Abstract
The objective of this study was to investigate confirmed progression independent of relapse activity in relapsing-remitting multiple sclerosis patients under long-term natalizumab treatment. We performed a retrospective, cross-sectional study of clinical data captured between 1994 and 2019 at two German multiple sclerosis tertiary referral centres. Data files of all relapsing-remitting multiple sclerosis patients treated with natalizumab for ≥24 months were analysed. Confirmed progression independent of relapse activity was defined as ≥12 week confirmed disability progression on a roving Expanded Disability Status Scale reference score by 1 point in patients with an Expanded Disability Status Scale score ≤3 or 0.5 in patients with an Expanded Disability Status Scale score ≥3.5 in the absence of a relapse. Cox proportional hazard models were used to analyse the probability of developing confirmed progression independent of relapse activity depending on both disease and natalizumab treatment duration. Among the 184 patients identified, 44 (24%) developed confirmed progression independent of relapse activity under natalizumab irrespective of the Expanded Disability Status Scale score at natalizumab onset. Time to confirmed progression independent of relapse activity was not affected by Expanded Disability Status Scale at natalizumab onset (categorized by Expanded Disability Status Scale score ≤3.5 versus >3.5) nor by duration of disease nor by duration of therapy. Confirmed progression independent of relapse activity occurred earlier in the disease course in patients with an earlier natalizumab therapy onset with regard to disease duration. A stepwise forward regression analysis revealed disease duration as the main factor for confirmed progression independent of relapse activity development (P = 0.005). Taken together, confirmed progression independent of relapse activity occurs in a substantial proportion of patients on long-term natalizumab treatment and independent of Expanded Disability Status Scale score at natalizumab onset. Our findings suggest that patients who are initiated on natalizumab early during disease course, usually in order to treat an aggressive clinical phenotype, have a higher risk of early confirmed progression independent of relapse activity.
Keywords: multiple sclerosis, natalizumab, disease progression, cPIRA, long-term treatment
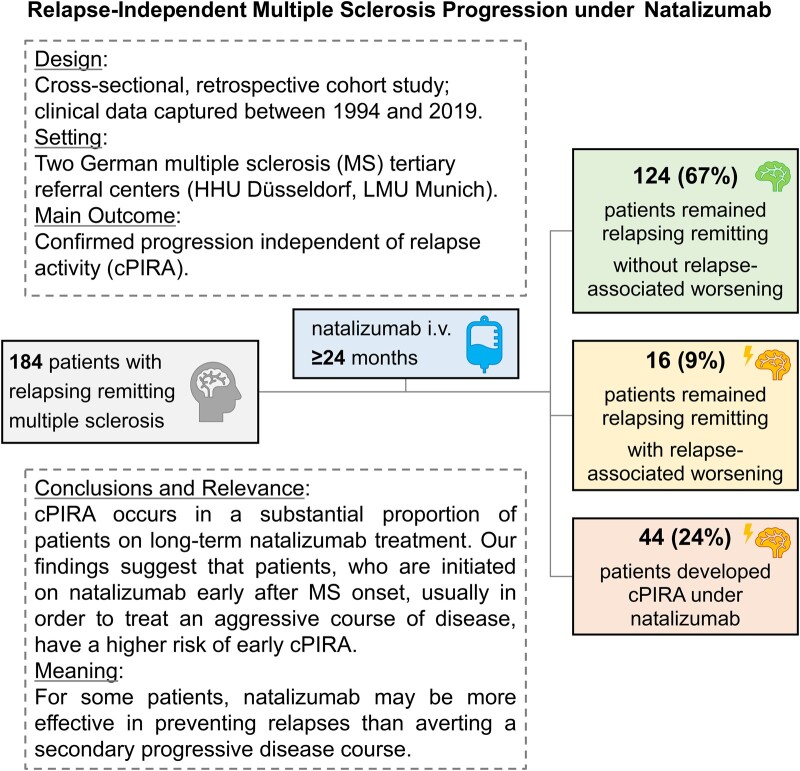
Graf et al. aimed to assess the relevance of relapse-independent disease progression in relapsing remitting multiple sclerosis patients under long-term natalizumab treatment. In this study, more than 20% of multiple sclerosis patients developed relapse-independent disease progression, mostly linked to a more active disease course prior to natalizumab onset.
Graphical Abstract
Graphical Abstract.
