Abstract
Eggshell translucency is a ubiquitous external eggshell quality problem caused by variations of eggshell ultrastructure or shell membrane. In previous studies, researchers have widely investigated this phenomenon with nutritional, environmental, and genetic perspectives in many breeds. However, most studies referring to phenotypic measurement of shell translucency have been performed using a relatively subjective two-, three-, or four-grading methods, which made it impossible to compare distribution of shell translucency among different breeds. In this study, we aimed to explore variations of translucent eggshell spots in different breeds and their distribution in blunt, middle, and sharp ends of eggshell using a relatively objective grayscale recognition method. We selected 45 eggs from each flock of pure lines, commercial strains, and Chinese local breeds (10 flocks, aged 60 to 70 wk), and stored them in a constant environment for 5 d. Then measured eggshell translucency using grayscale recognition method. Indicators of shell translucency included sum of spot areas on the whole eggshell (SUSA), sum area of the whole eggshell (SUSHA), RSS (ratio of SUSA to SUSHA), quantity of spots (QS), average spot area in eggshell (AAES), and diameter of spots in eggshell (DS). As results, in Hy-Line Brown, Brown-Egg Dwarf Layer, and Taihang (pink-shelled) breeds, phenotypic intensity of eggshell translucency was slight; in Rhode Island Red, Jingfen-1, and Dongxiang breeds, phenotypic intensity of eggshell translucency was relatively extensive; and in Jinghong-1, Hy-Line Sonia, White Leghorn, and Taihang (blue-shelled), phenotypic intensity of eggshell translucency was at an intermediate level. In general, the larger the RSS, the larger the QS, AAES, and DS. Of 3 ends for most breeds, eggshell translucency of blunt and sharp ends was usually greater than that of middle ends, and blunt ends seemed to have the most extensive eggshell translucency. Findings from this study could provide a reference for population selection to locate genes regulating shell translucency and to explore the physical structure mechanism for eggshell translucency formation.
Key words: hen, breed, translucent egg, egg quality, distribution
INTRODUCTION
Translucent eggs refer to eggs with watermark-like spots on the eggshell, specifically, spots that appear translucent in contrast to other areas that appear opaque when light penetrates the eggshell (Holst et al., 1932). Eggshell translucency is the appearance of moisture accumulation penetrating from the egg contents (Solomon, 1991), which is caused by genetic or vegetative ultrastructural variations in the eggshell or shell membrane (Jiang, 2015; Wang et al., 2017; Gautron et al., 2021), and the process may be accelerated or delayed by environmental factors (Fu et al., 2019; Zhang et al., 2019). It has been reported that hens laying translucent eggs do so continually, and a phenotypic discrepancy was found among individual hens (Baker and Curtiss, 1957). In our previous research, the heritability for eggshell translucency was firstly estimated to be 0.22 in a 63-wk Brown-Egg Dwarf Layer flock, which suggested the importance of genetic factors in the formation of eggshell translucency (Wang, 2017). In terms of nutrition, scientists have tried to increase the expression level of calcium-binding protein-d28kDa (CaBP-D28K) and plasma membrane calcium pump (PMCA) (Jiang, 2015; Attia et al., 2020) or the amount of medium-chain fatty acids (MCFAs) (Zhang et al., 2020) in the blood to improve the quality of the eggshell or shell membrane by the use of calcium and cholecalciferol, Acrespora terreticus, or balanced oil rich MCFA in diets; however, the phenotype of shell translucency was only relieved to a certain extent.
Scientists have conducted research on eggshell translucency in breeds of White Leghorn, Hy-Line, No. 3 of Nongda, and many other local breeds of laying hens, but most studies have measured eggshell translucency using subjective two-, three-, or four-grading methods, with no uniform grading standard. This made it impossible to compare the distribution of shell translucency among different breeds. In a previous study, we evaluated the accuracy of a four-grading method and found a 50% chance of misclassification for eggshell translucency; we then explored the grayscale recognition method for measuring eggshell translucency in terms of quantity and area of translucent spots in the eggshell (Wang et al., 2019). The aims of the current study were to explore the variations in translucent eggshell spots in different breeds and their distribution in blunt, middle, and sharp ends of the eggshell using a relatively objective grayscale recognition method. In perspective of commercial egg production, the study may reduce eggshell translucency by providing suitable population; in term of genetic and breeding, the study would provide flocks of available genetic variation, which were critical for gene location of eggshell translucency formation and further elimination of the trait.
