Abstract
Circadian rhythms are ~24-hour cycles of behavior and physiology that are generated by a network of molecular clocks located in nearly every tissue in the body. In mammals, the circadian system is organized hierarchically such that the suprachiasmatic nucleus (SCN) is the main circadian clock that receives light information from the eye and entrains to the light-dark cycle. The SCN then coordinates the timing of tissue clocks so internal rhythms are aligned with environmental cycles. Estrogens interact with the circadian system to regulate biological processes. At the molecular level, estrogens and circadian genes interact to regulate gene expression and cell biology. Estrogens also regulate circadian behavior across the estrous cycle. The timing of ovulation during the estrous cycle requires coincident estrogen and SCN signals. Studies using circadian gene reporter mice have also elucidated estrogen regulation of peripheral tissue clocks and metabolic rhythms. This review synthesizes current understanding of the interplay between estrogens and the circadian system, with a focus on female rodents, in regulating molecular, physiological, and behavioral processes.
Keywords: Circadian rhythm, estradiol, suprachiasmatic nucleus, mouse, clock genes, female
1. Introduction
Most biological processes, from gene expression to complex behaviors, fluctuate on a ~24-hour cycle and are called circadian rhythms (the Latin ‘circa diem’ means ‘around a day’) [1]. Circadian rhythms are generated by endogenous molecular clocks so they persist in the absence of external input. However, they also entrain to, or synchronize with, environmental cycles caused by the rotation of the earth on its axis [2]. In this way, circadian rhythms allow animals to anticipate predictable, daily changes in the environment to optimize the timing of behavior and physiology and improve fitness and longevity [3–5].
The mammalian circadian system is a multi-oscillator network organized hierarchically to coordinate internal rhythms with external cycles [6]. Nearly every cell in the body has a circadian rhythm and timing of cellular clocks are coordinated within tissues and organs [7]. In mammals, the suprachiasmatic nucleus (SCN) in the hypothalamus is at the top of the hierarchy. The SCN receives input about ambient lighting from the retina and entrains to the light-dark cycle. The SCN then coordinates the timing, or phases, of circadian clocks in central and peripheral tissues in the body.
Estrogens regulate a myriad of biological processes from gene expression to reproduction to complex behaviors [8–11]. Estrogens are steroid hormones mostly produced by ovaries, but also by the brain, adipose tissue, stomach, bone, and skin in female rodents [10]. Estrogen signaling is mediated by several types of estrogen receptors that are expressed throughout the body [8].
Decades of research have demonstrated that the circadian and endocrine systems interact to coordinate the timing of hormone production and secretion with reproductive physiology and behaviors [11–13]. Recent studies, many of them using estrogen receptor knockout and circadian reporter mice, have elucidated additional interactions between estrogens and the circadian system. In this article, we synthesize current understanding of the interplay between estrogens and the circadian system with a focus on female rodents.
2. Early discoveries of the interplay between circadian timing and estrogens
The estrous cycle is 4 to 5 days in female mice (Fig. 1) [14]. The four stages of the estrous cycle-metestrus, diestrus, proestrus, and estrus-are characterized by distinct hormonal milieus that drive changes in vaginal cell typology. Ovulation and the induction of estrus (“heat”) are caused by a surge of luteinizing hormone (LH) released from the anterior pituitary during the late afternoon/early night of proestrus [11]. The precise timing of the LH surge was early evidence that the circadian system regulated reproduction in female rodents. Herein we describe these early studies of the interplay between the circadian system and estrogens.
Figure 1. The luteinizing hormone (LH) surge and ovulation are precisely timed during the estrous cycle in female mice.

The estrous cycle is 4–5 days in female mice. A 5-day estrous is shown as female mice often remain in diestrus for >24h when they are housed without a male. Circulating estrogens increase during diestrus and proestrus and peak at the LH surge. The LH surge occurs in the late afternoon/early night (ZT12 in mice) of proestrus and signals the beginning of estrus, or heat. Ovulation occurs ~12h later, coincident with copulatory behavior.
2.1. First studies of circadian timing and estrogens
Early studies characterized the timing of reproduction in rodents [15, 16]. Ovulation and mating occurred during the late night or early morning of estrus and the timing was reversed by inverting the light-dark cycle [17]. The LH surge occurred reliably in the late afternoon/early night of proestrus [Zeitgeber time (ZT) 10 in rats and ZT12 in mice, where ZT0 is lights on]. [18]. In 1949, Sawyer, Everett, and Markee proposed that the LH surge and ovulation depended on a “neural timing factor” [19]. This hypothesis was supported in the seminal study by Everett and Sawyer in 1950 that found the neural “LH release apparatus” in rats must occur in the early afternoon for the LH surge and subsequent ovulation to appropriately occur (Fig. 2) [20]. They administered pentobarbital, which depressed neuronal excitability and thus blocked neural signals, at different times of day. In rats, the LH surge typically occurred in the late afternoon (~16:00) of proestrus, and ovulation occurred 9–11 hours later (Fig. 2A). When pentobarbital was injected at 14:00, the LH surge and ovulation did not occur later that day and instead were delayed by 24 hours (Fig. 2B). However, when pentobarbital was injected at 16:00, the LH surge and ovulation occurred as usual (Fig. 2C). These studies provided definitive evidence that a neurogenic signal temporally gated the LH surge in female rodents [17]. Thirty years later, this neural factor was shown to be the main circadian clock in the SCN (see Section 5).
Figure 2. Everett and Sawyer showed that a neural timing signal on the afternoon of proestrus was necessary for ovulation.

Female rats housed in 14L:10D had LH surges at 16:00 and ovulated 9–11 hours later at 01:00 (A). When the rats were treated with Nembutal (pentobarbital) at 14:00, the LH surge and ovulation were delayed by 24h (B). When the rats were treated with Nembutal at 16:00, the LH surge and ovulation occurred at the usual times (C). These experiments showed that the “LH release apparatus” was a timed neural signal that acted during the “time limits of pituitary activation” to induce the LH surge [20].
Circadian regulation of reproductive cycles was also apparent in experiments that manipulated the period of the circadian rhythm. Conditions changing the period of the circadian rhythm coordinately altered the duration of the estrous cycle. For example, housing hamsters or mice in constant lighting conditions or giving them deuterated water lengthened the period of the circadian rhythm and coordinately lengthened the estrous cycle [21–23].
2.2. Scalloping: Variations in locomotor activity across the estrous cycle
In 1977, Morin, Fitzgerald, and Zucker described “scalloping” of wheel-running activity of female hamsters, because the onset of activity fluctuated in a scalloped pattern every 4 days [24]. The phase of activity was advanced during proestrus, and this scalloping was eliminated in ovariectomized hamsters. Treatment of ovariectomized hamsters with estradiol (but not progesterone) caused the onset of activity to be persistently advanced, which was likely due to shortening of the endogenous period by estradiol.
Rats also display scalloping. In 1923, Wang found that peaks of activity were linked to the estrous cycle since sexually immature and ovariectomized rats did not have 4-day cycles of activity peaks [25]. In 1981, Albers et al. formally described that the phase of activity onset was advanced >30 minutes in rats during estrus [26]. Like hamsters, this phase advance was attributed to shortening of the endogenous period by estradiol in rats [27]. The duration of activity (α) was also ~1 hour longer during estrus compared to the other phases [26, 28, 29].
Female mice also show variations in activity over the estrous cycle, but their phases of activity onset do not vary. Instead, like rats, the amount of spontaneous wheel-running activity increases on the night of proestrus [30–33]. In mice, this variation in activity across the estrous cycle varies by strain and diminishes with aging, is most clearly observed with wheel-running activity, and is sometimes not observed [30, 31, 34]. Together, these studies show that fluctuations in estrogen levels across the estrous cycle overtly alter locomotor activity rhythms in female rodents.
2.3. The SCN is required for the circadian timing of the LH surge and ovulation
Even before the SCN was identified as the main circadian clock, many studies had implicated the anterior hypothalamus as a site of regulation of the estrous cycle and ovulation (reviewed in [35]). After the discovery of the SCN in 1976, several labs showed that the SCN in the anterior hypothalamus was necessary for ovulation in female rodents [36, 37]. It was later shown that SCN lesions abolished the circadian timing of the LH surge (see Section 5) [37, 38]. Additionally, hamsters housed in constant light with “split” activity rhythms, or two bouts of activity per day, also had two LH surges a day [39]. The split activity rhythms were later shown to reflect antiphase oscillations of the right and left SCN, further demonstrating that the timing of the LH surge is gated by the SCN [40]. These classical studies were the beginning of a field of study that continues today to investigate SCN control of the LH surge.
