Abstract
Introduction
Peripheral blood leukocytes are a suitable cell model for science research. However, blood samples from healthy volunteers are limited in volume and difficult to obtain due to the complexity of volunteer recruitment.
Objective
Therefore, it is urgent to find an alternative source of peripheral blood leukocytes.
Method
One of the possibilities is the use of leukocyte reduction filters (LRFs) in blood banks that is used for preparation of leukoreduced blood products. More than 90% of the leukocytes are trapped in the leukofilters allowing the desired blood product to pass through.
Results
It has been reported that the biological function of leukocytes collected from the filters are no different from those isolated from buffy coats, leukapheresis products and whole blood (WB) cells. Moreover, LRFs are waste products that are discarded after leukoreduction.
Conclusion
Thus, leukofilters represent an economic source of human cell populations that can be used for a variety of investigative purposes, with no cost. In the present study, we reviewed the different usage of LRFs in the research, clinical and commercial applications.
Keywords: Leukocyte reduction filters, Peripheral blood leukocytes, Usage
Introduction
Leukoreduction is the removal of leukocytes from blood components. Each leukoreduced product had residual leukocyte counts of less than 5 × 106 cells per unit.1 At present, leukoreduction is performed using Leukocyte Reduction Filters (LRFs) that trap leukocytes, while allowing the desired blood product to pass through.2 It has been demonstrated that leukoreduction can reduce adverse transfusion reactions such as febrile non-hemolytic transfusion reactions (FNHTR), alloimmunization against human leukocyte antigens (HLA) and human platelet antigens (HPA), which may cause refractoriness to platelet transfusion, and transmission of infectious diseases.3, 4, 5 In addition, leukoreduction can prevent the release of leukocyte metabolites, such as cytokines and histamine. These metabolites contribute to adverse reactions and increase the percentage of hemolysis in red blood cell (RBC) products.6 There are different commercial leukofilters, such as the Leukoflex LST-1 (MacoPharma), Leukoflex LCR5 (MacoPharma), Leukotrap WBF-3 (Pall Medical) Compoflex T3908 (Fresenius Hemocare), Optipure RZ 2000 (Baxter) and TACSI system (Terumo BCT). Leukoreduction via filtration is based on both barrier retention and adsorption of cells onto the membrane.7, 8, 9 Although commercially available LRFs vary in physical characteristics, all of them can trap leukocytes in their matrix using gravity flow.10, 11 The LRF material is comprised of a novel polymer fiber with a membrane-like structure, including porous foam material or non-woven fibers or woven mesh-shaped fibers. Due to the different pore size of the polymer fiber, leukocytes are filtered by mechanical block and cell adsorption. 12, 13, 14 Concerning the mechanism of leukocyte removal, it has been proposed that the most important mechanism is the adhesion of leukocytes to the filtration material. The efficiency of filtration is dependent on different factors, such as the area of contact of the blood cells with the filtration material, the overall thickness of the foam material and the diameter of the fiber. Other factors are charge and hydrophilicity.15, 16 Previous studies have been introduced LRFs as an alternative source of viable and functional human cells,8, 17, 18 Traditionally, the most common source of human cells for laboratory usage has been the buffy coats. However, blood samples from healthy volunteers are limited in volume and difficult to obtain due to the complexity of volunteer recruitment. There are several advantages in using leukofilters. The first advantage is that it is easy to obtain LRFs from blood banks without the need to perform phlebotomy, as this had already been performed at the time of blood donation. Another benefit stems from the attainment of informed consent from blood donors, therefore making it possible to use blood cells for research without ethical barriers. A further advantage is the safety in obtaining cells due to the pre-screening of blood donors for viral infections.11 Indeed, the whole process, from the donor selection to the delivery of the LRFs to the researchers, is controlled by the blood bank. These filters are discarded after use and, being waste products, represent an available source of human cell populations that can be used for a variety of investigative purposes with no cost (Fig. 1). In the present study, we reviewed different usages of LRFs as an economic source of cells in research, clinical and commercial applications.