MATERIALS AND METHODS
Hen Selection
The experiment collected 10 flocks of hens aged 60 to 70 wk, including Rhode Island Red (64 wk), Brown-Egg Dwarf Layer (60 wk), Hy-Line Brown (60 wk), Jinghong-1 (64 wk), White Leghorn (60 wk), laying white eggs, Hy-Line Sonia (66 wk), Jingfen-1 (66 wk), 2 lines of Taihang (pink-shelled, 71 wk; blue-shelled, 64 wk), and Dongxiang (64 wk). The 10 flocks contained pure lines, commercial strains, and Chinese local breeds, and laid eggs of brown, pink, white, and blue shell colors. Among all the flocks, the White Leghorn, Rhode Island Red, and brown-egg dwarf layer are pure lines of worldwide standard breeds; Hy-Line Brown, Hy-Line Sonia, Jinghong-1, and Jingfen-1 are specialized commercial strains; and Dongxiang and Taihang (pink- and blue-shelled) are Chinese local breeds. Further, White Leghorn was provided by Dawu Poultry Breeding Co. Ltd. (Baoding, China), Rhode Island Red and brown-egg dwarf layer were provided by CAU Poultry Breeding Corp. (Beijing, China), Hy-Line Brown and Hy-Line Sonia were from the Juncan Farm Cooperative (Baoding, China), Jinghong-1 and Jingfen-1 were from the Qingyuan Shunda Layer Farm (Baoding, China), Taihang (pink- and blue-shelled) was from the Zanhuang Natural Agricultural Products Development Corp. (Baoding, China), and Dongxiang was from the Zhuozhou Agricultural Science and Technology Garden of Chinese Agricultural University (Baoding, China). All breeds were raised in a similar environment, that is, caged, in fully enclosed coops, fed specialized diets with free diet intake, free water intake, and a 16 h:8 h light/dark cycle.
We selected 45 qualified eggs, which had a normal appearance on the laying day for each breed, excluding the broken and deformed eggs, and then marked each egg on the middle of the eggshell with specific Arabic numerals. Images of the eggs are shown in Figure 1. First, we measured the basic external egg qualities such as egg weight, eggshell index (Attia et al., 1994), and shell color. Egg weight was measured using an electronic balance with an accuracy of 0.01 g, ranging from 0 to 6,200 g (ML-T, METTLER TOLEDO Corp., Zurich, Switzerland). The long and short diameters of the eggshells were measured using an egg quality analyzer (NFN384, FHK, Tokyo, Japan), and the ratio of long to short diameter was the egg index. The eggshell color of the blunt/middle/sharp ends was measured using a portable spectrophotometer (CM-2600d, Konica Minolta Inc., Tokyo, Japan), and the shell color of each egg was the average value of the 3 ends. After collection of the phenotypic data on the external egg quality, all eggs were stored in a constant environment, specifically, at a relative temperature of 20 to 25°C and humidity of 50 to 60% for 5 d, and then the eggshell translucency and internal eggshell quality were measured.
Figure 1.
The appearance of eggs of 10 hen breeds.
Measurement of Eggshell Translucency
The eggshell translucency was identified using the grayscale recognition method based on our previous study (Wang et al., 2019). First, a light-emitting diode (LED) cold source (HLK, Tongfa Corp., Dezhou, China) penetrating the egg was required to enhance the contrast between the translucent and opaque areas on the eggshell. Images of the blunt, middle, and sharp ends of each egg (Figure 1) were captured using a digital camera (ILCE-5000 L, Sony Corp., Thailand, Japan), of which, pictures of blunt end and sharp end were captured from position perpendicular to equatorial plane of eggshell, 15 cm away from 2 end points, and middle end refers to the entire area from equator to blunt and sharp points of eggshell (a random side of eggshell perpendicular to equatorial plane of eggshell). After all pictures were acquired, translucent spot indicators were analyzed, including the sum area of the whole eggshell (SUSHA), sum of spot areas on the whole eggshell (SUSA), ratio of SUSA to SUSHA (RSS), quantity of spots (QS), average spot area in the eggshell (AAES), and diameter of spots in the eggshell (DS) on 3 ends of the eggshell of the 10 flocks. Then, the basic internal eggshell quality was measured immediately in case of deterioration of the egg whites and yolks.