Introduction
Multiple sclerosis is an immune-mediated disease of the CNS with a complex, diverse disease course. Clinically, there are two different disease subtypes of multiple sclerosis: relapsing multiple sclerosis and progressive multiple sclerosis either manifesting itself as a primary progressive form with gradual worsening of neurologic disability from symptom onset or a secondary progressive disease course with or without (prior) relapse activity.1 Pathologically, both inflammation and degeneration play an important role in both relapsing and progressive multiple sclerosis, and compartmentalized inflammation and degeneration in the CNS is considered to be of particular relevance in the progressive disease forms.2 Natalizumab was early licensed as a disease modifying therapy (DMT) for the treatment of highly active relapsing remitting multiple sclerosis and its efficacy in reducing the relapse rate has been demonstrated in multiple studies.3–8 However, natalizumab failed to meet the primary composite endpoint at 2 years in a phase 3 trial performed in secondary progressive multiple sclerosis.9 A detailed analysis suggested a potential benefit in certain subtests, particularly for function of the upper extremities, and the open label extension demonstrated efficacy on the primary outcome at year three.9 A possible explanation for the difference in efficacy of natalizumab in relapsing and progressive multiple sclerosis may be the inability to reach the compartmentalized pathology. To date, the effect of natalizumab on preventing conversion to secondary progressive multiple sclerosis is still unclear. In a recently published prospective cohort, the number of natalizumab infusions was associated with a decrease of relapse rate, but no association was found with the progression of disability, the accumulation of lesion burden or the magnitude of brain volume loss, suggesting an uncertain benefit of prolonged natalizumab use on clinical and MRI outcomes of disease progression.10 Another large real-world evidence study demonstrated that early natalizumab treatment during disease course reduced the risk of conversion to secondary progressive multiple sclerosis.11
We aimed to assess the relevance of relapse-independent disease progression as an indicator for secondary progressive multiple sclerosis conversion in two independent real-world cohorts of multiple sclerosis patients under long-term natalizumab treatment. It has to be kept in mind that there is still no consensus regarding the definition of secondary progressive multiple sclerosis. Among the most discussed guidelines are the Lublin criteria1,12 and the secondary progressive multiple sclerosis definition developed by Lorscheider et al.,13 but especially the time period of progression required for defining secondary progressive multiple sclerosis is heterogeneous in the literature.14–17 In a large prospective study, long-term disability progression was associated with brain volume loss but not relapse rate.18 The concept of progression independent of relapses (PIRA) has recently been introduced,19 and that of confirmed progression independent of relapse activity (cPIRA) in combination with the use of a roving EDSS reference score may be an adequate approach for this unmet clinical need.
Materials and methods
Patients and recruitment
We conducted a retrospective chart review study at two centres: the Multiple Sclerosis Centers of the Heinrich-Heine-University Düsseldorf and of the Institute of Clinical Neuroimmunology, LMU Hospital, Ludwig-Maximilians University, Munich. We documented all relevant epidemiological, clinical and paraclinical information about the selected patients, such as age, sex, disease duration, relapses, Expanded Disability Status Scale (EDSS), MRI, previous and current therapies. The data were assessed during clinical routine visits and retrospectively collected from the hospital information system [MEDICO, Cerner/CGM (Düsseldorf)] and clinical charts (LMU Hospital). Inclusion criteria were diagnosis of relapsing remitting multiple sclerosis according to McDonald criteria 2010,20 a continuous natalizumab therapy for ≥24 months, and availability of longitudinal EDSS and relapse data (at least three EDSS scores and information on relapse dates documented) for ≥24 months. The only exclusion criteria were cPIRA onset before natalizumab treatment and a disability change due to other causes than multiple sclerosis such as stroke, polyneuropathy or neurodegenerative disease leading to clinical impairment or deficit.
In this study, we used cPIRA as an indicator for secondary progressive multiple sclerosis conversion. It was defined as a ≥12 week confirmed disability progression (CDP) independent of relapse activity and evaluated in all patients including those who discontinued natalizumab therapy in the follow-up. Disability progression was defined as a worsening of 1 point on the EDSS in patients with a baseline EDSS ≤3 or 0.5 EDSS steps in patients with a baseline EDSS ≥3.5 in the absence of a relapse using a roving EDSS reference score.21 We chose to define EDSS progression based on a cut-off value of 3.5 as EDSS steps up to 3.5 are mainly dominated by single functional system scores and rather sensitive to inter-rater variability, while from scores above 3.0 relevant disability in more than one system is required and above 4.0 the walking disability becomes increasingly relevant. The relapse-free interval relevant for cPIRA was defined as a time interval without relapses for a minimum of 12 consecutive months. All patients with an EDSS worsening according to the aforementioned definition of disease progression were included in the cPIRA group when the relapse unrelated EDSS worsening (PIRA) could be confirmed in the next clinical follow-up at least 12 weeks later (cPIRA). Relapses occurring after the development of cPIRA were classified as superimposed relapses (SIR).
To include a maximum of data and to analyse the relevance of events outside of the natalizumab treatment interval, we did not limit the follow-up length, but instead included all available EDSS and MRI data from the multiple sclerosis first diagnosis to the last documented visit. Therefore, cPIRA evaluation began with the first EDSS documented, e.g. at the time of multiple sclerosis diagnosis.
Relapse data were extracted from the clinical databases and by chart review. Relapses had been identified and classified during the clinical routine by experienced multiple sclerosis specialists at our tertiary referral centres based on patient interviews and clinical examination. Relapses were defined as a neurologic deficit compatible with an acute CNS inflammatory demyelinating event lasting at least 24 h in the absence of fever. Disability progression observed in visits with a relapse in between was considered as relapse-associated worsening (RAW) and not considered for analysis of cPIRA. Furthermore, in order to avoid the risk of carry over EDSS progression resulting from prior relapses, all follow-up intervals with relapse activity within one month prior to the baseline examination were excluded from the cPIRA analysis.
MRI activity was defined as presence of gadolinium enhancing lesions on T1 imaging or the development of new or enlarging T2 lesions in comparison to the previous MRI. MRI data and findings were collected retrospectively during the observational period. Owing to impaired comparability of different and non-standardized MRI protocols performed on different scanners in the clinical routine, we had to limit our analysis to the occurrence of inflammatory lesions and were not able to analyse brain volume and/or brain atrophy patterns.
