Abstract
Background
Cancer is the second-leading cause of death in the United States. Clinical trials translate basic science discoveries into treatments needed by cancer patients. Inadequate accrual of trial participants is one of the most significant barriers to the completion of oncology clinical trials.
Objective
The purpose of this study was to investigate trial-level factors that affect accrual and/or completion of oncology clinical trials, identify gaps in the literature, and indicate opportunities for future research.
Design
A systematic review of the literature on trial-level factors that affect accrual and/or completion of oncology clinical trials was performed. Searches in PubMed and Scopus identified 6582 studies. Based on eligibility criteria, 16 studies were selected for the review. Results were analyzed according to the following: a) background factors, b) disease-related, c) treatment-related, and d) trial design.
Results
Background factors that were investigated in relation to oncology clinical trial accrual and/or completion included sponsor, number and location of participating institutions, competing trials, time of trial opening, and fast-track status. Disease-related factors included the annual incidence and type(s) of targeted cancer. Several types of treatment such as drugs, radiation and surgery were examined in the studies. Trial design factors included trial development time, eligibility criteria, randomization, sample size, trial phase, placebo use, and required protocol procedures and their timing.
Conclusion
With low patient participation rates in oncology clinical trials that hold promise for future treatments, it is imperative that trial-level factors affecting accrual be identified and addressed to facilitate the completion of trials.
Keywords: Clinical trial, Oncology, Cancer, Enrollment, Accrual
1. Introduction
Cancer is the second-leading cause of death in the United States with approximately 606,520 deaths expected in 2020 [1]. As pressure has escalated to expeditiously translate basic science discoveries into treatments that are urgently needed by cancer patients, the increased number of oncology clinical trials and exorbitant costs of conducting these trials have resulted in challenges to their completion. According to ClinicalTrials.gov, approximately 2800 oncology clinical trials opened in 2015. This number grew to over 4600 in 2019 [2]. The median cost of clinical trials for oncology drugs approved by the Federal Drug Administration (FDA) in 2015–2017 was $37.1 million per trial (interquartile range = $17.0 - $60.4 million) [3].
With growth in the number of oncology clinical trials and limited resources to support the conduct of these trials, inadequate accrual of trial participants has become one of the most significant barriers to the completion of clinical trials. Only 3–8% of adult oncology patients participate in clinical trials [4]. In addition, approximately 20% of oncology clinical trials fail to complete because of inadequate accrual [4]. Patient accrual is a significant metric in determining the success of a clinical trial, as achieving the targeted sample size is required for valid results [5]. Clinical trials are too frequently terminated early or extended due to inadequate accrual. This adversely impacts the financial and other resources of cancer trial sponsors and participating sites [6]. Most importantly, trials that are delayed or terminated early impede the ultimate goal of providing new effective cancer therapies to patients who urgently need them.
In 2010, the Institute of Medicine (IOM) called for a substantial improvement in the efficiency, completion, and prioritization of clinical trials [7]. To accomplish these objectives, precise predictions about a trial's accrual and completion are vital in this time of limited research funding for governmental, academic, and corporate entities [8]. These precise predictions to meet the IOM's objectives are only possible through a comprehensive understanding of the factors that affect accrual and completion of oncology clinical trials. The literature demonstrates that factors impacting accrual and completion of oncology clinical trials operate at the individual, interpersonal, organizational, community, and policy levels. Although many researchers have investigated factors at these levels and developed interventions such as patient navigation and communication training to address barriers, accrual and completion of clinical trials remain inadequate [[9], [10], [11], [12], [13], [14], [15]]. It is unclear whether studies have adequately explored factors at the trial level that may affect successful accrual and trial completion, e.g., eligibility criteria, planned sample size, phase of study, study design, and use of randomization.
The purpose of this systematic review was to examine the empirical literature to investigate trial-level factors that affect accrual and/or completion of oncology clinical trials, identify gaps in the literature, and indicate potential opportunities for future research. The following research question guided the review: Among studies that analyzed large data sets of clinical trials, which trial-level factors influenced accrual and/or completion of oncology trials?
2. Methods
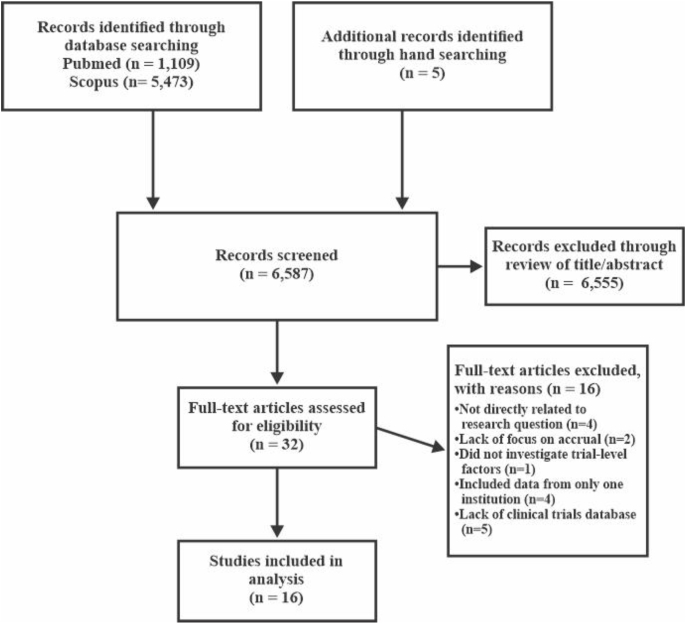
The authors consulted with a medical reference librarian to determine the best approach to search the literature for applicable studies. The PRISMA statement guided the systematic selection of literature included in the sample, and a PRISMA flow chart detailing the process was created (see Fig. 1) [16]. PubMed and Scopus databases were searched on February 24, 2020 for relevant publications. There were no date delimiters. The following search terms with appropriate Boolean operators in titles and abstracts were applied: (cancer OR oncology) AND (“clinical trials” OR “clinical research trials” OR “therapeutic trials”) AND (enrollment OR accrual OR recruitment) AND (“eligibility criteria” OR inclusion OR exclusion OR methodology OR design OR “randomized controlled trials” OR “randomized control trials” OR RCTs OR barriers OR challenges OR facilitators OR “facilitating factors” OR factors OR correlates OR pragmatic OR feasibility). Publications were limited to the English language published in peer-reviewed journals. The reference lists of retrieved publications were also hand searched for additional applicable primary sources.
