Abstract
Background
Frequent outbreaks around the globe and endemic appearance in different parts of the world emphasize the substantial risk of hantavirus diseases. Increasing incidence rates, trends of changing distribution of hantavirus species and new insights into clinical courses of hantavirus diseases call for multinational surveillance. Furthermore, evidence-based guidelines for the management of hantavirus diseases and scoring systems, which allow stratification of patients into risk categories, are lacking.
Methods
Hantavirus registry (HantaReg) is a novel registry platform facilitating multinational research of hantavirus-caused diseases, such as haemorrhagic fever with renal syndrome (HFRS) and hantavirus cardiopulmonary syndrome (HCPS). HantaReg provides an electronic case report form and uses the General Data Protection Regulation compliant platform clinicalsurveys.net, which can be accessed from any internet browser in the world. Having a modular structure, the registry platform is designed to display or hide questions and items according to the documented case (e.g. patient with HFRS versus HCPS) to facilitate fast, but standardized, data entry. Information categories documented in HantaReg are demographics, pre-existing diseases, clinical presentation, diagnostic and therapeutic approaches, as well as outcome.
Conclusions
HantaReg is a novel, ready-to-use platform for clinical and epidemiological studies on hantavirus diseases and facilitates the documentation of the disease course associated with hantavirus infections. HantaReg is expected to promote international collaboration and contributes to improving patient care through the analysis of diagnostic and treatment pathways for hantavirus diseases, providing evidence for robust treatment recommendations. Moreover, HantaReg enables the development of prognosis-indicating scoring systems for patients with hantavirus disease.
Keywords: epidemiology, hantavirus cardiopulmonary syndrome, HCPS, hantavirus disease, haemorrhagic fever with renal syndrome, HFRS, nephropathia epidemica, outbreak, registry
INTRODUCTION
Hantavirus-associated disease is a worldwide emerging zoonosis that remains a clinical challenge with increasing incidence and multiple serious outbreak situations in many parts of the world within the last years [1–7]. Approximately 30 000 humans are infected annually and the numbers of hantavirus case reports and affected countries are rising [8]. Hantaviruses are carried and transmitted to humans by persistently infected rodents, insectivore hosts and bats, although person-to-person transmissions of the Andes virus have been reported via the respiratory and saliva pathways in Latin America and Puumala virus antigen has been found in the saliva of patients [9–13].
Furthermore, climate change and landscape alteration have a strong effect on a regional level and highly affect hantavirus transmission [14, 15]. In Europe, a strong association of hantavirus infections, rodent food availability and ambient temperature of previous autumn/winter has been found which, in parallel with the recent change of climate, resulted in more frequent and more severe outbreaks [16, 17]. Additionally, novel hantaviruses are currently found in insectivore hosts, with unknown pathogenic impacts [6, 9].
Hantavirus disease comprises two distinct clinical syndromes, haemorrhagic fever with renal syndrome (HFRS) and hantavirus cardiopulmonary syndrome (HCPS) caused by the so-called Old World and New World hantaviruses, respectively [9, 18–21]. Depending on the species, courses of disease due to HFRS can range from mild to severe. HFRS, caused by Hantaan virus, Amur virus and Dobrava–Belgrade virus, is characterized by a severe clinical course, with a mortality rate of 10–15%, whereas Puumala virus infections usually lead to mild disease courses, also called ‘nephropathia epidemica’, with mortality rates ˂1% [18, 22, 23]. HCPS is characterized by the development of acute respiratory distress syndrome and cardiac arrhythmias, with attributable mortality ranging from 30% to 50% [24]. However, since the features of HFRS and HCPS can be highly similar, the presenting clinical picture is often perceived as an interconnected syndrome [25–28].
