Abstract
Tobacco smoking is a frequent problem affecting many kidney transplant (KT) candidates and recipients. The negative impact of active smoking on KT outcomes has been demonstrated. Consequently, most guidelines strongly recommend quitting smoking before considering kidney transplantation. However, nicotine addiction is a complex multifactorial disease and only 3–5% of the patients who try to quit by themselves achieve prolonged abstinence. Smoking cessation programmes (SCPs) have proven their efficacy in the general population to increase the rate of quitting and should therefore be proposed to all smoking KT candidates and recipients. Nevertheless, SCPs have not been evaluated in the KT field and not all KT centres have easy access to these programmes. In this work, we aim to review the current knowledge on the subject and provide an overview of the available interventions to help smoking patients quit. We detail non-pharmaceutical and pharmaceutical approaches and discuss their use in KT candidates and recipients.
Keywords: bupropion, kidney transplantation, nicotine replacement therapy, smoking cessation, varenicline
INTRODUCTION
Tobacco smoking is one of the major drivers of premature death and disability. It was responsible for 7.1 million deaths worldwide in 2017 [1]. In the kidney transplant (KT) field, there is high-quality evidence that smokers have poorer outcomes after transplantation compared with non-smokers [2–7]. Consequently, smoking cessation is strongly recommended in KT candidates and recipients [8–13].
Smoking cessation programmes (SCPs) have proven their efficacy and safety in young adults [14], in patients with cardiovascular diseases (CVDs) [15] and in patients with chronic obstructive pulmonary disease [16]. Three meta-analyses have confirmed the effectiveness of SCPs to aid smoking cessation [17–19]. Therefore the recently published Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [8] recommend offering SCPs to all KT candidates who are using tobacco products. Unfortunately, not all KT centres have access to SCPs [20]. The present work aims to review the current knowledge on this topic, detail available SCPs and discuss their application for smoking KT candidates/recipients.
EPIDEMIOLOGY OF SMOKING KT CANDIDATES/RECIPIENTS
Cigarette smoking is an independent risk factor for kidney failure (KF) [21, 22]. Moreover, chronic kidney disease (CKD) progression is accelerated by active smoking, most likely because of endothelial dysfunction caused by vascular production of reactive oxygen species, as well as transient increases in blood pressure accompanied by a decrease in both glomerular filtration rate and effective renal plasma flow [23–25]. Data derived from two Dialysis Outcomes and Practice Patterns Study analyses showed an incidence of active smokers in dialysis units of ∼15% [26, 27]. Rates of active smokers among KT candidates were reported at between 24% and 33%, with 90% who continue to smoke after KT [28, 29]. Moreover, it has been suggested that 12% of patients who stopped smoking before KT relapsed after [30]. A meta-analysis has shown that younger individuals, men and those with a lower body mass index were more likely to smoke after KT [3]. Other studies, however, suggest that these incidences might be lower. Indeed, Van Laecke et al. [31] reported in a single centre retrospective study including 1013 KT recipients (KTRs) an incidence of active smokers after transplantation of 7%. Also, a study including 4110 KTRs revealed an incidence of current smokers of 11% [32]. Though lower, these incidence rates are not optimal considering the well-established and highly unfavourable outcome of active smoking after kidney transplantation (see below). So there is room for improvement. Moreover, these incidences might be underestimated, as they arise mainly from self-reported smoking history, which depends on patient honesty. Thus a study aiming to confront the cotinine serum level (the gold standard for the detection of active smokers) and self-reported smoking history in a single-centre cohort of KT recipients showed that 34% of patients were diagnosed as current smokers despite claiming to be non-smokers [33].
IMPACT OF TOBACCO USE ON KTR OUTCOMES
Cigarette smoking has a dramatic impact on the outcome of KTRs. The correlation between post-KT CVD and cigarette smoking has been demonstrated. Kasiske et al. [34] showed that KTRs who smoke have a 90% increased risk of developing coronary artery disease. Ponticelli et al. [35] also showed that the risk of CVD is linked to the pack-years of smoking per year. Indeed, the relative risks for a major CVD event after KT were 1.5 and 2.14 in 11–25 and >25 pack-years smokers at transplantation, respectively. Active smoking KTRs are also more commonly affected by other atherogenic risk factors such as diabetes, hypertension and dyslipidaemia [36].
Active smoking negatively impacts allograft survival [34, 37–39], with reported relative risks of 1.3–2.3 for graft loss [37, 40]. Interestingly, quitting cigarette smoking for >5 years before KT was associated with a 34% relative risk reduction for graft failure [34]. In addition, although former smokers have increased long-term graft and death-censored graft loss rates compared with never smokers, this association is much stronger in patients who restarted or continued smoking after KT [31].
The mortality rate is also impacted by tobacco use in KTRs [5, 34, 41], with a 2.26-fold increased risk of death after KT [5]. However, the effect of cigarette smoking on mortality vanishes after 5 years of quitting [34].
Cigarette smoking is also a risk factor for post-KT invasive malignancies, mostly lung [34] and bladder cancers [42]. Cancer risk increases by 1.12 and 2.56 after 10 and 25 pack-years smoking history, respectively [43]. Cigarette smoking has also been associated with vascular renal problems such as fibrous intimal thickening of small arteries [44] and allograft rejection [28].
EXCLUSION OF SMOKERS FROM KT PROGRAMMES?
Some transplant programmes are very strict regarding smoking cessation and temporarily block active smokers from being listed [45, 46]. Indeed, growing evidence shows that not only KTRs, but also lung, liver and heart transplant recipients who smoke have poorer outcomes than non-smokers [3]. Several arguments could justify such a strict policy for smoking candidates. First, considering the scarcity of organs and the poor clinical outcomes associated with cigarette smoking, it may seem logical to allocate precious organs to patients who will benefit most—non-smoker or former smoker patients. Second, the perspective of organ transplantation is an important event in life. So, it can be postulated that smoker candidates will show high motivation to quit, especially if the demand is an official one for listing rather than a gentle suggestion. Stricter policies for organ transplantation for smoker candidates might lead to more frequent referral to SCPs, as reported for liver transplantation [47]. Another issue is the association between cigarette smoking and non-adherence that has been suggested in kidney transplantation [30] and heart transplantation [48]. This is a concern regarding the association between non-adherence and poorer allograft outcomes [49].