3. Molecular links between estrogens and circadian rhythms
3.1. The molecular circadian timekeeping mechanism
The circadian timekeeping mechanism is a self-sustained transcriptional-translational feedback loop of circadian genes (Figure 3A) [41]. This molecular mechanism, or cellular “clock,” is present in nearly every differentiated cell in mice. The transcription factors BMAL1 and CLOCK (or paralog NPAS2) heterodimerize and bind to E-box enhancer elements to drive Period (Per1, Per2, and Per3) and Cryptochrome (Cry1 and Cry 2) gene expression in mice. The Per and Cry transcripts translocate to the cytoplasm, where they are translated. PER and CRY then dimerize, and their stability is regulated by phosphorylation. Then, PER-CRY complexes inhibit transactivation by BMAL1-CLOCK, thereby inhibiting their own transcription. This transcription-translation loop takes approximately 24h to complete one cycle and thus sets the circadian period of cells. Bmal1 transcription is also modulated by an accessory loop, where nuclear receptors RORα and REV-ERBα upregulate or inhibit Bmal1 expression, respectively.
Figure 3. The molecular circadian timekeeping mechanism and clock output in mammals.
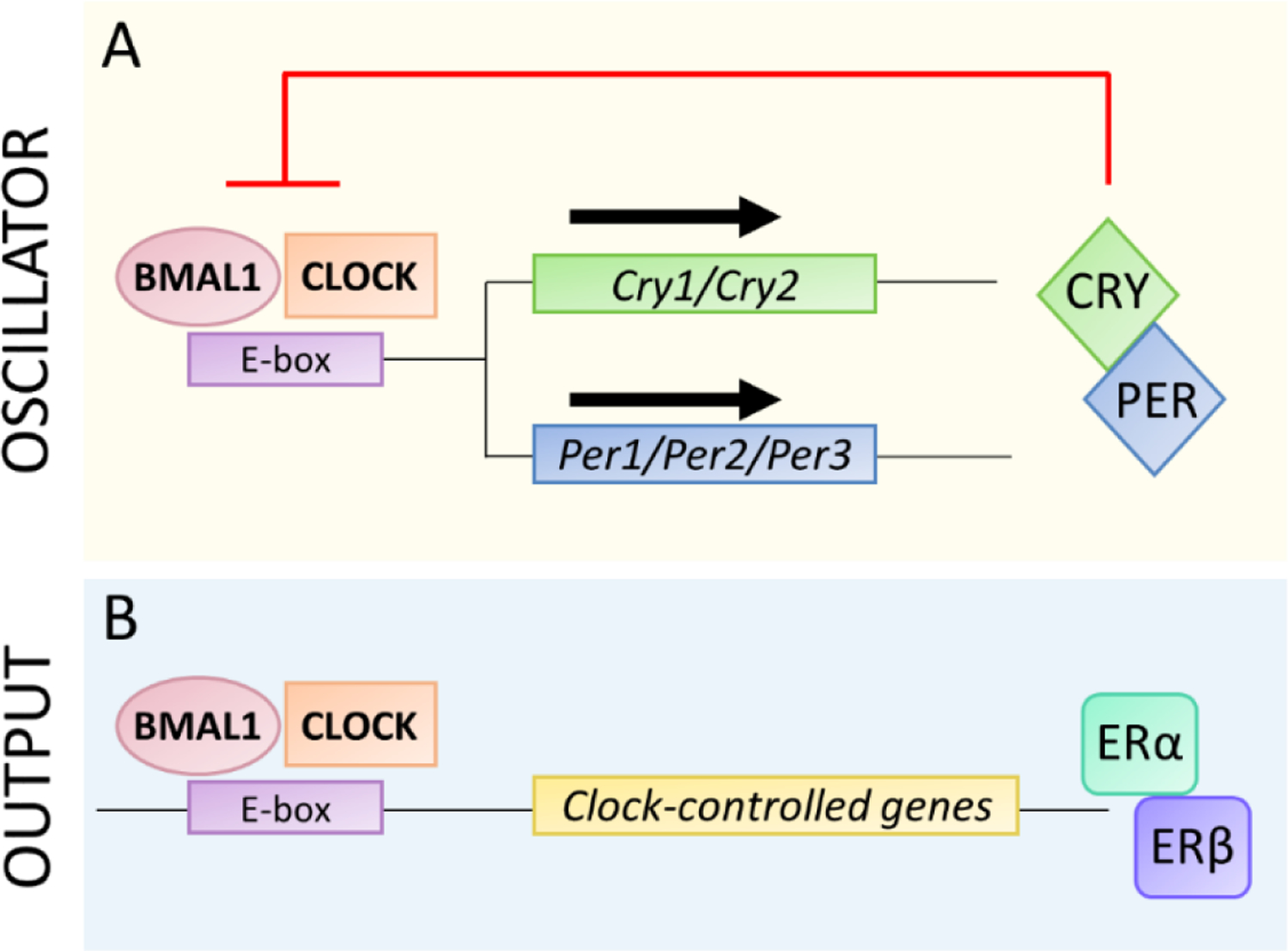
A. The transcription factors BMAL1 and CLOCK heterodimerize and bind to E-boxes in the promotor regions of the Cry and Per genes, which are then transcribed and translated. CRY and PER heterodimerize, are phosphorylated (not shown), and then feedback to inhibit transactivation by BMAL1-CLOCK.
B. BMAL1-CLOCK also binds to E-boxes to drive rhythmic transcription of many clock-controlled genes, including ERα and ERβ, which are outputs of the molecular clock.
Many genes contain E-boxes, thus rhythmic transcriptional activity of the BMAL1-CLOCK heterodimer also results in rhythmic expression of thousands of genes, called clock-controlled genes, that are outputs of circadian clocks (Figure 3B). This widespread E-box-mediated gene regulation serves as a link between the core circadian timekeeping mechanism and regulation of rhythms throughout the body.
3.2. Estrogen signaling mechanisms
Molecular estrogen signaling mechanisms in mammals are complex and are typically categorized genomic and non-genomic (reviewed in [8]). Genomic estrogen signaling occurs when dimers of nuclear receptors ERα and/or ERβ bind to specific DNA sequences, called estrogen response elements (EREs), in the regulatory regions of genes and assemble transcription regulation complexes. Non-genomic, rapid signaling occurs when estrogens bind to membrane-bound ERs, including ERα, ERβ, GPER1, Gq-mER, and ER-X [42]. Studies investigating the interplay between circadian rhythms and estrogen signaling have thus far focused on direct genomic estrogen regulation of circadian gene expression via EREs.
3.3. Direct genomic estrogen regulation of circadian gene expression
Estrogens directly regulate the expression of some circadian genes via the estrogen response element (ERE), which is a palindromic in inverted repeat (5’-GGTCAnnnTGACC-3’) [43]. Several circadian genes are regulated by estrogens, but direct regulation via the ERE has been demonstrated for only the Period2 and Clock genes.
Human and mouse Period2 genes have imperfect EREs in their 5’ flanking regulatory regions [44, 45]. Studies in human mammary epithelial and breast cancer cell lines showed that Per2 gene expression is increased when estrogen-ERα binds to the Per2 ERE [44, 46]. The interplay between PER2 and ERα signaling is necessary for normal morphogenesis of breast acinar cells, as knocking down either PER2 or ERα disrupts this process [46]. Thus, estrogens directly regulate Per2 expression rhythms via the ERE in human cells.
Estrogen regulates Per2 expression rhythms in rodents, but the effects vary by species and tissue [45]. Chronic estradiol treatment, compared to cholesterol treatment, of ovariectomized rats altered Per2 expression rhythms in liver, uterus, and kidney, but not in SCN and cortex [45]. The amplitude of the PER2 rhythm in the rat limbic forebrain was low during metestrus and diestrus and estradiol treatment of ovariectomized rats reduced PER2 expression in the limbic forebrain [47]. Notably, estrogen inhibited Per2 expression in rat tissues, but induced Per2 expression in human epithelial cells. In mice, estradiol treatment of uterus, but not SCN, shortened the period of the PERIOD2::LUCIFERASE rhythm [48]. Thus, estrogen regulation of Per2 expression is complex and varies by tissue and species.
The human Clock gene promoter contains one ERE and one ERE-half site [49]. Treatment of ERα-positive breast cancer cell lines increased the expression of Clock mRNA and protein, and knockdown of ERα inhibited this effect [49]. Chromatin immunoprecipitation also showed that ERα bound to the Clock promoter at regions containing the half-ERE and ERE sites [49]. Point mutations in either the half-ERE or ERE site reduced estradiol-ERα activation of a Clock promoter-luciferase reporter [49]. However, estradiol-ERα was still able to upregulate, albeit to a lesser degree, Clock promoter-luciferase activity, even when both ERE sites were mutated. Together, these data suggest that Clock expression is upregulated by estradiol-ERα via both direct binding to the ERE and via indirect mechanisms.