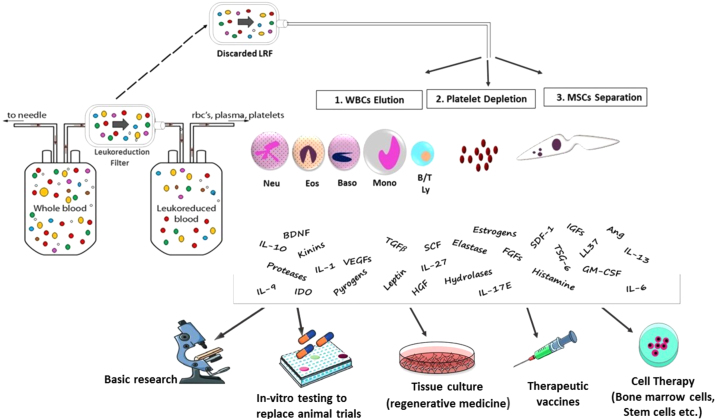
Figure 1.
Overview of the new application of Leukocyte Reduction Filters (LRFs). Discarded LRFs are an economic source of viable functional cells and bioactive molecules that can play a role in basic research, animal models, cell therapy and tissue engineering.
B/T Ly: lymphocytes B and T, LRF: leuko-reduction filter, Mono: monocytes, RBCs: red blood cells, WBCs: white blood cells, BDNF; Brain Derived Neurotrophic Factor, FGFs; Fibroblast Growth Factor, HGF; Hepatocyte Growth Factor, IGFs; Insulin-Like Growth Factor, VEGFs ;Vascular Endothelial Derived Growth Factor, TGFβ; Transforming Growth Factor Beta, IL-6; Interleukin 6, IL-10; Interleukin 10, IL-27; Interleukin 27, IL-17E; Interleukin 17, IL-13; Interleukin 13, IL-9; Interleukin 9, IL-1; Interleukin-1, Ang; Angiopoietin, GM-CSF; Granulocyte Macrophage CSF, SCF; Stem Cell Factor, SDF-1; Stromal Cell-Derived Factor 1, TSG-6; (TNF)-Stimulated Gene-6.
LRFs as a new source for research
Leukocytes are used for a variety of research purposes. In recent years, the demand for primary human cells has been increased. The gold standard to obtain these cells is leukapheresis.18, 19 However, the use of buffy coats or leukapheresis products for research purposes is highly expensive and is not available at every blood bank. Several groups have examined the phenotype and function of immune cells isolated from LRFs, compared to standard buffy coats. They observed that the percentages of T cells, B cells, monocytes and dendritic cells (DCs) were largely similar between peripheral blood mononuclear cells (PBMCs) isolated from LRFs and buffy coats.8, 18, 20
The number of cells recovered from different LFRs is dependent on the volume of phosphate buffered saline (PBS) used to flush back them from the filter. In a study by Izquierdo et al.,21 blood bags from healthy donors were less than 24 h old when processed through an LRF by gravity flow at room temperature (22 °C). Cells were recovered from filters at high numbers (about 5 × 108 PBMCs), with a viability >95%, and the phenotypic and functional experiments confirmed that they were similar to those cells obtained from buffy coats. In a study by Peytour et al.,22 discarded LRFs were processed through a rapid (<5 h) and easy 4-step procedure (WBC elution, platelet depletion, MNC separation and CD34+ cell selection) less than 24 h after blood donation. They obtained 0.4 ± 0.05 × 105 highly enriched CD34+ cells per LRF. The functional properties, such as cell cycle status, growth rate and myeloid differentiation in liquid culture, were rather similar to steady state peripheral blood (SSPB) and umbilical cord blood (CB) CD34+ cells. Ebner et al.,23 conducted a study to isolate CD14+ precursors from LRFs to generate DCs and compared them with isolated cells from buffy coats. They revealed that the yield of PBMCs obtained from LRFs is higher than that from buffy coats. The generated DCs had short culture and maturation periods, similar to the DCs obtained from the leukapheresis procedure. Thus, they concluded that LRFs have the potential to become a standard procedure for preparation of large quantities of DCs. Weidinger et al.,19 showed that PBMCs obtained from LRFs have a lower fraction of apoptotic and necrotic cells than PBMCs from leukapheresis. Furthermore, in a study by Pfeiffer et al.,24 PBMCs and especially high-quality monocyte-derived dendritic cells (moDCs) were generated from LRFs, comparable to those derived from leukapheresis products or whole blood. Néron et al.,25 demonstrated that cells recovered from WBF2 filters represent a good source of viable PBMCs because each unit can provide 2 × 108 to 3 × 108 PBMCs that can be used to prepare cryopreserved samples for phenotypic and functional investigations. Recently, we performed a study26 on the isolation of cells from Leukoflex LST-1 filters and approximately, 4.4 × 108 leukocytes (mononuclear cells + granulocytes) were obtained from each filter. Teleron et al.,17 designed a study to evaluate the capacity of LRFs as a source of peripheral blood-derived endothelial progenitor cells (EPCs). They displayed that the mean yield of EPCs from one LRF is 14-fold higher than that from fresh blood, probably due to the greater numbers of PBMCs obtained from each LRF. Accordingly, they concluded that LRFs can provide a safe and cheap source of EPCs.