However, although cigarette cessation should undoubtedly be the ultimate goal for smoking in KT candidates, a systematic exclusion of patients who failed quitting might not be ethically justifiable, and the policies worldwide have progressively adapted their recommendations.
In patients with KF, the KDIGO international guidelines strongly recommend smoking cessation at least 1 month before waitlisting but do not call for excluding smokers from being transplanted [8]. Likewise, the Canadian guidelines consider patients who continue to smoke to be eligible for KT with full informed consent regarding their increased risk of poorer outcomes [12]. Nevertheless, KT centres around the world apply individual policies when considering smoking candidates for kidney transplantation. A US survey [20] revealed that only 38% of KT centres considered smoking as an absolute contraindication for waitlisting. When faced with this dilemma, many factors should be carefully weighed. First, survival of active smokers in dialysis versus active smokers after KT has not yet been addressed. Thus, despite worse post-KT outcomes in smokers compared with non-smokers, KT might still offer to active smokers a survival advantage compared with dialysis. Moreover, participating in active SCPs before listing can also be associated with adverse effects, such as prolonged waiting time for deceased-donor transplantation. Likhitsup et al. [47] recently showed that the modification of their tobacco policy in liver transplant candidates from restrictive (smoking cessation required only for patients with a history of CVD and lung disease) to prohibitive (smoking cessation required for all liver transplant candidates) led to a significant increase in the median time to listing from 65 to 122 days. Nevertheless, this has to be balanced with the health benefit after transplantation for smoking patients who quit [31] and the expected lower recurrence of active smoking if the demands for smoking cessation are more strictly enforced.
Second, smoking is detected by self-reporting in the vast majority of KT centres and not by measurement of serum/urine cotinine or exhaled carbon monoxide (CO) [20]. The sensitivity of self-reporting depends on patient honesty. Denying access to transplantation to honest patients while giving it to undisclosed smokers is unfair. Third, smoking cessation therapy increases the chances of smoking cessation but ∼25% of organ transplant centres do not have access to these programmes [20]. Fourth, non-adherence is a complex multifactorial problem and cigarette smoking has to be interpreted among many other behavioural risk factors for non-compliance [49, 50]. Moreover, caution is required in order not to stigmatize all smoking candidates as ‘future nonadherent patients’. For example, it can be hypothesized that a smoking candidate who has demonstrated his motivation to stop (by entering into an SCP for instance) and has failed quitting is less likely susceptible to be non-adherent than a smoking candidate who simply refuses to try quitting.
In summary, cigarette cessation should undoubtedly be the ultimate goal for smoking KT candidates, but, in our opinion, a systematic exclusion of patients who failed quitting is not ethically justifiable. Smoking cessation intervention can help and should be offered by transplant centres.
NICOTINE ADDICTION: A CHRONIC MULTIFACTORIAL DISEASE
Nicotine addiction is a multifactorial disease involving physical, psychological and behavioural dependence. Physical dependence is the need for a person to have a certain level of nicotinaemia in order to function properly. Below this level, withdrawal symptoms appear, including anhedonia, insomnia, craving, irritability, depressed mood, restlessness and anxiety [51]. Nicotine acts on the brain’s reward system, releasing dopamine after binding to its high-affinity nicotine cholinergic receptor. In regular smokers, the binding induces an increase in the number of nicotine binding sites, but the exact mechanisms of up-regulation remain unclear [52]. Physical dependence can be easily and practically evaluated by questionnaires like the Fargerström test [53], but also by the measurement of exhaled CO [54], carboxyhaemoglobin in the blood or serum/urinary/salivary cotinine (Table 1). However, cotinine values have to be interpreted carefully, especially in patients with KF. Indeed, cotinine is the major metabolite (70%) of nicotine and is primarily metabolized by the liver enzyme cytochrome P450 2A6 (CYP2A6). Compared with nicotine, cotinine has a longer half-life (15–19 versus 2–3 h) and is eliminated over a longer period of time [55]. Different assays are available and slightly differ in their diagnostic performance [55]. A recent study has shown a sensitivity of 99.5% with a cotinine urinary test to detect active smokers in the general population [56]. However, specificity was only ∼90%, meaning a 10% rate of false-positive results, secondary to mainly environmental tobacco smoke. If poorly investigated, it can be anticipated that the false-positive rate might be even higher in KT candidates with KF. Indeed, it has been demonstrated that KF is associated with decreased elimination of cotinine and higher levels in blood compared with healthy people with the same level of tobacco consumption [57]. Moreover, interindividual variability in plasma concentrations of nicotine and cotinine is important among individuals with similar kidney function taking similar doses of nicotine. Indeed, a number of CYP2A6 gene variants have been described, resulting in impaired or enhanced ability to metabolize nicotine [58]. In addition, some drugs can either inhibit (amiodarone, amlodipine, clofibrate, fenofibrate, isoniazid etc.) or induce (barbiturates, rifampicin) CYP2A6 [59] and consequently influence cotinine levels. Furthermore, diet, ethnicity, sex and contraceptive use can influence urinary cotinine and/or nicotine metabolism, especially in adolescence [60, 61]. Hence KT physicians should be aware of these issues, especially if cotinine measures have borderline values.
Table 1.