The mouse Per1 promoter contains half-ERE sequences [45]. Chronic estradiol treatment of ovariectomized rats did not affect the Per1 expression rhythm in SCN and cortex but increased the amplitude and delayed the phase of the Per1 expression rhythm in liver and kidney [45]. Estradiol treatment in ovariectomized rats also increased Per1 expression in uterus and uterine stromal cells [50]. It is not known whether estrogen regulates Per1 expression directly via the half-ERE sites, or indirectly. The Bmal1 gene does not have an ERE [46]. In human epithelial cell lines, Bmal1 expression is altered by estrogen treatment, but this is likely an indirect effect downstream of estrogen regulation of Per2 expression [46].
3.4. Direct circadian regulation of estrogen receptor gene expression
The circadian system could directly regulate estrogen signaling if estrogen receptors contain E-boxes in their regulatory regions. CLOCK/BMAL1 heterodimers rhythmically bind to E-boxes [41]. Thus, genes containing E-boxes are often expressed with a daily or circadian rhythm.
The human Esr1 (ERα) gene has an imperfect E-box and it is expressed with a circadian rhythm in human mammary epithelial cell lines that have circadian clocks [51, 52]. In contrast, ERα is not rhythmic in human mammary cancer cell lines that lack circadian rhythms of the canonical circadian genes [51]. Additionally, mutation of the E-box in the ERα ERE reduced activation of a luciferase reporter in MCF-7 cells [52]. These data suggest that CLOCK:BMAL1 regulates ERα expression via its E-box, but this direct regulation has not been tested experimentally.
The Esr2 (ERβ) gene promoters in mouse, human, chimpanzee, and macaque contain E-boxes [53]. In mouse lung, ERβ mRNA is expressed with a circadian rhythm when in constant dark conditions [53]. ERβ mRNA is also rhythmic in mouse skeletal muscle, and this rhythm was abolished in BMAL1 KO mice [53]. In vitro studies further showed that mutating the E-box in the mouse ERβ promoter inhibited CLOCK/BMAL1 regulation of ERβ transcription [53]. Together these studies showed that ERβ expression is directly regulated by the circadian clock via its E-box in mice.
To our knowledge, no study has investigated whether the other estrogen receptors, GPER1, Gq-mER, and ER-X, are expressed with circadian rhythms or whether they contain E-boxes.
3.5. Reciprocal regulation between circadian and estrogen signaling
There is also reciprocal regulation between circadian rhythms and estrogen signaling that do not rely on EREs or E-boxes. These interactions instead involve direct binding of circadian and ER proteins and post-translational modifications of circadian genes. PER2 directly binds to ERα in human breast cancer MCF-7 cells [44]. Binding of PER2 to ERα enhances ERα degradation and knockdown of PER2 stabilizes ERα. In this way PER2 may impact mammary epithelial cell growth, since overexpression of PER2 inhibits growth in MCF-7 cells.
CLOCK directly binds to ERα and enhances estrogen-ERα transcriptional activity [54]. Estradiol treatment promoted the colocalization of CLOCK and ERα in the nucleus of MCF-7 cells [54]. Overexpression of CLOCK and ERα in MCF-7 cells treated with estradiol exacerbated ERE-luciferase activity compared to overexpression of either CLOCK or ERα alone. In addition, siRNA knockdown of CLOCK expression inhibited ERα-mediated ERE-luciferase activity. These data suggest that CLOCK may act a co-factor of to enhance estradiol-ERα transcriptional activity.
Estrogen induces post-translational modifications of CLOCK to regulate its transcriptional activity [54]. Sumoylation is a post-translational modification that alters localization, stability, or function of proteins. Estradiol treatment increased sumoylation of CLOCK and the transcription of luciferase reporter by CLOCK [54]. Sumoylation directly regulated CLOCK transcription factor activity since desumoylation with a protease reduced CLOCK transactivation. Thus, estrogens may regulate circadian functions by modulating transcriptional activity of CLOCK via post-translational modifications.
4. Estrogens and the SCN
4.1. The SCN is the main circadian pacemaker
The bilateral SCN in the anteroventral hypothalamus has approximately 20,000 neurons in mice [41]. Individual SCN neurons have cell-autonomous, endogenous rhythms generated by the transcriptional-translational feedback loop of circadian gene expression (Fig. 3). These individual cellular oscillators are coupled to each other to generate a robust, coherent rhythm from the whole SCN. The SCN is heterogeneous and is divided into core and shell regions. The ventral core region sits just above the optic chiasm and receives input directly from the retina. The core is anatomically defined by abundant vasoactive intestinal peptide (VIP) neurons. The shell wraps around and receives input from core neurons and is anatomically defined by arginine vasopressin (AVP) neurons. Other neurons in the SCN express a variety of neurochemicals and outputs of the SCN are neuronal and humoral.
4.2. Estrogens and SCN molecular timekeeping
Several lines of evidence suggest that estrogens do not have marked, direct effects on SCN timekeeping. First, radiolabeled estradiol does not accumulate in high concentrations in SCN of hamsters and rats [55, 56]. Second, ER expression is sparse in the SCN. ERα and ERβ mRNA and protein are absent or sparse in the SCN of adult female rats and hamsters [57–61]. In female mice, ERβ is highly expressed in the SCN (mostly in the shell), while ERα expression is sparse [62–65]. GPER is highly expressed in the mouse SCN, but this receptor regulates rapid effects that have not been studied in the SCN [66]. Third, estradiol treatment of SCN explants in culture does not alter circadian rhythms of expression of canonical circadian gene reporters [48, 67].
The effects of estrogens on circadian gene expression rhythms in the SCN are subtle. Cry 2, but not Cry 1, expression was decreased by a single estradiol injection in ovariectomized rats [68]. However, this study examined Cry expression at only one timepoint, but not the rhythm of Cry2 expression. Treatment of ovariectomized rats with supraphysiological estradiol levels (via Silastic tubing implants) did not affect Per1 mRNA rhythms [45]. In addition, the amplitude and phase of PER2::LUC expression in the mouse SCN did not vary across the estrous cycle [69]. In vivo estradiol (supraphysiological) treatment of ovariectomized rats advanced the phase of Per2 mRNA expression by ~2.5h in the SCN, but in vitro estradiol treatment of rodent SCN explants did not affect Per1-luc or PER2::LUC rhythms [45, 48, 67]. Together these data suggest that the effects of estradiol on circadian gene expression in the SCN are indirect, may occur only with supraphysiological levels of circulating estradiol, and may be mediated by extra-SCN brain loci since the effects are only observed in vivo.
Estradiol directly alters neuronal activity in the SCN. In vitro estradiol treatment of SCN explants from male rats increased the spontaneous firing frequency and excitatory synaptic transmission of neurons in the ventromedial SCN [70]. This estradiol-mediated increase in SCN neuron excitability was blocked by the estrogen receptor antagonist, ICI182780. Thus, there is a direct and rapid effect of estradiol on SCN cell excitability that is mediated by estrogen receptors in vitro. Estradiol also regulates c-Fos expression, a marker of neuronal activation, in SCN neurons. c-Fos expression in the SCN is rhythmic and peaks during the light phase. Ovariectomized rats have reduced c-Fos expression in the SCN during the daytime, and estradiol treatment (single injections or chronic implants) of ovariectomized rats restored high c-Fos expression during the light phase [71, 72]. Thus, estradiol increases SCN neuron activation.
4.3. Estrogens and the free-running activity rhythm
The endogenous period (τ) of animals is measured when they are housed in constant conditions (e.g., constant darkness), and is called the free-running rhythm. The SCN encodes the period of the free-running activity rhythm in rodents [73]. In hamsters, rats, and mice, ovariectomy lengthened and estradiol treatment shortened the free-running period of the activity rhythm [24, 27, 28, 74–76]. The effect of estrogens on the period of the activity rhythm likely underlies the phase advance during proestrus/estrus in hamsters and rats, resulting in scalloping (see Section 2.2) [24, 26]. Estrogen regulation of free-running period occurs in female, but not male, hamsters and rats and requires sexual differentiation of the brain to the female phenotype [27, 74]. When female hamsters and rats were exposed to androgens during development to masculinize their brains, estradiol treatment in adulthood did not consistently shorten the periods of their activity rhythms [27, 74].
Studies in estrogen receptor mutant mice have begun to elucidate that molecular mechanisms underlying estrogen regulation of free-running period. In mice, estradiol shortened free-running wheel activity rhythms via ERα, since treatment with an ERα agonist shortened the period [75, 76]. High, but not low, doses of ERβ agonists also shortened free-running period in mice, but the high dose may not mimic physiological activation of ERβ [76]. Non-genomic ERα signaling, and perhaps genomic ERα signaling, mediate estradiol regulation of free-running period, since estradiol did not affect period in global ERα KO and non-classical estrogen knock-in (NERKI, lack genomic signaling via ERE) female mice [75].