Statistical analysis
Statistical analyses were performed using SPSS 20 (IBM, Armonk, NY) and were run for all 184 patients included even if some of them stopped the natalizumab treatment and switched to another therapy during the follow-up. Table 1 summarizes the descriptive statistics listing the median and interquartile range for each variable of the different groups. A Mann–Whitney U-test was used to identify significant differences between the groups and a power analysis was conducted to define the correlation coefficient r. A Kruskal–Wallis test with Bonferroni posthoc test was used for to identify significant EDSS differences between groups. P-values below 0.05 were considered significant. A logistic regression analysis was performed to analyse which factors could have had an influence on cPIRA development in the overall cohort as well as in the single Munich and Düsseldorf cohorts separately. We opted for this regression model despite the risk of overfitting in order to decrease the chance of missing a signal from our defined variables. The following factors were included in the regression model: age and sex at natalizumab onset, the number and the class of DMTs prior to treatment with natalizumab as well as the duration of natalizumab therapy in years. DMTs were classified as first or second line as followed: as first line treatments we considered beta-interferon, glatiramer acetate, teriflunomide and dimethyl fumarate while second line treatments were mitoxantrone, alemtuzumab, fingolimod, natalizumab, rituximab, ocrelizumab and azathioprine. For the comparison of the demographic and clinical characteristics between the Düsseldorf and the Munich cohorts, we used the Mann–Whitney U two-sample rank-sum test. The probabilities of developing cPIRA were estimated using a Kaplan–Meier analysis. In order to facilitate interpretation and presentation of the results we divided the patients in equally sized subgroups based on the natalizumab treatment onset (≤8.6 years and >8.6 years), the EDSS score (≤3.5 and >3.5), the number of DMTs prior to natalizumab therapy onset (≤2 and >2) and the number of relapses that had occurred prior to the natalizumab treatment onset (<1, 1–2 and >2). Cox proportional hazard models correcting for age and sex reporting the hazard ratios [HR; Exp(B)] were used to compare the probability to develop cPIRA for these subgroups. Reporting followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline.
Table 1.
Demographic and clinical characteristics of the patientsa
| Baseline characteristics Medians (interquartile range) | Group 1: No-cPIRAb under natalizumabc (n = 140) | Group 2: cPIRA under natalizumabc (n = 44) | P-valuesd Group 1 vs Group 2 |
|---|---|---|---|
| Age at natalizumab onset—year | 33.5 (27; 42) | 38.5 (29; 45) | n/s |
| Female sex—no (%) | 92 (65.7) | 26 (59.1) | n/s |
| Therapy duration natalizumabe—year | 4.8 (3; 7.6) | 5 (3; 9) | n/s |
| Disease duration since first manifestation—year | 14 (10; 19) | 18 (14; 25) | 0.004 |
| r < 0.3 | |||
| Disease duration since first diagnosis—year | 12 (9; 17) | 15.5 (12; 19.8) | 0.004 |
| r < 0.3 | |||
| Disease duration between first manifestation and natalizumab onset—year | 6 (3; 11) | 8 (3; 17) | n/s |
| Disease duration between first diagnosis and natalizumab onset—year | 5 (2; 8) | 4.5 (2; 12.5) | n/s |
| Number of other therapiesf—no at study inclusion | 3 (2; 4) | 3 (2; 4) | n/s |
| Number of DMTsg prior to natalizumab—no | 2 (1; 3) | 2 (1; 3) | n/s |
| Annualized relapse rate under natalizumabh—no | 0 (0; 0.3) | 0 (0; 0.3) | n/s |
| Number of visitsi—no | 12 (8; 16) | 16 (12; 22) | 0.009 |
| r < 0.3 | |||
| EDSSj-changek in relapse free interval—no | 0 (−0.5; 0) | 1.5 (0.5; 2.5) | <0.001 |
| r > 0.5 |
Data include only patients who have been treated with natalizumab for a minimum of 2 consecutive years (main inclusion criterium) and whom EDSS values were available. All patient information is from the electronic database MEDICO (for the Düsseldorf Cohort) and from the patient files, which include clinical examinations and investigations results such as MRI findings, CSF and blood tests that have been collected before 01.01.2018 for the Düsseldorf cohort and before 07.08.2019 for the München cohort.
Confirmed Disability Progression independent of Relapse Activity.
The patient groups were defined as follows: patients who still experienced relapses during the observation time without EDSS worsening were included in the Group 1; Patients who developed a secondary progression under the natalizumab treatment were assigned to Group 2. The secondary progression was defined as an EDSS worsening of ≥1.0 point from the baseline EDSS score for patients with baseline score of 3.0 or less, or ≥0.5 for patients with baseline score of 3.5 or more that cannot be attributable to recent relapse activity. For each variable we provide the median of a given group with the corresponding interquartile range.
P-values reflect Mann–Whitney U-test. A power analysis was conducted to obtain the correlation coefficient r. P-values for the comparison of Group 1 with Group 2 showed significant differences (P < 0.05) for the following variables: Disease duration since first manifestation, Disease duration since first diagnosis, Number of visits and EDSS-Change in relapse-free interval.
The minimal natalizumab therapy duration is 2 years according to the inclusion criteria.
This category includes all documented therapeutic measures from relapse-treatments to DMTs which have been taken since the first MS manifestation.
Disease Modifying Therapies.