Evaluation of physical dependence
| Methods | Diagnostic thresholds |
|
1. How soon after you wake up do you smoke your first cigarette? (0) >60 min; (1) 31–60 min; (2) 6–30 min; (3) ≤5min 2. Do you find it difficult to refrain from smoking in places where it is forbidden? (0) No; (1) Yes 3. Which cigarette would you hate most to give up? (1) The first in the morning; (0) Any other 4. How many cigarettes per day do you smoke? (0) ≤10; (1) 11–20; (2) 21–30; (3) >30 5. Do you smoke more frequently during the first hours after awakening than during the rest of the day? (0) No; (1) Yes 6. Do you smoke even if you are so ill that you are in bed most of the day? (0) No; (1) Yes |
| Exhaled CO |
|
| Carboxyhaemoglobin | >1.7% in the blood: active smoker |
| Urinary cotinine |
|
Psychological dependence is mainly due to the relief of withdrawal symptoms during smoking. It gives the false belief to the smoker that smoking increases mood, concentration and performance [62]. Finally, conditioned behaviours of smokers are the third aspect of nicotine addiction. The smoker associates emotional, environmental and social stimuli with cigarette smoke, like after a meal, with a coffee or alcohol or sharing a moment with friends [63]. All these features of addiction should be considered in the SCP to avoid relapses.
SMOKING CESSATION PROGRAMME SCPs
Among smokers who try to quit without treatment, only 3–5% achieve a prolonged abstinence (for 6–12 months) [64]. Typically, SCPs offer a pluridisciplinary team, including doctors, nurses, social workers, psychologists and dieticians, who have access to drugs and medical facilities (at least exhaled CO measurement and cotinine measurement in blood and urine). SCPs have a cost, but are cost-effective, as there is strong evidence that cigarette smoking generates low productivity and smoking-attributable healthcare expenditures, affecting the patient and society. This specific subject has been recently summarized in a surgeon general’s report in 2020 [65]. In Belgium, tobacco specialist counselling is fully reimbursed (free for the patient). As it has a social purpose, financial issues should not limit access to SCPs.
Individual and group sessions are generally proposed. In our experience, KT candidates and recipients are usually referred by a nephrologist, but sometimes patients take the initiative on their own. Figure 1 proposes a practical clinical algorithm to take care of smoking KT candidates/recipients. Regular follow-up must be scheduled with the patient to monitor side effects and the efficacy of the treatment and to positively reinforce the motivations of the patient. In case of treatment failure, another therapeutic approach is proposed. The treatment options are detailed below.
FIGURE 1:
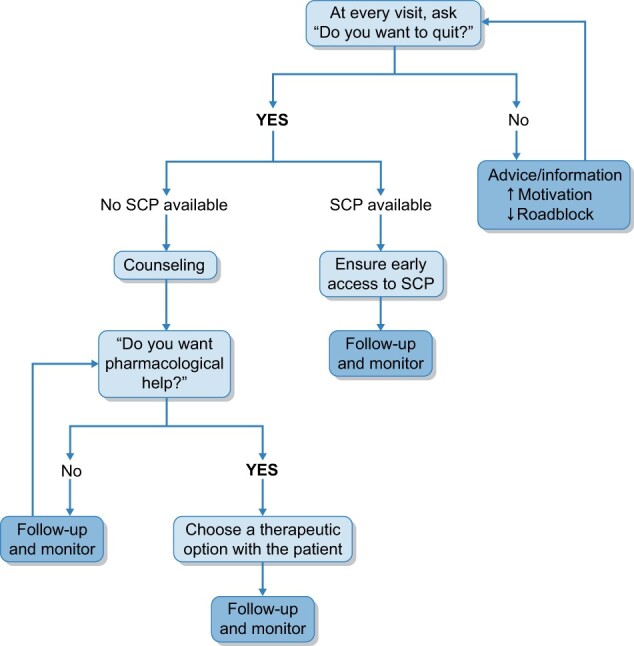
Practical clinical algorithm to take care of smoking KT candidates/recipients.
Counselling
In our centre, the smoking status of every KT candidate or recipient is assessed (by self-reporting) at every appointment. An SCP is offered to every smoking patient. The first approach of SCPs is usually non-pharmaceutical, using behavioural, motivational and cognitive interviewing of the patient (counselling). As a framework, the 5As (ask, assess, advise, assist, and arrange follow-up) is the gold standard intervention [66] (Table 2) and is efficient to increase the quitting rate [67]. After a general overview of the medical history (including medications), the smoking history is carefully reviewed: the smoking start date, the number of cigarettes smoked per day (to calculate the number of pack-years) and a typical day of the patient during the week and the weekend to evaluate smoking habits. Previous smoking cessation attempts are discussed; notably previous non-pharmacological methods, medications or side effects (like weight gain) are recorded. Polyaddiction (alcohol, coffee, soda, cannabis, cocaine, heroin etc.) is evaluated, as well as feelings of the ‘negative’ and ‘positive’ impacts of smoking for the patient, in order to remove false beliefs. Familial and professional status and physical and dietary habits are recorded. The physical, psychological and behavioural addiction to nicotine and motivation of the patient are evaluated via questionnaires available online [e.g. Richmond test (Tables 1 and 3)] [68] and through face-to-face contact. Anxiety and depression are tracked by the Hospital Anxiety Depression questionnaire, which helps in choosing the most appropriate medication (Figure 2) [69]. At the end of this first meeting, some tools and tips are given to the patient to aid his/her smoking cessation attempt (written advices, books, websites), notably to modify automatic behaviour and help the patient understand his/her physical and psychological addiction. A regular follow-up is then arranged (one visit per month, but this can be adapted to each patient). Then a pharmaceutical approach is generally proposed and must be adapted to the patient’s medical history and expectations. Indeed, our approach is to discuss with the patient the available products and choose with him/her the therapeutic option that would be most appropriate. All approved pharmacologic products improve the probability of quitting [70].
Table 2.
5A’s methods
| Ask | Systematically identify the smoking status at every visit |
|---|---|
| Advice | Provide a very brief, non-threatening recommendations to quit |
| Assess | Evaluate if the patient is ready to stop |
| Assist | Offer practical help for quitting |
| Arrange | Ensure the follow-up of the patient |
Table 3.
