Abstract
In the recent years, it was recognized that type-2 inflammation links many forms of nasal polyposis with severe asthma. Thus, some biological drugs developed for severe asthma appeared to exert an effect on nasal polyposis. So far, there are several trials supporting this concept; therefore, some monoclonal antibodies for severe asthma were assessed also in polyposis, with promising results. Since different specialists are involved in the management of nasal polyposis (eg, pulmonologists, ENT, allergists), it was felt that an educational and informative document was needed to better identify the indications of biologicals in nasal polyposis. We collected the main Italian Scientific Societies, and prepared (under the Allergic Rhinitis and its Impact on Asthma, ARIA) a document endorsed by all Societies, to provide a provisional statement for the future use of monoclonal antibodies as a medical treatment for polyposis. It is the first nationwide endorsed document on this aspect. The current pathogenic knowledge and the experimental evidence are herein reviewed, and some suggestions for a correct prescription and follow-up are provided.
Keywords: Nasal polyps, CRSwNP, Biological antibodies, ARIA, Personalized medicine
Introduction
Chronic rhinosinusitis with nasal polyposis (CRSwNP) is a worldwide, highly prevalent disease which may have important health implications and high social costs.1 Despite the fact that CRSwNP usually is a relatively easy to diagnose disease, several aspects of the pathology remains poorly known, such as the etiology, association with several asthma phenotypes, and epidemiology. In addition, even today treatment remains a challenge for physicians: up to now, the available therapeutic options are essentially topical or oral systemic corticosteroids (OCS) and surgery.
Recently, with a more detailed knowledge of the pathogenic mechanisms responsible, for the disease, several biological agents (monoclonal antibodies, mAbs) have become available for the treatment of severe asthma. These mAbs, acting on the so-called “type 2” inflammation, common to most forms of CRSwNP as well, have been shown effective also in nasal polyposis (NP), and the research on this topic is currently particularly active.
Specific randomized clinical trials (RCTs), or real-life observations, are ongoing, or have been published, with the aim of assessing the effect of omalizumab, benralizumab, mepolizumab, reslizumab, and dupilumab in CRSwNP, with promising results.
Due to the overlapping clinical nature of CRSwNP, different specialists can be involved, variably, in the clinical management: allergists, pulmonologists, otolaryngologists, clinical immunologists and, in part, pediatricians. Therefore it is important to ensure that all of them have knowledge of the pathogenic mechanisms and the new therapeutic perspectives, promoting a common approach to the use and prescribing of mAbs therapy in NP.
In this context, the Italian panel of ARIA experts (Allergic Rhinitis and its Impact on Asthma) invited the different Scientific Societies, involved in the field, to participate in the preparation of an informative and educational document on the use of MAbs in CRSwNP. Each Society participated with its own experts in developing t his Consensus Statement, and approved it in its final version.
Framework and extent of the problem
Chronic rhinosinusitis is an inflammatory disease of the nose and paranasal sinuses, which can present with (CRSwNP) or without NP (CRSsNP). NP is a part of the CRSwNP framework, as indicated by the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) position paper;, hence, the terms NP or CRSwNP can be considered substantially equivalent.2 However, when referring to NP, it is important to keep in mind that it is part of a wider spectrum of pathologies, where inflammation is frequently the pivotal part of the problem.
Data from epidemiological studies, in general, show that the prevalence of CRS varies from 2% to 14%, depending on the geographical area.3, 4, 5, 6, 7 Concerning CRSwNP, the estimated prevalence is around 1–5%, again with a variability based on geographical area.8 RCTs and Real Life data, suggest that asthma is present in 30–60% of individuals with NP, while NP is present in up to 70% of patients with severe asthma.9, 10, 11 The overlap and influence of various factors and comorbidities, including asthma, aspirin sensitivity, atopic dermatitis, allergy, and cigarette smoking, make a precise classification of NP difficult. In addition, also other diseases, such as fungal rhinosinusitis, some forms of vasculitis, and cystic fibrosis can be associated with NP.
The role of allergy in NP has been the subject of a long-standing debate. Up until a century ago, allergy (allergic rhinitis, in particular) was thought to be a direct cause of NP, but more recent studies have shown that NP is present in the same proportion of allergic individuals (approximately 2–4%) as it is in the general population.12,13 In contrast, there is a high prevalence of atopy in individuals with NP, but there is not enough evidence to conclude that it plays a causal role in the pathogenesis.14 At present, the only “strong” correlations observed are those between rhinitis and asthma and between severe asthma and NP, but not between NP and rhinitis.
