Following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, the large majority of haemodialysis (HD) patients rapidly seroconvert [1–3], with robust and durable serological responses up to 10 months [4]. These observations appear non-sequitur in view of the well-known immune dysfunction of HD patients, resulting in slower viral clearance and higher case fatality rates of SARS-CoV-2 infection, as well as impaired responses to SARS-CoV-2 vaccination [5]. A potential explanation may be that dialysis patients develop more severe disease with prolonged viral shedding and higher levels of pro-inflammatory cytokines, resulting in more intense immune stimulation. In a study of 2215 HD patients, older and diabetic patients had slightly higher antibody levels, but data on disease severity were not available [6].
In the general population, robust T-cell responses have been observed in asymptomatic patients even in the absence of detectable antibodies, suggesting that T-cell immunity develops independently of disease severity [7]. A cohort of 14 HD patients, sampled a mean of 40 days after coronavirus disease 2019 diagnosis, displayed T-cell responses of similar intensity to those of patients with normal renal function [8]. Currently there are no data on the durability and correlation with disease severity of T-cell immunity in HD patients.
Of the 569 patients on HD in March 2021, 41 had a history of polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infection, of which 24 had asymptomatic or mild disease and 17 had severe disease requiring hospitalization (Supplementary data, Table S1). Patients were stratified by days between the PCR test and blood sampling into Period 1 [mean 348 days (range 327–378)] and Period 2 [mean 125 days (range 48–200)].
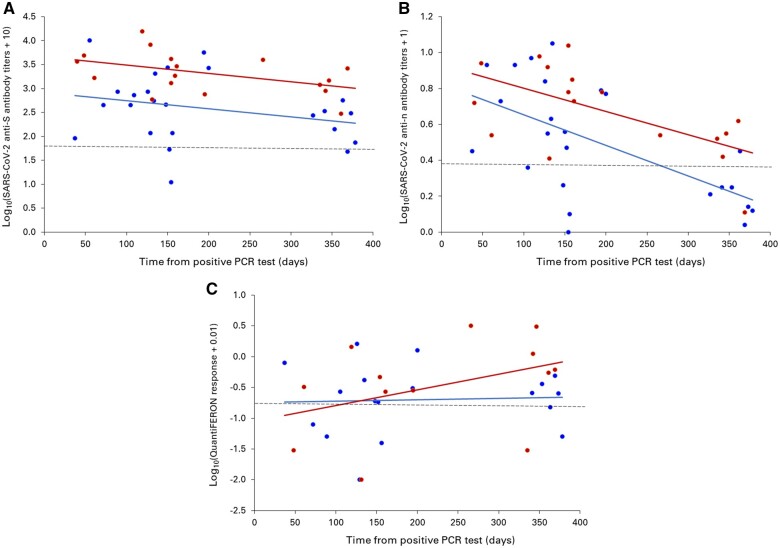
Serological responses persisted up to >1 year, with a faster decay of anti-nucleocapsid protein (anti-N) immunoglobulin G (IgG) than anti-spike protein (anti-S) IgG (Figure 1A and B). Patients with severe disease had higher antibody levels (Table 1), but the decay trajectory was similar. Interferon-γ production by peripheral blood CD4+ and CD8+ lymphocytes was unaffected by disease severity (Table 1) and could be detected up to >1 year after infection but did not display a clear pattern of evolution over time (Figure 1C).
FIGURE 1:
SARS-CoV-2 (A) anti-S IgG titres, (B) anti-N IgG titres and (C) QuantiFERON response in HD patients with a history of PCR-confirmed SARS-CoV-2 infection that had asymptomatic or mild disease (blue circles) or severe disease (red circles) as a function of the time interval between the lowest cycle threshold value and serum sampling. Anti-S IgG was measured by a chemiluminescent microparticle immunoassay (CMIA) on the ARCHITECT i System (SARS-CoV-2 IgG II Quant assay, Abbott Laboratories, Abbott Park, IL, USA). The cut-off for positivity (dashed line) was 50 AU/mL and the conversion factor to the World Health Organization binding antibody units (BAU/mL) is BAU/mL = 0.142 × AU/mL. Anti-N IgG was measured by a CMIA on the same ARCHITECT i analyser (SARS-CoV-2 IgG assay, Abbott). A signal:cut-off ratio ≥1.4 (dashed line) was interpreted as reactive. The QuantiFERON SARS-CoV-2 test (Qiagen, Venlo, The Netherlands) measures the secretion of interferon-γ by peripheral blood CD4+ and CD8+ lymphocytes upon SARS-CoV-2 glycoprotein stimulation. The threshold for positivity (dashed line) is 0.15 IU/mL.