Richmond test
| 1. Would you like to quit smoking if you could do it easily? (0) No; (1) Yes 2. Do you really want to quit smoking? (0) Not a bit; (1) A little; (2) Moderately; (3) Very Much 3. Do you think that you can quit smoking in the following 2 weeks? (0) Not a bit; (1) A little; (2) Moderately; (3) Very Much 4. Do you think that you will still be a former smoker in 6 months? (0) Not a bit; (1) A little; (2) Moderately; (3) Very Much |
Interpretation:
|
FIGURE 2:
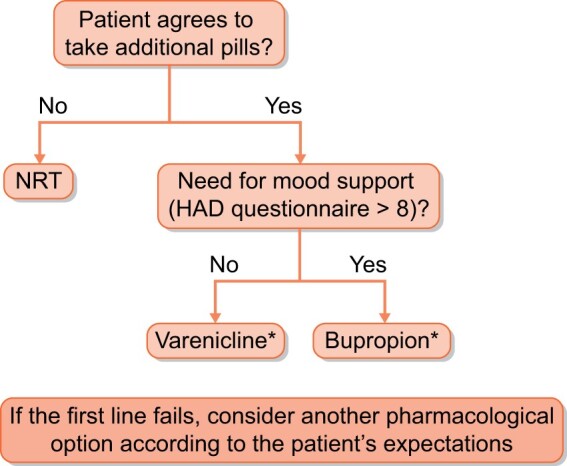
Local pharmacological management for smoking KT candidates and recipients.
Nicotine replacement therapy (NRT)
NRT has no contraindications and can be in a slow (like patches) or rapid delivery form (spray, gum, tablet and inhaler) [18]. Adverse effects include skin irritation from patches and mouth irritation from gums, inhalers and tablets. High-quality studies have shown that all forms of NRT increase the chance of quitting smoking by 50–60% [71].
Bupropion
Bupropion is an antidepressant that acts by inhibition of norepinephrine and dopamine reuptake [72]. Its efficiency is similar to NRT [73]. Its use is more difficult in daily practice due to drug interactions (Table 4), dose adjustments in CKD Stages 4 and 5 (Table 4) and adverse effects (including dry mouth, rash, headache, dizziness, sleep disorder) [73]. Moreover, previous risk of epilepsy, bipolar disorder, severe liver cirrhosis and the use of monoamine oxidase inhibitors are absolute contraindications for this drug.
Table 4.
Dose adjustment and drug interactions with pharmacologic drugs used for smoking cessation
| NRT | Bupropion | Cytisine | Varenicline | |
|---|---|---|---|---|
| GFR: >50 mL/min | No adjustment | No adjustment | No adjustment | No adjustment |
| GFR: 50–30 mL/min | No adjustment | No adjustment | No data | No adjustment |
| GFR: <30 mL/min | No adjustment | Maximum 150 mg 1×/day | No data | Maximum 1 mg 1×/day |
| Drug interactions | No interaction |
|
Caution with anti-tuberculosis medications; clozapine; ropinirole; oral contraception | Cimetidine |
Nicotinic cholinergic receptor partial agonist: varenicline and cytisine
The third category includes nicotinic cholinergic receptor partial agonists: varenicline and cytisine. Cytisine is widely used in Eastern Europe [74] and seems to be more efficient than placebo [75] and NRT [76] for smoking cessation. However, a recent placebo-controlled trial did not support its efficacy in tuberculosis patients [77]. Varenicline is the most effective available drug on the market (even though no studies comparing cytisine and varenicline are available). There is evidence that it enhances the chances of successful long-term smoking cessation between 2- and 3-fold [78]. The main side effects of cytisine and varenicline are nausea, vomiting and sleep disorders. Varenicline was initially feared to increase the risk of depression and suicide [79], but the Evaluating Adverse Events in a Global Smoking Cessation Study and meta-analysis have shown that even in psychiatric patients, neither varenicline, bupropion nor NRT caused more psychiatric events than a placebo [80, 81].
All these drugs (with the exception of cystisine, as there are no data) can be used in patients with CKD (Table 4). However, only scarce data are available for chronic dialysed patients (Table 5) [82–84]. Varenicline is exclusively excreted by the kidney (minimally metabolized) and has almost no drug interactions (except for cimetidine). The dose should be reduced only in severe renal failure and the concomitant administration of cimetidine should be avoided because it induces reduced renal clearance of varenicline [85]. There are no published data about their use in KTRs or potential interactions with immunosuppressive drugs. However, their metabolism (Table 5) makes this possibility unlikely.
Table 5.
Dose adjustment in dialysed patients
| Drug | Metabolism | PD | CVVH | HD-HDF |
|---|---|---|---|---|
| Bupropion |
|
|
|
|
| Cytisine | Renal elimination | No data | No data | No data |
| Varenicline | Renal elimination |
|
|
|
| Nicotine |
|
Not dialysed | Not dialysed | Not dialysed |
CVVH, continuous veno-venous haemofiltration; HD, haemodialysis; HDF, haemodiafiltration; PD, peritoneal dialysis.
In our centre, the first-line treatment is individualized for every patient according to his/her expectations and clinical situation. Figure 2 depicts our local pharmacological management for smoking patients (applicable for both candidates and recipients). As cytisine is not currently available in our country, it is not included in the algorithm. In our experience, 5% of our patients transplanted with a kidney in the last 2 years are followed in our SCP, of whom 80% achieved prolonged cessation. All were treated with varenicline without any side effects or drug interactions (especially with immunosuppressive drugs).
A place for electronic cigarette and heat-not-burn products?