NP reduces the quality of life (QoL) of those affected, and the symptom of “nasal obstruction” is certainly the one most responsible for the discomfort felt in everyday life, associated with hyposmia/anosmia and sleep disturbances. We should also not forget the costs related to the need for repeated surgery (although surgery has also demonstrated, in some cases, good cost-effectiveness), and those due to the side effects of oral corticosteroids (OCS), often taken for long periods: osteoporosis, diabetes, cataracts, obesity, hypertension, glaucoma (and delayed growth in childhood).
Clinical aspects and current therapy
The main symptoms/signs variably associated with NP, are: nasal obstruction, anterior/posterior nasal discharge (purulent), facial pain (frontal, maxillary), hypo/anosmia and sleep disturbances. Dysgeusia, a sense of ear muffling and coughing can also be present. As mentioned above asthma, often severe, is present in a significant proportion of patients, and hypersensitivity to aspirin or NSAIDs is present in about one third of them.1,15 The characteristic clinical presentation and evolution of the disease is that of a progressive worsening over time. Hyposmia/anosmia often occurs early and could be a sign of massive polyposis or of high inflammation. By definition, 2 or more symptoms, lasting at least 12 weeks, must be present: one of them must be nasal obstruction or rhinorrhea (anterior or posterior), added to which is facial pain or hyposmia.16 (Table 1).
Table 1.
Diagnostic criteria for CRSwNP
| Inflammation of the nose and paranasal sinuses, which is characterized by two or more symptoms. One between: |
|
| Other symptoms: |
| with or without facial pain with or without reduction or loss of sense of smell with endoscopic signs of NP or evidence of NP on CT scan |
In addition to the above-mentioned clinical aspects, objective findings are mandatory for the diagnosis. Nasal endoscopy and/or CT scan of the paranasal sinuses are required, while conventional X-Ray of the facial region is neither useful nor diagnostic.1 In this regard, there are now standard staging systems to quantify the severity of the disease. Those most commonly used are the Nasal Polyp Score (NPS) (endoscopic) and the Lund-McKay (CT-scan) scale (Table 2 A-B).17,18 In the latest clinical trials, exploring the efficacy of biologicals in CRSwNP, the disease was usually defined as NPS ≥5 (with a score of ≥2 for each nasal cavity).
Table 2.
A) Nasal polyp score (NPS). B) Lund-Mackay CT staging. Legend: 0 = no abnormality; 1 = partial opacification; 2 = total opacification; ∗ 0 = not occluded, 2 = occluded; max. score = 12 per side
| A) Nasal polyp score (NPS) |
| Endoscopic findings (score each side separately) |
| 0 = no polyps |
| 1 = small polyps in the middle meatus/edema |
| 2 = middle meatus blocked |
| 3 = polyps extending beyond the middle meatus, without a complete obstruction, or extending to the sphenoethmoidal recess |
| 4 = massive nasal polyposis |
| B) Lund-Mackay CT staging |
| Paranasal sinuses (score each side separately) |
| Maxillary (0,1,2) |
| Anterior ethmoid (0,1,2) |
| Posterior ethmoid (0,1,2) |
| Sphenoid (0,1,2) |
| Frontal (0,1,2) |
| Osteomeatal complex (0,2)∗ |
The parameter defined and validated in the literature and most frequently used to determine the impact of symptoms on patients’ QoL and establish whether the disease is uncontrolled is the 22-item Sino-Nasal Outcome Test (SNOT-22). This questionnaire is self-completed by the patient. The maximum score is 110 (greatest disease impact) and the minimum clinically important difference is 8.9 points.19 A score >50 usually indicates severe NP.
Other methods of evaluation can be used in association with the main criteria to quantify the severity of polyposis and its perceived impact:
-
-
VAS: visual analog scale; a continuous graphic scale of severity from 0 to 10.
-
-
UPSIT/Sniffing test: evaluation of the sense of smell by the recognition or not of standard aromas.
-
-
PNIF: peak nasal inspiratory flow.
The current usual treatment of NP is based on nasal irrigation with saline, nasal steroids, systemic steroids, and endoscopic surgery (ESS).1 In principle, nasal steroids are used to slow down the growth of the polyps, delay surgery, or prevent relapse after surgery.20 Oral corticosteroids (OCS), in treatment cycles or as continuous treatment, are used in the event of massive, relapsing, or disabling polyposis. OCS usually are effective at treating symptoms and hyposmia but, due to their known side effects, a continuous use is not recommended.21 Despite the well-known long term side effects of this kind of therapeutic approach, repeated use of OCS in clinical practice is very common, used far more frequently and for much longer periods than recommended by the EPOS 2012 guidelines (≤2 weeks).7
Nevertheless, endoscopic surgery (ESS) remains the standard treatment to improve patency of the paranasal sinus ostia, followed by treatment with a nasal steroid. The aim of surgical approach is limited to unblocking the nasal cavities and widening the ostia of the paranasal sinuses, to best restore respiratory function and allow intranasal corticosteroids to reach the mucous membrane of the sinuses. The underlying problem is the high incidence of relapse after surgery, which in turn requires the use of systemic steroids.