Another application of LRFs is using them in genetic analysis. Cook et al.,27 reported that the LRF is a source of large quantities of DNA for the study of genetic variations in human populations. A mean of 1520 μg (ranging from 450 to 3750 μg) of DNA per sample was obtained. Also, they indicated that the LRF-recovered DNA remains suitable for polymerase chain reaction (PCR) and sequencing methods after 2 years of storage at −40 °C to 4 °C. They concluded that this procedure allows for the attainment of sufficient quantities of genomic DNA for many experiments. However, these genetic experiments require the specific consent of the donor and ethical approval.
LRFs as a new source for cell therapy
Peytour et al22 introduced a procedure to obtain 0.4 ± 0.05 × 105 highly enriched CD34+ cells per LRF. They showed that CD34+ cells trapped in filters are functionally similar to those harvested from other sources, such as bone marrow (BM), cord blood or peripheral blood. Therefore, LRFs can be an alternative source of stem cells for hematopoietic transplantation. Ivanovic et al28 conducted a study to investigate the viability and the functional properties of mononuclear cells (MNCs) and CD34+ cells recovered from LRFs. The results displayed that the functionality of LRF-recovered CD34+ cells was well preserved with a significant ability of ex vivo expansion. They also confirmed the ability of these cells to differentiate into erythroid, megakaryocytic, and myelomonocytic lineages, upon the addition of certain cytokines in their liquid cultures. The problem of the relatively low amount of CD34+ cells in each LRF can be resolved by pooling cells recovered from several LRFs utilized for a continuous donor. Hence, LRF-recovered CD34+ cells can be applied to clinical procedures to generate mature ex vivo cells. Some studies disclosed that peripheral blood MNCs can differentiate into the CD34+ progenitors and they may differentiate into the various non-hematopoietic cell lineages.29, 30 Therefore, it is possible that LRF-recovered MNCs become a main source of cells for cell-based regenerative therapy in future. In addition, it is suggested that LRF CD34+ cells are good candidates for cell/tissue engineering, such as cardiac cell therapy,31, 32 and the attainment of pluripotent stem cells (iPS cells).33
The PBMNs are frequently used for the DC culture in adoptive immunotherapies. Today the gold standard to obtain monocytes for DC therapies is leukapheresis. In a study by Izquierdo et al.,21 monocytes recovered from WBF3 filters differentiated into in vitro DCs. The main feature of these cells is the capacity to stimulate native T cells and initiate primary immune responses.34 This characteristic has led to the use of DCs in cancer immunotherapy.35, 36
LRFs as a new source for drug production
Human α-defensins, also called human neutrophil peptides 1–3 (HNP 1–3), are cationic antimicrobial peptides with three disulfide bridges.37, 38, 39, 40 These peptides are effector molecules of the innate immunity. They also show chemotactic activity for monocytes and T cells. Defensins are mainly produced by neutrophils, but their production by other leukocytes, such as NK cells, B cells, T cells, monocytes and macrophages, has been reported.41, 42, 43 Previous studies have also demonstrated that defensins display anticancer activity.44, 45, 46 Synthesis of α-defensins using chemical and recombinant approaches is challenging due to the essential role of the disulfide bonds for its function. Recently LRFs have been introduced as a major production source for natural defensins.26, 47 We performed a study48 at the Iranian Blood Transfusion Organization (IBTO) and found that α-defensins purified from neutrophils trapped in LST-1 filters have anticancer activity that can be used for clinical applications in the future. Vossier L et al49 have also found that human defensins extracted from LRFs have high antimicrobial activity with minimal inhibitory concentration (MIC) values between 0.12 and 1 μg/mL. In addition, other polypeptides with antimicrobial activity have been found in neutrophils, such as calprotectin and LL-37,50 that can be extracted from the neutrophils trapped in leukofilters.