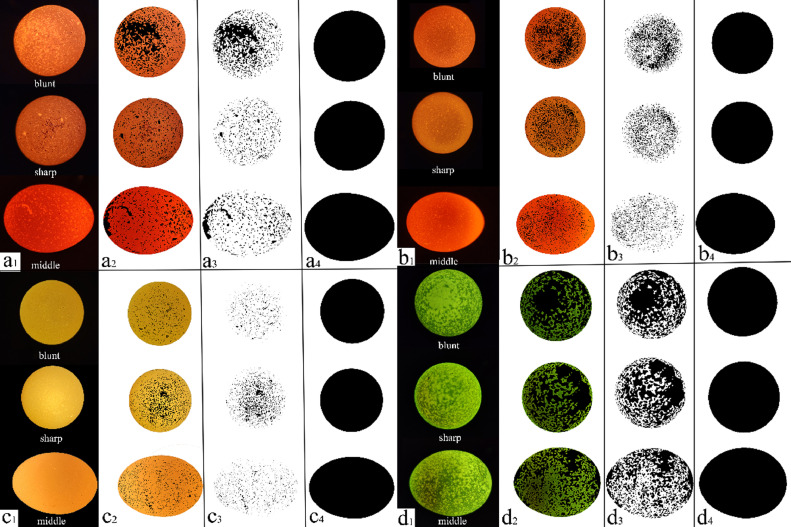
The basic procedure used for the measurement of the translucent spot indicators is as follows (Figure 2): 1) the pixels of the long and short diameters of the eggshell in the picture was measured using Microsoft Paint software (mspaint.exe, Microsoft Corp., Redmond, WA) to determine the scale of unit pixel and practical length of the shell diameter; 2) all translucent spots on the eggshell were filled in manually using Photoshop software (Photoshop CC 2018, Adobe Systems Corp., San Jose, CA) to enhance the contrast between the translucent spots and opaque areas; 3) the translucent spots and opaque areas were automatically spotted by setting up different color thresholds using Image-Pro Plus software (version 6.0, Media Cybernetics Corp., Silver Spring, MD); and 4) indicators of the translucent spots and eggshells, such as QS, SUSA, AAES, DS, and RSS, at the 3 ends of eggshell, using ImageJ software (version 1.41, National Institutes of Health, MD) were determined. The eggshell strength was measured using an eggshell force gauge (EFR 01, ORKA Corp., Israel); the eggshell thickness was measured using a micrometer (06030022, TESA Corp., Lausanne, Switzerland), with an accuracy of 0.1 μm; the albumen height, Haugh unit, and yolk color were automatically measured using an egg quality analyzer (EA 01, ORKA Corp., Israel), and Haugh unit was calculated based on the egg weight (W) and albumen height (H) using the formula: 100 × Log(H–1.7W0.37 + 7.57), (H, mm; W, ɡ) (Haugh, 1937). The yolk weight was measured using an electronic balance.
Figure 2.
Illustration of processing on translucent eggshell spots for 4 kinds of color and 3 ends of eggshell by Grayscale Recognition Method. a: brown shelled; b: pink shelled; c: white shelled; d: blue shelled; a1: eggshell profiles of blunt, sharp and middle ends by candling; a2: translucent spots were painted black manually by Photoshop CC 2018 software to enhance the contrast between translucent spots and opaque areas; a3: all spots on eggshell were extracted by Image-Pro Plus 6.0 software automatically; a4: profiles of the whole eggshells of blunt, sharp, and middle ends.
Statistical Analysis
All measurements of shell translucency and egg quality were quality-controlled to avoid the effects of outliers, and values outside the mean ± 3 standard deviations (SDs) were excluded as outliers. The RSS of the whole eggshell was weighted by the RSSs of the blunt, middle, and sharp ends using Equation 1:
| (1) |
where Sblunt, Smid, and Ssharp are the areas of the blunt end, middle end, and sharp end of the eggshell, respectively.
In addition, most RSS values of percentage data were below 30%, which might have caused the data to be non-normally distributed and affected the efficiency of hypothesis testing. The RSS values were adjusted using Equation 2:
| (2) |
where X is the initial value of the RSS, and Y represents the RSS values after formulation.
The inter- and intragroup variance of the shell translucency and egg quality indicators among the 10 flocks were analyzed using SPSS software (version 23.0, SPSS Inc., Chicago, IL) in the general linear model (GLM), and the model used is shown in Equation 3:
| (3) |
where is the measurement value of the different indicators, is the overall average, is the effect of groups, and is the residual error. Differences in the average values among groups for each indicator were compared by Duncan's multiple range test, and the significance level was set at 0.05.
Ethics Statement
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institutional Animal Care and Use Committee of Hebei Agricultural University, China.
RESULTS AND DISCUSSION
The external and internal egg quality indicators of the different breeds are shown in Table 1. In terms of the external egg quality, the egg weight (EW) of the local breeds, including Taihang (pink- and blue-shelled) and Dongxiang, were approximately 50 g, which were significantly lower than those of the pure lines and commercial strains (P < 0.05). The egg weight is positively correlated with the body weight; thus, the lower egg weight of the local breeds may have been due to the lower body weight (Liao et al., 2016; Yue et al., 2020). The differences in appearance of the eggshells are shown in Figure 1, and there were 4 eggshell colors, namely, brown, pink, white, and blue. In this study, shell color was evaluated using the LAB color model (León et al., 2006), wherein L represents the transition of shell color from dark to white (0–100), A represents the transition of shell color from green to gray to red (–128 to 127), and B represents the transition of shell color from blue to gray to yellow (–128 to 127). The white eggshell (White Leghorn) had the largest L value and moderate A and B values; the brown eggshell (Hy-Line Brown, Jinghong-1, Rhode Island Red, and Brown-Egg Dwarf) had the least L value and the largest A and B values, corresponding to dark red and yellow eggshells; the blue eggshells (Dongxiang and blue-shelled Taihang) had the least A value and moderate L and B values; the pink eggshell (Hy-Line Sonia, Jingfen-1, and Taihang) had moderate L, A, and B values.