Table 1.
Humoral and cellular response to SARS-CoV-2 infection stratified by disease severity and interval between infection and sampling
| Mild disease (95% CI) | Severe disease (95% CI) | P-valuea | |
|---|---|---|---|
| Period 1 (16 March 2020–30 August 2020) | n = 7 | n = 6 | |
| Anti-S IgG (GMT) | 187 (96–362) | 1306 (633–2695) | 0.003 |
| Anti-N IgG (GMT) | 1.62 (1.29–2.03) | 2.87 (2.05–4.03) | 0.016 |
| QuantiFERON (GMC) | 0.21 (0.11–0.40) | 0.69 (0.18–2.74) | 0.154 |
| Period 2 (31 August 2020–2 March 2021) | n = 17 | n = 11 | |
| Anti-S IgG (GMT) | 525 (227–1215) | 2658 (1483–4763) | 0.010 |
| Anti-N IgG (GMT) | 4.10 (2.93–5.73) | 6.18 (4.77–8.00) | 0.097 |
| QuantiFERON (GMC) | 0.20 (0.08–0.46) | 0.17 (0.05–0.62) | 0.880 |
According to linear models for log10-transformed outcomes. GMT, geometric mean titre; GMC, geometric mean concentration.
In conclusion, the humoral response to SARS-CoV-2 infection in HD patients is a marker of disease severity. Prolonged viral antigen exposure and production of inflammatory cytokines due to a more severe disease course may explain the intense and long-lasting antibody production in HD patients. Conversely, the cellular immune response is unaffected by disease severity and appears to be governed by as yet undetermined factors.
FUNDING
This research was supported by a grant of Amgen (DONAT ION-331036). The funding source had no role in the design, conduct, or analysis of the study or the decision to submit the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
Contributor Information
An S De Vriese, Division of Nephrology and Infectious Diseases, AZ Sint-Jan Brugge-Oostende AV, Brugge, Belgium; Division of Medical Microbiology, AZ Sint-Jan Brugge-Oostende AV, Brugge, Belgium.
Jens Van Praet, Division of Nephrology and Infectious Diseases, AZ Sint-Jan Brugge-Oostende AV, Brugge, Belgium; Division of Medical Microbiology, AZ Sint-Jan Brugge-Oostende AV, Brugge, Belgium.
Marijke Reynders, Department of Internal Medicine, Ghent University, Ghent, Belgium.
Line Heylen, Division of Nephrology, Ziekenhuis Oost-Limburg, Genk, Belgium; Faculty of Medicine and Life Sciences, Hasselt University, Diepenbeek, Belgium.
Liesbeth Viaene, Division of Nephrology, AZ Groeninge, Kortrijk, Belgium.
Rogier Caluwé, Division of Nephrology, OLV Hospital, Aalst, Belgium.
Melanie Schoutteten, Division of Nephrology, Ziekenhuis Oost-Limburg, Genk, Belgium; Faculty of Medicine and Life Sciences, Hasselt University, Diepenbeek, Belgium.
Dirk De Bacquer, Department of Public Health and Primary Care, Ghent University, Ghent, Belgium.
REFERENCES
- 1. De Vriese AS, Reynders M.. IgG antibodyresponse to SARS-CoV-2 infection and viral RNA persistence in patients on maintenance hemodialysis. Am J Kidney Dis 2020; 76: 440–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakhi H, Dahmane D, Attias Pet al. Kinetics of anti-SARS-CoV-2 IgG antibodies in hemodialysis patients six months after infection. J Am Soc Nephrol 2021; 32: 1033–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clarke CL, Prendecki M, Dhutia Aet al. Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection. Kidney Int 2021; 99: 1470–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dudreuilh C, Roper T, Breen Cet al. IgG SARS-CoV-2 antibodies persist at least for 10 months in patients on hemodialysis. Kidney Int Rep 2021; 6: 1961–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ikizler TA, Coates PT, Rovin BHet al. Immune response to SARS-CoV-2 infection and vaccination in patients receiving kidney replacement therapy. Kidney Int 2021; 99: 1275–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anand S, Montez-Rath ME, Han Jet al. Serial SARS-CoV-2 receptor-binding domain antibody responses in patients receiving dialysis. Ann Intern Med 2021; 174: 1073–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sekine T, Perez-Potti A, Rivera-Ballesteros Oet al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020; 183: 158–168.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anft M, Blazquez-Navarro A, Paniskaki Ket al. SARS-CoV-2-reactive cellular and humoral immunity in hemodialysis population. Kidney Int 2021; 99: 1489–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.