The European, American and Australian scientific societies do not support the use of electronic cigarette (e-cigarette) and heat-not-burn products for smoking cessation [86–89] and are against their recreational use by youths and young adults. E-cigarettes were reported to be 2 times more effective than NRT for smoking cessation with behavioural support at 52 weeks [90]. However, 80% of e-cigarette users continued their use at 52 weeks, compared with 9% in the NRT group [90], suggesting that the nicotine addiction was not resolved. Adding e-cigarettes to nicotine patches slightly increases the rate of abstinence versus patches alone [91]. But no difference for long-term abstinence was observed in studies comparing nicotine e-cigarettes plus counselling versus counselling alone [92] and nicotine e-cigarettes versus NRT [93]. Consequently the use of e-cigarettes in SCPs is currently not recommended [94]. Furthermore, although the long-term effects are unknown, short-term respiratory side effects of e-cigarettes (life-threatening e-cigarette or vaping-associated lung injury) have been described in the USA [95], Europe [96] and the UK [97]. Finally, some animal studies have shown that e-cigarette refill liquid is nephrotoxic in rats [98]. In summary, we do not propose e-cigarettes in KT candidates/recipients because of all these uncertainties and the lack of data regarding its use in these patients.
Other interventions
Numerous technological interventions (websites, applications, SMS, video games, social media) are emerging on the market to help smokers quit. Websites [99] offer free applications to support smokers. Advancing faster than the evidence, the efficacy seems moderate (also due to low engagement), and probably lower than the medications, but may help some smokers [100, 101]. Taylor et al. [102] reviewed 68 randomized controlled trials (some of them with a high risk of bias), suggesting that interactive and tailored Internet-based interventions are moderately more effective than non-active controls at ≥6 months.
CONCLUSION
Smoking has a negative impact on kidney graft outcomes and patient survival after kidney transplantation. Therefore smoking cessation is strongly recommended in KT candidates and recipients. However, nicotine addiction is complex and the rate of successful prolonged abstinence without any intervention is dramatically low. Different therapeutic approaches for smoking patients are available and have proven their efficacy. They should be offered whenever possible to all KT candidates/recipients suffering from smoking addiction.
FUNDING
None.
AUTHORS’ CONTRIBUTIONS
A.D. and S.G. developed the concept and design of the research and drafted and wrote the manuscript. A.R. provided the data for dialysed patients. N.K. revised and edited the manuscript. All authors approved the final version.
CONFLICT OF INTEREST STATEMENT
S.G. declares congress travel fees and educational events from Pfizer (payment to her institution) and drug samples for patients from Johnson & Johnson and Omega. A.D., A.R. and N.K. declare no conflicts of interest.
Contributor Information
Arnaud Devresse, Nephrology Department, Cliniques Universitaires Saint-Luc, Brussels, Belgium; Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, Belgium.
Sophie Gohy, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, Belgium; Pneumology Department, Cliniques Universitaires Saint-Luc, Brussels, Belgium; Cystic Fibrosis Reference Center, Cliniques Universitaires Saint-Luc, Brussels, Belgium; Pole of Pneumology, ENT and Dermatology, Université Catholique de Louvain, Brussels, Belgium.
Arnaud Robert, Nephrology Department, Cliniques Universitaires Saint-Luc, Brussels, Belgium.
Nada Kanaan, Nephrology Department, Cliniques Universitaires Saint-Luc, Brussels, Belgium; Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, Belgium.
REFERENCES
- 1. GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1923–1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thomsen T, Villebro N, Møller AM.. Interventions for preoperative smoking cessation. Cochrane Database Syst Rev 2014; 3: CD002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duerinckx N, Burkhalter H, Engberg SJet al. Correlates and outcomes of posttransplant smoking in solid organ transplant recipients: a systematic literature review and meta-analysis. Transplantation 2016; 100: 2252–2263 [DOI] [PubMed] [Google Scholar]
- 4. Nourbala MH, Nemati E, Rostami Zet al. Impact of cigarette smoking on kidney transplant recipients: a systematic review. Iran J Kidney Dis 2011; 5: 141–148 [PubMed] [Google Scholar]
- 5. Corbett C, Armstrong MJ, Neuberger J.. Tobacco smoking and solid organ transplantation. Transplantation 2012; 94: 979–987 [DOI] [PubMed] [Google Scholar]
- 6. Weinrauch LA, Claggett B, Liu Jet al. Smoking and outcomes in kidney transplant recipients: a post hoc survival analysis of the FAVORIT trial. Int J Nephrol Renovasc Dis 2018; 11: 155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anis KH, Weinrauch LA, D'Elia JA.. Effects of smoking on solid organ transplantation outcomes. Am J Med 2019; 132: 413–419 [DOI] [PubMed] [Google Scholar]
- 8. Chadban SJ, Ahn C, Axelrod DAet al. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation 2020; 104: S11–S103 [DOI] [PubMed] [Google Scholar]
- 9. Dudley C, Harden P.. Renal Association clinical practice guideline on the assessment of the potential kidney transplant recipient. Nephron Clin Pract 2011; 118(Suppl 1): c209–c224 [DOI] [PubMed] [Google Scholar]
- 10. Campbell S, Pilmore H, Gracey Det al. KHA-CARI guideline: recipient assessment for transplantation. Nephrology (Carlton) 2013; 18: 455–462 [DOI] [PubMed] [Google Scholar]