LRFs as an alternative to animal models
The preclinical studies for drug screening involve the use of animals, which is expensive and leads to the suffering of these animals. A number of alternative systems have been developed for use in research, such as tissue culture, stem cells, microbiological systems, DNA chips and computer analysis models. The benefits of these alternatives include the decrease in the number of animals used, reduction in the costs and control of the variables of the experiment.51, 52 In addition, it is reported that mouse models may poorly mimic human disorders, such as inflammatory disease.53, 54 The LRFs can be used as a culture medium with a valuable source of primary human cells. Therefore, it can be used as an alternative to animal models.
Conclusion
Until recently, only a little attention has been paid to the discarding cells present in leukofilters. A variety of viable and functional leukocytes are recoverable from blood filters. These filters are easy to obtain from blood transfusion centers, without the need to perform venipuncture for experimental purposes. This entails simpler handling, when compared with whole blood or leukapheresis, and generates a high yield of functional active cells. Hence, for economic, ethical and practical reasons LRFs represent a major alternative to human cells. This article provides an overview of the applications of LRFs. Moreover, filters can be used for other applications, such as the induction of cytokine release from cultured human leukocytes.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors acknowledge the blood transfusion research center, the High Institute for Research and Education in Transfusion Medicine, Tehran, Iran.
References
- 1.Roback J., Combs M., Grossman B., Hillyer C. Vol. 376. American Association of Blood Banks; Bethesda, MD: 2008. p. 471. (American association of blood banks technical manual). [Google Scholar]
- 2.Sharma R., Marwaha N. Leukoreduced blood components: advantages and strategies for its implementation in developing countries. Asian J Transfus Sci. 2010;4(1):3–8. doi: 10.4103/0973-6247.59384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fergusson D., Khanna M.P., Tinmouth A., Hébert P.C. Transfusion of leukoreduced red blood cells may decrease postoperative infections: two meta-analyses of randomized controlled trials. Can J Anaesth. 2004;51(5):417–424. doi: 10.1007/BF03018302. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg N., Zhao H., Wang H., Messing S., Heal J.M., Lyman G.H. The intention‐to‐treat principle in clinical trials and meta‐analyses of leukoreduced blood transfusions in surgical patients. Transfusion. 2007;47(4):573–581. doi: 10.1111/j.1537-2995.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- 5.Pennington J., Taylor G.P., Sutherland J., Davis R.E., Seghatchian J., Allain J.-P. Persistence of HTLV-I in blood components after leukocyte depletion. Blood. 2002;100(2):677–681. doi: 10.1182/blood.v100.2.677. [DOI] [PubMed] [Google Scholar]
- 6.Rapaille A., Moore G., Siquet J., Flament J., Sondag‐Thull D. Prestorage leukocyte reduction with in‐line filtration of whole blood: evaluation of red cells and plasma storage. Vox Sang. 1997;73(1):28–35. doi: 10.1046/j.1423-0410.1997.7310028.x. [DOI] [PubMed] [Google Scholar]
- 7.Sen A., Khetarpal Sm A., Jetley S. Comparative study of predeposit and bedside leucodepletion filters. Med J Armed Forces India. 2010;66(2):142–146. doi: 10.1016/S0377-1237(10)80127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer T., Zehnter I., Hofmann B., Zaisserer J., Burkhart J., Rapp S. Filter Buffy Coats (FBC): a source of peripheral blood leukocytes recovered from leukocyte depletion filters. J Immunol Methods. 2005;307(1-2):150–166. doi: 10.1016/j.jim.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Mönninghoff J., Moog R. Investigation of a new in-line leukocyte reduction filter for packed red blood cells. Transfus Apher Sci. 2012;46(3):253–256. doi: 10.1016/j.transci.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Singh S., Kumar A. Leukocyte depletion for safe blood transfusion. Biotechnol J. 2009;4(8):1140–1151. doi: 10.1002/biot.200800182. [DOI] [PubMed] [Google Scholar]