Regarding children under 10 years of age, nasal polyps are rare, and their presence should prompt an assessment, in the first instance, for the presence of congenital diseases (ie, cystic fibrosis and ciliary dyskinesia). The cornerstone of CRS therapy in children is medical treatment with appropriate antibiotic therapy and treatment of comorbidities such as allergic rhinitis and asthma. Surgery is justified only in a small percentage of children. Antibiotic therapy is the same as that for acute rhinosinusitis, but for a longer duration, typically 3–4 weeks. The choice of antimicrobial should include active agents against staphylococcus.
Biologic drugs, at present, represent a possible beneficial integration or improvement of the standard therapy, obviously under certain conditions.
Pathophysiology and immunology
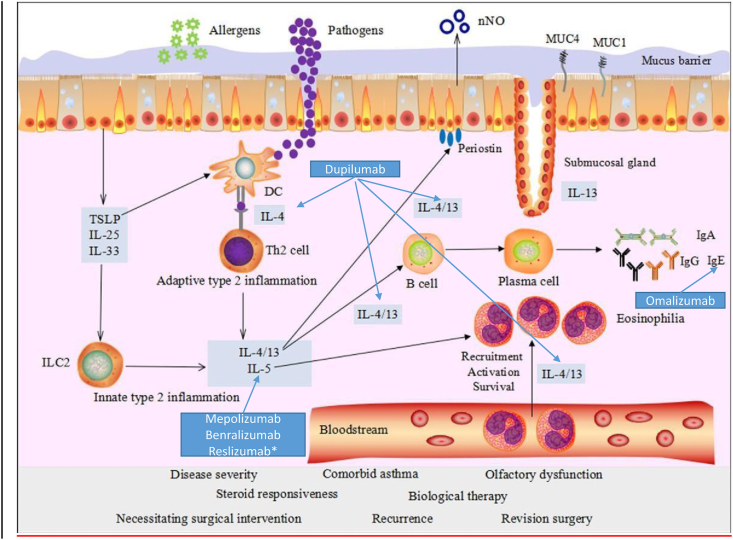
In the recent years, our understanding of the pathophysiological and immunological mechanisms underlying NP rapidly evolved, and “type 2” inflammation is now recognized as the common denominator of some forms of NP and asthma. Type 2 inflammation is driven by both parts of the immune system: the innate immune system (innate lymphoid cells 2, ILC2) and the acquired immune system (Th2 cells).
Studies carried out on severe asthma22, 23, 24 have identified specific characteristics linked to the T2-prevalent “component” of the disease: the involvement of ILC2 and of cytokines (TSLP, IL-25, IL-23), as early effectors in response to the possible epithelial damage. The complex immunological machine is then activated, with T helper 2 lymphocytes and their typical cytokines (IL-4, IL-5, and IL-13). In particular:
-
-
Specific IgE: they are the trigger of the allergic reaction and activate mast cell degranulation following contact with the allergen;
-
-
IL-4 regulates the differentiation of naive T cells into Th2 cells;
-
-
IL-5 promotes the maturation, activation and survival of eosinophils;
-
-
IL-13 is involved in hyperplasia of goblet cells, in mucus production, and in the mucociliary differentiation of nasal epithelial cells;
-
-
IL-4 and IL-13 both play a role in the class switching of B cells to IgE production; they stimulate eosinophil trafficking to the tissues, induce synthesis of chemokines (eotaxine-3, TARC) and expression of adhesion molecules that promote migration of inflammatory cells to the site of inflammation.
In this context, allergy, superantigens, and aspirin intolerance mechanisms can be involved.25, 26, 27, 28 The described inflammation mechanisms, despite there is now a “grey zone”, are common inflammatory paths (namely, type 2) shared by some forms of asthma and nasal polyposis (Fig. 1). A distinctive feature is the presence and activation of eosinophils (which predominantly infiltrate the polyps in this type of inflammation). This characteristic is not observed, for instance, in antrochoanal polyps and polyps associated with cystic fibrosis or ciliary dyskinesia, where a TH1/TH17 polarization with neutrophilic inflammation tends to prevail.29 Type 2 inflammation, common to both asthma and NP, is the conceptual basis for the use of biologics in the treatment of NP.
Fig. 1.