Table 1.
Basic external and internal indictors of egg quality of 10 hen breeds.
| ESC (N = 174) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breed | Day | N | EW (g) | L | A | B | ESI | ESS (kg/cm2) | EST (µm) | HU | YR (%) | YC |
| Hy-Line Brown | 421 | 45 | 67.0 ± 3.57a | 56.4 ± 3.18a | 19.9 ± 1.45a | 30.2 ± 1.18a | 1.33 ± 0.05ab | 4.87 ± 1.13a | 364.7 ± 19.3a | 75.1 ± 7.17a | 27.7 ± 2.86abc | 8.77 ± 0.82ab |
| Jinghong-1 | 450 | 45 | 63.3 ± 5.65bc | 63.6 ± 3.19b | 15.6 ± 2.44b | 27.5 ± 2.36b | 1.33 ± 0.04ab | 4.13 ± 0.67bc | 352.7 ± 25.2ab | 79.2 ± 8.18b | 26.0 ± 6.05a | 9.70 ± 0.84cd |
| Rhode Island Red | 450 | 45 | 68.2 ± 4.27d | 64.6 ± 4.88b | 15.1 ± 2.73b | 28.4 ± 3.34b | 1.33 ± 0.05ab | 4.01 ± 0.95c | 347.7 ± 29.8bc | 77.0 ± 4.91ab | 27.4 ± 2.59ab | 8.66 ± 0.94ab |
| Brown-Egg Dwarf layer | 421 | 45 | 61.4 ± 5.72c | 71.5 ± 6.21c | 11.5 ± 2.78c | 23.2 ± 3.41c | 1.36 ± 0.06cd | 3.03 ± 0.73d | 300.3 ± 23.7de | 77.0 ± 6.68ab | 30.9 ± 4.00d | 8.90 ± 1.03ab |
| Hy-Line Sonia | 462 | 45 | 63.2 ± 5.26bc | 81.6 ± 7.46d | 5.40 ± 1.96d | 18.0 ± 3.37d | 1.31 ± 0.05ab | 4.32 ± 0.93bc | 339.0 ± 19.0bc | 82.7 ± 6.19c | 29.1 ± 3.56bcd | 10.2 ± 0.79d |
| Jingfen-1 | 460 | 45 | 62.6 ± 4.66b | 85.0 ± 3.42e | 3.58 ± 2.13e | 16.2 ± 3.68e | 1.33 ± 0.04bc | 3.88 ± 0.82ce | 340.7 ± 33.4bc | 76.0 ± 6.92ab | 28.2 ± 3.82abc | 6.97 ± 0.72e |
| Taihang (Pink shelled) | 497 | 45 | 50.2 ± 4.36e | 86.4 ± 4.34f | 3.39 ± 2.72e | 13.9 ± 4.84f | 1.34 ± 0.07bc | 4.61 ± 1.29ab | 318.0 ± 29.4ef | 67.6 ± 7.44d | 35.6 ± 5.73e | 12.7 ± 0.99f |
| White Leghorn | 421 | 45 | 62.2 ± 4.80bc | 94.2 ± 0.83g | −0.46 ± 0.13f | 1.90 ± 1.15g | 1.31 ± 0.05a | 3.28 ± 0.95d | 310.3 ± 26.8de | 73.8 ± 6.63a | 30.0 ± 3.82cd | 8.47 ± 1.90a |
| Dongxiang | 421 | 45 | 47.2 ± 4.20f | 79.7 ± 4.20h | −5.72 ± 1.65g | 13.5 ± 3.40f | 1.38 ± 0.06d | 4.04 ± 1.04c | 304.8 ± 32.4de | 76.9 ± 7.76ab | 36.7 ± 4.91e | 9.47 ± 0.68cg |
| Taihang (Blue shelled) | 450 | 45 | 49.3 ± 5.49e | 84.4 ± 4.30e | −5.17 ± 2.70g | 11.9 ± 4.12h | 1.36 ± 0.07cd | 4.67 ± 0.55ab | 332.3 ± 33.0cf | 73.6 ± 7.84a | 30.9 ± 5.19d | 9.17 ± 1.12bg |
Abbreviations: EST, eggshell thickness; EW, egg weight; ESC, eggshell color; ESI, eggshell index, ratio of long diameter of eggshell to short diameter; ESS, eggshell strength; HU, Haugh unit; YR, yolk weight/egg weight × 100%; YC, yolk color.
L represents black to white, + is white, - is dark. A represents red to green, + is red, - is green. B represents yellow to blue, + is yellow, - is blue. L, A, B are average values of blunt, middle and sharp ends of eggshell.
Among 10 groups mean without a common superscript differ (P < 0.05).