- 11. Kasiske BL, Cangro CB, Hariharan Set al. The evaluation of renal transplantation candidates: clinical practice guidelines. Am J Transplant 2001; 1: 3–95 [PubMed] [Google Scholar]
- 12. Knoll G, Cockfield S, Blydt-Hansen Tet al. Canadian Society of Transplantation: consensus guidelines on eligibility for kidney transplantation. Can Med Assoc J 2005; 173: S1–S25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abramowicz D, Cochat P, Claas FHJet al. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dial Transplant 2015; 30: 1790–1797 [DOI] [PubMed] [Google Scholar]
- 14. Suls JM, Luger TM, Curry SJet al. Efficacy of smoking-cessation interventions for young adults: a meta-analysis. Am J Prev Med 2012; 42: 655–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suissa K, Larivière J, Eisenberg MJet al. Efficacy and safety of smoking cessation interventions in patients with cardiovascular disease: a network meta-analysis of randomized controlled trials. Circ Cardiovasc Qual Outcomes 2017; 10: e002458. [DOI] [PubMed] [Google Scholar]
- 16. Ruiz CAJ, Pinedo AR, Guerrero ACet al. Characteristics of COPD smokers and effectiveness and safety of smoking cessation medications. Nicotine Tob Res 2012; 14: 1035–1039 [DOI] [PubMed] [Google Scholar]
- 17. Silagy C, Mant D, Fowler Get al. Meta-analysis on efficacy of nicotine replacement therapies in smoking cessation. Lancet 1994; 343: 139–142 [DOI] [PubMed] [Google Scholar]
- 18. Stead LF, Perera R, Bullen Cet al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2008; 1: CD000146. [DOI] [PubMed] [Google Scholar]
- 19. Strassmann R, Bausch B, Spaar Aet al. Smoking cessation interventions in COPD: a network meta-analysis of randomised trials. Eur Respir J 2009; 34: 634–640 [DOI] [PubMed] [Google Scholar]
- 20. Cote DR, Chirichella TJ, Noon KAet al. Abdominal organ transplant center tobacco use policies vary by organ program type. Transplant Proc 2016; 48: 1920–1926 [DOI] [PubMed] [Google Scholar]
- 21. Xia J, Wang L, Ma Zet al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant 2017; 32: 475–487 [DOI] [PubMed] [Google Scholar]
- 22. Choi HS, Han KD, Oh TRet al. Smoking and risk of incident end-stage kidney disease in general population: a nationwide population-based cohort study from Korea. Sci Rep 2019; 9: 19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orth SR, Hallan SI.. Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients—absence of evidence or evidence of absence? Clin J Am Soc Nephrol 2008; 3: 226–236 [DOI] [PubMed] [Google Scholar]
- 24. Raij L, DeMaster EG, Jaimes EA.. Cigarette smoke-induced endothelium dysfunction: role of superoxide anion. J Hypertens 2001; 19: 891–897 [DOI] [PubMed] [Google Scholar]
- 25. Halimi JM, Philippon C, Mimran A.. Contrasting renal effects of nicotine in smokers and non-smokers. Nephrol Dial Transplant 1998; 13: 940–944 [DOI] [PubMed] [Google Scholar]
- 26. Rajagopalan S, Dellegrottaglie S, Furniss ALet al. Peripheral arterial disease in patients with end-stage renal disease: observations from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Circulation 2006; 114: 1914–1922 [DOI] [PubMed] [Google Scholar]
- 27. Combe C, Albert JM, Bragg-Gresham JLet al. The burden of amputation among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2009; 54: 680–692 [DOI] [PubMed] [Google Scholar]
- 28. Sung RS, Althoen M, Howell TAet al. Excess risk of renal allograft loss associated with cigarette smoking. Transplantation 2001; 71: 1752–1757 [DOI] [PubMed] [Google Scholar]
- 29. Nogueira JM, Haririan A, Jacobs SCet al. Cigarette smoking, kidney function, and mortality after live donor kidney transplant. Am J Kidney Dis 2010; 55: 907–915 [DOI] [PubMed] [Google Scholar]
- 30. Yavuz A, Tuncer M, Gürkan Aet al. Cigarette smoking in renal transplant recipients. Transplant Proc 2004; 36: 108–110 [DOI] [PubMed] [Google Scholar]
- 31. Van Laecke S, Nagler EV, Peeters Pet al. Former smoking and early and long-term graft outcome in renal transplant recipients: a retrospective cohort study. Transpl Int 2017; 30: 187–195 [DOI] [PubMed] [Google Scholar]
- 32. Weinrauch LA, Claggett B, Liu Jet al. Smoking and outcomes in kidney transplant recipients: a post hoc survival analysis of the FAVORIT trial. Int J Nephrol Renovasc Dis 2018; 11: 155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nguyen PTH, Galanti L, Pirson Yet al. Identification of current smokers among renal transplant recipients. Nephrol Dial Transplant 2007; 22: 1974–1978 [DOI] [PubMed] [Google Scholar]
- 34. Kasiske BL, Klinger D.. Cigarette smoking in renal transplant recipients. J Am Soc Nephrol 2000; 11: 753–759 [DOI] [PubMed] [Google Scholar]
- 35. Ponticelli C, Villa M, Cesana Bet al. Risk factors for late kidney allograft failure. Kidney Int 2002; 62: 1848–1854 [DOI] [PubMed] [Google Scholar]
- 36. Kheradmand A, Shahbazian H.. The role of pretransplant smoking on allograft survival in kidney recipients. Urol J 2005; 2: 36–39 [PubMed] [Google Scholar]
- 37. Lin S, Koford JK, Baird BCet al. Effect of donors' intravenous drug use, cigarette smoking, and alcohol dependence on kidney transplant outcome. Transplantation 2005; 80: 482–486 [DOI] [PubMed] [Google Scholar]
- 38. Cosio FG, Falkenhain ME, Pesavento TEet al. Patient survival after renal transplantation: II. The impact of smoking. J Clin Transplant 1999; 13: 336–341 [DOI] [PubMed] [Google Scholar]
- 39. Agarwal PK, Hellemons ME, Zelle DMet al. Smoking is a risk factor for graft failure and mortality after renal transplantation. Am J Nephrol 2011; 34: 26–31 [DOI] [PubMed] [Google Scholar]
- 40. Hurst FP, Altieri M, Patel PPet al. Effect of smoking on kidney transplant outcomes: analysis of the United States Renal Data System. Transplantation 2011; 92: 1101–1107 [DOI] [PubMed] [Google Scholar]