Main mechanisms of type 2 inflammation (Modified from: Yao Y et al. Eur Arch Oto-Laringology 2017; 274: 3559)
Biologic drugs in nasal polyposis
As previously mentioned, asthma is a disease usually sustained by a type 2 inflammatory cascade, and it represents the study prototype in precision medicine for identifying and characterizing the best treatments. In this model, the cytokines IL-4, IL-5, and IL-13 are essentially involved, as well as immunoglobulin E (IgE). Because many forms of NP also share this same type of inflammation, the biologic drugs currently available for severe asthma (anti-IgE, anti-IL4Rα, anti-IL5, and anti-IL5Rα) are undergoing investigation also in NP, in RCTs and phase II and III clinical trials. Two drugs, dupilumab and omalizumab, have concluded phase III studies, and dupilumab is already authorized in NP by both European Medicines Agency (EMA) and U.S. Food and Drug Administration (FDA) and in many countries. In the case of NP, the experimental data are often based on case reports (off-label use), but there are also randomized double-blind placebo control (RDBPC) studies that are starting to provide indications for use in clinical practice (Table 3). Of note, there is no experience with biologic drugs currently available for the treatment of NP in children.
Table 3.
RCTs with MAbs in NP
| AUTHOR, Year (ref) | DRUG (status) | DOSE | DURATION | AGE, years | Active/Placebo | MAIN FINDINGS |
|---|---|---|---|---|---|---|
| Gevaert, 201330 | Omalizumab Phase III concluded |
150–375 mg/month (SC) | 16 weeks | 42–56 | 15/8 | Reduction in total NPS (−2.67), Reduction in Lund-Mackay CT score (−4.0) |
| Gevaert, 202032 | Omalizumab Phase III Concluded (POLYP 1 and 2) |
75–600 mg/every 2 or 4 weeks (SC) | 24 weeks | 18–75 | 72/66 62/65 |
Reduction of NPS (−1.08 and −0.90), SNOT-22 score (−24.7 and −21.6) |
| Gevaert, 201133 | Mepolizumab Phase II in progress |
750 mg i.v. 2 injections 28 days apart |
8 weeks | 35–50 | 20/10 | Reduction in total NPS (−1.30) |
| Bachert, 201634 | Mepolizumab | 750 mg i.v. every 4 weeks | 25 weeks | 18–70 | 53/54 | Reduction in proportion of patients requiring surgical re-intervention. Reduction in VAS, total NPS (>-1), SNOT-22 score (−13.2) |
| Gevaert, 200635 | Reslizumab No registration trials in progress |
1–3 mg/kg i.v. single dose | 12 weeks | 18–63 | 16/8 | Reduction in total NPS (−1) in only half of the patients and only for 4 weeks |
| Bachert 201636 | Dupilumab Approved EMA-FDA |
600 mg SC load + 300 mg/week | 16 weeks | 35–65 | 30/30 | Reduction in total NPS (−1.9), Reduction in Lund-Mackay CT score (−8.8) |
| Bachert 201937 | Dupilumab | 300 mg SC every 2 weeks | 24 weeks | 30–65 | 143/133 | Significant reduction in NPS (−2.06), Lund-Mackay CT (−7.44), SNOT-22 (−17.36) and VAS at 6 months |
| Bachert 201937 | Dupilumab | 300 mg SC every 2 weeks OR 300 mg SC every 2 weeks for 24 weeks, then every 4 weeks |
52 weeks | 30–65 | 150-153/153 | Significant reduction in NPS (−1.80), Lund-Mackay CT (−5.13), SNOT-22 (−20.96) and VAS at 6 months. Further improvement at 12 months. Reduction in number of surgery interventions, reduction in use of OCS |
Anti-IgE (Omalizumab)
A RDBPC study30 that assessed the efficacy of omalizumab in patients with NP, evaluating both the endoscopic and CT indices, observed a reduction in both scores only in the active group. No differences emerged (though the sample size was small) between allergic and non-allergic subjects. A recent systematic review and meta-analysis showed that omalizumab appeared to be more effective, in terms of reducing the nasal endoscopic score (and hence the size of the nasal polyps themselves), in patients with concomitant bronchial asthma.31 A phase III clinical study has now been concluded and the results show a significant improvement of endoscopic, clinical and patient-reported outcomes, in patients with CRSwNP with an inadequate response to intranasal corticosteroids treated with omalizumab.32
Anti-IL5 (Mepolizumab)
Mepolizumab, the first mAb approved in Italy for the treatment of severe asthma with hypereosinophilia, was tested also in NP, given the pathophysiological importance of eosinophilic inflammation in the development of NP itself. In a RDBPC study, Gevaert et al33 treated 30 patients with severe or relapsing polyposis (2 i.v. injections 28 days apart of 750 mg of mepolizumab or placebo). The NPS and paranasal CT scores were assessed up to the second month. Twelve patients on active treatment showed an improvement, while no patient in the placebo arm improved. A more recent study in over 100 patients with relapsing nasal polyposis showed that mepolizumab (750 mg q4week), in addition to a significant improve in clinical outcomes and quality of life, reduced or delayed the need for surgical therapy.34 A phase III clinical study is currently ongoing (NCT03085797), and the results are expected within a short time.