- 11.Wegehaupt A.K., Roufs E.K., Hewitt C.R., Killian M.L., Gorbatenko O., Anderson C.M. Recovery and assessment of leukocytes from LR Express filters. Biologicals. 2017;49:15–22. doi: 10.1016/j.biologicals.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujii Y. The potential of the novel leukocyte removal filter in cardiopulmonary bypass. Expert Rev Med Devices. 2016;13(1):5–14. doi: 10.1586/17434440.2016.1126179. [DOI] [PubMed] [Google Scholar]
- 13.Singh S., Kumar A. Leukocyte depletion for safe blood transfusion. Biotechnol J. 2009;4(August (8)):1140–1151. doi: 10.1002/biot.200800182. PubMed PMID: 19418471. Epub 2009/05/07. eng. [DOI] [PubMed] [Google Scholar]
- 14.Barbe L., Boval B., Wautier M.P., Wautier J.L. Human promyelocytic cell line: a convenient tool for studying the molecular basis of WBC filtration. Transfusion. 2000;40(October (10)):1250–1256. doi: 10.1046/j.1537-2995.2000.40101250.x. PubMed PMID: 11061864. Epub 2000/11/03. eng. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi H., Nishiuchi T., Tamura H., Takeda K. Transfusion-associated graft-versus-host disease caused by leukocyte-filtered stored blood. Anesthesiology. 1993;79(6):1419–1421. doi: 10.1097/00000542-199312000-00034. PubMed PMID: 8267214. Epub 1993/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 16.Akahoshi M., Takanashi M., Masuda M., Yamashita H., Hidano A., Hasegawa K. A case of transfusion-associated graft-versus-host disease not prevented by white cell-reduction filters. Transfusion. 1992;32(2):169–172. doi: 10.1046/j.1537-2995.1992.32292180149.x. [DOI] [PubMed] [Google Scholar]
- 17.Teleron A.A., Carlson B., Young P.P. Blood donor white blood cell reduction filters as a source of human peripheral blood–derived endothelial progenitor cells. Transfusion. 2005;45(1):21–25. doi: 10.1111/j.1537-2995.2005.04191.x. [DOI] [PubMed] [Google Scholar]
- 18.Ebner S., Neyer S., Hofer S., Nussbaumer W., Romani N., Heufler C. Generation of large numbers of human dendritic cells from whole blood passaged through leukocyte removal filters: an alternative to standard buffy coats. J Immunol Methods. 2001;252(1–2):93–104. doi: 10.1016/s0022-1759(01)00337-4. [DOI] [PubMed] [Google Scholar]
- 19.Weidinger T.M., Keller A.K., Weiss D., Zimmermann R., Eckstein R., Strasser E.F. Peripheral blood mononuclear cells obtained from leukoreduction system chambers show better viability than those from leukapheresis. Transfusion. 2011;51(9):2047–2049. doi: 10.1111/j.1537-2995.2011.03238.x. [DOI] [PubMed] [Google Scholar]
- 20.Dietz A.B., Bulur P.A., Emery R.L., Winters J.L., Epps D.E., Zubair A.C. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006;46(12):2083–2089. doi: 10.1111/j.1537-2995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 21.Izquierdo N., Naranjo M., Fernández M., Cos J., Massuet L., Martínez-Picado J. Leukocyte Reduction Filters: an alternative source of Peripheral Blood Mononuclear Cells. Inmunología. 2003;22(3):255–262. [Google Scholar]
- 22.Peytour Y., Guitart A., Villacreces A., Chevaleyre J., Lacombe F., Ivanovic Z. Obtaining of CD34+ cells from healthy blood donors: development of a rapid and efficient procedure using leukoreduction filters. Transfusion. 2010;50(10):2152–2157. doi: 10.1111/j.1537-2995.2010.02683.x. [DOI] [PubMed] [Google Scholar]