Triggered by the rising numbers of hantavirus diseases in Europe and by frequent serious outbreak situations in many parts of the world, the novel multicentre register project Hantavirus registry (HantaReg) was established in February 2020 [1, 3, 7, 18, 29–32]. Considering the difficulties in obtaining sufficient data in a rare and transient disease, such an international approach is of utmost importance to allow for structured surveillance of hantavirus infections. HantaReg aims to overcome the lack of knowledge on the epidemiology and disease course, as well as diagnostic and therapeutic approaches concerning hantavirus diseases. Additionally, HantaReg serves as a platform for the surveillance of outbreak situations as well as endemic situations of hantavirus diseases. The specific objectives of HantaReg include the exact determination of the incidence of hantavirus diseases, the local and global monitoring of epidemiological trends, the identification of patient groups at risk and of patient-specific prognostic factors, as well as the assessment of attributable mortality and costs.
Herein, we describe the set-up, design and maintenance of the novel HantaReg.
MATERIALS AND METHODS
Study design
The registry project HantaReg was founded in February 2020 and is projected to continue data collection without a defined endpoint. HantaReg is an open registry and invites treating physicians from various specialties (e.g. nephrology or infectious diseases) and virologists to enter epidemiological and clinical data from cases of hantavirus diseases from any part of the world retrospectively. HantaReg utilizes an electronic case report form (eCRF), which was programmed using the online survey software EFS Leadership 7.0 version 1.2 (Questback GmbH, Cologne, Germany) accessible at www.clinicalsurveys.net. To provide the user with a simple but structured online documentation system, clinicalsurveys.net uses a customized version of Questback’s internationally approved EFS Survey and Leadership technology. HantaReg is an intuitive, high-performance web-based documentation platform for hantavirus diseases accessible through highly encrypted communication. HantaReg has a modular structure and the platform is designed to display or hide questions and items depending on the documented clinical course (e.g. patient with HFRS versus patient with HCPS), facilitating structured but fast documentation of cases even in outbreak situations.
Study population and case recruitment
Since HantaReg is a web-based registry, it enables worldwide enrolment of patients with hantavirus disease voluntarily. Further information is accessible at www.kidneyinfection.org. For inclusion of clinical cases into HantaReg, hantavirus infection must be confirmed by either serological, immunohistochemical or direct ribonucleic acid evidence, and clinical signs of HFRS or HCPS. Entered cases are further specified regarding the clinical course, e.g. signs of HFRS or HCPS resulting in a dedicated eCRF. Eligible cases to be included in HantaReg are identified at participating sites responsible for diagnosis, treatment and follow-up of hantavirus diseases in their region.
Moreover, HantaReg provides the opportunity to also capture data on matched reference patients to implement health economic aspects as well as data on attributable hospitalization and mortality analyses using a case–control design. Case–controls will be included from the same hospitals that enrol patient cases into HantaReg and matched for gender, age and pre-existing diseases.
Until January 2021, a total of 154 cases from six German centres were enrolled in HantaReg. After quality control, 150 patient entries were considered valid and are eligible for further inclusion.
Data collection and analysis
HantaReg collects demographic data (such as age group, time of diagnosis, sex, ethnic origin, weight, occupation, year and month of infection, the region of infection and outbreak situation), as well as pre-existing medical conditions, clinical signs and symptoms being present at the first clinical presentation, and virology and/or imaging procedures allowing diagnosis of hantavirus disease (Table 1). Details on disease course and therapeutic approaches including admission to the intensive care unit (ICU), antiviral and diuretic therapy (drug, dose, duration, administration, the reason to stop and adverse events), renal placement therapy (indication, mode of dialysis, duration and adverse events), mechanical ventilation (duration) and extracorporeal membrane oxygenation (ECMO) are recorded. Disease outcome (i.e. overall and attributable mortality), time of hospitalization and readmission to the hospital due to hantavirus disease are documented (Table 1). Additionally, if available, long-term kidney function using creatinine measurements after hantavirus disease is recorded and evaluated [33]. Autopsy results are documented as well.
Table 1.