Fig. 1.
Flow diagram for literature selection and inclusion.
The initial search produced 6582 citations (PubMed = 1109 and Scopus = 5473). Five additional citations for peer-reviewed articles were identified from hand searching. The titles and abstracts of the publications were evaluated for relevancy based on inclusion and exclusion criteria. Studies were included if they were: a) empirical studies that analyzed trial-level factors that influenced accrual and/or completion of oncology trials and b) studies that analyzed data from state, regional, national, or international clinical trial databases. Excluded were studies that investigated both oncological and non-oncological clinical trials, utilized a clinical trials database specific to a single institution or network of local institutions, or only examined individuals’ perceptions of trial-level factors that influenced accrual and/or completion of oncology clinical trials. Also excluded were qualitative studies, literature reviews, meta-analyses, dissertations, narratives, commentaries, workshop proceedings, and expert recommendations addressing trial-level factors. Upon evaluation, 6555 citations were removed due to ineligibility based on the review of titles and abstracts. Of the 32 remaining full-text publications, 16 met criteria to be included in the study sample. Of note, Scopus did not contain any eligible publications that were not already found in PubMed.
The results and discussion of this review were organized according to the themes of Bennette et al.‘s [17] conceptual model of trial-level factors associated with low trial accrual. The model's main themes encompass the following: a) background factors, b) disease-related, c) treatment-related, and d) trial design. Background includes factors such as competition from other clinical trials and insurance coverage of patient procedures associated with clinical trials. Disease-related include factors such as annual incidence of cancer and cancer stage. Treatment-related include factors such as type of treatment (e.g. chemotherapy or surgical) and use of a single modality (e.g. radiation) as opposed to multiple modalities (e.g. chemotherapy and radiation). Trial design includes factors such as eligibility criteria and use of randomization.
3. Results
3.1. General overview
Trial-related factors that impact a study's accrual and/or completion were examined in several contexts such as study design, population, type of cancer, sample size, trial phase, and database (Appendix 1). Fifteen studies were quantitative, and one study had a mixed methods design. All studies (n = 16) were at level 4 (e.g. retrospective cohort study) according to Melnyk's hierarchy of evidence [5]. Also, all studies examined oncology clinical trials for adults, with only three including trials for pediatrics. The majority of the studies (n = 10) did not limit inquiry to a specific type(s) of cancer. Three studies specified multiple types of cancer. The remaining studies (n = 3) specified one type of cancer, two of which were lung cancer. Sample size ranged from 16 to 12,875 clinical trials. Almost half of the studies (n = 7) included a sample of phase I, II, and III trials. Most of the remaining studies had a sample of phase I and II trials (n = 2) or phase II and III trials (n = 3). Two studies had a sample of only phase III trials. All studies (n = 16) used a national database(s) as the source of clinical trial data. The most commonly used database (n = 8) was ClinicalTrials.gov. Only one study utilized a theoretical or conceptual framework, which was Bennette et al.‘s [17] conceptual model of trial-level factors associated with low trial accrual.
3.2. Conceptual model of trial-level factors associated with low trial accrual
Of the 16 studies included in the final analysis, the following themes of Bennette et al.‘s [17] model were addressed: background factors (n = 10), 8 disease-related (n = 11), 5 treatment-related (n = 8), and trial design (n = 14).
3.2.1. Background factors
Background factors affecting oncology clinical trial accrual and/or completion were reported in the literature. Sponsor/funder was one of the examined background factors. Amongst published phase III oncology clinical trials, industry sponsored trials were among the fastest accruing [18]. Also, with poor accrual being the most common cause of early terminated clinical trials, industry sponsored immune checkpoint inhibitor trials were significantly less likely to terminate early compared with those that were sponsored by federal and academic institutions [19]. Worldwide, industry sponsored trials were also significantly more likely to attain accrual sufficiency than government funded trials [20]. Consequently, government sponsorship was a predictor of study failure of randomized clinical trials in radiation oncology [21].
Clinical trial development time was another examined background factor. Cheng et al. [22] measured trial development time from initial submission of the trial to the NCI Cancer Therapy Evaluation Program (CTEP) to the opening of the trial. Oncology clinical trials developed in <12 months were significantly more likely to meet accrual targets than those developed in 12–18 months. In contrast, oncology clinical trials developed in >24 months were significantly less likely to meet accrual targets than those developed in <12 months and 12–18 months.
Other background factors affecting oncology clinical trial accrual and/or completion were the number and location of participating institutions. Clinical trials conducted at a single institution were more likely to fail to complete than those conducted at multiple institutions [21,23]. Regarding location of participating sites, data from one study suggested that trials performed outside of the United States or both within and outside of the United States were more likely to complete than those conducted solely in the United States [23]. Findings from another study demonstrated that the continental location of the principal investigator and trials conducted internationally were not significantly associated with study failure [21]. Multinational trials were among the fastest accruing. However, there were no significant differences in accrual time between trials conducted in the United States compared to Europe among phase III oncology clinical trials [18].