- 23.Ebner S., Neyer S., Hofer S., Nussbaumer W., Romani N., Heufler C. Generation of large numbers of human dendritic cells from whole blood passaged through leukocyte removal filters: an alternative to standard buffy coats. J Immunol Methods. 2001;252(1–2):93–104. doi: 10.1016/s0022-1759(01)00337-4. [DOI] [PubMed] [Google Scholar]
- 24.Pfeiffer I.A., Zinser E., Strasser E., Stein M.F., Dörrie J., Schaft N. Leukoreduction system chambers are an efficient, valid, and economic source of functional monocyte-derived dendritic cells and lymphocytes. Immunobiology. 2013;218(11):1392–1401. doi: 10.1016/j.imbio.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Néron S., Dussault N., Racine C. Whole‐blood leukoreduction filters are a source for cryopreserved cells for phenotypic and functional investigations on peripheral blood lymphocytes. Transfusion. 2006;46(4):537–544. doi: 10.1111/j.1537-2995.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 26.Ferdowsi S., Pourfathollah A.A. Leukocyte reduction filters: A source of peripheral blood leukocytes for research and drug production. Glob J Transfus Med. 2019;4:122–123. doi: 10.1016/j.htct.2020.10.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook M.A., Jobson S.E., Atkinson D.C., Lowe D.P., Farmer S.L., Alvi-Ali W.J. Used leucodepletion filters as a source of large quantities of DNA suitable for the study of genetic variations in human populations. Transfus Med. 2003;13(2):77–81. doi: 10.1046/j.1365-3148.2003.00423.x. [DOI] [PubMed] [Google Scholar]
- 28.Ivanovic Z., Duchez P., Morgan D.A., Hermitte F., Lafarge X., Chevaleyre J. Whole‐blood leukodepletion filters as a source of CD34+ progenitors potentially usable in cell therapy. Transfusion. 2006;46(1):118–125. doi: 10.1111/j.1537-2995.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 29.Abuljadayel I.S. Induction of stem cell-like plasticity in mononuclear cells derived from unmobilised adult human peripheral blood. Curr Med Res Opin. 2003;19(5):355–375. doi: 10.1185/030079903125001901. [DOI] [PubMed] [Google Scholar]
- 30.Abuljadayel I.S., Afghan R.K., McCaffrey T.A., Lundergan C., Hawley T.S., Hawley R.G. SCID repopulating cells derived from unmobilised adult human peripheral blood. Curr Med Res Opin. 2004;20(1):87–100. doi: 10.1185/030079903125002766. [DOI] [PubMed] [Google Scholar]
- 31.Losordo D.W., Henry T.D., Davidson C., Sup Lee J., Costa M.A., Bass T. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109(4):428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peytour Y., Villacreces A., Chevaleyre J., Ivanovic Z., Praloran V. Discarded leukoreduction filters: a new source of stem cells for research, cell engineering and therapy? Stem Cell Res. 2013;11(2):736–742. doi: 10.1016/j.scr.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Chou B.-K., Mali P., Huang X., Ye Z., Dowey S.N., Resar L.M. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21(3):518. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinman R.M. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt Sinai J Med. 2001;68(3):160–166. [PubMed] [Google Scholar]
- 35.Nencioni A., Grünebach F., Schmidt S.M., Müller M.R., Boy D., Patrone F. The use of dendritic cells in cancer immunotherapy. Crit Rev Oncol Hematol. 2008;65(3):191–199. doi: 10.1016/j.critrevonc.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Banchereau J., Palucka A.K. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5(4):296. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 37.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3(9):710. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 38.Selsted M.E., Ouellette A.J. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6(6):551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 39.Niyonsaba F., Nagaoka I., Ogawa H. Human defensins and cathelicidins in the skin: beyond direct antimicrobial properties. Crit Rev Immunol. 2006;26(6):545–576. doi: 10.1615/critrevimmunol.v26.i6.60. [DOI] [PubMed] [Google Scholar]