As reported, eggshell colors are determined by protoporphyrin IX (Samiullah et al., 2015) and biliverdin (Wang et al., 2013); however, the presence of white, pink, brown, and blue eggshells and color uniformity are affected by species and dosage of pigments. The values of L*A*B provide objective measurements for various breeds. The eggshell strength of White Leghorn and Brown-Egg Dwarf was significantly lower than that of commercial strains and local breeds (P < 0.05). Compared to commercial strains, this may be due to thicker eggshells resulting from heterosis of the commercial strain (Isa et al., 2020). Compared to local breeds, the similar eggshell thickness, lower egg weight, and high surface-area-to-volume ratio of local breeds may account for higher eggshell strength (Sirri et al., 2018). In terms of interior egg quality, the yolk ratios of local breeds were significantly higher than those of pure lines and commercial strains (P < 0.05); for yolk color and Haugh unit, there were no significant differences among most breeds. Given the ages of the hens, the environmental factors of the 10 flocks were similar, and food was provided following the nutritional requirements of the different breeds. Thus, the variations in the external and interior egg quality largely represent the diversity and specificity of pure lines, commercial strains, and local breeds.
The RSS, as one of the most important indicators of eggshell translucency, is shown in Table 2. The RSSs of the Rhode Island Red, Jingfen-1, and Dongxiang hens were all above 10%, corresponding to the fourth-grade (most severe) translucent egg according to the grading method (Wang et al., 2019), which were significantly higher than those of the other 7 breeds (P < 0.05); the RSSs of Hy-Line Brown, Brown-Egg Dwarf, and Taihang (pink-shelled) were approximately 3%, corresponding to the second grade and were significantly lower than those of the other 7 breeds (P < 0.05); the RSSs of Jinghong-1, Hy-Line Sonia, White Leghorn, and Taihang (Blue-shelled) were at an intermediate level, with values of approximately 6%, corresponding to the third grade. From the distribution of the RSSs in the different flocks, it appears that eggshell translucency widely exists in different eggshell colors, pure lines, commercial strains, and local breeds. Previous studies have reported eggshell translucency in White Leghorn (Baker and Curtiss, 1957; Fu et al., 2019), Brown-Egg Dwarf (Wang et al., 2017), Pink-Egg Dwarf (Nie, 2013), and Hy-Line Brown (Jiang, 2015) hens, which are consistent with the current study. In addition, in this study, the RSS of blue-shelled Dongxiang hens was significantly higher than that of pink-shelled Dongxiang hens, suggesting a linkage effect between functional genes of eggshell translucency and eggshell color. This result is consistent with that of a genome-wide association study (GWAS) study, wherein the single-nucleotide polymorphisms (SNPs) of eggshell translucency were in Chr 2 and located in the QTL of eggshell color (Wang, 2017).
Table 2.
Difference of RSSs among 10 breeds before and after data conversion.
| Breed | Eggshell color | N | RSS1 (%) | P1 | RSS2 (%) | P2 |
|---|---|---|---|---|---|---|
| Hy-Line Brown | Brown | 45 | 3.36 ± 1.67a | 0.01 | 17.9 ± 4.59a | 0.29 |
| Jinghong-1 | Brown | 45 | 6.56 ± 3.85b | 0.01 | 24.9 ± 7.73b | 0.52 |
| Rhode Island Red | Brown | 45 | 10.2 ± 7.96c | <0.01 | 29.7 ± 11.7c | 0.09 |
| Brown-Egg Dwarf layers | Brown | 45 | 3.32 ± 1.81a | 0.01 | 17.7 ± 5.04a | 0.38 |
| Hy-Line Sonia | Pink | 45 | 5.80 ± 2.55b | 0.09 | 23.8 ± 5.45b | 0.78 |
| Jingfen-1 | Pink | 45 | 12.5 ± 9.45d | <0.01 | 32.3 ± 13.1cd | 0.29 |
| Taihang (Pink shelled) | Pink | 45 | 3.03 ± 1.36a | 0.37 | 17.6 ± 4.08a | 0.88 |
| White Leghorn | White | 45 | 6.79 ± 4.23b | <0.01 | 25.3 ± 7.89b | 0.03 |
| Dongxiang | Blue | 45 | 13.2 ± 7.73d | <0.01 | 35.2 ± 10.4d | 0.30 |
| Taihang (Blue shelled) | Blue | 45 | 6.79 ± 3.38b | 0.22 | 25.5 ± 6.98b | 0.51 |
RSS, ratio of sum of spots areas on the whole eggshell to sum area of the whole eggshell.
RSS1 are initial value; RSS2 are conversed values and the formula: Y Arc sin ((Sqrt)X) Where X are values of RSS1, and Y are values of RSS2.; P1 and P2 are calculated by Shapiro-Wilk test, where P>0.05 suggesting it conforms to the normally distributed and P < 0.05 is not.
Among 10 groups mean without a common superscript differ (P < 0.05).