Anti-IL5 (Reslizumab)
Reslizumab is a humanized MAb that blocks circulating IL-5 (not commercially available in Italy). In a pilot study of 24 subjects treated with reslizumab, a significant reduction in NP became apparent after a single intravenous injection at 1 mg/kg,35 but in only in 50% of patients. A post-hoc analysis also documented that an elevated IL-5 level in nasal secretions was able to predict the response to treatment.
Anti-IL5 receptor (Benralizumab)
Benralizumab is a humanized MAb that, unlike previous humanized monoclonal antibodies, can block the IL-5 receptor. Binding to the cell surface of eosinophils and basophils, it not only inhibits the action of IL-5, but also causes an “antibody-dependent, cell-mediated cytotoxicity” effect with consequent apoptosis of eosinophils. There are currently no published RDBPC studies, but phase II-III trials are ongoing (OSTRO NCT03401229; ORCHID NCT04157335; NCT03450083).
Anti-IL4/IL13 (Dupilumab)
Dupilumab is a fully human monoclonal antibody that targets the α chain (IL-4Rα) common to the IL-4 (IL-4Rα/ɣc) and IL-13 (IL-4Rα/IL-13Rα) receptors. Through antagonism to the shared part of IL-4 and IL-13 receptors, it blocks the biological effects of both cytokines, which are involved in type 2 inflammation and therefore also in NP. The FDA approved the use of Dupilumab on June 26, 2019, and it was the first approved biological treatment for the treatment of patients with inadequately controlled CRSwNP. In Europe, the EMA granted approval to dupilumab on October 29, 2019 as an adjunctive therapy to intranasal corticosteroids for the treatment of adults with severe CRSwNP in whom therapy with systemic corticosteroids and/or surgery did not provide adequate disease control.
In a phase II RDBPC study, dupilumab was tested in 60 patients with CRSwNP refractory to intranasal corticosteroids.36 Patients were randomized to subcutaneous dupilumab or placebo once weekly for 16 weeks; 51 patients completed the study. The dupilumab group showed a significant reduction in polyp growth (primary end point) that was clinically evident from the fourth week of treatment. Two further multi-center randomized placebo phase III clinical trials, one lasting 24 weeks (SINUS-24, 276 patients), the other 52 weeks (SINUS-52, 448 patients), were recently concluded with very promising results for the use of dupilumab in NP.37 The 2 studies compared subcutaneous Dupilumab 300 mg vs. placebo, evaluating the change in NPS and the Lund-McKay score from baseline. The number of patients was 276 (143 active and 133 placebo) in the 24-week study and 448 (150/145 active with 2 different administration protocols; and 153 placebo).
In these studies dupilumab significantly improved the key outcomes of disease and reached all primary and secondary endpoints in both the 24-week and 52-week studies. At week 24, dupilumab-treated patients showed significantly greater improvements in all primary and secondary endpoints compared to placebo. The improvements for SINUS-24 and SINUS-52, respectively, were: a) 57% and 51% improvement in nasal congestion/obstruction severity vs 19% and 15% improvement with placebo; b) 33% and 27% reduction in NPS vs. 7% and 4% increase with placebo; c) 42% and 27% improvement in sinus opacification vs. 4% and 0% with placebo; d) 52% and 45% improvement in loss of smell vs 12% and 10% improvement for placebo. Finally, there was a significant reduction in the re-surgery rate and in the use of OCS. In the SINUS-24 study, discontinuation of dupilumab vs placebo treatment at week 24 resulted in a loss of efficacy in all the endpoints observed up to week 48.
Current situation and unmet needs
It is recognized that NP remained until now a very “sectorial” disease, characterized by the ultra-specialist (ENT) context and an essentially surgical approach. Nonetheless, the current knowledge, showing that the features of type 2 inflammation are common to both asthma and NP, opened up new perspectives for introducing biologics into the “medical” treatment of the disease,38, 39, 40 as underlined by the recent EUFOREA Consensus.41 The studies conducted so far (Table 3) are encouraging, but they also raise many questions, given the complete novelty of the approach: a) when to use them? b) in which patients? c) which biological should be used? d) for how long? e) when a patient can be considered as responder or not? The available data provide only rough indications,41 which require further refinement in the future. Hence, the present document should be understood simply as offering a suggestion based on experience, clinical observations, experimental evidence and considerations of socio-economic benefit.