Recorded between the first and the last recorded relapse in the period of time under natalizumab treatment.
Meant is the number of visits which took place in the Universitätsklinikum Düsseldorf (UKD) or in the Universitätsklinikum München (LMU).
Expanded Disability Status Scale.
Recorded between the first and the last recorded EDSS value during the relapse-free period.
Ethics approval and consent to participate
The study was approved by the local ethics committee at the Heinrich Heine University of Düsseldorf (registry number 6083R) and at the Ludwig-Maximilians University Munich (Nr. 19116). Owing to the retrospective design of the study, informed consent was not necessary according to the local ethics committee.
Data availability statement, responsibility and analysis
Philipp Albrecht, the corresponding author, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The datasets supporting the conclusions of this article are included within the article and its additional files. Raw data generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Results
Comparison of the cPIRA rate depending on baseline parameters
Out of the 271 relapsing remitting multiple sclerosis patients identified with natalizumab therapy at the two investigating centres (Figs 1 and 2, Table 1 and Supplementary Table 1), 184 patients met the inclusion criteria, while 77 were excluded from the data analysis due to lack of EDSS and relapse data (27) or insufficient follow-up data (≤24 months of natalizumab therapy) (50). Furthermore, 10 patients were excluded due to cPIRA onset outside a natalizumab treatment interval, e.g. before initiation of natalizumab or development of cPIRA during a pause of natalizumab treatment. Of these 184 patients, 140 patients remained relapsing remitting (76%), while 44 developed cPIRA as an indicator for secondary progressive multiple sclerosis (24%). Under the 140 relapsing remitting patients, 16 patients (9%) presented a relapse-associated worsening (RAW) with relevant EDSS increase. The median time on natalizumab therapy until cPIRA occurred was 10 ± 1 years.
Figure 1.
Overview of the total cohort. Flowchart of the total cohort. *In the confirmed progression independent of relapse activity (cPIRA) group, the ‘+’ indicates the presence of relapse and MRI activity before or after the cPIRA defining interval.
Figure 2.
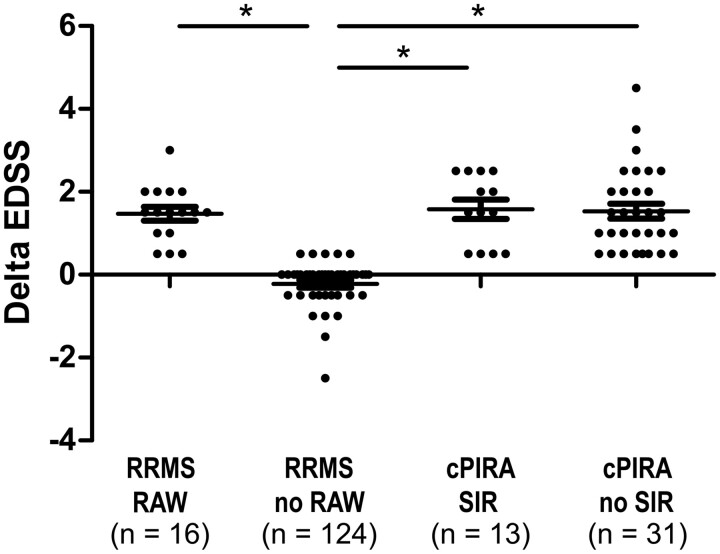
Disability change of the total cohort. EDSS change of confirmed progression independent of relapse activity (cPIRA) patients [with and without superimposed relapses (SIR)] and relapsing remitting multiple sclerosis patients [with and without relapse-associated worsening (RAW)], *P < 0.05, Kruskall–Wallis with Bonferroni post hoc test.
Investigation of factors responsible for time to cPIRA
Information on MRI activity was available for all patients but not for all follow-up intervals due to the heterogeneity of follow-up in the real-world setting. Approximately half of the 184 included patients had neither MRI nor relapse activity, and patients with relapses and MRI activity were less common (Fig. 1). In the No-cPIRA under natalizumab group, 70 of 140 patients (50%) had neither relapse nor MRI activity, as compared to 26 of 44 patients (59%) in the cPIRA under natalizumab group. On the other hand, 20 of 140 patients (14.3%) in the No-cPIRA under natalizumab and 2 of 44 patients (4.5%) in the cPIRA under natalizumab group had both MRI and relapse activity. Overall, disease duration was significantly longer, the number of hospital visits prior to natalizumab and the increase of EDSS in the relapse-free period were significantly higher in patients with cPIRA under natalizumab compared to No-cPIRA patients (Table 1). The EDSS remained stable and showed a tendency to improvement in No-cPIRA relapsing remitting multiple sclerosis patients without RAW (mean change of −0.2 ± 0.6 over a mean of 5.5 years) while patients who developed cPIRA with and without superimposed relapses (SIR) as well as relapsing remitting multiple sclerosis patients with RAW (Fig. 1) presented a significant deterioration of EDSS with a mean of 1.5 ± 0.9 over a mean of 5.8 years (Fig. 2). A significant difference with regards to MRI and CSF parameters was not detected (Supplementary Table 1). The regression analysis revealed disease duration as the main factor for cPIRA development (P = 0.005). Other factors (like sex, age, class of treatment and number of prior therapies as well as natalizumab therapy duration) had no additional influence on the development of cPIRA.