Details on the indicators of eggshell translucency, namely, SUSHA, SUSA, QS, AAES, and DS, are displayed in Table 3. For breeds with higher RSSs (>10%), the values of SUSA, QS, AAES, and DS tended to be higher than those with lower RSSs (3%). For flocks of intermediate RSSs, the QS was similar to those with higher RSSs, and the AAES and DS were similar to those with lower RSSs. For the SUSA and AAES in the different breeds with high RSSs, the coefficients of variation (ratio of standard deviation to mean) were relatively large and exceeded 0.5, which may have been mainly caused by flake connections of translucent spots on the eggshells. In previous studies, scientists have reported eggshell translucency in White Leghorn, Hy-Line Brown, and many other breeds (Baker and Curtiss, 1957; Fu et al., 2019; Jiang, 2015); however, most researchers have measured shell translucency using the grading method according to parts of eggshell, which made it difficult to compare the severity of eggshell translucency in different breeds. The current study is the first to objectively compare shell translucency of the whole egg (blunt, middle, and sharp ends) among multiple varieties of hen breeds using the grayscale recognition method.
Table 3.
Quantitative indicators of translucent spots on eggshell of 10 hen breeds.
| Breed | N | SUSHA (cm2) | SUSA (cm2) | QS | AAES (× 10−3 cm2) | DS (× 10−2 cm) |
|---|---|---|---|---|---|---|
| Hy-Line Brown | 45 | 52.55 ± 1.76a | 1.78 ± 0.91a | 1085.4 ± 346.7a | 1.55 ± 0.67abc | 4.38 ± 0.82ab |
| Jinghong-1 | 45 | 51.46 ± 3.38ab | 3.02 ± 1.65b | 2140.6 ± 843.5bc | 1.41 ± 0.75abc | 4.15 ± 0.84ab |
| Rhode Island Red | 45 | 56.27 ± 2.15c | 5.43 ± 4.69c | 2193.4 ± 640.4bc | 2.34 ± 2.44c | 5.62 ± 2.96c |
| Brown-Egg Dwarf Layer | 45 | 49.51 ± 3.69d | 1.66 ± 0.85a | 1418.0 ± 610.3a | 1.21 ± 0.57ad | 3.83 ± 0.96ad |
| Hy-Line Sonia | 45 | 49.27 ± 4.73d | 3.06 ± 1.48b | 2869.2 ± 2290.4d | 1.38 ± 0.09abc | 4.06 ± 1.04ab |
| Jingfen-1 | 45 | 49.61 ± 2.42de | 4.93 ± 3.45c | 3077.6 ± 1649.1d | 1.87 ± 1.48bc | 4.66 ± 1.41b |
| Taihang (Pink shelled) | 45 | 42.90 ± 2.01f | 1.29 ± 0.55a | 1349.3 ± 439.0a | 0.89 ± 0.42d | 3.41 ± 0.60d |
| White Leghorn | 45 | 50.85 ± 2.22be | 3.25 ± 2.04b | 2652.5 ± 1417.0cd | 1.56 ± 1.28bc | 4.36 ± 2.37ab |
| Dongxiang | 45 | 40.55 ± 2.00g | 5.48 ± 3.26c | 1567.4 ± 472.6ae | 2.64 ± 1.73e | 5.46 ± 2.10c |
| Taihang (Blue shell) | 45 | 41.78 ± 3.19fg | 2.88 ± 1.27b | 2028.1 ± 709.4be | 1.42 ± 0.67abd | 4.08 ± 0.80ad |
Abbreviations: AAES, average spots area in eggshell; DS, diameters of spots on eggshell; SUSHA, sum area of the whole eggshell (blunt + middle + sharp); SUSA, sum of spots areas on the whole eggshell; QS, quantity of spots on eggshell.
Among 10 groups mean without a common superscript differ (P < 0.05).
