Abstract
Quorum sensing (QS) plays an essential role in virulence factor production, biofilm formation, and antimicrobial resistance. As a potent QS inhibitor, hordenine can inhibit both QS and biofilm formation in Pseudomonas aeruginosa and Serratia marcescens. In this work, we tested the QS inhibitory potential of 27 hordenine analogs against QS and biofilm formation in P. aeruginosa and S. marcescens. Among the tested analogs, seven (12, 28, 27, 26, 2, 23, and 7) exhibited strong QS inhibitory activity against P. aeruginosa, five of which (12, 28, 27, 26, and 2) showed better inhibitory activity than hordenine. In addition, seven analogs (28, 12, 23, 7, 26, 2, and 27) exhibited better biofilm inhibition against P. aeruginosa than hordenine. Four analogs (7, 28, 2, and 12) showed QS inhibitory activity against S. marcescens, two of which (7 and 28) demonstrated better inhibitory activity than hordenine. Furthermore, analog 7 showed similar biofilm inhibition against S. marcescens as hordenine. Structure-activity relationship (SAR) analysis indicated that the inhibitory activities of the analogs were related to four factors, i.e., carbon chain length, presence or absence of an α,β-C C bond, amino group with/without lipophilic group, such as methyl group, and hydroxyl group in benzene ring.
Keywords: Hordenine analogs, Pseudomonas aeruginosa, Serratia marcescens, Quorum sensing inhibition, QSAR, Biofilm
1. Introduction
Infections by antibiotic-resistant bacteria pose a serious threat to human health [1,2]. Biofilm formation is considered vital to drug resistance in bacteria, with the biofilm matrix acting as a natural barrier to drug penetration and activation. Unicellular bacteria are capable of coordinating population-level behavior through a process termed quorum sensing (QS) [3]. Regulation of QS is linked to biofilm formation in many bacteria. Furthermore, various physiological functions in bacteria, such as virulence factor expression, bacterial movement and adhesion, and secondary metabolite synthesis, are regulated by QS [4].
Pseudomonas aeruginosa is an opportunistic pathogen and important cause of hospital-acquired airway and wound infections [5]. Infection can occur via the skin, oral cavity, mucous membrane, and blood, and can cause infection in various tissues and organs in the human body [6]. Studies have shown that P. aeruginosa regulates biofilm formation through the las QS system [7] and the synthesis of pyocyanin through the acylated homoserine lactone(AHL)system [8]. Pyocyanin is a crucial virulence factor of P. aeruginosa and plays an important role in host cell infection and disease [9].
As a ubiquitous environmental bacterium, Serratia marcescens is found in water and soil and in association with plants and animals, resulting in crop infections and food-borne diseases [[10], [11], [12]]. Serratia marcescens uses AHLs as QS signals to mediate the expression of various genes and participate in a variety of physiological activities, including virulence factor production and biofilm formation [13]. The virulence factor prodigiosin plays a critical role in host invasion and pathogenicity [14].
Hordenine is the primary bioactive alkaloid in malt (0.24 mg/g) and has been used as a vasoconstrictor and indirect adrenergic agent [15,16]. We previously reported that hordenine exhibits potent QS inhibitory activities against P. aeruginosa and S. marcescens [17,18]. However, few studies on hordenine analogs have been reported, especially on the inhibition of QS. Therefore, in the present study, we evaluated the QS and biofilm inhibitory activities of hordenine analogs against P. aeruginosa and S. marcescens.
2. Materials and methods
To investigate the QS inhibitory activities of hordenine analogs and their potential structure-activity relationships (SARs), we obtained 27 analogs (purity >98%) (Fig. S1), including seven analogs (2, 3, 5, 13, 14, 26, and 28) chemically synthesized in our laboratory (Fig. S2-8) and other compounds purchased from the Hainan Haidawson Company (Hainan, China).
The P. aeruginosa PAO1 was a kind gift from Q. Gong (Ocean University of China, Qingdao, China) and the S. marcescens NJ01 was kindly provided by W. Wang (Nanjing Agricultural University, Nanjing, China).
2.1. QS inhibitory screening of 27 hordenine analogs
We initially evaluated the QS inhibitory activities of the 27 hordenine analogs against P. aeruginosa PAO1 and S. marcescens NJ01 according to published methods [17,18]. Stock solutions were prepared by dissolving hordenine and the analogs in dimethyl sulfoxide (DMSO). Hordenine was used as a positive control and DMSO was used as a negative control.
2.2. Measurement of minimum inhibitory concentrations (MICs) of seven potential QS inhibitors (QSIs)
After confirming the QS inhibitory activities of hordenine analogs, the MICs of seven potential QSIs were determined as per Zhou et al. [17], with slight modification. In brief, 200 μL of each hordenine analog was added to 96-well plates and cultured at 37 °C and 150 rpm for 24 h. Absorbance at OD620 were determined using a microplate reader (BioTek Elx800, Winooski, VT, USA), with DMSO used as a negative control. The MICs of seven analogs (2, 7, 12, 23, 26, 27, and 28) (0.30–10 mg/mL) against P. aeruginosa PAO1 and four analogs (2, 7, 12, and 28) (0.30–10 mg/mL) against S. marcescens NJ01 were determined using two-fold serial dilution with an inoculum of 1 × 105 colony-forming units (CFU)/mL in Müller-Hinton broth (Sangon Biotech, Shanghai, China).