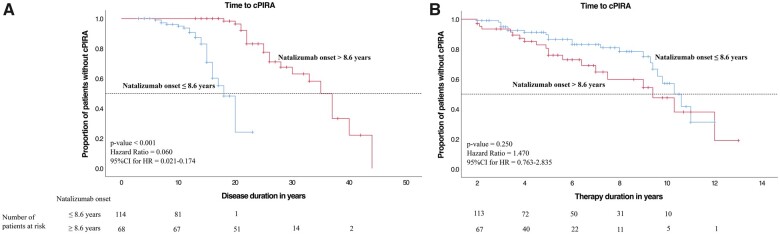
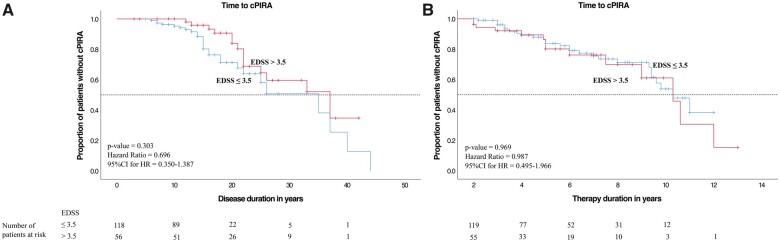
Cox proportional hazard models were used to compare the cPIRA-free survival between subgroups over time (Figs 3–7), and the number of remaining patients under observation at a given timepoint are indicated as ‘patients at risk’ below the x-axes in Figs 3–7. cPIRA occurred earlier in the course of disease in patients with an earlier onset of natalizumab therapy [≤8.6 versus >8.6 years, P < 0.001, HR Exp(B) = 0.060, 95%CI = 0.021–0.174] but considering only the time on natalizumab the onset of cPIRA did not differ between both groups [P = 0.250, HR Exp(B) = 1.470, 95%CI = 0.763–2.835]. Time to cPIRA did not differ between patients with EDSS ≤3.5 and >3.5 neither considering the duration of disease [P = 0.303, HR Exp(B) = 0.696, 95%CI = 0.350–1.387] nor the duration of therapy [P = 0.969, HR Exp(B) = 0.987, 95%CI = 0.495–1.966]. Furthermore, patients with >2 DMTs before natalizumab show no difference in the development of cPIRA over the disease course with respect to patients with ≤2 DMTs [P = 0.640, HR Exp(B) = 1.170, 95%CI = 0.607–2.255]. However, considering only the period of natalizumab therapy, patients with >2 DMTs prior to natalizumab developed cPIRA significantly earlier than patients with ≤2 DMTs [P = 0.031, HR Exp(B) = 1.996, 95% CI = 1.064–3.744]. The annualized relapse rate (ARR) did not differ between patients who developed cPIRA and those who did not. However, the mean EDSS deterioration was significantly higher in natalizumab treated patients who developed cPIRA (Fig. 2).
Figure 3.
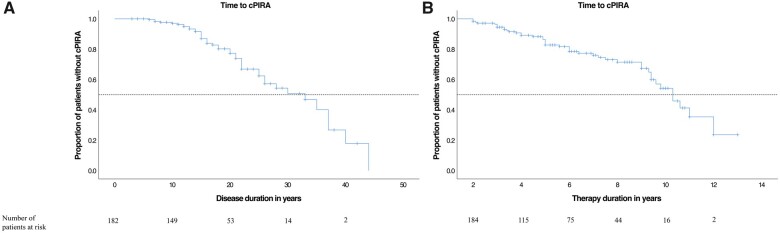
Kaplan–Meier curves of the total cohort. (A) Kaplan–Meier curves for the time (in years) of disease duration from the first multiple sclerosis manifestation and (B) natalizumab treatment duration until the outcome confirmed progression independent of relapse activity (cPIRA) occured. cPIRA was defined as an EDSS increase of ≥1.0 point from the baseline EDSS score for patients with baseline score of 3.0 or less, or ≥0.5 for patients with baseline score of 3.5 or more.
Figure 4.
Kaplan–Meier curves of the total cohort, disease duration at natalizumab onset subanalysis. (A) Kaplan–Meier curve for the time of disease duration and (B) natalizumab therapy duration (in years) to onset of confirmed progression independent of relapse activity (cPIRA). Progression free survival in the patients with natalizumab therapy onset is compared within (blue curve) and after (red curve) 8.6 years from multiple sclerosis first manifestation to first natalizumab dose. The discriminatory value of 8.6 years corresponds to the mean duration between the first multiple sclerosis manifestation and the first received natalizumab dose of the total cohort. P-values for the comparison of the two groups were obtained with Cox proportional hazard models correcting for age and sex.
Figure 5.
Kaplan–Meier curves of the total cohort, diseases severity subanalysis. (A) Kaplan–Meier curve for the time of disease duration and (B) natalizumab therapy duration (in years) to onset of confirmed progression independent of relapse activity (cPIRA). The analysis was performed after the patients have been divided into two groups according to the EDSS Score performed at the time of natalizumab therapy onset. The red curve represents the patients with an EDSS score greater than 3.5, while the blue curve the patients with an EDSS score of 3.5 or less. P-values for the comparison of the two groups were obtained with Cox proportional hazard model correcting for age and sex.
Figure 6.