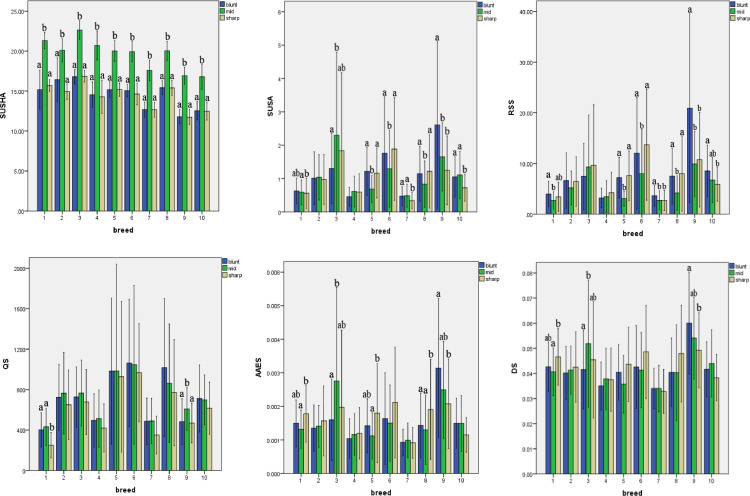
Further, Figure 3 illustrated the distribution of translucent spots on the blunt, middle, and sharp ends of the eggshell. For all breeds, the SUSHA of middle end was significantly larger than those of the other 2 ends (P < 0.05), and given that pictures of the blunt and sharp ends were captured from the 2 sides perpendicular to the long diameter of each egg, the 2 ends shared same SUSHA. For SUSA, the sharp ends of the Hy-Line Brown, Rhode Island Red, Taihang (pink- and blue-shelled), and Dongxiang hens were almost the smallest of the 3 ends; the middle ends of the Hy-Line Sonia, Jingfen-1, and White Leghorn hens were the smallest of the 3 ends (P < 0.05); and the blunt ends of most breeds displayed the largest SUSAs among the 3 ends. As RSS is determined by both SUSHA and SUSA, the middle ends of most breeds showed the smallest RSS, and the blunt ends of most breeds showed the largest RSS among the 3 ends. Given that the trait of shell translucency is a phenomenon of penetration and accumulation of moisture in the eggshell (Solomon, 1991; Wang, 2017), the special structure of air chamber in the blunt end may play an important role in the formation of translucent spots (Mao et al., 2007). For AAES, the sharp end of Hy-Line Brown, Hy-Line Sonia, and White Leghorn hens were significantly larger than those of the middle ends (P < 0.05), and there were no significant differences in the sharp and blunt ends among most breeds. For QS and DS, no trend of obvious difference existed among the 3 ends of all breeds. Given that the pictures of the 3 ends showed deformities in comparison to the real three-dimensional eggshell, the SUSHA, SUSA, AAES, and DS values were smaller than that of the actual value; however, the RSS and QS should remain consistent with the actual value to a large extent (Wang et al., 2019). Overall, these findings suggest that the eggshell translucency of the blunt and sharp ends were greater than that of the middle end, and blunt ends seemed to have the most severe eggshell translucency.
Figure 3.
Difference of SUSHA, SUSA, RSS, QS, AAES, DS among 3 ends of 10 flocks a,bamong 3 ends mean without a common superscript differ (P < 0.05) From 1 to 10 of abscissas represent different breeds: 1: Hy-Line Brown, 2: Jinghong-1, 3: Rhode Island Red, 3: Brown-Egg Dwarf Layer, 5: Hy-Line Sonia, 6: Jingfen-1, 7: Taihang (Pink-shelled); 8: White Leghorn, 9: Dongxiang, 10: Taihang (Blue-shelled). Abbreviations: AAES (cm2), average spots area in eggshell; DS (cm), diameters of spots on eggshell; QS, quantity of spots on eggshell; RSS(%), ratio of SUSA to SUSHA; SUSHA (cm2), sum area of the whole eggshell; SUSA (cm2), sum of spots areas on the whole eggshell.
Summary
This study is the first to investigate the distribution of eggshell translucent spots in different breeds using an objective method, the grayscale recognition method. The differences of internal and external egg quality imply that the 10 flocks largely represent different breeds in production of laying hens. In this study, it revealed that eggshell translucency is ubiquitous in pure lines, commercial strains, and local breeds of hens and that the phenotypic severity is specific to certain breeds. In Hy-Line Brown, Brown-Egg Dwarf Layer, and Taihang (pink-shelled), the phenotypic intensity of shell translucency was slight, and in Rhode Island Red, Jingfen-1, and Dongxiang, the phenotypic intensity was relatively extensive. In general, the RSS, as the key indicator for the measurement of shell translucency, the greater the shell translucency, the larger the RSS, and larger the QS, AAES, and DS. Furthermore, the shell translucency in the blunt and sharp ends was usually greater than that in the middle ends, and blunt ends seemed to have the most extensive shell translucency. This study may provide a reference for population selection to locate genes regulating eggshell translucency and to explore the physical structure mechanism for eggshell translucency formation.
Acknowledgments
ACKNOWLEDGMENTS
The work was supported by the National Natural Science Foundation of China (31902141), Key R&D Program Project of Hebei Province-Target Project of Modern Breeding Science and Technology (20326631D); Innovation and Entrepreneurship Training Program of College Student of Hebei Province (2020031).