HantaReg information categories documented.
| Category | Subcategory |
|---|---|
| Epidemiology | Age group at diagnosis, sex, weight, year and place of infection, ethnicity, occupation |
| Pre-existing diseases | Malignancy, HIV/AIDS, solid organ transplantation, chronic renal disease, chronic liver disease, chronic cardiovascular disease, chronic pulmonary disease and autoimmune disorder |
| Clinical presentation | Signs and symptoms including vital signs at admission, after 24 and 72 h and at the day of discharge, disease course, HFRS, HCPS |
| Diagnostics | Virological analyses and imaging procedures for the diagnosis of hantavirus infection, laboratory blood and urine results |
| Therapeutic approach | Admission to ICU; antiviral and diuretic treatment approaches (drug, dose, duration, administration and adverse effects), renal replacement therapy (indication, mode of dialysis, duration and adverse effects), mechanical ventilation (duration), ECMO |
| Outcome | Outcome of hantavirus diseases, time of hospitalization, readmission to the hospital, long term outcome on kidney function, development of chronic disease after hantavirus disease |
AIDS, acquired immunodeficiency syndrome; ECMO, extracorporeal membrane oxygenation; HCPS, hantavirus cardiopulmonary syndrome; HFRS, haemorrhagic fever with renal syndrome; HIV, human immunodeficiency virus; ICU, intensive care unit.
The export of documented cases entered into HantaReg is performed in SPSS-labelled data files with a binary format facilitating univariate and multivariate statistical analyses.
Objectives, analysis and use of data
The main objectives to be investigated are the following: characterization of disease course and clinical features due to hantavirus infection, development of HFRS and/or HCPS, diagnostic approaches to establish a diagnosis of HFRS and/or HCPS, identification of baseline prognostic factors, description of antiviral and diuretic therapy regimens, description of renal replacement therapy, mechanical ventilation and extracorporeal support systems (e.g. ECMO), as well as patient survival and long-term outcome. Moreover, HantaReg aims to monitor hantavirus disease incidence globally and locally over time, and functions as a registry platform for outbreak situations facilitating identification of sources of transmission and description of the impact of counteractions that were initiated. Furthermore, HantaReg may aid in developing and modifying existing clinical screening strategies, as well as diagnostic and treatment pathways, which may help to improve patient care and to inform future consensus guidelines.
Quality control
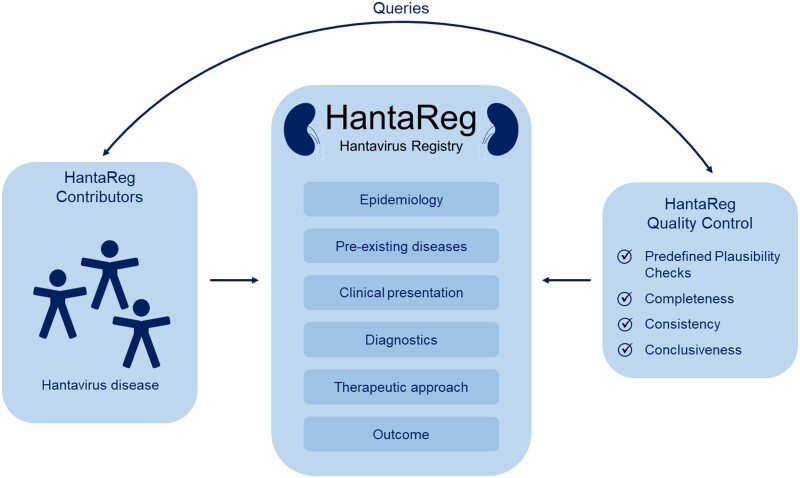
Automated entry checks, such as predefined plausibility checks and mandatory data input with regard to case definitions, minimize the risk for erroneous data entries. Additionally, a team of physicians reviews clinical cases entered into HantaReg to ensure completeness, consistency and conclusiveness. Queries concerning missing or unclear data are sent electronically to the collaborators (Figure 1). Changes upon queries will be made by each participating party, respectively. Upon resolution of all queries, cases are considered valid and available for statistical analysis.
FIGURE 1:
HantaReg. Information flow and quality control measures. The investigators document epidemiological and clinical data of hantavirus disease into the online electronic case report form. The HantaReg quality control team validates cases and clarifies relevant questions with the contributor, if necessary.
Furthermore, a structured peer review looking for implausibility or doubt with regard to medical history, diagnostic and therapeutic pathways, and queries is conducted at random time points and regular interim analyses. Importantly, the anonymity of the patient is ensured at all time.