2.3. Biofilm inhibition against P. aeruginosa PAO1 and S. marcescens NJ01
Biofilms were cultivated in Luria-Bertani (LB) broth supplemented with or without the hordenine analogs in 24-well polystyrene plates (Costar 3524, Corning, Corning, NY, USA) using previous methods with minor modifications [19]. After 24 h of static incubation, cultures and planktonic cells were removed and sessile cells were stained with 0.05% crystal violet, with the excess rinsed off using distilled water. After dissolution with 95% ethanol, biofilm biomass was determined at OD570 [20].
To investigate cell viability, biofilms were washed with phosphate-buffered saline (PBS) and digested with dextranase (5 units, D8144, Sigma-Aldrich, St. Louis, MO, USA), followed by 30 s of sonication, as described previously [21]. For S. marcescens NJ01, viable cells in the treated biofilms were counted by plating at 28 °C for 24 h. For P. aeruginosa PAO1, viable cells in the treated biofilms were counted by plating at 37 °C for 24 h.
To observe the biofilm more intuitively, samples were observed using confocal laser scanning microscopy (CLSM, LEICA TCS SP8, LEICA, Germany). Biofilms formed on circular glass coverslips were washed with PBS and subsequently stained with acridine orange (AO). Excess dye was removed using PBS. Stained biofilms were then visualized by CLSM (excitation, 488 nm; emission filter, 501–545 nm).
2.4. Effects of seven potential QSIs on pyocyanin
A single colony of P. aeruginosa PAO1 was selected and activated in 5 mL of LB medium for 17 h as a seed solution. Seed liquid was inoculated into new LB medium with 0.1% inoculum volume, and 200 μg/mL hordenine analog was added. DMSO was used as the negative control and 200 μg/mL hordenine was used as the positive control. The cells were cultured at 37 °C and 180 rpm for 17 h. The culture medium was filtered using a 0.22-μm sterile filter to obtain a sterile filtrate for standby. Determination of pyocyanin content followed previous methods [22]. After culture, the supernatant was extracted with chloroform (5/3, V/V). After standing and stratification, the lower organic phase was taken and 1 mL of 0.2 M HCl was added and mixed evenly, followed by centrifugation at 4 °C and 10 000 rpm for 10 min, with the upper 200 μL of solution taken to determine OD520.
2.5. Effects of four potential QSIs on prodigiosin
Serratia marcescens NJ01 was inoculated in fresh LB medium at 28 °C and 180 rpm overnight (17 h). After this, 1% inoculum was added to 2 mL of LB medium and 50 μg/mL hordenine analog. DMSO was used as a negative control and 50 μg/mL hordenine was used as a positive control. The solution was cultured at 28 °C and 180 rpm for 16–24 h, with 1 mL of each bacterial solution then placed in a centrifuge tube for centrifugation at 10 000 rpm at 25 °C for 10 min. The resulting supernatant was collected for standby (or filtered with a 0.22-μm sterile filter to obtain a sterile filtrate), with 1 mL of acidified ethanol (4%, 1 M HCl in ethanol) also added to the centrifuge tube for centrifugation at 10 000 rpm at 25 °C for 10 min. The supernatant was then transferred to a 96-well plate for measurement at OD534 [23].
2.6. Microscopy measurements
For the biofilm inhibitory experiments, P. aeruginosa and S. marcescens were selected and added to 5 mL of LB medium for 17 h of activation as the seed solution. The seed liquid was inoculated into TSB medium with 0.1% inoculum volume, after which the hordenine analogs (125 μg/mL and 50 μg/mL) were added and mixed in P. aeruginosa and S. marcescens, respectively. 125 μg/mL and 50 μg/mL DMSO were used as controls. The mixed bacterial solution (1 mL) was added to a flat bottom 24-well plate with a circular glass coverslip at the bottom and incubated at 37 °C for 24 h. After incubation, the circular glass coverslips were washed with PBS to remove floating bacteria.
For the membrane destruction experiment, the operation steps were the same as for the biofilm inhibitory experiment.
The biofilms on the circular glass coverslips were fixed with 2.5% glutaraldehyde for 12 h, then gradient dehydration was carried out with 50%, 70%, 80%, 90%, and 100% ethanol, and freeze-drying was carried out in a freeze dryer. After gold spraying, the biofilms were observed with scanning electron microscopy (SEM, JSM6360, JEOL, Tokyo, Japan) [24].
3. Results
3.1. MICs of analogs with QS inhibitory activity
After screening the QS inhibitory activities of all 27 analogs, seven analogs (28, 12, 23, 7, 26, 2, and 27) inhibited QS and biofilm formation of P. aeruginosa PAO1 (Fig. 1), with five analogs (12, 28, 27, 26, and 2) showing better QS inhibitory activity than that of hordenine. Four analogs (7, 2, 28, and 12) inhibited QS and biofilm formation of S. marcescens NJ01 (Fig. 2), with two analogs (7 and 28) showing better QS inhibitory activity than that of hordenine. The determined MICs are listed in Table 1, Table 2.
Fig. 1.
Seven hordenine analogs with QS inhibitory activity against P. aeruginosa PAO1.