4.4. Estrogens and circadian responses to light
The light responses of the circadian system vary across the 24-h cycle. Light pulses during the early night cause phase delays, light pulses during late night cause phase advances, and light during the subjective day causes no phase shift, of activity rhythms [77]. Circadian responses to light are rarely studied in females, but several studies have suggested a role for estrogens [78]. Ovariectomized mice had smaller phase delays compared to cycling female mice [79]. Likewise, female ERα knockout mice had decreased phase delays compared to wild-types [80]. Together, these data suggested that ERα signaling accentuated light-induced phase delays. However, subsequent studies reported conflicting results. Estradiol treatment of ovariectomized mice reduced phase delays [76]. Additionally, Mizuta et al. found that phase responses to light did not differ across the estrous cycle [33]. Thus, estrogen regulation of circadian responses to light may be observed only when hormonal milieus are disrupted by ovariectomy or ER knockout.
5. Circadian timing and reproductive functions of estrogens
5.1. Circadian timing in the female hypothalamic-pituitary-gonadal axis
The hypothalamic-pituitary-gonadal (HPG) axis in female rodents governs the estrous cycle and reproduction (Figure 4) [81]. Gonadotropin-releasing hormone (GnRH) is secreted into the hypophyseal portal circulation to stimulate the release of follicle stimulating hormone (FSH) and luteinizing hormone (LH) from gonadotropes in the anterior pituitary into systemic circulation. These gonadotropins promote follicle development and thus production of estrogens and progesterone. For most of the estrous cycle, estrogens negatively feedback to inhibit the release of LH, and consequently inhibit development of more follicles. However, on the afternoon of proestrus, when circulating estrogen levels are the highest, estrogen feedback to GnRH neurons switches from negative to positive [82]. This switch causes rapid firing of GnRH neurons, the release of a GnRH surge into portal circulation, and an LH surge just before dark. The LH surge stimulates ovulation about 12h later during estrus, or heat. The precise timing of the GnRH and LH surges are therefore critical to align fertility with sexual receptivity. This section will focus on the mechanisms by which the circadian system interacts with estrogen signaling to precisely time GnRH and LH surges during the estrous cycle. Other hormones (e.g., FSH, progesterone, prolactin) that function in the HPG axis are beyond the scope of this review [11, 81].
Figure 4. Estrogens and the circadian system interact in the HPG axis.

The SCN regulates activation of GnRH neurons via direct VIPergic projections to the POA and indirect AVPergic projections to kisspeptin (KISS) neurons in the AVPV. As the ovarian follicle develops, it releases increasing amounts of estrogens. On the afternoon of proestrus, high levels of estrogens coincide with the SCN-dependent daily timing signal to stimulate the GnRH surge, and subsequently the LH surge and ovulation.
5.2. Estrogens and the SCN act coordinately to regulate the timing of GnRH and LH surges
Circadian timing of GnRH release is necessary for the preovulatory LH surge [83]. In mice, GnRH neuron cell bodies are dispersed, but located primarily in the hypothalamic preoptic area (POA) and medial septum (Figure 4) [84]. The processes of GnRH neurons extend into the mediobasal hypothalamus where they release GnRH into the hypophyseal portal circulation. For most of the estrous cycle, GnRH release is pulsatile [81]. However, during proestrus a GnRH surge is released during the afternoon [83]. The release of the GnRH surge during a defined time window depends on two factors. First, the level of circulating estrogens must be elevated. Second, the SCN gates the time window of GnRH release.
High levels of circulating estrogens are required for the GnRH surge on the afternoon of proestrus in female mice. The developing ovarian follicle produces estrogens that peak during proestrus (Figure 1). Low levels of estrogens do not affect the firing rate of GnRH neurons; high physiological levels of estrogens (e.g. proestrus preovulatory levels) differentially affect GnRH neurons depending on time of day [82, 85]. In the morning, high estradiol suppressed firing rates in GnRH neurons in brain slice cultures. In the late afternoon, high estradiol increased the firing rate of GnRH neurons, resulting in greater GnRH released at the median eminence in slice culture [86]. Ovariectomized mice do not have a daily rhythm in GnRH firing rate in the POA and median eminence, thus estrogens are necessary for regulating GnRH neuronal activation [82]. ERα, but not ERβ, is necessary and sufficient for positive estrogen feedback on the GnRH surge in female mice [87, 88]. Estrogen-induced LH surges and c-Fos expression in GnRH neurons were absent in ERα KO female mice and restored in ovariectomized mice treated with ERα agonist [87–89]. GnRH neurons in mice and rats do not express ERα, so estrogen-ERα regulation of GnRH cells must be indirect [90, 91]. In sum, there is a daily rhythm of estrogen feedback, via ERα, on GnRH neuron activation that promotes the GnRH surge on the afternoon of proestrus when estrogen levels are elevated.
The SCN is required to gate the time window of GnRH release. SCN lesions eliminate the circadian timing of the LH surge in rats and mice [37, 38, 92]. Every day, at the end of the light phase, the SCN signals to GnRH neurons to make them sensitive to activation by high levels of estrogens. Since estrogens only reach this critical level on proestrus, the GnRH surge is released only on late afternoon of proestrus. However, if ovariectomized rodents are treated with constitutively high estradiol, they have an LH surge every afternoon, demonstrating that the SCN signal occurs daily [18, 82, 93].
Several lines of evidence show that the SCN temporally gates GnRH release via neural, rather than paracrine, mechanisms. First, pentobarbital administered during the afternoon of proestrus blocked the GnRH-induced LH surge, even in the presence of high circulating estradiol levels (Fig. 2) [20]. Second, complete deafferentation of the SCN with knife cuts, which severs nerves but does not block release of diffusible factors, abolished the LH surge [94]. Third, there are direct and indirect, estrogen-sensitive neuronal pathways from the SCN to GnRH neurons [95, 96]. Herein we focus on the role of the SCN in regulating the timing of GnRH and LH surges (see [97] for other regulators of the GnRH surge).
5.3. Communication of the SCN with GnRH neurons
The SCN regulates the timing of GnRH and LH surges via direct (monosynaptic) and indirect neural pathways (Fig. 4). VIP-containing SCN neurons project directly to the POA and synapse on or near GnRH neurons [84]. Inhibition of VIP expression or VIP function in the SCN of estradiol-treated female rats altered the timing and reduced the amplitude of the LH surge [98, 99]. Likewise, inhibition of the VIP receptor, VPAC2, inhibited the afternoon increase in GnRH firing rate of estradiol-treated ovariectomized mice [100]. Thus, the direct, VIPergic neural pathway from the SCN to the POA is required for normal LH surges.
The indirect neural pathway is also critical for the GnRH surge. AVP-containing SCN neurons project to kisspeptin neurons in the anterior periventricular nucleus (AVPV), and these kisspeptin neurons then project to GnRH neurons in the POA [101, 102]. Multiple lines of evidence indicate that kisspeptin neurons in the AVPV integrate estrogen and SCN signals to gate the GnRH surge. First, AVPV lesions, like SCN lesions, inhibited LH surges in rats [38]. Second, AVP injections into the medial preoptic area (which includes the AVPV) of SCN-lesioned, estradiol-treated, ovariectomized rats induced LH surges [103]. Third, intracerebroventricular infusion of AVP receptor antagonist on the afternoon of proestrus attenuated the LH surge [104]. Fourth, the peak of the circadian rhythm of AVP release (but not VIP release) from the SCN coincided with the peak of GnRH release in brain slice co-cultures of the SCN and POA, suggesting that AVP drives GnRH secretion [105]. Fifth, kisspeptin neurons are estrogen-responsive and express ERα, which is necessary for the LH surge [88]. Elevated estrogens, via ERα, increased kisspeptin expression in the AVPV, and antiestrogen treatment in the medial preoptic area blocked LH surges [106, 107]. Finally, kisspeptin signaling is necessary and sufficient for the LH surge [108–110]. Kisspeptin receptor knockout female mice lacked GnRH-dependent LH surges and intracerebroventricular injection of kisspeptin induced a GnRH-dependent LH surge [108–110]. Together these data suggest that positive estrogen feedback on the afternoon of proestrus converges with the daily timing signal from the SCN in kisspeptin neurons in the AVPV to stimulate the GnRH surge. However, AVPV cells express other neuropeptides which could also regulate the temporal gating of the GnRH surge.