Ethical and data protection regulation considerations
The local Ethics Committee and Review Board of the University of Cologne approved HantaReg (Identifier of the Ethics Committee of the University of Cologne: 19-1652) and the study is registered at ClinicalTrials.gov (Identifier: NCT04323904). HantaReg uses the General Data Protection Regulation compliant platform clinicalsurveys.net (Questback GmbH, Cologne, Germany) and all Good Epidemiological Practice Requirement specifications are met [34]. All clinical data fall under the regulations of medical confidentially and administration of the collected data and the eCRF itself is restricted to selected and named administrators at the University Hospital of Cologne. Contributors to HantaReg log in by username and password and can only view and modify the cases submitted by themselves. All documented cases are automatically collected anonymously in the database on Questback servers in Cologne, Germany. Since HantaReg allows the anonymous documentation of cases, informed consent is waived.
DISCUSSION
Hantavirus-caused infections are orphan diseases with substantial morbidity and mortality [9, 18]. Although rare so far, hantavirus diseases are progressively emerging globally, particularly in Europe, and new hantaviruses with as yet unknown pathogenic impact are found in their rodent and insectivore hosts [1, 5, 6, 9, 35]. Due to the worldwide burden of hantavirus infections, there is an urgent need for international cooperation to increase specific knowledge of the diseases to improve the overall outcome. HantaReg serves as a novel registry project simplifying international cooperation, epidemiological analyses and further studies of disease course, prognostic factors and attributable mortality. HantaReg enables multinational surveillance studies to detect worrisome epidemiological shifts. Due to the modular structure of HantaReg, rapid but structured case documentation is feasible. Several serious outbreak situations of hantavirus diseases have occurred in recent years and HantaReg’s lean design facilitates easy and fast entry of patient cases and, thus, real-time monitoring of epidemiological trends during such outbreak situations [1–3, 36]. From February 2020 until October 2020, six German founding centres included a pilot cohort of 150 cases with hantavirus-associated diseases into HantaReg to prove the registry’s functionality and everyday feasibility. Rapid case entry was supported by the registry’s intuitive and modular design.
Due to the new insights into organ tropism of hantaviruses infection, HFRS and HCPS are believed to be interconnected syndromes [6, 25–28]. However, knowledge regarding the frequency and the impact of cardiopulmonary manifestations caused by Old World hantaviruses originate from single case reports, while data from larger cohorts are lacking. Through the standardized documentation of the clinical course of hantavirus disease and by the documentation of the identified hantavirus species, HantaReg aims to determine hantavirus disease progression beyond the denominations of HFRS and HCPS.
Furthermore, HantaReg facilitates the collection of real-life data together with long-term observations. Retrospective long-term analyses in patients with Puumala-caused acute kidney injury revealed mixed results with regard to the development of hypertension and proteinuria [37–39]. Currently, studies identifying the long-term impact of hantavirus diseases besides renal function are not available. Therefore, the long-term impact of hantavirus diseases regarding development of any chronic disease, especially cardiovascular diseases, are collected and further analysed.
The limitations of HantaReg must be divided into general shortcomings of registries and specific limitations applying to HantaReg. To protect the privacy of enrolled patients and to comply with current data protection guidelines, data acquisition is performed retrospectively and in an anonymized manner, resulting in reduced quality of data. Although HantaReg aims for long-time observation of included patients, loss of follow-up may hamper the standardized documentation of the long-term impact of hantavirus diseases. In addition, reporting bias and selection bias have to be taken into account. However, since hantavirus-caused diseases are rare, pooling patient cases on a global scale facilitating large cohort analyses will significantly increase the quality of evidence and may provide the base for future clinical trials. Therefore, we would like to invite physicians around the world who are dealing with hantavirus diseases to participate in HantaReg and to contribute to this joint effort to better understand this disease.
In summary, the HantaReg serves as a novel platform promoting multi-centric collaboration and leading to increased knowledge of hantavirus diseases and their clinical courses. To date, specific guidelines for hantavirus diseases are lacking. By publishing individual patient data, HantaReg facilitates controlled or uncontrolled level II evidence, which may help to establish specific guidelines for hantavirus infections and may contribute to an overall improvement in patient care [40, 41].