Competing trials, time of trial opening, and fast-track status were background factors that were investigated in relation to oncology clinical trial accrual and/or completion. Among adult National Clinical Trials Network (NCTN) (cooperative group) cancer clinical trials, the number of competing trials was a predictor of low accrual, with a higher number of competing trials associated with low accrual [17]. Nguyen et al. [21] examined completed and incomplete randomized clinical trials in radiation oncology that opened in consecutive time periods. Significantly more trials failed during each consecutive time period (11.8% before 2007, 34% in 2007–2008, and 39.5% in 2009–2012). Hernandez-Torres et al. [24] found trial start date prior to 2003 was associated with lower accrual of older adults. Fast track review status designated by the Food and Drug Administration (FDA) was not associated with low accrual [17].
3.2.2. Disease-related
Lower annual incidence of the targeted type(s) of cancer and larger required enrollment fraction of the eligible patient population were predictors of low accrual [17]. Among NCI Cooperative Group phase III clinical trials, fewer breast cancer trials terminated due to inadequate accrual [25]. Also, Ruther et al. [18] found the fastest accruing trials among phase III oncology clinical trials were those for breast cancer. However, Hernandez-Torres et al. [24] demonstrated breast cancer clinical trials were associated with lower accrual of older adults. Among the older population, clinical trials for central nervous system cancers were associated with higher accrual [24]. There was no significant difference in adequate accrual between urological and nonurological trials. However, kidney cancer trials accrued the best, whereas bladder cancer trials accrued the worst among urological trials [20]. Predictors of low accrual were trials for common solid cancers as opposed to rare solid or liquid tumors and those with inclusion criteria that targeted multiple types of cancer [17].
There were mixed results for the association between accrual and metastatic disease. In two studies, metastatic disease, compared to nonmetastatic disease, was a predictor of low accrual [17,26]. Also, early stage cancer was significantly associated with enrollment of older persons [27]. However, in another study accrual was better for trials that involved advanced disease [28].
3.2.3. Treatment-related
Treatment-related factors were investigated in the literature. Clinical trials that investigated immune checkpoint inhibitors were less likely to terminate early compared to those that investigated other types of oncology drugs, but the results were not statistically significant [19]. Predictors of low accrual included non-targeted therapy and radiation therapy [17]. Accrual was poorer for Radiation Therapy Oncology Group trials than other cooperative groups and for multimodality trials that did not primarily include systemic treatment [28]. Whereas Bennette et al. [17] found the use of an investigational new drug to be a predictor of low accrual, other researchers [25,28] found no significant difference in inadequate accrual between clinical trials that involved a new investigational therapy and those that did not. Clinical trials involving standard therapy, with or without a new therapy, had better accrual than those that did not incorporate standard therapy [28]. Trials that compared surgery to other types of therapies such as drugs were associated with low accrual and/or trial failure, and multimodality clinical trials were associated with low accrual [17,21].
3.2.4. Trial design
Eligibility criteria, randomization, sample size, trial phase, placebo use, and required protocol procedures and their timing affect accrual and/or completion of oncology clinical trials. The main reported reasons for slow accrual for phase I oncology clinical trials were safety/toxicity (48%), design/protocol issues (42%) and eligibility criteria (41%). In addition, the main reasons for slow accrual for phase II oncology clinical trials were eligibility criteria (35%) and design/protocol issues such as required procedures, treatment schedule, and overall complexity of the trial (33%) [29]. Increased trial complexity defined by a higher number of targeted diseases in inclusion criteria, interventions and study locations was associated with low accrual [17].
Sample size and phase of the clinical trial were two trial design factors that affected accrual and/or completion of oncology clinical trials, although with mixed results in studies. Bennette et al. [17] found larger sample size was a predictor of low accrual. However, Khunger et al. [19] demonstrated the sample size goal (not reported) was higher for completed trials with a median sample goal of 47 compared with that of terminated trials with a median of 9. They also found phase II and phase III trials were significantly less likely to terminate early compared with phase I trials, with low accrual being the most common reason for early termination for all trials. However, Bennette et al. [17] demonstrated phase III was a predictor of low accrual. Other studies did not show accrual varied by trial phase [20].
Eligibility is another trial design factor that affects oncology clinical trial accrual. Overall, eligibility criteria that place burdens on patients, such as those that require the collection of tissues that are not involved with standard of care, were associated with low accrual [17]. In a study of phase I to III molecular trials, the total number of eligibility criteria was significantly associated with the enrollment period's duration in trials that had at least 35 enrolled patients [30].
Specific types of eligibility criteria, which have the potential to considerably limit accrual, were examined in the literature. In a study utilizing ClinicalTrials.gov, the following exclusion criteria were in early phase clinical trials for breast, colorectal, or lung cancers: age >75 years (6%), history of prior malignancies (86%), autoimmune disease with exceptions of vitiligo and alopecia (48%), any central nervous system (CNS) metastasis (38%), symptomatic CNS metastasis (34%), human immunodeficiency virus (31%), hepatitis B or C (21%), and atrial fibrillation (20%). Renal and hepatic eligibility criteria were prevalent, such as creatinine <1.5 of the upper limit of normal (ULN) (35%). Compared to targeted therapy clinical trials, chemotherapy clinical trials were more likely to have exclusion criteria pertaining to CNS metastasis and history of other malignancies. Industry-sponsored trials were more likely to have liver function exclusion criteria than those with other types of sponsors such as the NCI or universities [31].