5.4. A network of clocks in the HPG axis
Studies of genetic circadian clock mutants (e.g., ClockΔ19 mutant, Bmal1 KO) have shown that disabling circadian clocks disrupts the estrous cycle and the timing of the LH surge, and compromises fertility [111–113]. However, these effects could be due to disabling the clock in the SCN and/or other body clocks. Tissues at all levels of the HPG axis contain circadian clocks. In the hypothalamus, GnRH neurons in the POA and medial septum and kisspeptin neurons in the AVPV express ~24-hour rhythms of circadian proteins in vivo, suggesting they have functional circadian clocks [114–116]. Ex vivo AVPV brain slices also maintained circadian reporter rhythms in culture, and the period of the rhythm was lengthened by estradiol treatment, demonstrating that the AVPV is an estrogen-sensitive self-sustained clock [116]. The timing of the LH surge was disrupted in mice lacking circadian clocks in GnRH and kisspeptin neurons (cell-specific Bmal1 KO’s) [117]. Thus, circadian clocks in GnRH and kisspeptin neurons are required for proper timing of the LH surge.
The anterior pituitary is also a self-sustained circadian clock because it rhythmically expresses PER1 in vivo, and pituitary explants maintain circadian reporter rhythms in culture [67, 113, 118]. Gonadotrope-specific Bmal1 KO mice had normal LH surges, thus the pituitary circadian clock in gonadotropes is not required for the proper timing of the LH surge [113].
The ovaries are circadian clocks that rhythmically express canonical circadian genes in vivo and ex vivo [69, 119, 120]. The diurnal rhythms of circadian gene expression varied across the estrous cycle in rats, but there was no direct effect of estradiol treatment on circadian reporter rhythms in ovary explants [69]. Female mice with Bmal1 KO in either ovarian thecal or granulosa cells had normal estrous cycles and LH surges [121]. However, the timing of ovulation was disrupted in thecal cell-Bmal1 KO females, and this was likely due to the loss of phasic responsiveness of the follicle to LH [121]. Thus, the circadian clock in ovarian thecal cells is critical for properly timed LH induction of ovulation.
In sum, circadian clocks in the SCN and GnRH and kisspeptin neurons are required for proper timing of the LH surge, while circadian clocks in ovarian thecal cells are required for the responsiveness to LH that results in proper timing of ovulation.
6. Estrogens and metabolic circadian rhythms
The circadian system regulates metabolism by coordinating the timing of food intake with activity and glucose and fat metabolism in peripheral tissues [122]. Estrogens are also potent regulators of metabolism [10]. Recent studies have shown that estrogens impact metabolism by regulating circadian metabolic rhythms.
6.1. Estrogens regulate the circadian rhythm of eating
Estrogens regulate the daily eating rhythm in female rodents. In rats, ovariectomy increased food intake only during the light phase; thus, the daily eating rhythm was blunted and estradiol treatment restored the rhythm [72, 123, 124]. In mice fed low-fat diet, ovariectomy reduced the amplitude and advanced the phase of the eating rhythm [125]. The effects of estrogens on eating rhythms are more drastic when female mice are nutritionally challenged with a high-fat diet. Ovariectomy abolished the high-fat diet eating behavior rhythm, and estradiol treatment after ovariectomy restored the robust eating rhythm [126, 127]. In addition to the effects of estrogens, sex chromosomes (e.g., XX in females) also affected eating rhythms [125, 128]. Thus, estradiol increases the amplitude of the daily eating rhythm, and its effects are amplified under nutritional challenges.
6.1. Estrogens and liver circadian rhythms
The liver is an estrogen-responsive tissue that highly expresses ERα and has low levels of ERβ [10]. ERα is expressed with a circadian rhythm that peaks during the dark phase in male rats, but circadian expression of ERs has not been studied in females [129]. The liver is exposed to estrogens from systemic circulation, and to estrogens synthesized by parietal cells that enter the portal vein [130]. In male rats, estradiol levels in the hepatic artery and portal vein were rhythmic and peaked during the dark phase, but females were not studied [130].
Estrogens regulate the liver circadian clock. Treatment of ovariectomized rats with supraphysiological estradiol levels (via Silastic tubing implants) increased the amplitude and delayed the phase of Per1 mRNA rhythms, but did not alter Per2 mRNA rhythms [45]. Diurnal rhythms of Per1, Per2, and Bmal1 mRNA in the liver also varied across the estrous cycle in rats [69]. The phase of the Bmal1 rhythm was advanced during proestrus and the amplitude of the Per1 rhythm was reduced during estrus, which could be a consequence of rising estradiol levels during diestrus. In vivo, ovariectomized rats had delayed Per1-luc rhythms compared to cycling rats [67]. In sum, circulating estrogens subtly alter circadian gene expression rhythms in the liver.
Striking effects of estrogens on the liver circadian clock are revealed when female mice are challenged with high-fat diet feeding. The liver PER2::LUC rhythm was advanced 4h in ovariectomized compared to gonadally intact female mice fed high-fat diet [126]. The phase of the liver circadian clock rhythm was restored to normal when ovariectomized females were treated with physiological levels of estradiol [127]. Thus, estrogens markedly alter the liver circadian clock under nutritional stress.
7. Conclusions
Decades of research have revealed that estrogens and the circadian system interact at all levels of biological function, from gene expression to complex behaviors. However, many questions remain. The ways in which estrogen fluctuations across the estrous cycle impact circadian clocks in peripheral tissues are largely unknown. In addition, little is known about the role of classical estrogen receptor signaling in peripheral tissue clocks, and there are no studies investigating non-genomic estrogen receptor signaling in central and peripheral circadian clocks. These emerging studies of estrogens and circadian rhythms have the potential to advance our understanding of metabolism, cancer progression, and reproduction in females.
Figure 5. Estradiol regulates daily metabolic rhythms in female mice.

Female C57BL/6J mice were cycling (gonadally intact: A,D), ovariectomized (OVX: B,E) or ovariectomized and implanted with Silastic tubing that delivered physiological levels of estradiol (C,F). A-C. Representative actograms of eating behavior rhythms recorded during fed low-fat diet (LFD) and high-fat diet (HFD) feeding. D-F. Circular histograms show the amplitude of the eating behavior rhythm was reduced by HFD in OVX, but not in cycling females or in OVX females treated with estradiol (length of arrow is amplitude). G. The phase of the liver PER2::LUC rhythm was advanced 4h by HFD in OVX females compared to cycling and estradiol-treated OVX females. *p<0.05. Adapted from [126, 127].
Table 1.
Estrogen regulation of circadian gene expression.
| Circadian gene | ERE | Regulated by E2* | Tissues/Cell lines | References |
|---|---|---|---|---|
| Bmal1 | No | Yes | Human mammary cell lines | [46] |
| Clock | Yes (ERE and ERE-half site) | Yes (direct) | Human breast cancer cell line | [49] |
| Period1 | Yes (ERE-half sites) | Yes | Rat liver, kidney, uterus | [45, 50] |
| Period2 | Yes (imperfect ERE) | Yes (direct) | Rat SCN, liver, uterus, kidney; mouse uterus; human mammary cell lines | [44–48] |
| Period3 | NR | NR | NR | NR |
| Cry1 | NR | NR | NR | NR |
| Cry2 | NR | NR | NR | NR |
ERE: estrogen response element; NR: not reported
Regulation could be via direct via the ERE or indirect. Direct regulation was shown only for Clock and Period2.
Acknowledgments
Funding:
JSP was supported by the National Institutes of Health R01DK124774 and P30DK020579 and the University of Kentucky.