ACKNOWLEDGEMENTS
The authors thank Lea Münker for contributing cases to HantaReg at the University Hospital of Cologne, Germany.
FUNDING
Set-up and maintenance of HantaReg were supported by the Maria-Pesch Stiftung, Cologne, Germany. Participating centres do not receive any financial support or compensation for participation in HantaReg.
AUTHORS’ CONTRIBUTIONS
F.C.K., R.-U.M. and V.B. conceived the project idea, designed HantaReg, contributed cases, drafted the manuscript, and revised, discussed and approved the final manuscript. L.B., T.T.B., S.B., O.A.C., O.D., V.C., S.D., L.E., E.H., K.J.R.H.-A., S.R., M.R.S. and M.W. contributed cases, revised, discussed and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
F.C.K. has received a grant from the Maria-Pesch Stiftung, Cologne, Germany for the conduct of this study. O.A.C. has received research grants from Actelion, Amplyx, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, Janssen, Medicines Company, Melinta, Merck/MSD, Octapharma, Pfizer and Scynexis, is a consultant to Actelion, Allecra, Amplyx, Astellas, Basilea, Biosys, Cidara, Da Volterra, Entasis, F2G, Gilead, Matinas, MedPace, Menarini, Merck/MSD, Mylan, Nabriva, Noxxon, Octapharma, Paratek, Pfizer, PSI, Roche Diagnostics, Scynexis and Shionogi, and received lecture honoraria from Al-Jazeera Pharmaceuticals, Astellas, Basilea, Gilead, Grupo Biotoscana, Merck/MSD and Pfizer. All other authors have nothing to disclose. The results presented in this paper have not been published previously in whole or in part.
EXECUTIVE COMMITTEE: HantaReg was initiated and is coordinated by F.C.K., R.-U.M. and V.B. at the University Hospital of Cologne, Germany. An executive committee consisting of the following members is responsible for the governance of HantaReg: T.T.B., S.B., V.B., S.D., L.E., F.C.K. and S.R. (in alphabetical order). The executive committee protects and manages all data entered into the registry’s database. In addition, the executive committee handles requests for data and research proposals.
Contributor Information
Felix C Koehler, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Emergency Department, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Linda Blomberg, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Thomas Theo Brehm, I. Department of Internal Medicine, Division of Infectious Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Centre for Infection Research (DZIF), Partner Site Hamburg-Lübeck-Borstel-Riems, Germany.
Stefan Büttner, Department I of Internal Medicine, Klinikum Aschaffenburg-Alzenau, Aschaffenburg, Germany.
Oliver A Cornely, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Department I of Internal Medicine, Faculty of Medicine and University Hospital Cologne, Excellence Center for Medical Mycology (ECMM), University of Cologne, Cologne, Germany; Clinical Trials Centre Cologne (ZKS Köln), Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Olaf Degen, I. Department of Internal Medicine, Division of Infectious Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Centre for Infection Research (DZIF), Partner Site Hamburg-Lübeck-Borstel-Riems, Germany.
Veronica Di Cristanziano, Institute of Virology, Faculty of Medicine and University Hospital of Cologne, University of Cologne, Cologne, Germany.
Sebastian Dolff, Department of Infectious Diseases, University Hospital Essen, Essen, Germany.
Lukas Eberwein, 4th Department of Internal Medicine, Klinikum Leverkusen gGmbH, Leverkusen, Germany.
Elion Hoxha, III. Department of Medicine, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
K Johanna R Hoyer-Allo, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Sarah Rudolf, Department of Nephrology, Medical Clinic III, University Hospital Frankfurt, Frankfurt, Germany.