We examined other specific types of eligibility criteria. In a study of Eastern Cooperative Oncology Group (ECOG) -affiliated lung cancer clinical trials, 80% excluded a prior cancer diagnosis: active cancer (16%), any prior cancer (14%), within 5 years (43%), and within 2–3 years (7%). These exclusions were more common for phase II and III clinical trials (85%) compared to pilot/phase I clinical trials (25%). Estimated proportion of excluded prior lung cancer patients was up to 18% (>5% for 2/3 of clinical trials and >10% for approximately 1/3 of clinical trials). Exclusion criteria related to prior cancer treatment were present in 39% (20) of clinical trials, with 29% (15) excluding chemotherapy or other therapy and 10% (5) excluding both that and radiotherapy [32]. Although in one study [17] performance status (function, symptom burden, need for care) in exclusion criteria was not found to be associated with poor accrual in adult oncology clinical trials, performance status in exclusion criteria was significantly associated with enrollment of older persons in another study [27]. However, exclusion criteria related to renal dysfunction were associated with lower accrual of older adults [24].
Randomization and use of placebo were other trial factors studied regarding accrual and/or trial completion. Bennette et al. [17] found the use of randomization to be associated with low accrual. This was further supported by pediatric nonrandomized clinical trials having adequate accrual [25]. However, in another study, randomization was not found to affect accrual or the early termination of studies [20]. The use of a placebo also had mixed results. In a study of breast cancer clinical trials by Lemieux et al. [26], trials with no placebo were associated with better recruitment than those with a placebo. However, Bennette et al. [17] found no associations between low accrual and placebo use. Also, Ruther et al. [18] reported there were no significant differences in accrual time between placebo and non-placebo use in published phase III oncology clinical trials.
Required protocol procedures and their timing affected accrual in oncology clinical trials. The requirement of obtaining a tissue sample to assess eligibility was a predictor of low accrual [17]. Better recruitment was associated with an allowed 12 week or more interval vs. less time from diagnosis, surgery, or end of previous therapy for nonmetastatic clinical trials [26]. There was no association between blinding and length of follow-up and poor accrual [17].
Other trial design factors were investigated in the literature. There were no associations for accrual related to age group, sex, intervention model, therapeutic compared with nontherapeutic treatment, masking compared with open label, primary purpose, and specialty [20]. Among randomized clinical trials in radiation oncology, lack of accrual was the main reason for trial failure, and a safety endpoint as an outcome was associated with trial failure [21].
4. Discussion
In this systematic review, we examined the empirical literature to investigate trial-level factors that affect accrual and/or completion of oncology clinical trials, identified gaps in the literature, and suggest potential opportunities for future research. One of the most striking findings was the limited number of studies that utilized large databases, lest ClinicalTrials.gov, to examine trial-level factors that affect accrual and/or completion of oncology clinical trials. Researchers are no longer limited to studying clinical trials merely as a single trial or trials which involved a single or few institutions. ClinicalTrials.gov allows researchers to investigate clinical trials as an enterprise since it is the largest and most comprehensive clinical trial database in the world [33].
There was the lack of a standard definition of adequate or inadequate accrual. For example, Paul et al. [20] suggested insufficient accrual as anything less than 100% of the trial's minimum projected sample size whereas Bennette et al. [17] defined low accrual as less than 50% of the target sample size. Different definitions for the outcome variable of adequate or inadequate accrual may partially explain discrepant results in the examined studies' results.
Background factors that were investigated in relation to oncology clinical trial accrual and/or completion included sponsor, number of participating institutions, location of the institutions, competing trials, time of trial opening, and fast-track status. The literature consistently demonstrated that industry-sponsored trials outperformed trials sponsored by other entities in accrual and completion. The pharmaceutical industry may have more financial resources to manage clinical trials at multiple worldwide institutions and invest in accrual strategies such as advertising and participant incentives such as travel reimbursements. Unsurprisingly, a higher number of NCTN-sponsored competing trials was associated with low accrual. Fast track review status designated by the FDA was not associated with low accrual which would be expected, given that fast tracking involves having study sponsors and the FDA working closely together to prioritize and expedite the conduct of clinical trials to get the investigational therapy approved and released to the market.
The type of cancer and its annual incidence were disease-related factors that were investigated. Except among the older population, clinical trials for breast cancer trials consistently outperformed those for other types of cancers in accrual, possibly resulting from the high incidence of breast cancer and public awareness campaigns for these clinical trials. Predictors of low accrual were common solid cancers as opposed to rare solid or liquid tumors. Overall, there are more standard therapies available for common solid cancers than liquid and rare solid tumors. Therefore, patients with common solid cancers have more standard therapy options and do not have to rely on an investigational therapy, resulting in lower accrual in clinical trials.
Several types of treatment were examined in the studies. Clinical trials involving radiation and surgery face challenges with accrual and/or completion. Patients may choose drug regimens, whether as standard therapy or in trials involving only drugs, to avoid the invasiveness and potential complications of a surgical procedure. Also, the proposed surgical procedure in a clinical trial may not have established efficacy in itself or compared to marketed drugs. In addition, patients may prefer drug regimens over radiation clinical trials because they do not want to complete frequent visits to a radiation facility as radiation therapy often entails daily administrations for many weeks. There were mixed results about accrual between clinical trials that involved a new investigational therapy and those that did not, likely due to the difference in toxicity profiles of the investigational agents.
The following trial design factors were investigated: trial development time, eligibility criteria, randomization, sample size, trial phase, placebo use, and required protocol procedures and their timing. Eligibility criteria was the most frequently investigated factor. Although they are necessary to exclude patients who have negative prognostic factors and a high risk of adverse events, eligibility criteria can adversely impact accrual and/or trial completion. Each eligibility criterion needs to be evaluated to ensure it is supported by the scientific literature and not included just because it was contained in previous protocols [34]. Duma et al. [31] also recommends eligibility criteria to be relaxed once a drug's toxicity profile is better understood.