Patients who are not candidates for biologic therapy are: patients with unilateral polyposis, antrochoanal polyps, allergic fungal rhinosinusitis, cystic fibrosis, ciliary dyskinesia, because in such conditions there is no evidence of a type 2 inflammation.
Obviously, the aforementioned biologic drugs available in Italy can be prescribed for severe asthma with the recommendation to always verify the coexistence of NP, in accordance with the guidelines,1 and to objectively evaluate the effects on NP (as well as on asthma) at regular intervals (which could be those programmed for reviewing the treatment plan). It would be appropriate to perform an endoscopic evaluation (and/or CT scan) using the appropriate scoring systems (Table 2, Table 3) and/or a subjective evaluation with SNOT-22.
If one or more of these drugs is approved in Italy also for the NP indication, independently of severe asthma, it should not be prescribed as first choice, since endoscopic surgery (ESS) is the choice of preference, in any case, to restore the patency of the sinus ostia, which is essential for the correct and physiological ventilation of the paranasal sinuses, one of the cornerstone of the management of rhinosinusitis. However, we know that relapses are numerous42 and related to the presence of type 2 inflammation, so a criterion for starting treatment with a biologic drug could be a relapse despite therapy with nasal steroids. As in severe asthma, OCS are also used in NP for relapses or exacerbations, often in an abusive manner or for prolonged periods that expose the patient to side effects,43 and this could be an additional reason to start treatment with biologics. A further reason could be a persistent negative impact on QoL, despite surgery and topical treatment. Obviously, features suggestive of a type 2 inflammation must be present: peripheral/local eosinophilia, allergic sensitization with high total IgE, high exhaled nitric oxide (in the case of asthma). Currently, we can say little or nothing about the duration of treatment or the existence of biomarkers capable of predicting the response, or about when eventually to stop the treatment, based on the subjective and objective response. It should be emphasized that in a severe form of NP refractory to standard care, treatment with biologics, where approved, could also be considered as a first-line therapeutic approach.
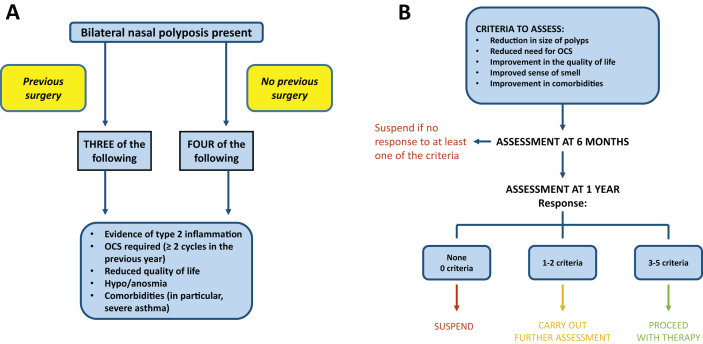
Based on the considerations and suggestions of the EUFOREA statement,41 this Consensus also proposes a scheme of indications (Fig. 2A-B), which should be considered only as a suggestion, and subject to modifications even in the short term.
Fig. 2.
A) Possible criteria for the use of biologic drugs in NP (from 41, modified); B) Assessment of the response to biologicals (from 41, modified)
Abbreviations
CRSwNP: Chronic rhinosinusitis with nasal polyposis; CRSsNP: Chronic rhinosinusitis sine nasal polyposis; CRS: chronic rhinosinusitis; DBRPCT: double blind randomized placebo controlled trial; ESS: endoscopic sinus surgery; MAb: monoclonal antibody; NP: nasal polyposis; NPS: nasal polyp score; OCS: oral corticosteroids; QoL: quality of life; SNOT: sino-nasal outcome test.
Funding
Not applicable.
Availability of data and materials
Not available
Author contributions
Diego Bagnasco and Giovanni Passalacqua drafted and wrote manuscript; all other authors reviewed and corrected the manuscript.
Consent for publication
All authors agreed to publication of the work in World Allergy Organization Journal.
Ethics approval
Not applicable.
Declaration of competing interest
There are no conflicts of interest reported for any of the authors.
Acknowledgements
InfoMed for publication charges.