Abbreviations:
- BMAL1
Brain and Muscle ARNT-Like 1
- CLOCK
Circadian Locomotor Output Cycles Kaput
- Cry
Cryptochrome
- ERα
Estrogen receptor alpha protein
- ERβ
Estrogen receptor beta protein
- Esr1
mouse estrogen receptor 1 gene that encodes ERα
- Esr2
mouse estrogen receptor 2 gene that encodes ERβ
- FSH
follicle-stimulating hormone
- GnRH
Gonadotropin-releasing hormone
- LH
Luteinizing hormone
- Per
Period
- SCN
suprachiasmatic nucleus
- ZT
Zeitgeber time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None
References
- [1].Halberg F, [Physiologic 24-hour periodicity; general and procedural considerations with reference to the adrenal cycle], Int Z Vitaminforsch Beih 10 (1959) 225–96. [PubMed] [Google Scholar]
- [2].Pittendrigh CS, Daan S, A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and lability of spontaneous frequency., J Comp Physiol 106 (1976) 333–55. [Google Scholar]
- [3].DeCoursey PJ, Krulas JR, Behavior of SCN-lesioned chipmunks in natural habitat: a pilot study, J Biol Rhythms 13(3) (1998) 229–44. [DOI] [PubMed] [Google Scholar]
- [4].Hurd MW, Ralph MR, The significance of circadian organization for longevity in the golden hamster, J Biol Rhythms 13(5) (1998) 430–6. [DOI] [PubMed] [Google Scholar]
- [5].Spoelstra K, Wikelski M, Daan S, Loudon AS, Hau M, Natural selection against a circadian clock gene mutation in mice, Proceedings of the National Academy of Sciences of the United States of America 113(3) (2016) 686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H, Resetting central and peripheral circadian oscillators in transgenic rats, Science 288(5466) (2000) 682–5. [DOI] [PubMed] [Google Scholar]
- [7].Welsh DK, Logothetis DE, Meister M, Reppert SM, Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms, Neuron 14(4) (1995) 697–706. [DOI] [PubMed] [Google Scholar]
- [8].Hewitt SC, Korach KS, Estrogen Receptors: New Directions in the New Millennium, Endocr Rev 39(5) (2018) 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rivera HM, Stincic TL, Estradiol and the control of feeding behavior, Steroids 133 (2018) 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mauvais-Jarvis F, Clegg DJ, Hevener AL, The role of estrogens in control of energy balance and glucose homeostasis, Endocr Rev 34(3) (2013) 309–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Williams WP 3rd, Kriegsfeld LJ, Circadian control of neuroendocrine circuits regulating female reproductive function, Front Endocrinol (Lausanne) 3 (2012) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kriegsfeld LJ, Silver R, The regulation of neuroendocrine function: Timing is everything, Horm Behav 49(5) (2006) 557–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miller BH, Takahashi JS, Central circadian control of female reproductive function, Front Endocrinol (Lausanne) 4 (2013) 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Allen E, The oestrous cycle in the mouse, American Journal of Anatomy 30 (1922) 297–371. [Google Scholar]
- [15].Braden AW, Austin CR, Fertilization of the mouse egg and the effect of delayed coitus and of hot-shock treatment, Aust J Biol Sci 7(4) (1954) 552–65. [DOI] [PubMed] [Google Scholar]
- [16].Snell GD, Fekete E, Hummel KP, Law LW, The Relation of Mating, Ovulation, and the Estrous Smear in the House Mouse to Time of Day, The Anatomical Record 76(1) (1940). [Google Scholar]
- [17].Bingel AS, Schwartz NB, Timing of LH release and ovulation in the cyclic mouse, J Reprod Fertil 19(2) (1969) 223–9. [DOI] [PubMed] [Google Scholar]
- [18].Legan SJ, Coon GA, Karsch FJ, Role of estrogen as initiator of daily LH surges in the ovariectomized rat, Endocrinology 96(1) (1975) 50–6. [DOI] [PubMed] [Google Scholar]
- [19].Sawyer CH, Everett JW, Markee JE, A neural factor in the mechanism by which estrogen induces the release of luteinizing hormone in the rat, Endocrinology 44(3) (1949) 218–33. [DOI] [PubMed] [Google Scholar]
- [20].Everett JW, Sawyer CH, A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation, Endocrinology 47(3) (1950) 198–218. [DOI] [PubMed] [Google Scholar]
- [21].Campbell CS, Ryan KD, Schwartz NB, Estrous cycles in the mouse: relative influence of continuous light and the presence of a male, Biol Reprod 14(3) (1976) 292–9. [DOI] [PubMed] [Google Scholar]
- [22].Alleva JJ, Waleski MV, Alleva FR, A biological clock controlling the estrous cycle of the hamster, Endocrinology 88(6) (1971) 1368–79. [DOI] [PubMed] [Google Scholar]
- [23].Fitzgerald K, Zucker I, Circadian organization of the estrous cycle of the golden hamster, Proceedings of the National Academy of Sciences of the United States of America 73(8) (1976) 2923–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Morin LP, Fitzgerald KM, Zucker I, Estradiol shortens the period of hamster circadian rhythms, Science 196(4287) (1977) 305–7. [DOI] [PubMed] [Google Scholar]
- [25].Wang GH, The relation between ‘spontaneous’ activity and oestrous cycle in the white rat, Comparative psychology monographs, Baltimore, Williams & Wilkins Company1923
- [26].Albers HE, Gerall AA, Axelson JF, Effect of reproductive state on circadian periodicity in the rat, Physiol Behav 26(1) (1981) 21–5. [DOI] [PubMed] [Google Scholar]
- [27].Albers HE, Gonadal hormones organize and modulate the circadian system of the rat, Am J Physiol 241(1) (1981) R62–6. [DOI] [PubMed] [Google Scholar]
- [28].Wollnik F, Turek FW, Estrous correlated modulations of circadian and ultradian wheel-running activity rhythms in LEW/Ztm rats, Physiol Behav 43(3) (1988) 389–96. [DOI] [PubMed] [Google Scholar]
- [29].Yokoyama A, Ramirez VD, Sawyer CH, Sleep and wakefulness in female rats under various hormonal and physiological conditions, General and Comparative Endocrinology 7 (1966) 10–17. [Google Scholar]
- [30].Takasu NN, Nakamura TJ, Tokuda IT, Todo T, Block GD, Nakamura W, Recovery from Age-Related Infertility under Environmental Light-Dark Cycles Adjusted to the Intrinsic Circadian Period, Cell Rep 12(9) (2015) 1407–13. [DOI] [PubMed] [Google Scholar]
- [31].Kopp C, Ressel V, Wigger E, Tobler I, Influence of estrus cycle and ageing on activity patterns in two inbred mouse strains, Behav Brain Res 167(1) (2006) 165–74. [DOI] [PubMed] [Google Scholar]
- [32].Juarez-Tapia C, Miranda-Anaya M, Ovariectomy influences the circadian rhythm of locomotor activity and the photic phase shifts in the volcano mouse, Physiol Behav 182 (2017) 77–85. [DOI] [PubMed] [Google Scholar]
- [33].Mizuta S, Sugiyama M, Tokuda IT, Nakamura W, Nakamura TJ, Photic phase-response curves for cycling female mice, Horm Behav 105 (2018) 41–46. [DOI] [PubMed] [Google Scholar]
- [34].Blattner MS, Mahoney MM, Circadian parameters are altered in two strains of mice with transgenic modifications of estrogen receptor subtype 1, Genes Brain Behav 11(7) (2012) 828–36. [DOI] [PubMed] [Google Scholar]
- [35].Everett JW, Central Neural Control of Reproductive Functions of the Adenohypophysis, Physiol Rev 44 (1964) 373–431. [DOI] [PubMed] [Google Scholar]
- [36].Brown-Grant K, Raisman G, Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats, Proc R Soc Lond B Biol Sci 198(1132) (1977) 279–96. [DOI] [PubMed] [Google Scholar]
- [37].Wiegand SJ, Terasawa E, Bridson WE, Goy RW, Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion, Neuroendocrinology 31(2) (1980) 147–57. [DOI] [PubMed] [Google Scholar]
- [38].Wiegand SJ, Terasawa E, Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat, Neuroendocrinology 34(6) (1982) 395–404. [DOI] [PubMed] [Google Scholar]
- [39].Swann JM, Turek FW, Multiple circadian oscillators regulate the timing of behavioral and endocrine rhythms in female golden hamsters, Science 228(4701) (1985) 898–900. [DOI] [PubMed] [Google Scholar]
- [40].de la Iglesia HO, Meyer J, Carpino A Jr., Schwartz WJ, Antiphase oscillation of the left and right suprachiasmatic nuclei, Science 290(5492) (2000) 799–801. [DOI] [PubMed] [Google Scholar]
- [41].Patke A, Young MW, Axelrod S, Molecular mechanisms and physiological importance of circadian rhythms, Nat Rev Mol Cell Biol 21(2) (2020) 67–84. [DOI] [PubMed] [Google Scholar]
- [42].Santollo J, Daniels D, Multiple estrogen receptor subtypes influence ingestive behavior in female rodents, Physiol Behav 152(Pt B) (2015) 431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Klinge CM, Estrogen receptor interaction with estrogen response elements, Nucleic Acids Res 29(14) (2001) 2905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gery S, Virk RK, Chumakov K, Yu A, Koeffler HP, The clock gene Per2 links the circadian system to the estrogen receptor, Oncogene 26(57) (2007) 7916–20. [DOI] [PubMed] [Google Scholar]
- [45].Nakamura TJ, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S, Shinohara K, Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats, J Neurosci Res 82(5) (2005) 622–30. [DOI] [PubMed] [Google Scholar]