Although trial-level factors that affect accrual and completion of oncology clinical trials have been discussed in publications, there remain gaps in the literature. Several trial-level factors have not yet been investigated utilizing ClinicalTrials.gov outside of studies that are sponsored by NCTN, focus on urological and non-urological solid cancers, and investigate radiation. These trial-level factors include primary purpose, randomization, blinding, and placebo use. In addition, there is a need for studies that characterize the relative importance of various trial-level factors driving clinical trial accrual and/or trial completion and to test the impact of including and excluding these driving trial-level factors on accrual. Research is needed to determine if trial protocols developed to minimize the inclusion of trial-related factors known to be significant barriers result in successful accrual. The reviewed studies did not indicate if some trial-related factors were more influential than others based on the type of cancer targeted in clinical trials. In addition, although this systematic review examined diverse trial-related factors, the review did not address influential trial-related factors specific to patient demographics, except for older adults. Trial-related factors may differ in the way they affect accrual in clinical trials focused on different types of cancers or populations, such as pediatrics. Interventions to improve accrual may need to be tailored to clinical trials for specific types of cancers and populations.
Studies utilizing a mixed methods design may increase knowledge about trial-level factors that affect accrual and/or study completion. Mixed methods studies could explore participants’ views of, and experiences with, trial-related factors to improve accrual and/or trial completion. This knowledge could assist researchers in developing and implementing efficient trial designs and effective interventions to increase accrual and completion of oncology clinical trials. These data would be helpful in determining which trial-related factors are modifiable.
We found that several of the examined studies had conflicting results about the association between trial-level factors and accrual and/or completion of oncology clinical trials. Therefore, more research is required to further elucidate these associations. Only eight of the sample articles utilized ClinicalTrials.gov, thus future researchers should consider use of this database when studying trial-level factors that affect accrual as having a larger sample sizes of clinical trials would increase generalizability of results. Furthermore, clinical trials for different types of cancer encounter distinct challenges to successful accrual. The majority of studies included in this systematic review did not specify a specific cancer, so future research is vital to address trial-level barriers to accrual associated with individual types of cancer. Also, since most of the studies in this review focused on adult oncology clinical trials, similar research is needed for clinical trials for other populations such as pediatrics. Finally, focused efforts on the development and implementation of interventions to address the trial-level factors that adversely impact accrual are needed. This research will need to involve careful reflection about the modifiability of trial-level factors. Improved accrual may contribute to successful completion of oncology clinical trials in a timely manner, reducing the waste of financial and other resources.
Several papers were unclear about how early discontinuation of trials due to protocol-defined safety and interim analyses was managed. Some trials are prematurely discontinued due to reasons unrelated to accrual issues such as toxicity, lack of futility, or inadequate efficacy. It appears in some papers that the researchers assumed that trial-level factors were the root cause of premature trial discontinuations when a trial did not achieve full accrual. However, the trials may have actually been prematurely terminated due to toxicity, lack of futility, or insufficient efficacy at a pre-planned interim analysis. Future studies should include the aforementioned details to facilitate the validity of published findings and subsequent systematic reviews of trials.
This systematic review has limitations. The literature search may not have included all available studies in the published literature because additional terms describing trial-level factors may have been omitted inadvertently. Moreover, since one investigator conducted the review, selected studies included in the final review could not be assessed for inter-rater reliability based on the inclusion and exclusion criteria.
5. Conclusion
With low adult patient participation rates in the increasing number of oncology clinical trials, it is imperative that trial-level factors affecting accrual be identified and interventions addressing these challenges be developed to facilitate the completion of trials. Following a theory-based evaluation and synthesis of research on trial-related factors that influence accrual in oncology clinical trials, this systematic review identified gaps in research in this area. In particular, there remain conflicting results about the associations among trial-level factors, accrual rates, and trial completion. To address the gaps in the literature, theoretically-based studies evaluating the association between trial-level factors and accrual/trial completion should be conducted. The use of theory guides the evaluation, analysis, and organization of an individual study's data by providing the underlying reasoning for key drivers and processes of accrual and completion of trials. In addition, researchers should simultaneously address background, disease-related, treatment-related, and trial design factors that influence accrual using innovative approaches, focusing on specific types of cancer and populations.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Contributor Information
Cherie L. Hauck, Email: cherie023@msn.com.
Teresa J. Kelechi, Email: kelechtj@musc.edu.
Kathleen B. Cartmell, Email: kcartme@clemson.edu.
Martina Mueller, Email: muellerm@musc.edu.