- 40.Rehaume L.M., Hancock R.E. Neutrophil-derived defensins as modulators of innate immune function. Crit Rev Immunol. 2008;28(3):185–200. doi: 10.1615/critrevimmunol.v28.i3.10. [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez‐García M., Oliva H., Climent N., García F., Gatell J.M., Gallart T. Human immature monocyte‐derived dendritic cells produce and secrete α‐defensins 1–3. J Leukoc Biol. 2007;82(5):1143–1146. doi: 10.1189/jlb.0507295. [DOI] [PubMed] [Google Scholar]
- 42.Mackewicz C.E., Yuan J., Tran P., Diaz L., Mack E., Selsted M.E. α-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. Aids. 2003;17(14):F23–F32. doi: 10.1097/00002030-200309260-00001. [DOI] [PubMed] [Google Scholar]
- 43.Klotman M.E., Chang T.L. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6(6):447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 44.Lichtenstein A., Ganz T., Selsted M.E., Lehrer R.I. In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood. 1986;68(6):1407–1410. [PubMed] [Google Scholar]
- 45.Lichtenstein A., Ganz T., Nguyen T., Selsted M., Lehrer R. Mechanism of target cytolysis by peptide defensins. Target cell metabolic activities, possibly involving endocytosis, are crucial for expression of cytotoxicity. J Immunol. 1988;140(8):2686–2694. [PubMed] [Google Scholar]
- 46.McKeown S.T., Lundy F.T., Nelson J., Lockhart D., Irwin C.R., Cowan C.G. The cytotoxic effects of human neutrophil peptide-1 (HNP1) and lactoferrin on oral squamous cell carcinoma (OSCC) in vitro. Oral oncology. 2006;42(7):685–690. doi: 10.1016/j.oraloncology.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Vossier L., Leon F., Bachelier C., Marchandin H., Lehmann S., Leonetti J.P. An innovative biologic recycling process of leukoreduction filters to produce active human antimicrobial peptides. Transfusion. 2014;54(5):1332–1339. doi: 10.1111/trf.12444. [DOI] [PubMed] [Google Scholar]
- 48.Ferdowsi S., Pourfathollah A.A., Amiri F., Rafiee M.H., Aghaei A. Evaluation of anticancer activity of alpha-defensins purified from neutrophils trapped in leukoreduction filters. Life Sci. 2019 May;1(224):249–254. doi: 10.1016/j.lfs.2019.03.072. PubMed PMID: 30935951. Epub 2019/04/03. eng. [DOI] [PubMed] [Google Scholar]
- 49.Vossier L., Leon F., Bachelier C., Marchandin H., Lehmann S., Leonetti J.P. An innovative biologic recycling process of leukoreduction filters to produce active human antimicrobial peptides. Transfusion. 2014;5(54) doi: 10.1111/trf.12444. 1332-9. [DOI] [PubMed] [Google Scholar]
- 50.Cederlund A., Agerberth B., Bergman P. Specificity in killing pathogens is mediated by distinct repertoires of human neutrophil peptides. J Innate Immun. 2010;2(6):508–521. doi: 10.1159/000317665. [DOI] [PubMed] [Google Scholar]
- 51.Gallup G.G., Jr., Suarez S.D. Alternatives to the use of animals in psychological research. Am Psychol. 1985;40(October (10)):1104–1111. doi: 10.1037//0003-066x.40.10.1104. PubMed PMID: 3907439. Epub 1985/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 52.Richmond J. Refinement, reduction, and replacement of animal use for regulatory testing: future improvements and implementation within the regulatory framework. Ilar j. 2002;43(Suppl):S63–S68. doi: 10.1093/ilar.43.suppl_1.s63. PubMed PMID: 12388854. Epub 2002/10/22. eng. [DOI] [PubMed] [Google Scholar]
- 53.Seok J., Warren H.S., Cuenca A.G., Mindrinos M.N., Baker H.V., Xu W. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leist M., Hartung T. Inflammatory findings on species extrapolations: humans are definitely no 70-kg mice. Arch Toxicol. 2013;87(4):563–567. doi: 10.1007/s00204-013-1038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]