Fig. 2.
Four hordenine analogs with QS inhibitory activity against S. marcescens NJ01.
Table 1.
MICs of seven hordenine analogs against P. aeruginosa PAO1 (μg/mL).
| Analog | 2 | 7 | 23 | 12 | 26 | 27 | 28 |
|---|---|---|---|---|---|---|---|
| Concentration | 400 | 600 | 400 | 300 | 500 | 550 | 250 |
Table 2.
MICs of four hordenine analogs against S. marcescens NJ01 (μg/mL).
| Analog | 2 | 7 | 12 | 28 |
|---|---|---|---|---|
| Concentration | 1000 | 1000 | 1000 | 500 |
3.2. Biofilm inhibition
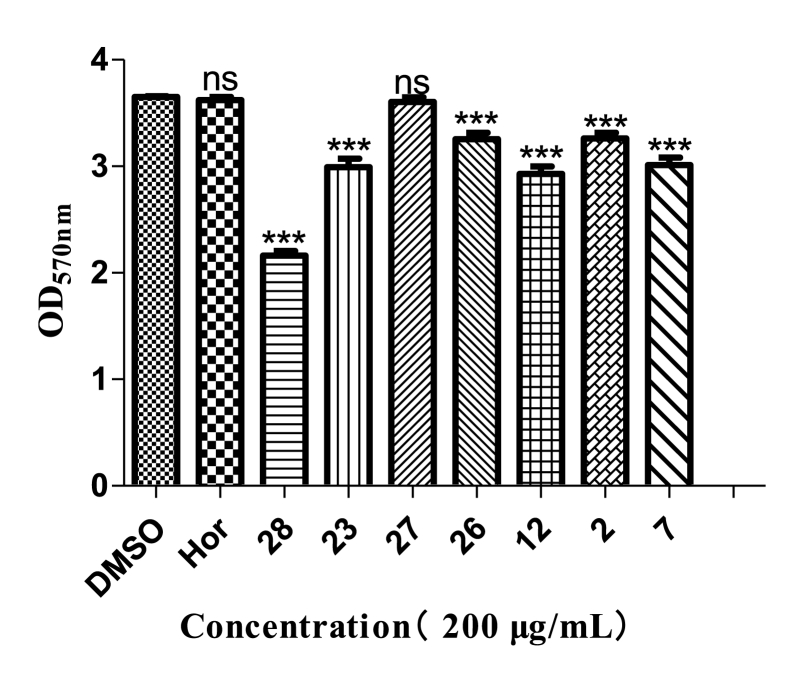
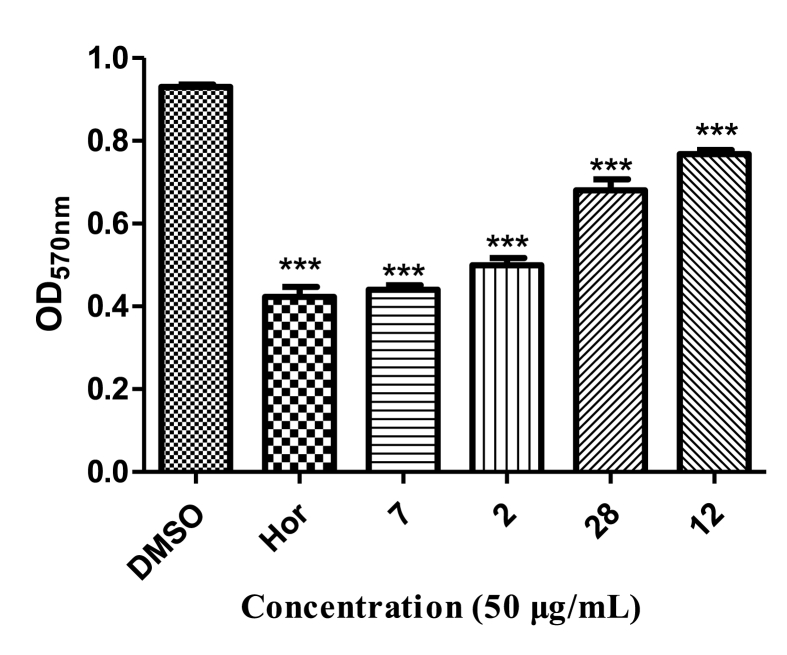
Biofilms are one of the main causes of antibiotic resistance [25]. Pseudomonas aeruginosa can produce highly structured and dense biofilms, which are mostly related to QS [26]. Here, analogs 28, 12, 23, 7, 26, 2, and 27 reduced biofilms by 40.8%, 19.7%, 18.1%, 17.5%, 10.8%, 10.7%, and 1.2%, respectively, thus showing better inhibitory activity than hordenine (0.08%) (Fig. 3).
Fig. 3.
Biofilm inhibitory activity of seven analogs against P. aeruginosa PAO1. Hordenine was used as a positive control and DMSO as a negative control. Statistical differences were determined by GraphPad Prism. (∗∗∗) p < 0.001 vs DMSO control. (∗∗) p < 0.01 vs DMSO control.