Appendix 1. Literature matrix for trial-level factors affecting accrual and/or completion of oncology clinical trials
| Author, Date | Study Purpose(s) Specific to Trial Factors and Accrual | Type(s) of Cancer | Sample Description, Size | Focused within NCI sponsored cooperative group setting? | Phase(s) of CTs | Database | Trial-related Factors | Results Specific to Trial Factors |
|---|---|---|---|---|---|---|---|---|
| Bennette et al., 2016 | Evaluate associations and predictors between trial-level factors and low accrual in adult cooperative group cancer CTs (clinical trials) | Multiple | 787 interventional, late phase, cooperative group adult oncology CTs that started in 2000–2011 | Yes | II, III | Aggregate Analysis of ClinicalTrials.gov (AACT), Drugs@FDA Database, Surveillance, Epidemiology, and End Results (SEER) Program | Number of competing trials, treatment setting, intervention modality, therapeutic, targeted therapy, new investigational agent, priority status, metastatic setting, clinical setting, sample size, randomized design, phase, placebo, number of interventions, more than one condition, blinded, number of participating sites, eligibility limited by performance status, eligibility limited by age |
|
| Cheng et al., 2010 | Investigate trial development time on accrual to oncology CTs | Multiple | 419 therapeutic, non-pediatric oncology CTs activated between 2000 and 2004 and sponsored by National Cancer Institute (NCI) Cancer Therapy Evaluation Program (CTEP) | Yes | I, I/II, II, III | CTEP Protocol and Information Office database with input from Clinical Data Update System and Clinical Trials Monitoring Service | Trial development time |
|
| Duma et al., 2019 | Identify comorbidities that adversely impact recruitment of patients with breast, colorectal, or lung cancers in early phase CTs | Breast, colorectal, lung | 1103 early phase therapeutic cancer CTs from 2000 to 2015 | No | I, Ib/II, II | ClinicalTrials.gov | Trial phase, target disease, anticancer therapy, line of therapy, location, sponsor, inclusion and exclusion criteria (age limits, comorbidities, organ function) |
|
| Gerber et al., 2014 | Determine prevalence of prior cancer-related exclusion criteria and their impact on lung cancer CT accrual | Lung | 51 lung cancer CTs sponsored or endorsed by the Eastern Cooperative Oncology Group (ECOG) thoracic committee | Yes | I/pilot, II, III | ECOG thoracic committee website; linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database | Eligibility criteria related to prior cancer and its treatment | = 41 (80%) of ECOG -affiliated lung cancer CTs excluded prior cancer diagnosis: active cancer (16%), any prior cancer (14%), within 5 years (43%), within 2–3 years (7%)).
|
| Gross et al., 2005 | Ascertain the effect of protocol factors on enrollment of older patients in cancer CTs | Lung, breast, colorectal, prostate | 36,167 patients enrolled in 33 National Cancer Institute (NCI)-sponsored cooperative group cancer CTs in 1996–2002 | Yes | Unspecified | NCI Clinical Trial Evaluation Program database; NCI Physician Data Query (PDQ) clinical trial database | Cancer type, performance status, comorbidities excluded stage |
|
| Hernandez-Torres et al., 2020 | Determine if exclusion criteria are associated with low accrual of older adults to cancer CTs | Multiple | 69 Canadian Cancer Trials Group studies that started in 1990–2010 | No | III and randomized phase II | Canadian Socioeconomic Management System database | CT start date, cancer type, and exclusion criteria | = The following CT factors were associated with lower accrual of older adults: start date prior to 2003, breast cancer indication, and exclusion criteria related to renal dysfunction. = Central nervous system CTs were associated with higher accrual of older adults. |
| Khunger et al., 2018 | Ascertain the frequency and factors associated with withdrawal and early termination of oncology CTs, focusing on immune checkpoint inhibitor (ICI) trials | Multiple | 12,875 adult, interventional, randomized oncology trials; 350 ICI trials (2011–2015) | No | I, I/II, II, II/III, III | ClinicalTrials.gov | Type of cancer, type of treatment, sponsor, phase, accrual goal |
|
| Kim et al., 2015 | Investigate implications of eligibility criteria in phase I to III molecular trials | Multiple | 67 CTs conducted by Novartis Oncology in the United States from 2006 to 2013 | No | I, II, III (only II and III in final analysis) | Use of ClinicalTrials.gov was not successful; Manual review of trials | Number and characteristics of eligibility criteria | Overall, the total number of eligibility criteria did not affect enrollment duration. However, it was significantly associated with the enrollment period's duration in trials that had at least 35 patients. |
| Korn et al., 2010 | Examine accrual for National Cancer Institute (NCI) Cooperative Group phase III CTs between 2000 and 2007 | Multiple | 191 CTs activated in 2000–2007 *includes 42 pediatric CTs |
Yes | III | Unspecified | Disease site, use of randomization, use of investigational new drug | An estimated 22.0% of all adult and pediatric CTs would be terminated due to inadequate accrual, with 1.7% (2991) of the total enrolled accrued patients being on these CTs. Fewer breast cancer CTs terminate due to inadequate accrual. 2 of 42 pediatric trials had poor accrual. None of the pediatric nonrandomized CTs had inadequate accrual. There was no significant difference in inadequate accrual between CTs that involved an investigational new drug and those that did not. |