Footnotes
Document approved by: AAIITO: Ass. Allergologi Immunologi Italiani Territoriali e Ospedalieri; AICNA: Accademia Italiana di Citologia Nasale; AIPO: Associazione Italiana Pneumologi Ospedalieri; IAR: Italian Academy of Rhinology; SIAAIC: Soc. Italiana di Allergologia Asma e Immunologia Clinica; SIAIP: Soc. Italiana di Allergologia e Immunologia Pediatrica; SIICA: Soc. Italiana di Immunologia Clinica e Allergologia; SIMRI: Soc. Italiana Malattie Respiratorie Infantili; SIO: Soc. Italiana di Otorinolaringoiatria; SIP: Soc. Italiana di Pediatria; SIP/IRS: Soc. Italiana di Pneumologia/Italian Respiratory Society
Note: The version from Italian to English was performed by Dr. Diego Bagnasco and Prof. Giovanni Passalacqua, and it was approved by all the participating Scientific Societies. The original document is available at the official Italian ARIA website (https://www.progettolibra.it/)
Full list of author information is available at the end of the article.
References
- 1.Hopkins C. Chronic rhinosinusitis with nasal polyps. NEJM. 2019;381(1):55–63. doi: 10.1056/NEJMcp1800215. [DOI] [PubMed] [Google Scholar]
- 2.Fokkens W.J., Lund V.J., Mullol J. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23:1–298. [PubMed] [Google Scholar]
- 3.Hulse K.E., Stevens W.W., Tan B.K., Schleimer R.P. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45:328–346. doi: 10.1111/cea.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hastan D., Fokkens W.J., Bachert C. Chronic rhinosinusitis in Europe–an underestimated disease. A GA2LEN study. Allergy. 2011;66:1216–1223. doi: 10.1111/j.1398-9995.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch A.G., Stewart W.F., Sundaresan A.S. Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy. 2017;72:274–281. doi: 10.1111/all.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hedman J. Int J Epidemiol. 1999;28:717–722. doi: 10.1093/ije/28.4.717. [DOI] [PubMed] [Google Scholar]
- 7.Khan A., Vandeplas G., Huynh T. The global allergy and asthma European network (GALEN rhinosinusitis cohort: a large European cross-sectional study of chronic rhinosinusitis patients with and without nasal polyps. Rhinology. 2019;57:32–42. doi: 10.4193/Rhin17.255. [DOI] [PubMed] [Google Scholar]
- 8.Langdon C., Mullol J. Nasal polyps in patients with asthma: prevalence, impact, and management challenges. J Asthma Allergy. 2016;9:45–53. doi: 10.2147/JAA.S86251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin D.C., Chandra R.K., Tan B.K. Association between severity of asthma and degree of chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25:205–208. doi: 10.2500/ajra.2011.25.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw D.E., Sousa A.R., Fowler S.J. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46:1308–1321. doi: 10.1183/13993003.00779-2015. [DOI] [PubMed] [Google Scholar]
- 11.Bagnasco D., Milanese M., Rolla G. Anti-IL-5 therapy in real life. The North-Western Italian experience and comparison with the regulatory trials. World Allergy Organ J. 2018;11(1):34. doi: 10.1186/s40413-018-0210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Settipane G.A., Chafee F.H. Nasal polyps in asthma and rhinitis. A review of 6,037 patients. J Allergy Clin Immunol. 1977;59:17–21. doi: 10.1016/0091-6749(77)90171-3. [DOI] [PubMed] [Google Scholar]
- 13.Hedman J., Kaprio J., Poussa T. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol. 1999;28:717–722. doi: 10.1093/ije/28.4.717. [DOI] [PubMed] [Google Scholar]
- 14.Munoz del Castillo F., Jurado-Ramos A., Fernandez-Conde B.L. Allergenic profile of nasal polyposis. J Investig Allergy Clin Immunol. 2009;19:110–116. [PubMed] [Google Scholar]
- 15.Orlandi R.R., Kingdom T.T., Hwang P.H. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–S209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 16.Fokkens W.J., Lund V.J., Mullol J. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;3:1–298. [PubMed] [Google Scholar]
- 17.Meltzer E.O., Hamilos D.L., Hadley J.A. Rhinosinusitis: developing guidance for clinical trials. Otolaryngol Head Neck Surg. 2006;135(5 Supp):S31–S80. doi: 10.1016/j.otohns.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Lund V.J., Mackay I.S. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–184. [PubMed] [Google Scholar]
- 19.Hopkins C., Rudmik L., Lund V.J. The predictive value of the preoperative Sinonasal outcome test-22 score in patients undergoing endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope. 2015;125:1779–1784. doi: 10.1002/lary.25318. [DOI] [PubMed] [Google Scholar]