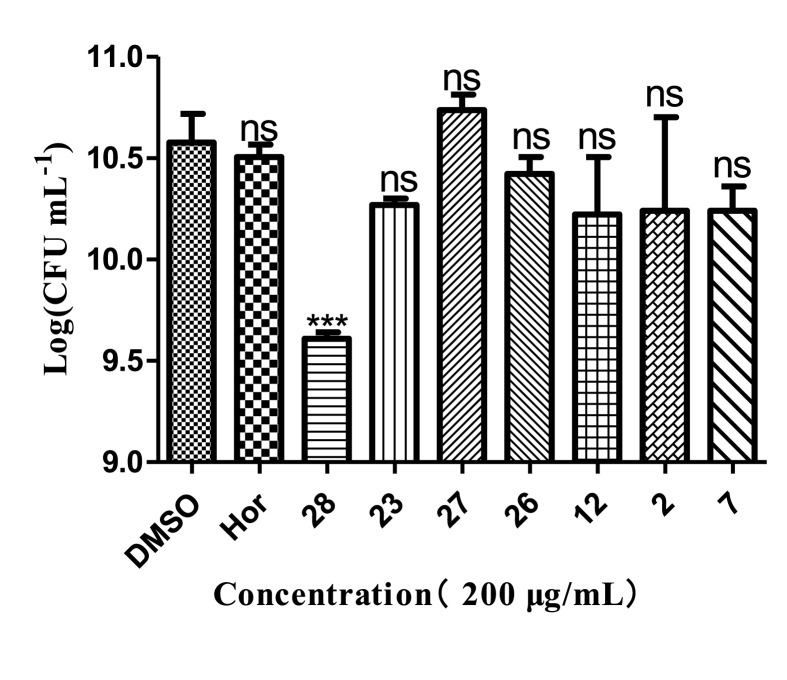
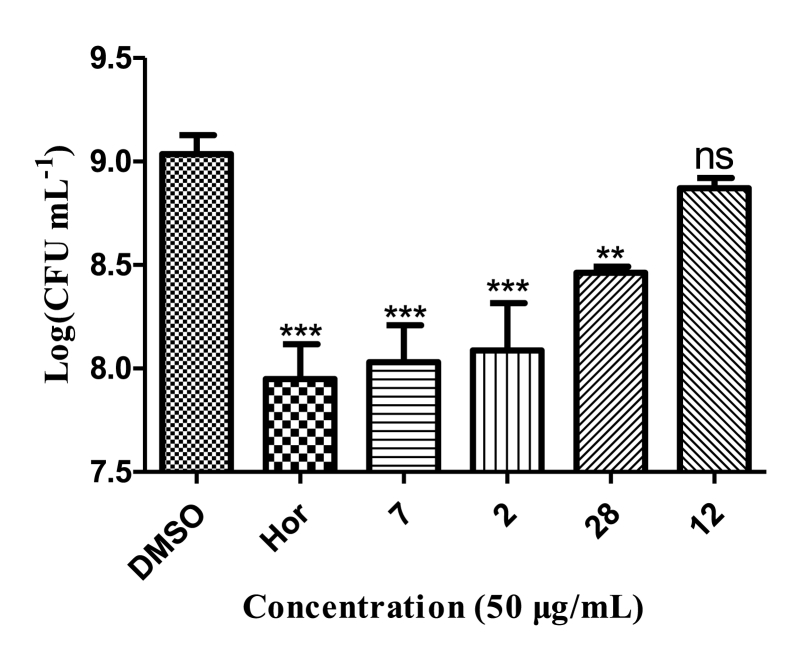
Cell survival determination indicated that analogs 28, 23, 26, 12, 2, and 7 (200 μg/mL) had stronger effect on planktonic cell viability than hordenine, especially analog 28 (200 μg/mL) resulted in more than 50% reduction in viable cells (Fig. 4).
Fig. 4.
Effects of seven analogs on cell viability of treated biofilms of P. aeruginosa PAO1. Hordenine was used as a positive control and DMSO as a negative control. Statistical differences were determined by GraphPad Prism. (∗∗∗) p < 0.001 vs DMSO control. (∗∗) p < 0.01 vs DMSO control.
In addition to quantitative analysis, treated biofilms were also visualized using CLSM. The CLSM images showed reduced thickness in the analog-treated biofilms. In addition, treatment with analogs 28, 23, 26, 12, and 7 resulted in major disruption to biofilm architecture (Fig. 5).
Fig. 5.
CLSM images of P. aeruginosa PAO1 biofilms treated with seven analogs (200 μg/mL). Hordenine was used as a positive control and DMSO as a negative control.
We also determined the inhibitory activities of four hordenine analogs against S. marcescens NJ01 biofilms by crystal violet staining (Fig. 6). Treatment with analogs 7, 2, 28, and 12 (50 μg/mL) reduced biofilms by 52.7%, 46.3%, 26.9%, and 17.5%, respectively. In contrast, hordenine (50 μg/mL) treatment reduced biofilms by 54.6%.
Fig. 6.
Biofilm inhibitory activities of four analogs against S. marcescens NJ01. Hordenine was used as a positive control and DMSO as a negative control. Statistical differences were determined by GraphPad Prism. (∗∗∗) p < 0.001 vs DMSO control. (∗∗) p < 0.01 vs DMSO control.
We also detected viable cells in the treated biofilms. Cell survival determination indicated that treatment with hordenine and analogs 7, 2, and 28 (50 μg/mL) affected planktonic cell viability, but analog 12 (50 μg/mL) had no effect (Fig. 7).