| Lemieux et al., 2008 | Identify protocol characteristics of breast cancer CTs associated with poor recruitment | Breast | 688 CTs opened between 1997 and 2002 in Ontario | No | I, II (or I and II), III (or (II and III) | Questionnaires to cooperative groups and pharmaceutical companies; missing data obtained from publications (ClinicalTrials.gov and websites for cooperative groups and pharmaceutical companies were used only to verify if trials should be included if no completed questionnaire received) | Phase, randomization, control group, blinding, intervention, intervention available outside the study, sponsor, location, number of participating sites, menopausal status, metastasis, minimal age limit, maximal age limit, number of eligibility criteria, premature dosing, maximum interval between diagnosis/surgery/end of therapy and enrollment, extra baseline tests, extra follow-up tests | The following protocol factors were associated with better recruitment: no placebo vs. placebo, nonmetastatic vs. metastatic, and allowed 12 week or more interval vs. less from diagnosis, surgery, or end of previous therapy for nonmetastatic CTs. |
| Lyss & Lilenbaum, 2009 | Ascertain accrual patterns among cooperative group non-small cell lung cancer CTs | Non-Small Cell Lung | 16 randomized CTs sponsored by the main cooperative groups in North America that closed accrual between 2000 and 2005 | Yes | II, III | Community Oncology and Prevention Trials Research Group; National Cancer Institute of Canada | Extent of disease, trial phase, # of modalities |
|
| Massett et al., 2016 | Determine reasons for slow accrual in early phase trials sponsored by the National Cancer Institute | Multiple | 135 corrective action plans from 2011 to 2013 *11 (8%) were pediatric trials and 5 (4%) were for trials for both adults and children |
Yes | I, II | Corrective action plans and NCI Cancer Therapy Evaluation Program (CTEP) database | Study design/protocol, eligibility |
|
| Nguyen et al., 2018 | Compare characteristics of completed and incomplete randomized CTs in radiation oncology and identify predictors of trial failure | Multiple | 134 trials that were registered from 2007 to 2010 | No | I, II, III | ClinicalTrials.gov | Cooperative group involvement, sponsor, PI location, number of open institutions, international study, PI's h-index, disease site, age, sex, main comparators, number of study arms, masking, blinding, primary purpose, anticipated enrollment, final enrollment, primary outcome |
|
| Paul et al., 2019 | Determine predictors of adequate accrual in urological and nonurological solid cancer trials | Prostate, colorectal, kidney, bladder, testicular, breast, lung | 326 trials in 2000–2006 | No | III and IV | ClinicalTrials.gov; International Standard Randomized Controlled Trial Number Registry (United Kingdom based); online databases such as PubMed and Google Scholar | Age group, nonrandomized vs randomized, funding source, sex, intervention model, therapeutic vs nontherapeutic, masking vs open label, primary purpose, specialty, phase |
|
| Ruther et al., 2015 | Determine accrual speed in published phase III oncology CTs across geographical locations and identify its influential factors | Multiple | 546 phase III oncology therapeutic CTs published in 2006–2010 *included 4% pediatric/young adult CTs |
III | OVID-Medline | Country, type of cancer, funder, arms, and result |
|
|
| Stensland et al., 2014 | Evaluate study factors associated with trials that fail to complete | Multiple | 7776 adult interventional cancer trials | No | I/II, II, III | ClinicalTrials.gov | Number of sites, sponsor, location |
|
CT = clinical trials.
References
- 1.American Cancer Society Cancer facts & figures 2020. 2020. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html accessed.
- 2.ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2020. https://clinicaltrials.gov/ct2/search/advanced?cond=&term=&cntry=&state=&city=&dist= [cited 2020 Aug 4]. Available from: [Google Scholar]
- 3.Hsiue E.H., Moore T.J., Alexander G.C. Estimated costs of pivotal trials for U.S. Food and Drug Administration-approved cancer drugs, 2015-2017. Clin. Trials. 2020 doi: 10.1177/1740774520907609. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society Cancer Action Network . ACS CAN; Atlanta (GA): 2018. Barriers to Patient Enrollment in Therapeutic Clinical Trials to Cancer – A Landscape Report.https://www.fightcancer.org/sites/default/files/National%20Documents/Clinical-Trials-Landscape-Report.pdf [Internet] [cited 2020 Aug 5]. Available from: [Google Scholar]
- 5.Melnyk B.M., Morrison-Beedy D. Springer; New York: 2012. Intervention Research: Designing, Conducting, Analyzing, and Funding. [Google Scholar]
- 6.Steinman G.L., Smith W.B., Westrick M.L., Greenberg H.E. Hot button protocol and operational issues between sponsors and sites in clinical pharmacology studies: a moderated forum session. Ther Innov Regul Sci. 2017;51(3):298–302. doi: 10.1177/2168479017705688. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine (U.S.) National Academies Press; Washington, D.C.: 2010. Committee on Cancer Clinical Trials, National Academies Press (U.S.), Institute of Medicine (U.S.). Board on Health Care Services., NCI Cooperative Group Program (National Cancer Institute). A National Cancer Clinical Trials System for the 21st Century : Reinvigorating the NCI Cooperative Group Program. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroen A.T., Petroni G.R., Hongkun W., Gray R., Wang X.F., Cronin W. Preliminary evaluation of factors associated with premature trial closure and feasibility of accrual benchmarks in phase III oncology trials. Clin. Trials. 2010;7(4):312–321. doi: 10.1177/1740774510374973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahaghotu C., Tyler R., Sartor O. African American participation in oncology clinical trials--focus on prostate cancer: implications, barriers, and potential solutions. Clin. Genitourin. Cancer. 2016;14(2):105–116. doi: 10.1016/j.clgc.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Fouad M.N., Acemgil A., Bae S., Forero A., Lisovicz N., Martin M.Y. Patient navigation as a model to increase participation of african Americans in cancer clinical trials. J Oncol Pract. 2016;12(6):556–563. doi: 10.1200/JOP.2015.008946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurd T.C., Kaplan C.D., Cook E.D., Chilton J.A., Lytton J.S., Hawk E.T. Building trust and diversity in patient-centered oncology clinical trials: an integrated model. Clin. Trials. 2017;14(2):170–179. doi: 10.1177/1740774516688860. [DOI] [PubMed] [Google Scholar]