Kaplan–Meier curves of the total cohort, previous treatment subanalysis. (A) Kaplan–Meier curve for the time of disease duration and (B) natalizumab therapy duration (in years) to onset of confirmed progression independent of relapse activity (cPIRA). Compared is the progression free survival in the patients with less (blue curve) and more (red curve) than 2 DMTs prior to natalizumab therapy onset. P-values for the comparison of the two groups were obtained with Cox proportional hazard model correcting for age and sex.
Figure 7.
Kaplan–Meier curves of the total cohort, relapse rate subanalysis. (A) Kaplan–Meier curve for the time of disease duration and (B) natalizumab therapy duration (in years) to onset of confirmed progression independent of relapse activity (cPIRA). The survival analysis was performed for subgroups stratified based on the rate of annualized relapse rate (ARR) prior to natalizumab treatment onset. P-values for the comparison of the groups were calculated with Cox proportional hazard model correcting for age and sex.
An overview of the Düsseldorf and Munich cohorts including separate sub-analyses is provided in Supplementary Table 2 (for the subgroup of cPIRA patients under natalizumab) and Supplementary Fig. 1. Comparing the Düsseldorf and Munich cohorts revealed significant differences regarding the time between first manifestation and first diagnosis, current therapy, Measles Rubella and Varicella Zoster (MRZ)-reaction and natalizumab discontinuation, but no differences regarding age, sex, EDSS at first diagnosis and disease duration. A stepwise forward regression analysis revealed that no variable influenced the occurrence of cPIRA in the Munich cohort while for the Düsseldorf cohort age and prior therapies had a significant influence on secondary progressive multiple sclerosis development. In the Munich cohort, 14 of 70 included patients (20%) and in the Düsseldorf cohort, 30 out of 114 included patients (26.3%) developed secondary progressive multiple sclerosis according to our cPIRA definition (Supplementary Fig. 1). We observed no significant difference regarding the EDSS change between the Munich and Düsseldorf cohorts.
Discussion
With over a decade of experience treating multiple sclerosis patients with natalizumab, it is now possible to explore the long-term effects in a real-world setting. Our study indicates that natalizumab does not change disability progression in progressive MS, which is in line with the results from a randomized controlled phase 3 trial.9
Interestingly, a recent pooled analysis of phase 3 relapsing remitting multiple sclerosis trials revealed that most of the accumulated disease progression is not relapse-associated.22 Furthermore, data from the Tysabri® Observational Program (TOP) suggest that the probability of disability worsening under natalizumab is 27.8%.23 In our study, we applied a modified version of the PIRA concept19,22 with confirmation of disability progression at a variable timepoint >12 weeks after the previous assessment. Furthermore, we defined progression as an EDSS increase of 0.5 points beyond 3.5 and not beyond 5.5 as used in previous studies.19,22 This may be interpreted as a limitation for reason of comparability. However, we consider the phase of moderate disability with EDSS 3.5–5.5 to be particularly relevant for cPIRA development and therefore chose to increase the sensitivity for change in this range.
Overall, our findings are in line with previous studies.22,23 Since these findings have not been directly correlated, caution is required to imply a cause-and-effect relationship.
Our data suggest that almost 80% of patients do not develop cPIRA and remain stable despite a mean disease duration of 15.6 ± 7.5 years. Of note, natalizumab was not completely ineffective in a phase 3 secondary progressive multiple sclerosis trial as it may positively influence upper limb function.9 However, more than 20% of our patients did develop cPIRA and by definition, the EDSS of these patients increased under natalizumab with mean change of 1.4 ± 0.9 compared to baseline. When comparing our results with existing natural disease course data, the rate for conversion to a secondary progressive disease course may be reduced by 50% under natalizumab.14,24,25 However, the secondary progressive multiple sclerosis conversion rate in our study was not inferior to the rates reported for other DMTs.26–28 As natalizumab failed to reduce EDSS progression in a phase 3 secondary progressive multiple sclerosis trial,9 its mechanism of preventing leukocyte trafficking may not be of sufficient relevance for halting progression. Moreover, the relevance of preventing relapses in order to prevent secondary progressive multiple sclerosis may not be as relevant as expected.29
In a recent real-world evidence study on the long-term effects of immunotherapies,11 secondary progressive multiple sclerosis conversion was significantly lower in natalizumab-treated patients, as compared to untreated patients. Considering our data, this effect may rather be associated with disease duration. The exact reasons for cPIRA under natalizumab despite good control of relapses remain open. The positive effect on progression reported for siponimod versus placebo30 suggests that tissue penetrance may be of relevance, e.g. to target CNS resident cells such as microglia. More mechanistic studies comparing therapeutics and including advanced imaging and biomarkers are warranted. Basic MRI and CSF may not be suitable to determine patients’ risk for developing cPIRA. Neuroimaging studies suggest that the estimated rate of lesion growth31 and of atrophied brain T2 lesion volume32,33 are associated with secondary progressive multiple sclerosis conversion risk. However, our study is limited by its retrospective design and by an indication bias favouring more active patients to receive natalizumab. Furthermore, 32% (87 of 271) patients were excluded due to inclusion/exclusion criteria and missing EDSS data, and our study design does not allow us to account for these patients. This dropout rate is due to the fact that patients often decide to receive natalizumab treatment in a private practice setting and sometimes do not return to the university centres. We do not expect these to have a different course and therefore do not anticipate a major source of bias.