- [46].Rossetti S, Corlazzoli F, Gregorski A, Azmi NH, Sacchi N, Identification of an estrogen-regulated circadian mechanism necessary for breast acinar morphogenesis, Cell Cycle 11(19) (2012) 3691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Perrin JS, Segall LA, Harbour VL, Woodside B, Amir S, The expression of the clock protein PER2 in the limbic forebrain is modulated by the estrous cycle, Proceedings of the National Academy of Sciences of the United States of America 103(14) (2006) 5591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nakamura TJ, Sellix MT, Menaker M, Block GD, Estrogen directly modulates circadian rhythms of PER2 expression in the uterus, Am J Physiol Endocrinol Metab 295(5) (2008) E1025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Xiao L, Chang AK, Zang MX, Bi H, Li S, Wang M, Xing X, Wu H, Induction of the CLOCK gene by E2-ERalpha signaling promotes the proliferation of breast cancer cells, PloS one 9(5) (2014) e95878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].He PJ, Hirata M, Yamauchi N, Hattori MA, Up-regulation of Per1 expression by estradiol and progesterone in the rat uterus, J Endocrinol 194(3) (2007) 511–9. [DOI] [PubMed] [Google Scholar]
- [51].Rossetti S, Esposito J, Corlazzoli F, Gregorski A, Sacchi N, Entrainment of breast (cancer) epithelial cells detects distinct circadian oscillation patterns for clock and hormone receptor genes, Cell Cycle 11(2) (2012) 350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].deGraffenried LA, Hilsenbeck SG, Fuqua SA, Sp1 is essential for estrogen receptor alpha gene transcription, J Steroid Biochem Mol Biol 82(1) (2002) 7–18. [DOI] [PubMed] [Google Scholar]
- [53].Cai W, Rambaud J, Teboul M, Masse I, Benoit G, Gustafsson JA, Delaunay F, Laudet V, Pongratz I, Expression levels of estrogen receptor beta are modulated by components of the molecular clock, Molecular and cellular biology 28(2) (2008) 784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Li S, Wang M, Ao X, Chang AK, Yang C, Zhao F, Bi H, Liu Y, Xiao L, Wu H, CLOCK is a substrate of SUMO and sumoylation of CLOCK upregulates the transcriptional activity of estrogen receptor-alpha, Oncogene 32(41) (2013) 4883–91. [DOI] [PubMed] [Google Scholar]
- [55].Krieger MS, Morrell JI, Pfaff DW, Autoradiographic localization of estradiol-concentrating cells in the female hamster brain, Neuroendocrinology 22(3) (1976) 193–205. [DOI] [PubMed] [Google Scholar]
- [56].Pfaff D, Keiner M, Atlas of estradiol-concentrating cells in the central nervous system of the female rat, J Comp Neurol 151(2) (1973) 121–58. [DOI] [PubMed] [Google Scholar]
- [57].Cintra A, Fuxe K, Harfstrand A, Agnati LF, Miller LS, Greene JL, Gustafsson JA, Rapid important paper on the cellular localization and distribution of estrogen receptors in the rat tel-and diencephalon using monoclonal antibodies to human estrogen receptor, Neurochem Int 8(4) (1986) 587–95. [DOI] [PubMed] [Google Scholar]
- [58].Li HY, Blaustein JD, De Vries GJ, Wade GN, Estrogen-receptor immunoreactivity in hamster brain: preoptic area, hypothalamus and amygdala, Brain research 631(2) (1993) 304–12. [DOI] [PubMed] [Google Scholar]
- [59].Simerly RB, Chang C, Muramatsu M, Swanson LW, Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study, J Comp Neurol 294(1) (1990) 76–95. [DOI] [PubMed] [Google Scholar]
- [60].Shughrue PJ, Lane MV, Merchenthaler I, Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system, J Comp Neurol 388(4) (1997) 507–25. [DOI] [PubMed] [Google Scholar]
- [61].Shughrue PJ, Merchenthaler I, Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system, J Comp Neurol 436(1) (2001) 64–81. [PubMed] [Google Scholar]
- [62].Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE, Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha, Endocrinology 144(5) (2003) 2055–67. [DOI] [PubMed] [Google Scholar]
- [63].Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I, The distribution of estrogen receptor-beta mRNA in forebrain regions of the estrogen receptor-alpha knockout mouse, Endocrinology 138(12) (1997) 5649–52. [DOI] [PubMed] [Google Scholar]
- [64].Ehret G, Buckenmaier J, Estrogen-receptor occurrence in the female mouse brain: effects of maternal experience, ovariectomy, estrogen and anosmia, J Physiol Paris 88(5) (1994) 315–29. [DOI] [PubMed] [Google Scholar]
- [65].Zuloaga DG, Zuloaga KL, Hinds LR, Carbone DL, Handa RJ, Estrogen receptor beta expression in the mouse forebrain: age and sex differences, J Comp Neurol 522(2) (2014) 358–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ, Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues, J Endocrinol 202(2) (2009) 223–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Murphy ZC, Pezuk P, Menaker M, Sellix MT, Effects of ovarian hormones on internal circadian organization in rats, Biol Reprod 89(2) (2013) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nakamura TJ, Shinohara K, Funabashi T, Kimura F, Effect of estrogen on the expression of Cry1 and Cry2 mRNAs in the suprachiasmatic nucleus of female rats, Neurosci Res 41(3) (2001) 251–5. [DOI] [PubMed] [Google Scholar]
- [69].Nakamura TJ, Sellix MT, Kudo T, Nakao N, Yoshimura T, Ebihara S, Colwell CS, Block GD, Influence of the estrous cycle on clock gene expression in reproductive tissues: effects of fluctuating ovarian steroid hormone levels, Steroids 75(3) (2010) 203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Fatehi M, Fatehi-Hassanabad Z, Effects of 17beta-estradiol on neuronal cell excitability and neurotransmission in the suprachiasmatic nucleus of rat, Neuropsychopharmacology 33(6) (2008) 1354–64. [DOI] [PubMed] [Google Scholar]
- [71].Peterfi Z, Churchill L, Hajdu I, Obal F Jr., Krueger JM, Parducz A, Fos-immunoreactivity in the hypothalamus: dependency on the diurnal rhythm, sleep, gender, and estrogen, Neuroscience 124(3) (2004) 695–707. [DOI] [PubMed] [Google Scholar]
- [72].Takamata A, Torii K, Miyake K, Morimoto K, Chronic oestrogen replacement in ovariectomised rats attenuates food intake and augments c-Fos expression in the suprachiasmatic nucleus specifically during the light phase, Br J Nutr 106(8) (2011) 1283–9. [DOI] [PubMed] [Google Scholar]
- [73].Ralph MR, Foster RG, Davis FC, Menaker M, Transplanted suprachiasmatic nucleus determines circadian period, Science 247(4945) (1990) 975–8. [DOI] [PubMed] [Google Scholar]
- [74].Zucker I, Fitzgerald KM, Morin LP, Sex differentiation of t-e circadian system in the golden hamster, Am J Physiol 238(1) (1980) R97–101. [DOI] [PubMed] [Google Scholar]
- [75].Blattner MS, Mahoney MM, Estrogen receptor 1 modulates circadian rhythms in adult female mice, Chronobiology international 31(5) (2014) 637–44. [DOI] [PubMed] [Google Scholar]
- [76].Royston SE, Yasui N, Kondilis AG, Lord SV, Katzenellenbogen JA, Mahoney MM, ESR1 and ESR2 differentially regulate daily and circadian activity rhythms in female mice, Endocrinology 155(7) (2014) 2613–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Schwartz WJ, Zimmerman P, Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains, The Journal of neuroscience : the official journal of the Society for Neuroscience 10(11) (1990) 3685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Davis FC, Darrow JM, Menaker M, Sex differences in the circadian control of hamster wheel-running activity, Am J Physiol 244(1) (1983) R93–105. [DOI] [PubMed] [Google Scholar]
- [79].Brockman R, Bunick D, Mahoney MM, Estradiol deficiency during development modulates the expression of circadian and daily rhythms in male and female aromatase knockout mice, Horm Behav 60(4) (2011) 439–47. [DOI] [PubMed] [Google Scholar]
- [80].Blattner MS, Mahoney MM, Photic phase-response curve in 2 strains of mice with impaired responsiveness to estrogens, J Biol Rhythms 28(4) (2013) 291–300. [DOI] [PubMed] [Google Scholar]
- [81].Plant TM, 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-pituitary-gonadal axis, J Endocrinol 226(2) (2015) T41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Christian CA, Mobley JL, Moenter SM, Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity, Proceedings of the National Academy of Sciences of the United States of America 102(43) (2005) 15682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Fink G, 60 YEARS OF NEUROENDOCRINOLOGY: MEMOIR: Harris’ neuroendocrine revolution: of portal vessels and self-priming, J Endocrinol 226(2) (2015) T13–24. [DOI] [PubMed] [Google Scholar]
- [84].Barry J, Immunohistochemistry of luteinizing hormone-releasing hormone-producing neurons of the vertebrates, Int Rev Cytol 60 (1979) 179–221. [DOI] [PubMed] [Google Scholar]
- [85].Chu Z, Andrade J, Shupnik MA, Moenter SM, Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype, The Journal of neuroscience : the official journal of the Society for Neuroscience 29(17) (2009) 5616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Glanowska KM, Venton BJ, Moenter SM, Fast scan cyclic voltammetry as a novel method for detection of real-time gonadotropin-releasing hormone release in mouse brain slices, The Journal of neuroscience : the official journal of the Society for Neuroscience 32(42) (2012) 14664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE, Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility, Neuron 52(2) (2006) 271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Couse JF, Yates MM, Walker VR, Korach KS, Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta, Mol Endocrinol 17(6) (2003) 1039–53. [DOI] [PubMed] [Google Scholar]