Fig. 7.
Effects of four analogs on cell viability of treated biofilms of S. marcescens NJ01. Hordenine was used as a positive control and DMSO as a negative control. Statistical differences were determined by GraphPad Prism. (∗∗∗) p < 0.001 vs DMSO control. (∗∗) p < 0.01 vs DMSO control.
Treated biofilms were also visualized using CLSM. The CLSM images indicated that treatment with analogs 7, 2, 28, and 12 (50 μg/mL) notably disrupted biofilm architecture (Fig. 8).
Fig. 8.
CLSM images of S. marcescens NJ01 biofilms treated with four analogs (50 μg/mL). Hordenine was used as a positive control and DMSO as a negative control.
3.3. Determination of pyocyanin
The synthesis of pyocyanin in P. aeruginosa is controlled by QS [27]. As shown in Fig. 9, a significant decrease in the concentration of pyocyanin was observed after treatment with hordenine and analogs 27, 24, 28, 29, 2, 23, and 7 (200 μg/mL). Analogs 27, 12, 26, 28, and 2 showed better inhibition of pyocyanin (26.8%, 34.6%, 24.0%, 28.3%, and 20.9%, respectively) than that of hordenine (15.5%). Analogs 23 and 7 inhibited pyocyanin by 14.5% and 3.2%, respectively.
Fig. 9.
Inhibitory activities of seven analogs against pyocyanin. Hordenine was used as a positive control and DMSO as a negative control. Statistical differences were determined by GraphPad Prism. (∗∗∗) p < 0.001 vs DMSO control. (∗∗) p < 0.01 vs DMSO control.
3.4. Determination of prodigiosin
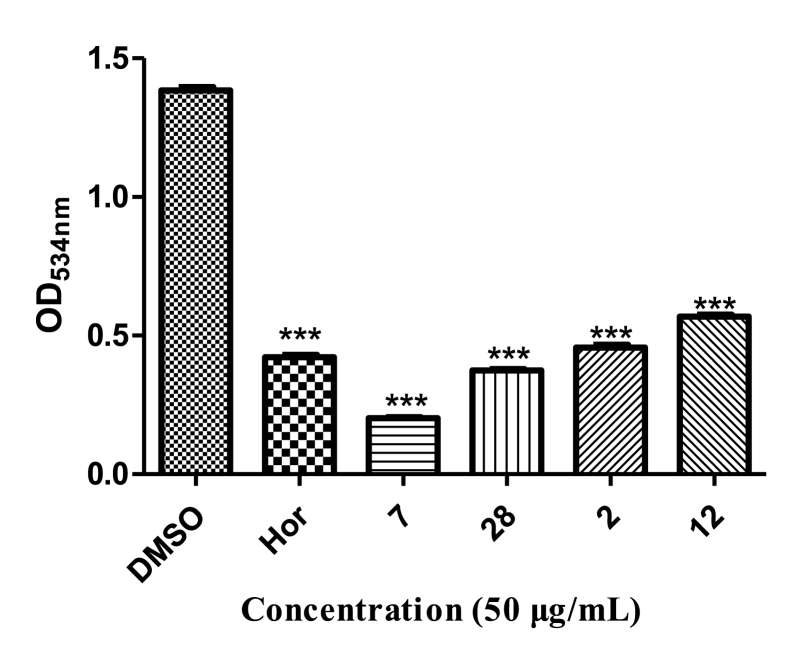
The synthesis of prodigiosin in S. marcescens is controlled by QS [18]. As shown in Fig. 10, treatment with hordenine and analogs 7, 28, 2, and 12 (50 μg/mL) resulted in a significant decrease in the concentration of prodigiosin. Analogs 7 and 28 showed stronger inhibition of prodigiosin production (85.4% and 73.0%, respectively) than that of hordenine (69.6%). Analogs 2 and 12 inhibited prodigiosin production by 68.0% and 59.0%, respectively.
Fig. 10.
Inhibitory activities of four analogs against prodigiosin. Hordenine was used as a positive control and DMSO as a negative control. Statistical differences were determined by GraphPad Prism. (∗∗∗) p < 0.001 vs DMSO control. (∗∗) p < 0.01 vs DMSO control.
3.5. SEM results
Among the hordenine analogs, analog 28 showed the best QS inhibitory activity against P. aeruginosa and analog 7 showed the best QS inhibitory activity against S. marcescens. Therefore, SEM images on the inhibitory and destructive biofilms of the two analogs were carried out (Fig. 11).
Fig. 11.
SEM images of analog 28 on P. aeruginosa PAO1 biofilm and analog 7 on S. marcescens NJ01 biofilm. (A) P. aeruginosa PAO1 treated with DMSO; (B) P. aeruginosa PAO1 treated with analog 28 (125 μg/mL) to inhibit biofilm formation; (C) P. aeruginosa PAO1-formed biofilm treated with analog 28 (125 μg/mL) was destroyed; (D) S. marcescens NJ01 treated with DMSO; (E) S. marcescens NJ01 treated with analog 7 (50 μg/mL) to inhibit biofilm formation; (F) S. marcescens NJ01-formed biofilm treated with analog 7 (50 μg/mL) was destroyed.
