meningococcal disease in the African meningitis belt. Wkly Epidemiol Rec. 2008;83:90–1. [PubMed]
- 145.Meningitis in Chad Niger and Nigeria: 2009 epidemic season. Wkly Epidemiol Rec. 2010;85:47–63. [PubMed] [Google Scholar]
- 146.World Health Organization. Outbreak news. Meningicoccal disease. https://www.who.int/emergencies/disease-outbreak-news. Accessed 24 June 2021.
- 147.Hossain MJ, Roca A, Mackenzie GA, et al. Serogroup W135 meningococcal disease, the Gambia, 2012. Emerg Infect Dis. 2013;19:1507–1510. doi: 10.3201/eid1909.130077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leimkugel J, Hodgson A, Forgor AA, et al. Clonal waves of Neisseria colonisation and disease in the African meningitis belt: eight- year longitudinal study in northern Ghana. PLoS Med. 2007;4:e101. [DOI] [PMC free article] [PubMed]
- 149.Souleymane C, Abdelaye K, Ibrehima G, et al. Outcome of epidemiological surveillance of bacterial meningitis in Mali from 1996 to 2016: what lesson to learn? Mol Pathol Epidemiol. 2017;2:1–6. [Google Scholar]
- 150.Sanogo YO, Guindo I, Diarra S, et al. A new sequence type of Neisseria meningitidis serogroup C associated with a 2016 meningitis outbreak in Mali. J Infect Dis. 2019;220:S190–S197. doi: 10.1093/infdis/jiz272. [DOI] [PubMed] [Google Scholar]
- 151.Djibo S, Nicolas P, Alonso JM, et al. Outbreaks of serogroup X meningococcal meningitis in Niger 1995–2000. Trop Med Int Health. 2003;8:1118–1123. doi: 10.1046/j.1360-2276.2003.01126.x. [DOI] [PubMed] [Google Scholar]
- 152.Sidikou F, Zaneidou M, Alkassoum I, et al. Emergence of epidemic Neisseria meningitidis serogroup C in Niger, 2015: an analysis of national surveillance data. Lancet Infect Dis. 2016;16:1288–1294. doi: 10.1016/S1473-3099(16)30253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bassey BE, Vaz RG, Gasasira AN, et al. Pattern of the meningococcal meningitis outbreak in Northern Nigeria, 2009. Int J Infect Dis. 2016;43:62–67. doi: 10.1016/j.ijid.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 154.Funk A, Uadiale K, Kamau C, et al. Sequential outbreaks due to a new strain of Neisseria meningitidis serogroup C in northern Nigeria, 2013–14. PLoS Curr. 2014;6. [DOI] [PMC free article] [PubMed]
- 155.Kwambana-Adams BA, Amaza RC, Okoi C, et al. Meningococcus serogroup C clonal complex ST-10217 outbreak in Zamfara State. Northern Nigeria Sci Rep. 2018;8:14194. doi: 10.1038/s41598-018-32475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Price S. Talk to patients about: meningococcal disease. Tex Med. 2018;114:46. [PubMed] [Google Scholar]
- 157.Aguilera JF, Perrocheau A, Meffre C, Hahné S. Outbreak of serogroup W135 meningococcal disease after the Hajj pilgrimage, Europe, 2000. Emerg Infect Dis. 2002;8:761–767. doi: 10.3201/eid0808.010422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Klaber R, Booy R, El Bashir H, Mifsud A, Taylor S. Sustained outbreak of W135 meningococcal disease in east London. UK Lancet. 2002;360:644. doi: 10.1016/s0140-6736(02)09795-7. [DOI] [PubMed] [Google Scholar]
- 159.Wilder-Smith A, Goh KT, Barkham T, Paton NI. Hajj-associated outbreak strain of Neisseria meningitidis serogroup W135: estimates of the attack rate in a defined population and the risk of invasive disease developing in carriers. Clin Infect Dis. 2003;36:679–683. doi: 10.1086/367858. [DOI] [PubMed] [Google Scholar]
- 160.World Health Organization. Enhanced surveillance of epidemic meningococcal meningitis in Africa: a three-year experience. Wkly Epidemiol Rec. 2005;80:313–20. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.