Martin R Späth, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Manuel Wanken, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Roman-Ulrich Müller, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Volker Burst, Department II of Internal Medicine and Center for Molecular Medicine Cologne, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany; Emergency Department, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
REFERENCES
- 1. Kruger DH, Figueiredo LT, Song JWet al. Hantaviruses–globally emerging pathogens. J Clin Virol 2015; 64: 128–136 [DOI] [PubMed] [Google Scholar]
- 2. Hofmann J, Meisel H, Klempa Bet al. Hantavirus outbreak, Germany, 2007. Emerg Infect Dis 2008; 14: 850–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ettinger J, Hofmann J, Enders Met al. Multiple synchronous outbreaks of Puumala virus, Germany, 2010. Emerg Infect Dis 2012; 18: 1461–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hartline J, Mierek C, Knutson Tet al. Hantavirus infection in North America: a clinical review. Am J Emerg Med 2013; 31: 978–982 [DOI] [PubMed] [Google Scholar]
- 5. Reusken C, Heyman P.. Factors driving hantavirus emergence in Europe. Curr Opin Virol 2013; 3: 92–99 [DOI] [PubMed] [Google Scholar]
- 6. Krautkramer E, Zeier M, Plyusnin A.. Hantavirus infection: an emerging infectious disease causing acute renal failure. Kidney Int 2013; 83: 23–27 [DOI] [PubMed] [Google Scholar]
- 7. Faber M, Krüger DH, Auste Bet al. Molecular and epidemiological characteristics of human Puumala and Dobrava-Belgrade hantavirus infections, Germany, 2001 to 2017. Euro Surveill 2019; 24: 1800675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watson DC, Sargianou M, Papa Aet al. Epidemiology of Hantavirus infections in humans: a comprehensive, global overview. Crit Rev Microbiol 2014; 40: 261–272 [DOI] [PubMed] [Google Scholar]
- 9. Avsic-Zupanc T, Saksida A, Korva M.. Hantavirus infections. Clin Microbiol Infect 2019; 21: e6–e16 [DOI] [PubMed] [Google Scholar]
- 10. Padula PJ, Edelstein A, Miguel SDet al. Hantavirus pulmonary syndrome outbreak in Argentina: molecular evidence for person-to-person transmission of Andes virus. Virology 1998; 241: 323–330 [DOI] [PubMed] [Google Scholar]
- 11. Wells RM, Sosa Estani S, Yadon ZEet al. An unusual hantavirus outbreak in southern Argentina: person-to-person transmission? Hantavirus pulmonary syndrome study group for Patagonia. Emerg Infect Dis 1997; 3: 171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pizarro E, Navarrete M, Mendez Cet al. Immunocytochemical and ultrastructural evidence supporting that Andes Hantavirus (ANDV) is transmitted person-to-person through the respiratory and/or salivary pathways. Front Microbiol 2019; 10: 2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pettersson L, Klingström J, Hardestam Jet al. Hantavirus RNA in saliva from patients with hemorrhagic fever with renal syndrome. Emerg Infect Dis 2008; 14: 406–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zeimes CB, Quoilin S, Henttonen Het al. Landscape and regional environmental analysis of the spatial distribution of hantavirus human cases in Europe. Front Public Health 2015; 3: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guterres A, de Lemos ERS.. Hantaviruses and a neglected environmental determinant. One Health 2018; 5: 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monchatre-Leroy E, Crespin L, Boué Fet al. Spatial and temporal epidemiology of nephropathia epidemica incidence and hantavirus seroprevalence in rodent hosts: identification of the main environmental factors in Europe. Transbound Emerg Dis 2017; 64: 1210–1228 [DOI] [PubMed] [Google Scholar]
- 17. Roda Gracia J, Schumann B, Seidler A.. Climate variability and the occurrence of human puumala hantavirus infections in Europe: a systematic review. Zoonoses Public Health 2015; 62: 465–478 [DOI] [PubMed] [Google Scholar]
- 18. Jonsson CB, Figueiredo LT, Vapalahti O.. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev 2010; 23: 412–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee PW, Amyx HL, Gajdusek DCet al. New hemorrhagic fever with renal syndrome-related virus in rodents in the United States. Lancet 1982; 2: 1405. [PubMed] [Google Scholar]