The different patient cohorts described in studies mentioned above cannot be compared. As disease duration was the only factor influencing cPIRA in our cohort, we cannot postulate a clear treatment associated mechanism. The fact that we investigated cPIRA in data obtained in clinical routine without standardized follow-up intervals and heterogeneous treatment duration bares the risk of bias.
One may assume that patients with a severe disease course are more likely to visit the outpatient clinic more frequently compared to clinically stable patients, which may lead to cPIRA overestimation. However, for all patients presenting confirmed EDSS progression information on relapse activity was available and therefore cPIRA and RAW could be evaluated. On the one hand, both cPIRA patients with and without SIR showed a significant EDSS increase compared to relapsing remitting multiple sclerosis patients without RAW. On the other hand, we have to acknowledge that RAW may be underrepresented since 10 patients form our centres with relapse activity discontinued natalizumab before reaching 24 months of follow-up and therefore did not meet the inclusion criteria.
In our cohort, cPIRA risk was not different between the EDSS ≤3 and ≥3.5 group. Therefore, a bias resulting from differential sensitivity in high and low ranges of the EDSS scoring system seems rather unlikely as relapse independent disease progression was equally distributed in these groups. Furthermore, using CDP at week 24 did not change the main result.
A strength of our study is the real-world setting with long follow-up times in two independent cohorts. We acknowledge that a comparison to patients on other long-term treatment would be of highest interest. In context with previous studies,21,22 we cannot exclude that mild exacerbations which do not fulfil the relapse definition may be a cause of cPIRA.
Our data suggest that patients with early natalizumab initiation (≤8.6 years after diagnosis) seem to be more likely to develop cPIRA than those with late initiation (Fig. 4A). However, since natalizumab therapy duration had no influence on progression regardless of the timing of initiation (Fig. 4B), we interpret this finding to be driven by disease severity: Patients with a severer disease course may receive natalizumab earlier.
The fact that 20% of our patients developed cPIRA highlights the need for close monitoring of clinical disability in patients under long-term natalizumab treatment as therapeutic consequences may be considered.
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
No funding was received for the preparation of this report. J.H. is (partially) funded by the German Federal Ministry of Education and Research [Grant Numbers 01ZZ1603[A-D] and 01ZZ1804[A-H] (DIFUTURE)]. The article publication charge was funded by the Open Access Fund of the Heinrich-Heine-University Düsseldorf.
Competing interests and conflict of interest
J.G. received travel/meeting/accommodation reimbursements from Biogen, Merck Serono, Sanofi-Genzyme, Grifols, and a Research Fellowship from the Deutsche Forschungsgemeinschaft (project number 438899010, GZ: GR 5665/1-1). V.I.L. received fees for speaking from Biogen, Genzyme, and Novartis and research support from Novartis. G.S. declares no relevant competing interests. K.L. declares no relevant competing interests. I.M. received travel expenses from MedDay and Roche Pharma, and compensation from Serono for participation in an advisory board. T.K. received travel expenses and speaker honoraria from Bayer Healthcare, Teva Pharma, Merck, Novartis Pharma, Sanofi-Aventis/Genzyme, CLB Behring, Roche Pharma and Biogen as well as grant support from Bayer-Schering AG, Novartis and Chugai Pharma. S.G.M. received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology, and by Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. H.P.H. (outside this work) received, with approval of the Rector of Heinrich-Heine University and the CEO of University of Düsseldorf Hospital honoraria for consulting, serving on steering committees and speaking from Alexion, Bayer Healthcare, Biogen, Celgene BMS, Geneuro, LFB, Medimmune, Merck Serono, Novartis, Octapharma, Roche, Sanofi Genzyme, TG Therapeutics and VielaBio. J.H. reports personal fees, research grants and non-financial support from Merck, Novartis, Roche, Santhera, Biogen, Alexion, Celgene, Sanofi Genzyme; and non-financial support of the Guthy-Jackson Charitable Foundation, all outside the submitted work. O.A. received, with approval of the Rector of Heinrich-Heine University, grants from the German Research Foundation (DFG), the German Ministry for Education and Research (BMBF) as part of the German Competence Network Multiple Sclerosis (KKNMS; for NEMOS NationNMO-PAT FKZ 01GI1602B), the Eugène Devic European Network (EU-FP7), honoraria and travel/accommodation/meeting expenses from Almirall, Bayer, Biogen, Medimmune, Merck Serono, Novartis, Roche, Sanofi-Genzyme, and Teva. P.A. received, with approval of the Rector of Heinrich-Heine University and the CEO of University of Düsseldorf Hospital, personal fees, research grants and non-financial support from Allergan, Biogen, Celgene, Ipsen, Merck Serono, Merz Pharmaceuticals, Novartis, and Roche, personal fees and non-financial support from Bayer Healthcare, and Sanofi-Aventis/Genzyme, outside the submitted work.