- 20.Kowalski M.L. Oral and nasal steroids for nasal polyps. Curr Allergy Asthma Rep. 2011;11:187–188. doi: 10.1007/s11882-011-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heffler E., Bagnasco D., Canonica G.W. Strategies to reduce corticosteroid-related adverse events in asthma. Curr Opin Allergy Clin Immunol. 2019;19:61–67. doi: 10.1097/ACI.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 22.Kaur R., Chupp G. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J Allergy Clin Immunol. 2019;144:1–12. doi: 10.1016/j.jaci.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 23.Caminati M., Pham D.L., Bagnasco D., Canonica G.W. Type 2 immunity in asthma. World Allergy Organ J. 2018;11(1):13. doi: 10.1186/s40413-018-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papi A., Brightling C., Pedersen S.E., Reddel H.K. Asthma. Lancet. 2018;391:78380. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 25.Wei B., Liu F., Zhang J. Multivariate analysis of inflammatory endotypes in recurrent nasal polyposis in a Chinese population. Rhinology. 2018;56:216–226. doi: 10.4193/Rhin17.240. [DOI] [PubMed] [Google Scholar]
- 26.Schleimer R.P. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol. 2017;12:331–357. doi: 10.1146/annurev-pathol-052016-100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muluk N.B., Altın F., Cingi C. Role of superantigens in allergic inflammation: their relationship to allergic rhinitis, chronic rhinosinusitis, asthma, and atopic dermatitis. Am J Rhinol Allergy. 2018;32:502–517. doi: 10.1177/1945892418801083. [DOI] [PubMed] [Google Scholar]
- 28.Vickery T.W., Ramakrishnan V.R., Suh J.D. The role of Staphylococcus aureus in patients with chronic sinusitis and nasal polyposis. Curr Allergy Asthma Rep. 2019;19:21–24. doi: 10.1007/s11882-019-0853-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X., Zhang N., Bo M. Diversity of Th cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. JACI. 2016;138:1344–1353. doi: 10.1016/j.jaci.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 30.Gevaert P., Calus L., Van Zele T. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131:110. doi: 10.1016/j.jaci.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 31.Rivero A., Liang J. Anti-IgE and anti-IL5 biologic therapy in the treatment of nasal polyposis: a systematic review and meta-analysis. Ann Otol Rhinol Laryngol. 2017;126:739–747. doi: 10.1177/0003489417731782. [DOI] [PubMed] [Google Scholar]
- 32.Gevaert P., Omachi T.A., Corren J. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020 Sep;146(3):595–605. doi: 10.1016/j.jaci.2020.05.032. [DOI] [PubMed] [Google Scholar]
- 33.Gevaert P., Van Bruaene N., Cattaert T. A Mepolizumab, a humanized anti–IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128:989–995. doi: 10.1016/j.jaci.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 34.Bachert C., Sousa A.R., Lund V.J. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol. 2017;140:1024–1031. doi: 10.1016/j.jaci.2017.05.044. [DOI] [PubMed] [Google Scholar]
- 35.Gevaert P., Lang-Loidolt D., Lackner A. Nasal IL-5 levels determine the response to antiIL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006;118:1133–1141. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 36.Bachert C., Mannent L., Naclerio R.M. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. J Am Med Assoc. 2016;315:469–479. doi: 10.1001/jama.2015.19330. [DOI] [PubMed] [Google Scholar]
- 37.Bachert C., Han J.K., Desrosiers M. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019:31881. doi: 10.1016/S0140-6736(19)31881-1. [DOI] [PubMed] [Google Scholar]
- 38.Bachert C., Zhang N., Hellings P.W., Bousquet J. Endotype-driven care pathways in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2018;141:1543–1551. doi: 10.1016/j.jaci.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Bachert C., Zhang L., Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015;136:14311440. doi: 10.1016/j.jaci.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Malvezzi L., Ferrando M., Puggioni F., Heffler E., Passalacqua G., Canonica G.W. A Focus on chronic rhinosinusitis with nasal polyposis: leaving aside endoscopic surgery, a step towards biologic therapies. J Otolaryngol ENT Res. 2017;7(2) [Google Scholar]
- 41.Fokkens W.J., Lund V., Bachert C. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy. 2019 May 15;74(12):2312–2319. doi: 10.1111/all.13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Veen J., Seys S.F., Timmermans M. Real-life study showing uncontrolled rhinosinusitis after sinus surgery in a tertiary referral centre. Allergy. 2017;72:282–290. doi: 10.1111/all.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canonica G.W., Colombo G.L., Bruno G.M. SANI Network Shadow cost of oral corticosteroids-related adverse events: a pharmacoeconomic evaluation applied to real-life data from the Severe Asthma Network in Italy (SANI) registry. World Allergy Organ J. 2019 Jan 26;12(1):100007. doi: 10.1016/j.waojou.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not available