4. Discussion
Bacterial QS is a distinct mechanism of cell-cell communication, whereby bacteria can ‘sense’ the density of cells in the surrounding environment, resulting in the expression or suppression of specific genes [28,29]. Many important bacterial features related to physiology, virulence, and biofilm production are mediated by QS-dependent gene expression. Due to its potential in human medicine applications, research on QS has garnered significant attention in the last 15–20 years [30]. In this study, we determined the QS and biofim inhibitory activity of 27 hordenine analogs towards P. aeruginosa and S. marcescens, and preliminarily investigated their SARs.
4.1. Pyocyanin inhibition effects of hordenine analogs and SAR analysis
Pyocyanin is an important indicator of QS operons in P. aeruginosa [17]. Therefore, pyocyanin reduction is necessary to inhibit QS. Here, we studied the pyocyanin inhibitory activities of seven hordenine analogs against P. aeruginosa at a concentration (200 μg/mL) much lower than the MIC. As seen in Fig. 9, six analogs significantly inhibited the secretion of pyocyanin. Thus, we analyzed the pyocyanin inhibition SARs of the seven analogs. Hordenine and its analogs are composed of a benzene ring, para-phenolic hydroxyl group, aliphatic amino group, and amino substituent. According to Fig. 9, the four structural elements of hordenine and its analogs determined their pyocyanin inhibition SARs. Notably, hordenine and analogs 3, 23, 26, and 27 are phenethylamines, while 2, 7, 12, and 28 are amphetamines.
-
(1)
The activity of analog 27 (26.8%) without a hydroxyl group on the benzene ring was better than that of analog 23 (14.5%) with a hydroxyl group. These results suggest that polar groups, such as hydroxyl groups, in the benzene ring have an antagonistic effect on the inhibition of pyocyanin.
-
(2)
The inhibition rate of analog 27 (26.8%) was also better than that of analog 26 (24.0%), indicating that the presence of a lipophilic group (such as methyl) on the amino group antagonizes the inhibition of pyocyanin. In addition, analog 3 did not inhibit pyocyanin, indicating that the hydroxyl group in the benzene ring decreased the production of pyocyanin.
-
(3)
Comparing the pyocyanin inhibition rates, we found that the activities of analogs 3 and 23 were lower than that of hordenine, showing different SARs from that of (2). These results indicate that besides the presence of hydroxyl groups in the benzene ring, other factors, such as the methyl group attached to the amino group, also affect pyocyanin production.
-
(4)
The inhibition rate of analog 2 (20.9%) was lower than that of analog 27 (26.8%) with the increase in amino aliphatic chain, indicating that inhibitory activity decreases with the increase in the length of carbon chain.
-
(5)
The inhibition rate of analog 12 (34.6%) was stronger than that of analog 2 (20.9%), indicating that the activity of amphetamine in the amino group bearing a lipophilic group (such as methyl group) is better than that of amphetamine in the amino group without an attached lipophilic group.
-
(6)
The inhibition rate of analog 2 (20.9%) was better than that of analog 7 (3.2%), indicating that the double bond may have antagonistic effects on the activity of amphetamines. The same SAR was found for the comparison of analogs 12 and 28 (inhibition rates of 34.6% and 28.3%, respectively).
4.2. Biofilm inhibition effects of hordenine analogs against P. aeruginosa and SAR analysis
We also studied the inhibition of the seven analogs on P. aeruginosa biofilm formation at a concentration (200 μg/mL) far lower than the MIC. As seen in Fig. 3, analog 28 significantly inhibited biofilm growth (40.8%), and its inhibitory activity was far better than that of hordenine (0.08%). Thus, we carried out SAR analysis of the inhibitory activities of the seven analogs on biofilm formation.
-
(1)
Analog 23 with a hydroxyl group on the benzene ring (18.1%) showed much stronger biofilm inhibitory activity than that of analog 27 (1.2%) without a hydroxyl group on the benzene ring.
-
(2)
Analog 23 (18.1%) showed much higher biofilm inhibitory activity than that of hordenine (0.08%), indicating that the amino group without methyl was better than that bearing a methyl group.
-
(3)
Analog 28 with one methyl group attached to the amino group (40.8%) showed better biofilm inhibitory activity than that of analog 7 without a methyl group attached to the amino group (17.5%).
-
(4)
Analog 28 showed much higher biofilm inhibitory activity than that of hordenine (0.08%). Similar trends were also found for analogs 26 (with a methyl group attached to the amino group, 10.8%) and 27 (with no methyl group attached to the amino group, 1.2%) and for analogs 12 (19.7%) and 2 (10.7%).
-
(5)
Analog 7 with olefins on the carbon chain (17.5%) showed stronger biofilm inhibitory activity than that of analog 2 without olefins on the carbon chain (10.7%). A similar trend was found for analogs 28 and 12.
Among all tested analogs, analog 28 showed potent pyocyanin inhibitory activity (28.3%) and biofilm inhibitory activity (40.8%) against P. aeruginosa. Thus, we performed SEM experiments to observe its effects on biofilm formation. For the biofilm destruction test, after treatment with analog 28 (125 μg/mL), P. aeruginosa biofilm formation decreased slightly, but the tight structure of the biofilm became very loose and filled with gaps. This would be conducive for the entry of antibiotics into the biofilm to reach single cells and recover bacterial sensitivity to antibiotics.