- [89].Hoffman GE, Smith MS, Verbalis JG, c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems, Front Neuroendocrinol 14(3) (1993) 173–213. [DOI] [PubMed] [Google Scholar]
- [90].Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL, Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain, Endocrinology 141(9) (2000) 3506–9. [DOI] [PubMed] [Google Scholar]
- [91].Herbison AE, Pape JR, New evidence for estrogen receptors in gonadotropin-releasing hormone neurons, Front Neuroendocrinol 22(4) (2001) 292–308. [DOI] [PubMed] [Google Scholar]
- [92].Gray GD, Soderstein P, Tallentire D, Davidson JM, Effects of lesions in various structures of the suprachiasmatic-preoptic region on LH regulation and sexual behavior in female rats, Neuroendocrinology 25(3) (1978) 174–91. [DOI] [PubMed] [Google Scholar]
- [93].Legan SJ, Karsch FJ, A daily signal for the LH surge in the rat, Endocrinology 96(1) (1975) 57–62. [DOI] [PubMed] [Google Scholar]
- [94].Nomura K, Takahashi S, Kawashima S, Maintenance of estrous cycle in female rats with anterior or posterior deafferentation of the suprachiasmatic nucleus, Neuroendocrinology 47(5) (1988) 444–52. [DOI] [PubMed] [Google Scholar]
- [95].Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM, Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies, J Comp Neurol 384(4) (1997) 569–79. [DOI] [PubMed] [Google Scholar]
- [96].Watts AG, Swanson LW, Sanchez-Watts G, Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat, J Comp Neurol 258(2) (1987) 204–29. [DOI] [PubMed] [Google Scholar]
- [97].Christian CA, Moenter SM, The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges, Endocr Rev 31(4) (2010) 544–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Harney JP, Scarbrough K, Rosewell KL, Wise PM, In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges, Endocrinology 137(9) (1996) 3696–701. [DOI] [PubMed] [Google Scholar]
- [99].van der Beek EM, Swarts HJ, Wiegant VM, Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats, Neuroendocrinology 69(4) (1999) 227–37. [DOI] [PubMed] [Google Scholar]
- [100].Christian CA, Moenter SM, Vasoactive intestinal polypeptide can excite gonadotropin-releasing hormone neurons in a manner dependent on estradiol and gated by time of day, Endocrinology 149(6) (2008) 3130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen CW, Liposits Z, Kallo I, Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen, J Neuroendocrinol 22(9) (2010) 1032–9. [DOI] [PubMed] [Google Scholar]
- [102].Gu GB, Simerly RB, Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat, J Comp Neurol 384(1) (1997) 142–64. [PubMed] [Google Scholar]
- [103].Palm IF, Van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A, Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus, Neuroscience 93(2) (1999) 659–66. [DOI] [PubMed] [Google Scholar]
- [104].Funabashi T, Aiba S, Sano A, Shinohara K, Kimura F, Intracerebroventricular injection of arginine-vasopressin V1 receptor antagonist attenuates the surge of luteinizing hormone and prolactin secretion in proestrous rats, Neurosci Lett 260(1) (1999) 37–40. [DOI] [PubMed] [Google Scholar]
- [105].Funabashi T, Shinohara K, Mitsushima D, Kimura F, Gonadotropin-releasing hormone exhibits circadian rhythm in phase with arginine-vasopressin in co-cultures of the female rat preoptic area and suprachiasmatic nucleus, J Neuroendocrinol 12(6) (2000) 521–8. [DOI] [PubMed] [Google Scholar]
- [106].Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA, Regulation of Kiss1 gene expression in the brain of the female mouse, Endocrinology 146(9) (2005) 3686–92. [DOI] [PubMed] [Google Scholar]
- [107].Petersen SL, Barraclough CA, Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain, Brain research 484(1–2) (1989) 279–89. [DOI] [PubMed] [Google Scholar]
- [108].Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA, A role for kisspeptins in the regulation of gonadotropin secretion in the mouse, Endocrinology 145(9) (2004) 4073–7. [DOI] [PubMed] [Google Scholar]
- [109].Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T, Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat, Biochemical and biophysical research communications 320(2) (2004) 383–8. [DOI] [PubMed] [Google Scholar]
- [110].Dror T, Franks J, Kauffman AS, Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling, Biol Reprod 88(6) (2013) 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS, Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy, Current biology : CB 14(15) (2004) 1367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ratajczak CK, Boehle KL, Muglia LJ, Impaired steroidogenesis and implantation failure in Bmal1−/− mice, Endocrinology 150(4) (2009) 1879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Chu A, Zhu L, Blum ID, Mai O, Leliavski A, Fahrenkrug J, Oster H, Boehm U, Storch KF, Global but not gonadotrope-specific disruption of Bmal1 abolishes the luteinizing hormone surge without affecting ovulation, Endocrinology 154(8) (2013) 2924–35. [DOI] [PubMed] [Google Scholar]
- [114].Hickok JR, Tischkau SA, In vivo circadian rhythms in gonadotropin-releasing hormone neurons, Neuroendocrinology 91(1) (2010) 110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Smarr BL, Gile JJ, de la Iglesia HO, Oestrogen-independent circadian clock gene expression in the anteroventral periventricular nucleus in female rats: possible role as an integrator for circadian and ovarian signals timing the luteinising hormone surge, J Neuroendocrinol 25(12) (2013) 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Chassard D, Bur I, Poirel VJ, Mendoza J, Simonneaux V, Evidence for a Putative Circadian Kiss-Clock in the Hypothalamic AVPV in Female Mice, Endocrinology 156(8) (2015) 2999–3011. [DOI] [PubMed] [Google Scholar]
- [117].Bittman EL, Circadian Function in Multiple Cell Types Is Necessary for Proper Timing of the Preovulatory LH Surge, J Biol Rhythms 34(6) (2019) 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD, Circadian rhythms in isolated brain regions, The Journal of neuroscience : the official journal of the Society for Neuroscience 22(1) (2002) 350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gras S, Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary, Endocrinology 147(8) (2006) 3769–76. [DOI] [PubMed] [Google Scholar]
- [120].Karman BN, Tischkau SA, Circadian clock gene expression in the ovary: Effects of luteinizing hormone, Biol Reprod 75(4) (2006) 624–32. [DOI] [PubMed] [Google Scholar]
- [121].Mereness AL, Murphy ZC, Forrestel AC, Butler S, Ko C, Richards JS, Sellix MT, Conditional Deletion of Bmal1 in Ovarian Theca Cells Disrupts Ovulation in Female Mice, Endocrinology 157(2) (2016) 913–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Green CB, Takahashi JS, Bass J, The meter of metabolism, Cell 134(5) (2008) 728–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Varma M, Chai JK, Meguid MM, Laviano A, Gleason JR, Yang ZJ, Blaha V, Effect of estradiol and progesterone on daily rhythm in food intake and feeding patterns in Fischer rats, Physiol Behav 68(1–2) (1999) 99–107. [DOI] [PubMed] [Google Scholar]
- [124].Nishimura Y, Mabuchi K, Omura N, Igarashi A, Miura M, Mima N, Negishi H, Morimoto K, Takamata A, Fluoxetine Mimics the Anorectic Action of Estrogen and Its Regulation of Circadian Feeding in Ovariectomized Female Rats, Nutrients 12(3) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chen X, Wang L, Loh DH, Colwell CS, Tache Y, Reue K, Arnold AP, Sex differences in diurnal rhythms of food intake in mice caused by gonadal hormones and complement of sex chromosomes, Horm Behav 75 (2015) 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Palmisano BT, Stafford JM, Pendergast JS, High-Fat Feeding Does Not Disrupt Daily Rhythms in Female Mice because of Protection by Ovarian Hormones, Front Endocrinol (Lausanne) 8 (2017) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Omotola O, Legan S, Slade E, Adekunle A, Pendergast JS, Estradiol regulates daily rhythms underlying diet-induced obesity in female mice, Am J Physiol Endocrinol Metab 317(6) (2019) E1172–E1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Link JC, Hasin-Brumshtein Y, Cantor RM, Chen X, Arnold AP, Lusis AJ, Reue K, Diet, gonadal sex, and sex chromosome complement influence white adipose tissue miRNA expression, BMC Genomics 18(1) (2017) 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Eagon PK, DiLeo A, Polimeno L, Francavilla A, Van Thiel DH, Guglielmi F, Starzl TE, Circadian rhythm of hepatic cytosolic and nuclear estrogen receptors, Chronobiology international 3(4) (1986) 207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kobayashi H, Estrogen synthesis in gastric parietal cells and secretion into portal vein, Anat Sci Int 95(1) (2020) 22–30. [DOI] [PubMed] [Google Scholar]