- 41. Underwood PW, Sheetz KH, Cron DCet al. Cigarette smoking in living kidney donors: donor and recipient outcomes. Clin Transplant 2014; 28: 419–422 [DOI] [PubMed] [Google Scholar]
- 42. Liu S, Chaudhry MR, Berrebi AAet al. Polyomavirus replication and smoking are independent risk factors for bladder cancer after renal transplantation. Transplantation 2017; 101: 1488–1494 [DOI] [PubMed] [Google Scholar]
- 43. Danpanich E, Kasiske BL.. Risk factors for cancer in renal transplant recipients. Transplantation 1999; 68: 1859–1864 [DOI] [PubMed] [Google Scholar]
- 44. Zitt N, Kollerits B, Neyer Uet al. Cigarette smoking and chronic allograft nephropathy. Nephrol Dial Transplant 2007; 22: 3034–3039 [DOI] [PubMed] [Google Scholar]
- 45. Weill D, Benden C, Corris PAet al. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015; 34: 1–15 [DOI] [PubMed] [Google Scholar]
- 46. Mehra MR, Canter CE, Hannan MMet al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant 2016; 35: 1–23 [DOI] [PubMed] [Google Scholar]
- 47. Likhitsup A, Hassan A, Mellinger Jet al. Impact of a prohibitive versus restrictive tobacco policy on liver transplant candidate outcomes. Liver Transpl 2019; 25: 1165–1176 [DOI] [PubMed] [Google Scholar]
- 48. Denhaerynck K, Berben L, Fabienne Dobbels Fet al. Multilevel factors are associated with immunosuppressant nonadherence in heart transplant recipients: the International BRIGHT study. Am J Transplant 2018; 18: 1447–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gokoel SRM, Gombert-Handoko KB, Zwart TCet al. Medication non-adherence after kidney transplantation: a critical appraisal and systematic review. Transplant Rev (Orlando) 2020; 34: 100511. [DOI] [PubMed] [Google Scholar]
- 50. Kuypers DRJ. From nonadherence to adherence. Transplantation 2020; 104: 1330–1340 [DOI] [PubMed] [Google Scholar]
- 51. Benowitz NL. Nicotine addiction. N Engl J Med 2010; 362: 2295–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Govind AP, Vezina P, Green WN.. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol 2009; 78: 756–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heatherton TF, Kozlowski LT, Frecker RCet al. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991; 86: 1119–1127 [DOI] [PubMed] [Google Scholar]
- 54. Seijo-Bestilleiro R, Seoane-Pillado T, Pertega-Diaz Set al. Randomized clinical trial to determine the effectiveness of CO-oximetry and anti-smoking brief advice in a cohort of kidney transplant patients who smoke. Int J Med Sci 2020; 17: 2673–2684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Florescu A, Ferrence R, Einarson Tet al. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit 2009; 31: 14–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Achilihu H, Feng J, Wang Let al. Tobacco use classification by inexpensive urinary cotinine immunoassay test strips. J Anal Toxicol 2019; 43: 149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perry RJ, Griffiths W, Dextraze Pet al. Elevated nicotine levels in patients undergoing hemodialysis: a role in cardiovascular mortality and morbidity? Am J Med 1984; 76: 241–246 [DOI] [PubMed] [Google Scholar]
- 58. Malaiyandi V, Sellers EM, Tyndale RF.. Implications of CYP2A6 genetic variation for smoking behaviors and nicotine dependence. Clin Pharmacol Ther 2005; 77: 145–158 [DOI] [PubMed] [Google Scholar]
- 59. Lacy CF, Armstrong LL, Goldman MPet al. Cytochrome P450 Enzymes: Substrates, Inhibitors, and Inducers. Hudson, OH: LexiComp, 2007:1899–1912 [Google Scholar]
- 60. Kandel DB, Hu MC, Schaffran Cet al. Urine nicotine metabolites and smoking behavior in a multiracial/multiethnic national sample of young adults. Am J Epidemiol 2007; 165: 901–910 [DOI] [PubMed] [Google Scholar]
- 61. Davis RA, Stiles MF, deBethizy JDet al. Dietary nicotine: a source of urinary cotinine. Food Chem Toxicol 1991; 29: 821–882 [DOI] [PubMed] [Google Scholar]
- 62. Pasetes SV, Ling PM, Apollonio DE.. Cognitive performance effects of nicotine and industry affiliation: a systematic review. Subst Abuse 2020; 14: 1178221820926545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Olausson P, Jentsch JD, Taylor JR.. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004; 173: 98–104 [DOI] [PubMed] [Google Scholar]
- 64. Hughes JR, Keely J, Naud S.. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99: 29–38 [DOI] [PubMed] [Google Scholar]
- 65. U.S. Public Health Service, Office of the Surgeon General. Smoking Cessation: A Report of the Surgeon General. https://www.cdc.gov/tobacco/data_statistics/sgr/2020-smoking-cessation/index.html (14 March 2021, date last accessed)
- 66. 2008 PHS Guideline Update Panel, Liaisons, and Staff. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir Care 2008; 53: 1217–1222 [PubMed] [Google Scholar]
- 67. Lancaster T, Stead LF.. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2017; 3: CD001292. [DOI] [PubMed] [Google Scholar]
- 68. Richmond RL, Kehoe LA, Webster IW.. Multivariate models for predicting abstention following intervention to stop smoking by general practitioners. Addiction 1993; 88: 1127–1135 [DOI] [PubMed] [Google Scholar]
- 69. Zigmond AS, Snaith RP.. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370 [DOI] [PubMed] [Google Scholar]
- 70. Cahill K, Lancaster T.. Workplace interventions for smoking cessation. Cochrane Database Syst Rev 2014; 2: CD003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hartmann-Boyce J, Chepkin SC, Ye Wet al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev 2018; 5: CD000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stahl SM, Pradko JF, Haight BRet al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry 2004; 6: 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Howes S, Hartmann-Boyce J, Livingstone-Banks Jet al. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2020; 4: CD000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zatoński W, Zatoński M.. Cytisine versus nicotine for smoking cessation. N Engl J Med 2015; 372: 1072. [DOI] [PubMed] [Google Scholar]