4.3. Prodigiosin inhibition effects of hordenine analogs and SAR analysis
We next studied the prodigiosin inhibitory activity of four hordenine analogs against S. marcescens at a concentration (50 μg/mL) much lower than the MIC. As seen in Fig. 10, analogs 2, 7, 12, and 28 (50 μg/mL) significantly inhibited the secretion of prodigiosin. These four analogs were identified as amphetamines. We also analyzed the SARs of these four analogs.
-
(1)
The inhibitory activity of analog 7 (85.4%) was better than that of analog 2 (68.0%), indicating that olefins on the carbon chain may be necessary to increase inhibitory activity. The same results were found for analogs 28 (73.0%) and 12 (59.0%).
-
(2)
The inhibitory activity of analog 7 (85.4%) was better than that of analog 28 (73.0%), indicating that a methyl group attached to the amino group is necessary for prodigiosin inhibition effects of hordenine analogs. The same results were found for analogs 2 (68.0%) and 12 (59.0%).
4.4. Biofilm inhibition effects of hordenine analogs against S. marcescens and SAR analysis
We next studied the inhibitory effects of four analogs on S. marcescens biofilm formation at a concentration (50 μg/mL) far lower than the MIC. As seen in Fig. 6, the inhibitory activities of analogs 7, 2, 28, and 12 (50 μg/mL) on biofilm growth were 52.7%, 46.3%, 26.9%, and 17.5%, respectively.
-
(1)
The biofilm inhibitory activity of analogs 7 and 28 with olefins in the carbon chain was better than that of analogs 2 and 12 without olefins in the carbon chain.
-
(2)
The biofilm inhibitory activity of analogs 7 and 2 with an amino group without a methyl group was better than that of analogs 28 and 12 with a methyl group attached to the amino group.
As analog 7 showed strong prodigiosin inhibitory activity (85.4%) and biofilm inhibitory activity (52.7%) against S. marcescens, we performed SEM experiments to observe its effects on biofilm formation. As seen in Fig. 11, analog 7 showed strong inhibitory activity and membrane destruction against S. marcescens, which would be conducive to allowing the entry of antibiotics into the single cells of S. marcescens.
Overall, SAR analysis showed that the QS and biofilm inhibitory activities of hordenine analogs are related to four factors, i.e., carbon chain length, presence or absence of an α,β-C C bond, and amino group with/without lipophilic group, such as methyl group, and hydroxyl group in benzene ring. Especially the presence of α,β-C C bond and amino group with methyl group could significantly promote the inhibition effect against QS and biofilm formation.
5. Conclusions
In this study, 27 hordenine analogs were tested to determine their QS and biofilm inhibitory activities against two pathogens, P. aeruginosa and S. marcescens. At sub-MIC concentrations, QS inhibitory activity was evaluated based on the inhibition of typical virulence factors of each bacterium, i.e., pyocyanin and prodigiosin. Seven analogs showed potential QS inhibitory activity against P. aeruginosa. Among them, five analogs (12, 28, 27, 26, and 2) exhibited higher inhibitory activity against pyocyanin than hordenine, and analog 28 showed higher biofilm inhibitory activity than hordenine. Analog 28 was the best QSI against P. aeruginosa. Four analogs showed QS inhibitory activity against S. marcescens, with analogs 7 and 28 exhibiting higher inhibitory activity against prodigiosin than hordenine. SAR analysis showed that four factors of the seven analogs were necessary, i.e., carbon chain length, presence or absence of an α,β-C C bond, and amino group with/without lipophilic group, such as methyl group, and hydroxyl group in benzene ring.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Data availability statement
All data generated and analyzed during this study are included in this published article (and its supplementary information files).
CRediT authorship contribution statement
All authors listed contributed greatly to this study. Yue Liu: Prepared and performed the experiments and wrote the paper. Jun-Jian Li: Provided technical support and designed the research. Hong-Yuan Li: Performed synthesis of partial compounds. Shi-Ming Deng: Provided technical support and designed the research. Ai-Qun Jia: Provided technical support and designed the research.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of Hainan Province (319QN165) and the National Natural Science Foundation of China (41766006).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.synbio.2021.09.010.
Contributor Information
Jun-Jian Li, Email: 993909@hainanu.edu.cn.
Shi-Ming Deng, Email: dsm701@126.com.