- 20. Hallin GW, Simpson SQ, Crowell REet al. Cardiopulmonary manifestations of hantavirus pulmonary syndrome. Crit Care Med 1996; 24: 252–258 [DOI] [PubMed] [Google Scholar]
- 21. Duchin JS, Koster FT, Peters CJet al. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The hantavirus study group. N Engl J Med 1994; 330: 949–955 [DOI] [PubMed] [Google Scholar]
- 22. Makary P, Kanerva M, Ollgren Jet al. Disease burden of Puumala virus infections, 1995–2008. Epidemiol Infect 2010; 138: 1484–1492 [DOI] [PubMed] [Google Scholar]
- 23. Hjertqvist M, Klein SL, Ahlm Cet al. Mortality rate patterns for hemorrhagic fever with renal syndrome caused by Puumala virus. Emerg Infect Dis 2010; 16: 1584–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de St Maurice A, Ervin E, Schumacher Met al. Exposure characteristics of hantavirus pulmonary syndrome patients, United States, 1993–2015. Emerg Infect Dis 2017; 23: 733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clement J, Maes P, Lagrou Ket al. A unifying hypothesis and a single name for a complex globally emerging infection: hantavirus disease. Eur J Clin Microbiol Infect Dis 2012; 31: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vollmar P, Lubnow M, Simon Met al. Hantavirus cardiopulmonary syndrome due to Puumala virus in Germany. J Clin Virol 2016; 84: 42–47 [DOI] [PubMed] [Google Scholar]
- 27. Rasmuson J, Andersson C, Norrman Eet al. Time to revise the paradigm of hantavirus syndromes? Hantavirus pulmonary syndrome caused by European hantavirus. Eur J Clin Microbiol Infect Dis 2011; 30: 685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rasmuson J, Lindqvist P, Sorensen Ket al. Cardiopulmonary involvement in Puumala hantavirus infection. BMC Infect Dis 2013; 13: 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Faber MS, Ulrich RG, Frank Cet al. Steep rise in notified hantavirus infections in Germany. Euro Surveill 2010; 2010: 15. [PubMed] [Google Scholar]
- 30. Klempa B. Hantaviruses and climate change. Clin Microbiol Infect 2009; 15: 518–523 [DOI] [PubMed] [Google Scholar]
- 31. Zhang YZ, Zou Y, Fu ZFet al. Hantavirus infections in humans and animals, China. Emerg Infect Dis 2010; 16: 1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knust B, Brown S, de St Maurice Aet al. Seoul virus infection and spread in US home-based ratteries-rat and human testing results from a multistate outbreak investigation. J Infect Dis 2020; 222: 1311–1319 [DOI] [PubMed] [Google Scholar]
- 33. Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830 [DOI] [PubMed] [Google Scholar]
- 34. Hoffmann W, Latza U, Baumeister SEet al. Guidelines and recommendations for ensuring Good Epidemiological Practice (GEP): a guideline developed by the German Society for Epidemiology. Eur J Epidemiol 2019; 34: 301–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmaljohn C, Hjelle B.. Hantaviruses: a global disease problem. Emerg Infect Dis 1997; 3: 95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lovric Z, Kolaric B, Kosanovic LMLet al. An outbreak of haemorrhagic fever with renal syndrome linked with mountain recreational activities in Zagreb, Croatia, 2017. Epidemiol Infect 2018; 146: 1236–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miettinen MH, Makela SM, Ala-Houhala IOet al. Ten-year prognosis of Puumala hantavirus-induced acute interstitial nephritis. Kidney Int 2006; 69: 2043–2048 [DOI] [PubMed] [Google Scholar]
- 38. Kleinknecht D, Rollin PE.. Hypertension after hemorrhagic fever with renal syndrome. Nephron 1992; 61: 121. [DOI] [PubMed] [Google Scholar]
- 39. Latus J, Schwab M, Tacconelli Eet al. Clinical course and long-term outcome of hantavirus-associated nephropathia epidemica. Emerg Infect Dis 2015; 21: 76–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA 1992; 268: 2420–2425 [DOI] [PubMed] [Google Scholar]
- 41. Wilson MC, Hayward RS, Tunis SRet al. Users' guides to the Medical Literature. VIII. How to use clinical practice guidelines. B. What are the recommendations and will they help you in caring for your patients? The Evidence-Based Medicine Working Group. JAMA 1995; 274: 1630–1632 [DOI] [PubMed] [Google Scholar]