- 12.Ling J., Rees E., Hardy J. What influences participation in clinical trials in palliative care in a cancer centre? Eur. J. Cancer. 2000;36(5):621–626. doi: 10.1016/s0959-8049(99)00330-5. [DOI] [PubMed] [Google Scholar]
- 13.National Conference of State Legislatures . NCSL; 2010. Clinical Trials: what Are States Doing?https://www.ncsl.org/research/health/clinical-trials-what-are-states-doing-2010.aspx [Internet] [cited 2020 Aug 5]. Available from: [Google Scholar]
- 14.Wuensch A., Goelz T., Ihorst G., Terris D.D., Bertz H., Bengel J. Effect of individualized communication skills training on physicians' discussion of clinical trials in oncology: results from a randomized controlled trial. BMC Cancer. 2017;17(1):264. doi: 10.1186/s12885-017-3238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yusuf S. Randomized clinical trials: slow death by a thousand unnecessary policies? CMAJ (Can. Med. Assoc. J.) 2004;171(8):889–892. doi: 10.1503/cmaj.1040884. ; discussion 92-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 17.Bennette C.S., Ramsey S.D., McDermott C.L., Carlson J.J., Basu A., Veenstra D.L. Predicting low accrual in the national cancer institute's cooperative group clinical trials. J. Natl. Cancer Inst. 2016;108(2) doi: 10.1093/jnci/djv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruther N.R., Mathiason M.A., Wee S.K., Emmel A.E., Go R.S. Speed of accrual into phase III oncology trials: a comparison across geographic locations. Am. J. Clin. Oncol. 2015;38(6):575–582. doi: 10.1097/01.coc.0000436087.69084.c6. [DOI] [PubMed] [Google Scholar]
- 19.Khunger M., Rakshit S., Hernandez A.V., Pasupuleti V., Glass K., Galsky M.D. Premature clinical trial discontinuation in the era of immune checkpoint inhibitors. Oncol. 2018;23(12):1494–1499. doi: 10.1634/theoncologist.2018-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul K., Sathianathen N., Dahm P., Le C., Konety B.R. Variation in accrual and race/ethnicity reporting in urological and nonurological related cancer trials. J. Urol. 2019;202(2):385–391. doi: 10.1097/JU.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen T.K., Nguyen E.K., Warner A., Louie A.V., Palma D.A. Failed randomized clinical trials in radiation oncology: what can we learn? Int. J. Radiat. Oncol. Biol. Phys. 2018;101(5):1018–1024. doi: 10.1016/j.ijrobp.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Cheng S.K., Dietrich M.S., Dilts D.M. A sense of urgency: evaluating the link between clinical trial development time and the accrual performance of cancer therapy evaluation program (NCI-CTEP) sponsored studies. Clin. Cancer Res. 2010;16(22):5557–5563. doi: 10.1158/1078-0432.CCR-10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stensland K.D., McBride R.B., Latif A., Wisnivesky J., Hendricks R., Roper N. Adult cancer clinical trials that fail to complete: an epidemic? J. Natl. Cancer Inst. 2014;106(9) doi: 10.1093/jnci/dju229. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Torres C., Cheung W.Y., Kong S., O'Callaghan C.J., Hsu T. Accrual of older adults to cancer clinical trials led by the Canadian cancer trials group - is trial design a barrier? J Geriatr Oncol. 2020;11(3):455–462. doi: 10.1016/j.jgo.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Korn E.L., Freidlin B., Mooney M., Abrams J.S. Accrual experience of national cancer Institute cooperative group phase III trials activated from 2000 to 2007. J. Clin. Oncol. 2010;28(35):5197–5201. doi: 10.1200/JCO.2010.31.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemieux J., Forget G., Brochu O., Provencher L., Cantin G., Desbiens C. Evaluation of eligibility and recruitment in breast cancer clinical trials. Breast. 2014;23(4):385–392. doi: 10.1016/j.breast.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Gross C.P., Herrin J., Wong N., Krumholz H.M. Enrolling older persons in cancer trials: the effect of sociodemographic, protocol, and recruitment center characteristics. J. Clin. Oncol. 2005;23(21):4755–4763. doi: 10.1200/JCO.2005.14.365. [DOI] [PubMed] [Google Scholar]
- 28.Lyss A.P., Lilenbaum R.C. Accrual to National Cancer Institute-sponsored non-small-cell lung cancer trials: insights and contributions from the CCOP program. Clin. Lung Cancer. 2009;10(6):410–413. doi: 10.3816/CLC.2009.n.077. [DOI] [PubMed] [Google Scholar]
- 29.Massett H.A., Mishkin G., Rubinstein L., Ivy S.P., Denicoff A., Godwin E. Challenges facing early phase trials sponsored by the national cancer Institute: an analysis of corrective action plans to improve accrual. Clin. Cancer Res. 2016;22(22):5408–5416. doi: 10.1158/1078-0432.CCR-16-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim E.S., Bernstein D., Hilsenbeck S.G., Chung C.H., Dicker A.P., Ersek J.L. Modernizing eligibility criteria for molecularly driven trials. J. Clin. Oncol. 2015;33(25):2815–2820. doi: 10.1200/JCO.2015.62.1854. [DOI] [PubMed] [Google Scholar]
- 31.Duma N., Kothadia S.M., Azam T.U., Yadav S., Paludo J., Vera Aguilera J. Characterization of comorbidities limiting the recruitment of patients in early phase clinical trials. Oncol. 2019;24(1):96–102. doi: 10.1634/theoncologist.2017-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerber D.E., Laccetti A.L., Xuan L., Halm E.A., Pruitt S.L. Impact of prior cancer on eligibility for lung cancer clinical trials. J. Natl. Cancer Inst. 2014;106(11) doi: 10.1093/jnci/dju302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fain K.M. National Library of Medicine; Bethesda (MD): 2018 Jan 23. ClinicalTrials.gov Moves toward Increased Transparency [Internet]https://nlmdirector.nlm.nih.gov/2018/01/23/clinicaltrials-gov-moves-toward-increased-transparency/ (cited 2020 Sep 12). Available from: [Google Scholar]
- 34.Malik L., Lu D. Eligibility criteria for phase I clinical trials: tight vs loose? Cancer Chemother. Pharmacol. 2019;83(5):999–1002. doi: 10.1007/s00280-019-03801-w. [DOI] [PubMed] [Google Scholar]