- 75. West R, Zatonski W, Cedzynska Met al. Placebo-controlled trial of cytisine for smoking cessation. N Engl J Med 2011; 365: 1193–1200 [DOI] [PubMed] [Google Scholar]
- 76. Walker N, Howe C, Glover Met al. Cytisine versus nicotine for smoking cessation. N Engl J Med 2014; 371: 2353–2362 [DOI] [PubMed] [Google Scholar]
- 77. Dogar O, Keding A, Gabe Ret al. Cytisine for smoking cessation in patients with tuberculosis: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet Glob Health 2020; 8: e1408–e1417 [DOI] [PubMed] [Google Scholar]
- 78. Cahill K, Lindson-Hawley N, Thomas KHet al. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2016; 5: CD006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jiménez-Ruiz C, Berlin I, Hering T.. Varenicline: a novel pharmacotherapy for smoking cessation. Drugs 2009; 69: 1319–1338 [DOI] [PubMed] [Google Scholar]
- 80. Anthenelli RM, Benowitz NL, West Ret al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet 2016; 387: 2507–2520 [DOI] [PubMed] [Google Scholar]
- 81. Thomas KH, Martin RM, Knipe DWet al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ 2015; 350: h1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Formanek P, Salisbury-Afshar E, Afshar M.. Helping patients with ESRD and earlier stages of CKD to quit smoking. Am J Kidney Dis 2018; 72: 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jeong SH, Newcombe D, Sheridan Jet al. Pharmacokinetics of cytisine, an α4 β2 nicotinic receptor partial agonist, in healthy smokers following a single dose. Drug Test Anal 2015; 7: 475–482 [DOI] [PubMed] [Google Scholar]
- 84. Svensson CK. Clinical pharmacokinetics of nicotine. Clin Pharmacokinet 1987; 12: 30–40 [DOI] [PubMed] [Google Scholar]
- 85. Feng B, Obach RS, Burstein AHet al. Effect of human renal cationic transporter inhibition on the pharmacokinetics of varenicline, a new therapy for smoking cessation: an in vitro-in vivo study. Clin Pharmacol Ther 2008; 83: 567–576 [DOI] [PubMed] [Google Scholar]
- 86. McDonald CF, Jones S, Beckert Let al. Electronic cigarettes: a position statement from the Thoracic Society of Australia and New Zealand. Respirology 2020; 25: 1082–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bals R, Boyd J, Esposito Set al. Electronic cigarettes: a task force report from the European Respiratory Society. Eur Respir J 2019; 53: 1801151. [DOI] [PubMed] [Google Scholar]
- 88. American Thoracic Society. Vaping: The Threat to Public Healthand the ATS Response. 2021. https://www.thoracic.org/professionals/clinical-resources/disease-related-resources/vaping-the-threat-to-public-health-and-the-ats-response.php (28 January 2021, date last accessed)
- 89. International Association for the Study of Lung Cancer. IASLC Policy Statement – Electronic Cigarettes. https://www.iaslc.org/iaslc-news/press-release/iaslc-policy-statement-electronic-cigarettes (28 January 2021, date last accessed)
- 90. Hajek P, Phillips-Waller A, Przulj Det al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019; 380: 629–637 [DOI] [PubMed] [Google Scholar]
- 91. Walker N, Parag V, Wong SFet al. Use of e-cigarettes and smoked tobacco in youth aged 14–15 years in New Zealand: findings from repeated cross-sectional studies (2014-19). Lancet Public Health 2020; 5: e204–e212 [DOI] [PubMed] [Google Scholar]
- 92. Eisenberg MJ, Hébert-Losier A, Windle SBet al. Effect of e-cigarettes plus counseling vs counseling alone on smoking cessation: a randomized clinical trial. JAMA 2020; 324: 1844–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Bullen C, Howe C, Laugesen Met al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382: 1629–1637 [DOI] [PubMed] [Google Scholar]
- 94. Hartmann-Boyce J, McRobbie H, Lindson Net al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 2020; 10: CD010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Layden JE, King BA, Meiman J.. Pulmonary illness related to e-cigarette use. Reply. N Engl J Med 2020; 382: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Marlière C, De Greef J, Gohy Set al. Fatal e-cigarette or vaping associated lung injury (EVALI): a first case report in Europe. Eur Respir J 2020; 56: 2000077. [DOI] [PubMed] [Google Scholar]
- 97. Nair N, Hurley M, Gates Set al. Life-threatening hypersensitivity pneumonitis secondary to e-cigarettes. Arch Dis Child 2020; 105: 1114–1116 [DOI] [PubMed] [Google Scholar]
- 98. El Golli N, Jrad-Lamine A, Neffati Het al. Impact of e-cigarette refill liquid exposure on rat kidney. Regul Toxicol Pharmacol 2016; 77: 109–116 [DOI] [PubMed] [Google Scholar]
- 99. Smokefree.gov. https://smokefree.gov/ (14 April 2021, date last accessed)
- 100. Wang MP, Luk TT, Wu Yet al. Chat-based instant messaging support integrated with brief interventions for smoking cessation: a community-based, pragmatic, cluster-randomised controlled trial. Lancet Digit Health 2019; 1: e183–e192 [DOI] [PubMed] [Google Scholar]
- 101. Das S, Tonelli M, Ziedonis D.. Update on smoking cessation: e-cigarettes, emerging tobacco products trends, and new technology-based interventions. Curr Psychiatry Rep 2016; 18: 51. [DOI] [PubMed] [Google Scholar]
- 102. Taylor GMJ, Dalili MN, Semwal Met al. Internet-based interventions for smoking cessation. Cochrane Database Syst Rev 2017; 9: CD007078. [DOI] [PMC free article] [PubMed] [Google Scholar]