Ai-Qun Jia, Email: ajia@hainanu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Gajdács M. Antibiotic resistance: from the bench to patients. J Antibiot. 2019;8(3):129. doi: 10.3390/antibiotics8030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cegelski L. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol. 2009;6(1):17–27. doi: 10.1038/nrmicro1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geske G.D. Small molecule inhibitors of bacterial quorum sensing and biofilm formation. J Am Chem Soc. 2005;127(37):12762–12763. doi: 10.1021/ja0530321. [DOI] [PubMed] [Google Scholar]
- 4.Hawver L.A. Specificity and complexity in bacterial quorum sensing systems. FEMS Microbiol Rev. 2016;40(5):738–752. doi: 10.1093/femsre/fuw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behzadi P. It's not easy being green: a narrative review on the microbiology, virulence and therapeutic prospects of multidrug-resistant Pseudomonas aeruginosa. J Antibiot. 2021;10(1):42. doi: 10.3390/antibiotics10010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodward T.C. Budget impact model of tobramycin inhalation solution for treatment of Pseudomonas aeruginosa in cystic fibrosis patients. J Med Econ. 2010;13(3):492–499. doi: 10.3111/13696998.2010.505863. [DOI] [PubMed] [Google Scholar]
- 7.Maddocks S.E. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology. 2008;154:3609–3623. doi: 10.1099/mic.0.2008/022772-0. [DOI] [PubMed] [Google Scholar]
- 8.Raina S. Microbial quorum sensing: a tool or a target for antimicrobial therapy? Appl Biochem Biotechnol. 2009;54(2):65–84. doi: 10.1042/BA20090072. [DOI] [PubMed] [Google Scholar]
- 9.Lau G.W. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med. 2004;10(12):599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Gajdács M. Resistance trends and epidemiology of citrobacter-enterobacter-Serratia in urinary tract infections of inpatients and outpatients (RECESUTI): a 10-year survey. Medicina. 2019;55(6):285. doi: 10.3390/medicina55060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morohoshi T. Inhibition of quorum sensing in Serratia marcescens AS-1 by synthetic analogs of N-acylhomoserine lactone. Appl Environ Microbiol. 2007;73:6339–6344. doi: 10.1128/AEM.00593-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruton B.D. Serratia marcescens, a phloem-colonizing, squash bug-transmitted bacterium: causal agent of cucurbit yellow vine disease. Plant Dis. 2003;87:937–944. doi: 10.1094/PDIS.2003.87.8.937. [DOI] [PubMed] [Google Scholar]
- 13.Truchado P. Inhibition of quorum sensing (QS) in Yersinia enterocolitica by an orange extract rich in glycosylated flavanones. J Agric Food Chem. 2012;60:8885–8894. doi: 10.1021/jf301365a. [DOI] [PubMed] [Google Scholar]
- 14.Liu G.Y. Color me bad: microbial pigments as virulence factors. Trends Microbiol. 2009;17:406–413. doi: 10.1016/j.tim.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou G. Separation and determination of hordenine in hordeum vulgare by capillary electrophoresis-electrochemical luminescence. Jiangsu Agric Sci. 2018;46(18):192–195. [Google Scholar]
- 16.Hapke H.J. Pharmakological effects of hordenine. Dtsch Tierarztl Wochenschr. 1995;102(6):228–232. [PubMed] [Google Scholar]
- 17.Zhou J.W. Hordenine: a novel quorum sensing inhibitor and anti-biofilm agent against Pseudomonas aeruginosa. J Agric Food Chem. 2018;66(7):1620–1628. doi: 10.1021/acs.jafc.7b05035. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J.W. Inhibition of quorum sensing and virulence in Serratia marcescens by hordenine. J Agric Food Chem. 2019;67(3):784–795. doi: 10.1021/acs.jafc.8b05922. [DOI] [PubMed] [Google Scholar]
- 19.Damiano S. Antioxidant and antibiofilm activities of secondary metabolites from Ziziphus jujuba leaves used for infusion preparation. Food Chem. 2017;230:24–29. doi: 10.1016/j.foodchem.2017.02.141. [DOI] [PubMed] [Google Scholar]
- 20.Senobar Tahaei S.A. Correlation between biofilm-formation and the antibiotic resistant phenotype in Staphylococcus aureus isolates: a laboratory-based study in Hungary and a review of the literature. Infect Drug Resist. 2021;14:1155–1168. doi: 10.2147/IDR.S303992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brackman G. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Chemother. 2011;55:2655–2661. doi: 10.1128/AAC.00045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar N.V. Synthesis and quorum sensing inhibitory activity of key phenolic compounds of ginger and their analogs. Food Chem. 2014;159:451–457. doi: 10.1016/j.foodchem.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan R. Piper betle and its bioactive metabolite phytol mitigates quorum sensing mediated virulence factors and biofilm of nosocomial pathogen Serratia marcescens in vitro. J Ethnopharmacol. 2016;193:592–603. doi: 10.1016/j.jep.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Ramanathan S. Inhibition of quorum sensing-dependent biofilm and virulence genes expression in environmental pathogen Serratia marcescens by petroselinic acid. Antonie Leeuwenhoek. 2018;111:501–515. doi: 10.1007/s10482-017-0971-y. [DOI] [PubMed] [Google Scholar]
- 25.Kim H.S. 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci Rep. 2015;5:8656. doi: 10.1038/srep08656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poole K. Aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49(2):479–487. doi: 10.1128/AAC.49.2.479-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutherford S.T. Bacterial quorum sensing: its rolein virulence and possibilities for its control. Cold Spring Harbor Perspect Med. 2012;2:a012427. doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller M.B. Quorum sensing in bacteria. Annu Nat Rev Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Q. Quorum sensing: a prospective therapeutic target for bacterial diseases. BioMed Res Int. 2019;7:1–15. doi: 10.1155/2019/2015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gajdács M. The role of drug repurposing in the development of novel antimicrobial drugs: non-antibiotic pharmacological agents as quorum sensing-inhibitors. J Antibiot. 2019;8(4):270. doi: 10.3390/antibiotics8040270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during this study are included in this published article (and its supplementary information files).