Supplementary Material
Glossary
- ARR =
annualized relapse rate
- CDP =
confirmed disability progression
- cPIRA =
confirmed progression independent of relapse activity
- DMTs =
disease modifying therapies
- EDSS =
Expanded Disability Status Scale
- LMU =
Ludwig-Maximilians University
- MRZ =
Measles Rubella and Varicella Zoster Reaction
References
- 1. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology. 2014;83(3):278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(Pt 5):1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348(1):15–23. [DOI] [PubMed] [Google Scholar]
- 4. Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. [DOI] [PubMed] [Google Scholar]
- 5. Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):911–923. [DOI] [PubMed] [Google Scholar]
- 6. Mazdeh M, Hosseini S, Taheri M, Ghafouri-Fard S.. The effect of natalizumab on disability score and relapse rate of multiple sclerosis patients: A prospective cohort study. Clin Transl Med. 2018;7(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhovtis Ryerson L, Frohman TC, Foley J, et al. Extended interval dosing of natalizumab in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(8):885–889. [DOI] [PubMed] [Google Scholar]
- 8. O'Connor P, Miller D, Riester K, et al. Relapse rates and enhancing lesions in a phase II trial of natalizumab in multiple sclerosis. Mult Scler. 2005;11(5):568–572. [DOI] [PubMed] [Google Scholar]
- 9. Kapoor R, Ho P-R, Campbell N, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): A phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405–415. [DOI] [PubMed] [Google Scholar]
- 10. Zivadinov R, Hojnacki D, Bergsland N, et al. Effect of natalizumab on brain atrophy and disability progression in multiple sclerosis patients over 5 years. Eur J Neurol. 2016;23(6):1101–1109. [DOI] [PubMed] [Google Scholar]
- 11. Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lublin FD, Coetzee T, Cohen JA, Marrie RA, Thompson AJ.. The 2013 clinical course descriptors for multiple sclerosis: A clarification. Neurology. 2020;94(24):1088–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lorscheider J, Buzzard K, Jokubaitis V, et al. Defining secondary progressive multiple sclerosis. Brain. 2016;139(Pt 9):2395–2405. [DOI] [PubMed] [Google Scholar]
- 14. Scalfari A, Neuhaus A, Daumer M, Ebers GC, Muraro PA.. Age and disability accumulation in multiple sclerosis. Neurology. 2011;77(13):1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Confavreux C, Vukusic S, Adeleine P.. Early clinical predictors and progression of irreversible disability in multiple sclerosis: An amnesic process. Brain. 2003;126(Pt 4):770–782. [DOI] [PubMed] [Google Scholar]
- 16. Confavreux C, Vukusic S, Moreau T, Adeleine P.. Relapses and progression of disability in multiple sclerosis. N Engl J Med. 2000;343(20):1430–1438. [DOI] [PubMed] [Google Scholar]
- 17. Tremlett H, Yousefi M, Devonshire V, Rieckmann P, Zhao Y.. Impact of multiple sclerosis relapses on progression diminishes with time. Neurology. 2009;73(20):1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cree BAC, Hollenbach JA, Bove R, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lorscheider J, Benkert P, Schädelin S, et al. Disability progression unrelated to relapses in relapsing-remitting multiple sclerosis: Insights from the Swiss multiple sclerosis cohort study. https://onlinelibrary.ectrims-congress.eu/ectrims/2019/stockholm/279536/johannes.lorscheider.disability.progression.unrelated.to.relapses.in.html?f=listing%3D3%2Abrowseby%3D8%2Asortby%3D1%2Amedia%3D1. Accessed 20 April 2021.
- 20. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kappos L, Butzkueven H, Wiendl H, et al. Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult Scler. 2018;24(7):963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Butzkueven H, Kappos L, Wiendl H, et al. Long-term safety and effectiveness of natalizumab treatment in clinical practice: 10 years of real-world data from the Tysabri Observational Program (TOP). J Neurol Neurosurg Psychiatry. 2020;91(6):660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tremlett H, Yinshan Z, Devonshire V.. Natural history of secondary-progressive multiple sclerosis. Mult Scler. 2008;14(3):314–324. [DOI] [PubMed] [Google Scholar]
- 25. Confavreux C, Vukusic S.. Natural history of multiple sclerosis: A unifying concept. Brain. 2006;129(Pt 3):606–616. [DOI] [PubMed] [Google Scholar]
- 26. Ford C, Goodman AD, Johnson K, et al. Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: Results from the 15-year analysis of the US prospective open-label study of glatiramer acetate. Mult Scler. 2010;16(3):342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kappos L, Edan G, Freedman MS, et al. The 11-year long-term follow-up study from the randomized BENEFIT CIS trial. Neurology. 2016;87(10):978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cree BAC, Gourraud P-A, Oksenberg JR, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016;80(4):499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fambiatos A, Jokubaitis V, Horakova D, et al. Risk of secondary progressive multiple sclerosis: A longitudinal study. Mult Scler. 2019;26(1):79–90. [DOI] [PubMed] [Google Scholar]
- 30. Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263–1273. [DOI] [PubMed] [Google Scholar]
- 31. Fisniku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: A 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008;131(Pt 3):808–817. [DOI] [PubMed] [Google Scholar]
- 32. Genovese AV, Hagemeier J, Bergsland N, et al. Atrophied brain T2 lesion volume at MRI is associated with disability progression and conversion to secondary progressive multiple sclerosis. Radiology. 2019;293(2):424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chung KK, Altmann D, Barkhof F, et al. A 30-year clinical and magnetic resonance imaging observational study of multiple sclerosis and clinically isolated syndromes. Ann Neurol. 2020;87(1):63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Philipp Albrecht, the corresponding author, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The datasets supporting the conclusions of this article are included within the article and its additional files. Raw data generated during and/or analysed during the current study are available from the corresponding author on reasonable request.