DISCLOSURES
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
REFERENCES
- Attia Y.A., Al-Harthi M.A., Abo El-Maaty H.M. Calcium and cholecalciferol levels in late-phase laying hens: effects on productive traits, egg quality, blood biochemistry, and immune responses. Front. Vet. Sci. 2020;7:389. doi: 10.3389/fvets.2020.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia Y.A., Burke W.H., Yamani K.A. Response of broiler breeder hens to forced molting by hormonal and dietary manipulations. Poult. Sci. 1994;73:245–258. doi: 10.3382/ps.0730245. [DOI] [PubMed] [Google Scholar]
- Baker R., Curtiss R. Individual hen differences in egg shell mottling and the relationship of shell mottling to clutch size, internal quality and weight loss. Poult. Sci. 1957;36:904–908. [Google Scholar]
- Fu L., Wu G.Q., Sun C.J., Zheng J.X., Li G.Q., Xu G.Y. Study on the cause of translucent eggshells. Chin. Poult. Sci. 2019;41:6–11. (In Chinese) [Google Scholar]
- Gautron J., Stapane L., Roy N.Le, Nys Y., Rodriguez-Navarro A.B., Hincke M.T. Avian eggshell biomineralization: an update on its structure, mineralogy and protein tool kit. BMC Mol. Cell Biol. 2021;22:11. doi: 10.1186/s12860-021-00350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh R. The haugh unit for measuring egg quality. US Egg Poult. Mag. 1937;43:552–555. [Google Scholar]
- Holst W.F., Almquist H.J., Lorenz F.W. A study of shell texture of the hen's egg. Poult. Sci. 1932;11:144–149. [Google Scholar]
- Isa A.M., Sun Y., Shi L., Jiang L., Li Y., Fan J., Wang P., Ni A., Huang Z., Ma H., Li D., Chen J. Hybrids generated by crossing elite laying chickens exhibited heterosis for clutch and egg quality traits. Poult. Sci. 2020;99:6332–6340. doi: 10.1016/j.psj.2020.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M.J. Shandong Agric. Univ.; Taian, China: 2015. Effect of calcium metabolism on eggshell quality in caged laying hens. [Google Scholar]
- León K., Mery D., Pedreschi F., León J. Color measurement in L*a*b* units from RGB digital images. Food Res. Int. 2006;39:1084–1091. [Google Scholar]
- Liao R., Zhang X., Chen Q., Wang Z., Wang Q., Yang C., Pan Y. Genome-wide association study reveals novel variants for growth and egg traits in Dongxiang blue-shelled and White Leghorn chickens. Anim. Genet. 2016;47:588–596. doi: 10.1111/age.12456. [DOI] [PubMed] [Google Scholar]
- Mao K.M., Murakami A., Iwasawa A., Yoshizaki N. The asymmetry of avian egg-shape: an adaptation for reproduction on dry land. J. Anat. 2007;210:741–748. doi: 10.1111/j.1469-7580.2007.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie W. China Agric. Univ.; Beijing, China: 2013. Effects of dietary phosphorus levels on laying performance egg shell quality and Ca and P absorption in laying hen. [Google Scholar]
- Samiullah S., Roberts J.R., Chousalkar K. Eggshell color in brown-egg laying hens – a review. Poult. Sci. 2015;94:2566–2575. doi: 10.3382/ps/pev202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirri F., Zampiga M., Berardinelli A., Meluzzi A. Variability and interaction of some egg physical and eggshell quality attributes during the entire laying hen cycle. Poult. Sci. 2018;97:1818–1823. doi: 10.3382/ps/pex456. [DOI] [PubMed] [Google Scholar]
- Solomon S.E. Pages 111–121 in Egg and Eggshell Quality. Wolfe Publishing Limited; Aylesbury, UK: 1991. Translucency. [Google Scholar]
- Wang D.H. China Agric. Univ.; Beijing, China: 2017. Mechanism exploration for translucent egg formation. [Google Scholar]
- Wang D.H., Chen H., Zhou R.Y., Huang C.X., Gao H.X., Fan B.L., Liu G.J., Ning Z.H. Study of measurement methods on phenotype of translucent eggs. Poult. Sci. 2019;98:6677–6683. doi: 10.3382/ps/pez539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.H., Li Y.J., Liu L., Liu J.S., Bao M., Yang N., Zhuo C.H., Ning Z.H. Traits of eggshells and shell membranes of translucent eggs. Poult. Sci. 2017;96:351–358. doi: 10.3382/ps/pew328. [DOI] [PubMed] [Google Scholar]
- Wang Z., Qu L., Yao J., Yang X., Li G., Zhang Y., Li J., Wang X., Bai J., Xu G., Deng X., Yang N., Wu C. An EAV-HP insertion in 5′ Flanking region of SLCO1B3 causes blue eggshell in the chicken. PLoS Genetics. 2013;9 doi: 10.1371/journal.pgen.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q.X., Chen H., Xu Y.J., Huang C.X., Xi J.Z., Zhou R.Y., Xu L.J., Wang H., Chen Y. Effect of housing system and age on products and bone properties of Taihang chickens. Poult. Sci. 2020;99:1341–1348. doi: 10.1016/j.psj.2019.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.L., Cheng X., Gao Q., Fan C.D., Xia A.S., Zang D., Ning Z.H. Effect of dietary blend oil on production performance and egg quality of laying hens. Chin. Poult. Sci. 2020;42:36–39. (In Chinese) [Google Scholar]
- Zhang M.K., Yang H.X., Zou X.T., Yan Y., Wei X.F., Shao G.G., Xu Z.G. Study on the factors affecting translucent egg. Acta Agric. Shanghai. 2019;35:72–76. (In Chinese) [Google Scholar]